Differently Prepared PbO2/Graphitic Carbon Nitride Composites for Efficient Electrochemical Removal of Reactive Black 5 Dye
Abstract
:1. Introduction
2. Results and Discussions
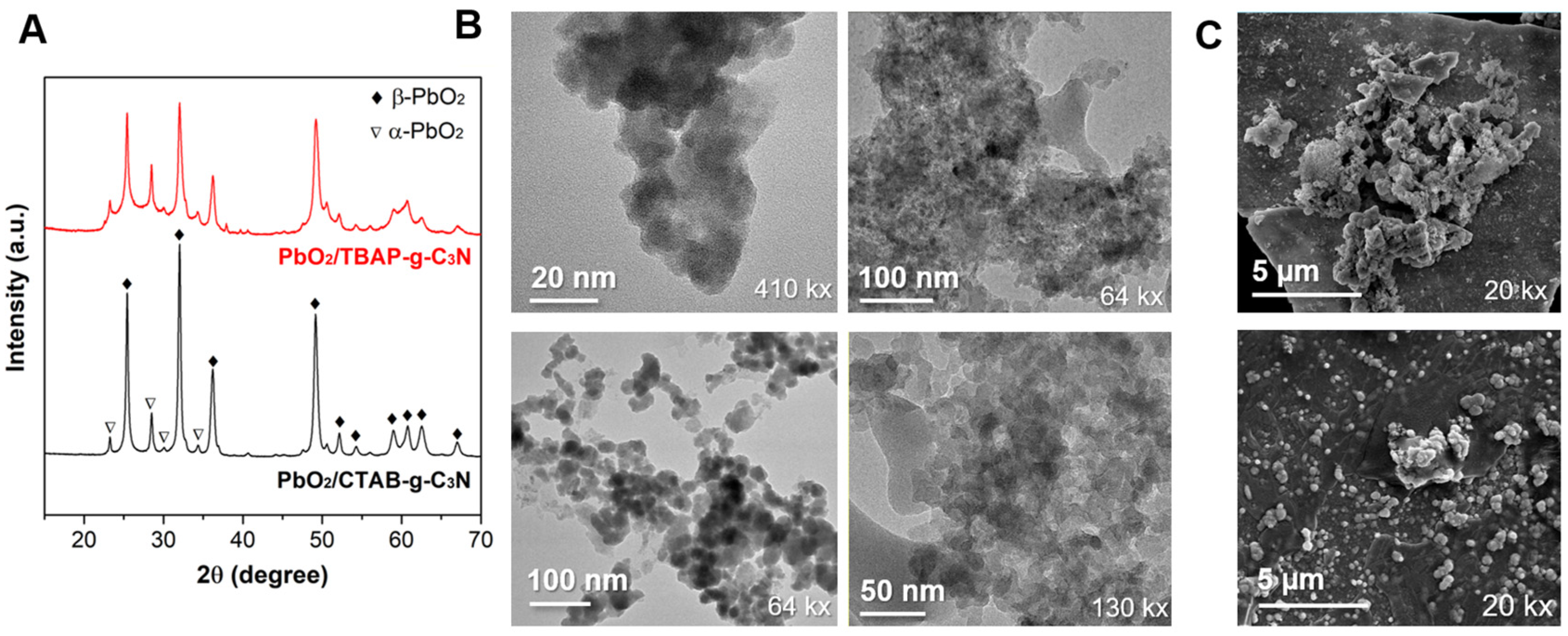
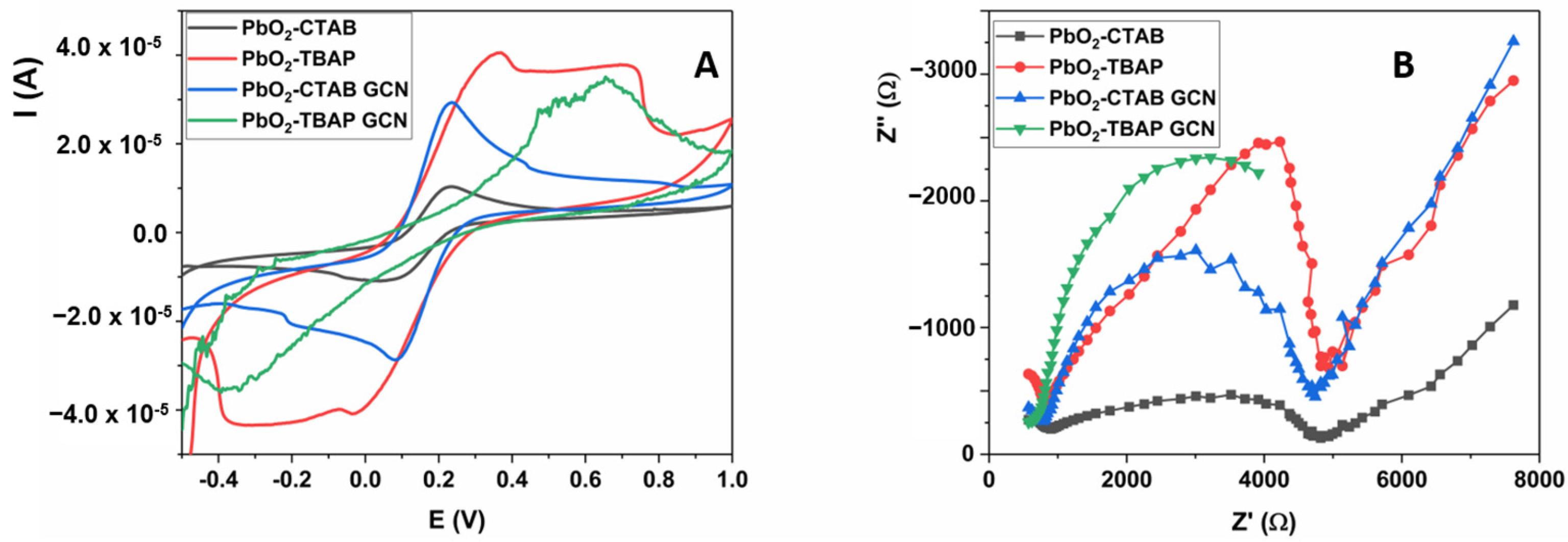
2.1. Morphological and Electrochemical Properties of Materials
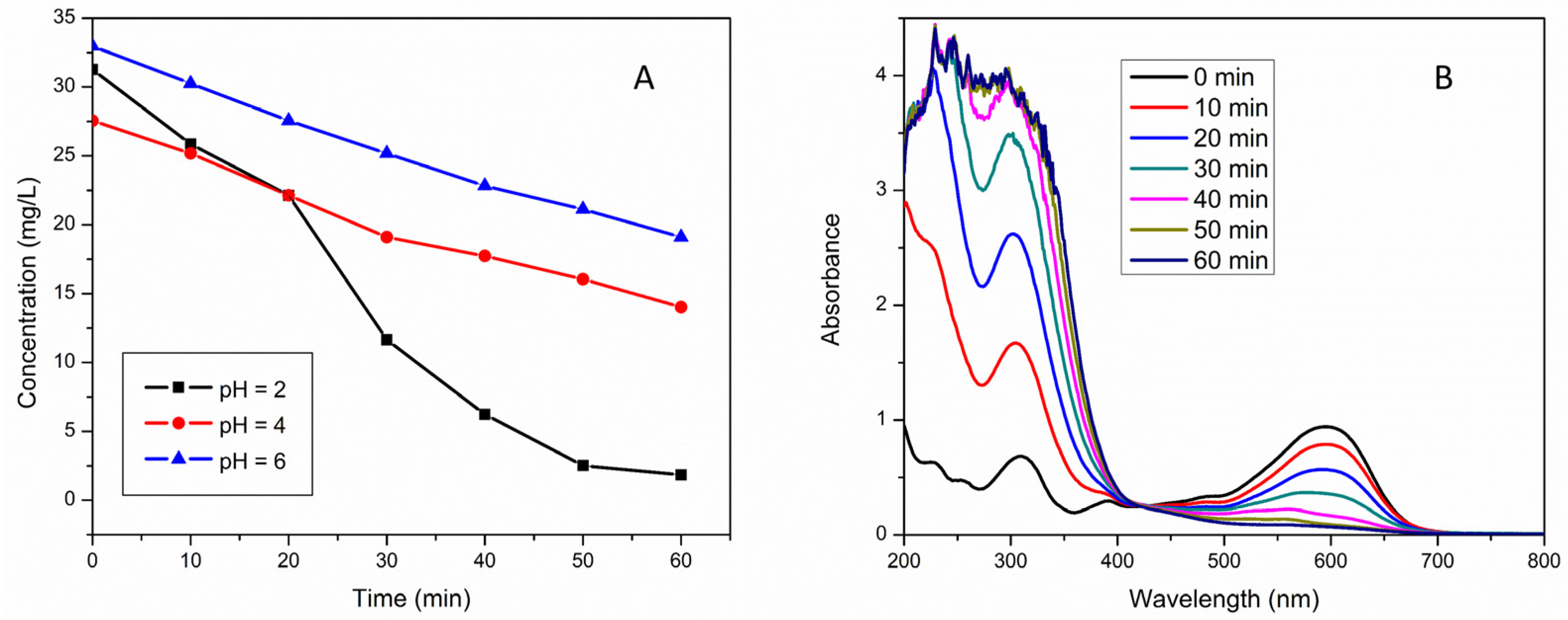
2.2. Application of Composites for the Electrochemical Removal of RB5
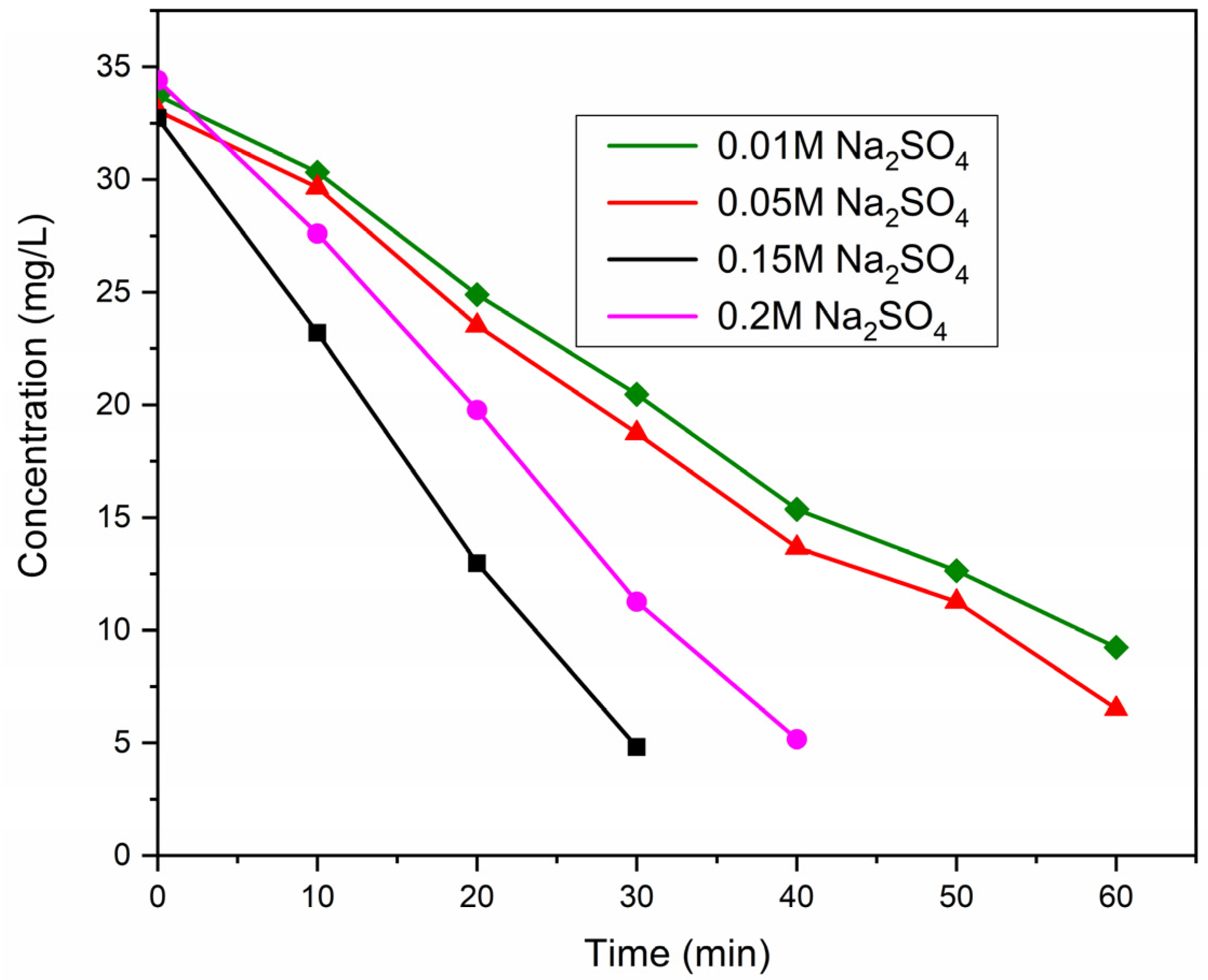
2.2.2. Optimization of Supporting Electrolyte Concentration
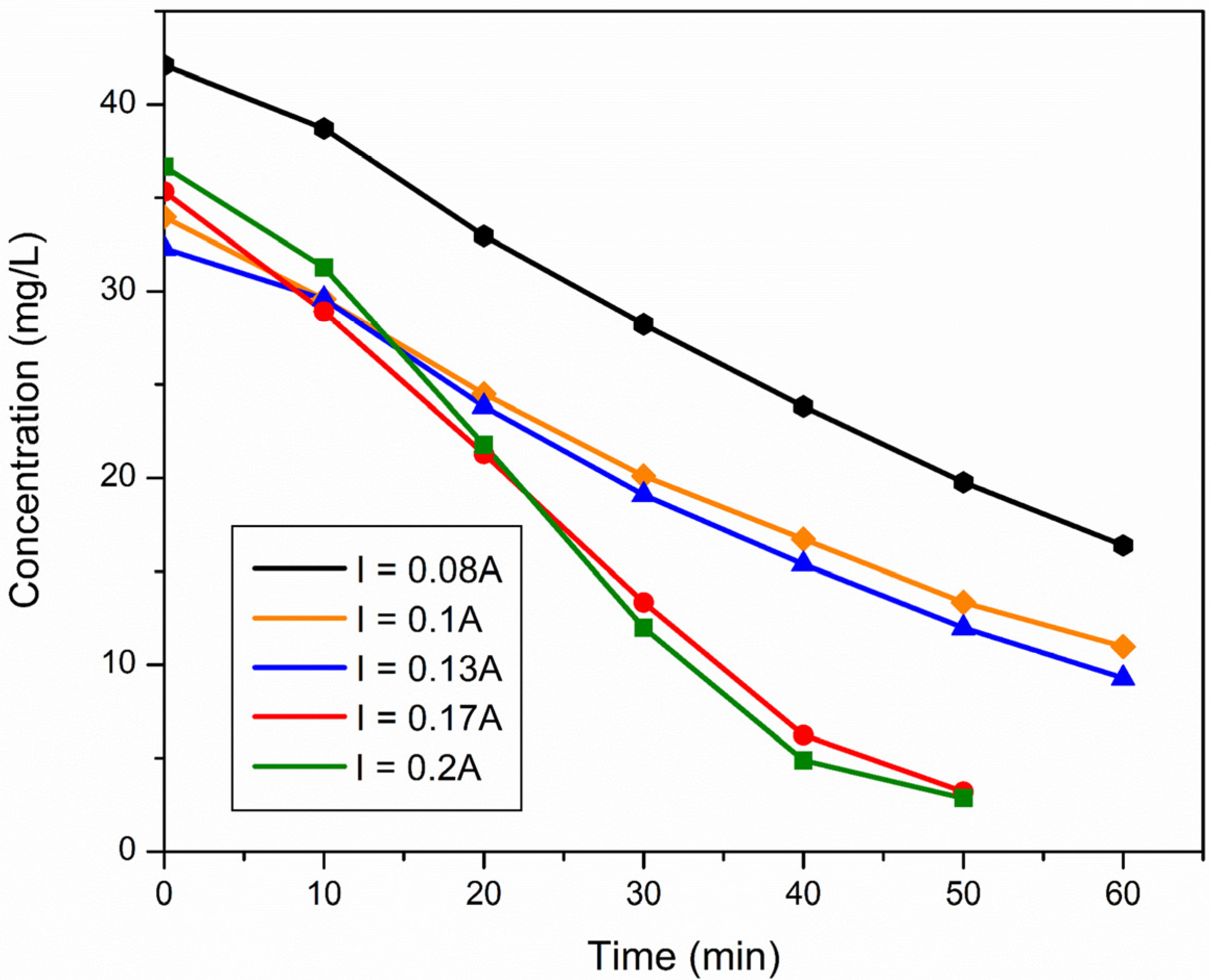
2.2.3. Optimization of Current Density-Applied Voltage
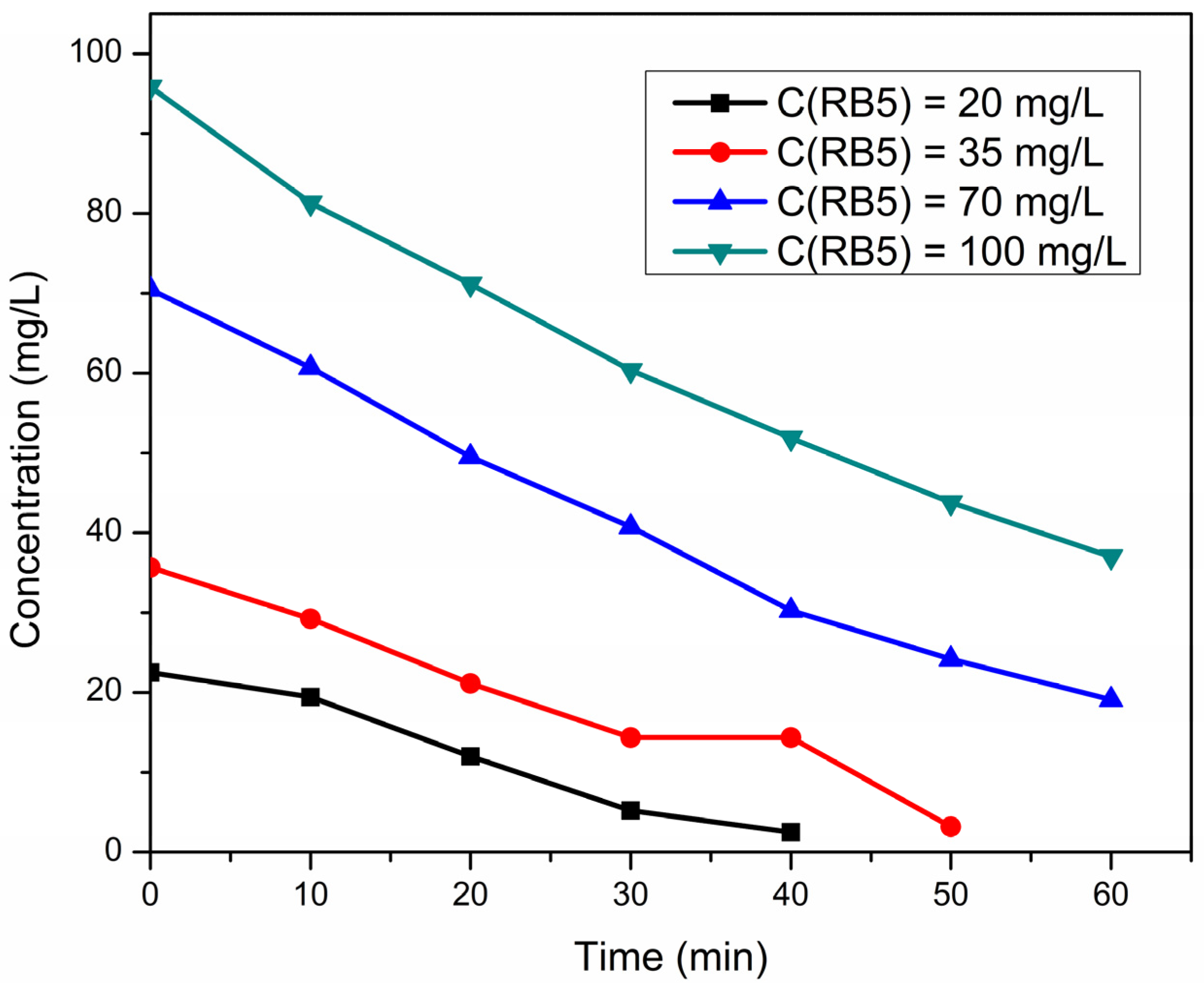
2.2.4. Optimization of RB5 Dye Concentration
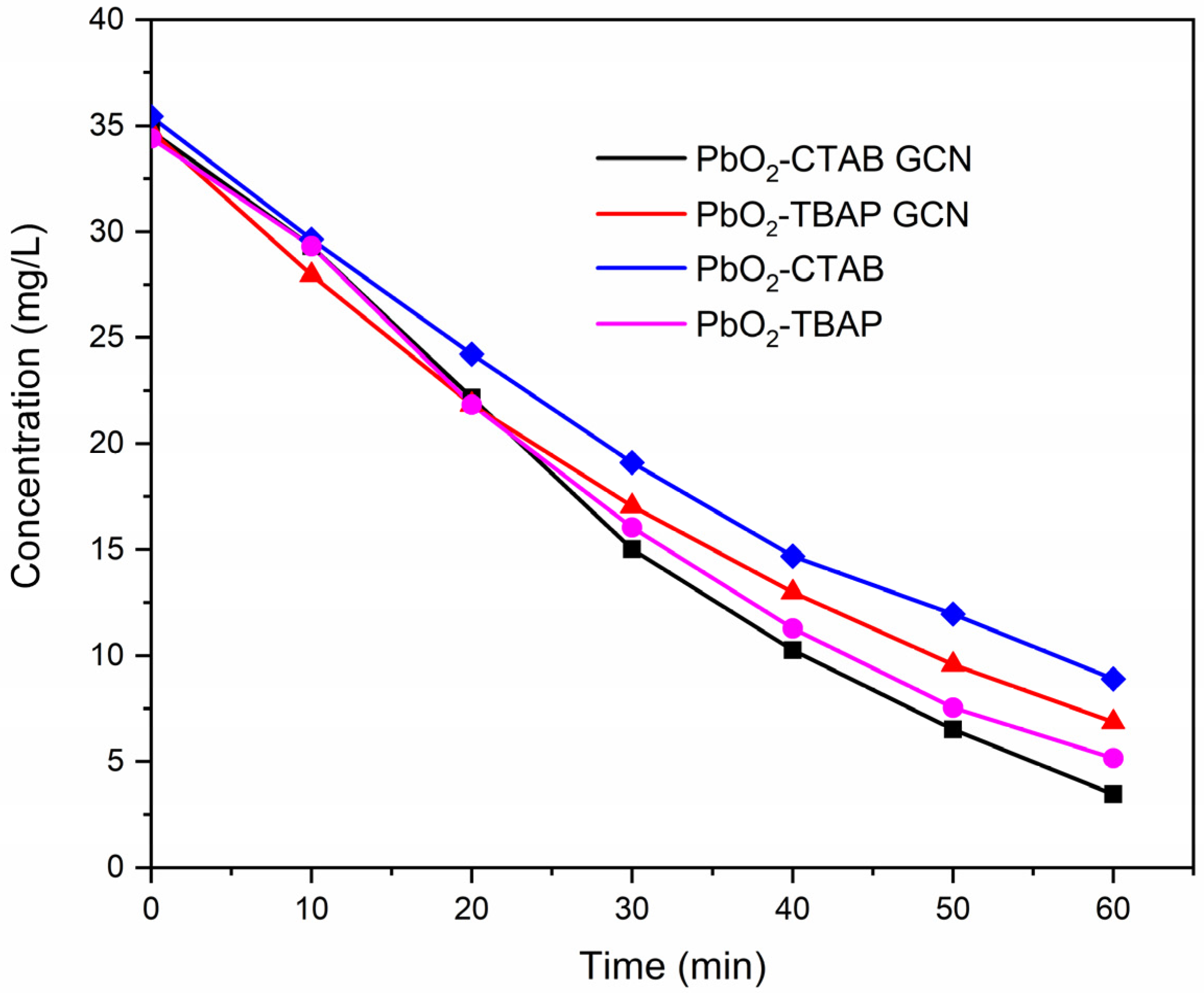
2.3. Performance Comparison of the Synthesized Materials and Composites
2.4. Specific Energy Consumption
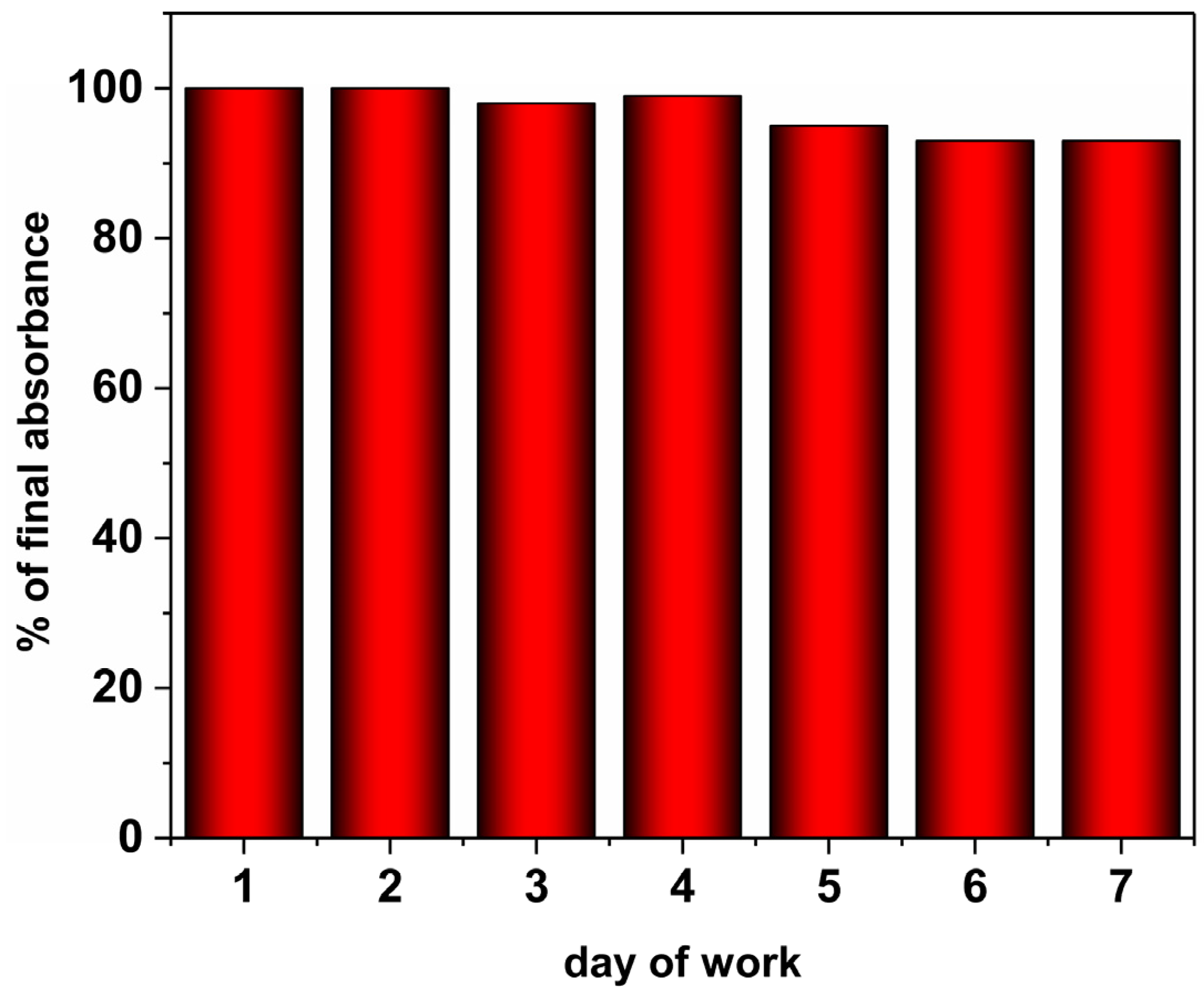
2.5. Stability and Longevity of the Method
3. Materials and Methods
3.1. Chemicals
3.2. Material Synthesis
3.3. Electrode Preparation
3.4. Electrochemical and Morphological Characterization
3.5. Experimental Setup
3.6. Specific Energy Consumption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El Bouraie, M.; El Din, W.S. Biodegradation of Reactive Black 5 by Aeromonas Hydrophila Strain Isolated from Dye-Contaminated Textile Wastewater. Sustain. Environ. Res. 2016, 26, 209–216. [Google Scholar] [CrossRef]
- Kothari, M.S.; Vegad, K.G.; Shah, K.A.; Aly Hassan, A. An Artificial Neural Network Combined with Response Surface Methodology Approach for Modelling and Optimization of the Electro-Coagulation for Cationic Dye. Heliyon 2022, 8, e08749. [Google Scholar] [CrossRef] [PubMed]
- Droguett, T.; Mora-Gómez, J.; García-Gabaldón, M.; Ortega, E.; Mestre, S.; Cifuentes, G.; Pérez-Herranz, V. Electrochemical Degradation of Reactive Black 5 Using Two-Different Reactor Configuration. Sci. Rep. 2020, 10, 4482. [Google Scholar] [CrossRef] [PubMed]
- Jager, D.; Kupka, D.; Vaclavikova, M.; Ivanicova, L.; Gallios, G. Degradation of Reactive Black 5 by Electrochemical Oxidation. Chemosphere 2018, 190, 405–416. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of Wastewaters Containing Synthetic Organic Dyes by Electrochemical Methods. An Updated Review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Yu, J.; Shu, S.; Wang, Q.; Gao, N.; Zhu, Y. Evaluation of Fe2+/Peracetic Acid to Degrade Three Typical Refractory Pollutants of Textile Wastewater. Catalysts 2022, 12, 684. [Google Scholar] [CrossRef]
