Hydrogen Production through Oxidative Steam Reforming of Acetic Acid over Ni Catalysts Supported on Ceria-Based Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
2.2. Catalytic Performance
3. Materials and Methods
3.1. Supports and Catalysts Preparation
3.2. Supports and Catalysts Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Vo, D.V.N.; Chelliapan, S.; Joo, S.W.; Vasseghian, Y. Latest eco-friendly avenues on hydrogen production towards a circular bioeconomy: Currents challenges, innovative insights, and future perspectives. Renew. Sustain. Energy Rev. 2022, 168, 112916. [Google Scholar] [CrossRef]
- International Energy Agency (IEA) The Future of Hydrogen; International Energy Agency: Paris, France, 2019.
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2021; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Pal, D.B.; Singh, A.; Bhatnagar, A. A review on biomass based hydrogen production technologies. Int. J. Hydrogen Energy 2022, 47, 1461–1480. [Google Scholar] [CrossRef]
- Nabgan, W.; Tuan Abdullah, T.A.; Mat, R.; Nabgan, B.; Gambo, Y.; Ibrahim, M.; Ahmad, A.; Jalil, A.A.; Triwahyono, S.; Saeh, I. Renewable hydrogen production from bio-oil derivative via catalytic steam reforming: An overview. Renew. Sustain. Energy Rev. 2017, 79, 347–357. [Google Scholar] [CrossRef]
- García-Gómez, N.; Valecillos, J.; Valle, B.; Remiro, A.; Bilbao, J.; Gayubo, A.G. Combined effect of bio-oil composition and temperature on the stability of Ni spinel derived catalyst for hydrogen production by steam reforming. Fuel 2022, 326, 124966. [Google Scholar] [CrossRef]
