Application of BiVO4/TiO2/CNT Composite Photocatalysts for Membrane Fouling Control and Photocatalytic Membrane Regeneration during Dairy Wastewater Treatment
Abstract
1. Introduction
2. Results
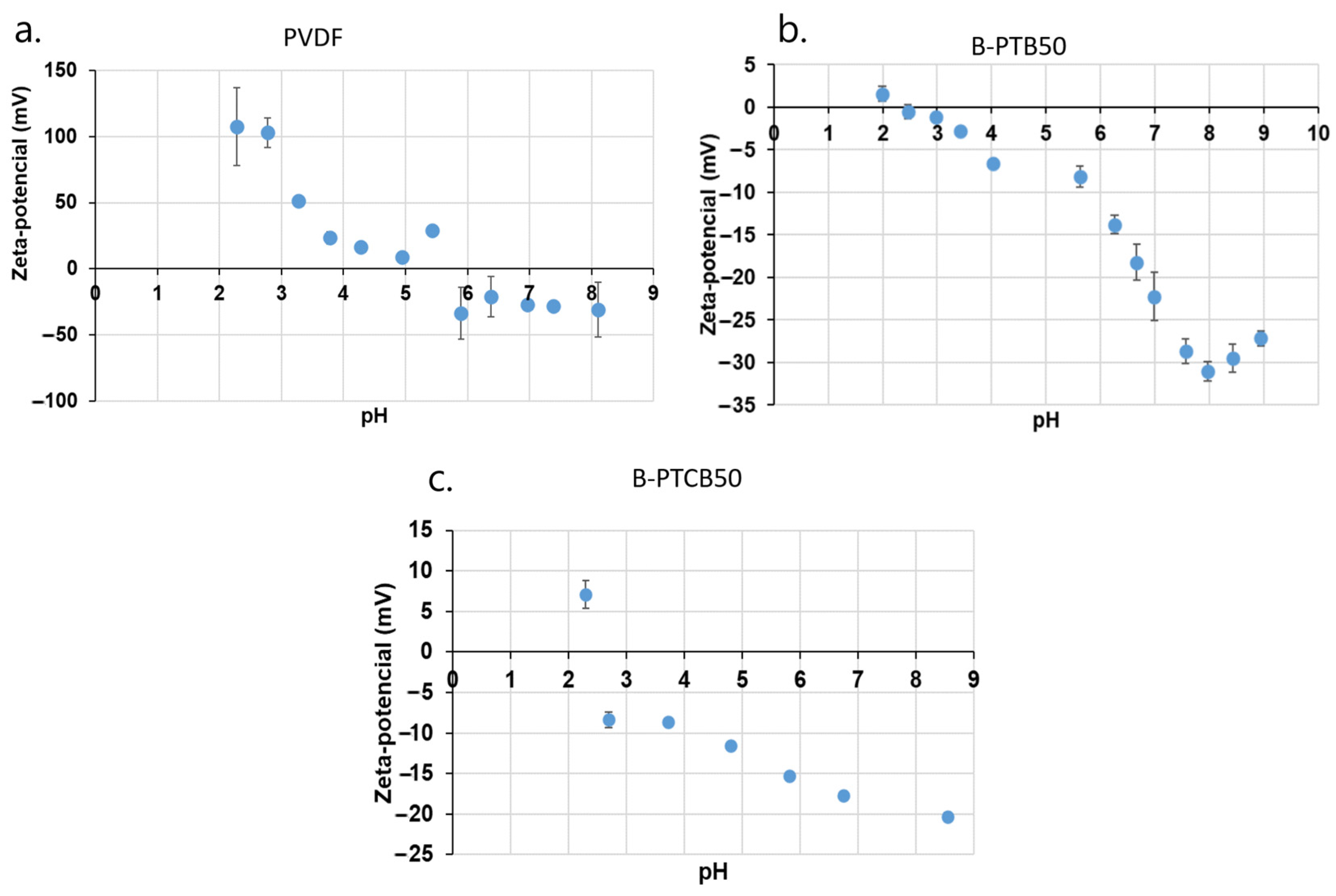
2.1. Zeta Potential of Prepared Membranes
2.2. Application of PTCB Membranes for Synthetic Dairy Wastewater Treatment
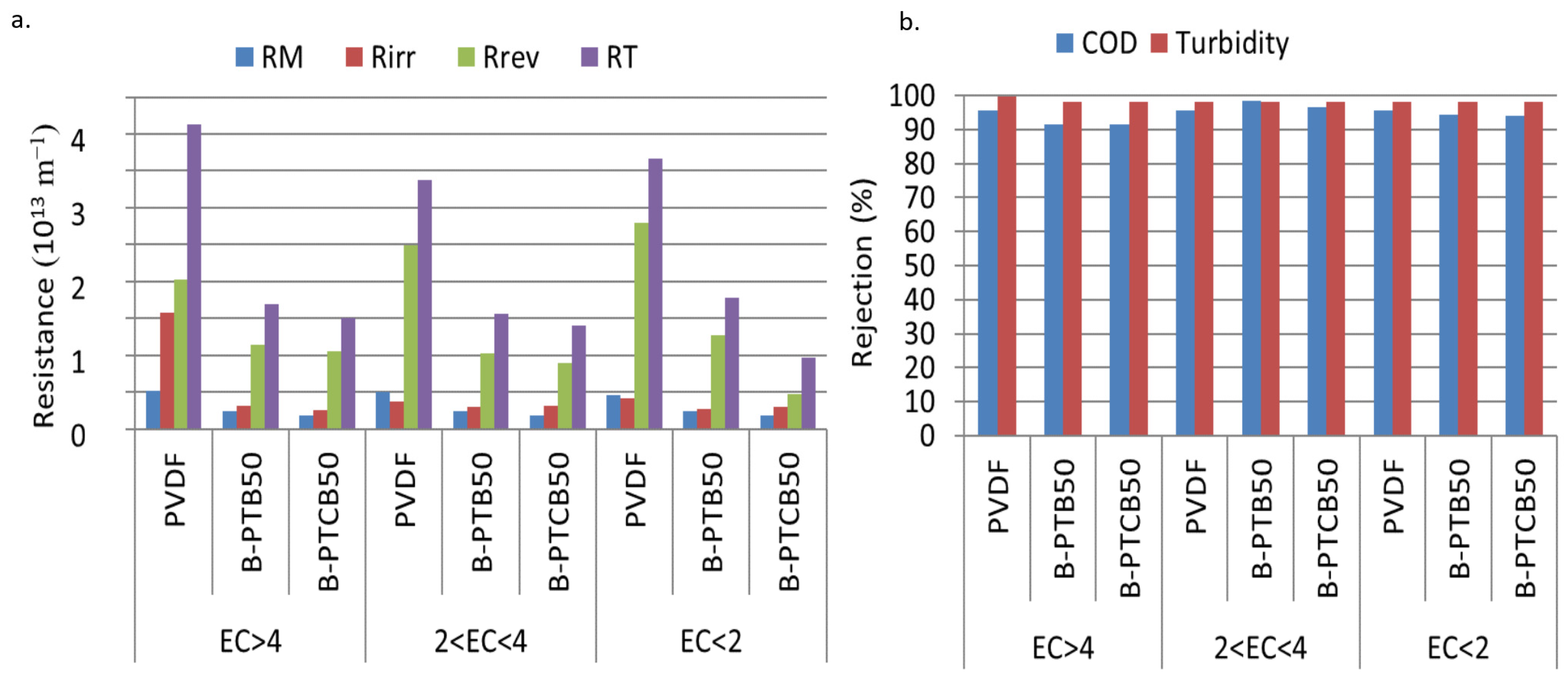
2.2.1. Effects of Salinity on Fouling and Retention
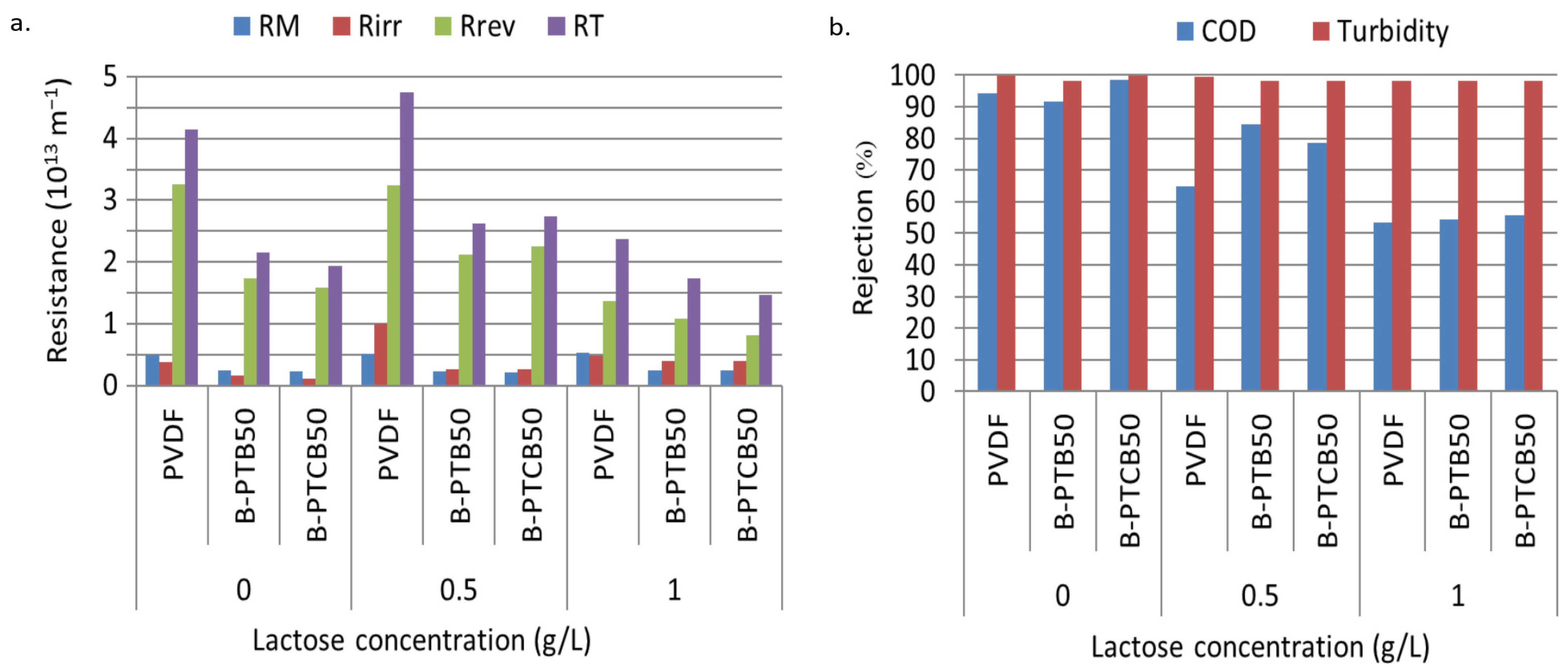
2.2.2. Effects of Lactose on Fouling and Retention
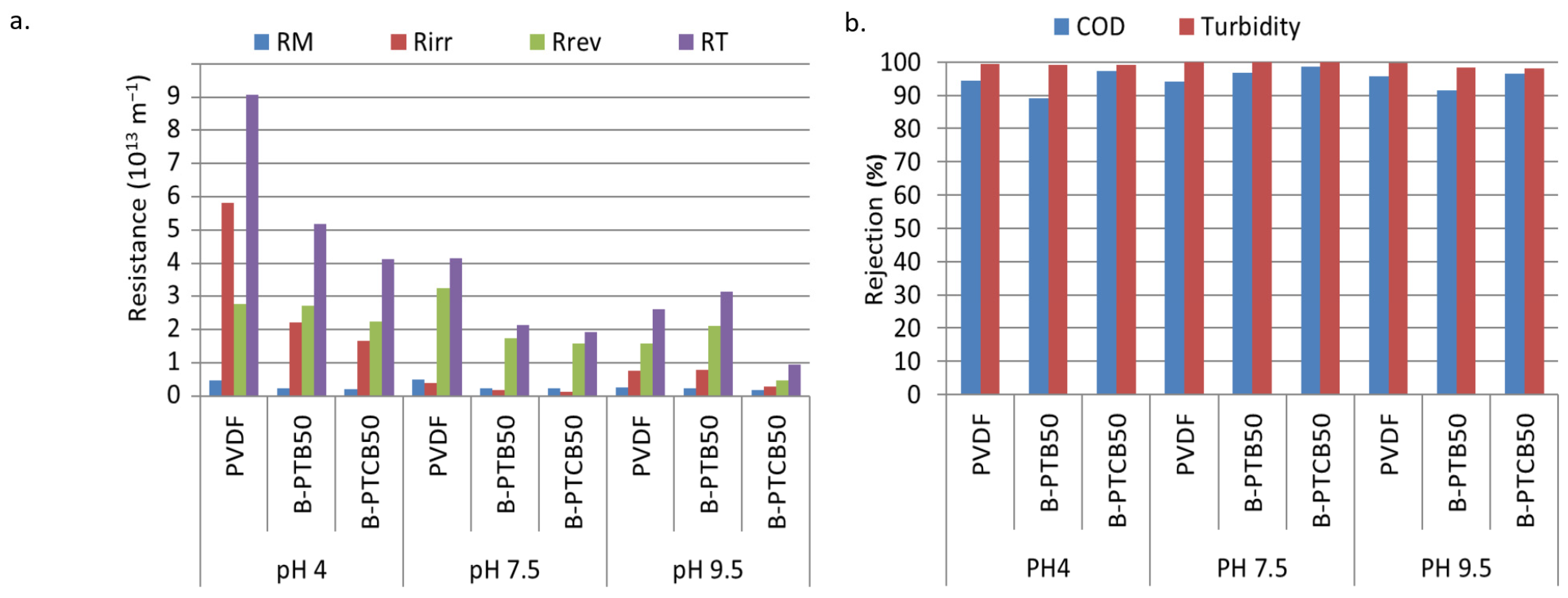
2.2.3. Effects of pH on Fouling and Rejection
2.3. Application of PTCB Membranes for Real Dairy Wastewater Treatment
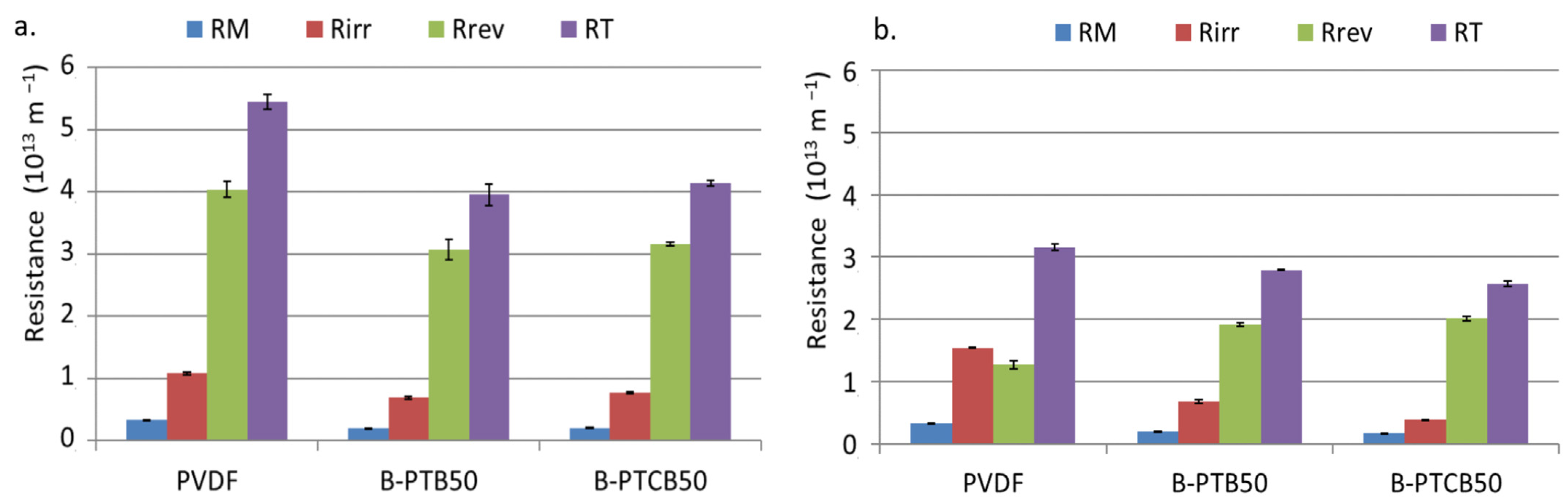
2.3.1. Filtration Resistances
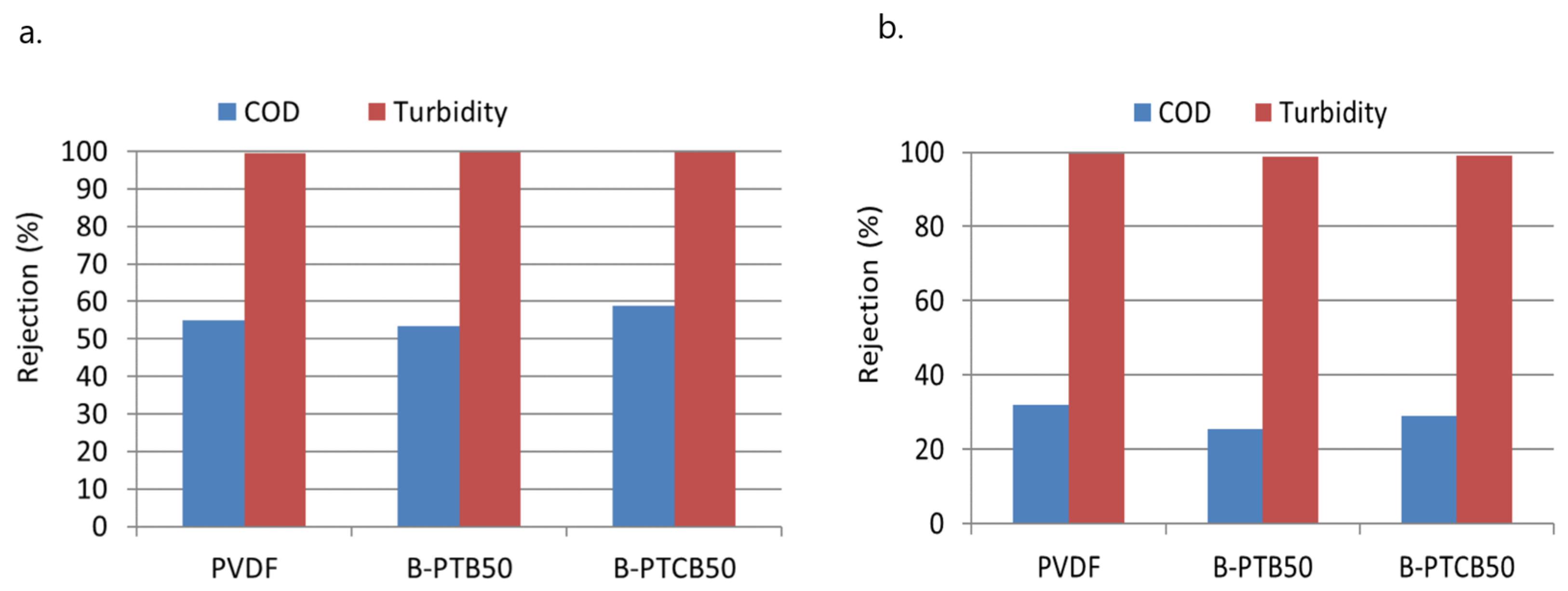
2.3.2. Rejection
2.3.3. Membrane Regeneration
3. Materials and Methods
3.1. Wastewater Collection and Preparation
3.2. Synthetic Dairy Wastewater
3.3. Real Dairy Wastewater
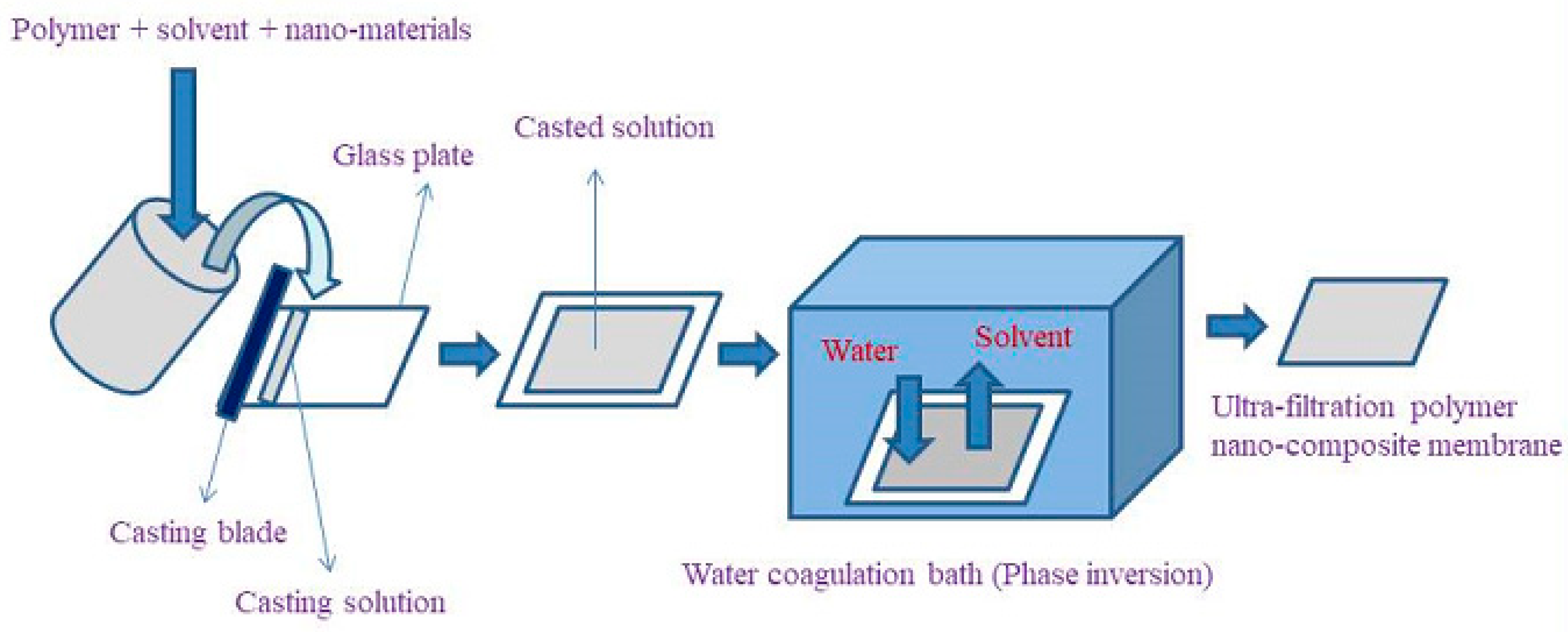
3.4. Membrane Preparation
3.5. Membrane Characterization
Zeta Potential Analysis
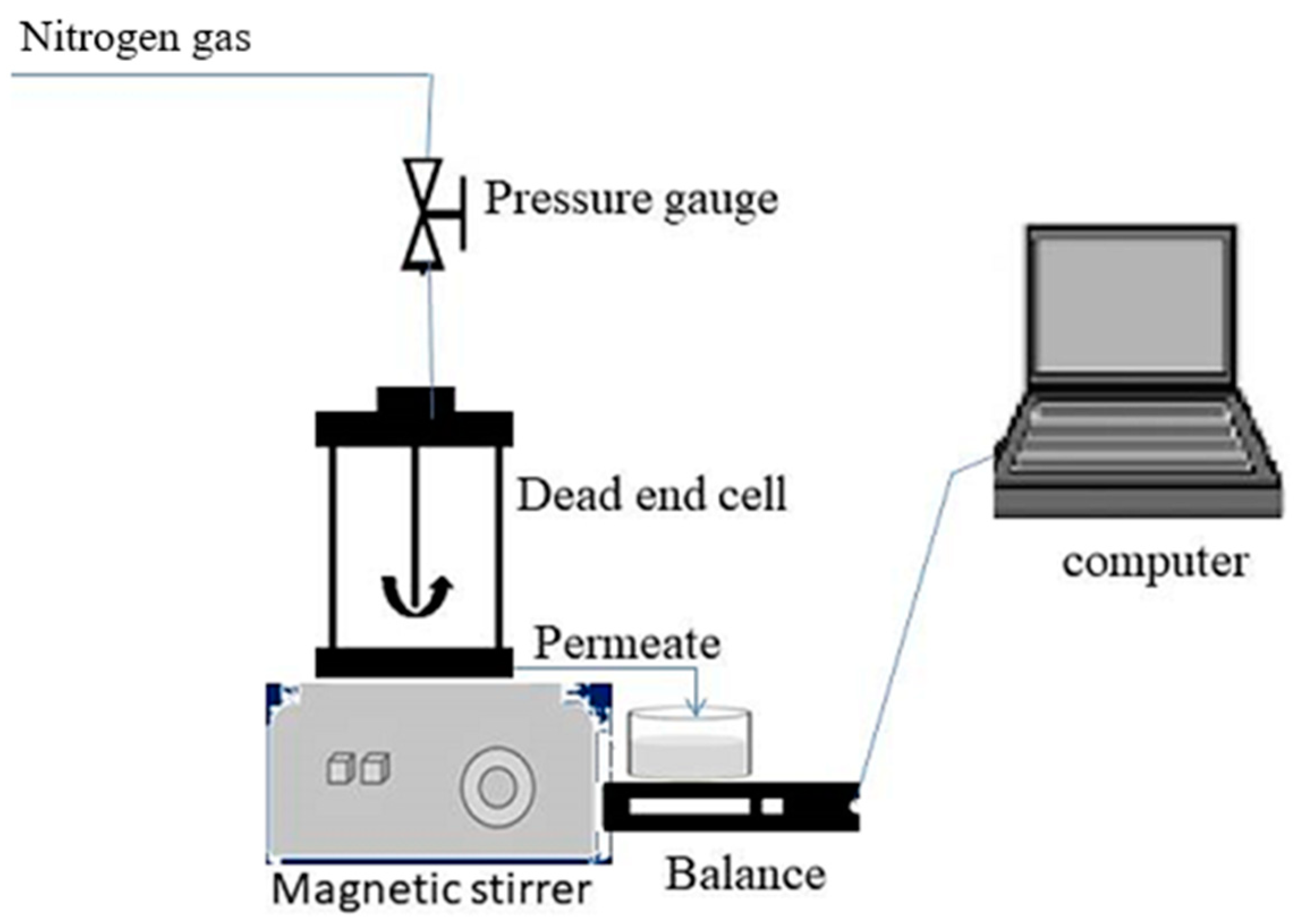
3.6. Membrane Filtration Experiments
3.6.1. Water Flux and Contaminant Rejection Performance
3.6.2. Fouling Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zinadini, S.; Vatanpour, V.; Zinatizadeh, A.A.; Rahimi, M.; Rahimi, Z.; Kian, M. Preparation and characterization of antifouling graphene oxide/polyethersulfone ultrafiltration membrane: Application in MBR for dairy wastewater treatment. J. Water Process Eng. 2015, 7, 280–294. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; ur Rahman, U.; Soares, B.C.V.; Souza, S.L.Q.; Cruz, A.G. Treatment and utilization of dairy industrial waste: A review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Muniz, G.L.; Borges, A.C.; da Silva, T.C.F. Performance of natural coagulants obtained from agro-industrial wastes in dairy wastewater treatment using dissolved air flotation. J. Water Process Eng. 2020, 37, 101453. [Google Scholar] [CrossRef]
- Catenacci, A.; Bellucci, M.; Yuan, T.; Malpei, F. Dairy wastewater treatment using composite membranes. In Current Trends and Future Developments on Bio Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 261–288. [Google Scholar] [CrossRef]
- Sobola, D.; Kaspar, P.; Částková, K.; Dallaev, R.; Papež, N.; Sedlák, P.; Trčka, T.; Orudzhev, F.; Kaštyl, J.; Weiser, A.; et al. PVDF Fibers Modification by Nitrate Salts Doping. Polymers 2021, 13, 2439. [Google Scholar] [CrossRef]
- Kaspar, P.; Sobola, D.; Částková, K.; Dallaev, R.; Šťastná, E.; Sedlák, P.; Knápek, A.; Trčka, T.; Holcman, V. Case Study of Polyvinylidene Fluoride Doping by Carbon Nanotubes. Materials 2021, 14, 1428. [Google Scholar] [CrossRef]
- Ji, J.; Liu, F.; Hashim, N.A.; Abed, M.R.M.; Li, K. Poly(vinylidene fluoride) (PVDF) membranes for fluid separation. React. Funct. Polym. 2015, 86, 134–153. [Google Scholar] [CrossRef]
- Fan, G.; Chen, C.; Chen, X.; Li, Z.; Bao, S.; Luo, J.; Yan, Z. Enhancing the antifouling and rejection properties of PVDF membrane by Ag3PO4-GO modification. Sci. Total Environ. 2021, 801, 149611. [Google Scholar] [CrossRef]