- Emadi, Z.; Sadeghi, R.; Forouzandeh, S.; Mohammadi-Moghadam, F.; Sadeghi, R.; Sadeghi, M. Simultaneous Anaerobic Decolorization/Degradation of Reactive Black-5 Azo Dye and Chromium(VI) Removal by Bacillus Cereus Strain MS038EH Followed by UV-C/H2O2 Post-Treatment for Detoxification of Biotransformed Products. Arch. Microbiol. 2021, 203, 4993–5009. [Google Scholar] [CrossRef]
- Saroyan, H.; Ntagiou, D.; Rekos, K.; Deliyanni, E. Reactive Black 5 Degradation on Manganese Oxides Supported on Sodium Hydroxide Modified Graphene Oxide. Appl. Sci. 2019, 9, 2167. [Google Scholar] [CrossRef]
- De Luca, P.; Nagy, B.J. Treatment of Water Contaminated with Reactive Black-5 Dye by Carbon Nanotubes. Materials 2020, 13, 5508. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Sun, J.; Fareed, M.F.; Kenawy, E.-R.; Ali, S.S. Ecofriendly Biodegradation of Reactive Black 5 by Newly Isolated Sterigmatomyces Halophilus SSA1575, Valued for Textile Azo Dye Wastewater Processing and Detoxification. Sci. Rep. 2020, 10, 12370. [Google Scholar] [CrossRef]
- Mandic, M.; Djokic, L.; Nikolaivits, E.; Prodanovic, R.; O’Connor, K.; Jeremic, S.; Topakas, E.; Nikodinovic-Runic, J. Identification and Characterization of New Laccase Biocatalysts from Pseudomonas Species Suitable for Degradation of Synthetic Textile Dyes. Catalysts 2019, 9, 629. [Google Scholar] [CrossRef]
- Khalik, W.F.; Ho, L.-N.; Ong, S.-A.; Voon, C.-H.; Wong, Y.-S.; Yusoff, N.; Lee, S.-L.; Yusuf, S.Y. Optimization of Degradation of Reactive Black 5 (RB5) and Electricity Generation in Solar Photocatalytic Fuel Cell System. Chemosphere 2017, 184, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X.; Liu, Z.; Miao, F.; Xu, Y.; Zhang, H. Peroxymonosulfate Enhanced Photocatalytic Degradation of Reactive Black 5 by ZnO-GAC: Key Influencing Factors, Stability and Response Surface Approach. Sep. Purif. Technol. 2021, 279, 119754. [Google Scholar] [CrossRef]
- Rosli, N.I.M.; Lam, S.-M.; Sin, J.-C.; Putri, L.K.; Mohamed, A.R. Comparative Study of G-C3N4/Ag-Based Metals (V, Mo, and Fe) Composites for Degradation of Reactive Black 5 (RB5) under Simulated Solar Light Irradiation. J. Environ. Chem. Eng. 2022, 10, 107308. [Google Scholar] [CrossRef]
