Pt1−xNix Alloy Nanoparticles Embedded in Self-Grown Carbon Nanofibers: Synthesis, Properties and Catalytic Activity in HER
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Pt1−xNix Alloys
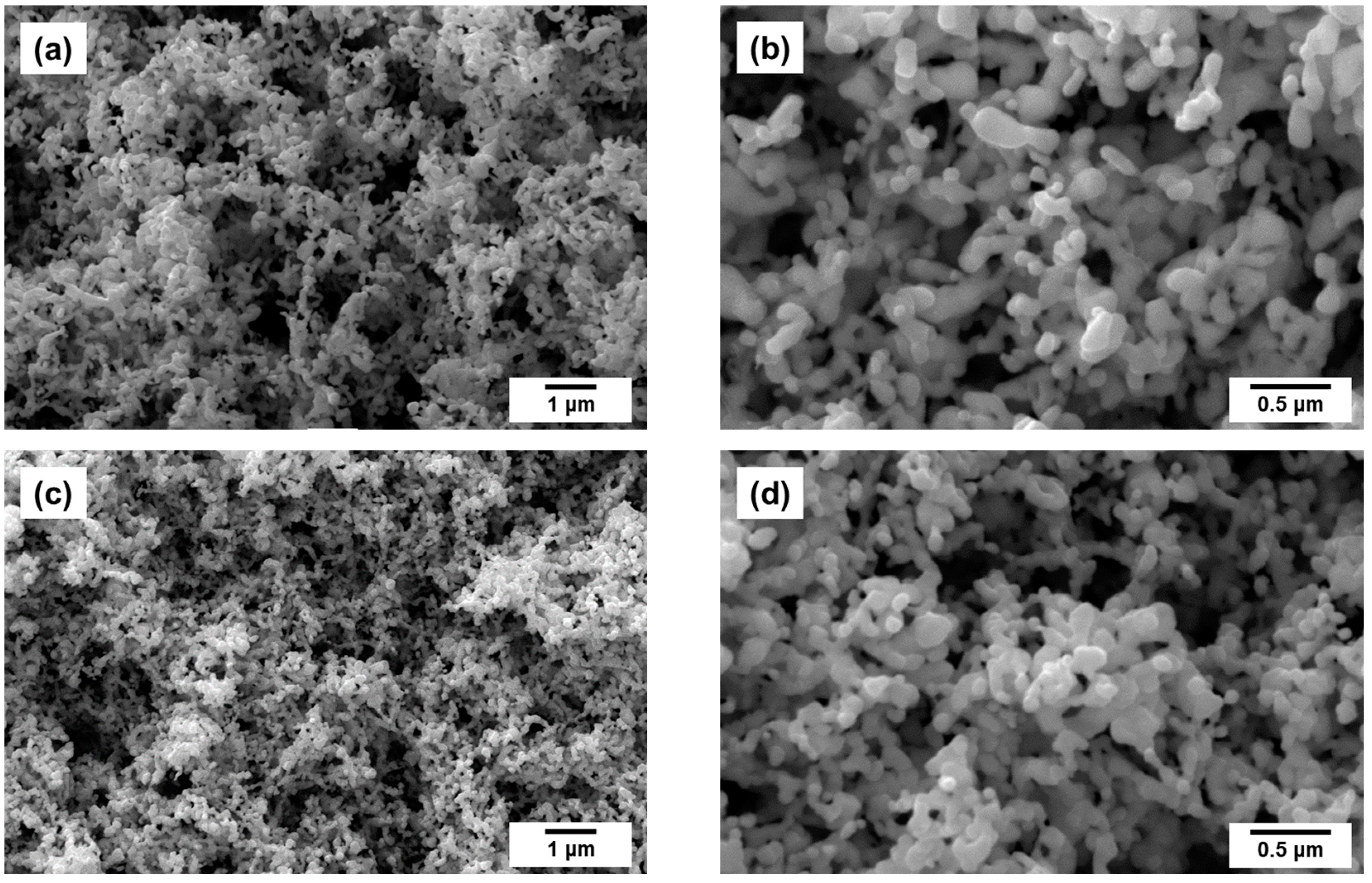
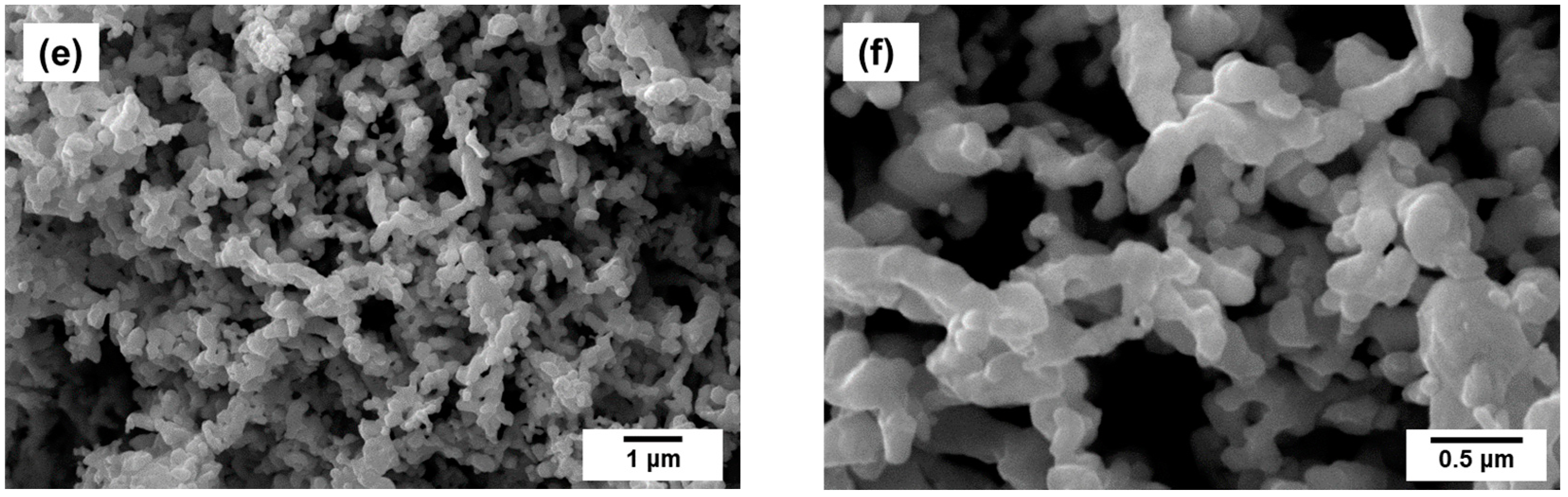
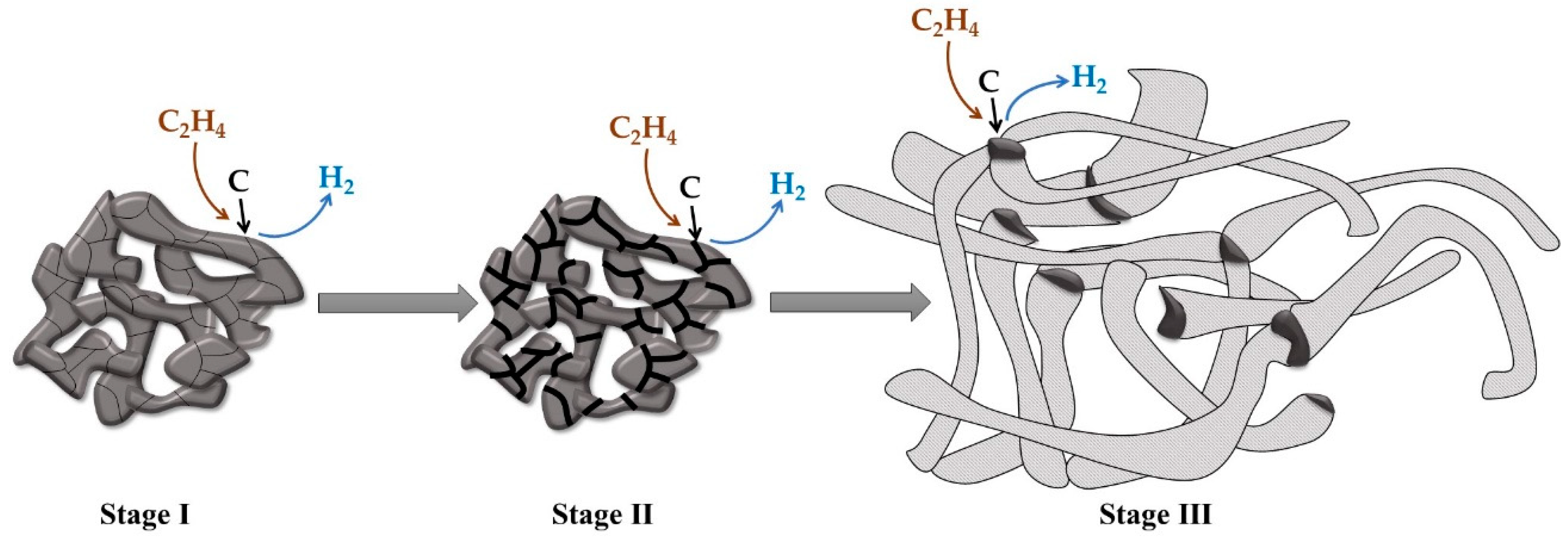
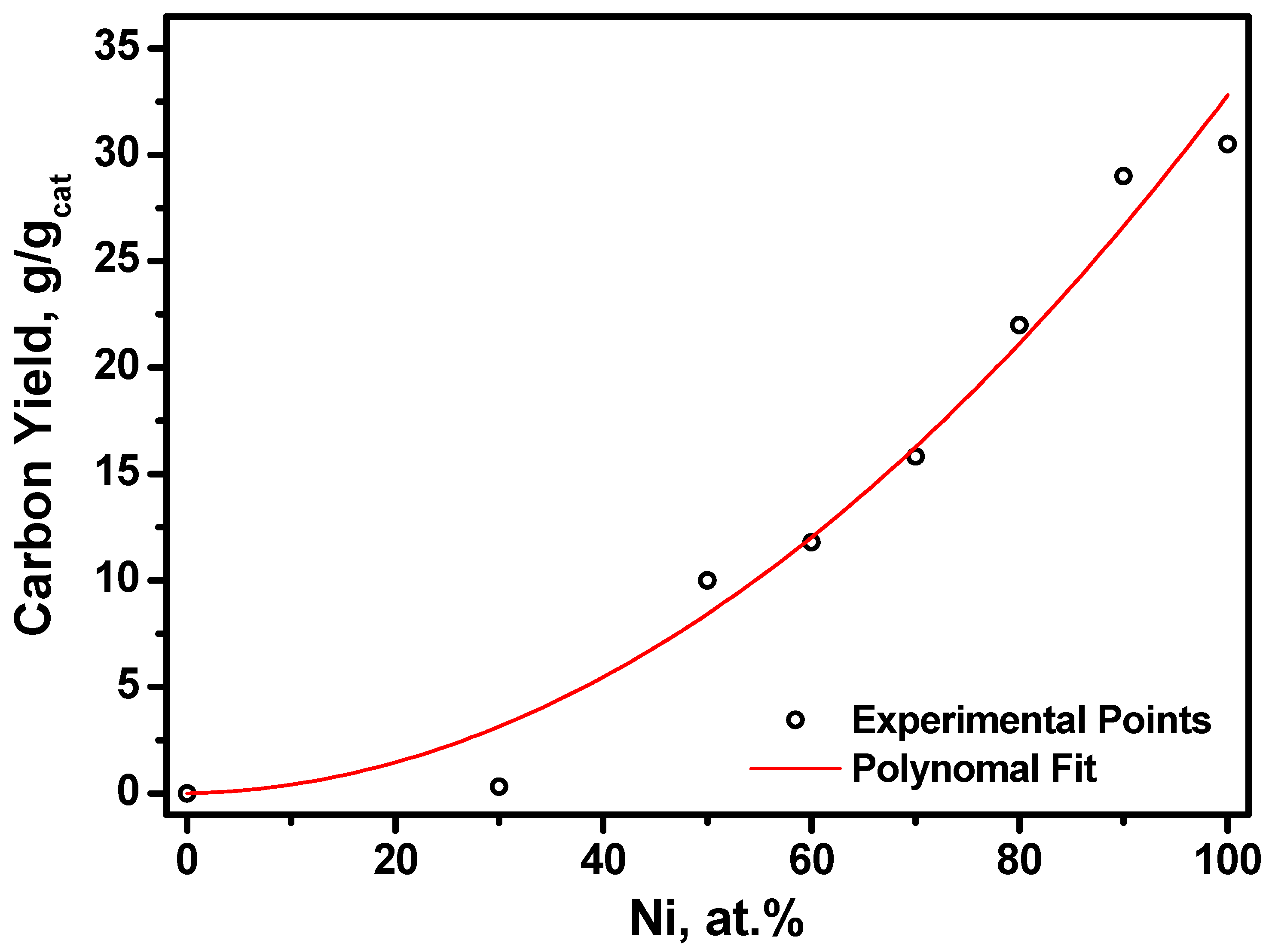
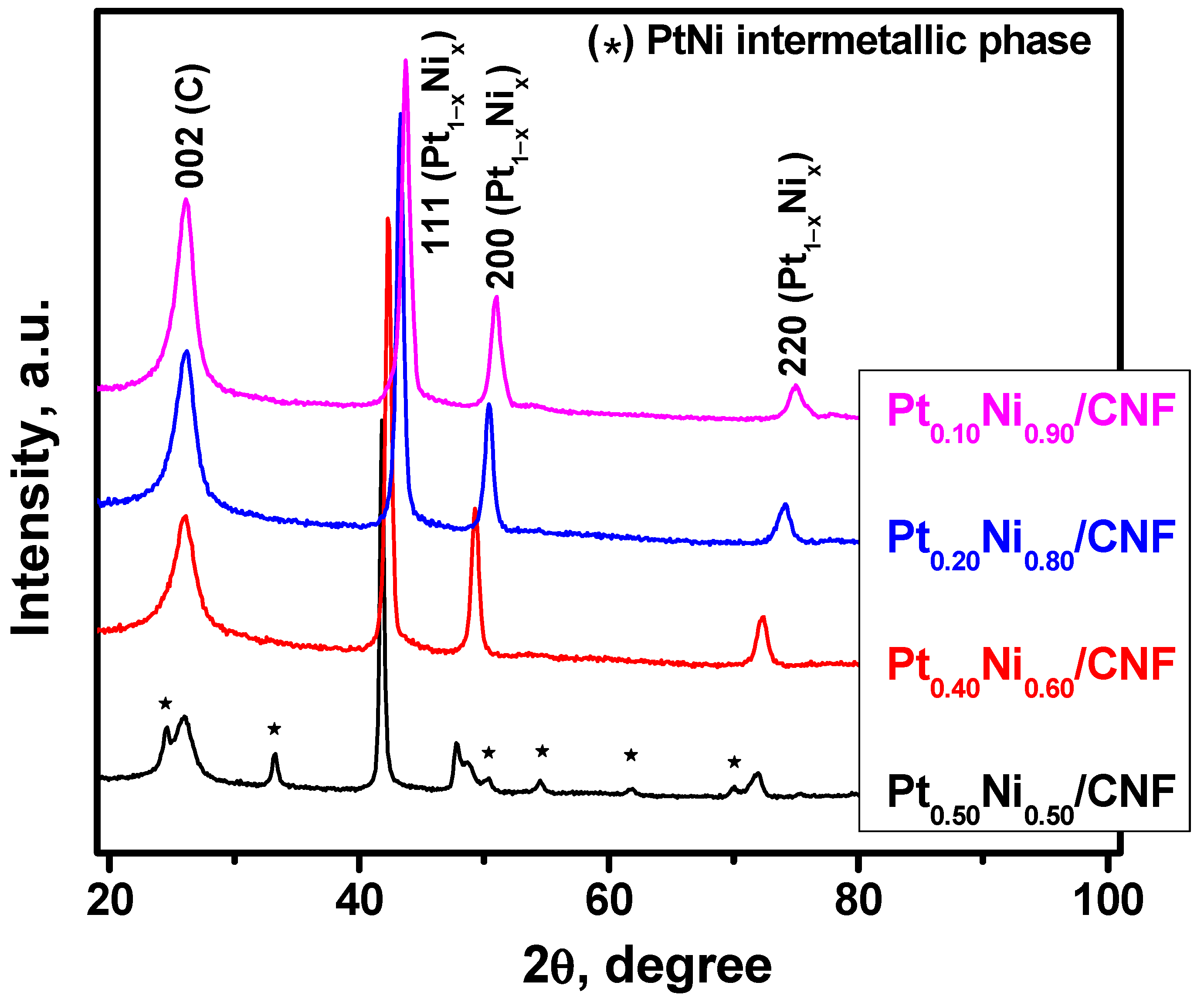
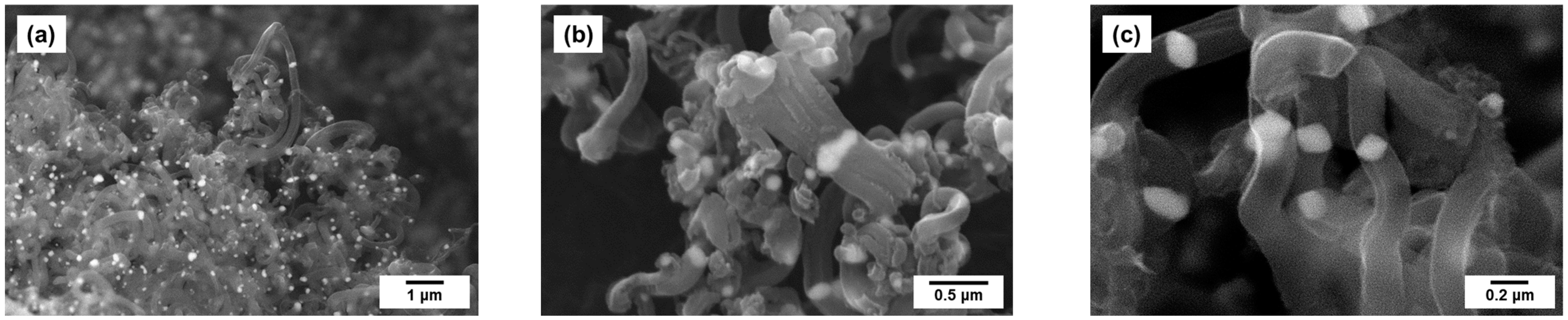
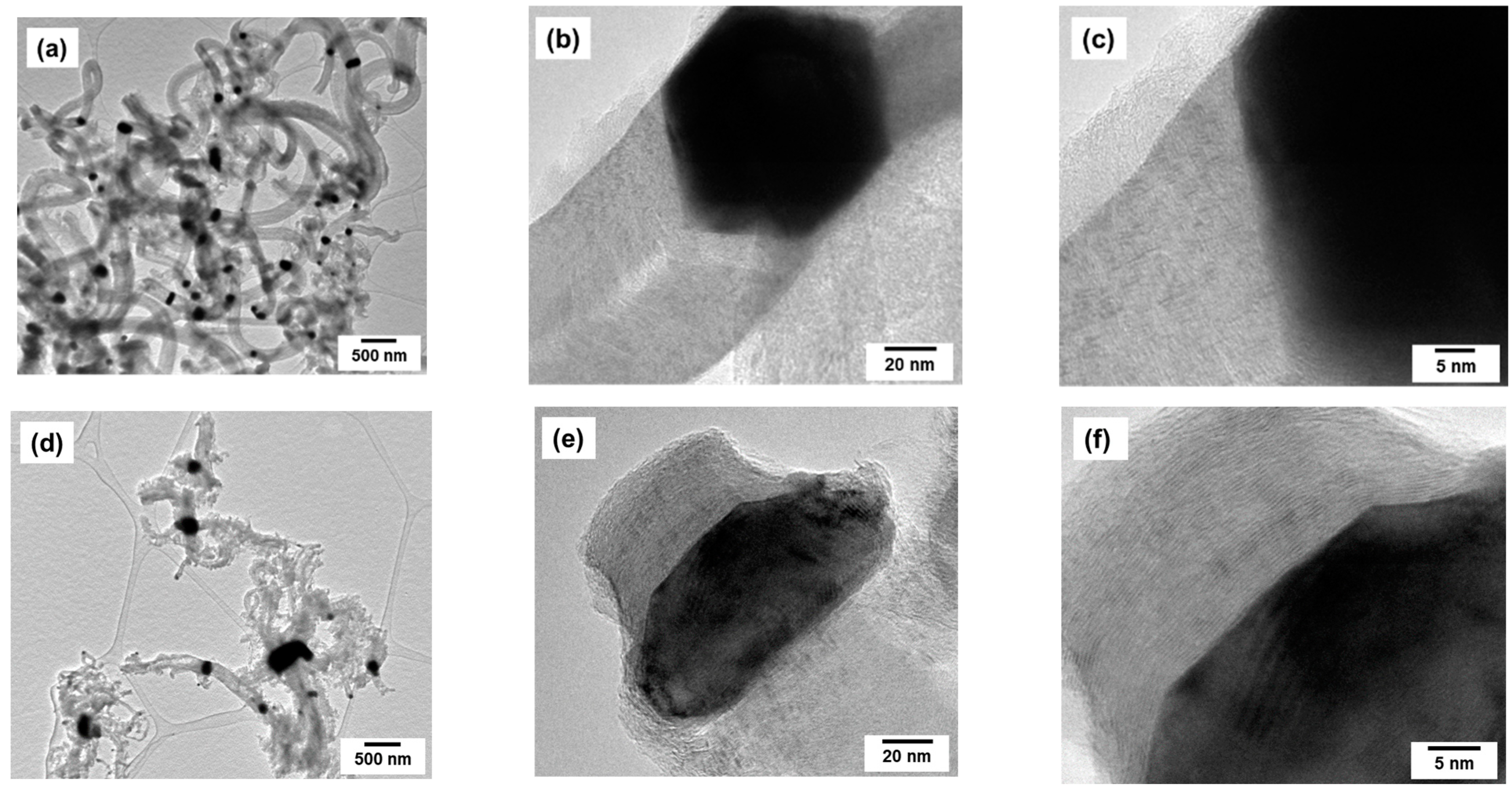
2.2. Preparation of Pt1−xNix/CNF Composites via Ethylene Decomposition over Pt1−xNix Alloys
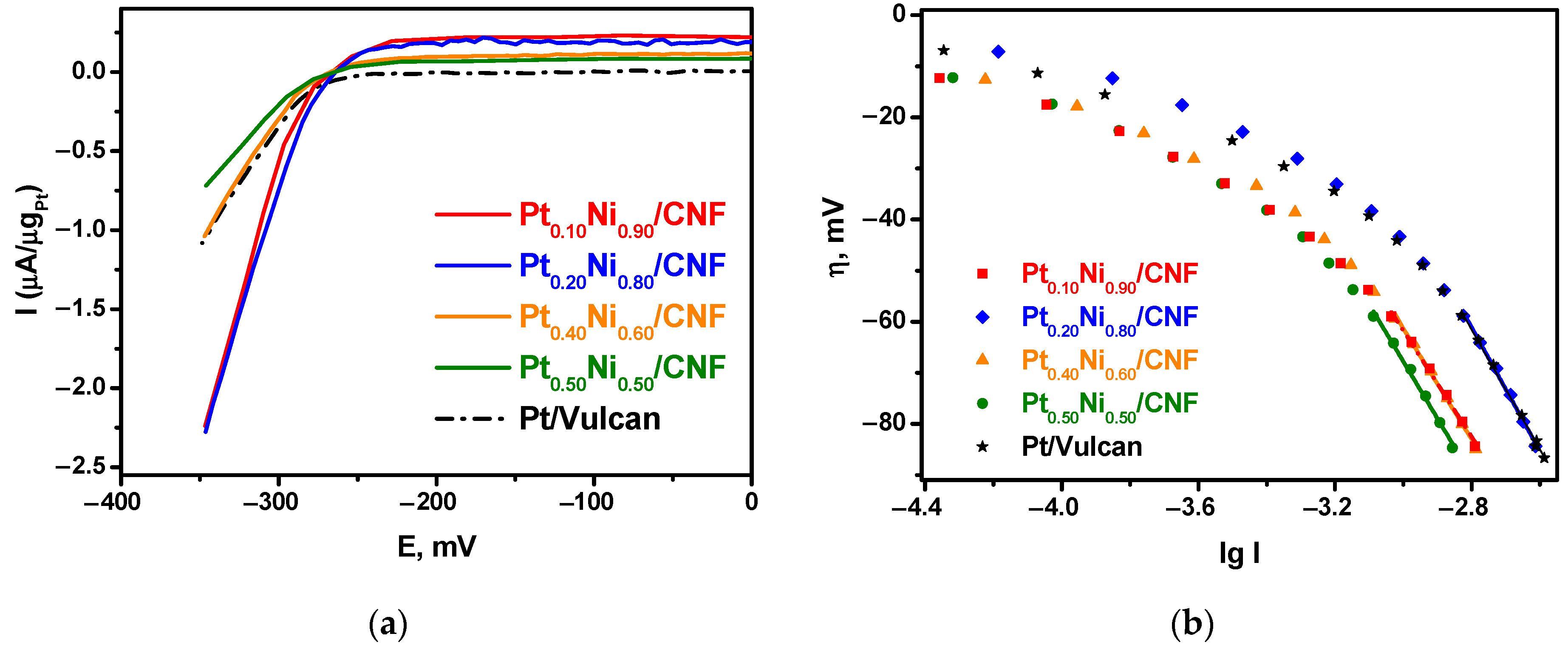
2.3. Electrocatalytic Testing of Pt1−xNix/CNF Composites
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Synthesis of Pt1−xNix Alloys
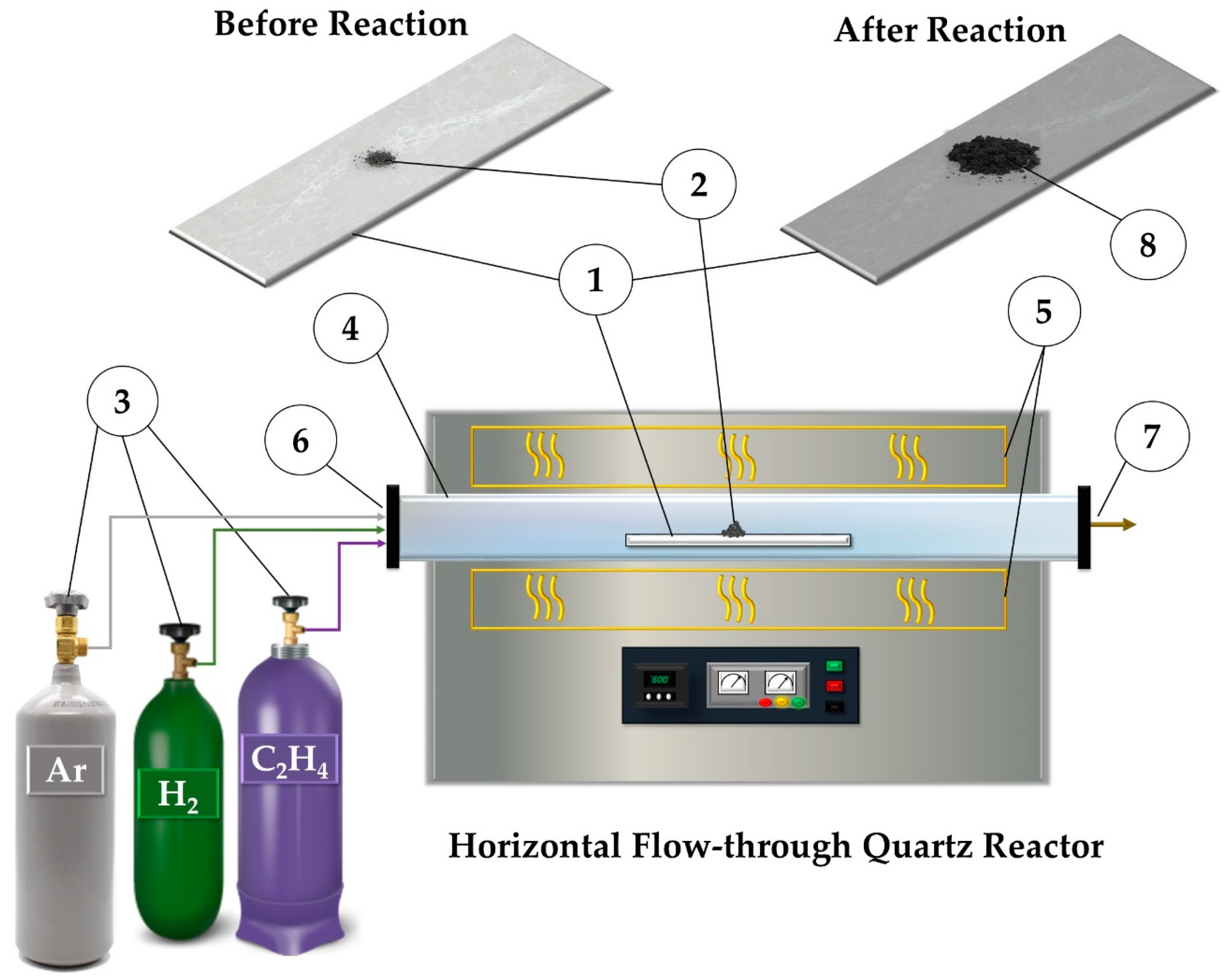
3.3. Synthesis of Pt1−xNix/CNF Composites
3.4. Characterization of Materials
3.5. Electrochemical Testing of Pt1−xNix/CNF Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel Cells: Principles, Types, Fuels, and Applications. ChemPhysChem 2000, 1, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Lucia, U. Overview on fuel cells. Renew. Sustain. Energy Rev. 2014, 30, 164–169. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.-C.; Wu, Z.-P.; Chen, G.; Yang, F.; Zhu, S.; Siddharth, K.; Kong, Z.; Lu, A.; Li, J.-C.; et al. Recent advances in electrocatalysts for proton exchange membrane fuel cells and alkaline membrane fuel cells. Adv. Mater. 2021, 33, 2006292. [Google Scholar] [CrossRef]
- Lü, X.; Qu, Y.; Wang, Y.; Qin, C.; Liu, G. A comprehensive review on hybrid power system for PEMFC-HEV: Issues and strategies. Energy Convers. Manag. 2018, 171, 1273–1291. [Google Scholar] [CrossRef]
