Reductive Catalytic Fractionation of Abies Wood into Bioliquids and Cellulose with Hydrogen in an Ethanol Medium over NiCuMo/SiO2 Catalyst
Abstract
1. Introduction
2. Results
2.1. Thermochemical Properties of Abies Wood
2.2. Kinetic Analysis of Abies Wood Pyrolysis
2.3. Hydrogenation of Abies Wood in Ethanol Medium
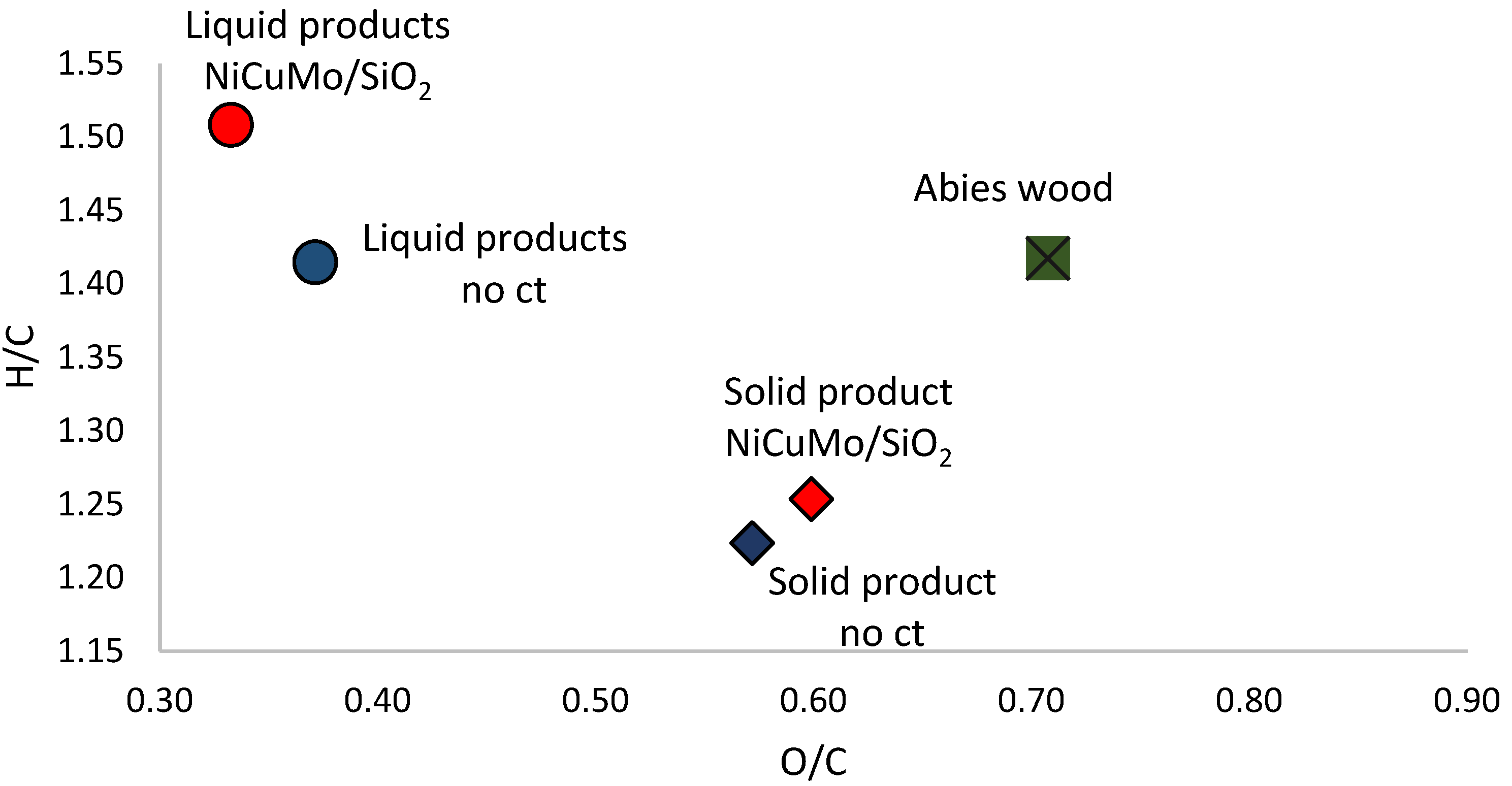
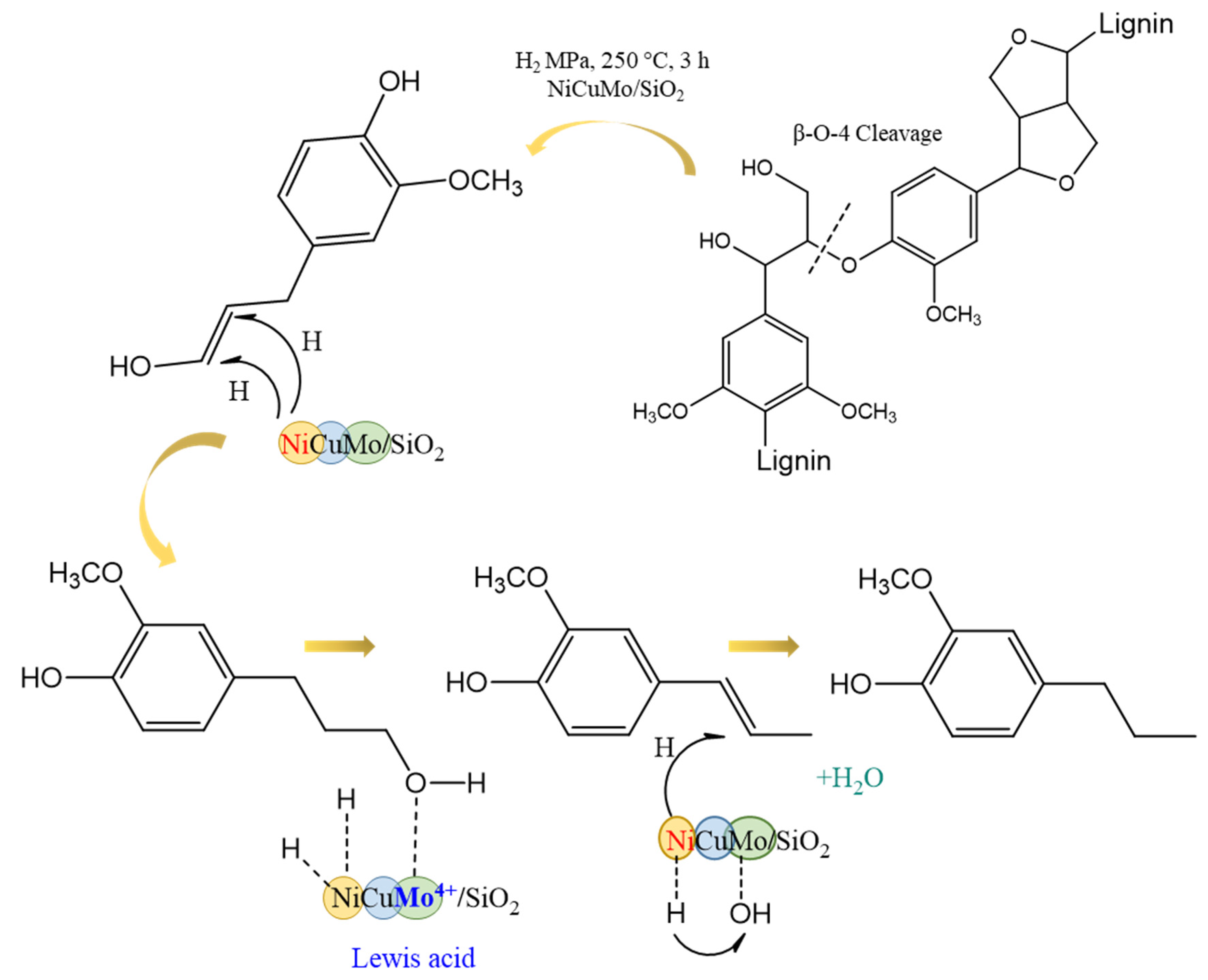
2.4. Composition of Liquid Products of Abies Wood Hydrogenation
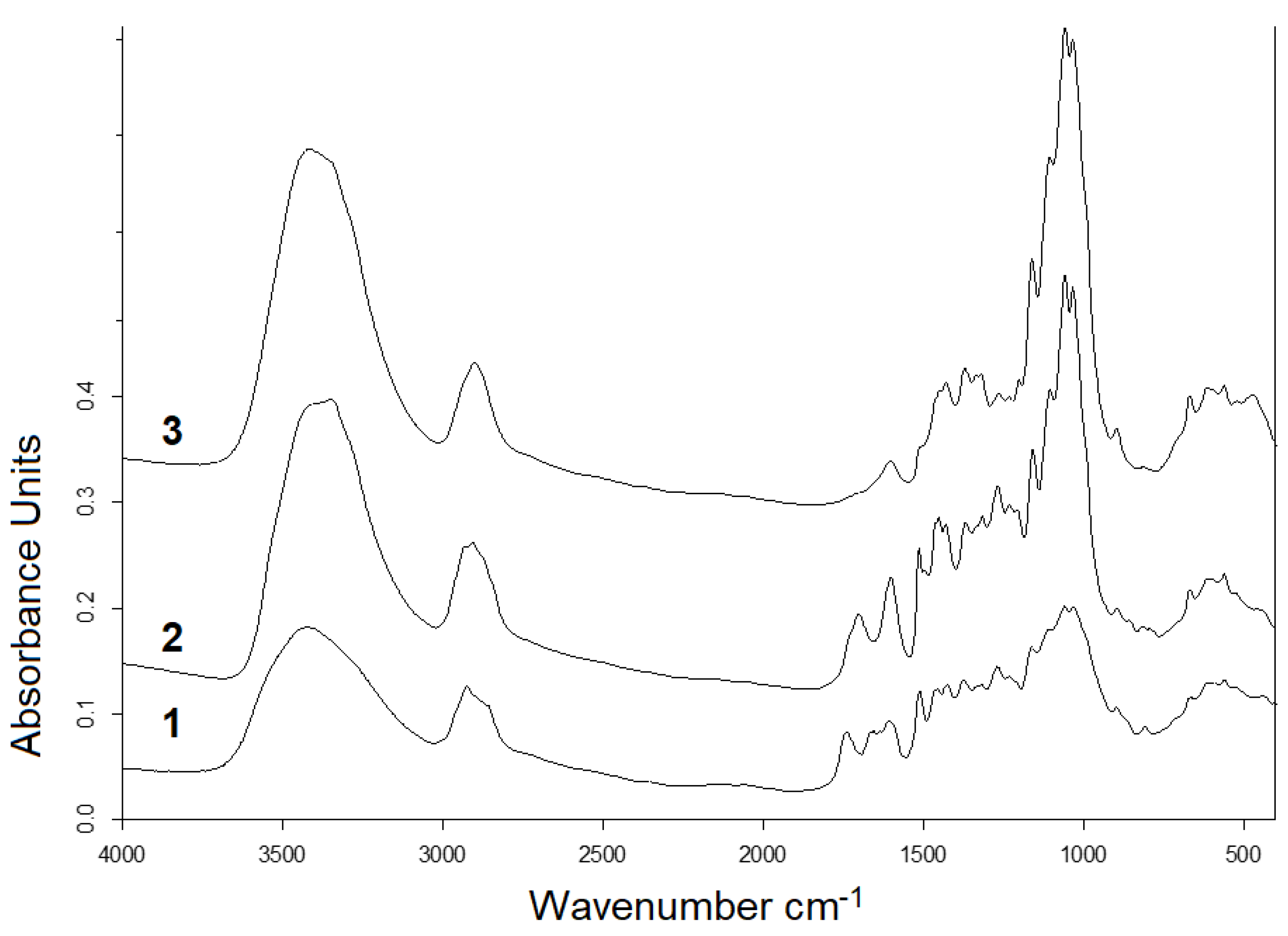
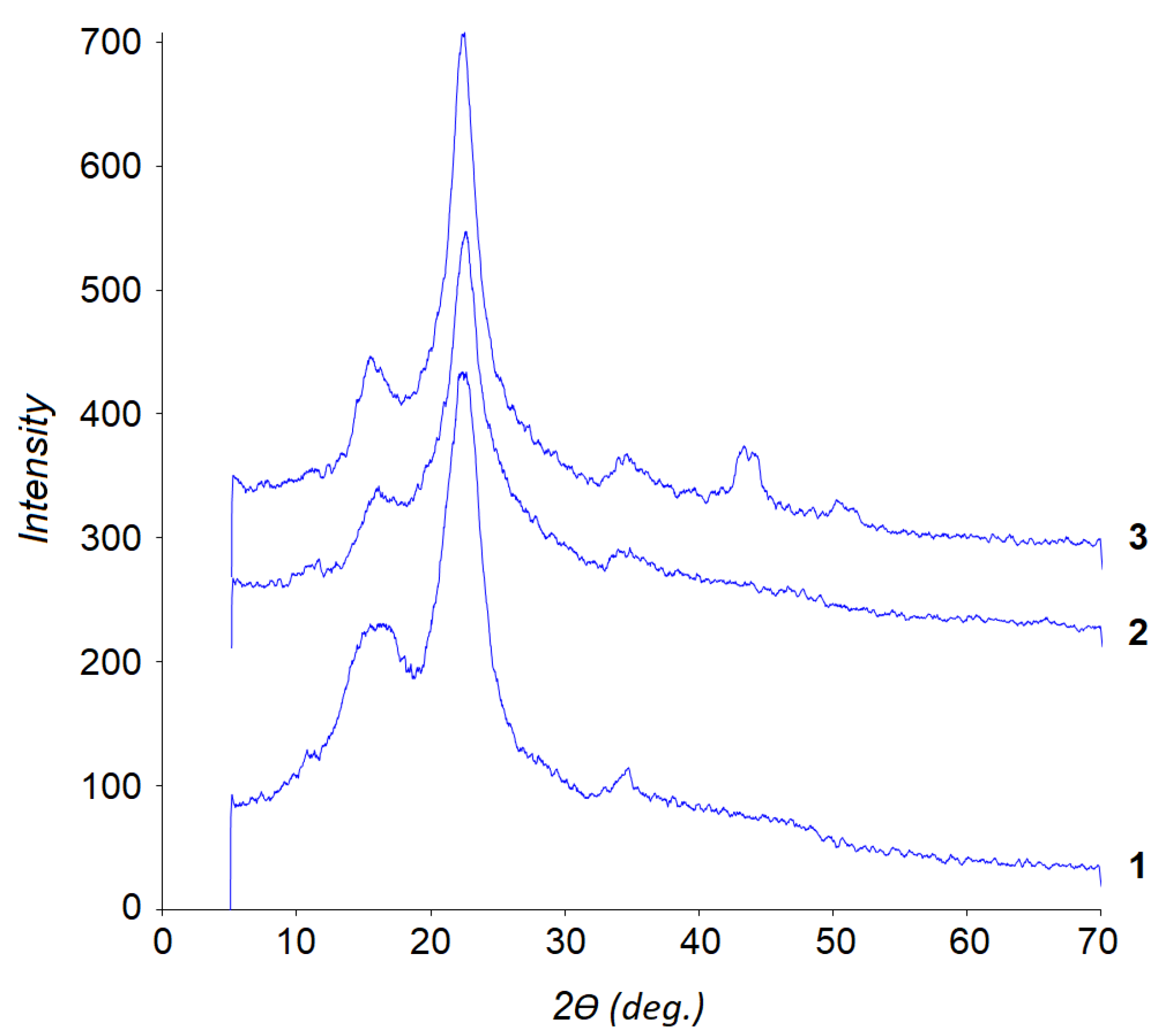
2.5. Composition and Structure of Solid Products of Abies Wood Hydrogenation
2.6. Composition of Gaseous Products of Abies Wood Hydrogenation
2.7. Fractionation of Abies Wood
3. Materials and Methods
3.1. Preparation of Abies Wood Samples
3.2. Catalyst Preparation
3.3. Hydrogenation of Abies Wood
3.4. Thermochemical Properties of Abies Wood
3.5. Composition and Structure of the Products of Abies Wood Hydrogenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braghiroli, F.L.; Passarini, L. Valorization of Biomass Residues from Forest Operations and Wood Manufacturing Presents a Wide Range of Sustainable and Innovative Possibilities. Curr. For. Rep. 2020, 6, 172–183. [Google Scholar] [CrossRef]
- Dulce María, D.-M. Valorization of Biomass as a Raw Material to Obtain Products of Industrial Interest. In Biomass, Biorefineries and Bioeconomy; Mohamed, S., Ed.; IntechOpen: Rijeka, Croatia, 2022; Chapter 11. [Google Scholar]
- Cesprini, E.; Greco, R.; Causin, V.; Urso, T.; Cavalli, R.; Zanetti, M. Quality assessment of pellets and briquettes made from glued wood waste. Eur. J. Wood Wood Prod. 2021, 79, 1153–1162. [Google Scholar] [CrossRef]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Luterbacher, J.S.; Martin Alonso, D.; Dumesic, J.A. Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Green Chem. 2014, 16, 4816–4838. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.; Fan, L.-S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Elangovan, S.; Afanasenko, A.; Haupenthal, J.; Sun, Z.; Liu, Y.; Hirsch, A.K.H.; Barta, K. From Wood to Tetrahydro-2-benzazepines in Three Waste-Free Steps: Modular Synthesis of Biologically Active Lignin-Derived Scaffolds. ACS Cent. Sci. 2019, 5, 1707–1716. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Korányi, T.I.; Fridrich, B.; Pineda, A.; Barta, K. Development of ‘Lignin-First’ Approaches for the Valorization of Lignocellulosic Biomass. Molecules 2020, 25, 2815. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Garyntseva, N.V.; Kondrasenko, A.A.; Pestunov, A.V.; Djakovitch, L.; Pinel, C. Catalytic peroxide fractionation processes for the green biorefinery of wood. React. Kinet. Mech. Catal. 2019, 126, 717–735. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Kaygorodov, K.L.; Skiba, E.A.; Tarabanko, N.E.; Chelbina, Y.V.; Baybakova, O.V.; Kuznetsov, B.N.; Djakovitch, L. Processing Pine Wood into Vanillin and Glucose by Sequential Catalytic Oxidation and Enzymatic Hydrolysis. Wood Chem. Technol. 2017, 37, 43–51. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.F.; Renders, T.; De Meester, B.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef]
- Baryshnikov, S.V.; Miroshnikova, A.V.; Kazachenko, A.S.; Malyar, Y.N.; Taran, O.P.; Lavrenov, A.V.; Djakovitch, L.; Kuznetsov, B.N. Hydrogenation of abies wood and ethanol lignin by hydrogen in supercritical ethanol in the presence of bifunctional catalyst Pt/ZrO2. J. Sib. Fed. Univ. Chem. 2019, 12, 550–561. [Google Scholar] [CrossRef]
- Taran, O.P.; Sharypov, V.I.; Baryshnikov, S.V.; Beregovtsova, N.G.; Miroshnikova, A.V.; Kazachenko, A.S.; Sychev, V.V.; Kuznetsov, B.N. Reductive fractionation of larch in a supercritical ethanol medium in the presence of bifunctional Ru/C catalyst and hydrogen donor. Katal. V Promyshlennosti 2020, 20, 127–139. [Google Scholar] [CrossRef]
- Taran, O.P.; Miroshnikova, A.V.; Baryshnikov, S.V.; Kazachenko, A.S.; Skripnikov, A.M.; Sychev, V.V.; Malyar, Y.N.; Kuznetsov, B.N. Reductive Catalytic Fractionation of Spruce Wood over Ru/C Bifunctional Catalyst in the Medium of Ethanol and Molecular Hydrogen. Catalysts 2022, 12, 1384. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Schutyser, W.; Sels, B.F. Lignin-first biomass fractionation: The advent of active stabilisation strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Miroshnikova, A.V.; Kazachenko, A.S.; Kuznetsov, B.N.; Taran, O.P. Reductive Catalytic Fractionation of Lignocellulosic Biomass: A New Promissing Method for Its Complex Processing. Catal. Ind. 2022, 14, 231–250. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Koelewijn, S.F.; Renders, T.; Courtin, C.M.; Sels, B.F. Tuning the lignin oil OH-content with Ru and Pd catalysts during lignin hydrogenolysis on birch wood. Chem. Commun. 2015, 51, 13158–13161. [Google Scholar] [CrossRef]