- Rao, M.P.; Ponnusamy, V.K.; Wu, J.J.; Asiri, A.M.; Anandan, S. Hierarchical CuO Microstructures Synthesis for Visible Light Driven Photocatalytic Degradation of Reactive Black-5 Dye. J. Environ. Chem. Eng. 2018, 6, 6059–6068. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, M.; Huang, C. Degradation of the Reactive Black 5 by Fenton and Fenton-like System. Procedia Eng. 2011, 15, 4835–4840. [Google Scholar] [CrossRef]
- Mengelizadeh, N.; Mohseni, E.; Dehghani, M.H. Heterogeneous Activation of Peroxymonosulfate by GO-CoFe2O4 for Degradation of Reactive Black 5 from Aqueous Solutions: Optimization, Mechanism, Degradation Intermediates and Toxicity. J. Mol. Liq. 2021, 327, 114838. [Google Scholar] [CrossRef]
- Fadaei, S.; Noorisepehr, M.; Pourzamani, H.; Salari, M.; Moradnia, M.; Darvishmotevalli, M.; Mengelizadeh, N. Heterogeneous Activation of Peroxymonosulfate with Fe3O4 Magnetic Nanoparticles for Degradation of Reactive Black 5: Batch and Column Study. J. Environ. Chem. Eng. 2021, 9, 105414. [Google Scholar] [CrossRef]
- López-Ramón, M.V.; Rivera-Utrilla, J.; Sánchez-Polo, M. Photocatalytic Degradation of Organic Wastes in Water. Catalysts 2022, 12, 114. [Google Scholar] [CrossRef]
- Ramírez, J.I.D.L.; Villegas, V.A.R.; Sicairos, S.P.; Guevara, E.H.; Brito Perea, M.D.C.; Sánchez, B.L. Synthesis and Characterization of Zinc Peroxide Nanoparticles for the Photodegradation of Nitrobenzene Assisted by UV-Light. Catalysts 2020, 10, 1041. [Google Scholar] [CrossRef]
- Fernández-Perales, M.; Rozalen, M.; Sánchez-Polo, M.; Rivera-Utrilla, J.; López-Ramón, M.V.; Álvarez, M.A. Solar Degradation of Sulfamethazine Using RGO/Bi Composite Photocatalysts. Catalysts 2020, 10, 573. [Google Scholar] [CrossRef]
- Amin Marsooli, M.; Rahimi Nasrabadi, M.; Fasihi-Ramandi, M.; Adib, K.; Pourmasoud, S.; Ahmadi, F.; Eghbali, M.; Sobhani Nasab, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Synthesis of Magnetic Fe3O4/ZnWO4 and Fe3O4/ZnWO4/CeVO4 Nanoparticles: The Photocatalytic Effects on Organic Pollutants upon Irradiation with UV-Vis Light. Catalysts 2020, 10, 494. [Google Scholar] [CrossRef]
- Jović, M.; Stanković, D.; Manojlović, D.; Anđelković, I.; Milić, A.; Dojčinović, B.; Roglić, G. Study of the Electrochemical Oxidation of Reactive Textile Dyes Using Platinum Electrode. Int. J. Electrochem. Sci. 2013, 8, 16. [Google Scholar]
