Photodynamic Light-Triggered Release of Curcumin from Hierarchical FAU Zeolite
Abstract
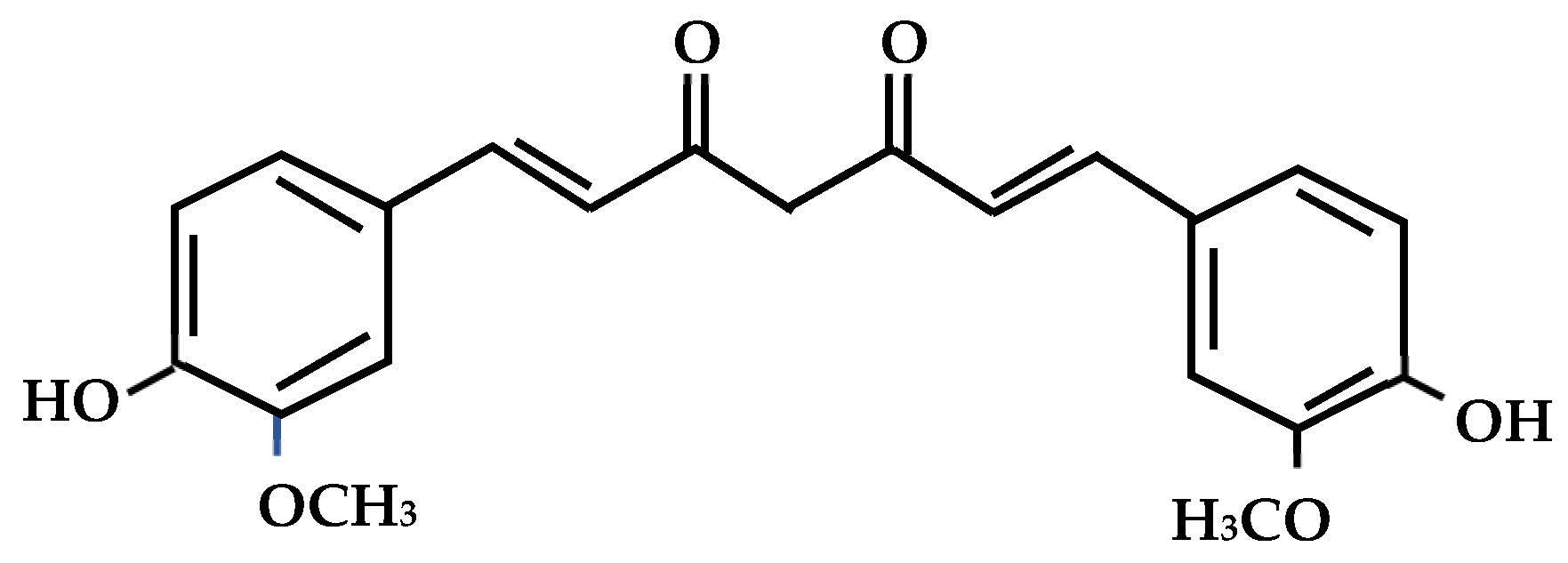
1. Introduction
2. Results
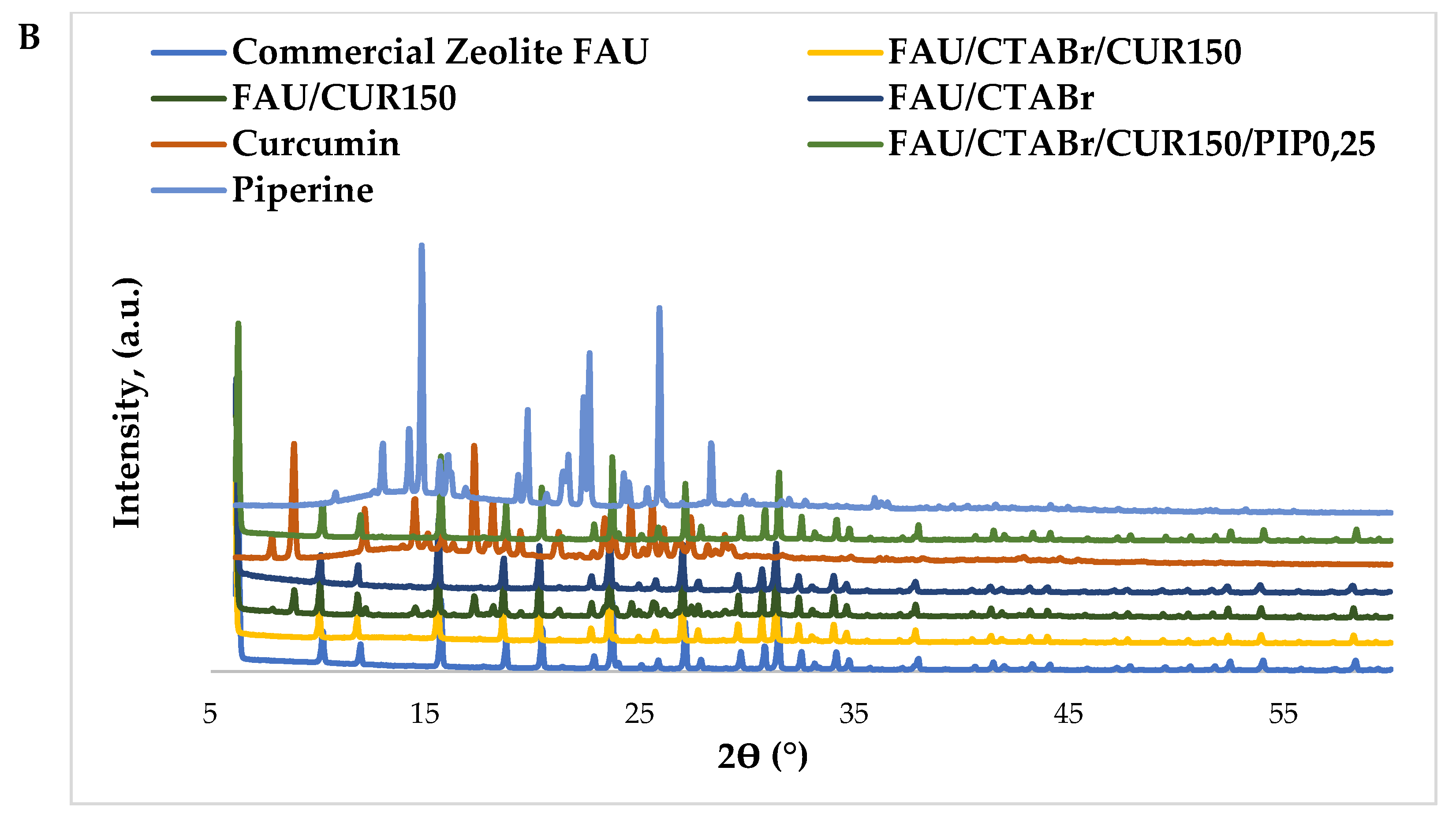
2.1. X-ray Diffraction Studies
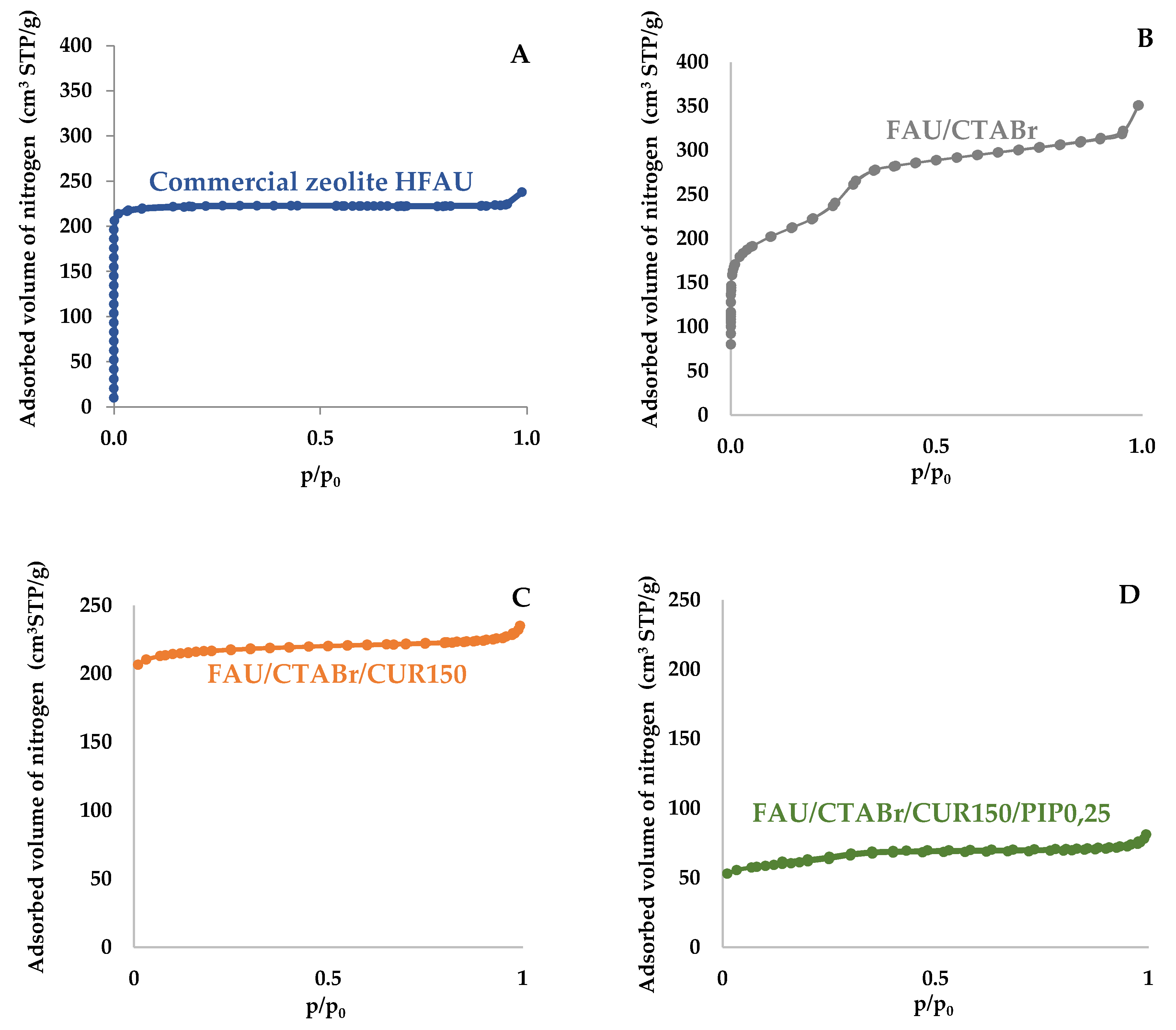
2.2. Nitrogen Adsorption/Desorption Isotherms
2.3. FT-IR Spectroscopy
2.4. Elemental Analysis
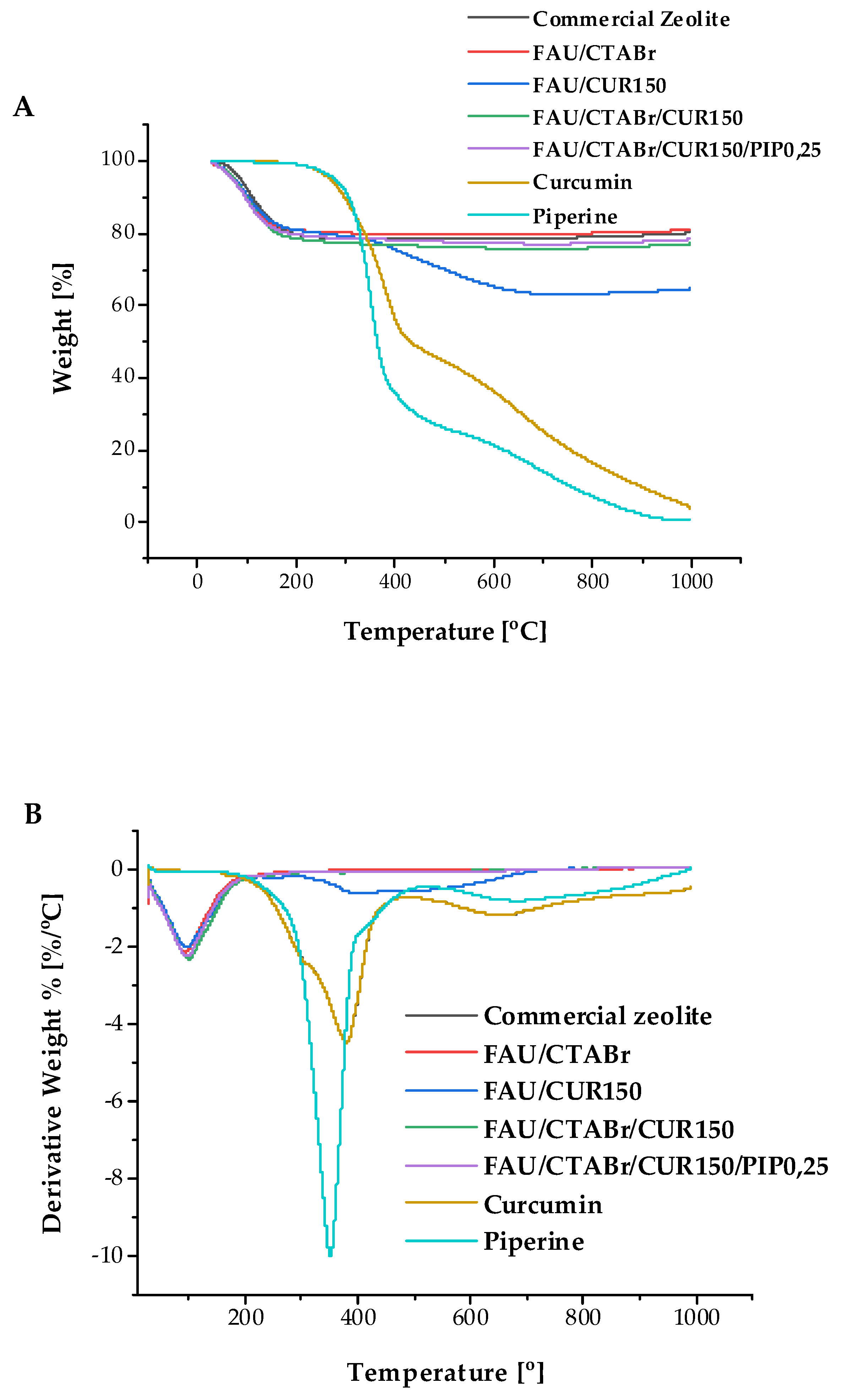
