In-Depth Kinetic Modeling and Chemical Analysis for the Epoxidation of Vegetable Oils in a Liquid–Liquid–Solid System
Abstract
1. Introduction
- −
- −
- −
2. Results and Discussion
2.1. Analysis, NMR and FTIR Results, and GPC
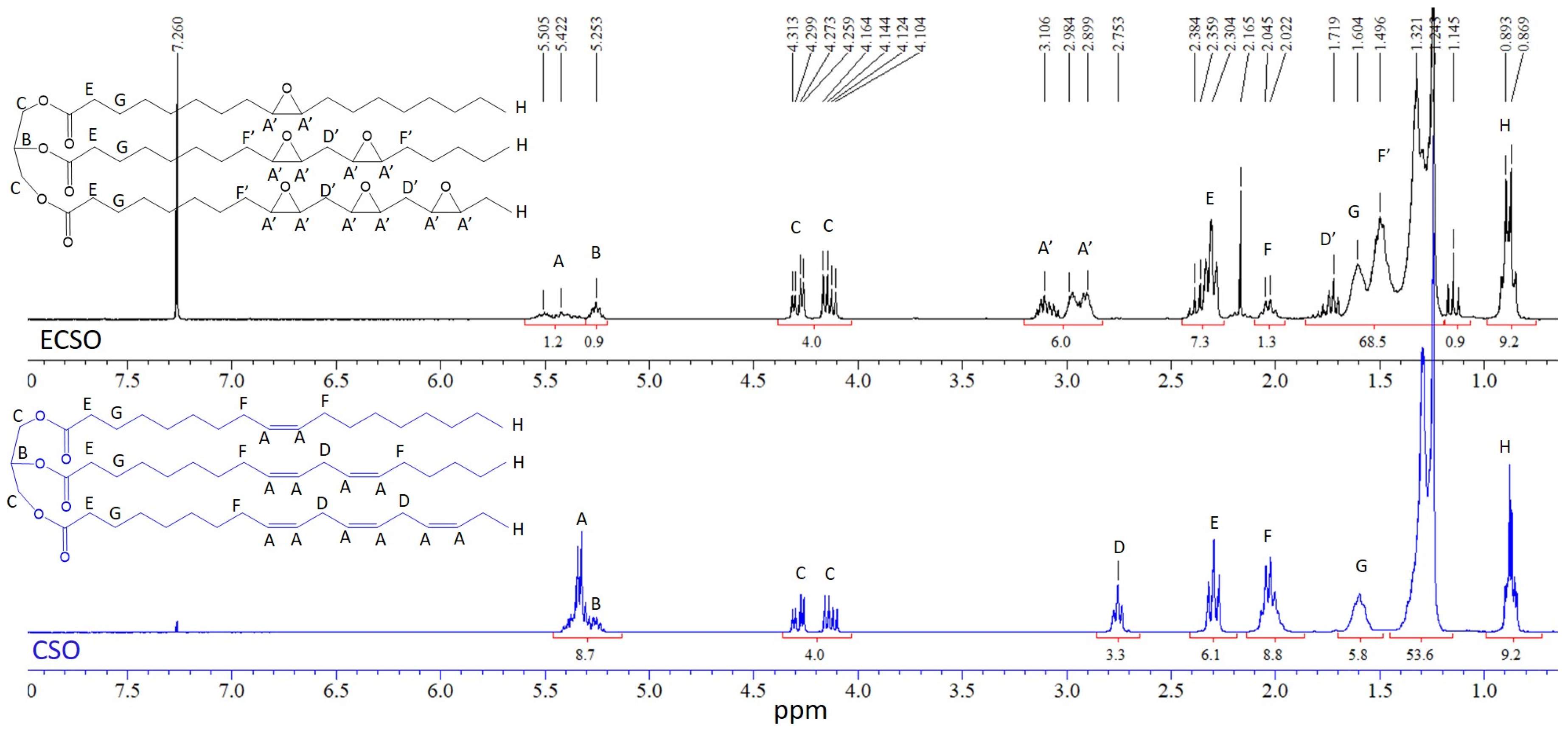
2.1.1. NMR Spectra
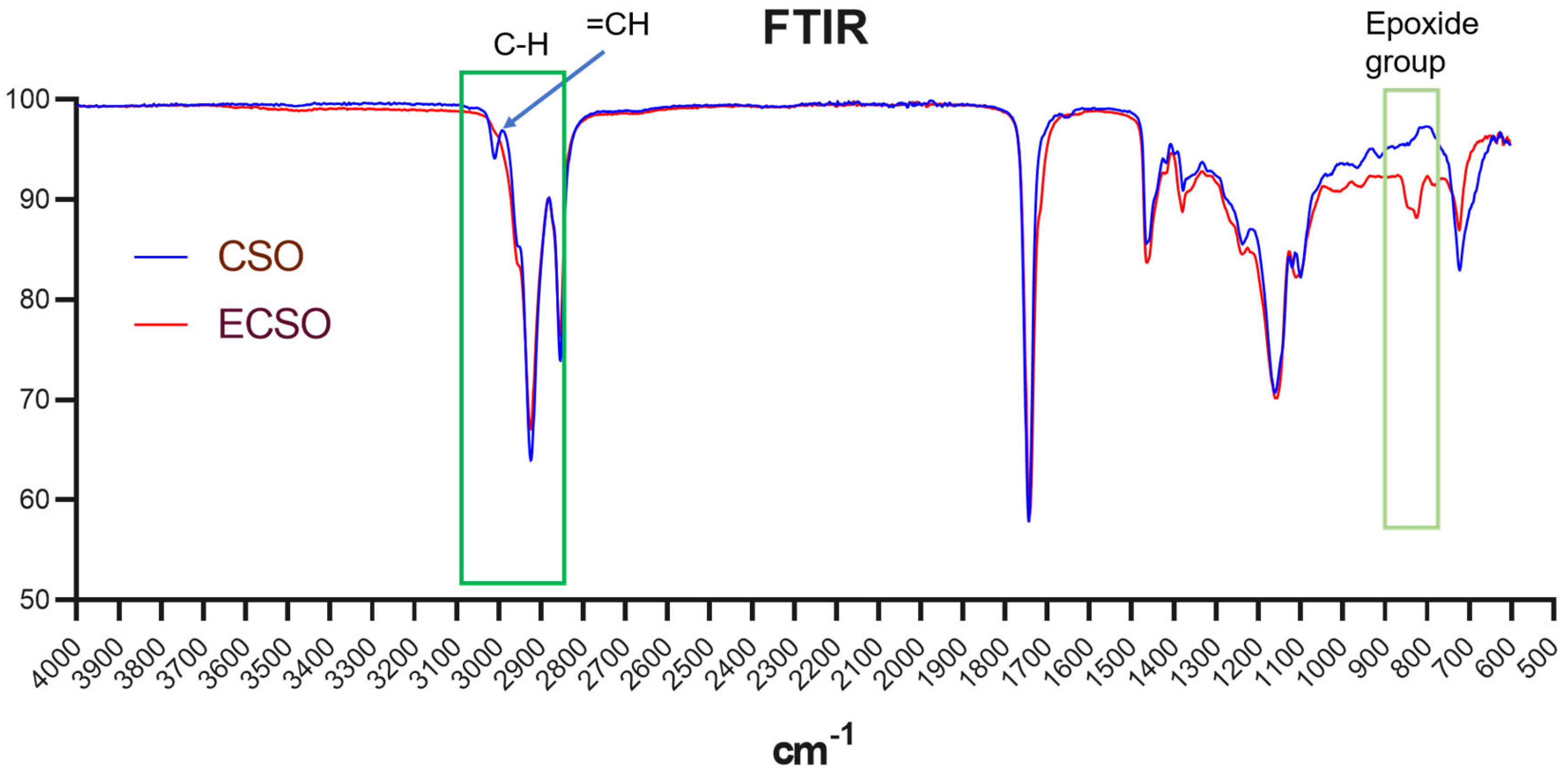
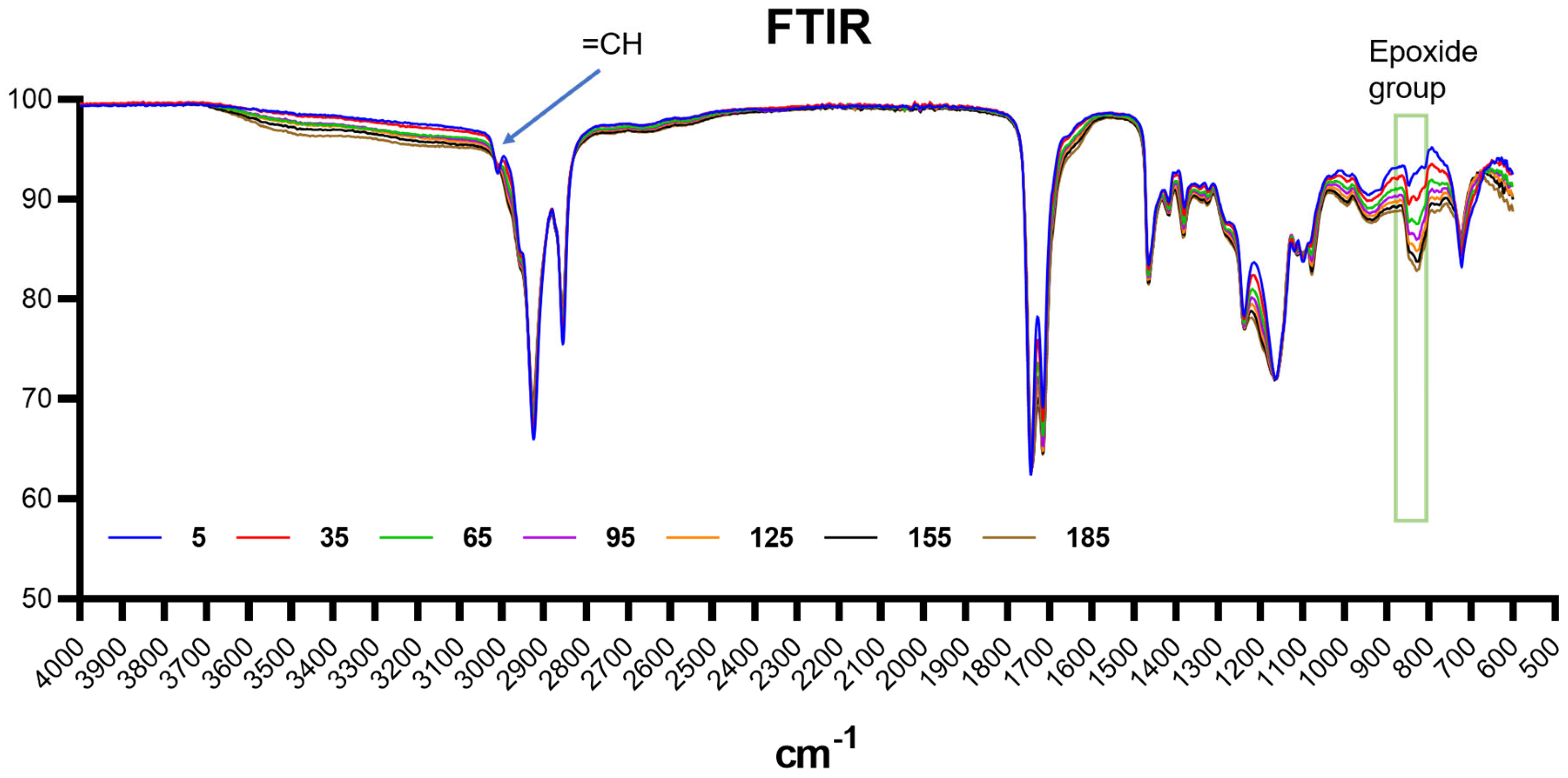
2.1.2. FTIR Spectra
2.1.3. GPC
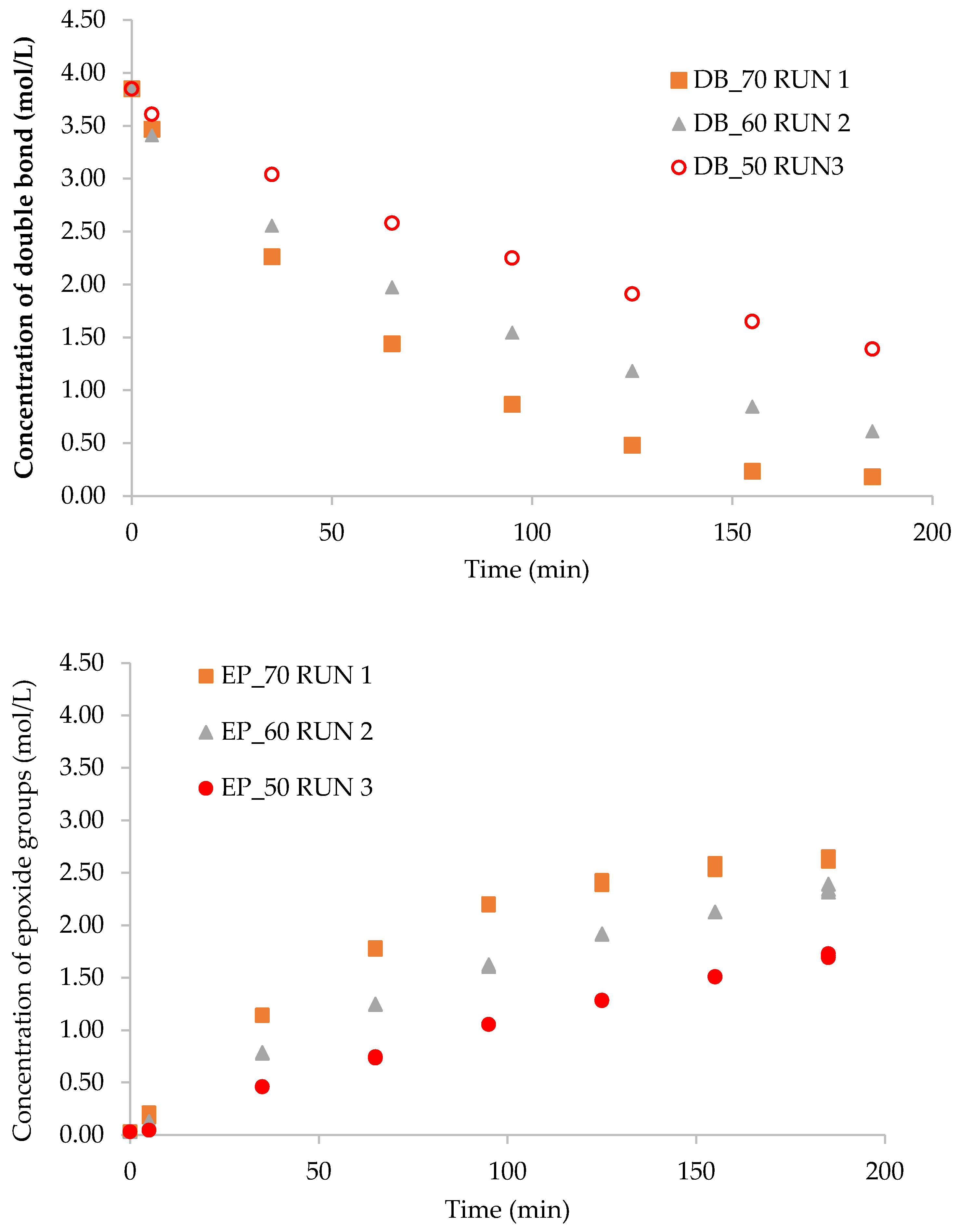
2.2. Effect of Reaction Temperature on the Kinetics of Epoxidation
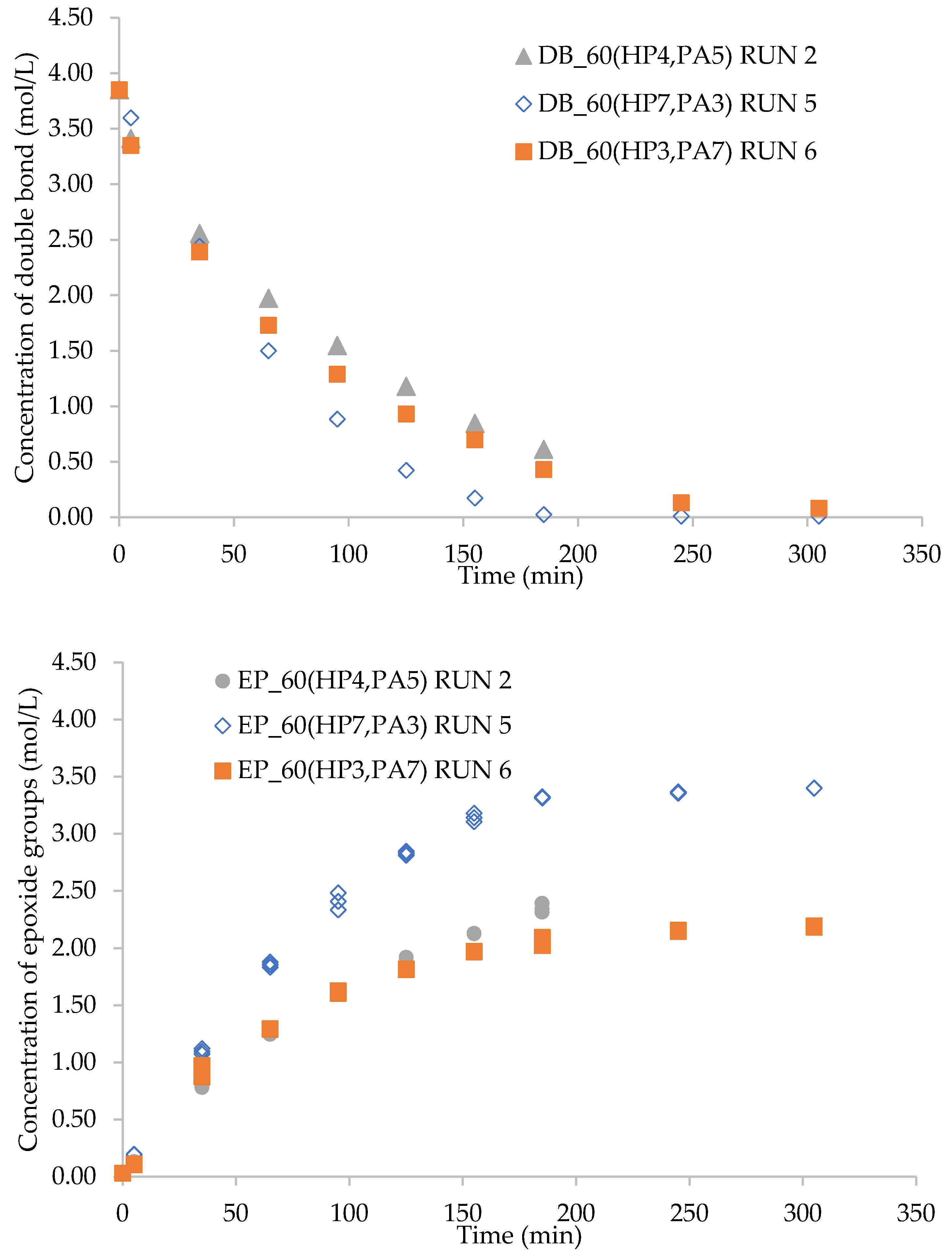
2.3. Effect of HP/PA Ratio on the Kinetics of Epoxidation
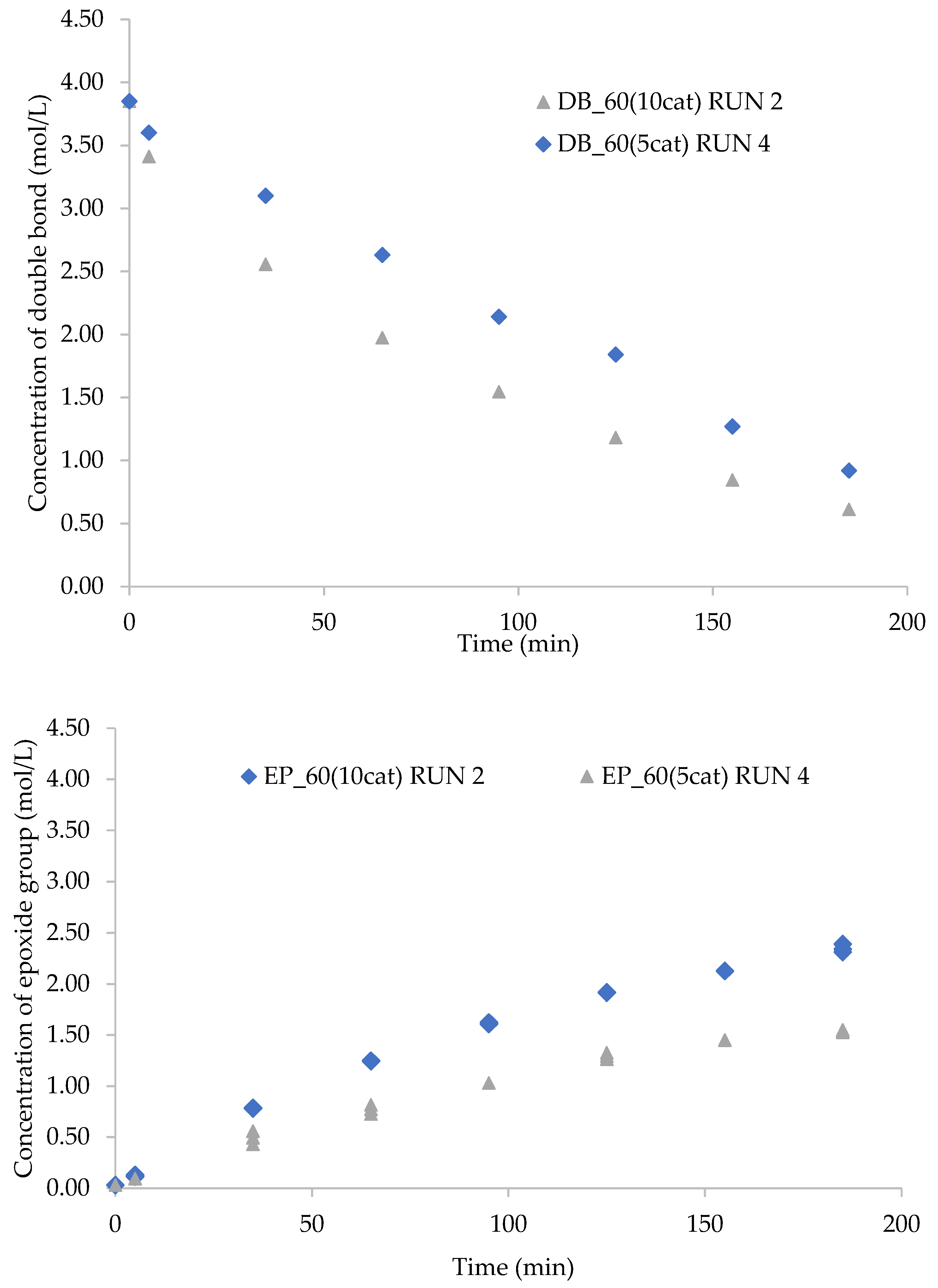
2.4. Catalyst Loading Effect on the Kinetics of Epoxidation
3. Kinetic Modeling
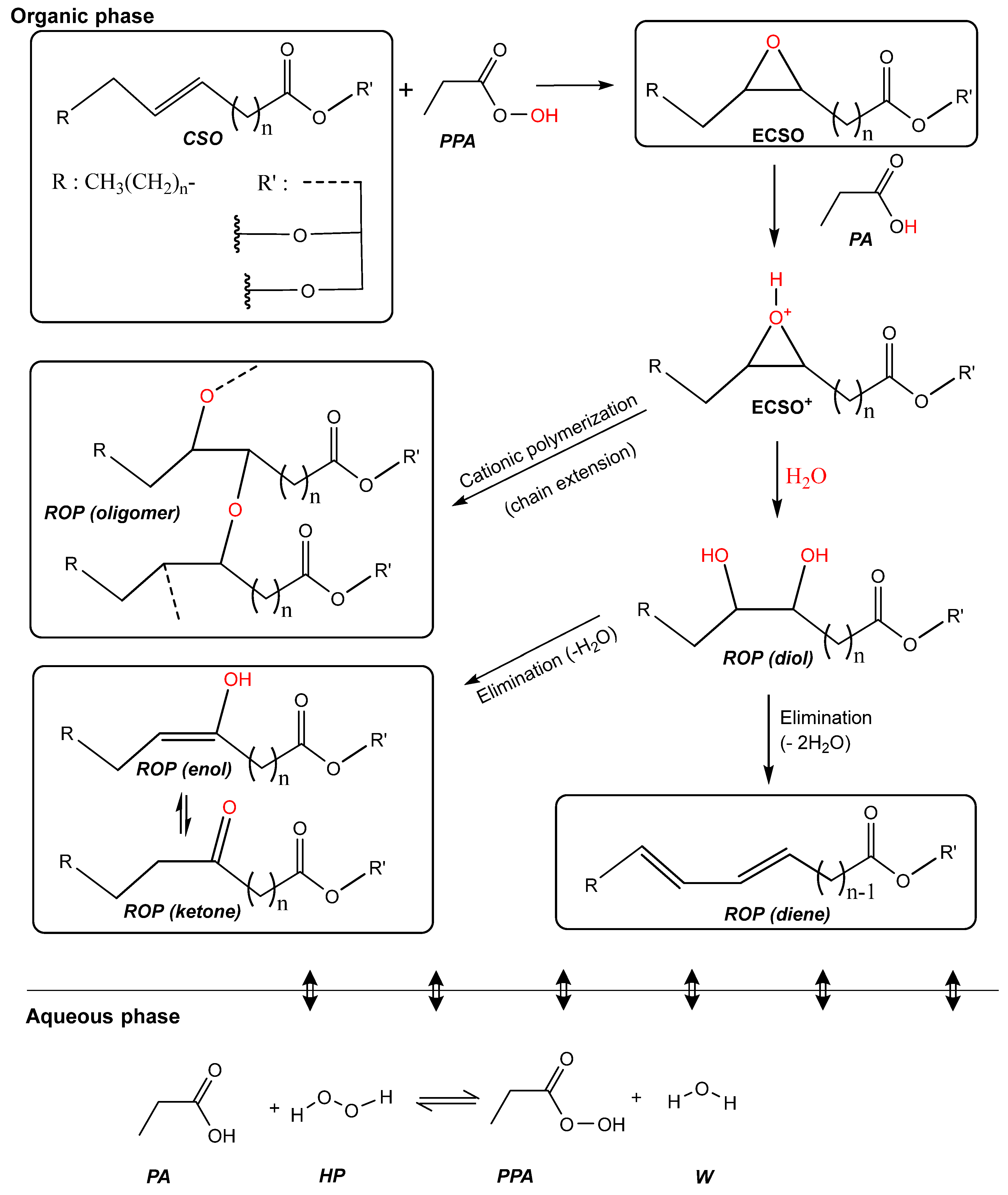
3.1. Kinetics
3.2. Material Balances
3.3. Regression
3.3.1. Kinetic Modeling of Propionic Acid Perhydrolysis
3.3.2. Kinetic Modeling of Epoxidation
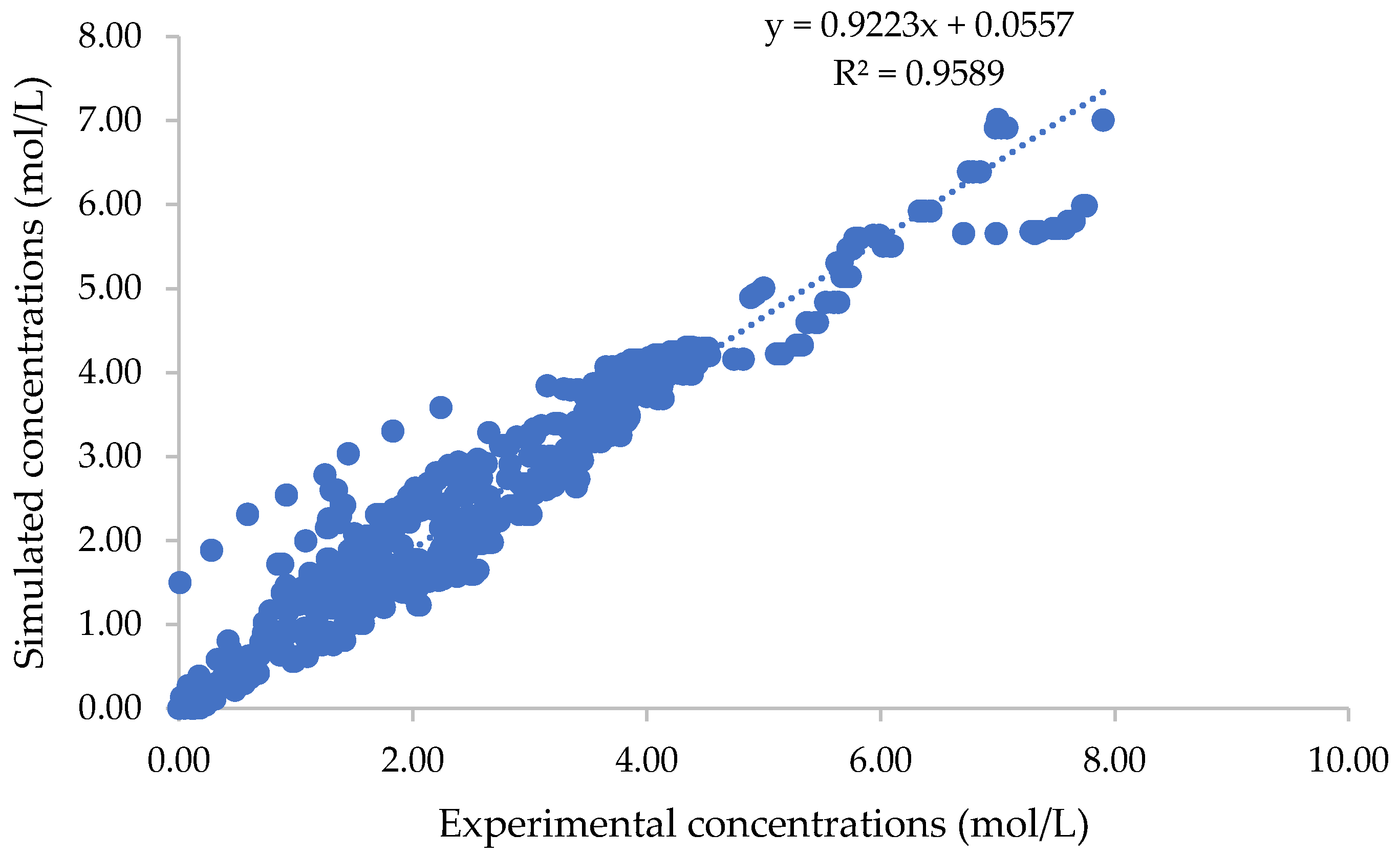
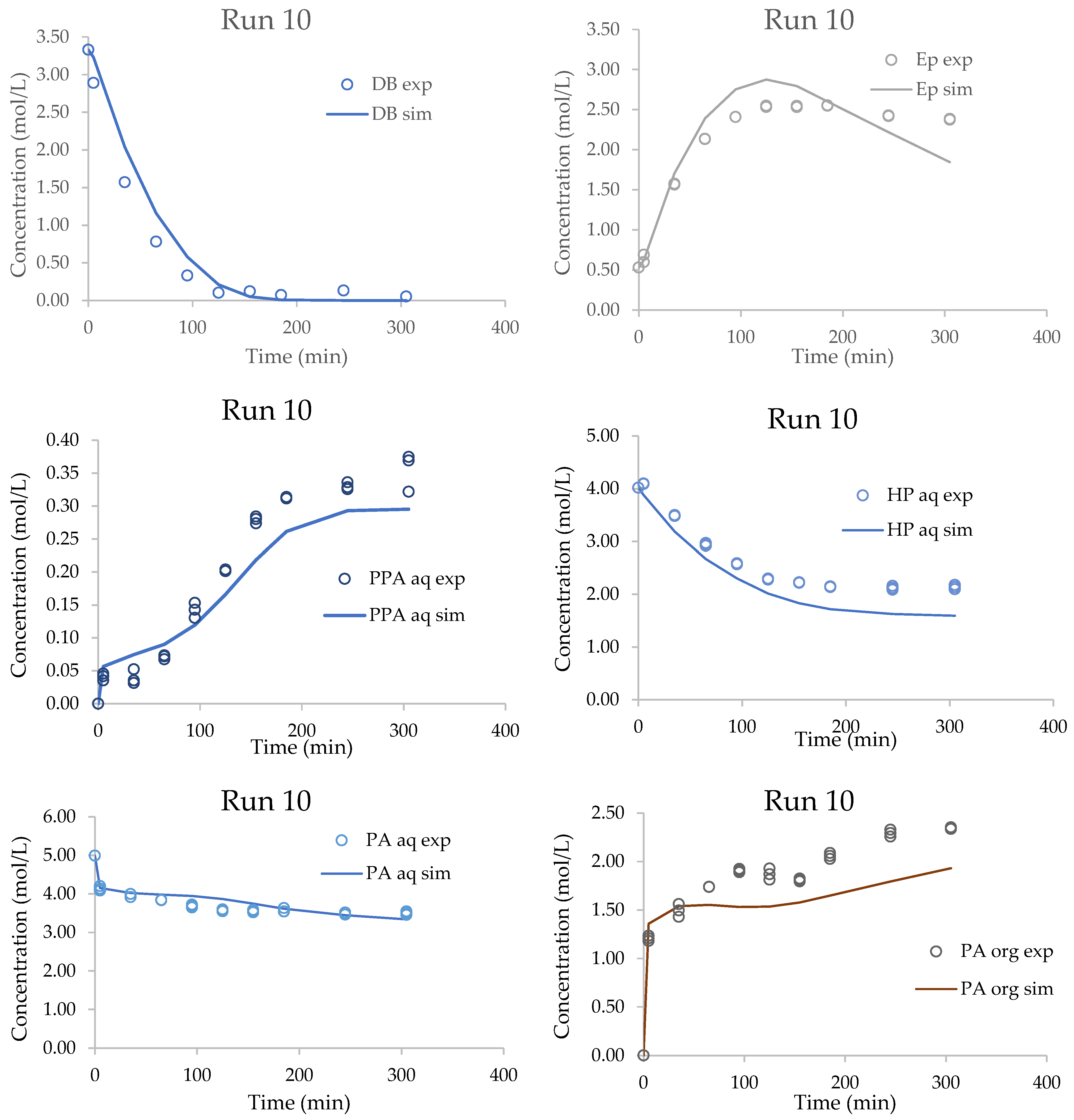
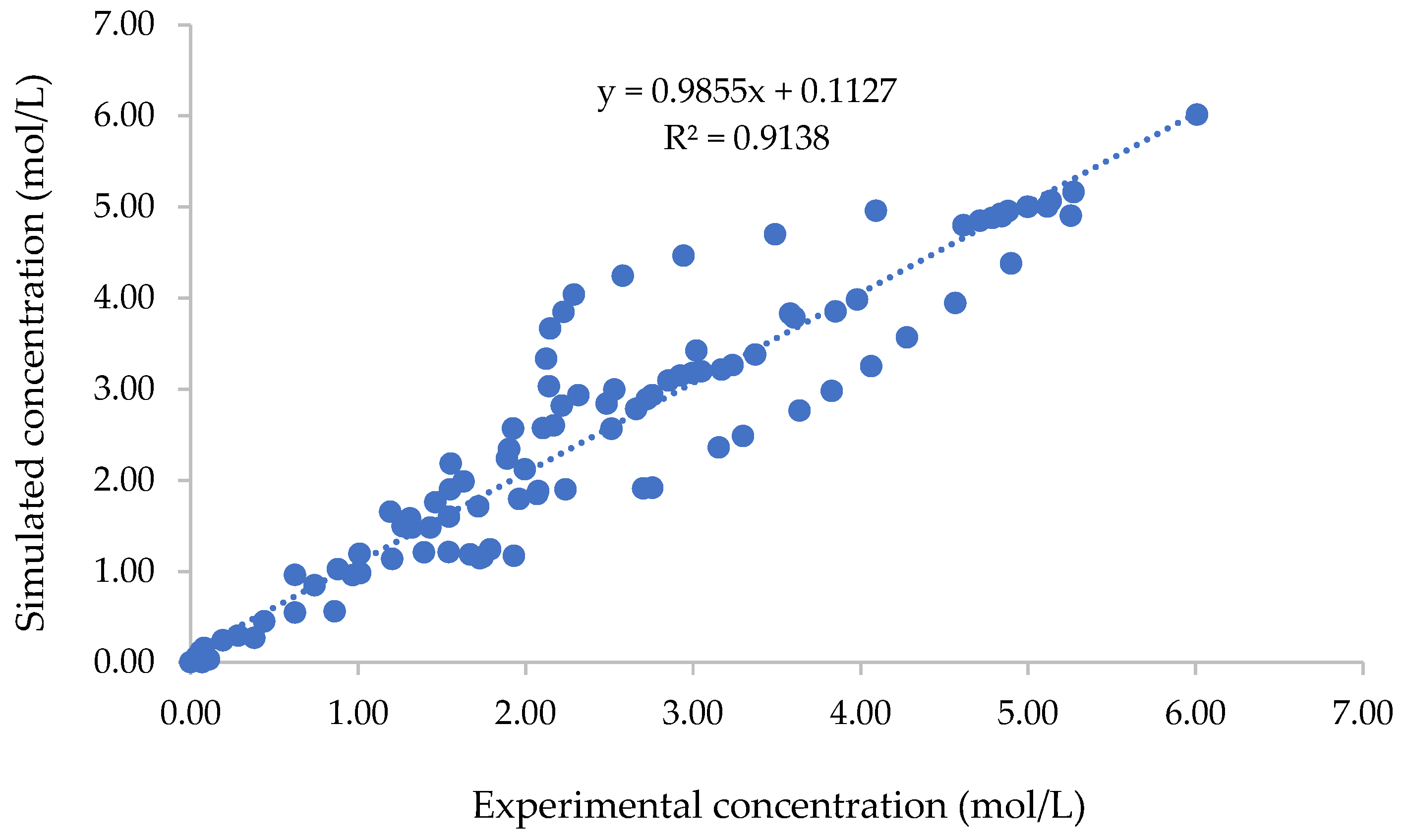
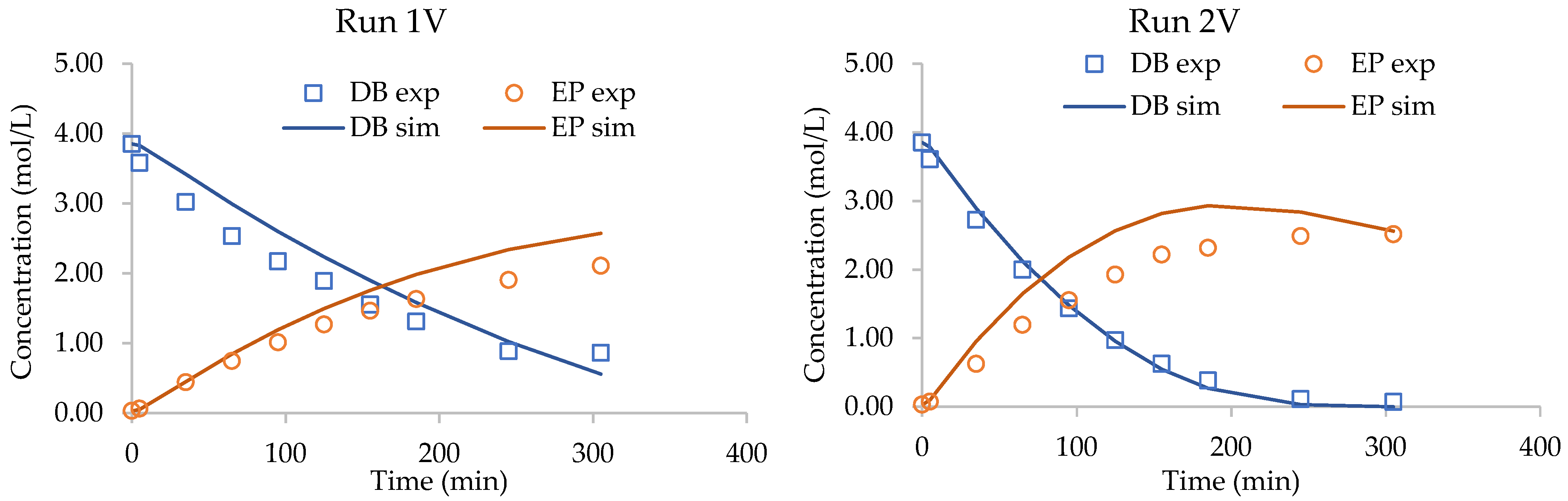
3.4. Validation
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Reaction
4.3. Analytical Methods
4.3.1. NMR
4.3.2. FTIR
4.3.3. GPC
4.3.4. Double Bond Content
4.3.5. Epoxide Content
4.3.6. Hydrogen Peroxide Content
4.3.7. Concentration of Acid
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gebremariam, S.N.; Marchetti, J.M. Biodiesel production technologies: Review. AIMS Energy 2017, 5, 425–457. [Google Scholar] [CrossRef]
- Mittal, V.; Talapatra, K.N.; Ghosh, U.K. A comprehensive review on biodiesel production from microalgae through nanocatalytic transesterification process: Lifecycle assessment and methodologies. Int. Nano Lett. 2022, 12, 351–378. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.M.A.; Soudagar, M.E.M.; Fattah, I.M.R. Current state and perspectives on transesterification of triglycerides for biodiesel production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Alsultan, A.G.; Asikin-Mijan, N.; Ibrahim, Z.; Yunus, R.; Razali, S.Z.; Mansir, N.; Islam, A.; Seenivasagam, S.; Taufiq-Yap, Y.H. A short review on catalyst, feedstock, modernised process, current state and challenges on biodiesel production. Catalysts 2021, 11, 1261. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Adekunle, K.F. A Review of Vegetable Oil-Based Polymers: Synthesis and Applications. Open J. Polym. Chem. 2015, 5, 34–40. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lamarzelle, O.; Rix, E.; Grau, E.; Cramail, H. Isocyanate-Free Routes to Polyurethanes and Poly(hydroxy Urethane)s. Chem. Rev. 2015, 115, 12407–12439. [Google Scholar] [CrossRef]
- Lligadas, G. Renewable polyols for polyurethane synthesis via thiol-ene/yne couplings of plant oils. Macromol. Chem. Phys. 2013, 214, 415–422. [Google Scholar] [CrossRef]
- Guzmán Agudelo, A.F.; Pérez-Sena, W.Y.; Kebir, N.; Salmi, T.; Ríos, L.A.; Leveneur, S. Influence of steric effects on the kinetics of cyclic-carbonate vegetable oils aminolysis. Chem. Eng. Sci. 2020, 228, 115954. [Google Scholar] [CrossRef]
- Pérez-Sena, W.Y.; Cai, X.; Kebir, N.; Vernières-Hassimi, L.; Serra, C.; Salmi, T.; Leveneur, S. Aminolysis of cyclic-carbonate vegetable oils as a non-isocyanate route for the synthesis of polyurethane: A kinetic and thermal study. Chem. Eng. J. 2018, 346, 271–280. [Google Scholar] [CrossRef]
- Meng, Y.; Taddeo, F.; Aguilera, A.F.; Cai, X.; Russo, V.; Tolvanen, P.; Leveneur, S. The lord of the chemical rings: Catalytic synthesis of important industrial epoxide compounds. Catalysts 2021, 11, 765. [Google Scholar] [CrossRef]
- Scotti, N.; Ravasio, N.; Psaro, R.; Evangelisti, C.; Dworakowska, S.; Bogdal, D.; Zaccheria, F. Copper mediated epoxidation of high oleic natural oils with a cumene-O2 system. Catal. Commun. 2015, 64, 80–85. [Google Scholar] [CrossRef]