- Lasia, A. Mechanism and kinetics of the hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 19484–19518. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions—A Review. Int. J. Hydrog. Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Seger, B.; Chorkendorff, I. Recent Development in Hydrogen Evolution Reaction Catalysts and Their Practical Implementation. J. Phys. Chem. Lett. 2015, 6, 951–957. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef]
- Sealy, C. The problem with platinum. Mater. Today 2008, 11, 65–68. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Sarapuu, A.; Solla-Gull, J.; Maia, G.; Kannan, A.M.; Alonso-Vante, N.; Tammeveski, K. Oxygen reduction reaction on nanostructured Pt-based electrocatalysts: A review. Int. J. Hydrog. Energy 2020, 45, 31775–31797. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15. [Google Scholar] [CrossRef]
- Watanabe, M.; Igarashi, H.; Fujino, T. Design of CO tolerant anode catalysts for polymer electrolyte fuel cell. Electrochemistry 1999, 67, 1194–1196. [Google Scholar] [CrossRef]
- Liu, J.; Lan, J.; Yang, L.; Wang, F.; Yin, J. PtM (M = Fe, Co, Ni) Bimetallic Nanoclusters as Active, Methanol-Tolerant, and Stable Catalysts toward the Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2019, 7, 6541–6549. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, D. Nano PtCu binary and PtCuAg ternary alloy catalysts for oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2015, 278, 396–403. [Google Scholar] [CrossRef]