- Savić, B.G.; Stanković, D.M.; Živković, S.M.; Ognjanović, M.R.; Tasić, G.S.; Mihajlović, I.J.; Brdarić, T.P. Electrochemical Oxidation of a Complex Mixture of Phenolic Compounds in the Base Media Using PbO2-GNRs Anodes. Appl. Surf. Sci. 2020, 529, 147120. [Google Scholar] [CrossRef]
- Žunić, M.J.; Milutinović-Nikolić, A.D.; Stanković, D.M.; Manojlović, D.D.; Jović-Jovičić, N.P.; Banković, P.T.; Mojović, Z.D.; Jovanović, D.M. Electrooxidation of P-Nitrophenol Using a Composite Organo-Smectite Clay Glassy Carbon Electrode. Appl. Surf. Sci. 2014, 313, 440–448. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Rodrigo, M.A. Renewable Energies Driven Electrochemical Wastewater/Soil Decontamination Technologies: A Critical Review of Fundamental Concepts and Applications. Appl. Catal. B Environ. 2020, 270, 118857. [Google Scholar] [CrossRef]
- Manojlović, D.; Lelek, K.; Roglić, G.; Zherebtsov, D.; Avdin, V.; Buskina, K.; Sakthidharan, C.; Sapozhnikov, S.; Samodurova, M.; Zakirov, R.; et al. Efficiency of Homely Synthesized Magnetite: Carbon Composite Anode toward Decolorization of Reactive Textile Dyes. Int. J. Environ. Sci. Technol. 2020, 17, 2455–2462. [Google Scholar] [CrossRef]
- Stanković, D.M.; Ognjanović, M.; Espinosa, A.; del Puerto Morales, M.; Bessais, L.; Zehani, K.; Antić, B.; Dojcinović, B. Iron Oxide Nanoflower–Based Screen Print Electrode for Enhancement Removal of Organic Dye Using Electrochemical Approach. Electrocatalysis 2019, 10, 663–671. [Google Scholar] [CrossRef]
- Jović, M.; Manojlović, D.; Stanković, D.; Dojčinović, B.; Obradović, B.; Gašić, U.; Roglić, G. Degradation of Triketone Herbicides, Mesotrione and Sulcotrione, Using Advanced Oxidation Processes. J. Hazard. Mater. 2013, 260, 1092–1099. [Google Scholar] [CrossRef]
- Marković, M.; Jović, M.; Stanković, D.; Kovačević, V.; Roglić, G.; Gojgić-Cvijović, G.; Manojlović, D. Application of Non-Thermal Plasma Reactor and Fenton Reaction for Degradation of Ibuprofen. Sci. Total Environ. 2015, 505, 1148–1155. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Liang, J.; Yue, L.; Li, T.; Luo, Y.; Liu, Q.; Lu, S.; Asiri, A.M.; Gong, Z.; et al. Anodic Oxidation for the Degradation of Organic Pollutants: Anode Materials, Operating Conditions and Mechanisms. A Mini Review. Electrochem. Commun. 2021, 123, 106912. [Google Scholar] [CrossRef]