- Perez-Sena, W.Y.; Wärnå, J.; Eränen, K.; Tolvanen, P.; Estel, L.; Leveneur, S.; Salmi, T. Use of semibatch reactor technology for the investigation of reaction mechanism and kinetics: Heterogeneously catalyzed epoxidation of fatty acid esters. Chem. Eng. Sci. 2021, 230, 116206. [Google Scholar] [CrossRef]
- Wisniak, J.; Cancino, A.; Vega, J.C. Epoxidation of Anchovy Oils. A Study of Variables. I&EC Prod. Res. Dev. 1964, 3, 306–311. [Google Scholar] [CrossRef]
- Wisniak, J.; Navarrete, E. Epoxidation Of Fish Oil Kinetic and Optimization Model. Ind. Eng. Chem. Prod. Res. Dev. 1970, 9, 33–41. [Google Scholar] [CrossRef]
- Prileschajew, N. Oxydation ungesättigter Verbindungen mittels organischer Superoxyde. Berichte der Dtsch. Chem. Gesellschaft 1909, 42, 4811–4815. [Google Scholar] [CrossRef]
- Wai, P.T.; Jiang, P.; Shen, Y.; Zhang, P.; Gu, Q.; Leng, Y. Catalytic developments in the epoxidation of vegetable oils and the analysis methods of epoxidized products. RSC Adv. 2019, 9, 38119–38136. [Google Scholar] [CrossRef]
- Sienkiewicz, A.M.; Czub, P. The unique activity of catalyst in the epoxidation of soybean oil and following reaction of epoxidized product with bisphenol A. Ind. Crops Prod. 2016, 83, 755–773. [Google Scholar] [CrossRef]
- Chen, J.; De Liedekerke Beaufort, M.; Gyurik, L.; Dorresteijn, J.; Otte, M.; Klein Gebbink, R.J.M. Highly efficient epoxidation of vegetable oils catalyzed by a manganese complex with hydrogen peroxide and acetic acid. Green Chem. 2019, 21, 2436–2447. [Google Scholar] [CrossRef]
- Aguilera, A.F.; Tolvanen, P.; Eränen, K.; Wärnå, J.; Leveneur, S.; Marchant, T.; Salmi, T. Kinetic modelling of Prileschajew epoxidation of oleic acid under conventional heating and microwave irradiation. Chem. Eng. Sci. 2019, 199, 426–438. [Google Scholar] [CrossRef]
- Almasi, S.; Ghobadian, B.; Najafi, G.; Dehghani Soufi, M. A novel approach for bio-lubricant production from rapeseed oil-based biodiesel using ultrasound irradiation: Multi-objective optimization. Sustain. Energy Technol. Assess. 2021, 43, 100960. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, Q.; Chen, T.; Wei, X.; Meng, L. Development and characterisation research on SnO2-Al2O3-NiO/SO42− catalysed epoxidation of soybean oil under hydraulic cavitation. Appl. Organomet. Chem. 2022, 36, e6617. [Google Scholar] [CrossRef]
