Abstract
The current work focuses on the photo degradation of organic pollutants, particularly methylene blue (MB) dye, and the production of hydrogen as green energy using a composite of silver phosphate Ag3PO4 (AP) and barium oxide/silver phosphate BaO@Ag3PO4 (APB) as a photocatalyst. This composite was successfully synthesized using a chemical co-precipitation approach. The physicochemical properties of the obtained samples were investigated using scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), ultraviolet–visible diffuse reflectance spectroscopy (UV–Vis/DRS), and photoluminescence (PL) spectrophotometry. From XRD, the average crystallite sizes of AP and APB are 39.1 and 46 nm, respectively, with a homogeneous morphology detected by SEM. UV and PL experiments showed that the compound is active under visible light, with an improvement in the lifetimes of the electrons and the holes in the presence of BaO with Ag3PO4. The as-synthesized APB photocatalyst sample showed a remarkably high degradation efficiency of MB (20 ppm, 50 mL) of around 94%, with a hydrogen production yield of around 7538 μmol/(h·g), after 120 min of illumination, which is greater than the degradation efficiency of the AP photocatalyst sample, which was about 88%. The high photodegradation efficiency was attributed to the electronic promotion effect of the BaO particles. The APB composite demonstrated an increased photocatalytic performance in effectively degrading an organic dye (MB) with no secondary pollutants when exposed to visible light irradiation.
1. Introduction
Water treatment is becoming a major global concern due to a scarcity of clean drinking water, a considerable growth in the population [1], and an increase in water-polluting industries such as oil refineries, dye factories, and hospitals [2]. There are three major types of waste pollutants: biological contaminants (bacteria, viruses, and fungi), mineral pollutants (sulphate, nitrate, and heavy metals), and organic contaminants (dung, medicinal, dyestuffs, oil, and parasites) [3]. Effective methods for removing organic compounds from water have sparked considerable interest. For the removal of organic pollutants from polluted water and wastewater, a variety of techniques, including coagulation, filtration with coagulation, precipitation, ozonation, adsorption, ion exchange, reverse osmosis, and advanced oxidation processes (AOPs), have been used.
The photocatalysis and AOPs subset is an environmentally benign method that utilizes clean sunlight to degrade organic pollutants in water. In another are of attention, hydrogen production is one of the most promising renewable energy sources. Its nature is eco-friendly (carbon-free fuel), limitless, efficient, and cost-attractive [4]. Meanwhile, environmental problems resulting from the combustion of non-renewable fossil fuels have compelled scientists to focus on exploring green and renewable alternative energy sources. Solar-driven hydrogen production has drawn considerable interest, as solar energy is a clean, inexhaustible energy resource. Due to the daily and seasonal variability of sunlight, the energy harvested from the sun needs to be efficiently converted into clean chemical fuel that can be stored, transported, and used upon demand.
Photocatalytic hydrogen generation is a clean, eco-friendly, and renewable source. Researchers are interested in semiconductor-based photocatalysts because they exhibit a high efficiency when exposed to visible light. Studies have shown that, when compared to the majority of other known photocatalysts, these photocatalysts have significantly improved photo-oxidative capabilities and a higher photocatalytic degradation efficiency [5]. Among of them, silver orthophosphate (Ag3PO4) is currently an important host material for activator ions in its lattice because of its exceptional chemical stability, greater product yield, and low annealing temperature, and because it has a suitable band gap of approximately 2.45 eV [6]. Furthermore, it has been identified as a promising photocatalyst activated by visible light irradiation and shows strong photo-oxidative activity for the production of O2 from water through the splitting and degradation of the organic pollutants as dyes [7]. However, there is one major disadvantage to applying pure Ag3PO4, and that is its sensitivity to photo illumination, as a result of the photo-generated corrosion electrons [8]. To overcome this limitation, Ag3PO4 has been conjugated with other semiconductor materials such as SnO2 [9], AgBr [10], In(OH)3 [11], CeO2 [12], TiO2 [13], NiFe2O4 [14], and ZnFe2O4 [15]. Another material that has received extensive interest as a means to improve the photo stability and photoactivity of Ag3PO4 is barium oxide (BaO) [16]. Whereas (BaO) is frequently used to improve catalyst activity and has previously been used as a dopant for producing efficient and reusable photocatalysts, it is also widely used in many applications such as artefacts, wall paintings [17], the biomedical field [18], catalysts [19], the pharmaceutical industry [20], radiation dosimetry [21], etc.
The work presented herein focused on the visible-light-driven photo degradation of a dye pollutant, in particular methylene blue (MB) dye, and on hydrogen production using the nanostructure systems of Ag3PO4 and BaO@Ag3PO4 (APB) compounds as a photocatalyst. Furthermore, the physicochemical properties of the obtained samples were extensively studied to explore their excellent photocatalytic activity for the degradation of MB under visible light irradiation and their greater rate of hydrogen production as compared to pure Ag3PO4. The synthesised APB proved to be an effective photocatalyst material for applications related to environmental protection.
2. Results and Discussion
The physicochemical properties of the obtained samples were characterized using scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), ultraviolet–visible diffuse reflectance spectroscopy (UV–Vis/DRS), and photoluminescence (PL) spectrophotometry.
2.1. Structural Analysis
2.1.1. Scanning Electron Microscopy (SEM) Analysis
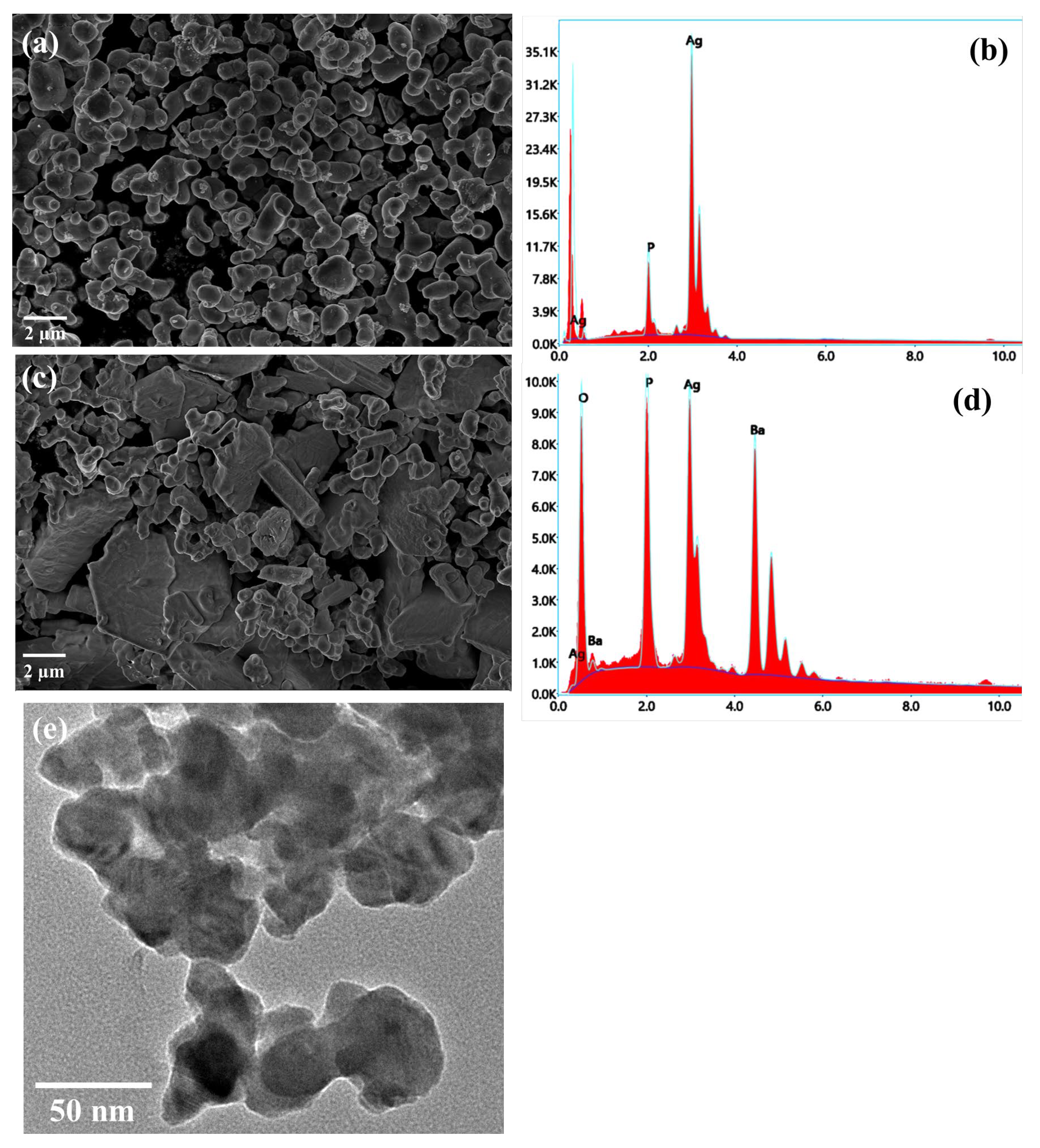
The surface morphologies of the prepared materials were analyzed using SEM. In order to observe the morphology of the composite sample, SEM images of the AP and APB samples were recorded and are shown in Figure 1. The SEM image of pure Ag3PO4 presents a nanorod-like structure with a small spherical particle on the surface (Figure 1a). In the presence of BaO, these plates became smoother and sharper (Figure 1c). However, compared with pure Ag3PO4, the particle length-to-width ratio is larger, and the rods appear to be more elongated. This result clearly indicates that BaO conjugation with Ag3PO4 changes the morphology of the pure Ag3PO4. Energy dispersive X-ray spectroscopy (EDX) was used to perform a quantitative element analysis of the samples. The EDX diagram of Ag3PO4 indicates that the main elements in the material are Ag, P, and O, and that no impurity elements are present, as shown in Figure 1b. This proves the formation of Ag3PO4. The EDX diagram of BaO@Ag3PO4 reveals that the main elements are Ag, P, O, and Ba, and that no impurity elements are present, as shown in Figure 1d. This indicates the successful incorporation between BaO and Ag3PO4. A TEM image shows that the particle size of the BaO@Ag3PO4 composite is around 40 nm (Figure 1e), which matches the XRD result.