- Wei, Q.; Wu, C.; Zhang, J.; Cui, Z.; Jiang, T.; Li, J. Fabrication of surface microstructure for the ultrafiltration membrane based on “active–passive” synergistic antifouling and its antifouling mechanism of protein. React. Funct. Polym. 2021, 169, 105068. [Google Scholar] [CrossRef]
- Gholami, S.; Llacuna, J.L.; Vatanpour, V.; Dehqan, A.; Paziresh, S.; Cortina, J.L. Impact of a new functionalization of multiwalled carbon nanotubes on antifouling and permeability of PVDF nanocomposite membranes for dye wastewater treatment. Chemosphere 2022, 294, 133699. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Vatanpour, V. A comprehensive study on the performance and antifouling enhancement of the PVDF mixed matrix membranes by embedding different nanoparticles: Clay, functionalized carbon nanotube, SiO2 and TiO2. Sep. Purif. Technol. 2018, 197, 372–381. [Google Scholar] [CrossRef]
- Coelho, F.E.B.; Deemter, D.; Candelario, V.M.; Boffa, V.; Malato, S.; Magnacca, G. Development of a photocatalytic zirconia-titania ultrafiltration membrane with antifouling and self-cleaning properties. J. Environ. Chem. Eng. 2021, 9, 106671. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Chen, P.; Li, F.; Hu, X.; Hua, T. Photocatalytic and antifouling properties of TiO2-based photocatalytic membranes. Mater. Today Chem. 2022, 23, 100650. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Yang, F.; Bai, W.; Zhang, X.; Li, H.; Duan, G.; Xu, Y.; Li, Y. A bioinspired antibacterial and photothermal membrane for stable and durable clean water remediation. Mater. Horiz. 2023, 10, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, J.; Zhang, X.; Yuan, D.; Duan, G.; Li, Y. Robust and multifunctional natural polyphenolic composites for water remediation. Mater. Horiz. 2022, 9, 2496–2517. [Google Scholar] [CrossRef]