- Maia, D.L.H.; Fernandes, F.A.N. Influence of carboxylic acid in the production of epoxidized soybean oil by conventional and ultrasound-assisted methods. Biomass Convers. Biorefinery 2020, 12, 5861–5868. [Google Scholar] [CrossRef]
- Leveneur, S.; Ledoux, A.; Estel, L.; Taouk, B.; Salmi, T. Epoxidation of vegetable oils under microwave irradiation. Chem. Eng. Res. Des. 2014, 92, 1495–1502. [Google Scholar] [CrossRef]
- Leveneur, S. Thermal Safety Assessment through the Concept of Structure-Reactivity: Application to Vegetable Oil Valorization. Org. Process Res. Dev. 2017, 21, 543–550. [Google Scholar] [CrossRef]
- Casson Moreno, V.; Russo, V.; Tesser, R.; Di Serio, M.; Salzano, E. Thermal risk in semi-batch reactors: The epoxidation of soybean oil. Process Saf. Environ. Prot. 2017, 109, 529–537. [Google Scholar] [CrossRef]
- Rakotondramaro, H.; Wärnå, J.; Estel, L.; Salmi, T.; Leveneur, S. Cooling and stirring failure for semi-batch reactor: Application to exothermic reactions in multiphase reactor. J. Loss Prev. Process Ind. 2016, 43, 147–157. [Google Scholar] [CrossRef]
- Leveneur, S.; Pinchard, M.; Rimbault, A.; Safdari Shadloo, M.; Meyer, T. Parameters affecting thermal risk through a kinetic model under adiabatic condition: Application to liquid-liquid reaction system. Thermochim. Acta 2018, 666, 10–17. [Google Scholar] [CrossRef]
- Cai, X.; Zheng, J.L.; Aguilera, A.F.; Vernières-Hassimi, L.; Tolvanen, P.; Salmi, T.; Leveneur, S. Influence of ring-opening reactions on the kinetics of cottonseed oil epoxidation. Int. J. Chem. Kinet. 2018, 50, 726–741. [Google Scholar] [CrossRef]
- Jalil, M.J.; Azmi, I.S.; Yamin, A.F.M.; Yeop, M.Z.; Hadi, A. Catalytic Epoxidation of Oleic Acid and Subsequent Ring-Opening by In Situ Hydrolysis for Production Dihydroxystearic Acid. J. Polym. Environ. 2022, 1–11. [Google Scholar] [CrossRef]