2.5. Thermogravimetric Studies
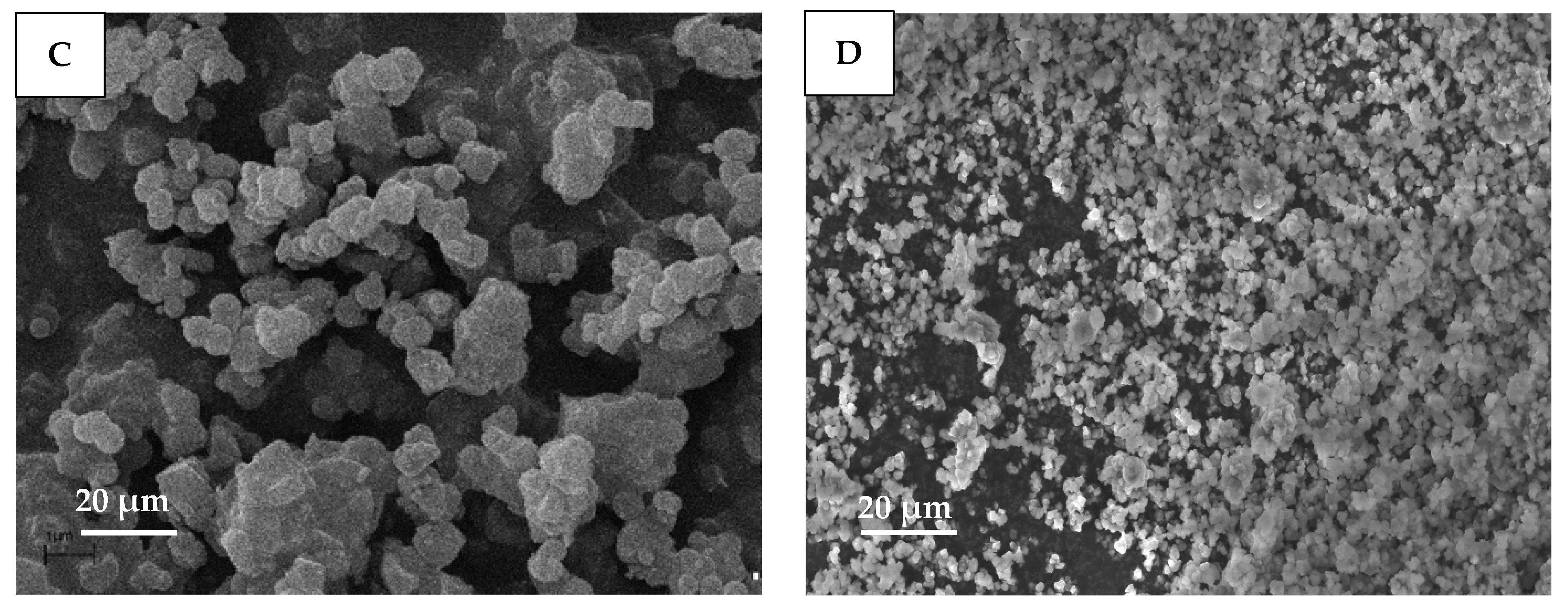
2.6. Scanning Electron Microscopy (SEM)
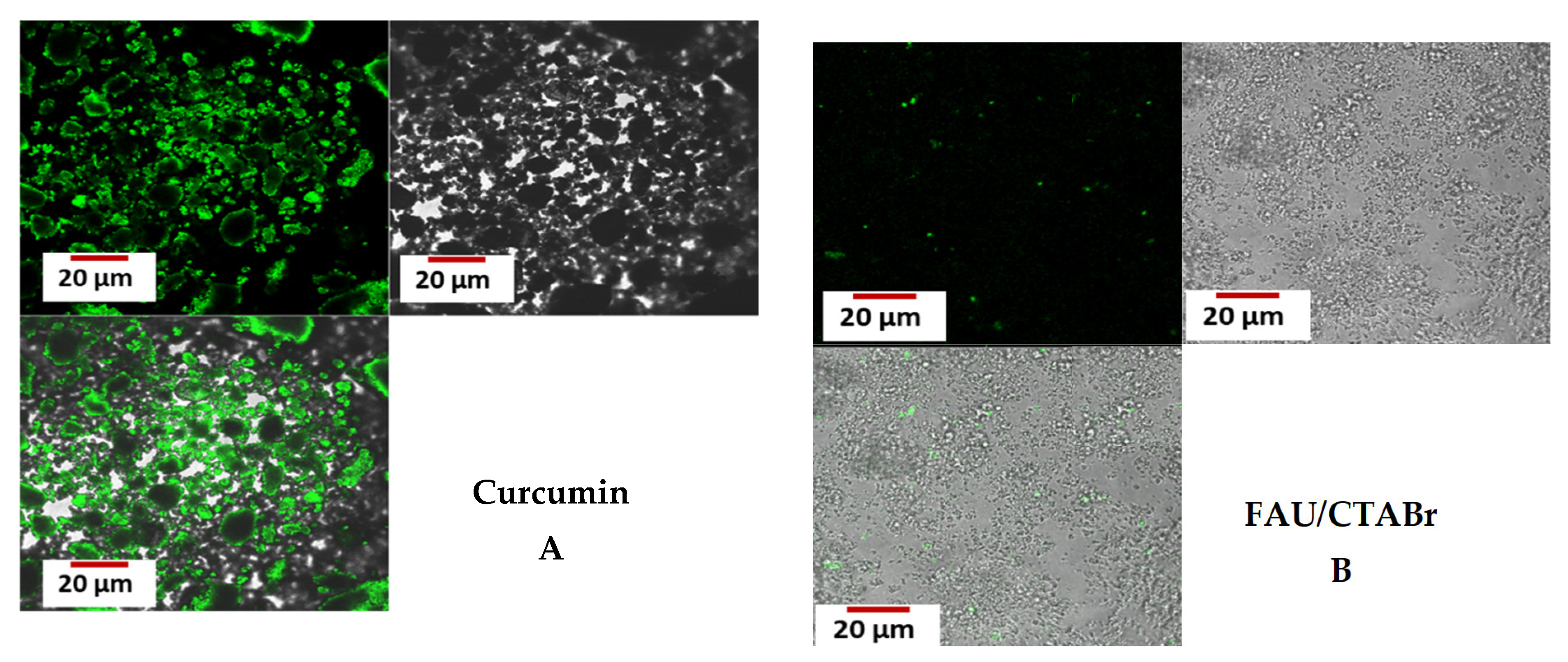
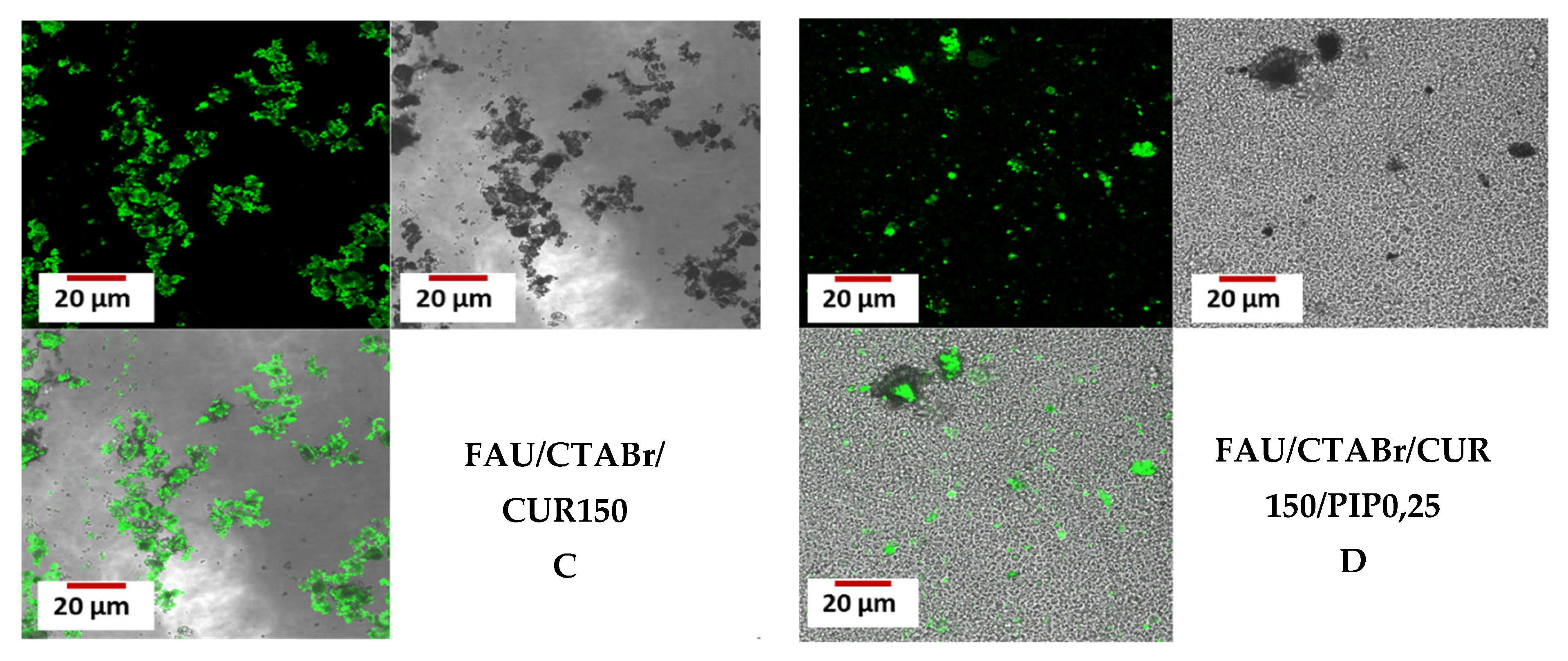
2.7. Confocal Microscopy
2.8. Encapsulation Efficiency and Loading Capacity of Curcumin
2.9. UV–Visible Spectroscopy
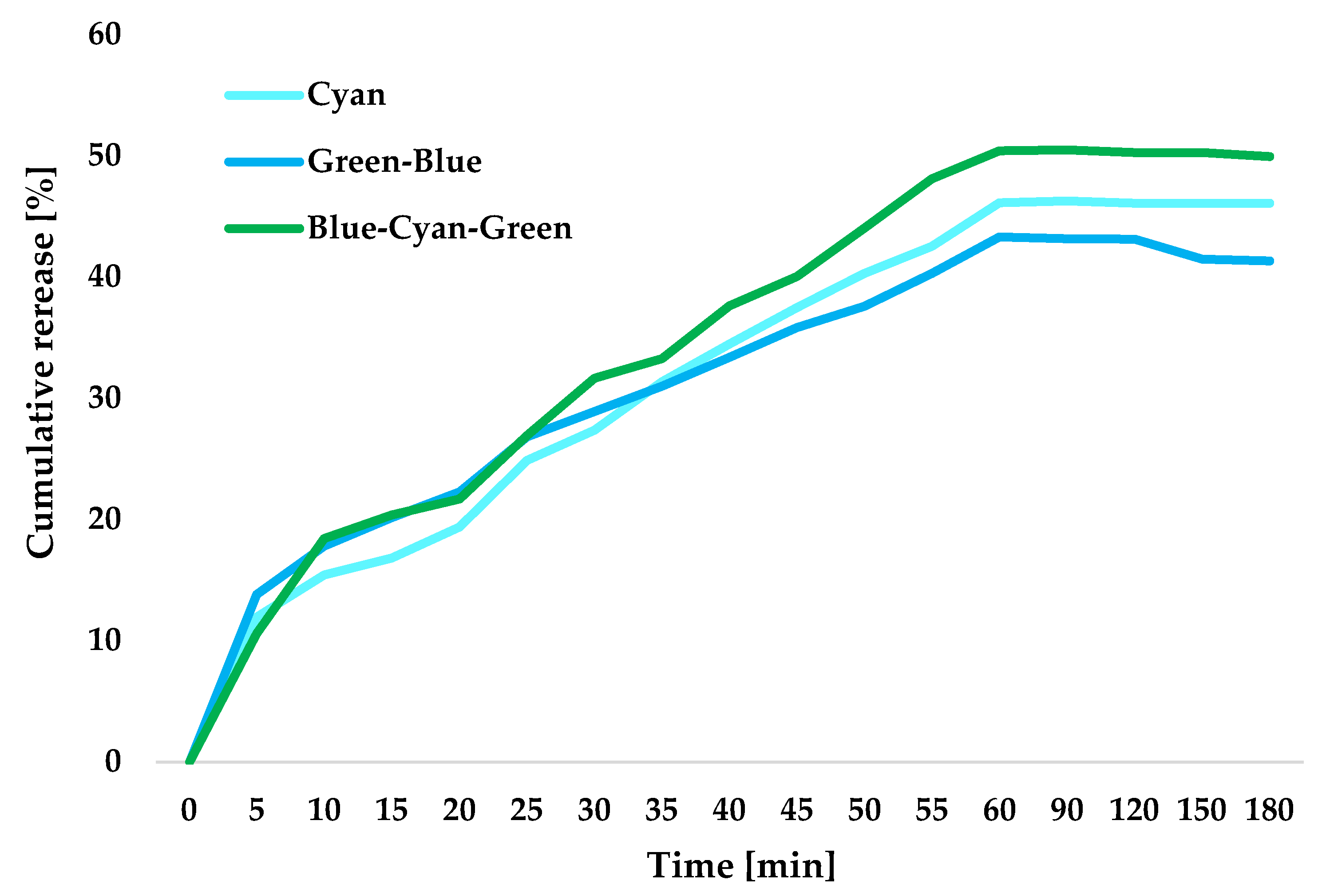
2.10. Open System Release Study of Curcumin