- Duan, P.; Qian, C.; Wang, X.; Jia, X.; Jiao, L.; Chen, Y. Fabrication and Characterization of Ti/Polyaniline-Co/PbO2–Co for Efficient Electrochemical Degradation of Cephalexin in Secondary Effluents. Environ. Res. 2022, 214, 113842. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, G.; Guo, L.; Dai, Q.; Ma, X. Electrochemical Oxidation of Acid Orange 7 Azo Dye Using a PbO2 Electrode: Parameter Optimization, Reaction Mechanism and Toxicity Evaluation. Chemosphere 2020, 241, 125010. [Google Scholar] [CrossRef] [PubMed]
- Shmychkova, O.; Luk’yanenko, T.; Dmitrikova, L.; Velichenko, A. Modified Lead Dioxide for Organic Wastewater Treatment: Physicochemical Properties and Electrocatalytic Activity. J. Serb. Chem. Soc. 2019, 84, 187–198. [Google Scholar] [CrossRef]
- Zhou, M.; Dai, Q.; Lei, L.; Ma, C.; Wang, D. Long Life Modified Lead Dioxide Anode for Organic Wastewater Treatment: Electrochemical Characteristics and Degradation Mechanism. Environ. Sci. Technol. 2005, 39, 363–370. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, A.; Sharma, G.; Du, B.; Naushad, M.; Stadler, F.J. Carbon Quantum Dots and Reduced Graphene Oxide Modified Self-Assembled S@C3N4/B@C3N4 Metal-Free Nano-Photocatalyst for High Performance Degradation of Chloramphenicol. J. Mol. Liq. 2020, 300, 112356. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, P.; An, W.; Liu, L.; Liang, Y.; Cui, W. In-Situ Fe-Doped g-C3N4 Heterogeneous Catalyst via Photocatalysis-Fenton Reaction with Enriched Photocatalytic Performance for Removal of Complex Wastewater. Appl. Catal. B Environ. 2019, 245, 130–142. [Google Scholar] [CrossRef]
- Mohammad, A.; Ahmad, K.; Qureshi, A.; Tauqeer, M.; Mobin, S.M. Zinc Oxide-Graphitic Carbon Nitride Nanohybrid as an Efficient Electrochemical Sensor and Photocatalyst. Sens. Actuators B Chem. 2018, 277, 467–476. [Google Scholar] [CrossRef]
- Ma, S.; Xue, J.; Zhou, Y.; Zhang, Z.; Cai, Z.; Zhu, D.; Liang, S. Facile Fabrication of a Mpg-C3N4/TiO2 Heterojunction Photocatalyst with Enhanced Visible Light Photoactivity toward Organic Pollutant Degradation. RSC Adv. 2015, 5, 64976–64982. [Google Scholar] [CrossRef]
- Duan, X.; Sui, X.; Wang, Q.; Wang, W.; Li, N.; Chang, L. Electrocatalytic Oxidation of PCP-Na by a Novel Nano-PbO2 Anode: Degradation Mechanism and Toxicity Assessment. Environ. Sci. Pollut. Res. 2020, 27, 43656–43669. [Google Scholar] [CrossRef]