- Soria, M.A.; Barros, D.; Madeira, L.M. Hydrogen production through steam reforming of bio-oils derived from biomass pyrolysis: Thermodynamic analysis including in situ CO2 and/or H2 separation. Fuel 2019, 244, 184–195. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stefanidis, S.D.; Lappas, A.A. Catalyst deactivation, ash accumulation and bio-oil deoxygenation during ex situ catalytic fast pyrolysis of biomass in a cascade thermal-catalytic reactor system. Fuel Process. Technol. 2019, 186, 99–109. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Kruse, A.; Ramos, F.; Ollero, P. Integral energy valorization of municipal solid waste reject fraction to biofuels. Energy Convers. Manag. 2019, 180, 1167–1184. [Google Scholar] [CrossRef]
- Remón, J.; Broust, F.; Volle, G.; García, L.; Arauzo, J. Hydrogen production from pine and poplar bio-oils by catalytic steam reforming. Influence of the bio-oil composition on the process. Int. J. Hydrogen Energy 2015, 40, 5593–5608. [Google Scholar] [CrossRef] [Green Version]
- Megía, P.J.; Vizcaíno, A.J.; Ruiz-Abad, M.; Calles, J.A.; Carrero, A. Coke evolution in simulated bio-oil aqueous fraction steam reforming using Co/SBA-15. Catal. Today 2021, 367, 145–152. [Google Scholar] [CrossRef]
- Cortese, M.; Ruocco, C.; Palma, V.; Megía, P.J.; Carrero, A.; Calles, J.A. On the Support Effect and the Cr Promotion of Co Based Catalysts for the Acetic Acid Steam Reforming. Catalysts 2021, 11, 133. [Google Scholar] [CrossRef]
- Lopez, G.; Garcia, I.; Arregi, A.; Santamaria, L.; Amutio, M.; Artetxe, M.; Bilbao, J.; Olazar, M. Thermodynamic assessment of the oxidative steam reforming of biomass fast pyrolysis volatiles. Energy Convers. Manag. 2020, 214, 112889. [Google Scholar] [CrossRef]