- Wang, X.X.; Swihart, M.T.; Wu, G. Achievements, Challenges and Perspectives on Cathode Catalysts in Proton Exchange Membrane Fuel Cells for Transportation. Nat. Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Colón-Mercado, H.R.; Kim, H.; Popov, B.N. Durability study of Pt3Ni1 catalysts as cathode in PEM fuel cells. Electrochem. Commun. 2004, 6, 795–799. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, J.; Zheng, Y.; Xiao, M.; Gao, R.; Zhang, Z.; Wen, G.; Dou, H.; Deng, Y.P.; Yu, A. Materials Engineering toward Durable Electrocatalysts for Proton Exchange Membrane Fuel Cells. Adv. Energy Mater. 2022, 12, 2102665. [Google Scholar] [CrossRef]
- Yang, H.; Coutanceau, C.; Léger, J.-M.; Alonso-Vante, N. Methanol tolerant oxygen reduction on carbon-supported Pt–Ni alloy nanoparticles. J. Electroanal. Chem. 2005, 576, 305–313. [Google Scholar] [CrossRef]
- Liu, S.-H.; Zheng, F.-S.; Wu, J.-R. Preparation of ordered mesoporous carbons containing well-dispersed and highly alloying Pt–Co bimetallic nanoparticles toward methanol-resistant oxygen reduction reaction. Appl. Catal. B Environ. 2011, 108, 81–89. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Dai, Y.; Wang, W.; Chen, S. Ni@Pt Core−Shell Nanoparticles: Synthesis, Structural and Electrochemical Properties. J. Phys. Chem. C 2008, 112, 1645–1649. [Google Scholar] [CrossRef]
- Shan, A.; Huang, S.; Zhao, H.; Jiang, W.; Teng, X.; Huang, Y.; Chen, C.; Wang, R.; Lau, W.-M. Atomic-scaled surface engineering Ni-Pt nanoalloys towards enhanced catalytic efficiency for methanol oxidation reaction. Nano Res. 2020, 13, 3088–3097. [Google Scholar] [CrossRef]
- Ali, S.; Khan, I.; Khan, S.A.; Sohail, M.; Ahmed, R.; Rehman, A.u.; Ansari, M.S.; Morsy, M.A. Electrocatalytic performance of Ni@Pt core–shell nanoparticles supported on carbon nanotubes for methanol oxidation reaction. J. Electroanal. Chem. 2017, 795, 17–25. [Google Scholar] [CrossRef]