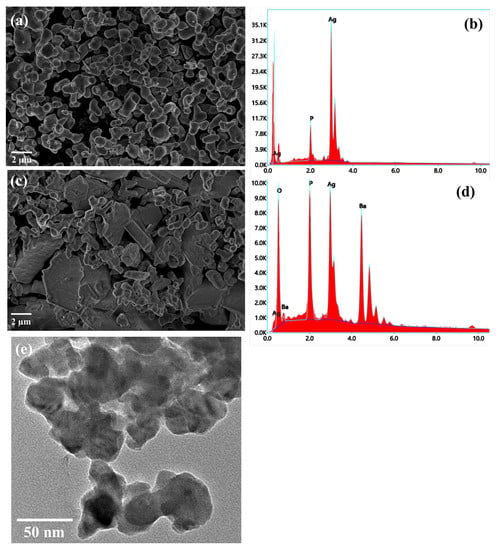
Figure 1.
(a) Top-view SEM image of Ag3PO4 sample and (b) its EDX spectroscopy; (c) SEM image of BaO@Ag3PO4 composite and (d) its EDX spectroscopy; and (e) TEM image of BaO@Ag3PO4 composite.
2.1.2. X-ray Diffraction (XRD) Analysis
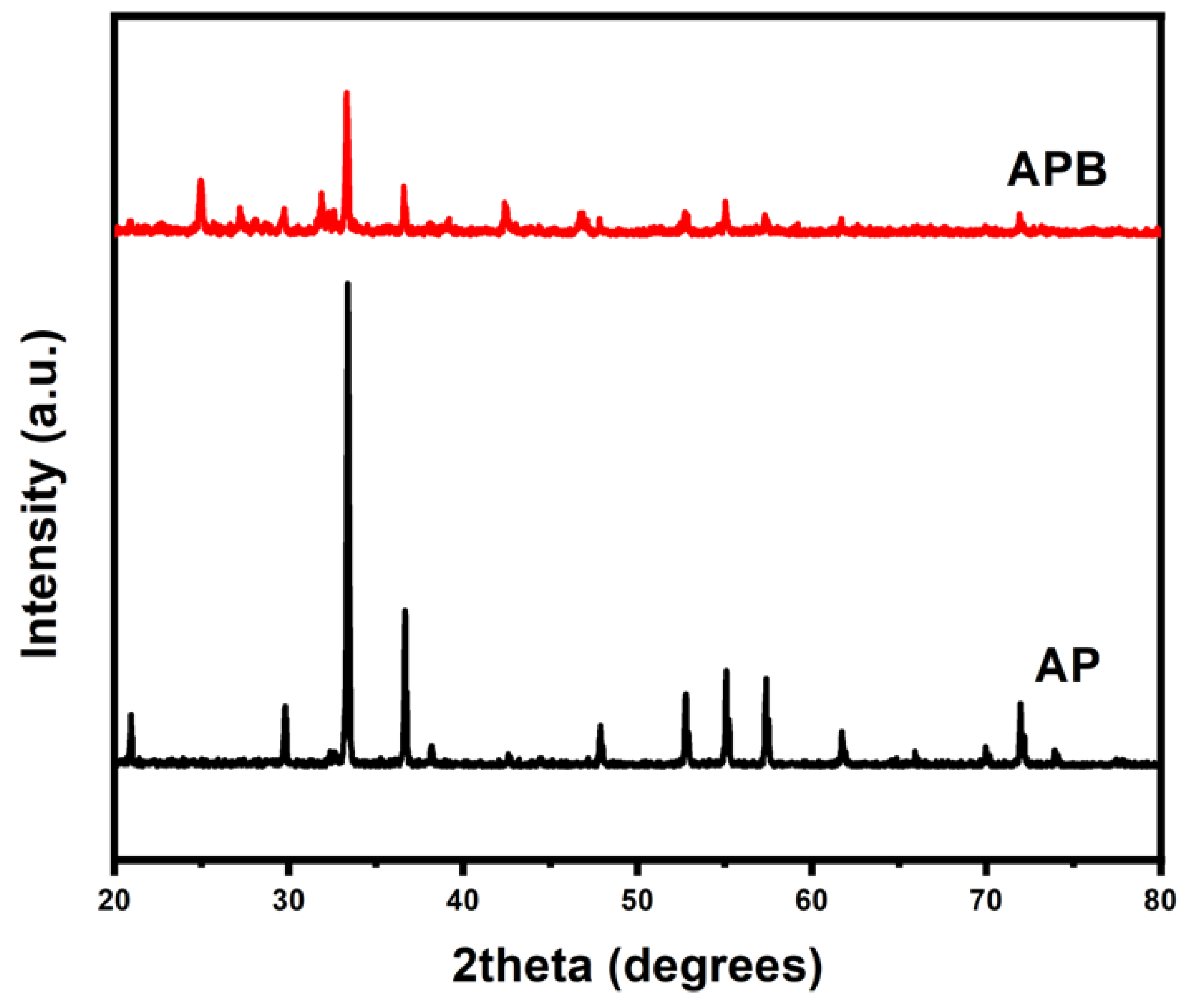
The XRD spectrum of the pure Ag3PO4 and the BaO@Ag3PO4 nanocomposite in the present study is shown in Figure 2. In the XRD pattern of the pure AP, the main diffraction peaks are shown at the 2θ positions [20.37°, 29.32°, 32.90°, 36.25°, 47.89°, 52.37° and 54.60°], which corresponds to plans (110), (200), (210), (211), (220), (310), and (222), those indexed to the pure body-centered cubic structure of AP according to the standard spectrum JCDPs No. (01-074-1876). In addition, the XRD patterns of the APB nanocomposite show peaks at the 2θ positions [24.91°, 27.19°, 29.61°, 31.90°, 33.57°, 36.57°,42.40°, 46.76°, 52.71°and 55.01°] of the synthesized material. The peaks were indexed by comparing them with the standard data available, JCDPs No. (00-033-16167), which confirmed the successful synthesis of the BaO@Ag3PO4 composite, and no impurity diffraction peaks were observed. The average crystallite sizes of the samples have been calculated from the full width at half maximum (FWHM) of the XRD pattern in Figure 2, using the Williamson–Hull formula [21],
where β is the FWHM in radians, λ is the wavelength of the X-rays used, θ is the scattering Bragg angle, D is the particle diameter, and S is the strain. The parameter K is the shape factor (taken as 0.94). The crystallite sizes of the samples were 39.1 and 40 nm for the pure AP sample and the APB composite, respectively.
β × cos(θ) = [K × λ/D] + [4 × S × sin(θ)]
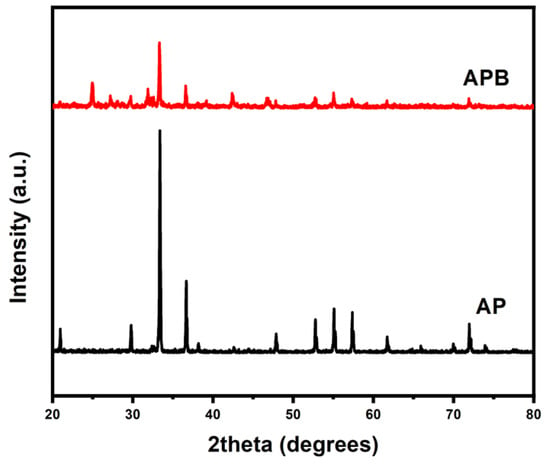
Figure 2.
The powder X-ray diffraction patterns of pure Ag3PO4 and BaO@Ag3PO4 nanocomposite.