- Yusuf, M.; Bazli, L.; Alam, M.A.; Masood, F.; Keong, L.K.; Noor, A.; Hellgardt, K.; Abdullah, B. Hydrogen production via natural gas reforming: A comparative study between DRM, SRM and BRM techniques. In Proceedings of the 2021 Third International Sustainability and Resilience Conference: Climate Change, Sakheer, Bahrain, 15–16 November 2021; pp. 155–158. [Google Scholar] [CrossRef]
- Ruocco, C.; Palma, V.; Ricca, A. Experimental and kinetic study of oxidative steam reforming of ethanol over fresh and spent bimetallic catalysts. Chem. Eng. J. 2019, 377, 119778. [Google Scholar] [CrossRef]
- Moreira, R.; Bimbela, F.; Gandía, L.M.; Ferreira, A.; Sánchez, J.L.; Portugal, A. Oxidative steam reforming of glycerol. A review. Renew. Sustain. Energy Rev. 2021, 148, 111299. [Google Scholar] [CrossRef]
- Nahar, G.; Dupont, V. Recent advances in hydrogen production via autothermal reforming process (ATR): A review of patents and research articles. Recent Pat. Chem. Eng. 2013, 6, 8–42. [Google Scholar] [CrossRef]
- Ekeoma, B.C.; Yusuf, M.; Johari, K.; Abdullah, B. Mesoporous silica supported Ni-based catalysts for methane dry reforming: A review of recent studies. Int. J. Hydrogen Energy 2022, 148, 111299. [Google Scholar] [CrossRef]
- Matus, E.; Sukhova, O.; Ismagilov, I.; Kerzhentsev, M.; Stonkus, O.; Ismagilov, Z. Hydrogen Production through Autothermal Reforming of Ethanol: Enhancement of Ni Catalyst Performance via Promotion. Energies 2021, 14, 5176. [Google Scholar] [CrossRef]
- Anil, S.; Indraja, S.; Singh, R.; Appari, S.; Roy, B. A review on ethanol steam reforming for hydrogen production over Ni/Al2O3 and Ni/CeO2 based catalyst powders. Int. J. Hydrogen Energy 2022, 47, 8177–8213. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, Q.; Hu, X.; Jia, X.; Huang, L. Zn-Al hydrotalcite-derived CoxZnyAlOz catalysts for hydrogen generation by auto-thermal reforming of acetic acid. Int. J. Energy Res. 2019, 43, 7075–7084. [Google Scholar] [CrossRef]
