Peroxymonosulfate Activation by CuO-Fe2O3-Modified Ni Foam: A 1O2 Dominated Process for Efficient and Stable Degradation of Tetracycline
Abstract
:1. Introduction
2. Results and Discussion
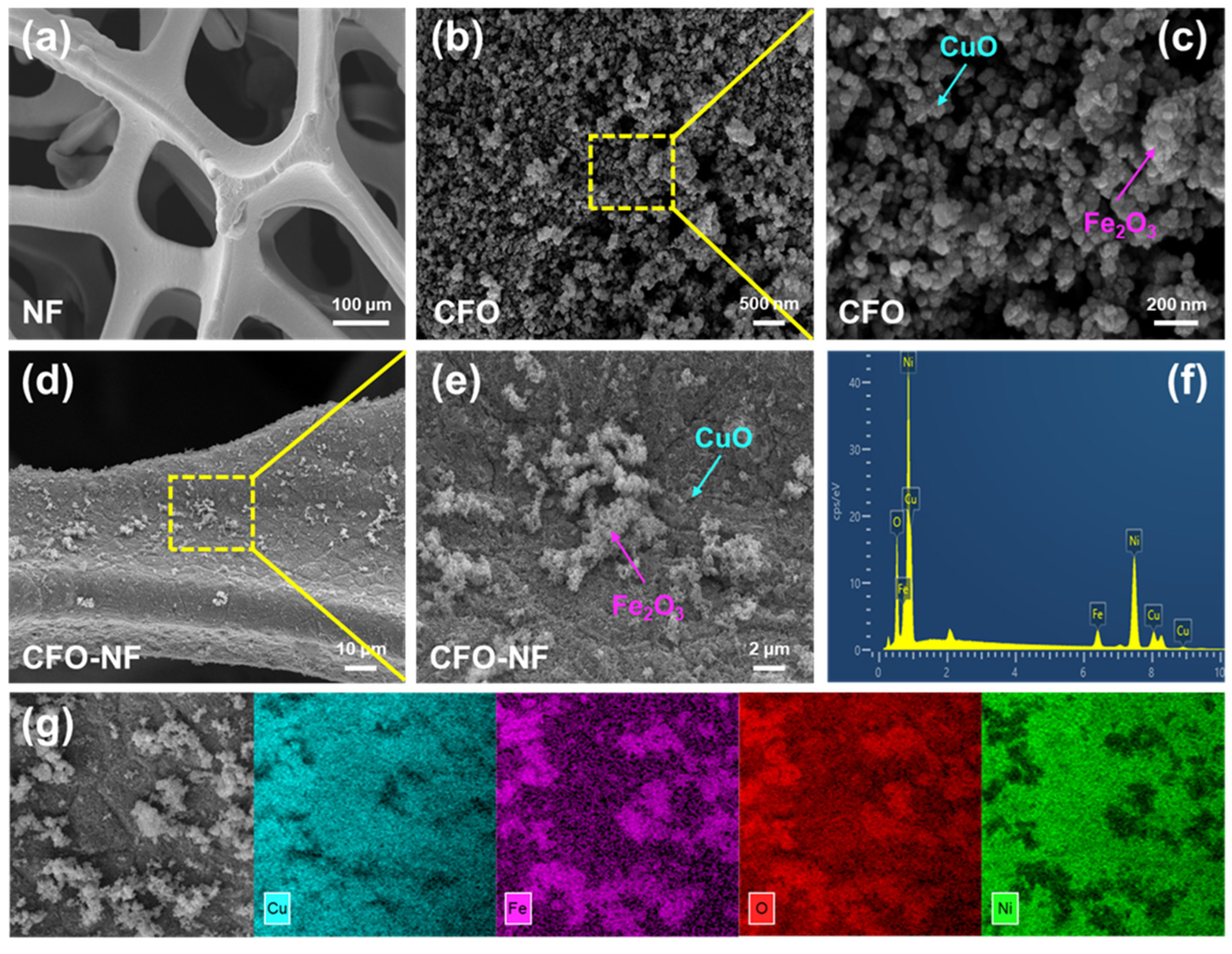
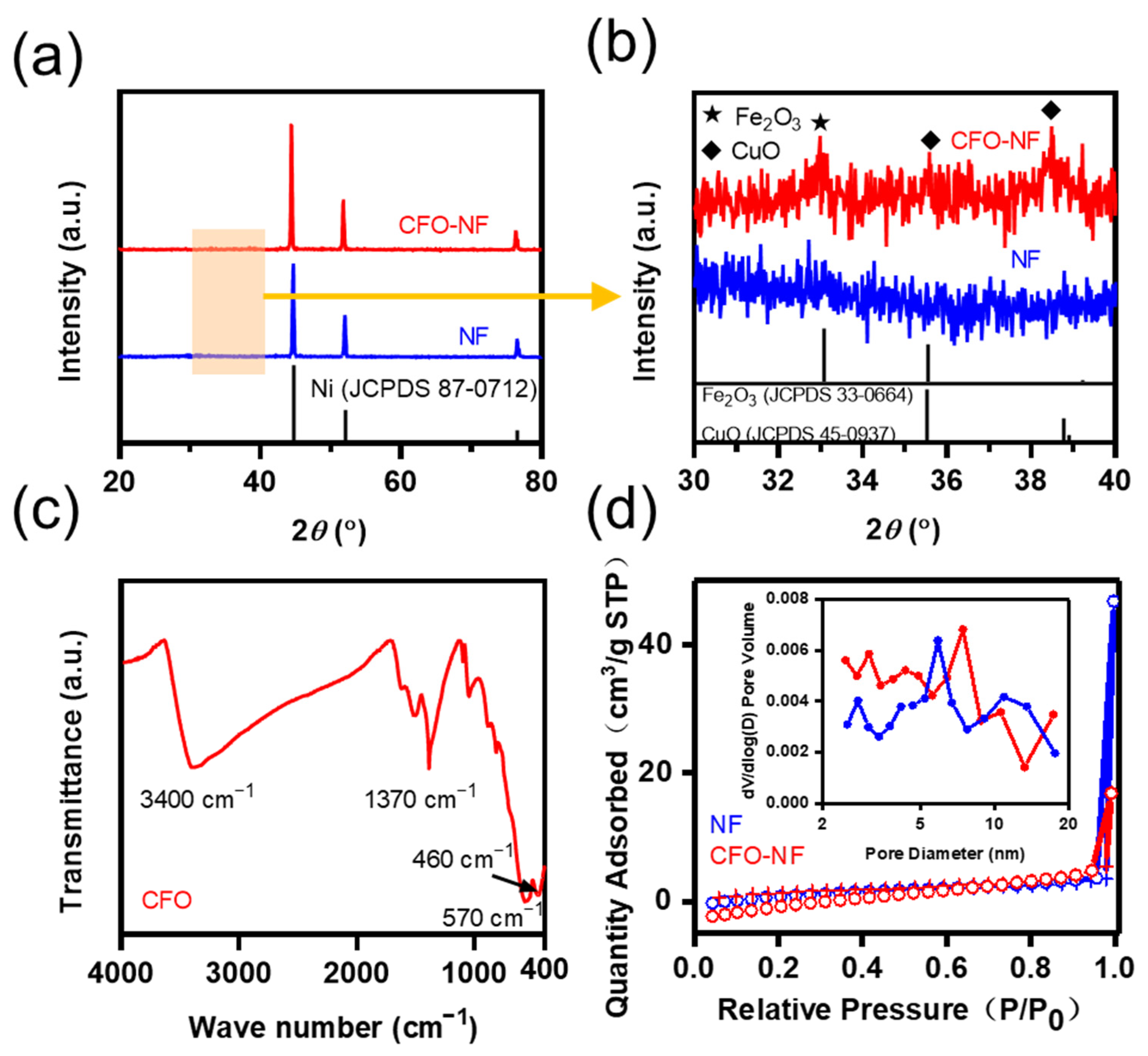
2.1. The Characterizations of CFO-NF Composites
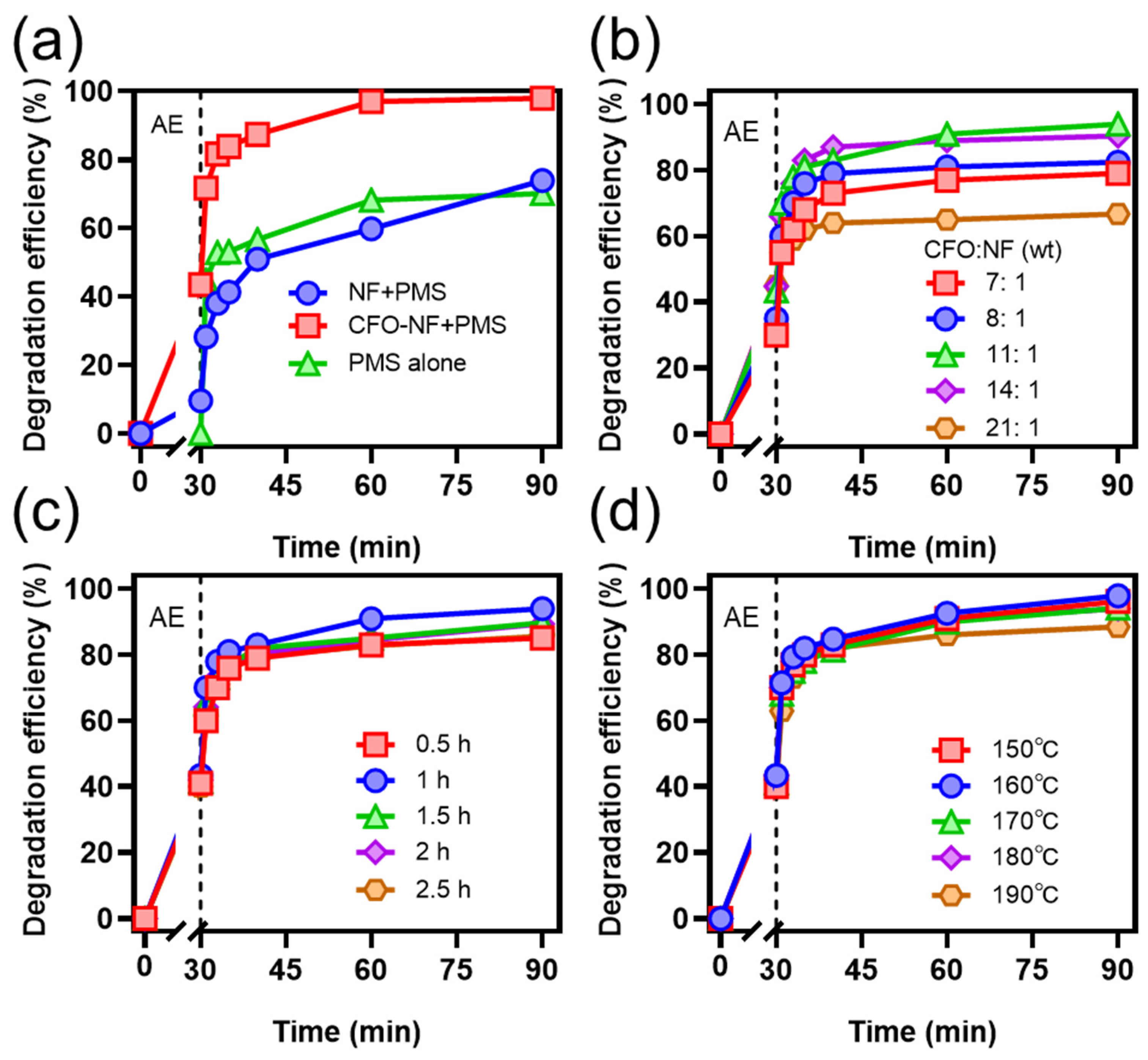
2.2. Performance of CFO-NF for TC Degradation
2.2.1. Effects of Synthesis Conditions on TC Degradation
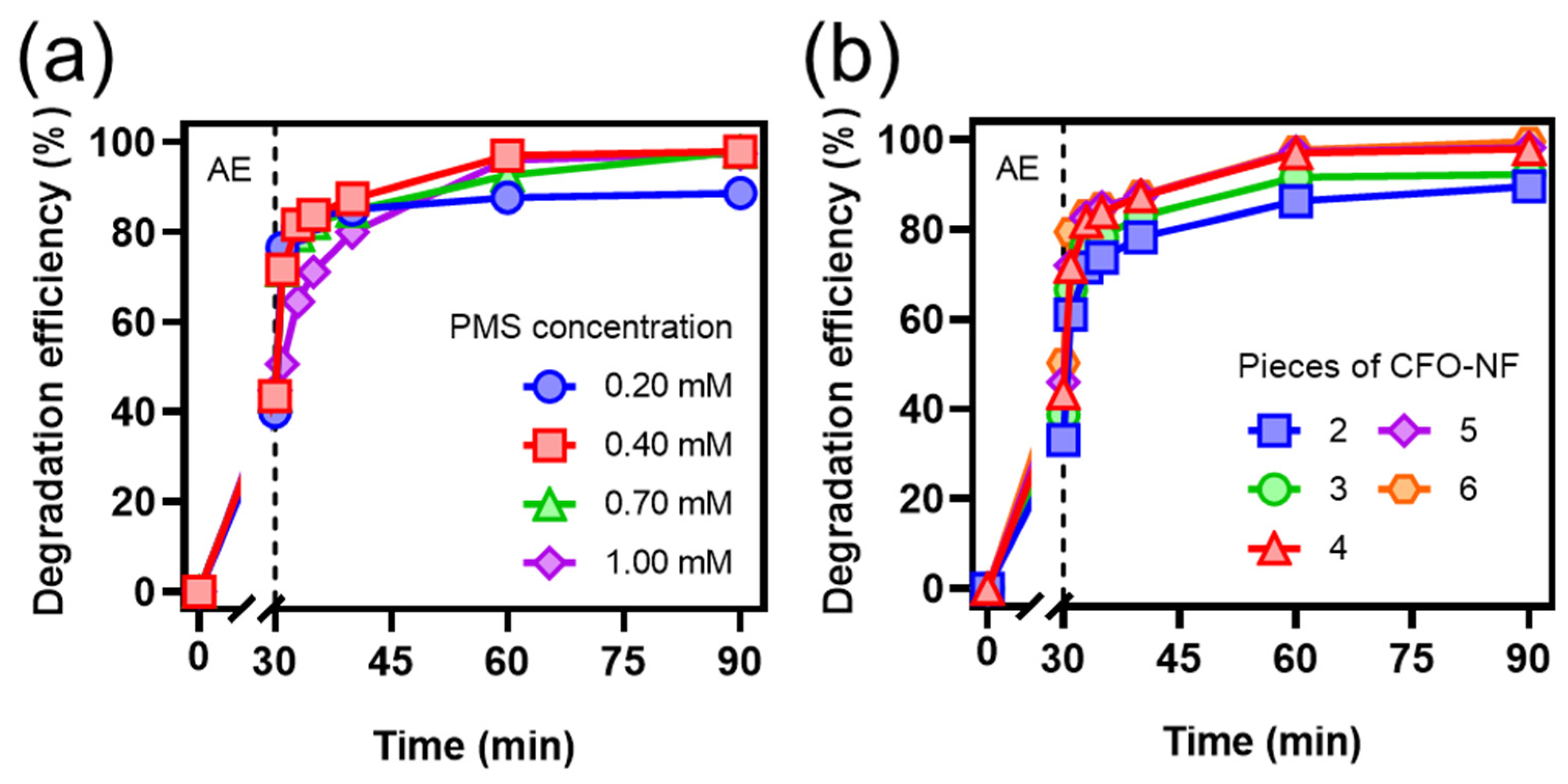
2.2.2. Effects of Reaction Conditions on TC Degradation
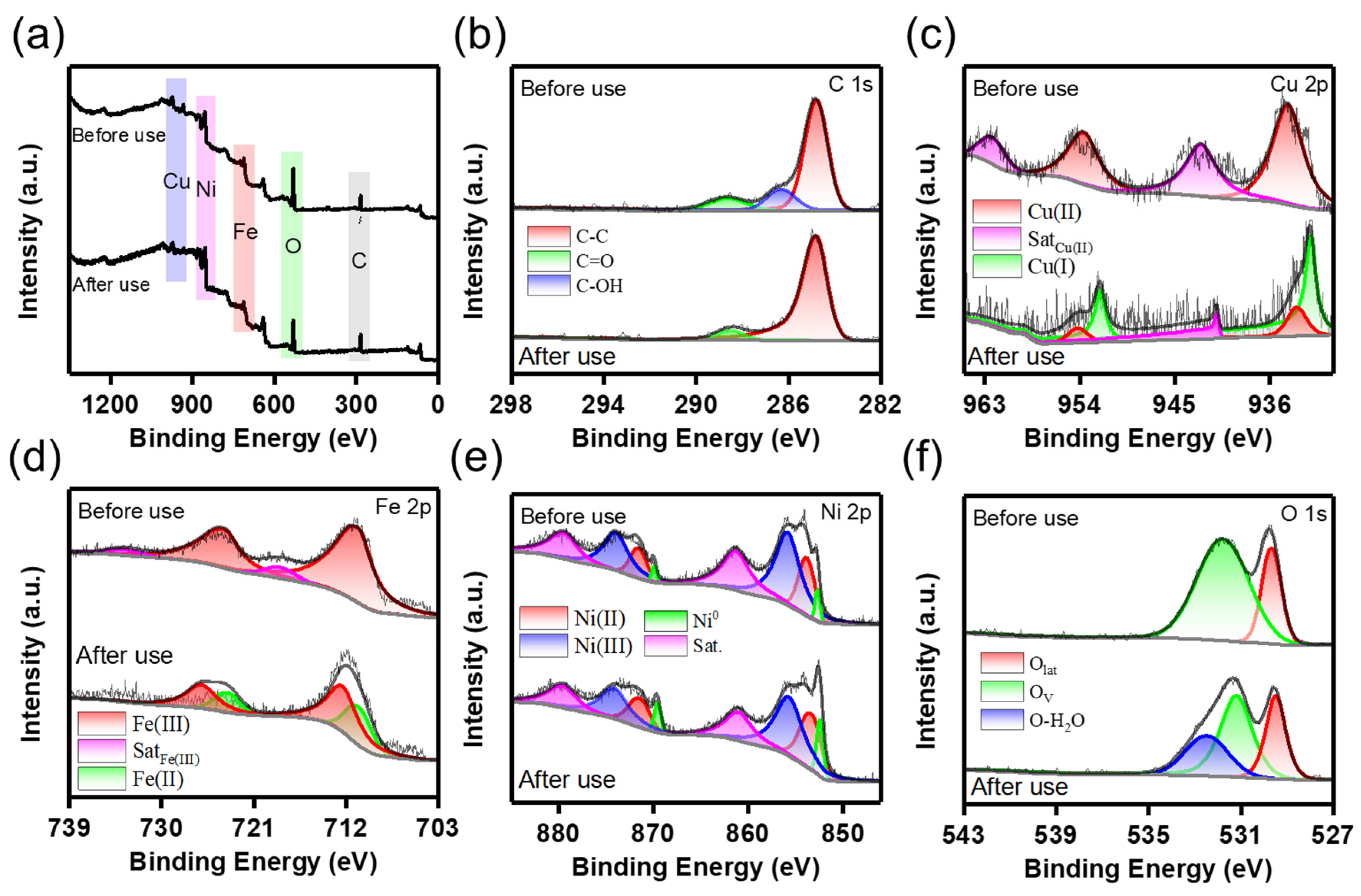
2.3. Stability of CFO-NF Composites
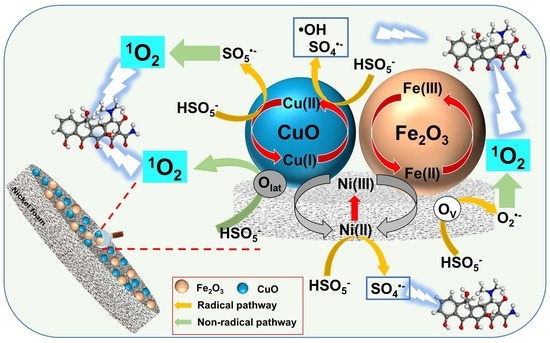
2.4. Mechanism for PMS Activation and TC Degradation
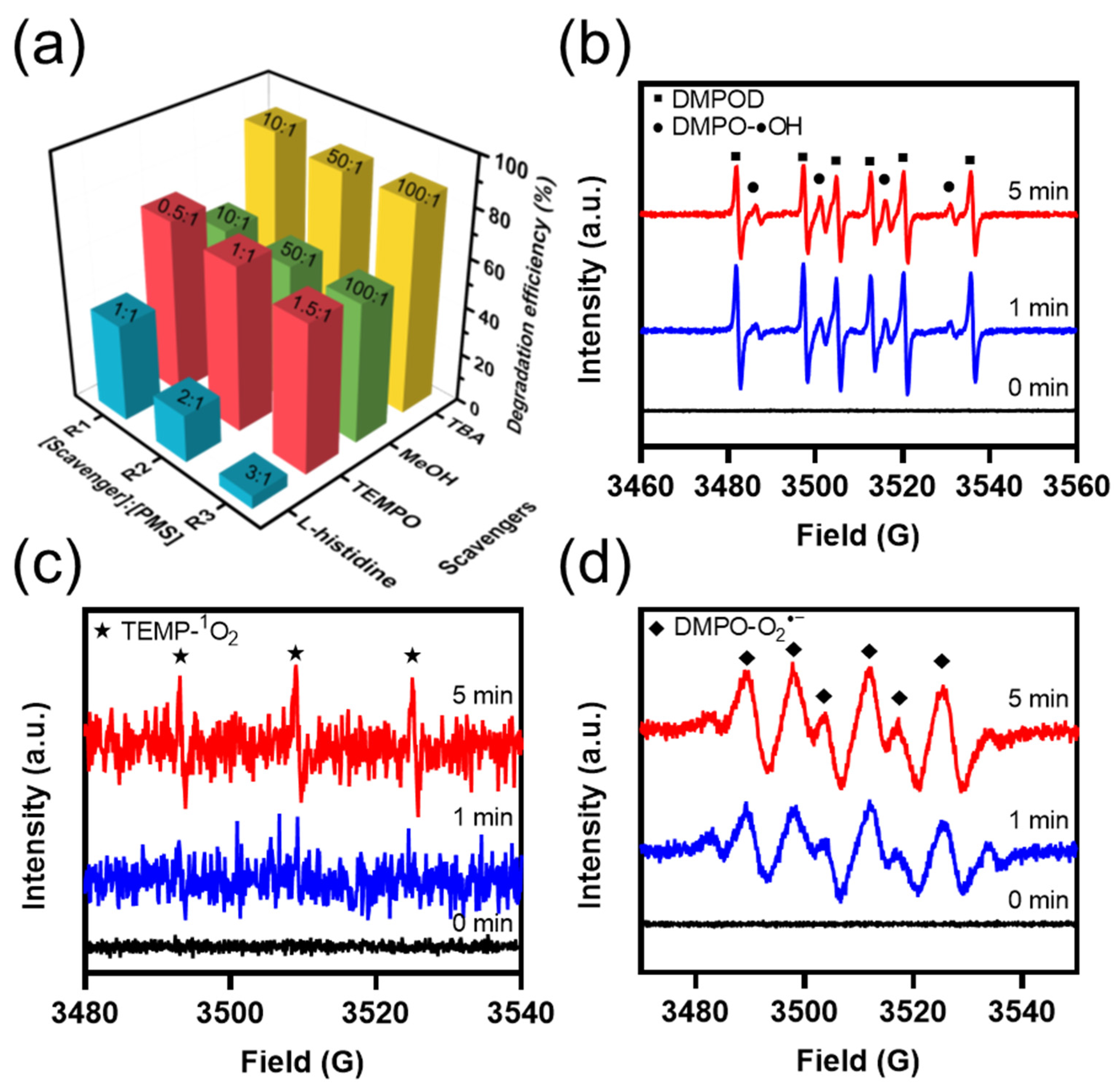
2.4.1. Identification of Actives Species
2.4.2. Catalytic Mechanisms of the CFO-NF/PMS System
3. Materials and Methods
3.1. Materials
3.2. Fabrication of CFO-NF Composites
3.3. Characterizations
3.4. Experimental Procedures
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, J.L.; Chen, L.; Luo, X.; Huang, L.; Li, S.Y.; Gong, X.B. Degradation of tetracycline hydrochloride through efficient peroxymonosulfate activation by B, N co-doped porous carbon materials derived from metal-organic frameworks: Nonradical pathway mechanism. Sep. Purif. Technol. 2022, 281, 119887. [Google Scholar] [CrossRef]
- Peng, X.M.; Wu, J.Q.; Zhao, Z.L.; Wang, X.; Dai, H.L.; Xu, L.; Xu, G.P.; Jian, Y.; Hu, F.P. Activation of peroxymonosulfate by single-atom Fe-g-C3N4 catalysts for high efficiency degradation of tetracycline via nonradical pathways: Role of high-valent iron-oxo species and Fe-NX sites. Chem. Eng. J. 2022, 427, 130803. [Google Scholar] [CrossRef]