- Huang, X.; Ouyang, X.; Hendriks, B.M.S.; Gonzalez, O.M.M.; Zhu, J.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Selective production of mono-aromatics from lignocellulose over Pd/C catalyst: The influence of acid co-catalysts. Faraday Discuss. 2017, 202, 141–156. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Renders, T.; Kennis, S.; Koelewijn, S.F.; Van den Bossche, G.; Vangeel, T.; Deneyer, A.; Depuydt, D.; Courtin, C.M.; Thevelein, J.M.; et al. Integrating lignin valorization and bio-ethanol production: On the role of Ni-Al2O3 catalyst pellets during lignin-first fractionation. Green Chem. 2017, 19, 3313–3326. [Google Scholar] [CrossRef]
- Miroshnikova, A.V.; Kazachenko, A.S.; Tarabanko, V.E.; Sychev, V.V.; Skripnikov, A.M.; Mikhlin, Y.L.; Kosivtsov, Y.; Chudina, A.I.; Taran, O.P. Hydrogenation of Flax Shives in Ethanol over a Ni/C Catalyst. Catalysts 2022, 12, 1177. [Google Scholar] [CrossRef]
- Chen, J.; Lu, F.; Si, X.; Nie, X.; Lu, R.; Xu, J. High Yield Production of Natural Phenolic Alcohols from Woody Biomass Using a Nickel-Based Catalyst. ChemSusChem 2016, 9, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Sturgeon, M.R.; O’Brien, M.H.; Ciesielski, P.N.; Katahira, R.; Kruger, J.S.; Chmely, S.C.; Hamlin, J.; Lawrence, K.; Hunsinger, G.B.; Foust, T.D.; et al. Lignin depolymerisation by nickel supported layered-double hydroxide catalysts. Green Chem. 2014, 16, 824–835. [Google Scholar] [CrossRef]
- Anderson, E.M.; Katahira, R.; Reed, M.; Resch, M.G.; Karp, E.M.; Beckham, G.T.; Román-Leshkov, Y. Reductive Catalytic Fractionation of Corn Stover Lignin. ACS Sustain. Chem. Eng. 2016, 4, 6940–6950. [Google Scholar] [CrossRef]
- Oregui, M.; Gandarias, I.; Miletic, N.; Simonsen, S.F.; Kronstad, A.; Arias, P.L.; Barth, T. Thermocatalytic conversion of lignin in an ethanol/formic acid medium with NiMo catalysts: Role of the metal and acid sites. Appl. Catal. B: Environ. 2017, 217, 353–364. [Google Scholar] [CrossRef]
- Horáček, J.; Homola, F.; Kubičková, I.; Kubička, D. Lignin to liquids over sulfided catalysts. Catal. Today 2012, 179, 191–198. [Google Scholar] [CrossRef]
- Sharypov, V.I.; Kuznetsov, B.N.; Yakovlev, V.A.; Beregovtsova, N.G.; Baryshnikov, S.V.; Djakovitch, L.; Pinel, C. Composition of Liquid Products of Acetonlignin Conversion Over NiCu/SiO2 Catalysts in Supercritical Butanol. J. Sib. Fed. Univ. Chem. 2015, 8, 465–475. [Google Scholar] [CrossRef]
- Yakovlev, V.A.; Khromova, S.A.; Sherstyuk, O.V.; Dundich, V.O.; Ermakov, D.Y.; Novopashina, V.M.; Lebedev, M.Y.; Bulavchenko, O.; Parmon, V.N. Development of new catalytic systems for upgraded bio-fuels production from bio-crude-oil and biodiesel. Catal. Today 2009, 144, 362–366. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Khromova, S.A.; Venderbosch, R.H.; Yakovlev, V.A.; Heeres, H.J. Catalytic hydrotreatment of fast-pyrolysis oil using non-sulfided bimetallic Ni-Cu catalysts on a δ-Al2O3 support. Appl. Catal. B Environ. 2012, 117–118, 105–117. [Google Scholar] [CrossRef]