- Azmi, I.S.; Ozir, T.A.Z.T.; Rasib, I.M.; Nurherdiana, S.D.; Jalil, M.J. Synergistic epoxidation of palm oleic acid using a hybrid oxygen carrier solution. Biomass Convers. Biorefinery 2022, 1–8. [Google Scholar] [CrossRef]
- Leveneur, S.; Wärnå, J.; Salmi, T.; Murzin, D.Y. Catalytic synthesis and decomposition of peroxycarboxylic acids—Green catalytic synthesis of green compounds. Trends Chem. Eng. 2010, 13, 17–52. [Google Scholar] [CrossRef]
- Santacesaria, E.; Tesser, R.; Di Serio, M.; Turco, R.; Russo, V.; Verde, D. A biphasic model describing soybean oil epoxidation with H2O2 in a fed-batch reactor. Chem. Eng. J. 2011, 173, 198–209. [Google Scholar] [CrossRef]
- Wu, Z.; Nie, Y.; Chen, W.; Wu, L.; Chen, P.; Lu, M.; Yu, F.; Ji, J. Mass transfer and reaction kinetics of soybean oil epoxidation in a formic acid-autocatalyzed reaction system. Can. J. Chem. Eng. 2016, 94, 1576–1582. [Google Scholar] [CrossRef]
- Salmi, T.; Russo, V.; Aguilera, A.F.; Tolvanen, P.; Wärnå, J.; Di Serio, M.; Tesser, R.; Cogliano, T.; Leveneur, S.; Eränen, K. A new perspective on vegetable oil epoxidation modeling: Reaction and mass transfer in a liquid–liquid–solid system. AIChE J. 2022, 68, e17626. [Google Scholar] [CrossRef]
- Zheng, J.L.; Wärnå, J.; Salmi, T.; Burel, F.; Taouk, B.; Leveneur, S. Kinetic modeling strategy for an exothermic multiphase reactor system: Application to vegetable oils epoxidation using Prileschajew method. AIChE J. 2016, 62, 726–741. [Google Scholar] [CrossRef]
- Janković, M.R.; Govedarica, O.M.; Sinadinović-Fišer, S.V. The epoxidation of linseed oil with in situ formed peracetic acid: A model with included influence of the oil fatty acid composition. Ind. Crops Prod. 2020, 143, 111881. [Google Scholar] [CrossRef]