2.1.3. FT-IR Spectral Analysis
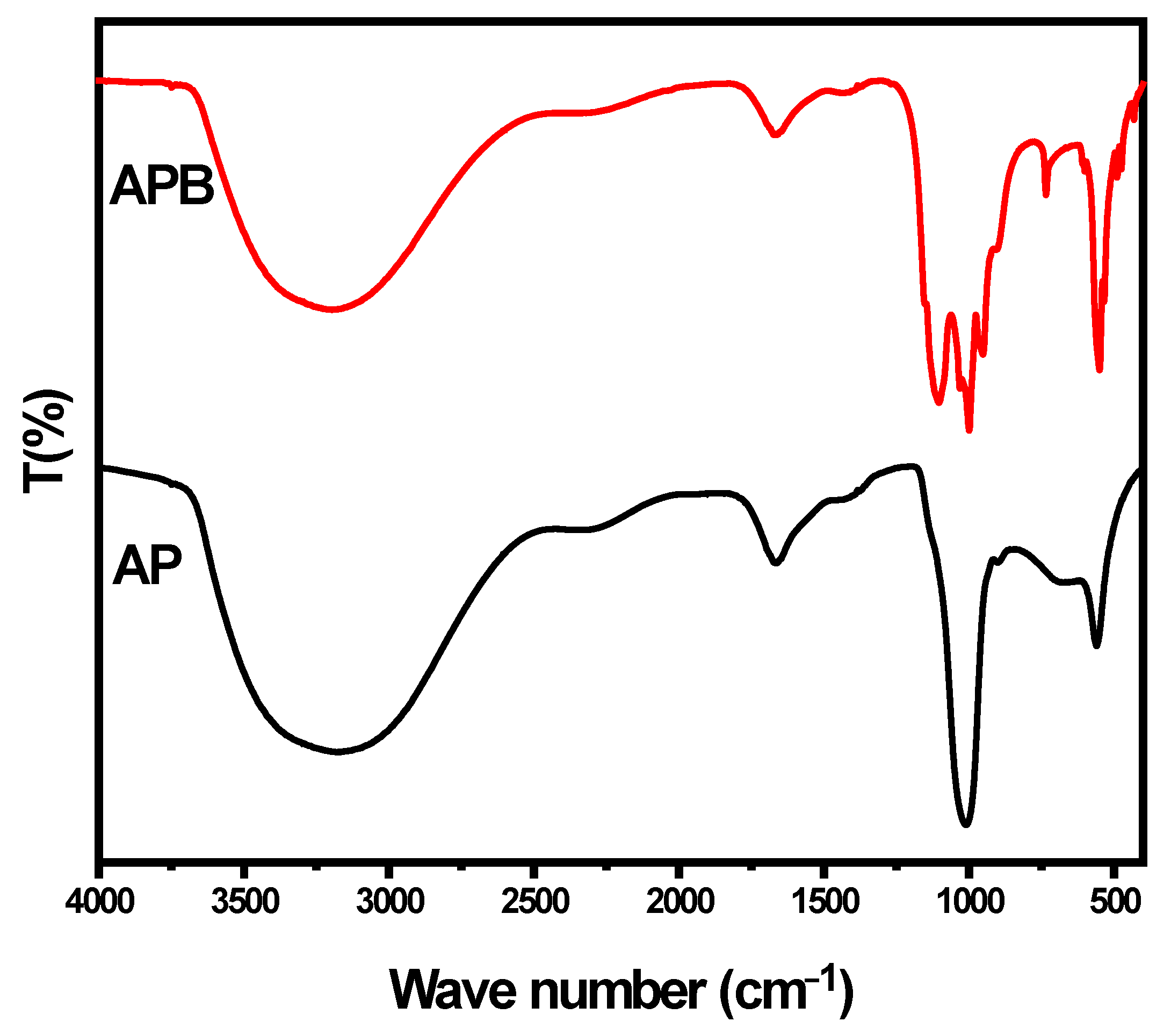
The FTIR spectra of the as-prepared sample, shown in Figure 3, were measured in the range of 400–4000 cm−1. The spectra of the pure Ag3PO4 exhibit strong bands at 3178 and 1669 cm−1 that may be attributed to the bending modes of the O-H groups (P–O–H bridges) and molecular water due to moisture absorption during the KBr pellet preparation [21]. The absorption band at 560 cm−1 corresponds to the symmetric stretching modes of the P–O–P linkages, while the band at 901.87 cm−1 represents the asymmetric stretching mode of P–O–P links [21]. The band appearing at 1010 cm−1 is attributed to P–O− groups, which are vibrational and asymmetric stretching modes of the chain terminating (PO3)2− [21]. In the presence of BaO, different vibrational bands are found to shift and present new bands at (534, 952, 734, 608, 489, and 431). This indicates that BaO changes the structure of the pure silver phosphate. On the basis of the FT-IR spectra, it can be inferred that, after the addition of BaO, the basic structure of Ag3PO4 is almost the same, but some P-O-Ag bonds present in the pure Ag3PO4 may be replaced by P-O-BaO bonds. It may be proposed that Ba2+ ions are attached to the negative end of the P-O bond. Thus, the conjugation metal ions form a P-O-Ba bond in the glass structure, and Ba2+ ions serve as ionic cross-links between the non-bridging oxygen of two different phosphate chains [21].
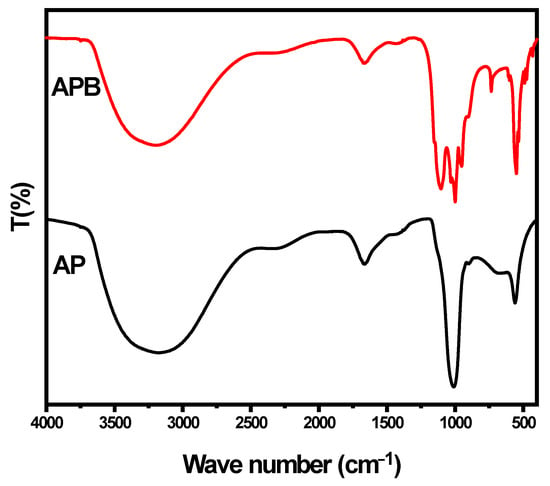
Figure 3.
FT-IR spectra of pure Ag3PO4 and BaO@Ag3PO4 nanocomposite.
2.2. Optical Analysis
The optical properties of the prepared samples were investigated using UV–Vis DRS and PL spectra at room temperature.
2.2.1. The DRS Spectra and Band Gap
There are several mechanisms that control photocatalytic activity: the production of electron–hole pairs, photo absorption, charge carrier transfer, and charge carrier utilization. The optimization of the photocatalytic activity depends on the efficiency of the product and the transfer of the e−/h+ pairs, which depends on the energy band gap (Eg) of the photocatalyst. The energy band gap values (Eg) of the samples were calculated according to the following Equation (2) from the reflectance curves [22,23]:
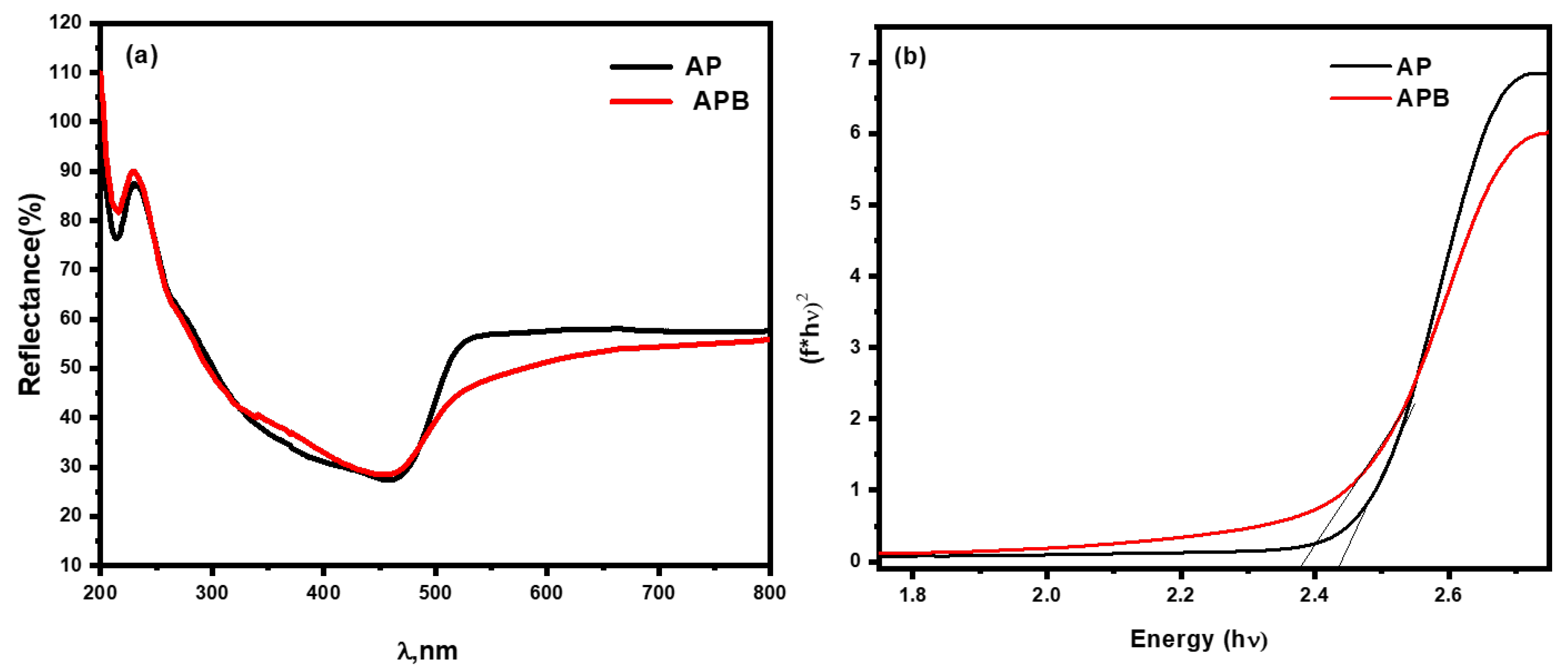
where α is the absorption coefficient, v is the frequency of light, and n is the constant of proportionality. The n value is determined by the transition of the semiconductor, i.e., the direct transition as in the prepared nanocomposite (n = 1). The diffuse reflection spectra of the as-prepared Ag3PO4 and BaO@Ag3PO4 composite were investigated using UV–Vis optical spectroscopy in the range of 200–800 nm, as shown in Figure 4. It is observed that the pure Ag3PO4 could absorb visible light as shown in Figure 4a, in agreement with the results previously reported [21]. In contrast, the BaO@Ag3PO4 composite has a higher absorbance than Ag3PO4 in the range of 500–800 nm. The band gap value of pure Ag3PO4 is 2.43 eV. However, the band gap of the BaO@Ag3PO4 nanocomposite is 2.36 eV shifted towards the red shift in the presence of barium oxide. The optical band gaps were calculated from (F(R) hʋ)1/2 against the photon energy (hʋ) plots as showed in Figure 4b. The conjugation of the two band gaps led to more stability between the e−/h+ pairs, as the band gap reduces the quantum confinement effect. Moreover, BaO@Ag3PO4 nanocomposites can be excited under visible light to give more electron–hole pairs. This remarkable absorption enhancement in the visible light region is beneficial for improving the photocatalytic activity in this irradiation region.