2.11. Study of Curcumin Release in a Closed System
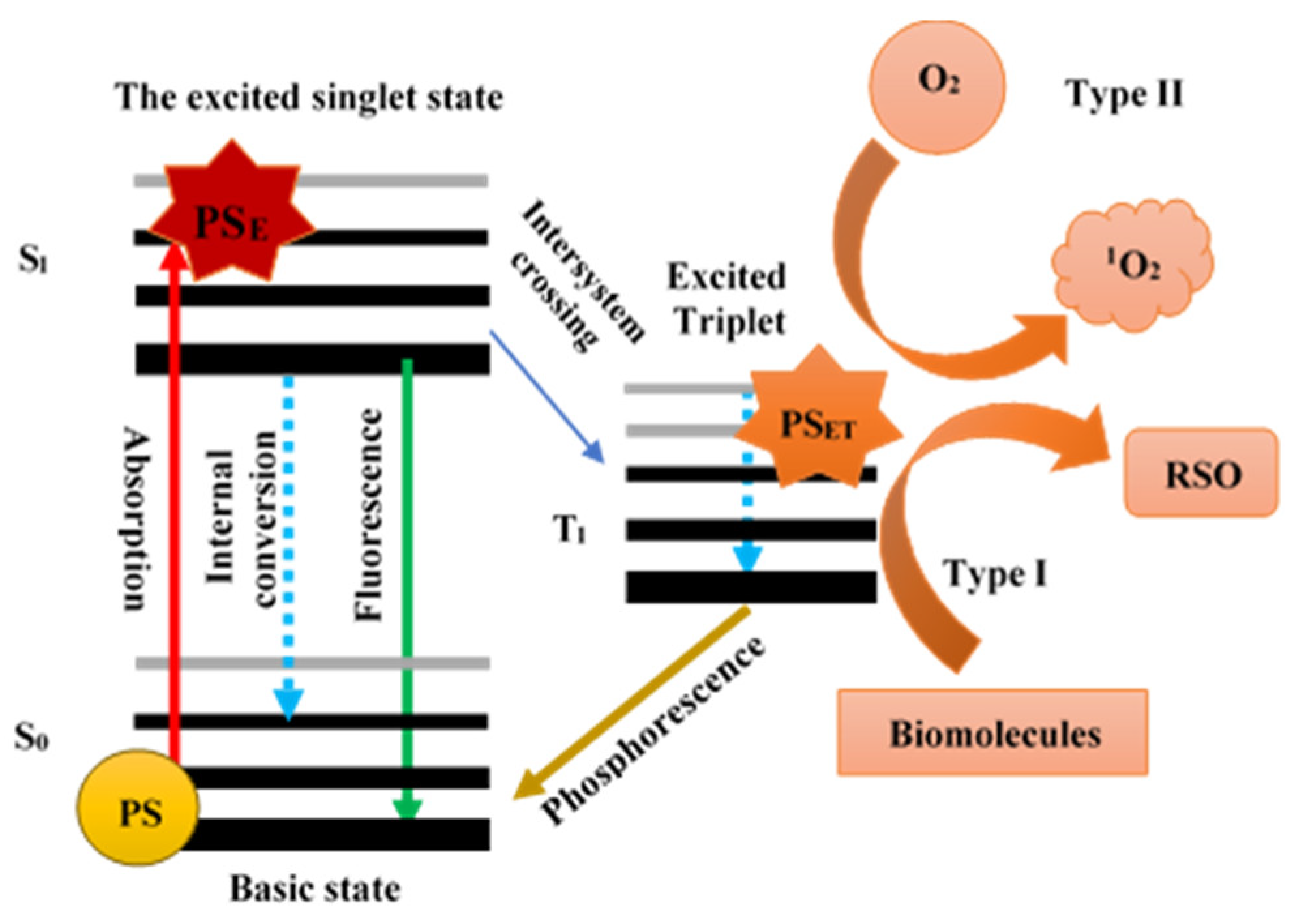
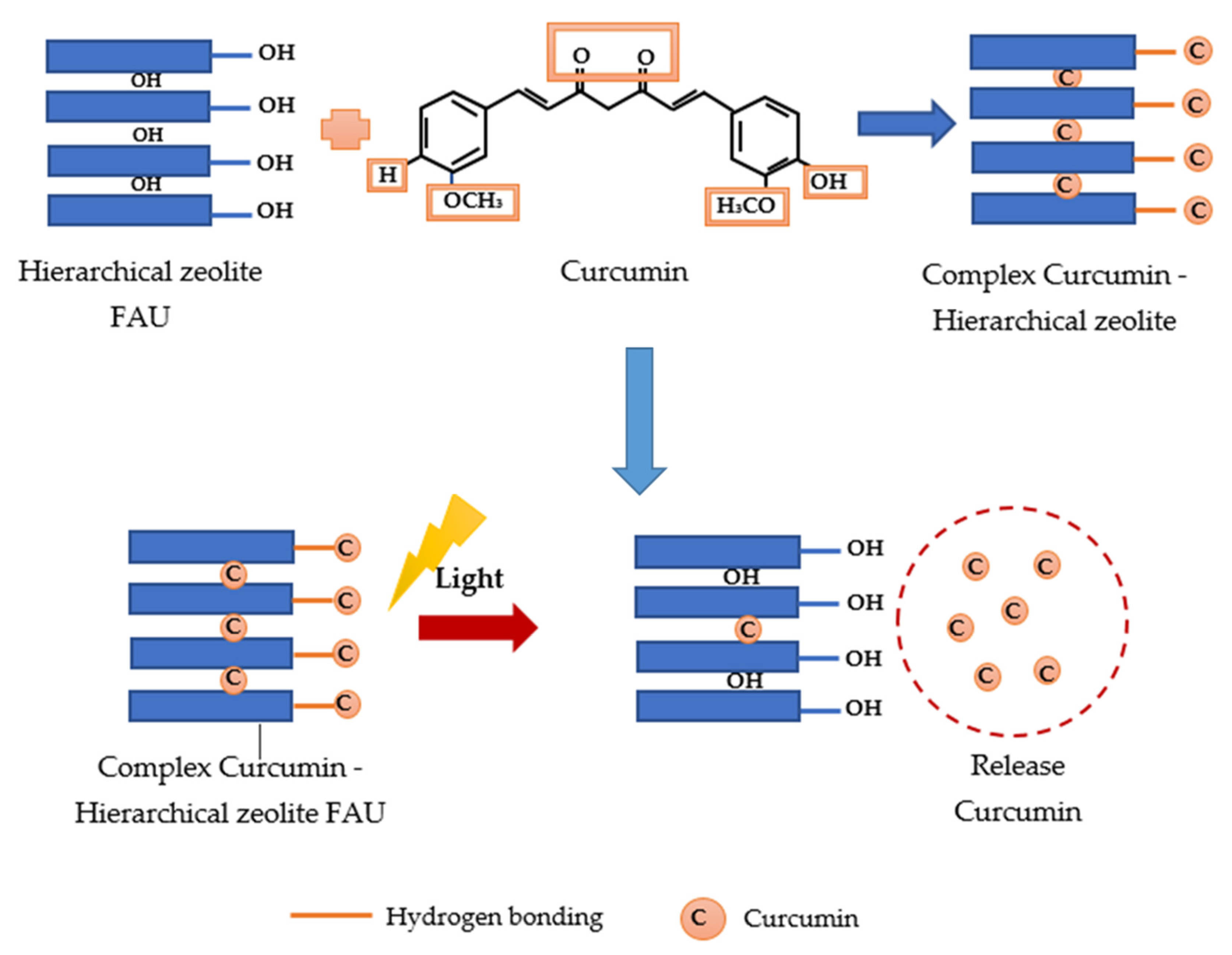
2.12. Potential Mechanism of Photodynamic Light-Triggered Release of Curcumin from Hierarchical Zeolites
2.13. Study of the Kinetics of Curcumin Release from Hierarchical Zeolite
3. Materials and Methods
3.1. Materials
3.2. Incorporation of Curcumin to Hierarchical Zeolite-Type FAU
3.3. Optimization of the Curcumin Release from Hierarchical Zeolites
3.4. Curcumin Release from Hierarchical Zeolites
3.4.1. Curcumin Release in an Open System
- Preparation Procedure for the Different Components of Curcumin Release
- Preparation of the Acceptor Fluid
- Sample preparation
3.4.2. Curcumin Release in a Closed System
- blue and green—450 and 525 nm;
- cyan and green—500–525 nm;
- blue and cyan—450–500 nm;
- blue, green and cyan—450–525 nm;
- yellow and red and yellow—595–630 nm.
3.5. Characterization of Materials
- X-ray diffraction (XRD);
- Low-temperature N2 adsorption/desorption;
- Fourier transform infrared spectroscopy (FT-IR);
- Elemental analysis;
- Thermogravimetric analysis (TG);
- Scanning electron microscopy (SEM);
- Confocal microscopy;
- Release of the active substance.