- Bykova, M.V.; Ermakov, D.Y.; Khromova, S.A.; Smirnov, A.A.; Lebedev, M.Y.; Yakovlev, V.A. Stabilized Ni-based catalysts for bio-oil hydrotreatment: Reactivity studies using guaiacol. Catal. Today 2014, 220–222, 21–31. [Google Scholar] [CrossRef]
- Sharypov, V.I.; Kusnetsov, B.N.; Yakovlev, V.A.; Beregovtsova, N.G.; Baryshnikov, S.V. Studying the thermal conversion of acetone lignin in supercritical butanol in the presence of NiCuMo/SiO2 catalysts. Catal. Ind. 2017, 9, 170–179. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sharypov, V.I.; Baryshnikov, S.V.; Miroshnikova, A.V.; Taran, O.P.; Yakovlev, V.A.; Lavrenov, A.V.; Djakovitch, L. Catalytic hydrogenolysis of native and organosolv lignins of aspen wood to liquid products in supercritical ethanol medium. Catal. Today 2021, 379, 114–123. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sharypov, V.I.; Baryshnikov, S.V.; Beregovtsova, N.G.; Yakovlev, V.A. Investigation of the Process of Microcrystalline Cellulose Hydrogenation in the Water Medium in the Presence of Catalysts NiCu/SiO2 and NiCuMo/SiO2. J. Sib. Fed. Univ. Chem. 2016, 9, 230–242. [Google Scholar] [CrossRef]
- Gazi, S. Valorization of Wood Biomass-Lignin via Selective Bond Scission: A Minireview. Appl. Catal. B Environ. 2019, 257, 117936. [Google Scholar] [CrossRef]
- Torr, K.M.; van de Pas, D.J.; Cazeils, E.; Suckling, I.D. Mild hydrogenolysis of in-situ and isolated Pinus radiata lignins. Bioresour. Technol. 2011, 102, 7608–7611. [Google Scholar] [CrossRef]
- Parsell, T.; Yohe, S.; Degenstein, J.; Jarrell, T.; Klein, I.; Gencer, E.; Hewetson, B.; Hurt, M.; Kim, J.I.; Choudhari, H.; et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem. 2015, 17, 1492–1499. [Google Scholar] [CrossRef]
- Tekin, K.; Hao, N.; Karagoz, S.; Ragauskas, A.J. Ethanol: A Promising Green Solvent for the Deconstruction of Lignocellulose. ChemSusChem 2018, 11, 3559–3575. [Google Scholar] [CrossRef]
- Mishra, G.; Kumar, J.; Bhaskar, T. Kinetic studies on the pyrolysis of pinewood. Bioresour. Technol. 2015, 182, 282–288. [Google Scholar] [CrossRef]
- Yan, B.; Lin, X.; Chen, Z.; Cai, Q.; Zhang, S. Selective production of phenolic monomers via high efficient lignin depolymerization with a carbon based nickel-iron-molybdenum carbide catalyst under mild conditions. Bioresour. Technol. 2021, 321, 124503. [Google Scholar] [CrossRef] [PubMed]