α hv = [A(hv-Eg)]^(n/2)
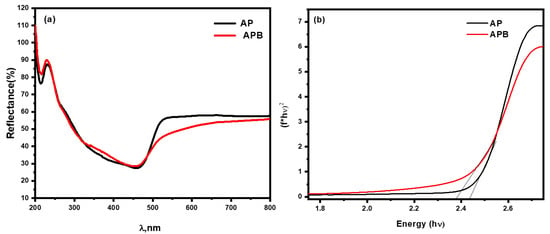
Figure 4.
(a) UV–Vis diffuse reflectance spectra of pure Ag3PO4 and BaO@Ag3PO4 nanocomposite. (b) Band gap spectra of pure Ag3PO4 and BaO@Ag3PO4 nanocomposite.
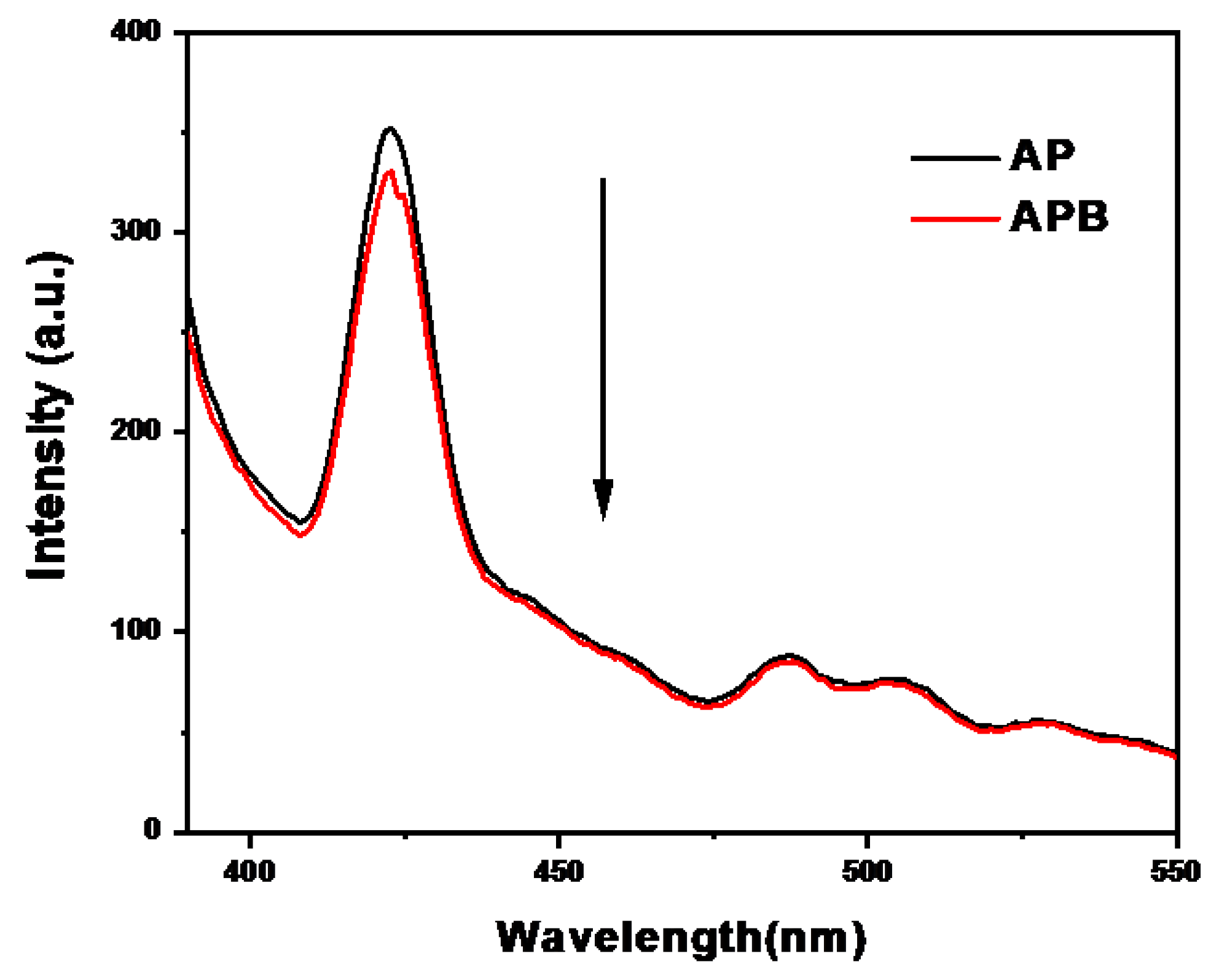
2.2.2. Photoluminescence (PL)
Figure 5 displays the room temperature photoluminescence (PL) spectra of the AP and the APB composite at an excitation wavelength of 370 nm. The spectra show the rate of recombination and charge separation within the photocatalysts. The PL spectrum of pure silver phosphate shows a strong emission band at 422 nm, while the APB composite shows a significant intensity decrease in PL as a result of the efficient charge transfer at the heterostructure interface, indicating that the recombination between the photo-electrons generated and the holes is reduced. Moreover, there is a synergistic effect between the AP and the BaO as a heterostructure that decreases the PL intensity, which leads to an increase in the lifetime of electron stability [21].
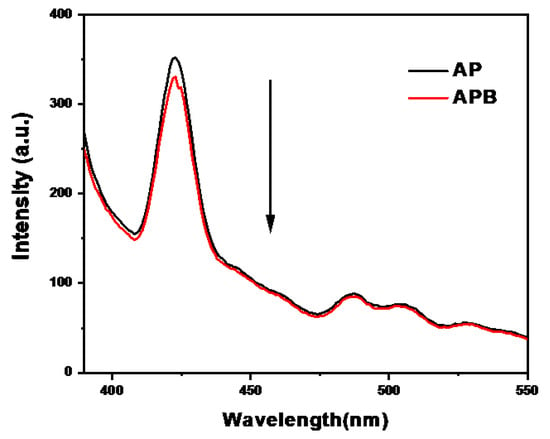
Figure 5.
Photoluminescence spectra of pure AP and APB composite.