- Greluk, M.; Rotko, M.; Turczyniak-Surdacka, S. Enhanced catalytic performance of La2O3 promoted Co/CeO2 and Ni/CeO2 catalysts for effective hydrogen production by ethanol steam reforming. Renew. Energy 2020, 155, 378–395. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, H.; Zhang, Y.; Pu, J. Highly active Ni/CeO2 for the steam reforming of acetic acid using CTAB as surfactant template. Int. J. Hydrogen Energy 2022, 47, 27493–27507. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.C.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Hydrogen production by ethanol steam reforming over Cu–Ni supported catalysts. Int. J. Hydrogen Energy 2007, 32, 1450–1461. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Megía, P.J.; Carrero, A.; Calles, J.A.; Vizcaíno, A.J. Hydrogen Production from Steam Reforming of Acetic Acid as a Model Compound of the Aqueous Fraction of Microalgae HTL Using Co-M/SBA-15 (M: Cu, Ag, Ce, Cr) Catalysts. Catalysts 2019, 9, 1013. [Google Scholar] [CrossRef] [Green Version]
- Roggenbuck, J.; Schäfer, H.; Tsoncheva, T.; Minchev, C.; Hanss, J.; Tiemann, M. Mesoporous CeO2: Synthesis by nanocasting, characterisation and catalytic properties. Microporous Mesoporous Mater. 2007, 101, 335–341. [Google Scholar] [CrossRef]
- Luo, L.; Oliver, C.C.; Joseph, I.M.; Gang, D.D.; Chen, M.; Hernandez, R.; Yan, H. Pore structure of ordered mesoporous Pt-CeO2 probed by CO via VT-DRIFTS. Appl. Surf. Sci. 2022, 588, 152866. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J. Ce and La modification of mesoporous Cu–Ni/SBA-15 catalysts for hydrogen production through ethanol steam reforming. Microporous Mesoporous Mater. 