- Xu, L.Y.; Zhang, H.; Xiong, P.; Zhu, Q.Q.; Liao, C.Y.; Jiang, G.B. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Xu, P.; Wang, P.; Li, X.; Wei, R.; Wang, X.; Yang, C.; Shen, T.; Zheng, T.; Zhang, G. Efficient peroxymonosulfate activation by CuO-Fe2O3/MXene composite for atrazine degradation: Performance, coexisting matter influence and mechanism. Chem. Eng. J. 2022, 440, 135863. [Google Scholar] [CrossRef]
- Qu, J.H.; Tian, X.; Zhang, X.B.; Yao, J.Y.; Xue, J.Q.; Li, K.G.; Zhang, B.; Wang, L.; Zhang, Y. Free radicals-triggered reductive and oxidative degradation of highly chlorinated compounds via regulation of heat-activated persulfate by low-molecular-weight organic acids. Appl. Catal. B-Environ. 2022, 310, 121359. [Google Scholar] [CrossRef]
- Milh, H.; Cabooter, D.; Dewil, R. Role of process parameters in the degradation of sulfamethoxazole by heat-activated peroxymonosulfate oxidation: Radical identification and elucidation of the degradation mechanism. Chem. Eng. J. 2021, 422, 130457. [Google Scholar] [CrossRef]
- Qu, J.H.; Xu, Y.; Zhang, X.B.; Sun, M.Z.; Tao, Y.; Zhang, X.M.; Zhang, G.S.; Ge, C.J.; Zhang, Y. Ball milling-assisted preparation of N-doped biochar loaded with ferrous sulfide as persulfate activator for phenol degradation: Multiple active sites-triggered radical/non-radical mechanism. Appl. Catal. B-Environ. 2022, 316, 121639. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Li, X.C.; Fang, J.Y.; Chen, L.W. Influence of pH on the Formation of Sulfate and Hydroxyl Radicals in the UV/Peroxymonosulfate System. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef]
- Chen, Y.X.; Lan, S.Y.; Zhu, M.S. Construction of piezoelectric BaTiO3/MoS2 heterojunction for boosting piezo-activation of peroxymonosulfate. Chin. Chem. Lett. 2021, 32, 2052–2056. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, G.S.; Chen, Y.R.; Han, B.; Xia, K.S.; Zhou, C.G. Utilizing cobalt-doped materials as heterogeneous catalysts to activate peroxymonosulfate for organic pollutant degradation: A critical review. Environ. Sci.-Wat. Res. Technol. 2021, 7, 1197–1211. [Google Scholar] [CrossRef]