3.5.1. X-ray Diffraction
3.5.2. Low-Temperature Nitrogen Adsorption/Desorption Isotherms
3.5.3. Fourier Transform IR Spectroscopy
3.5.4. Elemental Analysis
3.5.5. Thermogravimetric Analysis TG
3.5.6. Scanning Electron Microscopy SEM
3.5.7. Confocal Microscopy
3.5.8. Encapsulation Efficiency and Loading Capacity of Curcumin
3.5.9. UV–Visible Spectroscopy
3.5.10. Curcumin Release from Hierarchical Zeolites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Regi, M.V.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Regi, M.V. Ordered Mesoporous Materials in the Context of Drug Delivery Systems and Bone Tissue Engineering. Chem. Eur. J. 2006, 12, 5934–5943. [Google Scholar] [CrossRef]
- Rathbone, M.J.; Hadgraft, J.; Roberts, M.S.; Lane, M.E. Modified-Release Drug Delivery Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 303–321. [Google Scholar]
- Rámila, A.; Muňoz, B.; Pérez-Pariente, J.; Vallet-Regí, M. Mesoporous MCM-41 as drug host system. J. Sol-Gel Sci. Technol. 2003, 26, 1199–1202. [Google Scholar] [CrossRef]
- Horcajada, P.; Rámila, A.; Pérez-Pariente, J.; Vallet-Regí, M. Influence of Pore Size of MCM-41 Matrices on Drug Delivery Rate. Microporous Mesoporous Mater. 2004, 68, 105–109. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). IUPAC Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Andersson, J.; Rosenhoim, J.; Areva, S.; Linden, M. Influences of Material Characteristics on Ibuprofen Drug Loading and Release Profiles from Ordered Micro- and Mesoporous Silica Matrices. Chem. Mater. 2004, 16, 4160–4167. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A. Hierarchical zeolites: Synthesis and catalytic properties. Microporous Mesoporous Mater. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Jia, X.; Khan, W.; Wu, Z.; Choi, J.; Yip, A.C.K. Modern synthesis strategies for hierarchical zeolites: Bottom-up versus top-down strategies. Adv. Powder Technol. 2019, 30, 467–484. [Google Scholar] [CrossRef]
- Verboekend, D.; Milina, M.; Mitchell, S.; Ramírez, J.P. Hierarchical Zeolites by Desilication: Occurrence and Catalytic Impact of Recrystallization and Restructuring. Cryst. Growth Des. 2013, 13, 5025–5035. [Google Scholar] [CrossRef]
- Ding, K.; Corma, A.; Maciá-Agulló, J.A.; Hu, J.G.; Krämer, S.; Stair, P.C.; Stucky, G.D. Constructing hierarchical porous zeolites via kinetic regulation. J. Am. Chem. Soc. 2015, 137, 11238–11241. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 140–141. [Google Scholar]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine–a review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Machoke, A.G.; Schwieger, W. Catalytic test reactions for the evaluation of hierarchical zeolites. Chem. Soc. Rev. 2016, 45, 3313–3330. [Google Scholar] [CrossRef] [PubMed]
- Elsatar, A.G.A.; Farag, M.M.; Youssef, H.F.; Salih, S.A.; Mounier, M.M.; El-Meliegy, E. Different zeolite systems for colon cancer therapy: Monitoring of ion release, cytotoxicity and drug release behavior. Prog. Biomater. 2019, 8, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Kralj, M.; Pavelic, K. Medicine on a small scale: How molecular medicine can benefit from self-assembled and nanostructured materials. EMBO Rep. 2003, 4, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Thom, D.C.; Davies, J.E.; Santerre, J.P.; Friedman, S. The hemolytic and cytotoxic properties of a zeolite-containing root filling material in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 95, 101–108. [Google Scholar] [CrossRef]
- Piasek, A.; Bartoszek, A.; Namieśnik, J. Substancje pochodzenia roślinnego przeciwdziałające kardiotoksyczności towarzyszącej chemioterapii nowotworów. Adv. Hyg. Exp. Med. 2009, 63, 142–158. [Google Scholar]
- Goldman, P. Herbal medicines today and the roots of modern pharmacology. Ann. Intern. Med. 2001, 135, 594–600. [Google Scholar] [CrossRef]
- Robinson, M.M.; Zhang, X. The World Medicines Situation 2011. In Traditional Medicines: Global Situation, Issues and Challenges, 3rd ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological Activities of Curcumin and its Analogues (Congeners) Made by Man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Lakshmi, S.; Padmaja, G.; Remani, P. Antitumour Effects of Isocurcumenol Isolated from Curcuma zedoaria Rhizomes on Human and Murine Cancer Cells. Int. J. Med. Chem. 2011, 2011, 253962. [Google Scholar] [CrossRef]
- Itokawa, H.; Shi, Q.; Akiyama, T.; Morris-Natschke, S.L.; Lee, K.H. Recent advances in the investigation of curcuminoids. Chin. Med. 2008, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.; Prabhu, K.S.; Shirwaikar, A.; Shirwaikar, A. Curcuma zedoaria Rosc. (white turmeric): A review of its chemical, pharmacological and ethnomedicinal properties. J. Pharm. Pharmacol. 2009, 61, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, K.; Utsumi, T.; Kumano, T.; Maekawa, S.; Oyama, N.; Kawakami, J. Discovery of a new function of curcumin which enhances its anticancer therapeutic potency. Sci. Rep. 2016, 60, 30962. [Google Scholar] [CrossRef]
- Jun, X.L.; Xiang-Fe, H.; Hang, L.Z. Anti-Cancer Agents. Med. Chem. 2012, 12, 210–211. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Toden, S.; Goel, A. The Holy Grail of Curcumin and its Efficacy in Various Diseases: Is Bioavailability Truly a Big Concern? J. Restorat. Med. 2017, 6, 27–36. [Google Scholar] [CrossRef]
- Han, H.K. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert Opin. Drug Metab. Toxicol. 2011, 7, 721–729. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Photodynamic Therapy to Treat Cancer. Available online: http://www.cancer.gov/cancertopics/factsheet/Therapy/photodynamic (accessed on 10 January 2022).
- Silva, L.P.; Magalhães, C.M.; Montenegro, A.N.; Ferreira, P.J.; Duarte, D.; Rodríguez-Borges, J.E.; Vale, N.; Esteves da Silva, J.C.G. Study of the combination of self-activating photodynamic therapy and chemotherapy for cancer treatment. Biomolecules 2019, 9, 384. [Google Scholar] [CrossRef]
- Moret, F.; Reddi, E. Strategies for optimizing the delivery to tumors of macrocyclic photosensitizers used in photodynamic therapy (pdt). J. Porphyr. Phthalocyanines 2017, 21, 239–256. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2016, 6, 8785–8793. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shao, X.; Zhao, J.; Wu, M. Controllable photodynamic therapy implemented by regulating singlet oxygen efficiency. Adv. Sci. 2017, 4, 1700113. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.Y.; Corke, H. Antimicrobial and anticancer applications and related mechanisms of curcumin-mediated photodynamic treatments. Trends Food Sci. Technol. 2020, 97, 341–354. [Google Scholar] [CrossRef]
- Tuanwei, L.; Lifeng, Y. Functional Polymer Nanocarries for Photodynamic Therapy. Pharmaceuticals 2018, 11, 2–4. [Google Scholar]
- Lee, S.C.; Seong, Y.S.; Kim, S.S.; Koh, H.J.; Kwon, O.W. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica 2004, 218, 193–201. [Google Scholar] [CrossRef]
- Choudhary, S.; Nouri, K.; Elsaie, M.L. Photodynamic therapy in dermatology: A review. Lasers Med. Sci. 2009, 24, 971–980. [Google Scholar] [CrossRef]
- Chrepa, V.; Kotsakis, G.A.; Pagonis, T.C.; Hargreaves, K.M. The effect of photodynamic therapy in root canal disinfection: A systematic review. J. Endod. 2014, 40, 891–898. [Google Scholar] [CrossRef]
- Cieplik, F.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Hiller, K.A.; Maisch, T.; Karygianni, L. Antimicrobial photodynamic therapy as an adjunct for treatment of deep carious lesions-a systematic review. Photodiagn. Photodyn. Ther. 2017, 18, 54–62. [Google Scholar] [CrossRef]