2.3. Photocatalytic Activity of APB Composite
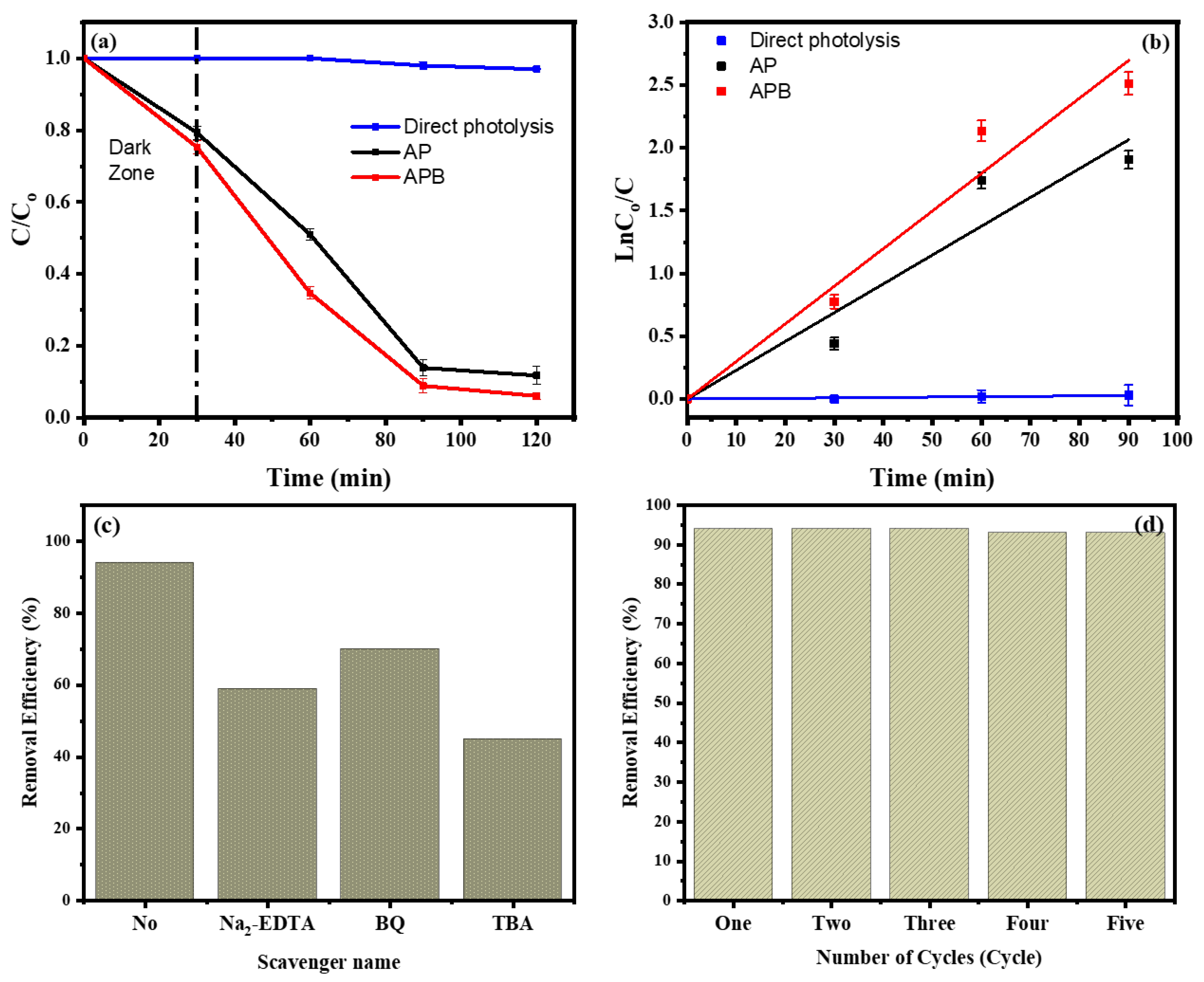
The photocatalytic degradation of MB dyes under visible light caused by the pure Ag3PO4 and the BaO@Ag3PO4 composite is shown in Figure 6a. As presented in this figure, it can clearly be seen that the APB composite has a higher photocatalytic activity for the degradation of MB than the Ag3PO4 photocatalyst. Not only did the BaO act as an electron promoter and improve the charge separation of electrons and holes that was created by light irradiation, but the BaO also improved the stability of the electron–hole pairs. Figure 6a measures the degradation at times of irradiation of 0, 30, 60, 90, and 120 min. Before the photocatalytic reaction, the photocatalyst’s MB solution was kept for 30 min in the dark to reach the adsorption/desorption equilibrium. This equation gives the efficiency of the degradation of MB:
where C0 is the initial concentration and C is the remaining concentration of MB after the reaction. As shown in Figure 6a, the efficiency of the MB removal in the presence of pure AP and of APB is about 88% and 94%, respectively, after 120 min. According to the L–H kinetics model, the kinetics of the MB degradation by the prepared nanocatalysts was evaluated. The equation of pseudo-first-order kinetics can be expressed as
where ka is the rate constant (min−1), C0 is the initial concentration (mg L−1), and C is the concentration of the MB solution at the irradiation time t (min). Figure 6b shows the relation between ln (C0/C) and time. Ka can be obtained from the linear relation between them, and it is (3.137 × 10−4, 0.02295 and 0.02996 min−1) for MB, AP, and APB, respectively. The rate constants are increased in the following order: APB ˃ AP ˃ MB. Based on these results, the APB composite shows the best activity. These characteristics increase their photocatalytic activity under visible light, and this is proved by their enhanced degradation of MB under visible light irradiation. Therefore, the as-prepared APB composites can work as effective photocatalysts for organic compound degradation with good stability. In addition, as shown in Table 1, BaO/Ag3PO4 had the highest photocatalytic activity under visible light illumination in comparison with the results of earlier studies.
D% = (C0−C/C0) × 100
ln(C0/C) = ka × t
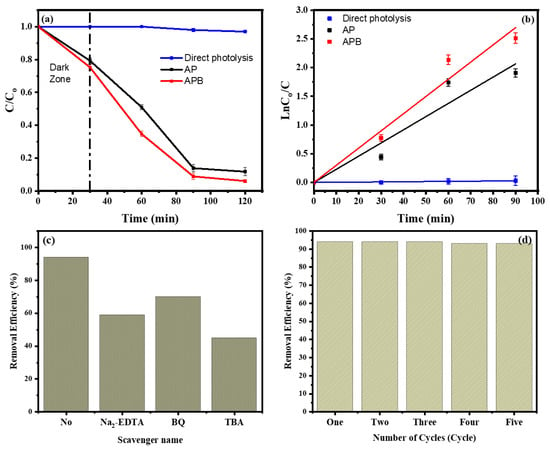
Figure 6.
(a) The removal curve of MB in the presence of pure Ag3PO4 and BaO@Ag3PO4 nanocomposite. (b) Kinetics of MB removal by pure Ag3PO4 and BaO@Ag3PO4 nanocomposite. (c) The influence of different scavengers on the removal efficiency of MB by BaO@Ag3PO4 and (d) the removal efficiency of BaO@Ag3PO4 as a catalyst for MB degradation throughout five consecutive cycles of filtration and reuse (after 2 h).

Table 1.
Removal of MB under visible light, with various photo catalysts.
In addition, we used several free radical trapping agents with the BaO@Ag3PO4 nanocomposite as described to make inferences about the roles of hydroxyl radicals (•OH), holes (h+), and superoxide anions (•O2−) in the efficiency of the photodegradation of MB (Figure 6c). The trapping agents were Tert-butyl alcohol (TBA), disodium ethylenediaminetetraacetic acid (Na2-EDTA), and p-benzoquinone (BQ), which were used to trap free radicals of hydroxyl radicals (•OH), holes (h+), and superoxide radicals (•O2−), respectively [24,25]. The photodegradation of MB differed depending on the sacrificial agent. The photocatalytic degradation was reduced to 45% in the presence of TBA (5 mM). As a result, during the photodegradation of MB, the •OH radical that was created by the photo-process was crucial [26]. Additionally, as shown in Figure 6d, the BaO@Ag3PO4 nanocomposite exhibits strong stability in its MB removal for up to five cycles. After five cycles, the photodegradation of MB was still stable at 93%. These BaO@Ag3PO4 nanocomposite results show a strong photodegradation of MB with a narrow energy band gap as well as a remarkable stability of catalytic efficiency.