- Tian, K.; Hu, L.M.; Li, L.T.; Zheng, Q.Z.; Xin, Y.J.; Zhang, G.S. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Ding, P.J.; Niu, J.R.; Chang, F.Q.; He, Z.; Wagberg, T.; Li, Z.X.; Hu, G.Z. NiCo2O4 hollow microsphere-mediated ultrafast peroxymonosulfate activation for dye degradation. Chin. Chem. Lett. 2021, 32, 2495–2498. [Google Scholar] [CrossRef]
- Hu, L.M.; Zhang, G.S.; Liu, M.; Wang, Q.; Wang, P. Enhanced degradation of Bisphenol A (BPA) by peroxymonosulfate with Co3O4-Bi2O3 catalyst activation: Effects of pH, inorganic anions, and water matrix. Chem. Eng. J. 2018, 338, 300–310. [Google Scholar] [CrossRef]
- Duan, L.X.; Zhou, X.J.; Liu, S.T.; Shi, P.H.; Yao, W.F. 3D-hierarchically structured Co3O4/graphene hydrogel for catalytic oxidation of Orange II solutions by activation of peroxymonosulfate. J. Taiwan Inst. Chem. Eng. 2017, 76, 101–108. [Google Scholar] [CrossRef]
- Yang, C.Y.; Wang, P.; Li, J.N.; Wang, Q.; Xu, P.; You, S.J.; Zheng, Q.Z.; Zhang, G.S. Photocatalytic PVDF ultrafiltration membrane blended with visible-light responsive Fe(III)-TiO2 catalyst: Degradation kinetics, catalytic performance and reusability. Chem. Eng. J. 2021, 417, 129340. [Google Scholar] [CrossRef]
- Chen, X.T.; Zhang, T.; Kan, M.; Song, D.G.; Jia, J.P.; Zhao, Y.X.; Qian, X.F. Binderless and Oxygen Vacancies Rich FeNi/Graphitized Mesoporous Carbon/Ni Foam for Electrocatalytic Reduction of Nitrate. Environ. Sci. Technol. 2020, 54, 13344–13353. [Google Scholar] [CrossRef]
- Yuan, R.X.; Hu, L.; Yu, P.; Wang, H.Y.; Wang, Z.H.; Fang, J.Y. Nanostructured Co3O4 grown on nickel foam: An efficient and readily recyclable 3D catalyst for heterogeneous peroxymonosulfate activation. Chemosphere 2018, 198, 204–215. [Google Scholar] [CrossRef]