- Fisher, T.; Hajaligol, M.; Waymack, B.; Kellogg, D. Pyrolysis Behaviour and Kinetics of Biomass Derived Materials. J. Anal. Appl. Pyrol. 2002, 62, 331–349. [Google Scholar] [CrossRef]
- Sebio-Puñal, T.; Naya, S.; López-Beceiro, J.; Tarrío-Saavedra, J.; Artiaga, R. Thermogravimetric analysis of wood, holocellulose, and lignin from five wood species. J. Therm. Anal. Calorim. 2012, 109, 1163–1167. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bossche, G.; Vangeel, T.; Van Aelst, K.; Sels, B. Reductive catalytic fractionation: State of the art of the lignin-first biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, P.; Yu, W.; Shen, D.; Gu, S. Catalytic hydrogenolysis of lignin in ethanol/isopropanol over an activated carbon supported nickel-copper catalyst. Bioresour. Technol. 2021, 319, 124238. [Google Scholar] [CrossRef]
- Hou, Q.; Qi, X.; Zhen, M.; Qian, H.; Nie, Y.; Bai, C.; Zhang, S.; Bai, X.; Ju, M. Biorefinery roadmap based on catalytic production and upgrading 5-hydroxymethylfurfural. Green Chem. 2021, 23, 119–231. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Xiao, L.-P.; Wang, B.; Sun, R.-C.; Song, G. Sequential utilization of bamboo biomass through reductive catalytic fractionation of lignin. Bioresour. Technol. 2019, 285, 121335. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, C.; Xu, G.; Ma, Y.; Liu, X.; Zhang, Y. Depolymerization of lignin via a non-precious Ni–Fe alloy catalyst supported on activated carbon. Green Chem. 2017, 19, 1895–1903. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Wang, S.; Li, H.; Li, Z.; Shi, Z.-J.; Xiao, L.; Sun, R.-C.; Fang, Y.; Song, G. Catalytic Hydrogenolysis of Lignins into Phenolic Compounds over Carbon Nanotube Supported Molybdenum Oxide. ACS Catal. 2017, 7, 7535–7542. [Google Scholar] [CrossRef]
- Parto, S.G.; Jørgensen, E.K.; Christensen, J.M.; Pedersen, L.S.; Larsen, D.B.; Duus, J.Ø.; Jensen, A.D. Solvent assisted catalytic conversion of beech wood and organosolv lignin over NiMo/γ-Al2O3. Sustain. Energy Fuels 2020, 4, 1844–1854. [Google Scholar] [CrossRef]
- Ricciardi, L.; Verboom, W.; Lange, J.-P.; Huskens, J. Production of furans from C5 and C6 sugars in the presence of polar organic solvents. Sustain. Energy Fuels 2022, 6, 11–28. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Baryshnikov, S.V.; Chudina, A.I.; Malyar, Y.N.; Sychev, V.V.; Taran, O.P.; Laurent, D.; Kuznetsov, B.N. Hydrogenation of abies wood and ethanol-lignin by molecular hydrogen in supercritical ethanol over bifunctional RU/C catalyst. Khimiya Rastit. Syr’ya 2019, 2, 15–26. [Google Scholar] [CrossRef]