- Velichenko, A.; Luk’yanenko, T.; Nikolenko, N.; Shmychkova, O.; Demchenko, P.; Gladyshevskii, R. Composite Electrodes PbO2-Nafion®. J. Electrochem. Soc. 2020, 167, 063501. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.; Singhal, V.; Giammar, D.E. Effects of PH and Carbonate Concentration on Dissolution Rates of the Lead Corrosion Product PbO2. Environ. Sci. Technol. 2010, 44, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Broda, B.; Inzelt, G. Investigation of the Electrochemical Behaviour of Lead Dioxide in Aqueous Sulfuric Acid Solutions by Using the in Situ EQCM Technique. J. Solid State Electrochem. 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Belal, R.M.; Zayed, M.A.; El-Sherif, R.M.; Abdel Ghany, N.A. Advanced Electrochemical Degradation of Basic Yellow 28 Textile Dye Using IrO2/Ti Meshed Electrode in Different Supporting Electrolytes. J. Electroanal. Chem. 2021, 882, 114979. [Google Scholar] [CrossRef]
- Jalali Sarvestani, M.R.; Doroudi, Z. Removal of Reactive Black 5 from Waste Waters by Adsorption: A Comprehensive Review. J. Water Environ. Nanotechnol. 2020, 5, 180–190. [Google Scholar] [CrossRef]
- Ejeta, S.Y.; Imae, T. Cobalt Incorporated Graphitic Carbon Nitride as a Bifunctional Catalyst for Electrochemical Water-Splitting Reactions in Acidic Media. Molecules 2022, 27, 6445. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. MXene-Based Photocatalysts in Degradation of Organic and Pharmaceutical Pollutants. Molecules 2022, 27, 6939. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Kamaruddin, M.N.; Ramli, Z.A.C.; Ahmad, K.N.; Al-Azzawi, W.K.; Al-Amiery, A. Decomposition of Formic Acid and Acetic Acid into Hydrogen Using Graphitic Carbon Nitride Supported Single Metal Catalyst. Sustainability 2022, 14, 3156. [Google Scholar] [CrossRef]
- Liu, J.; Guo, H.; Yin, H.; Nie, Q.; Zou, S. Accelerated Photodegradation of Organic Pollutants over BiOBr/Protonated g-C3N4. Catalysts 2022, 12, 1109. [Google Scholar] [CrossRef]
- Khan, H.; Kang, S.; Lee, C.S. Evaluation of Efficient and Noble-Metal-Free NiTiO3 Nanofibers Sensitized with Porous GC3N4 Sheets for Photocatalytic Applications. Catalysts 2021, 11, 385. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Shan, J.; Liu, J.; Huang, X. Preparation, Characterization of Graphitic Carbon Nitride Photo-Catalytic Nanocomposites and Their Application in Wastewater Remediation: A Review. Crystals 2021, 11, 723. [Google Scholar] [CrossRef]
- Shen, Y.; Dos santos-Garcia, A.J.; Martín de Vidales, M.J. Graphitic Carbon Nitride-Based Composite in Advanced Oxidation Processes for Aqueous Organic Pollutants Removal: A Review. Processes 2021, 9, 66. [Google Scholar] [CrossRef]