- Rosado, G.; Verde, Y.; Valenzuela-Muñiz, A.M.; Barbosa, R.; Miki Yoshida, M.; Escobar, B. Catalytic activity of Pt-Ni nanoparticles supported on multi-walled carbon nanotubes for the oxygen reduction reaction. Int. J. Hydrog. Energy 2016, 41, 23260–23271. [Google Scholar] [CrossRef]
- Chen, J.; Niu, Q.; Chen, G.; Nie, J.; Ma, G. Electrooxidation of Methanol on Pt@Ni Bimetallic Catalyst Supported on Porous Carbon Nanofibers. J. Phys. Chem. C 2017, 121, 1463–1471. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiao, L.; Yang, Y.; DiSalvo, F.J.; Abruña, H.D. High-loading intermetallic Pt3Co/C core–shell nanoparticles as enhanced activity electrocatalysts toward the oxygen reduction reaction (ORR). Chem. Mater. 2018, 30, 1532–1539. [Google Scholar] [CrossRef]
- Guerrero-Ortega, L.P.A.; Manzo-Robledo, A.; Ramírez-Meneses, E.; Mateos-Santiago, J.; Lartundo-Rojas, L.; Garibay-Febles, V. Methanol electro-oxidation reaction at the interface of (bi)-metallic (PtNi) synthesized nanoparticles supported on carbon Vulcan. Int. J. Hydrog. Energy 2018, 43, 6117–6130. [Google Scholar] [CrossRef]
- Kiani, M.; Zhang, J.; Luo, Y.; Chen, Y.; Chen, J.; Fan, J.; Wang, G.; Wang, R. Facile synthesis and enhanced catalytic activity of electrochemically dealloyed platinum–nickel nanoparticles towards formic acid electro-oxidation. J. Energy Chem. 2019, 35, 9–16. [Google Scholar] [CrossRef]