2.4. Photocatalysis Mechanism for Degradation of Organic Pollutant by APB Composite
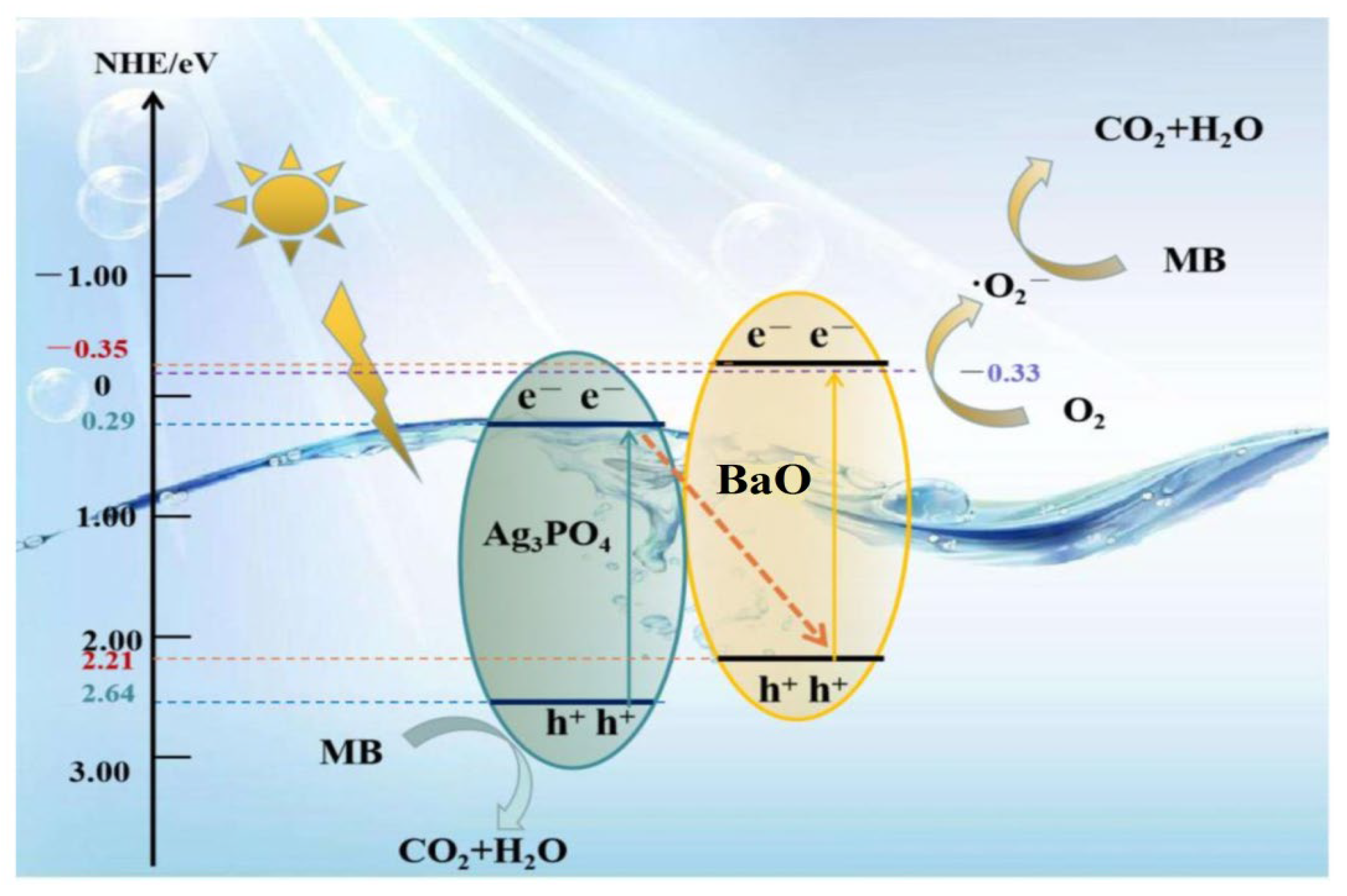
The mechanisms of APB photocatalysts presented in this part are based on all of the characterizing results described previously. The presence of BaO enhanced the light absorption of the catalyst to be active in the visible region. It has a small band gap energy which is active in the visible region. In addition, the junction between Ag3PO4 and BaO enhanced the stability between the electron–hole pairs. Moreover, BaO plays an important role in capturing the electrons from the conduction bands of the Ag3PO4 to increase the lifetime of the electrons and, subsequently, enhance the photocatalytic activity, as recorded in this study. Thus, the mechanism of the APB photocatalysts after irradiation under visible light is summarized by the following equations (Equations (5) to (12)) and illustrated in schematic the graph in Figure 7.
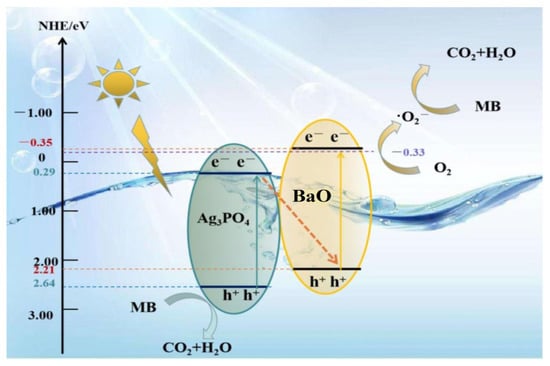
Figure 7.
Schematic diagram of the photocatalytic mechanism of BaO@Ag3PO4 nanocomposite under visible light irradiation.
The photocatalytic APB can be excited under visible light irradiation to generate electron–hole pairs (Equation (5)). The electrons in the CB of the Ag3PO4 were transferred to the surface of the BaO, where BaO acted as an electron acceptor and increased the formation of oxide radicals (•O2−) through a reduction process at the conduction band (Equation (6)). These oxide radicals played important roles in the degradation of organic pollutants and the formation of hydroxyl radicals (Equation (7)). The holes in the valance band at Ag3PO4 formed hydroxyl radicals with water molecules according to Equations (8)–(11). Finally, the organic pollutant was degraded by the hydroxyl radicals into environmentally friendly molecules: CO2 and H2O (Equation (12)).
Ag3PO4/BaO + hʋ → h+ + e−
Ag3PO4 (e−)/BaO + O2 → •O2−
•O2− + 2H2O → 4OH•
h+ + H2O → OH• + H+
H2O + H+ + •O2− → H2O2 + OH•
H2O2 + e− → OH− + OH•
h+ + OH− → OH•
OH• + organic pollutant → degradation to CO2 + H2O
2.5. Hydrogen Evolution Detected by Prepared Photocatalysts
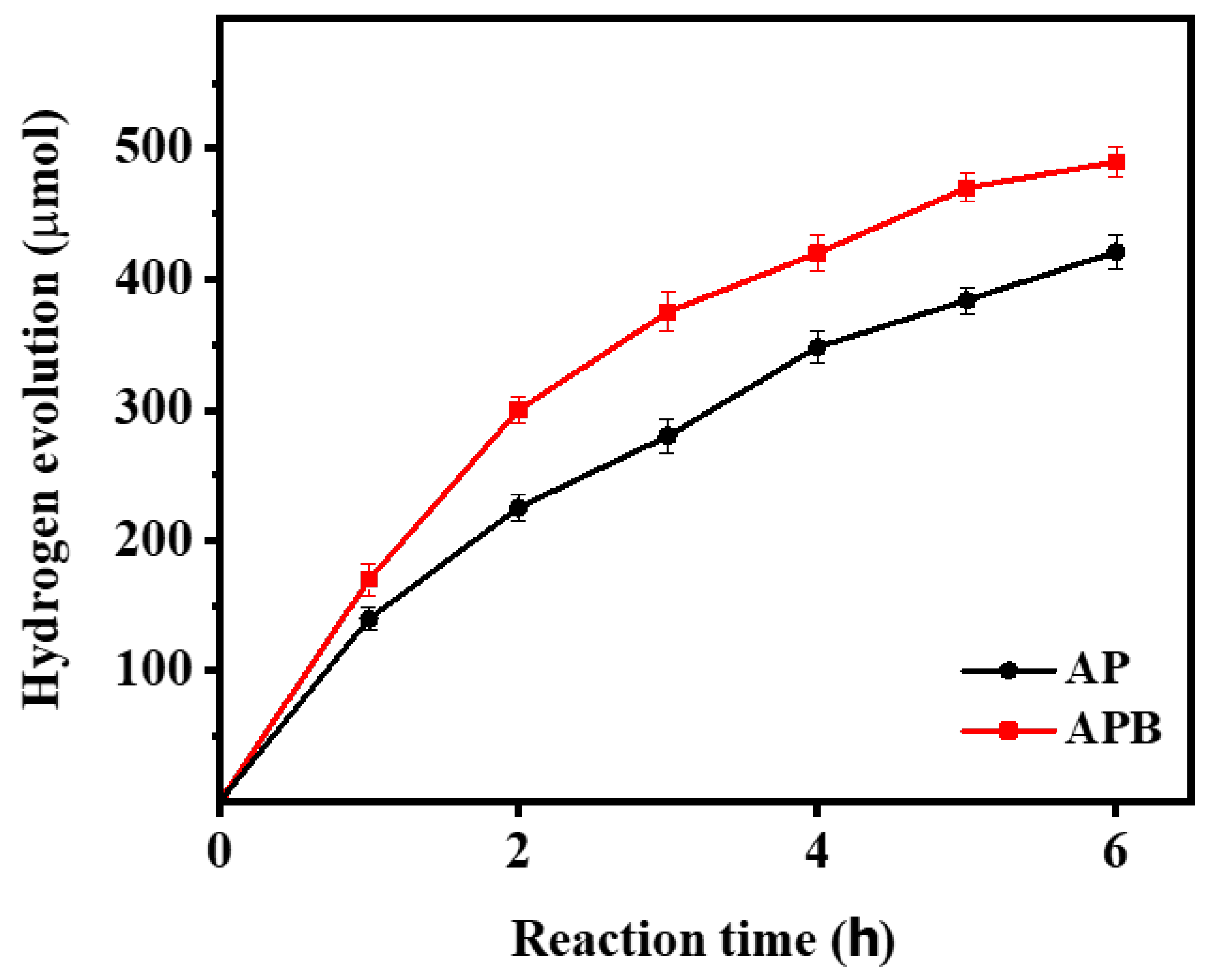
The photocatalytic H2 evolution of the pure Ag3PO4 and the BaO@Ag3PO4 nanocomposite under visible light irradiation is shown in Figure 8. Figure 8 shows that the BaO@Ag3PO4 photocatalyst achieves an H2-production rate of up to 490 µmol during 6 h under visible light irradiation. It is found that the photocatalytic activity of the composites is highly related to the content of the BaO and the synergism effect between the BaO and the Ag3PO4. To describe the true hydrogen production efficiency of a water-splitting reaction under visible light, we detected the quantum efficiency of the photocatalysts, and it is 5.3 and 4.5% for Ag3PO4 and BaO@Ag3PO4, respectively. The quantum efficiency was calculated from the conversion efficiency according to the following equation [23]:
where H2 is the hydrogen evolution rate, which was multiplied by the Gibbs free energy for generating one mole of hydrogen from water; Ptotal is the total power of the irradiation light; and Area is the area which was exposed to the incident light. Compared to earlier investigations of other nanocomposite photocatalysts, the obtained nanocomposite BaO@Ag3PO4 produces hydrogen with strong activity, as shown in Table 2.