- Janković, M.; Sinadinović-Fišer, S.; Govedarica, O.; Pavličević, J.; Budinski-Simendić, J. Kinetika epoksidovanja sojinog ulja persirćetnom kiselinom formiranom in situ u prisustvu jonoizmenjivačke smole: Pseudo-homogeni model. Chem. Ind. Chem. Eng. Q. 2017, 23, 97–111. [Google Scholar] [CrossRef]
- Leveneur, S.; Zheng, J.; Taouk, B.; Burel, F.; Wärnå, J.; Salmi, T. Interaction of thermal and kinetic parameters for a liquid-liquid reaction system: Application to vegetable oils epoxidation by peroxycarboxylic acid. J. Taiwan Inst. Chem. Eng. 2014, 45, 1449–1458. [Google Scholar] [CrossRef]
- Campanella, A.; Fontanini, C.; Baltanás, M.A. High yield epoxidation of fatty acid methyl esters with performic acid generated in situ. Chem. Eng. J. 2008, 144, 466–475. [Google Scholar] [CrossRef]
- Mungroo, R.; Goud, V.V.; Pradhan, N.C.; Dalai, A.K. Modification of epoxidised canola oil. Asia-Pacific, J. Chem. Eng. 2011, 6, 14–22. [Google Scholar] [CrossRef]
- Dinda, S.; Patwardhan, A.V.; Goud, V.V.; Pradhan, N.C. Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids. Bioresour. Technol. 2008, 99, 3737–3744. [Google Scholar] [CrossRef] [PubMed]
- Coud, V.V.; Pradhan, N.C.; Patwardhan, A.V. Epoxidation of karanja (Pongamia glabra) oil by H2O2. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 635–640. [Google Scholar] [CrossRef]
- Goud, V.V.; Patwardhan, A.V.; Pradhan, N.C. Studies on the epoxidation of mahua oil (Madhumica indica) by hydrogen peroxide. Bioresour. Technol. 2006, 97, 1365–1371. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Bakare, O.I.; Okieimen, C.O. Studies on the epoxidation of rubber seed oil. Ind. Crops Prod. 2002, 15, 139–144. [Google Scholar] [CrossRef]
- Abraham, M.E.; Benenati, R.F. Kinetics and mechanism of the epoxidation of unsaturated fatty acids. AIChE J. 1972, 18, 807–811. [Google Scholar] [CrossRef]
- Leveneur, S.; Salmi, T.; Musakka, N.; WärnÅ, J. Kinetic study of decomposition of peroxypropionic acid in liquid phase through direct analysis of decomposition products in gas phase. Chem. Eng. Sci. 2007, 62, 5007–5012. [Google Scholar] [CrossRef]