- Bai, H.; He, P.; Hao, L.; Fan, Z.; Niu, R.; Tang, T.; Gong, J. Waste-treating-waste: Upcycling discarded polyester into metal–organic framework nanorod for synergistic interfacial solar evaporation and sulfate-based advanced oxidation process. Chem. Eng. J. 2023, 456, 140994. [Google Scholar] [CrossRef]
- Chen, L.; Yang, B.; Zhou, P.; Xu, T.; He, C.; Xu, Y.; Zhao, W.; Zhao, C. A polyethersulfone composite ultrafiltration membrane with the in-situ generation of CdS nanoparticles for the effective removal of organic pollutants and photocatalytic self-cleaning. J. Membr. Sci. 2021, 638, 119715. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Luo, J.; Wan, Y. A novel paradigm of photocatalytic cleaning for membrane fouling removal. J. Membr. Sci. 2022, 641, 119859. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Ait Akbour, R.; Nidheesh, P.V.; Hamdani, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process. Process. Intensif. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Nasr, O.; Mohamed, O.; Al-Shirbini, A.-S.; Abdel-Wahab, A.-M. Photocatalytic degradation of acetaminophen over Ag, Au and Pt loaded TiO2 using solar light. J. Photochem. Photobiol. A Chem. 2019, 374, 185–193. [Google Scholar] [CrossRef]