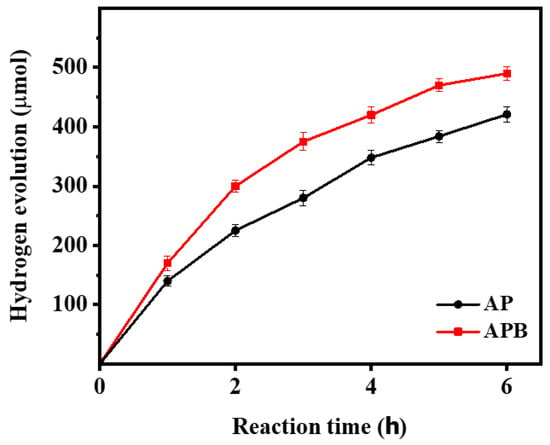
Figure 8.
Comparison of the photocatalytic activity of the pure Ag3PO4 and BaO@Ag3PO4 nanocomposite samples for photocatalytic hydrogen production.

Table 2.
Photocatalytic activity of various photocatalysts.
3. Materials and Methods
3.1. Chemicals
All of the chemicals used in this study were of analytical grade and were purchased from Sigma Aldrich (Cairo, Egypt). Silver nitrate (AgNO3), Ammonium dihydrogen phosphate (NH4H2PO4), Barium carbonate (BaCO3), and NaOH powder were obtained from Sigma-Aldrich (Cairo, Egypt). Methylene blue (MB) dye was purchased from Merck KGaA (Frankfurter, Germany). All solutions were prepared using freshly deionized water. All chemicals were of analytical grade and were used as received from the supplier.
3.2. Methods
3.2.1. Synthesis of Ag3PO4 Nanoparticles
The Ag3PO4 nanoparticles (NPs) sample was synthesized using the co-precipitation method according to the following steps: An appropriate amount of AgNO3 salt (0.5 gm) was dissolved completely in 50 mL of deionized water (DI) under continuous stirring at room temperature. An equal volume of NH4H2PO4 crystalline powder (0.5 gm) was dissolved in 50 mL of deionized water (DI) under continuous magnetic stirring at room temperature [18]. Then, the first solution containing the AgNO3 salt was added slowly to the second solution containing NH4H2PO4 under continuous stirring. After that, the resulting solution was sonicated for 15 min to avoid any agglomeration. As a result, a yellow precipitate was obtained and then collected and washed several times with distilled water. Finally, the precipitate was dried in an oven at 100 °C overnight, and then calcined at 400 °C for 1 hr to obtain the Ag3PO4 nanocrystalline sample [23].
3.2.2. Preparation of BaO@Ag3PO4 Composite
The BaO@Ag3PO4 composite was synthesized at room temperature using a co-precipitation technique through the following steps: An appropriate amount of BaCO3 salt (0.5 gm) was dissolved completely in 50 mL of deionized water (DI) under stirring at room temperature. Next, 0.25 gm of AgNO3 salt was added to 0.25 gm of NH4H2PO4 and dissolved in 50 mL of deionized water (DI) under stirring at room temperature. The first solution containing BaCO3 salt was added dropwise, in a slow manner, to the second solution under vigorous stirring at room temperature. Later on, the solution was sonicated for 15 min to avoid any agglomeration. After that, the obtained yellow precipitate was collected and washed several times with distilled water, then dried in an oven at 100 °C overnight, and then ground and calcined at 400 °C for 1 h to obtain the BaO@Ag3PO4 composite [18].
3.2.3. Characterization Techniques
The crystallographic patterns of the prepared nanocomposite were investigated using Philips X’Pert X-ray diffraction (XRD) with Cu-Ka radiation (λ = 1.54056° A) and an accelerating voltage of 40 kV and 40 mA. A typical 2θ scan was between 10–80° and conducted at a scan rate of 4°/min (Panalytical, Almelo, Netherlands). Fourier-transform infrared spectroscopy (FTIR) was performed using a Nicolet iS10 FTIR spectrometer to identify the surface functional groups in the synthesized materials (Perkin Elmer, Waltham, MA, USA). The morphological features were evaluated by scanning electron microscopy (SEM, Hitachi S-4800, Boise, USA). Ultraviolet-visible spectroscopy (UV–Vis, Jasco V-507) coupled with a Shimadzu IRS-2200 (Perkin Elmer Lambda 1050,Waltham, MA, USA) diffuse reflectance spectroscopy (DRS) was used to investigate the optical properties of the prepared materials. Solid-state photoluminescence (PL) analysis was also performed using a Perkin Elmer fluorometer (model LS-55) at room temperature.
3.2.4. Photocatalytic Activity Study and H2 Production
The photocatalytic activity of the synthesized nanocomposite was evaluated for the degradation of the MB dye as a model of organic pollutants under visible light irradiation. The experiments were carried out under visible light irradiation at room temperature by a halogen lamp with a power of 500 W, and the distance from the light source to the cell was 10 cm, with 80 mW/cm2 measured by X1-1 Optometer (Gigahertz-Optik) RW-3705-4 (400–1000 nm) calibrated detectors. In each experiment, 50 mL of MB dye solution with an initial concentration of 20 ppm was taken in a glass beaker, and 2.0 g/L of the prepared catalyst was added.
The solution was stirred in the darkness for 30 min until it reached an adsorption/desorption equilibrium between the MB dye molecule and the catalyst. Then, the experiment was carried out further with constant stirring under visible light irradiation. After that, we collected 3 mL of the solution 4 times every 30 min, and we separated the catalyst by centrifuge and then detected the concentration using a UV–visible spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) at the maximum absorption wavelength (622 nm). In order to test the catalyst’s activity, a dark reaction was performed. In the absence of the catalyst, a blank experiment was carried out, and it was observed that the dye degradation was limited.
For the H2 evolution test, 10 mg of photocatalyst was dispersed with a constant stirring in 50 mL of an aqueous solution of sacrificial reagent (0.1 M Na2S + 0.02 M Na2SO3). After a given irradiation time, the evolved hydrogen was collected and monitored using gas chromatography (micro-GCAgilent, Santa Clara, California, United States) with a 5 Å molecular sieve column (3 m × 2 mm). Each experimental run was performed in triplicate to observe the reproducibility, so the photocatalytic results presented in this work are the average of three replicates.
4. Conclusions
In summary, the APB composite has been successfully synthesized using a simple co-precipitation method as a novel catalyst for methylene blue (MB) degradation. Afterwards, the physicochemical properties of the resulting samples were studied through a series of characterizations. Through these characterizations, it was found that the addition of BaO slightly changed the morphology of the material; additionally, it greatly improved its bandgap structure and improved the range of its response to visible light. From UV–visible studies, it was shown that the optical band gaps are 2.43 and 2.36 eV for the Ag3PO4 and the APB, respectively, which indicates that the APB absorbs in both the UV and visible regions. Additionally, the composite has a high separation efficiency for the photo-generated electron and hole, and the major active species engaged in the reaction are O2− and h+. Furthermore, the as-synthesized photocatalyst exhibited excellent photocatalytic efficiency. Under visible light irradiation, nearly 94% of the MB (20 mg L−1, 50 mL) can be degraded by only 0.02 g of the catalyst in 120 min. In addition, the enhanced visible light mechanism of the Ag3PO4/BaO composite was proposed. Overall, a higher photocatalytic activity for MB degradation is obtained for the APB photocatalyst than for the Ag3PO4 photocatalyst, which is ascribed to the highly synergistic charge transfer between the BaO and the Ag3PO4 support. On the basis of these results, it can be concluded that the BaO@Ag3PO4 composite is a promising photocatalyst candidate for the photodegradation of hazardous organic materials in wastewater.