2009, 119, 200–207. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M.; Wang, Y.; Younesi, A.; Arandiyan, H. The evaluation of autothermal methane reforming for hydrogen production over Ni/CeO2 catalysts. Int. J. Hydrogen Energy 2018, 43, 22340–22346. [Google Scholar] [CrossRef]
- Ferreira, G.R.; Nogueira, F.G.E.; Lucrédio, A.F.; Assaf, E.M. Ethanol Steam Reforming by Ni Catalysts for H2 Production: Evaluation of Gd Effect in CeO2 Support. Catal. Lett. 2022, 152, 3125–3145. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; García-Moreno, L.; Megía, P.J. Steam Reforming of Model Bio-Oil Aqueous Fraction Using Ni-(Cu, Co, Cr)/SBA-15 Catalysts. Int. J. Mol. Sci. 2019, 20, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megía, P.J.; Calles, J.A.; Carrero, A.; Vizcaíno, A.J. Effect of the incorporation of reducibility promoters (Cu, Ce, Ag) in Co/CaSBA-15 catalysts for acetic acid steam reforming. Int. J. Energy Res. 2021, 45, 1685–1702. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Steinfeld, A. Thermodynamic analysis of cerium-based oxides for solar thermochemical fuel production. Energy Fuels 2012, 26, 1928–1936. [Google Scholar] [CrossRef]
- Batista da Silva, R.; Brandão, S.T.; Lucotti, A.; Tommasini, M.S.; Castiglioni, C.; Groppi, G.; Beretta, A. Chemical pathways in the partial oxidation and steam reforming of acetic acid over a Rh-Al2O3 catalyst. Catal. Today 2017, 289, 162–172. [Google Scholar] [CrossRef]
- Osorio-Vargas, P.; Flores-González, N.A.; Navarro, R.M.; Fierro, J.L.G.; Campos, C.H.; Reyes, P. Improved stability of Ni/Al2O3 catalysts by effect of promoters (La2O3, CeO2) for ethanol steam-reforming reaction. Catal. Today 2016, 259, 27–38. [Google Scholar] [CrossRef]