- Marchal, S.; Dolivet, G.; Lassalle, H.P.; Guillemin, F.; Bezdetnaya, L. Targeted photodynamic therapy in head and neck squamous cell carcinoma: Heading into the future. Lasers Med. Sci. 2015, 30, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.; Al-Kheraif, A.A.; Qadri, T.; Hassan, M.I.; Ahmed, A.; Warnakulasuriya, S.; Javed, F. Efficacy of photodynamic therapy in the management of oral premalignant lesions. A systematic review. Photodiagn. Photodyn. Ther. 2015, 12, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.; Heilweil, G.; Barak, A.; Loewenstein, A. Retinal pigment epithelial tear following photodynamic therapy for choroidal neovascularization secondary to AMD. Eye 2005, 19, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.; Figueira, J.; Cachulo, M.L.; Duarte, L.; Faria de Abreu, J.R.; Cunha-Vaz, J.G. Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Akaza, E.; Yuzawa, M.; Mori, R. Three-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Jpn. J. Ophthalmol. 2011, 55, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Muslubas, I.S.; Hocaoglu, M.; Arf, O.; Ozdemir, H.; Karacorlu, M. Treatment outcomes in patients with polypoidal choroidal vasculopathy. Turk. J. Ophthalmol. 2016, 46, 16–20. [Google Scholar] [CrossRef]
- Kachynski, A.V.; Pliss, A.; Kuzmin, A.N.; Ohulchanskyy, T.Y.; Baev, A.; Qu, J.; Prasad, P.N. Photodynamic therapy by in situ nonlinear photon conversion. Nat. Photon. 2014, 8, 455–461. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Dong, C.; Su, L.; Wang, H.; Chang, J. Smart ph-responsive upconversion nanoparticles for enhanced tumor cellular internalization and near-infrared light-triggered photodynamic therapy. Chem. Commun. 2015, 51, 406–408. [Google Scholar] [CrossRef]
- Wang, W.; Lin, L.; Ma, X.; Wang, B.; Liu, S.; Yan, X.; Li, S.; Tian, H.; Yu, X. Light-induced hypoxia-triggered living nanocarriers for synergistic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 19398–19407. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Yao, C.; Wang, W.; Zhao, M.; El-Toni, A.M.; Zhang, F. Orthogonal near-infrared upconversion co-regulated site-specific o2 delivery and photodynamic therapy for hypoxia tumor by using red blood cell microcarriers. Biomaterials 2017, 125, 90–100. [Google Scholar] [CrossRef]
- Li, J.; Meng, X.; Deng, J.; Lu, D.; Zhang, X.; Chen, Y.; Zhu, J.; Fan, A.; Ding, D.; Kong, D. Multifunctional micelles dually responsive to hypoxia and singlet oxygen: Enhanced photodynamic therapy via interactively triggered photosensitizer delivery. ACS Appl. Mater. Interface 2018, 10, 17117–17128. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, L.; Shi, H.; Du, W.; Qi, Y.; Qiu, C.; Liang, X.; Shi, W.; Liu, J. Endoplasmic reticulum-targeting photosensitizer hypericin confers chemo-sensitization towards oxaliplatin through inducing pro-death autophagy. Int. J. Biochem. Cell Biol. 2017, 87, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhu, Y.; Zhang, M.; Luo, L.; Wu, J.; Zhou, H.; Guan, L.; Battaglia, G.; Tian, Y. Localization matters: A nuclear targeting two-photon absorption iridium complex in photodynamic therapy. Chem. Commun. 2017, 53, 3303–3306. [Google Scholar] [CrossRef]
- Shibu, E.S.; Hamada, M.; Murase, N.; Biju, V. Nanomaterials formulations for photothermal and photodynamic therapy of cancer. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 53–72. [Google Scholar] [CrossRef]
- Voon, S.H.; Kiew, L.V.; Lee, H.B.; Lim, S.H.; Noordin, M.I.; Kamkaew, A.; Burgess, K.; Chung, L.Y. In vivo studies of nanostructure-based photosensitizers for photodynamic cancer therapy. Small 2014, 10, 4993–5013. [Google Scholar] [CrossRef] [PubMed]
- Musielak, E.; Feliczak-Guzik, A.; Jaroniec, M.; Nowak, I. Modification and Functionalization of Zeolites for Curcumin Uptake. Materials 2022, 15, 6316. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Lakdawala, D.H.; Shaikh, A.A.; Desai, A.R.; Choksi, H.H.; Vaidya, R.J.; Ranch, K.M.; Koli, A.R.; Vyas, B.A.; Shah, D.O. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J. Control. Release 2016, 226, 47–56. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Reinoso, F.R.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evalua-tion of Surface Area and Pore Size Distribu-tion (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kim, S.; Philippot, S.; Fontanay, S.; Duval, R.E.; Lamouroux, E.; Canilho, N.; Pasc, A. pH-and glutathione-responsive release of curcumin from mesoporous silica nanoparticles coated using tannic acid–Fe(III) complex. RSC Adv. 2015, 5, 90550–90558. [Google Scholar] [CrossRef]
- Moradi, P.; Hajjami, M.; Tahmasbi, B. Fabricated copper catalyst on biochar nanoparticles for the synthesis of tetrazoles as antimicrobial agents. Polyhedron 2020, 175, 114169–114180. [Google Scholar] [CrossRef]
- Chaudhuri, H.; Dash, S.; Ghorai, S.; Pal, S.; Sarkar, A. SBA-16: Application for the removal of neutral, cationic, and anionic dyes from aqueous medium. J. Environ. Chem. Eng. 2016, 4, 157–166. [Google Scholar] [CrossRef]
- Ameri, A.; Taghizadeh, T.; Kiakalaieh, A.T.; Forootanfar, H.; Mojtabavi, S.; Jahandar, H.; Tarighi, S.; Faramarzi, M.A. Bio-removal of phenol by the immobilized laccase on the fabricated parent and hierarchical NaY and ZSM-5 zeolites. J. Taiwan Inst. Chem. Eng. 2021, 120, 300–312. [Google Scholar] [CrossRef]
- Preisig, D.; Haid, D.; Varum, F.J.O.; Bravo, R.; Alles, R.; Huwyler, J.; Puchkov, M. Drug loading into porous calcium carbonate microparticles by solvent evaporation. Eur. J. Pharm. Biopharm. 2014, 87, 548–558. [Google Scholar] [CrossRef]
- Jayandran, M.; Haneefa, M.M.; Balasubramanian, V. Characterization and comparative studies of turmeric oleoresin derived from selected turmeric plants. Asian J. Pharm. Sci. Technol. 2015, 5, 18–21. [Google Scholar]
- Ribeiro, S.P.S.; Martins, R.C.; Barbosa, G.M.; Rocha, M.; Landesmann, A.; Nascimento, M. Influence of the zeolite acidity on its synergistic action with a flame-retarding polymeric intumescent formulation. J. Mat. Sci. 2020, 54, 619–630. [Google Scholar] [CrossRef]
- Karimi, M.; Habibizadeh, M.; Rostamizadeh, K.; Khatamian, M.; Divband, B. Preparation and characterization of nanocomposites based on different zeolite frameworks as carriers for anticancer drug: Zeolite Y versus ZSM-5. Pol. Bull. 2019, 76, 2233–2252. [Google Scholar] [CrossRef]
- Sun, X.Z.; Williams, G.R.; Hou, X.X.; Zhu, L.M. Electrospun curcumin-loaded fibers with potential biomedical applications. Carbohydr. Polym. 2013, 94, 147–153. [Google Scholar] [CrossRef]
- Chen, Z.; Xia, Y.; Liao, S.; Huang, Y.; Li, Y.; He, Y.; Tong, Z.; Li, B. Thermal degradation kinetics study of curcumin with nonlinear methods. Food Chem. 2014, 155, 81–86. [Google Scholar] [CrossRef]
- Amosa, M.; AlKhatib, M.; Jami, M.; Jimat, D.; Uthman, O.; Muyibi, S. Morphological synthesis and environmental application of ZSM-5 zeolite crystals from combined low-water and fluoride syntheses routes. Adv. Environ. Biol. 2014, 8, 613–625. [Google Scholar]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of Curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Ouyang, J.; Yang, H.; Chang, S. Chitosan modified halloysite nanotubes as emerging porous microspheres for drug carrier. Appl. Clay Sci. 2016, 126, 306–312. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Asghar, S.; Khan, I.U.; Iqbal, M.S.; Khalid, S.H. Development of the Amorphous Solid Dispersion of Curcumin: A Rational Selection of Polymers for Enhanced Solubility and Dissolution. Crystals 2022, 12, 1606. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, A.; Garg, N.; Randhawa, J.K. Curcumin encapsulated zeolitic imidazolate frameworks as stimuli responsive drug delivery system and their interaction with biomimetic environment. Sci. Rep. 2017, 7, 12598–12610. [Google Scholar] [CrossRef]
- Hudiyanti, D.; Al Khafiz, M.F.; Anam, K.; Siahaan, P.; Christa, S.M. In Vitro Evaluation of Curcumin Encapsulation in Gum Arabic Dispersions under Different Environments. Molecules 2022, 27, 3855. [Google Scholar] [CrossRef]
- Kuźmińska, J.; Sobczak, A.; Wierzchowski, M.; Gośliński, T.; Jelińska, A. Historia i chemia kurkuminy. Farmacja Współczesna 2021, 14, 140–145. [Google Scholar]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Prokopowicz, M.; Różycki, K.M. Innovation in cosmetics. World Sci. News 2017, 72, 448–456. [Google Scholar]
- Education in microscopy and digital imaging. Available online: https://zeiss-campus.magnet.fsu.edu/articles/lightsources/leds.html (accessed on 29 January 2023).