- de Haro, J.C.; Izarra, I.; Rodríguez, J.F.; Pérez, Á.; Carmona, M. Modelling the epoxidation reaction of grape seed oil by peracetic acid. J. Clean. Prod. 2016, 138, 70–76. [Google Scholar] [CrossRef]
- Musante, R.L.; Grau, R.J.; Baltanás, M.A. Kinetic of liquid-phase reactions catalyzed by acidic resins: The formation of peracetic acid for vegetable oil epoxidation. Appl. Catal. A Gen. 2000, 197, 165–173. [Google Scholar] [CrossRef]
- Hasdemir, D.; Hoefsloot, H.C.J.; Smilde, A.K. Validation and selection of ODE based systems biology models: How to arrive at more reliable decisions. BMC Syst. Biol. 2015, 9, 32. [Google Scholar] [CrossRef]
- Ozbuyukkaya, G.; Parker, R.S.; Veser, G. Determining robust reaction kinetics from limited data. AIChE J. 2022, 68, e17538. [Google Scholar] [CrossRef]
- Stewart, W.E.; Caracotsios, M. Computer-Aided Modeling of Reactive Systems; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 9780470274958. [Google Scholar]
- Leveneur, S.; De Araujo Filho, C.A.; Estel, L.; Salmi, T. Modeling of a liquid-liquid-solid heterogeneous reaction system: Model system and peroxyvaleric acid. Ind. Eng. Chem. Res. 2012, 51, 189–201. [Google Scholar] [CrossRef]
- Leveneur, S.; Wärnå, J.; Salmi, T.; Murzin, D.Y.; Estel, L. Interaction of intrinsic kinetics and internal mass transfer in porous ion-exchange catalysts: Green synthesis of peroxycarboxylic acids. Chem. Eng. Sci. 2009, 64, 4101–4114. [Google Scholar] [CrossRef]
- Leveneur, S.; Thönes, M.; Hébert, J.P.; Taouk, B.; Salmi, T. From kinetic study to thermal safety assessment: Application to peroxyformic acid synthesis. Ind. Eng. Chem. Res. 2012, 51, 13999–14007. [Google Scholar] [CrossRef]
- Cai, X.; Ait Aissa, K.; Estel, L.; Leveneur, S. Investigation of the Physicochemical Properties for Vegetable Oils and Their Epoxidized and Carbonated Derivatives. J. Chem. Eng. Data 2018, 63, 1524–1533. [Google Scholar] [CrossRef]