- Bereketidou, O.A.; Goula, M.A. Biogas reforming for syngas production over nickel supported on ceria–alumina catalysts. Catal. Today 2012, 195, 93–100. [Google Scholar] [CrossRef]
- Santos, A.C.S.F.; Damyanova, S.; Teixeira, G.N.R.; Mattos, L.V.; Noronha, F.B.; Passos, F.B.; Bueno, J.M.C. The effect of ceria content on the performance of Pt/CeO2/Al2O3 catalysts in the partial oxidation of methane. Appl. Catal. A Gen. 2005, 290, 123–132. [Google Scholar] [CrossRef]
- Trimm, D.L. Coke formation and minimisation during steam reforming reactions. Catal. Today 1997, 37, 233–238. [Google Scholar] [CrossRef]
- Ruocco, C.; Palma, V.; Ricca, A. Hydrogen production by oxidative reforming of ethanol in a fluidized bed reactor using a PtNi/CeO2SiO2 catalyst. Int. J. Hydrogen Energy 2019, 44, 12661–12670. [Google Scholar] [CrossRef]
- Lima, D.S.; Calgaro, C.O.; Perez-Lopez, O.W. Hydrogen production by glycerol steam reforming over Ni based catalysts prepared by different methods. Biomass Bioenergy 2019, 130, 105358. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lolli, A.; Amadori, R.; Lucarelli, C.; Cutrufello, M.G.; Rombi, E.; Cavani, F.; Albonetti, S. Hard-template preparation of Au/CeO2mesostructured catalysts and their activity for the selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Microporous Mesoporous Mater. 2016, 226, 466–475. [Google Scholar] [CrossRef]
- Deeprasertkul, C.; Longloilert, R.; Chaisuwan, T.; Wongkasemjit, S. Impressive low reduction temperature of synthesized mesoporous ceria via nanocasting. Mater. Lett. 2014, 130, 218–222. [Google Scholar] [CrossRef]
- Gu, D.; Schüth, F. Synthesis of non-siliceous mesoporous oxides. Chem. Soc. Rev. 2014, 43, 313–344. [Google Scholar] [CrossRef]