- Nair, A.K.; Jagadeesh Babu, P.E. Ag-TiO2 nanosheet embedded photocatalytic membrane for solar water treatment. J. Environ. Chem. Eng. 2017, 5, 4128–4133. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, M.; Wang, L.; Fei, Y.; Wang, S.; Núnez-Delgado, A.; Bokhari, A.; Race, M.; Khataee, A.; Klemes, J.; et al. Photocatalytic degradation of xanthate in flotation plant tailings by TiO2/ graphene nanocomposites. Chem. Eng. J. 2022, 431, 134104. [Google Scholar] [CrossRef]
- Trapalis, A.; Todorova, N.; Giannakopoulou, T.; Boukos, N.; Speliotis, T.; Dimotikali, D.; Yu, J. TiO2/graphene composite photocatalysts for NOx removal: A comparison of surfactant-stabilized graphene and reduced graphene oxide. Appl. Catal. B Environ. 2016, 180, 637–647. [Google Scholar] [CrossRef]
- Selvaraj, M.; Hai, A.; Banat, F.; Haija, M.A. Application and prospects of carbon nanostructured materials in water treatment: A review. J. Water Process Eng. 2020, 33, 100996. [Google Scholar] [CrossRef]
- Akhavan, O. Lasting antibacterial activities of Ag–TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J. Colloid Interface Sci. 2009, 336, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zouzelka, R.; Kusumawati, Y.; Remzova, M.; Rathousky, J.; Pauporté, T. Photocatalytic activity of porous multiwalled carbon nanotube-TiO2 composite layers for pollutant degradation. J. Hazard. Mater. 2016, 317, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Malathi, A.; Arunachalam, P.; Kirankumar, V.S.; Madhavan, J.; Al-Mayouf, A.M. An efficient visible light driven bismuth ferrite incorporated bismuth oxyiodide (BiFeO3/BiOI) composite photocatalytic material for degradation of pollutants. Opt. Mater. 2018, 84, 227–235. [Google Scholar] [CrossRef]