- Shi, J.; Xing, D.; Lia, J. FTIR Studies of the Changes in Wood Chemistry from Wood Forming Tissue under Inclined Treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef]
- Shi, J.-B.; Yang, Q.-L.; Lin, L.; Peng, L.-C. Fractionation and characterization of physicochemical and structural features of corn stalk hemicelluloses from yellow liquor of active oxygen cooking. Ind. Crops Prod. 2013, 44, 542–548. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, A.; Wang, S.; Zhang, Q. Effect of Different Heat Treatment Temperatures on the Chemical Composition and Structure of Chinese Fir Wood. BioResources 2016, 11, 4006–4016. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin–A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal latticed type. Part I. Spectra of lattice types I, II, III and of amorphous cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Boonyasuwat, S.; Omotoso, T.; Resasco, D.E.; Crossley, S.P. Conversion of Guaiacol over Supported Ru Catalysts. Catal. Lett. 2013, 143, 783–791. [Google Scholar] [CrossRef]
- Omotoso, T.; Boonyasuwat, S.; Crossley, S.P. Understanding the role of TiO2 crystal structure on the enhanced activity and stability of Ru/TiO2 catalysts for the conversion of lignin-derived oxygenates. Green Chem. 2014, 16, 645–652. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S. Lignin Valorization through Catalytic Lignocellulose Fractionation: A Fundamental Platform for the Future Biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Abu-Omar, M.M. Renewable Epoxy Networks Derived from Lignin-Based Monomers: Effect of Cross-Linking Density. ACS Sustain. Chem. Eng. 2016, 4, 6082–6089. [Google Scholar] [CrossRef]
- Koelewijn, S.F.; Van den Bosch, S.; Renders, T.; Schutyser, W.; Lagrain, B.; Smet, M.; Thomas, J.; Dehaen, W.; Van Puyvelde, P.; Witters, H.; et al. Sustainable bisphenols from renewable softwood lignin feedstock for polycarbonates and cyanate ester resins. Green Chem. 2017, 19, 2561–2570. [Google Scholar] [CrossRef]
- Lakani, S.A.; Hedjazi, S.; Abdulkhani, A. Chemical analysis and antioxidant activities of bark extracts from four endemic species of Hyrcanian forests in Iran. Holzforschung 2019, 73, 287–294. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Garyntseva, N.V.; Tarabanko, V.E.; Chesnokov, N.V.; Djakovitch, L.; Rataboul, F. Kinetic Studies and Optimization of Heterogeneous Catalytic Oxidation Processes for the Green Biorefinery of Wood. Top. Catal. 2020, 63, 229–242. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y. High-loaded nickel–silica catalysts for hydrogenation, prepared by sol–gel: Route: Structure and catalytic behavior. Appl. Catal. A Gen. 2003, 245, 277–288. [Google Scholar] [CrossRef]