- Jonoush, Z.A.; Rezaee, A.; Ghaffarinejad, A. Electrocatalytic nitrate reduction using Fe0/Fe3O4 nanoparticles immobilized on nickel foam: Selectivity and energy consumption studies. J. Clean Prod. 2020, 242, 118569. [Google Scholar] [CrossRef]
- Ye, Z.H.; Lin, L.Y.; Yu, C.F. Nickel precursor-free synthesis of nickel cobalt sulfide on Ni foam: Effects of the pH value on the morphology and the energy-storage ability. J. Energy Storage 2016, 8, 60–68. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.X.; Zhang, D.Y.; Lan, Y.Q.; Guo, J. CuO-Co3O4@CeO2 as a heterogeneous catalyst for efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate. J. Hazard. Mater. 2020, 381, 121209. [Google Scholar] [CrossRef]
- Ding, M.M.; Chen, W.; Xu, H.; Shen, Z.; Lin, T.; Hu, K.; Lu, C.H.; Xie, Z.L. Novel α-Fe2O3/MXene nanocomposite as heterogeneous activator of peroxymonosulfate for the degradation of salicylic acid. J. Hazard. Mater. 2020, 382, 121064. [Google Scholar] [CrossRef] [PubMed]
- Bousalah, D.; Zazoua, H.; Boudjemaa, A.; Benmounah, A.; Messaoud-Boureghda, M.Z.; Bachari, K. Enhanced reactivity of the CuO-Fe2O3 intimate heterojunction for the oxidation of quinoline yellow dye (E104). Environ. Sci. Pollut. Res. 2022, 29, 69988–69999. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.C.; Ge, M.; Hu, Z.; Guo, C.S. One-pot synthesis of magnetic CuO/Fe2O3/CuFe2O4 nanocomposite to activate persulfate for levofloxacin removal: Investigation of efficiency, mechanism and degradation route. Chem. Eng. J. 2020, 389, 124456. [Google Scholar] [CrossRef]
- Xu, P.; Wang, P.; Wang, Q.; Wei, R.; Li, Y.; Xin, Y.; Zheng, T.; Hu, L.; Wang, X.; Zhang, G. Facile synthesis of Ag2O/ZnO/rGO heterojunction with enhanced photocatalytic activity under simulated solar light: Kinetics and mechanism. J. Hazard. Mater. 2021, 403, 124011. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Park, Y.K.; Kwon, E.; Tsang, Y.F.; Thanh, B.X.; Khiem, T.C.; You, S.M.; Hu, C.C.; Lin, K.Y.A. Nanoneedle-Assembled Copper/Cobalt sulfides on nickel foam as an enhanced 3D hierarchical catalyst to activate monopersulfate for Rhodamine b degradation. J. Colloid Interf. Sci. 2022, 613, 168–181. [Google Scholar] [CrossRef]