- Ratova, M.; Redfern, J.; Verran, J.; Kelly, P.J. Highly efficient photocatalytic bismuth oxide coatings and their antimicrobial properties under visible light irradiation. Appl. Catal. B Environ. 2018, 239, 223–232. [Google Scholar] [CrossRef]
- Sisay, E.J.; Veréb, G.; Pap, Z.; Gyulavári, T.; Ágoston, Á.; Kopniczky, J.; Hodúr, C.; Arthanareeswaran, G.; Sivasundari, G.K.; László, Z. Visible-light driven photocatalytic PVDF-TiO2/CNT/BiVO4 hybrid nanocomposite ultrafiltration membrane for dairy wastewater treatment. Chemosphere 2022, 307, 135589. [Google Scholar] [CrossRef]
- Qazi, J.I.; Nadeem, M.; Baig, S.S.; Baig, S.; Syed, Q. Anaerobic fixed-film biotreatment of dairy wastewater. Middle East J. Sci. Res. 2011, 8, 590–593. [Google Scholar]
- Deshannavar, U.B.; Basavaraj, R.K.; Naik, N.M. High-rate digestion of dairy industry effluent by upflow anaerobic fixed-bed reactor. J. Chem. Pharm. Res. 2012, 4, 2895–2899. [Google Scholar]
- Srivastava, H.P.; Arthanareeswaran, G.; Anantharaman, N.; Starov, V.M. Performance of modified poly(vinylidene fluoride) membrane for textile wastewater ultrafiltration. Desalination 2011, 282, 87–94. [Google Scholar] [CrossRef]
- Lawrence, N.D.; Perera, J.M.; Iyer, M.; Hickey, M.W.; Stevens, G.W. The use of streaming potential measurements to study the fouling and cleaning of ultrafiltration membranes. Sep. Purif. Technol. 2006, 48, 106–112. [Google Scholar] [CrossRef]
- Vatanpour, V.; Yekavalangi, M.E.; Safarpour, M. Preparation and characterization of nanocomposite PVDF ultrafiltration membrane embedded with nanoporous SAPO-34 to improve permeability and antifouling performance. Sep. Purif. Technol. 2016, 163, 300–309. [Google Scholar] [CrossRef]