- Deng, X.; Chen, K.; Tüysüz, H. Protocol for the Nanocasting Method: Preparation of Ordered Mesoporous Metal Oxides. Chem. Mater. 2017, 29, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Yang, S.; Zhang, P.; Li, P.; Wu, P.; Li, M.; Chen, N.; Jie, K.; Huang, C.; Zhang, N.; et al. Facile Synthesis of Highly Porous Metal Oxides by Mechanochemical Nanocasting. Chem. Mater. 2018, 30, 2924–2929. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Martínez del Monte, D.; García-Rojas, E.; Alique, D.; Calles, J.A.; Sanz, R. Comprehensive permeation analysis and mechanical resistance of electroless pore-plated Pd-membranes with ordered mesoporous ceria as intermediate layer. Sep. Purif. Technol. 2021, 258, 118066. [Google Scholar] [CrossRef]
- Li, H.; Jia, X.; Wang, N.; Chen, B.; Xie, X.; Wang, Q.; Huang, L. Auto-thermal reforming of acetic acid over hydrotalcites-derived co-based catalyst: A stable and anti-coking Co/Sr-Alx-O catalyst. Appl. Catal. B Environ. 2020, 267, 118370. [Google Scholar] [CrossRef]
- Hu, X.; Yang, J.; Sun, W.; Wang, N.; An, S.; Wang, Q.; Zhang, Y.; Xie, X.; Huang, L. Y-Zr-O solid solution supported Ni-based catalysts for hydrogen production via auto-thermal reforming of acetic acid. Appl. Catal. B Environ. 2020, 278, 119264. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; García-Moreno, L.; Vizcaíno, A.J. Production of renewable hydrogen from glycerol steam reforming over bimetallic Ni-(Cu,Co,Cr) catalysts supported on SBA-15 silica. Catalysts 2017, 7, 55. [Google Scholar] [CrossRef]
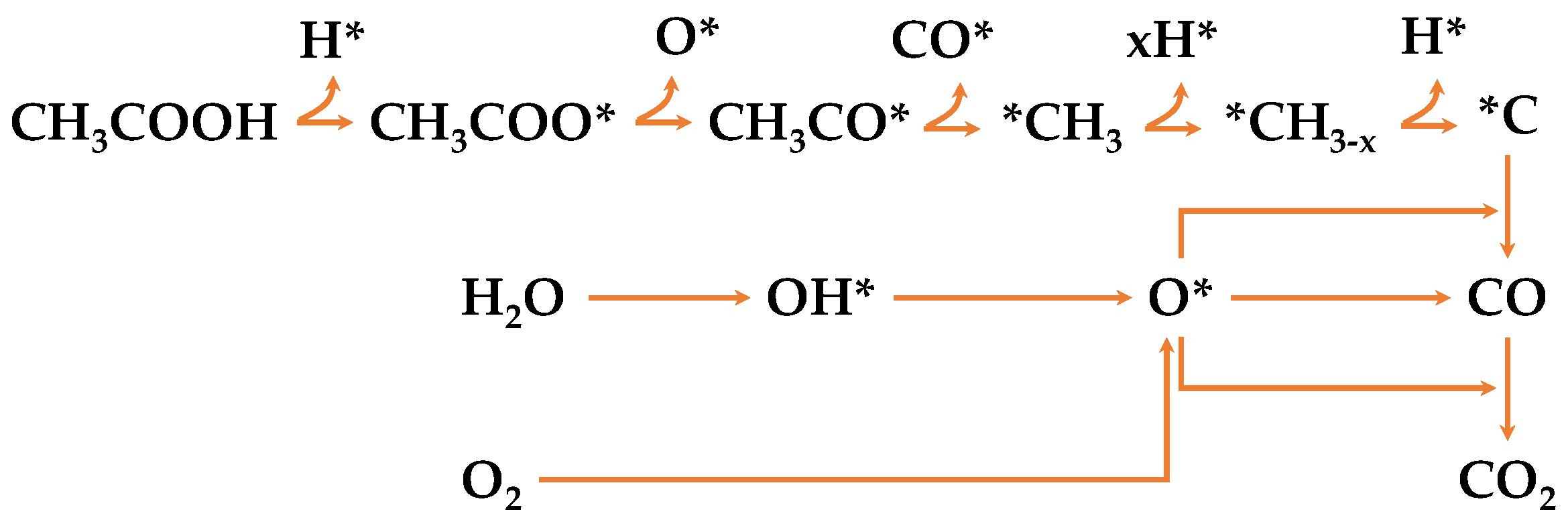

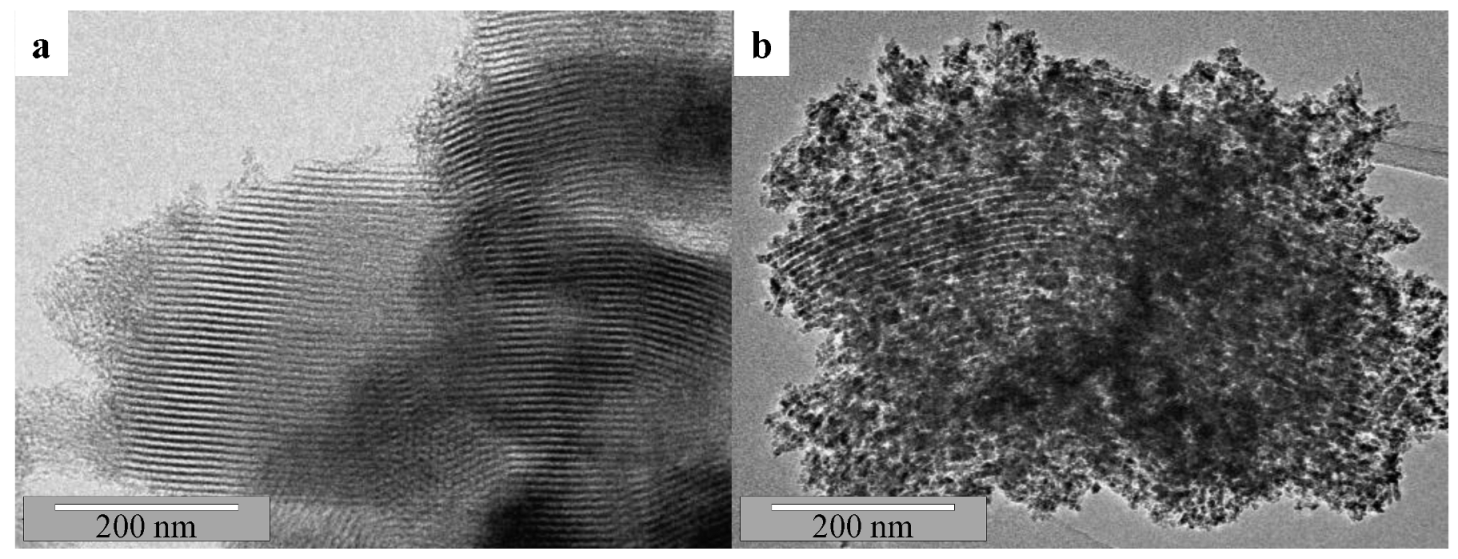

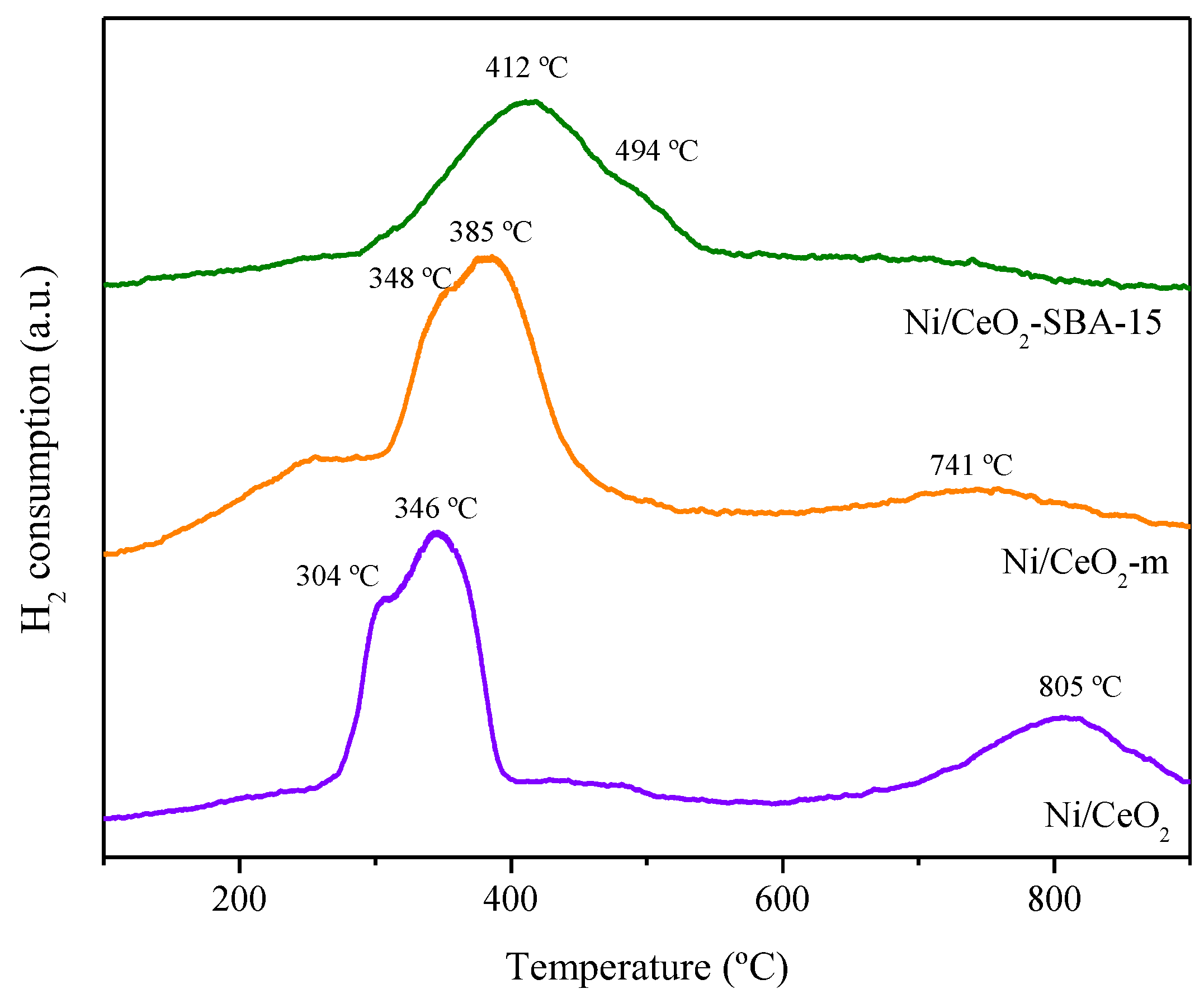


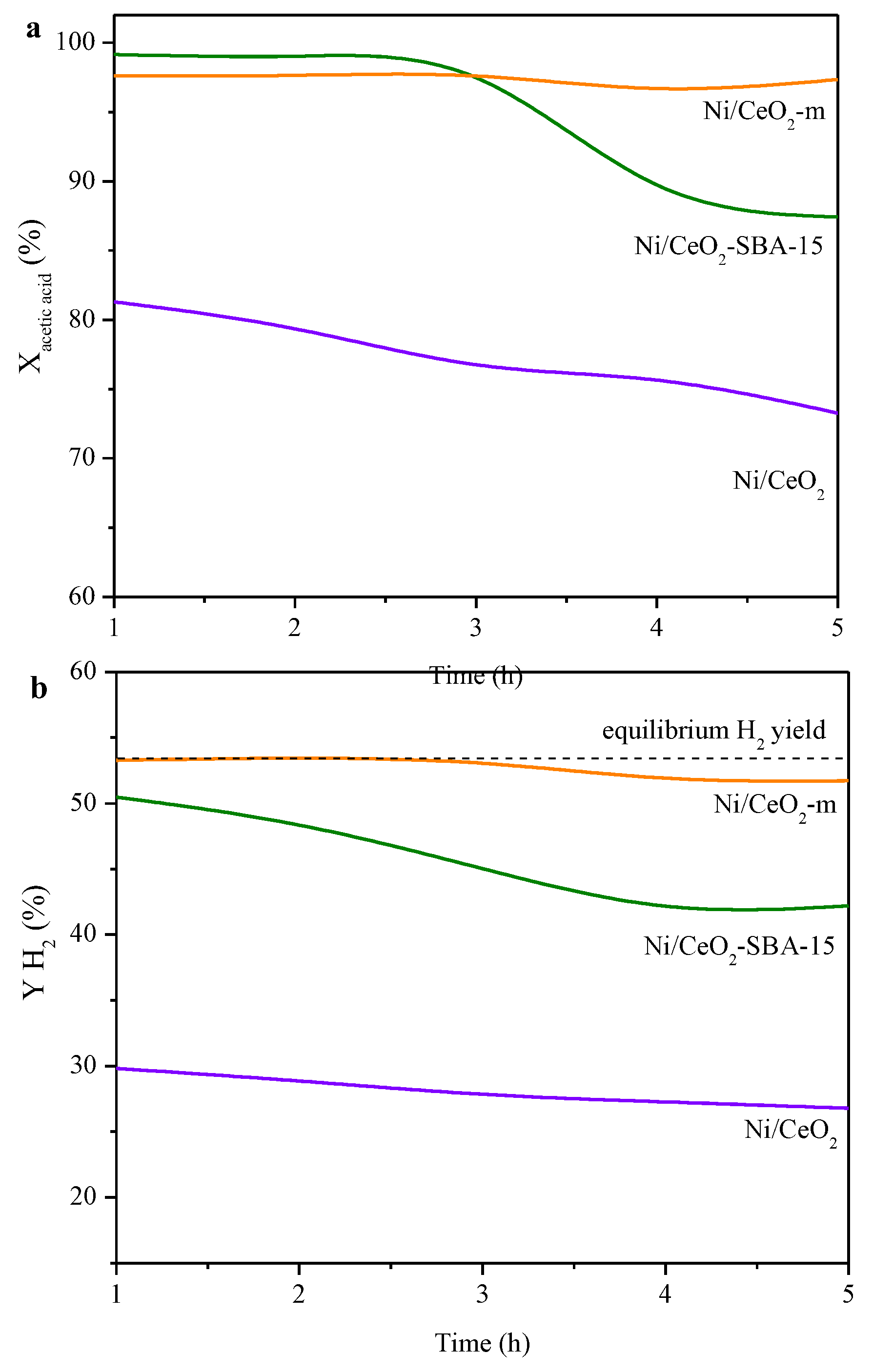