- Jiang, L.P.; Wei, Z.Y.; Ding, Y.H.; Ma, Y.Y.; Fu, X.; Sun, J.; Ma, M.; Zhu, W.X.; Wang, J.L. In-situ synthesis of self-standing cobalt-doped nickel sulfide nanoarray as a recyclable and integrated catalyst for peroxymonosulfate activation. Appl. Catal. B-Environ. 2022, 307, 121184. [Google Scholar] [CrossRef]
- Hu, L.M.; Zhang, G.S.; Liu, M.; Wang, Q.; Dong, S.Y.; Wang, P. Application of nickel foam-supported Co3O4-Bi2O3 as a heterogeneous catalyst for BPA removal by peroxymonosulfate activation. Sci. Total Environ. 2019, 647, 352–361. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.Y.; Zhang, J.L.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Guo, Y.G.; Lou, X.Y.; Fang, C.L.; Xiao, D.X.; Wang, Z.H.; Liu, J.S. Novel Photo-Sulfite System: Toward Simultaneous Transformations of Inorganic and Organic Pollutants. Environ. Sci. Technol. 2013, 47, 11174–11181. [Google Scholar] [CrossRef]
- Wang, Y.R.; Tian, D.F.; Chu, W.; Li, M.R.; Lu, X.W. Nanoscaled magnetic CuFe2O4 as an activator of peroxymonosulfate for the degradation of antibiotics norfloxacin. Sep. Purif. Technol. 2019, 212, 536–544. [Google Scholar] [CrossRef]
- Huang, Y.; Han, C.; Liu, Y.Q.; Nadagouda, M.N.; Machala, L.; O’Shea, K.E.; Sharma, V.K.; Dionysiou, D.D. Degradation of atrazine by ZnxCu1-xFe2O4 nanomaterial-catalyzed sulfite under UV-vis light irradiation: Green strategy to generate SO4·−. Appl. Catal. B-Environ. 2018, 221, 380–392. [Google Scholar] [CrossRef]
- Li, Y.; Ma, S.; Xu, S.; Fu, H.; Li, Z.; Li, K.; Sheng, K.; Du, J.; Lu, X.; Li, X.; et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: Emphasizing the synergistic effect between graphitized structure and CoFe2O4. Chem. Eng. J. 2020, 387, 124094. [Google Scholar] [CrossRef]