- Tang, J.; Cheng, Z.; Li, H.; Xiang, L. Electro-Chemical Degradation of Norfloxacin Using a PbO2-NF Anode Prepared by the Electrodeposition of PbO2 onto the Substrate of Nickel Foam. Catalysts 2022, 12, 1297. [Google Scholar] [CrossRef]
- Zheng, T.; Wei, C.; Chen, H.; Xu, J.; Wu, Y.; Xing, X. Fabrication of PbO2 Electrodes with Different Doses of Er Doping for Sulfonamides Degradation. Int. J. Environ. Res. Public. Health 2022, 19, 3503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, Z.; Yao, J. Enhancement of the Activity of Electrochemical Oxidation of BPS by Nd-Doped PbO2 Electrodes: Performance and Mechanism. Water 2020, 12, 1317. [Google Scholar] [CrossRef]
- Saxena, P.; Ruparelia, J. Influence of Supporting Electrolytes on Electrochemical Treatability of Reactive Black 5 Using Dimensionally Stable Anode. J. Inst. Eng. India Ser. A 2019, 100, 299–310. [Google Scholar] [CrossRef]
- Rivera, M.; Pazos, M.; Sanromán, M.Á. Development of an Electrochemical Cell for the Removal of Reactive Black 5. Desalination 2011, 274, 39–43. [Google Scholar] [CrossRef]
- Eguiluz, K.I.B.; Hernandez-Sanchez, N.K.; Dória, A.R.; Santos, G.O.S.; Salazar-Banda, G.R.; Ponce de Leon, C. Template-Made Tailored Mesoporous Ti/SnO2-Sb2O5-IrO2 Anodes with Enhanced Activity towards Dye Removal. J. Electroanal. Chem. 2022, 910, 116153. [Google Scholar] [CrossRef]
- Zambrano, J.; Min, B. Comparison on Efficiency of Electrochemical Phenol Oxidation in Two Different Supporting Electrolytes (NaCl and Na2SO4) Using Pt/Ti Electrode. Environ. Technol. Innov. 2019, 15, 100382. [Google Scholar] [CrossRef]
- Carneiro, J.F.; Aquino, J.M.; Silva, A.J.; Barreiro, J.C.; Cass, Q.B.; Rocha-Filho, R.C. The Effect of the Supporting Electrolyte on the Electrooxidation of Enrofloxacin Using a Flow Cell with a BDD Anode: Kinetics and Follow-up of Oxidation Intermediates and Antimicrobial Activity. Chemosphere 2018, 206, 674–681. [Google Scholar] [CrossRef]
- Xu, L.; Guo, Z.; Du, L.; He, J. Decolourization and Degradation of C.I. Acid Red 73 by Anodic Oxidation and the Synergy Technology of Anodic Oxidation Coupling Nanofiltration. Electrochim. Acta 2013, 97, 150–159. [Google Scholar] [CrossRef]
- Cao, M.; Hu, C.; Peng, G.; Qi, Y.; Wang, E. Selected-Control Synthesis of PbO2 and Pb3O4 Single-Crystalline Nanorods. J. Am. Chem. Soc. 2003, 125, 4982–4983. [Google Scholar] [CrossRef]