- Xu, C.; Li, Q.; Liu, Y.; Wang, J.; Geng, H. Hierarchical nanoporous PtFe alloy with multimodal size distributions and its catalytic performance toward methanol electrooxidation. Langmuir 2012, 28, 1886–1892. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Q.; Xu, G.-L.; Qin, X.; Hwang, I.; Sun, C.-J.; Liu, M.; Hua, W.; Wu, H.; Zhu, S.; et al. Atomically Dispersed Pt and Fe Sites and Pt–Fe Nanoparticles for Durable Proton Exchange Membrane Fuel Cells. Nat. Catal. 2022, 5, 503–512. [Google Scholar] [CrossRef]
- Mani, P.; Srivastava, R.; Strasser, P. Dealloyed binary PtM3 (M= Cu, Co, Ni) and ternary PtNi3M (M= Cu, Co, Fe, Cr) electrocatalysts for the oxygen reduction reaction: Performance in polymer electrolyte membrane fuel cells. J. Power Sources 2011, 196, 666–673. [Google Scholar] [CrossRef]
- Pavlets, A.; Pankov, I.; Alekseenko, A. Electrochemical Activation and Its Prolonged Effect on the Durability of Bimetallic Pt-Based Electrocatalysts for PEMFCs. Inorganics 2023, 11, 45. [Google Scholar] [CrossRef]
- Hasa, B.; Martino, E.; Tsatsos, S.; Vakros, J.; Kyriakou, G.; Katsaounis, A. Non-precious Sn as alternative substitute metal in graphene-based catalysts for methanol electrooxidation. J. Appl. Electrochem. 2022, 52, 509–520. [Google Scholar] [CrossRef]
- Cong, Y.; Chai, C.; Zhao, X.; Yi, B.; Song, Y. Pt0. 25Ru0. 75/N-C as Highly Active and Durable Electrocatalysts toward Alkaline Hydrogen Oxidation Reaction. Adv. Mater. Interf. 2020, 7, 2000310. [Google Scholar] [CrossRef]
- Matsumoto, F. Ethanol and Methanol Oxidation Activity of PtPb, PtBi, and PtBi2 Intermetallic Compounds in Alkaline Media. Electrochemistry 2012, 80, 132–138. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Chesnokov, V.V.; Buyanov, R.A.; Pakhomov, N.A. Decomposition of chlorinated hydrocarbons on iron-group metals. Kinet. Catal. 2001, 42, 543–548. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bauman, Y.I.; Korneev, D.V.; Vedyagin, A.A. Metal dusting as a route to produce active catalyst for processing chlorinated hydrocarbons into carbon nanomaterials. Top. Catal. 2013, 56, 1026–1032. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Valverde, J.L.; Keane, M.A. Catalytic growth of structured carbon from chloro-hydrocarbons. Appl. Catal. A Gen. 2007, 332, 237–246. [Google Scholar] [CrossRef]
- Afonnikova, S.D.; Popov, A.A.; Bauman, Y.I.; Plyusnin, P.E.; Mishakov, I.V.; Trenikhin, M.V.; Shubin, Y.V.; Vedyagin, A.A.; Korenev, S.V. Porous Co-Pt Nanoalloys for Production of Carbon Nanofibers and Composites. Materials 2022, 15, 7456. [Google Scholar] [CrossRef]
- Grabke, H.J. Metal dusting. Mater. Corros. 2003, 54, 736–746. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Mishakov, I.V.; Rudneva, Y.V.; Popov, A.A.; Rieder, D.; Korneev, D.V.; Serkova, A.N.; Shubin, Y.V.; Vedyagin, A.A. Catalytic synthesis of segmented carbon filaments via decomposition of chlorinated hydrocarbons on Ni-Pt alloys. Catal. Today 2020, 348, 102–110. [Google Scholar] [CrossRef]
- Popov, A.A.; Shubin, Y.V.; Bauman, Y.I.; Plyusnin, P.E.; Mishakov, I.V.; Sharafutdinov, M.R.; Maksimovskiy, E.A.; Korenev, S.V.; Vedyagin, A.A. Preparation of porous Co-Pt alloys for catalytic synthesis of carbon nanofibers. Nanotechnology 2020, 31, 495604. [Google Scholar] [CrossRef] [PubMed]
- International Centre for Diffraction Data. Powder Diffraction File, PDF-2/Release; International Centre for Diffraction Data: Newtown Township, PA, USA, 2009; Available online: https://www.icdd.com/pdf-2/ (accessed on 25 October 2022).