Author Contributions
Conceptualization, H.S., E.R.S. and A.A.N.; methodology H.S., R.E. and H.H.E.-M.; validation, all authors; formal analysis, H.S., R.E. and H.H.E.-M.; investigation, H.S, E.R.S., H.H.E.-M., P.R. and A.A.N.; resources, E.R.S. and H.S.; data curation, H.S. and A.A.N.; writing—review and editing, H.S., E.R.S., H.H.E.-M., R.E., P.R. and A.A.N.; visualization H.S., E.R.S., R.E., P.R., H.H.E.-M. and A.A.N.; supervision, A.A.N. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Liu, B.; Fang, Y.; Li, Z.; Xu, S. Visible-light nanostructured photocatalysts—A review. J. Nanosci. Nanotechnol. 2015, 15, 889–920. [Google Scholar] [CrossRef]
- Taghdiri, M. Selective adsorption and photocatalytic degradation of dyes using polyoxometalate hybrid supported on magnetic activated carbon nanoparticles under sunlight, visible, and UV irradiation. Int. J. Photoenergy 2017, 2017, 8575096. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Armor, J. Catalysis and the hydrogen economy. Catal. Lett. 2005, 101, 131–135. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.W.; Choi, W. Platinized WO3 as an environmental photocatalyst that generates OH radicals under visible light. Environ. Sci. Technol. 2010, 44, 6849–6854. [Google Scholar] [CrossRef]
- Liu, J.; Fu, X.; Chen, S.; Zhu, Y. Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method. Appl. Phys. Lett. 2011, 99, 191903. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef]
- Agbe, H.; Raza, N.; Dodoo-Arhin, D.; Chauhan, A.; Kumar, R.V. H2O2 rejuvenation-mediated synthesis of stable mixed-morphology Ag3PO4 photocatalysts. Heliyon 2018, 4, e00599. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Huang, H.; Liu, Y.; Kang, Z. Ag3PO4/SnO2 semiconductor nanocomposites with enhanced photocatalytic activity and stability. New J. Chem. 2012, 36, 1541–1544. [Google Scholar] [CrossRef]
- Jinfeng, Z.; Tao, Z. Preparation and characterization of highly efficient and stable visible-light-responsive photocatalyst AgBr/Ag3PO4. J. Nanomater. 2013, 2013, 6. [Google Scholar] [CrossRef]
- Guo, J.; Ouyang, S.; Zhou, H.; Kako, T.; Ye, J. Ag3PO4/In (OH) 3 composite photocatalysts with adjustable surface-electric property for efficient photodegradation of organic dyes under simulated solar-light irradiation. J. Phys. Chem. C 2013, 117, 17716–17724. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, H.; Chen, Z.; Wang, W.; Huang, L.; Xu, H.; Li, H. The CeO2/Ag3PO4 photocatalyst with stability and high photocatalytic activity under visible light irradiation. Phys. Status Solidi 2016, 213, 2356–2363. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Zhang, X. One-dimensional Ag3PO4/TiO2 heterostructure with enhanced photocatalytic activity for the degradation of 4-nitrophenol. RSC Adv. 2015, 5, 29693–29697. [Google Scholar] [CrossRef]
- Patil, S.S.; Tamboli, M.S.; Deonikar, V.G.; Umarji, G.G.; Ambekar, J.D.; Kulkarni, M.V.; Kolekar, S.S.; Kale, B.B.; Patil, D.R. Magnetically separable Ag3PO4/NiFe2O4 composites with enhanced photocatalytic activity. Dalton Trans. 2015, 44, 20426–20434. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Chen, Y.; Liu, M.; Li, M. Synthesis of magnetically separable Ag3PO4/ZnFe2O4 composite photocatalysts for dye degradation under visible LED light irradiation. J. Environ. Chem. Eng. 2015, 3, 2809–2815. [Google Scholar] [CrossRef]
- El-Adawy, A.; Hussein, A.; Sheha, E.; Abdel-Samad, S.; Hassan, A.A.; Al-Abyad, M. Synthesis and Thermoluminescence of Novel Ag3PO4: Ba2+ Nanophosphor as Gamma Radiations Detector. Egypt. J. Chem. 2021, 64, 3681–3690. [Google Scholar]
- Saoud, K.M.; Ibala, I.; Ladki, D.E.; Ezzeldeen, O.; Saeed, S. Microwave assisted preparation of calcium hydroxide and barium hydroxide nanoparticles and their application for conservation of cultural heritage. In Proceedings of the Euro-Mediterranean Conference, Limassol, Cyprus, 3–8 November 2014; pp. 342–352. [Google Scholar]
- Alarifi, S.; Ali, D.; Al-Bishri, W. In vitro apoptotic and DNA damaging potential of nanobarium oxide. Int. J. Nanomed. 2016, 11, 249. [Google Scholar]
- Yang, L.; Choi, Y.; Qin, W.; Chen, H.; Blinn, K.; Liu, M.; Liu, P.; Bai, J.; Tyson, T.A.; Liu, M. Promotion of water-mediated carbon removal by nanostructured barium oxide/nickel interfaces in solid oxide fuel cells. Nat. Commun. 2011, 2, 357. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Jha, R.; Jana, P. Epoxidation of styrene by TBHP to styrene oxide using barium oxide as a highly active/selective and reusable solid catalyst. Green Chem. 2006, 8, 689–690. [Google Scholar] [CrossRef]
- Raj, R.B.; Umadevi, M.; Parimaladevi, R.; Anuratha, M. ZnO/BaO nanocomposites: A promising photocatalyst in degrading anionic and cationic dyes under UV and visible light and an efficient antibacterial agent. J. Sol-Gel Sci. Technol. 2022, 102, 628–636. [Google Scholar] [CrossRef]
- Saravanan, R.; Manoj, D.; Qin, J.; Naushad, M.; Gracia, F.; Lee, A.F.; Khan, M.M.; Gracia-Pinilla, M. Mechanothermal synthesis of Ag/TiO2 for photocatalytic methyl orange degradation and hydrogen production. Process Saf. Environ. Prot. 2018, 120, 339–347. [Google Scholar] [CrossRef]
- Diab, K.R.; El-Maghrabi, H.H.; Nada, A.A.; Youssef, A.M.; Hamdy, A.; Roualdes, S.; Abd El-Wahab, S. Facile fabrication of NiTiO3/graphene nanocomposites for photocatalytic hydrogen generation. J. Photochem. Photobiol. A Chem. 2018, 365, 86–93. [Google Scholar] [CrossRef]
- Nada, E.A.; El-Maghrabi, H.H.; Ali, H.R.; Abd El-Wahab, S.; Sabry, D.Y.; Moustafa, Y.M.; Nada, A.A. Superior photodegradation activity of MoS2/TiO2 nanofibers for phenol under visible light irradiation. Desalination Water Treat. 2022, 255, 236–241. [Google Scholar] [CrossRef]
- Nada, E.A.; El-Maghrabi, H.H.; Raynaud, P.; Ali, H.R.; Abd El-Wahab, S.; Sabry, D.Y.; Moustafa, Y.M.; Nada, A.A. Enhanced Photocatalytic Activity of WS2/TiO2 Nanofibers for Degradation of Phenol under Visible Light Irradiation. Inorganics 2022, 10, 54. [Google Scholar] [CrossRef]
- Elshypany, R.; Selim, H.; Zakaria, K.; Moustafa, A.H.; Sadeek, S.A.; Sharaa, S.; Raynaud, P.; Nada, A.A. Elaboration of Fe3O4/ZnO nanocomposite with highly performance photocatalytic activity for degradation methylene blue under visible light irradiation. Environ. Technol. Innov. 2021, 23, 101710. [Google Scholar] [CrossRef]
- Ismail, A.A.; Faisal, M.; Al-Haddad, A. Mesoporous WO3-graphene photocatalyst for photocatalytic degradation of Methylene Blue dye under visible light illumination. J. Environ. Sci. 2018, 66, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Amin Marsooli, M.; Rahimi Nasrabadi, M.; Fasihi-Ramandi, M.; Adib, K.; Pourmasoud, S.; Ahmadi, F.; Eghbali, M.; Sobhani Nasab, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Synthesis of magnetic Fe3O4/ZnWO4 and Fe3O4/ZnWO4/CeVO4 nanoparticles: The photocatalytic effects on organic pollutants upon irradiation with UV-Vis light. Catalysts 2020, 10, 494. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, L.; Bai, Y.; Zhao, J.; Wang, F.; Zhang, R.; Huang, H.; Su, B. Zn3 (OH) 2V2O7·2H2O/g-C3N4: A novel composite for efficient photodegradation of methylene blue under visible-light irradiation. Appl. Surf. Sci. 2015, 347, 602–609. [Google Scholar] [CrossRef]
- Marsooli, M.A.; Fasihi-Ramandi, M.; Adib, K.; Pourmasoud, S.; Ahmadi, F.; Ganjali, M.R.; Sobhani Nasab, A.; Rahimi Nasrabadi, M.; Plonska-Brzezinska, M.E. Preparation and characterization of magnetic Fe3O4/CdWO4 and Fe3O4/CdWO4/PrVO4 nanoparticles and investigation of their photocatalytic and anticancer properties on PANC1 cells. Materials 2019, 12, 3274. [Google Scholar] [CrossRef]
- Patil, S.S.; Patil, D.R.; Apte, S.K.; Kulkarni, M.V.; Ambekar, J.D.; Park, C.-J.; Gosavi, S.W.; Kolekar, S.S.; Kale, B.B. Confinement of Ag3PO4 nanoparticles supported by surface plasmon resonance of Ag in glass: Efficient nanoscale photocatalyst for solar H2 production from waste H2S. Appl. Catal. B Environ. 2016, 190, 75–84. [Google Scholar] [CrossRef]
- Chava, R.K.; Do, J.Y.; Kang, M. Fabrication of CdS-Ag3PO4 heteronanostructures for improved visible photocatalytic hydrogen evolution. J. Alloys Compd. 2017, 727, 86–93. [Google Scholar] [CrossRef]
- Liu, C.-F.; Perng, T.-P. Fabrication and band structure of Ag3PO4–TiO2 heterojunction with enhanced photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2020, 45, 149–159. [Google Scholar] [CrossRef]
- Ghorbanloo, M.; Nada, A.A.; El-Maghrabi, H.H.; Bekheet, M.F.; Riedel, W.; Djamel, B.; Viter, R.; Roualdes, S.; Soliman, F.S.; Moustafa, Y.M.; et al. Superior efficiency of BN/Ce2O3/TiO2 nanofibers for photocatalytic hydrogen generation reactions. Appl. Surf. Sci. 2022, 594, 153438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).