- Dong, X.B.; Ren, B.X.; Sun, Z.M.; Li, C.Q.; Zhang, X.W.; Kong, M.H.; Zheng, S.L.; Dionysiou, D.D. Monodispersed CuFe2O4 nanoparticles anchored on natural kaolinite as highly efficient peroxymonosulfate catalyst for bisphenol A degradation. Appl. Catal. B-Environ. 2019, 253, 206–217. [Google Scholar] [CrossRef]
- Fedorov, K.; Plata-Gryl, M.; Khan, J.A.; Boczkaj, G. Ultrasound-assisted heterogeneous activation of persulfate and peroxymonosulfate by asphaltenes for the degradation of BTEX in water. J. Hazard. Mater. 2020, 397, 122804. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Jiang, J.; Shen, Y.; Pang, S.; Luo, C.; Zhao, X. Carbon Materials Inhibit Formation of Nitrated Aromatic Products in Treatment of Phenolic Compounds by Thermal Activation of Peroxydisulfate in the Presence of Nitrite. Environ. Sci. Technol. 2019, 53, 9054–9062. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Dai, Z.; Meng, H.; Duan, X.; Qin, Y.; Zhou, Y.; Ao, Z.; Wang, S.; An, T. Peroxydisulfate activation by positively polarized carbocatalyst for enhanced removal of aqueous organic pollutants. Water Res. 2019, 166, 115043. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, C.; Lv, W.; Liu, G. Incorporating Oxygen Atoms in a SnS2 Atomic Layer to Simultaneously Stabilize Atomic Hydrogen and Accelerate the Generation of Hydroxyl Radicals for Water Decontamination. Environ. Sci. Technol. 2022, 56, 4980–4987. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Q.; Gao, N.Y.; Deng, Y.; Deng, J.; Zhou, S.Q.; Li, J.; Xin, X.Y. Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate. J. Hazard. Mater. 2014, 276, 452–460. [Google Scholar] [CrossRef]