| Number | Chemicals | Concentration in g/L |
|---|---|---|
| 1 | BSA | 1 |
| 2 | Ammonium chloride (NH4Cl) | 0.5833 |
| 3 | Sodium dihydrogen phosphate (NaH2PO4) | 0.9 |
| 4 | Sodium bicarbonate (NaHCO3) | 1.560 |
| 5 | Magnesium sulfate heptahydrate (MgSO4 × H2O) | 0.6 |
| 6 | Ferrous sulfate heptahydrate (Fe(SO4) × 7H2O) | 0.024 |
| 7 | Manganese sulfate monohydrate (MnSO4 × H2O) | 0.024 |
| 8 | Calcium chloride (CaCl2 without water) | 0.036 |
| Level of Salinity | COD | Turbidity | EC (mS) | SAL | TDS (g/L) | pH |
|---|---|---|---|---|---|---|
| High salinity (EC > 4) | 1154 | 46.67 | 4.14 | 2.2 | 2.165 | 7.5 |
| Medium salinity (2 < EC < 4) | 1148 | 21.83 | 2.33 | 1.1 | 1.25 | 7.5 |
| Low salinity (EC < 2) | 1155 | 8.16 | 1.59 | 0.8 | 0.85 | 7.5 |
| Level of Lactose (g/L) | COD | Turbidity | EC (mS) | SAL | TDS (g/L) | pH |
|---|---|---|---|---|---|---|
| 0 | 1154 | 46.67 | 4.14 | 2.2 | 2.165 | 7.5 |
| 0.5 | 1653 | 158.67 | 3.83 | 2.1 | 2.07 | 7.88 |
| 1 | 2316 | 188.33 | 3.80 | 2.1 | 2.23 | 7.81 |
| Parameter | Average | SD |
|---|---|---|
| pH | 7.09 | 0.02 |
| Color | milky white | |
| EC (mS) | 2.1 | 0.01 |
| TDS (g/L) | 1.11 | 0.01 |
| BOD (mg/L) | 2181 | 70.71 |
| COD (mg/L) | 3770 | 20.00 |
| Ca (mg/L) | 159.67 | 4.04 |
| CaO (mg/L) | 231 | 1.00 |
| CaCO3 (mg/L) | 412.33 | 2.52 |
| NH4 (mg/L) | 56.87 | 0.31 |
| NH4N (mg/L) | 44.15 | 0.39 |
| NH3 (mg/L) | 53.31 | 0.38 |
| NO3 (mg/L) | 10.05 | 0.83 |
| NO2N (mg/L) | 2.2 | 0.20 |
| TOTAL N (mg/L) | 74.33 | 1.15 |
| PO43– (mg/L) | 1178.33 | 3.51 |
| PO4-P (mg/L) | 40.43 | 2.60 |
| P2P5 (mg/L) | 89.61 | 1.65 |