- Popov, A.A.; Varygin, A.D.; Plyusnin, P.E.; Sharafutdinov, M.R.; Korenev, S.V.; Serkova, A.N.; Shubin, Y.V. X-ray diffraction reinvestigation of the Ni-Pt phase diagram. J. Alloys Compd. 2022, 891, 161974. [Google Scholar] [CrossRef]
- Nash, P.; Singleton, M.F. The Ni-Pt (nickel-platinum) system. Bull. Alloy Phase Diagr. 1989, 10, 258–262. [Google Scholar] [CrossRef]
- Shubin, Y.V.; Bauman, Y.I.; Plyusnin, P.E.; Mishakov, I.V.; Tarasenko, M.S.; Mel’gunov, M.S.; Stoyanovskii, V.O.; Vedyagin, A.A. Facile synthesis of triple Ni-Mo-W alloys and their catalytic properties in chemical vapor deposition of chlorinated hydrocarbons. J. Alloys Compd. 2021, 866, 158778. [Google Scholar] [CrossRef]
- Rudneva, Y.V.; Shubin, Y.V.; Plyusnin, P.E.; Bauman, Y.I.; Mishakov, I.V.; Korenev, S.V.; Vedyagin, A.A. Preparation of highly dispersed Ni1-xPdx alloys for the decomposition of chlorinated hydrocarbons. J. Alloys Compd. 2019, 782, 716–722. [Google Scholar] [CrossRef]
- Tripathi, S. Role of Nanocatalysts in Synthesis of Carbon Nanofiber. In Carbon Nanofibers: Fundamentals and Applications; Scrivener Publishing LLC.: Beverly, MA, USA, 2021; pp. 49–74. [Google Scholar]
- Yu, Z.; Chen, D.; Tøtdal, B.; Holmen, A. Parametric study of carbon nanofiber growth by catalytic ethylene decomposition on hydrotalcite derived catalysts. Mater. Chem. Phys. 2005, 92, 71–81. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Buyanov, R.A. The formation of carbon filaments upon decomposition of hydrocarbons catalysed by iron subgroup metals and their alloys. Russ. Chem. Rev. 2000, 69, 623–638. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Kutaev, N.V.; Bauman, Y.I.; Shubin, Y.V.; Koskin, A.P.; Serkova, A.N.; Vedyagin, A.A. Mechanochemical synthesis, structure, and catalytic activity of Ni-Cu, Ni-Fe, and Ni-Mo alloys in the preparation of carbon nanofibers during the decomposition of chlorohydrocarbons. J. Struct. Chem. 2020, 61, 769–779. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Mishakov, I.V.; Vedyagin, A.A.; Ramakrishna, S. Synthesis of bimodal carbon structures via metal dusting of Ni-based alloys. Mater. Lett. 2017, 201, 70–73. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Afonnikova, S.D.; Bauman, Y.I.; Shubin, Y.V.; Trenikhin, M.V.; Serkova, A.N.; Vedyagin, A.A. Carbon Erosion of a Bulk Nickel–Copper Alloy as an Effective Tool to Synthesize Carbon Nanofibers from Hydrocarbons. Kinet. Catal. 2022, 63, 97–107. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Korneev, D.V.; Bauman, Y.I.; Vedyagin, A.A.; Nalivaiko, A.Y.; Shubin, Y.V.; Gromov, A.A. Interaction of chlorinated hydrocarbons with nichrome alloy: From surface transformations to complete dusting. Surf. Interf. 2022, 30, 101914. [Google Scholar] [CrossRef]
- Zeng, Z.; Natesan, K. Control of metal dusting corrosion in Ni-based alloys. Int. J. Hydrog. Energy 2007, 32, 3640–3647. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Tang, X.; Peng, X.; Deng, H. Facile synthesis of carbon quantum dot-carbon nanotube composites on an eggshell-derived catalyst by one-step chemical vapor deposition. Diam. Relat. Mater. 2021, 120, 108657. [Google Scholar] [CrossRef]
- Shaikjee, A.; Coville, N.J. Catalyst restructuring studies: The facile synthesis of tripod-like carbon fibers by the decomposition of trichloroethylene. Mater. Lett. 2012, 68, 273–276. [Google Scholar] [CrossRef]
- Franke, P.; Neuschütz, D. Binary Systems. Part 5: Binary Systems Supplement 1; Springer: Berlin/Heidelberg, Germany, 2007; Volume 19. [Google Scholar]
- Esconjauregui, S.; Whelan, C.M.; Maex, K. The reasons why metals catalyze the nucleation and growth of carbon nanotubes and other carbon nanomorphologies. Carbon 2009, 47, 659–669. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Mishakov, I.V.; Rudneva, Y.V.; Plyusnin, P.E.; Shubin, Y.V.; Korneev, D.V.; Vedyagin, A.A. Formation of Active Sites of Carbon Nanofibers Growth in Self-Organizing Ni–Pd Catalyst during Hydrogen-Assisted Decomposition of 1,2-Dichloroethane. Ind. Eng. Chem. Res. 2018, 58, 685–694. [Google Scholar] [CrossRef]