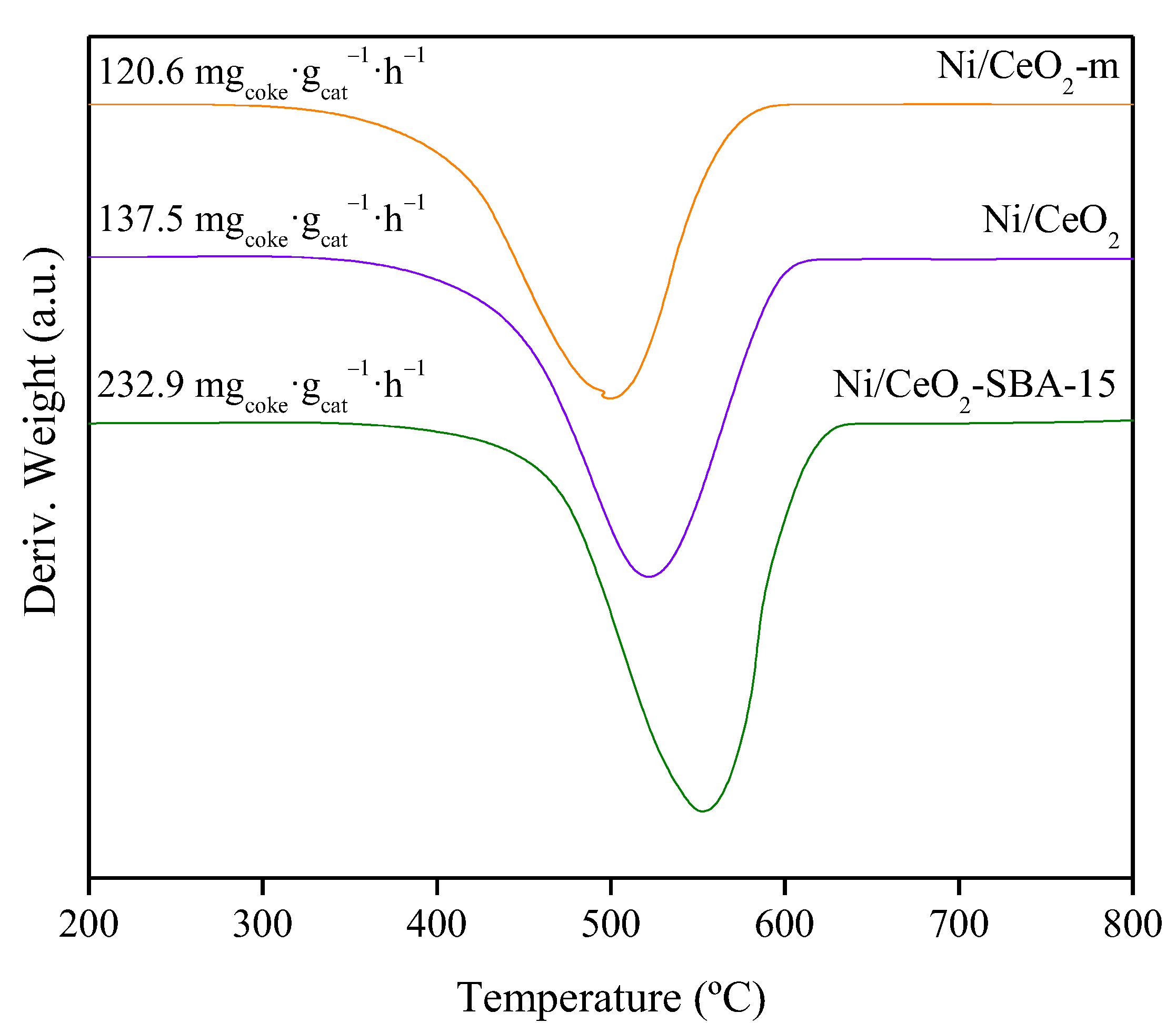
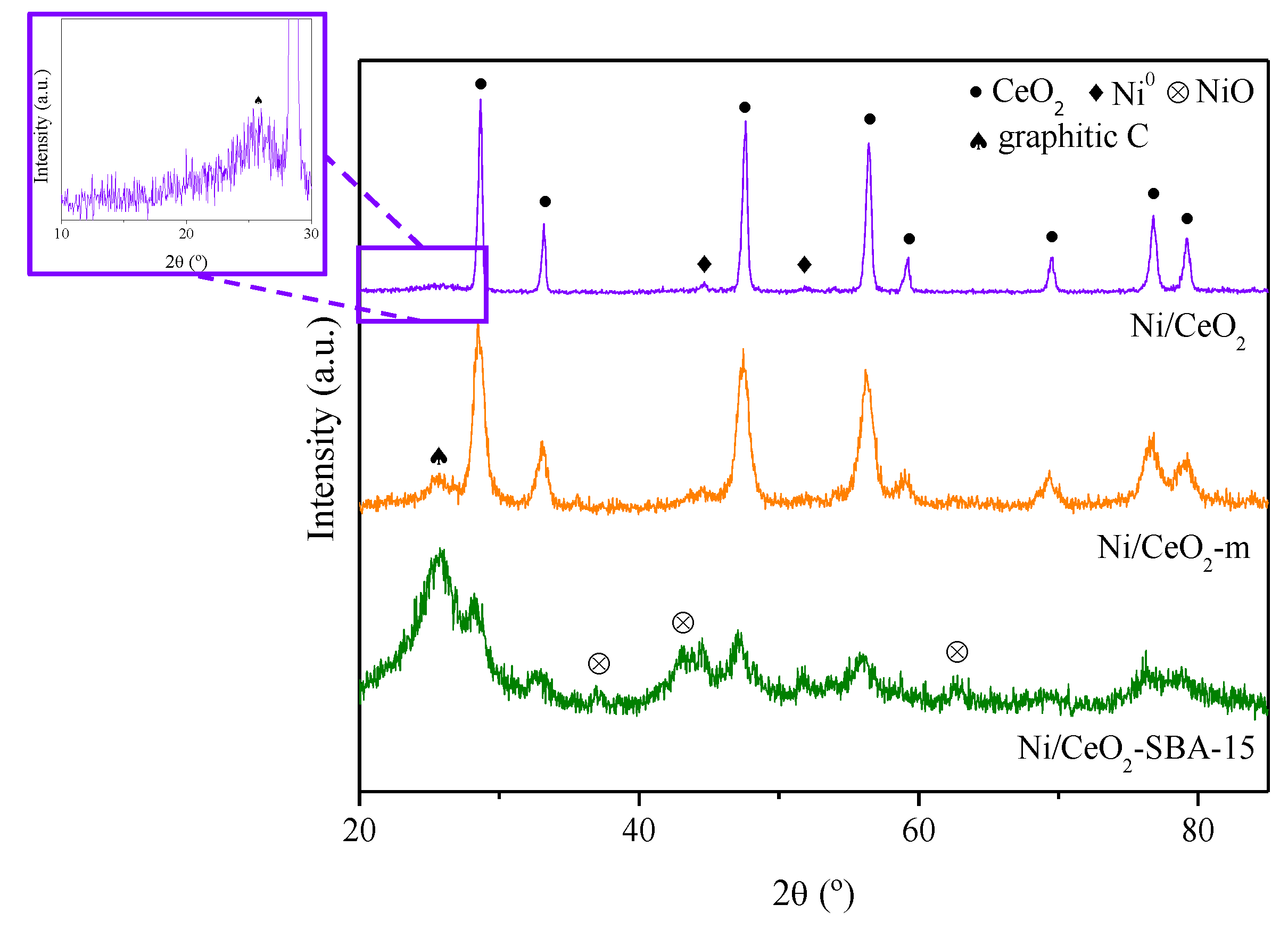
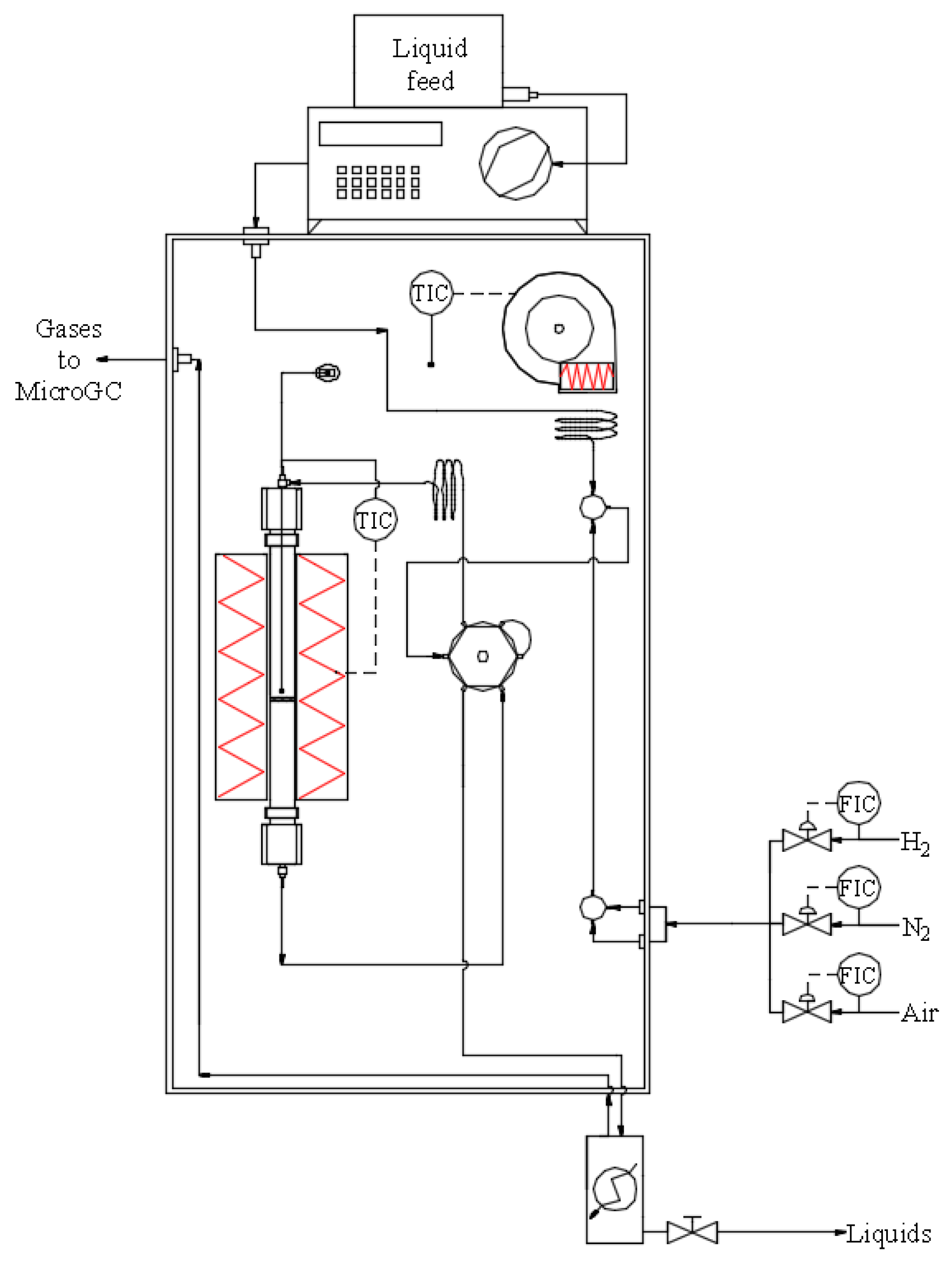
| Sample | Nia (wt.%) | Cea (wt.%) | SBET (m2/g) | Vpb (cm3/g) | Dpc (nm) | DNiOd (nm) | DNie (nm) |
|---|---|---|---|---|---|---|---|
| CeO2 | - | - | - | - | - | - | - |
| Ni/CeO2 | 6.2 | - | - | - | - | 32.5 | 28.4 |
| CeO2-m | - | - | 114 | 0.29 | 7.3 | - | - |
| Ni/CeO2-m | 6.7 | - | 98 | 0.26 | 8.1 | 12.5 | 12.2 |
| SBA-15 | - | - | 601 | 0.91 | 7.7 | - | - |
| CeO2-SBA-15 | - | 9.1 | 490 | 0.72 | 7.2 | - | - |
| Ni/CeO2-SBA-15 | 6.3 | 8.5 | 432 | 0.63 | 7.3 | 11.2 | 11.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megía, P.J.; Morales, A.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production through Oxidative Steam Reforming of Acetic Acid over Ni Catalysts Supported on Ceria-Based Materials. Catalysts 2022, 12, 1526. https://doi.org/10.3390/catal12121526
Megía PJ, Morales A, Vizcaíno AJ, Calles JA, Carrero A. Hydrogen Production through Oxidative Steam Reforming of Acetic Acid over Ni Catalysts Supported on Ceria-Based Materials. Catalysts. 2022; 12(12):1526. https://doi.org/10.3390/catal12121526
Chicago/Turabian StyleMegía, Pedro J., Anabel Morales, Arturo J. Vizcaíno, José A. Calles, and Alicia Carrero. 2022. "Hydrogen Production through Oxidative Steam Reforming of Acetic Acid over Ni Catalysts Supported on Ceria-Based Materials" Catalysts 12, no. 12: 1526. https://doi.org/10.3390/catal12121526
APA StyleMegía, P. J., Morales, A., Vizcaíno, A. J., Calles, J. A., & Carrero, A. (2022). Hydrogen Production through Oxidative Steam Reforming of Acetic Acid over Ni Catalysts Supported on Ceria-Based Materials. Catalysts, 12(12), 1526. https://doi.org/10.3390/catal12121526