- Hasanudin, H.; Asri, W.R.; Fanani, Z.; Adisti, S.J.; Hadiah, F.; Maryana, R.; Al Muttaqii, M.; Zhu, Z.; Machado, N.T. Facile Fabrication of SiO2/Zr Assisted with EDTA Complexed-Impregnation and Templated Methods for Crude Palm Oil to Biofuels Conversion via Catalytic Hydrocracking. Catalysts 2022, 12, 1522. [Google Scholar] [CrossRef]
- Gulyaeva, Y.K.; Alekseeva, M.V.; Ermakov, D.Y.; Bulavchenko, O.A.; Zaikina, O.O.; Yakovlev, V.A. High-Loaded Nickel Based Sol–Gel Catalysts for Methylcyclohexane Dehydrogenation. Catalysts 2020, 10, 1198. [Google Scholar] [CrossRef]
- Alekseeva, M.V.; Otyuskaya, D.S.; Rekhtina, M.A.; Bulavchenko, O.A.; Stonkus, O.A.; Kaichev, V.V.; Zavarukhin, S.G.; Thybaut, J.W.; Alexiadis, V.; Venderbosch, R.H.; et al. NiCuMo-SiO2 catalyst for pyrolysis oil upgrading: Model acidic treatment study. Appl. Catal. A: Gen. 2019, 573, 1–12. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional Analysis of Lignocellulosic Feedstocks. 1. Review and Description of Methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, E.; Alén, R. Analytical Methods in Wood Chemistry, Pulping, and Papermaking; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]





| Catalyst | Yield, wt% | |||||
|---|---|---|---|---|---|---|
| Liquid Products | Solid Products | Gases | ||||
| CO | CO2 | CH4 | Total | |||
| No catalyst | 36.0 | 48.0 | 0.2 | 1.5 | 3.1 | 4.8 |
| NiCuMo/SiO2 | 42.0 | 39.5 | 7.4 | 2.6 | 1.8 | 11.8 |
| Catalyst | Mn a (Da) | Mw b (Da) | PD c |
|---|---|---|---|
| No catalyst | 620 | 1870 | 3.01 |
| NiCuMo/SiO2 | 510 | 1370 | 2.66 |
| RT | Compound | Structure | Yield, wt% * | |
|---|---|---|---|---|
| No Catalyst | NiCuMo/SiO2 | |||
| 18.2 | guaiacol | 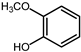 | 1.5 | 0.4 |
| 21.7 | 4-methylguaiacol |  | 0.3 | 0.7 |
| 24.4 | 4-ethylguaiacol | 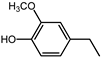 | 2.2 | 4.7 |
| 26.8 | 4-propylguaiacol | 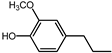 | 1.3 | 11.9 |
| 29.0 | 4-propenylguaiacol |  | 2.8 | trace |
| 33.9 | 4-propanolguaiacol | 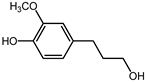 | 0.8 | 16.1 |
| Other monomer compounds | 0.7 | 1.9 | ||
| Dimer compounds | 0.1 | 1.1 | ||
| Total yield | 9.7 | 36.8 | ||