| I (A) | U (V) |
|---|---|
| 0.08 | ~3.63 |
| 0.10 | ~3.65 |
| 0.13 | ~4.2 |
| 0.17 | ~4.8–4.9 |
| 0.20 | ~5.1–5.3 |
| Material | Supporting Electrolyte | % of RB5 Removal and Time | Energy Consumption | Ref |
|---|---|---|---|---|
| RuO2/IrO2/TiO2@DSA® | 0.008 M NaCl | ~100% in 15 min | Not reported | [4] |
| Ti/CoOx–RuO2–SnO2–Sb2O5 | 0.07 M Na2SO4 | ~40% in 2 h | 34.5 kWh/kg a | [56] |
| Ti/SnO2-Sb2O5-IrO2 | 0.1 M Na2SO4 | ~60% in 2 h | Not reported | [58] |
| Graphite | 0.1 M Na2SO4 | ~100% in 3 h | Not reported | [57] |
| PbO2-CTAB/g-C3N4 on SS | 0.15 M Na2SO4 | ~90% in 1 h | 0.4374 kWh/g | This study |
| T (min) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
|---|---|---|---|---|---|---|---|---|
| electrode | RB5 degradation rate (%) | SEC (kWh/g) | ||||||
| I | 0.00 | 16.45 | 37.43 | 57.69 | 70.19 | 81.01 | 88.53 | 0.4374 |
| II | 0.00 | 15.81 | 31.31 | 45.49 | 58.15 | 67.64 | 74.89 | 0.5173 |
| III | 0.00 | 19.94 | 37.52 | 50.93 | 62.79 | 72.14 | 80.24 | 0.4895 |
| IV | 0.00 | 14.83 | 36.57 | 53.38 | 67.21 | 78.09 | 85.01 | 0.4698 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, A.; Savić, S.; Kukuruzar, A.; Konya, Z.; Manojlović, D.; Ognjanović, M.; Stanković, D.M. Differently Prepared PbO2/Graphitic Carbon Nitride Composites for Efficient Electrochemical Removal of Reactive Black 5 Dye. Catalysts 2023, 13, 328. https://doi.org/10.3390/catal13020328
Marković A, Savić S, Kukuruzar A, Konya Z, Manojlović D, Ognjanović M, Stanković DM. Differently Prepared PbO2/Graphitic Carbon Nitride Composites for Efficient Electrochemical Removal of Reactive Black 5 Dye. Catalysts. 2023; 13(2):328. https://doi.org/10.3390/catal13020328
Chicago/Turabian StyleMarković, Aleksandar, Slađana Savić, Andrej Kukuruzar, Zoltan Konya, Dragan Manojlović, Miloš Ognjanović, and Dalibor M. Stanković. 2023. "Differently Prepared PbO2/Graphitic Carbon Nitride Composites for Efficient Electrochemical Removal of Reactive Black 5 Dye" Catalysts 13, no. 2: 328. https://doi.org/10.3390/catal13020328
APA StyleMarković, A., Savić, S., Kukuruzar, A., Konya, Z., Manojlović, D., Ognjanović, M., & Stanković, D. M. (2023). Differently Prepared PbO2/Graphitic Carbon Nitride Composites for Efficient Electrochemical Removal of Reactive Black 5 Dye. Catalysts, 13(2), 328. https://doi.org/10.3390/catal13020328