- Jun, L.Y.; Mubarak, N.M.; Yee, M.J.; Yon, L.S.; Bing, C.H.; Khalid, M.; Abdullah, E.C. An overview of functionalised carbon nanomaterial for organic pollutant removal. J. Ind. Eng. Chem. 2018, 67, 175–186. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Lim, S.; Hong, S.-h.; Qiao, W.; Whitehurst, D.D.; Mochida, I.; An, B.; Yokogawa, K. A conceptual model for the structure of catalytically grown carbon nano-fibers. Carbon 2005, 43, 1828–1838. [Google Scholar] [CrossRef]
- Monthioux, M.; Noé, L.; Dussault, L.; Dupin, J.C.; Latorre, N.; Ubieto, T.; Romeo, E.; Royo, C.; Monzón, A.; Guimon, C. Texturising and structurising mechanisms of carbon nanofilaments during growth. J. Mater. Chem. 2007, 17, 4611–4618. [Google Scholar] [CrossRef]
- Shaikjee, A.; Coville, N.J. The role of the hydrocarbon source on the growth of carbon materials. Carbon 2012, 50, 3376–3398. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis of hydrogen evolution: Progress in cathode activation. Adv. Electrochem. Sci. Eng. 1992, 2, 1–85. [Google Scholar]
- Brauer, G. Handbuch der Präparativen Anorganischen Chemie; Enke: Stuttgart, Germany, 1975; Volume 2. [Google Scholar]
- Kraus, W.; Nolze, G. PowderCell—A program to visualize crystal structures, calculate the corresponding powder patterns and refine experimental curves. J. Appl. Cryst. 1996, 29, 301–303. [Google Scholar] [CrossRef]
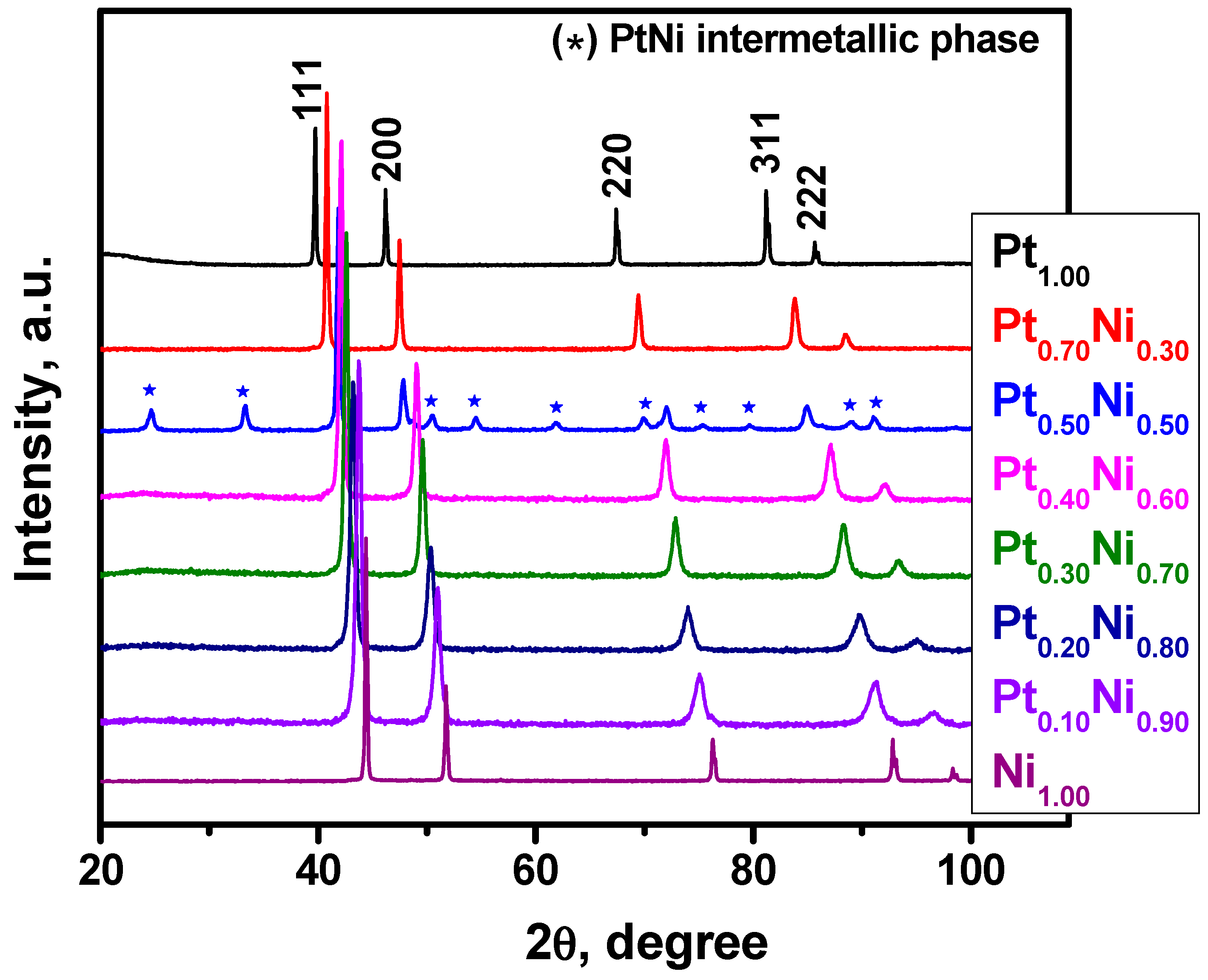

| # | Preset Composition, at.% Ni | Nominal Composition, at.% Ni | Phase | Phase Structure | Lattice Parameter, Å |
|---|---|---|---|---|---|
| 1 | 0 | 0 | Pt | fcc | 3.9232(5) |
| 2 | 30 | 29 ± 2 | Pt0.70Ni0.30 | fcc | 3.8245(5) |
| 3 | 50 | 51 ± 4 | PtNi | tetragonal | a = 2.690(2) c = 3.617(2) |
| Pt0.47Ni0.53 | fcc | 3.739(2) | |||
| 4 | 60 | 61 ± 5 | Pt0.40Ni0.60 | fcc | 3.708(2) |
| 5 | 70 | 70 ± 5 | Pt0.30Ni0.70 | fcc | 3.667(2) |
| 6 | 80 | 80 ± 6 | Pt0.19Ni0.81 | fcc | 3.619(2) |
| 7 | 90 | 90 ± 7 | Pt0.10Ni0.90 | fcc | 3.575(2) |
| 8 | 100 | 100 | Ni | fcc | 3.5242(5) |
| Initial Alloy | Ethylene Decomposition Reaction Time, min | Carbon Yield, g/gcat | Total Metal Content in Pt1-xNix/CNF Composite, wt.% | Lattice Parameter of the Initial Alloy, Å | Lattice Parameter of the Alloy after the Reaction, Å |
|---|---|---|---|---|---|
| Pt0.10Ni0.90 | 3 | 4.3 | 19 | 3.575 (2) | 3.58 (1) |
| Pt0.20Ni0.80 | 4 | 4.3 | 19 | 3.619 (2) | 3.62 (1) |
| Pt0.40Ni0.60 | 5 | 4.5 | 18 | 3.708 (2) | 3.70 (1) |
| Pt0.50Ni0.50 | 15 | 3.6 | 22 | a = 2.690 (2) c = 3.617 (2) | a = 2.69 (1) c = 3.62 (1) |
| Sample | Composition, at.% Ni | |
|---|---|---|
| Preset | EDX * | |
| Pt0.50Ni0.50/CNF | 50 | 49 ± 5 |
| Pt0.20Ni0.80/CNF | 80 | 80 ± 3 |
| Sample | η = a + b∙lg I | |
|---|---|---|
| a (mV) | b (mV) | |
| Pt0.10Ni0.90/CNF | −373 ± 11 | −104 ± 4 |
| Pt0.20Ni0.80/CNF | −400 ± 10 | −121 ± 4 |
| Pt0.40Ni0.60/CNF | −387 ± 8 | −109 ± 3 |
| Pt0.50Ni0.50/CNF | −403 ± 13 | −112 ± 5 |
| Pt/Vulcan | −390 ± 8 | −117 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, A.A.; Afonnikova, S.D.; Varygin, A.D.; Bauman, Y.I.; Trenikhin, M.V.; Plyusnin, P.E.; Shubin, Y.V.; Vedyagin, A.A.; Mishakov, I.V. Pt1−xNix Alloy Nanoparticles Embedded in Self-Grown Carbon Nanofibers: Synthesis, Properties and Catalytic Activity in HER. Catalysts 2023, 13, 599. https://doi.org/10.3390/catal13030599
Popov AA, Afonnikova SD, Varygin AD, Bauman YI, Trenikhin MV, Plyusnin PE, Shubin YV, Vedyagin AA, Mishakov IV. Pt1−xNix Alloy Nanoparticles Embedded in Self-Grown Carbon Nanofibers: Synthesis, Properties and Catalytic Activity in HER. Catalysts. 2023; 13(3):599. https://doi.org/10.3390/catal13030599
Chicago/Turabian StylePopov, Anton A., Sofya D. Afonnikova, Andrey D. Varygin, Yury I. Bauman, Mikhail V. Trenikhin, Pavel E. Plyusnin, Yury V. Shubin, Aleksey A. Vedyagin, and Ilya V. Mishakov. 2023. "Pt1−xNix Alloy Nanoparticles Embedded in Self-Grown Carbon Nanofibers: Synthesis, Properties and Catalytic Activity in HER" Catalysts 13, no. 3: 599. https://doi.org/10.3390/catal13030599
APA StylePopov, A. A., Afonnikova, S. D., Varygin, A. D., Bauman, Y. I., Trenikhin, M. V., Plyusnin, P. E., Shubin, Y. V., Vedyagin, A. A., & Mishakov, I. V. (2023). Pt1−xNix Alloy Nanoparticles Embedded in Self-Grown Carbon Nanofibers: Synthesis, Properties and Catalytic Activity in HER. Catalysts, 13(3), 599. https://doi.org/10.3390/catal13030599