- Chen, L.; Duan, J.; Du, P.H.; Sun, W.L.; Lai, B.; Liu, W. Accurate identification of radicals by in-situ electron paramagnetic resonance in ultraviolet-based homogenous advanced oxidation processes. Water Res. 2022, 221, 118747. [Google Scholar] [CrossRef]
- Wen, Y.H.; Sharma, V.K.; Ma, X.M. Activation of Peroxymonosulfate by Phosphate and Carbonate for the Abatement of Atrazine: Roles of Radical and Nonradical Species. ACS ES&T Wat. 2022, 2, 635–643. [Google Scholar] [CrossRef]
- Zhen, J.Y.; Zhang, S.S.; Zhuang, X.M.; Ahmad, S.; Lee, T.; Si, H.Y.; Cao, C.B.; Ni, S.Q. Sulfate radicals based heterogeneous peroxymonosulfate system catalyzed by CuO-Fe3O4-Biochar nanocomposite for bisphenol A degradation. J. Water Process. Eng. 2021, 41, 102078. [Google Scholar] [CrossRef]
- Chai, H.; Yang, C.Y.; Xu, P.; Wang, P.; Qu, J.H.; Zhang, G.S. Enhanced visible-light photocatalytic activity with Fe2O3-ZnO@C/g-C3N4 heterojunction: Characterization, kinetics, and mechanisms. J. Clean Prod. 2022, 377, 134511. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.J.; Yang, W.; Fida, H.; You, L.M.; Zhou, K. Scalable synthesis of Ca-doped α-Fe2O3 with abundant oxygen vacancies for enhanced degradation of organic pollutants through peroxymonosulfate activation. Appl. Catal. B-Environ. 2020, 262, 118250. [Google Scholar] [CrossRef]
- Tan, L.; Yu, J.T.; Wang, H.Y.; Gao, H.T.; Liu, X.; Wang, L.; She, X.L.; Zhan, T.R. Controllable synthesis and phase-dependent catalytic performance of dual-phase nickel selenides on Ni foam for overall water splitting. Appl. Catal. B-Environ. 2022, 303, 120915. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Wu, D.; Chen, M.; Liang, Y.; Wang, Q.; Wang, L.; Fu, X.-Z.; Luo, J.-L. In situ facile fabrication of Ni(OH)2 nanosheet arrays for electrocatalytic co-production of formate and hydrogen from methanol in alkaline solution. Appl. Catal. B-Environ. 2021, 281, 119510. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhou, W. Cu-doped Ni-LDH with abundant oxygen vacancies for enhanced methyl 4-hydroxybenzoate degradation via peroxymonosulfate activation: Key role of superoxide radicals. J. Colloid Interface Sci. 2022, 610, 504–517. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.G.; Zhang, Y.L.; Tang, W.H.; Cheng, X.; Li, W. Heterogeneous activation of peroxymonosulfate by sillenite Bi25FeO40: Singlet oxygen generation and degradation for aquatic levofloxacin. Chem. Eng. J. 2018, 343, 128–137. [Google Scholar] [CrossRef]
- Zhang, L.S.; Jiang, X.H.; Zhong, Z.A.; Tian, L.; Sun, Q.; Cui, Y.T.; Lu, X.; Zou, J.P.; Luo, S.L. Carbon Nitride Supported High-Loading Fe Single-Atom Catalyst for Activation of Peroxymonosulfate to Generate 1O2 with 100 % Selectivity. Angew. Chem.-Int. Edit. 2021, 60, 21751–21755. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Xu, P.; Tian, K.; Cao, M.; Shi, F.; Zhang, G. Peroxymonosulfate Activation by CuO-Fe2O3-Modified Ni Foam: A 1O2 Dominated Process for Efficient and Stable Degradation of Tetracycline. Catalysts 2023, 13, 329. https://doi.org/10.3390/catal13020329
Ren X, Xu P, Tian K, Cao M, Shi F, Zhang G. Peroxymonosulfate Activation by CuO-Fe2O3-Modified Ni Foam: A 1O2 Dominated Process for Efficient and Stable Degradation of Tetracycline. Catalysts. 2023; 13(2):329. https://doi.org/10.3390/catal13020329
Chicago/Turabian StyleRen, Xueqing, Peng Xu, Ke Tian, Menghan Cao, Fengyin Shi, and Guangshan Zhang. 2023. "Peroxymonosulfate Activation by CuO-Fe2O3-Modified Ni Foam: A 1O2 Dominated Process for Efficient and Stable Degradation of Tetracycline" Catalysts 13, no. 2: 329. https://doi.org/10.3390/catal13020329
APA StyleRen, X., Xu, P., Tian, K., Cao, M., Shi, F., & Zhang, G. (2023). Peroxymonosulfate Activation by CuO-Fe2O3-Modified Ni Foam: A 1O2 Dominated Process for Efficient and Stable Degradation of Tetracycline. Catalysts, 13(2), 329. https://doi.org/10.3390/catal13020329