- Al Khafiz, M.F.; Hikmahwati, Y.; Anam, K.; Hudiyanti, D. Key conditions of alpha-tocopherol encapsulation in gum Arabic dispersions. ScopeIndex 2019, 10, 2622–2627. [Google Scholar]






| Materials | Surface Area (m2/g) | Pore Volume (cm3/g) | ||
|---|---|---|---|---|
| SBET | Total Pore Volume | Volume of Micropores | Mesopore Volume | |
| Commercial zeolite FAU | 718 | 0.37 | 0.30 | 0.05 |
| FAU/CTABr | 892 | 0.49 | 0.19 | 0.30 |
| FAU/CTABr/CUR150 | 688 | 0.35 | 0.31 | 0.04 |
| FAU/CTABr/CUR150/ PIP0,25 | 203 | 0.12 | 0.06 | 0.06 |
| Sample Name | %C | %H |
|---|---|---|
| Commercial zeolite FAU | 0.74 | 0.35 |
| Curcumin | 30.26 | 4.61 |
| FAU/CTABr | 0.02 | 2.28 |
| FAU/CUR150 | 5.69 | 2.64 |
| FAU/CTABr/CUR150 | 17.30 | 3.24 |
| FAU/CTABr/CUR150/PIP0.25 | 19.23 | 2.52 |
| Sample | Active Substance | EE (% ± SD) | LC (% ± SD) |
|---|---|---|---|
| FAU/CUR150 | Curcumin | 55.8 ± 0.62 | 9.7 ± 0.52 |
| FAU/CTABr/CUR150 | Curcumin | 69.6 ± 0.12 | 14.7 ± 0.22 |
| FAU/CTABr/CUR150/PIP0,25 | Curcumin and Piperine | 78.9 ± 0.45 | 20.4 ± 0.16 |
| Wavelength | Color | % Release | ±SD |
|---|---|---|---|
| 365–370 | UV | 12.64% | ±0.70 |
| 450 | Blue | 18.28% | ±0.27 |
| 500 | Cyan | 47.99% | ±0.18 |
| 525 | Green | 36.98% | ±0.43 |
| 595 | Yellow | 18.38% | ±0.32 |
| 620–630 | Red | 25.11% | ±0.26 |
| Color | Wavelength | % Release | ±SD |
|---|---|---|---|
| Blue–Green | 450 and 525 nm | 45.89% | ±0.21 |
| Cyan–Green | 500–525 nm | 51.20% | ±0.33 |
| Blue–Cyan–Green | 430–550 | 53.24% | ±0.54 |
| Yellow–Red | 450–525 nm | 31.07% | ±0.12 |
| Multi-colored | 365–630 nm | 23.48% | ±0.61 |
| Material | Kinetic Model | Total Amount Released [%] | ||
|---|---|---|---|---|
| 0 Order | 1st Order | Higuhi | ||
| R2 | R2 | R2 | ||
| Open system | ||||
| K/G5/T37/LNA | 0.7745 | 0.7953 | 0.7745 | 23.28 |
| K/G5/T37/PIP/LNA | 0.8103 | 0.8195 | 0.8103 | 10.57 |
| K/G5/T37/WL | 0.8151 | 0.8262 | 0.8144 | 12.84 |
| K/G5/T37/PIP/WL | 0.6675 | 0.6795 | 0.6675 | 9.65 |
| K/G5/T37/LM | 0.6693 | 0.6961 | 0.6693 | 21.35 |
| K/G5/T37/PIP/LM | 0.5836 | 0.6044 | 0.5836 | 14.13 |
| Fotodynamic release | ||||
| Cyan | 0.6063 | 0.6486 | 0.6063 | 46.26 |
| Green-Blue | 0.5360 | 0.5747 | 0.5360 | 41.29 |
| Blue-Cyan-Green | 0.5939 | 0.6398 | 0.5939 | 50.46 |
| Sample | Stabilizer | Stabilizer Quantity (mL) | Process Temperature (°C) | Type of Light |
|---|---|---|---|---|
| C/G1/T37/LNA | Glycerin | 1 | 37 | natural light artificial light |
| C/E1/T37/LNA | Ethyl alcohol | 1 | 37 | natural light artificial light |
| C/TW1/T37/LNA | Tween 80 | 1 | 37 | natural light artificial light |
| C/G5/T37/LM | Glycerin | 5 | 37 | natural light artificial light additional light source |
| C/G5/T37/NL | Glycerin | 5 | 37 | no light |
| C/G5/T43/LNA | Glycerin | 5 | 43 | natural light artificial light |
| C/G5/T37/PIP/LM | Glycerin | 5 | 37 | natural light artificial light additional light source |
| C/G5/T37/PIP/NL | Glycerin | 5 | 37 | no light |
| C/G5/T37/PIP/LNA | Glycerin | 5 | 43 | natural light artificial light additional light source |
| Measurement Parameter | Parameter Value |
|---|---|
| Process temperature | 37 °C |
| Agitator speed | 200 RPM/min |
| Testing time | 24 h |
| Media quantity | 2.00 mg |
| Amount of promoter | 5.00 mL |
| Buffer volume | 20.00 mL |
| Wavelength Range (nm) | Perceived Color |
|---|---|
| 365–370 | UV |
| 395–405 | UV |
| 450 | Blue |
| 500 | Cyan |
| 525 | Green |
| 595 | Yellow |
| 620–630 | Red |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musielak, E.; Feliczak-Guzik, A.; Jaroniec, M.; Nowak, I. Photodynamic Light-Triggered Release of Curcumin from Hierarchical FAU Zeolite. Catalysts 2023, 13, 394. https://doi.org/10.3390/catal13020394
Musielak E, Feliczak-Guzik A, Jaroniec M, Nowak I. Photodynamic Light-Triggered Release of Curcumin from Hierarchical FAU Zeolite. Catalysts. 2023; 13(2):394. https://doi.org/10.3390/catal13020394
Chicago/Turabian StyleMusielak, Ewelina, Agnieszka Feliczak-Guzik, Mietek Jaroniec, and Izabela Nowak. 2023. "Photodynamic Light-Triggered Release of Curcumin from Hierarchical FAU Zeolite" Catalysts 13, no. 2: 394. https://doi.org/10.3390/catal13020394
APA StyleMusielak, E., Feliczak-Guzik, A., Jaroniec, M., & Nowak, I. (2023). Photodynamic Light-Triggered Release of Curcumin from Hierarchical FAU Zeolite. Catalysts, 13(2), 394. https://doi.org/10.3390/catal13020394