| Compound | Yields, wt% * | |
|---|---|---|
| No Catalyst | NiCuMo/SiO2 | |
| Furan derivatives | 1.8 | 4.1 |
| Methyl esters of oxo and hydroxy acids | 1.4 | 0.1 |
| Alcohols and ketones | 1.2 | 0.3 |
| Total yield | 4.4 | 4.5 |
| Catalyst | Content, wt% | Conversion, wt% | ||||
|---|---|---|---|---|---|---|
| Cellulose | Hemicelluloses | Lignin | Cellulose | Hemicelluloses | Lignin | |
| No catalyst | 66.5 | 3.0 | 30.5 | 30.1 | 99.0 | 42.0 |
| NiCuMo/SiO2 | 73.2 | 2.4 | 24.4 | 40.0 | 99.2 | 70.2 |
| Sample | Crystallinity Index (XRD) |
|---|---|
| Abies wood | 0.54 |
| Solid product of non-catalytic hydrogenation | 0.68 |
| Solid product of hydrogenation in the presence of NiCuMo/SiO2 | 0.72 |
| Catalyst | Composition, wt% | Mo/Ni Atomic Ratio | SBET, m2/g a | V∑, cm3/g b | Vmicro, cm3/g c | <d>pore, Å d | |||
|---|---|---|---|---|---|---|---|---|---|
| Ni | Cu | Mo | Si | ||||||
| NiCuMo/SiO2 | 46 | 6.7 | 11.7 | 15.0 | 0.16 | 109.0 | 0.23 | 0.01 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsov, B.N.; Miroshnikova, A.V.; Kazachenko, A.S.; Baryshnikov, S.V.; Malyar, Y.N.; Yakovlev, V.A.; Skripnikov, A.M.; Fetisova, O.Y.; Xu, Y.; Taran, O.P. Reductive Catalytic Fractionation of Abies Wood into Bioliquids and Cellulose with Hydrogen in an Ethanol Medium over NiCuMo/SiO2 Catalyst. Catalysts 2023, 13, 413. https://doi.org/10.3390/catal13020413
Kuznetsov BN, Miroshnikova AV, Kazachenko AS, Baryshnikov SV, Malyar YN, Yakovlev VA, Skripnikov AM, Fetisova OY, Xu Y, Taran OP. Reductive Catalytic Fractionation of Abies Wood into Bioliquids and Cellulose with Hydrogen in an Ethanol Medium over NiCuMo/SiO2 Catalyst. Catalysts. 2023; 13(2):413. https://doi.org/10.3390/catal13020413
Chicago/Turabian StyleKuznetsov, Boris N., Angelina V. Miroshnikova, Aleksandr S. Kazachenko, Sergey V. Baryshnikov, Yuriy N. Malyar, Vadim A. Yakovlev, Andrey M. Skripnikov, Olga Yu. Fetisova, Yong Xu, and Oxana P. Taran. 2023. "Reductive Catalytic Fractionation of Abies Wood into Bioliquids and Cellulose with Hydrogen in an Ethanol Medium over NiCuMo/SiO2 Catalyst" Catalysts 13, no. 2: 413. https://doi.org/10.3390/catal13020413
APA StyleKuznetsov, B. N., Miroshnikova, A. V., Kazachenko, A. S., Baryshnikov, S. V., Malyar, Y. N., Yakovlev, V. A., Skripnikov, A. M., Fetisova, O. Y., Xu, Y., & Taran, O. P. (2023). Reductive Catalytic Fractionation of Abies Wood into Bioliquids and Cellulose with Hydrogen in an Ethanol Medium over NiCuMo/SiO2 Catalyst. Catalysts, 13(2), 413. https://doi.org/10.3390/catal13020413