| TOTAL P (mg/L) | 39.10 | 1.68 |
| Resistances (m−1) | Formula |
|---|---|
| Membrane resistance | |
| Reversible resistance | |
| Overall resistance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisay, E.J.; Kertész, S.; Fazekas, Á.; Jákói, Z.; Kedves, E.Z.; Gyulavári, T.; Ágoston, Á.; Veréb, G.; László, Z. Application of BiVO4/TiO2/CNT Composite Photocatalysts for Membrane Fouling Control and Photocatalytic Membrane Regeneration during Dairy Wastewater Treatment. Catalysts 2023, 13, 315. https://doi.org/10.3390/catal13020315
Sisay EJ, Kertész S, Fazekas Á, Jákói Z, Kedves EZ, Gyulavári T, Ágoston Á, Veréb G, László Z. Application of BiVO4/TiO2/CNT Composite Photocatalysts for Membrane Fouling Control and Photocatalytic Membrane Regeneration during Dairy Wastewater Treatment. Catalysts. 2023; 13(2):315. https://doi.org/10.3390/catal13020315
Chicago/Turabian StyleSisay, Elias Jigar, Szabolcs Kertész, Ákos Fazekas, Zoltán Jákói, Endre Zsolt Kedves, Tamás Gyulavári, Áron Ágoston, Gábor Veréb, and Zsuzsanna László. 2023. "Application of BiVO4/TiO2/CNT Composite Photocatalysts for Membrane Fouling Control and Photocatalytic Membrane Regeneration during Dairy Wastewater Treatment" Catalysts 13, no. 2: 315. https://doi.org/10.3390/catal13020315
APA StyleSisay, E. J., Kertész, S., Fazekas, Á., Jákói, Z., Kedves, E. Z., Gyulavári, T., Ágoston, Á., Veréb, G., & László, Z. (2023). Application of BiVO4/TiO2/CNT Composite Photocatalysts for Membrane Fouling Control and Photocatalytic Membrane Regeneration during Dairy Wastewater Treatment. Catalysts, 13(2), 315. https://doi.org/10.3390/catal13020315