- Cai, X.; Matos, M.; Leveneur, S. Structure–Reactivity: Comparison between the Carbonation of Epoxidized Vegetable Oils and the Corresponding Epoxidized Fatty Acid Methyl Ester. Ind. Eng. Chem. Res. 2019, 58, 1548–1560. [Google Scholar] [CrossRef]
- Caracotsios, M.; Stewart, W.E. Sensitivity analysis of initial value problems with mixed odes and algebraic equations. Comput. Chem. Eng. 1985, 9, 359–365. [Google Scholar] [CrossRef]
- Stewart, W.E.; Caracotsios, M.; Sørensen, J.P. Parameter estimation from multiresponse data. AIChE J. 1992, 38, 641–650. [Google Scholar] [CrossRef]
- Buzzi-Ferraris, G. Planning of experiments and kinetic analysis. Catal. Today 1999, 52, 125–132. [Google Scholar] [CrossRef]
- Leveneur, S.; Salmi, T.; Murzin, D.Y.; Estel, L.; Wärnå, J.; Musakka, N. Kinetic study and modeling of peroxypropionic acid synthesis from propionic acid and hydrogen peroxide using homogeneous catalysts. Ind. Eng. Chem. Res. 2008, 47, 656–664. [Google Scholar] [CrossRef]
- Toch, K.; Thybaut, J.W.; Marin, G.B. A systematic methodology for kinetic modeling of chemical reactions applied to n-hexane hydroisomerization. AIChE J. 2015, 61, 880–892. [Google Scholar] [CrossRef]
- Paquot, C. 2.504. Determination of thep-Anisidine Value (p-A.V.); Elsevier Science: Amsterdam, The Netherlands, 1979; ISBN 9781483280820. [Google Scholar]
- Maerker, G. Determination of oxirane content of derivatives of fats. J. Am. Oil Chem. Soc. 1965, 42, 329–332. [Google Scholar] [CrossRef]
- Greenspan, F.P.; MacKellah, D.G. Analysis of Aliphatic Per Acids. Anal. Chem. 1948, 20, 1061–1063. [Google Scholar] [CrossRef]




| Initial Mass (g) | Initial Concentration (mol/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | T (K) | Catalyst | CSO | 33% HP | PA | H2O | [H2O]aq | [DB]org | [Ep]org | [HP]aq | [PA]aq |
| 1 | 341.05 | 10.20 | 100.10 | 85.30 | 73.00 | 42.10 | 27.51 | 3.85 | 0.03 | 4.13 | 4.92 |
| 2 | 331.84 | 9.80 | 100.10 | 84.50 | 74.00 | 44.00 | 27.60 | 3.85 | 0.03 | 4.05 | 4.93 |
| 3 | 323.15 | 10 | 100.10 | 86.30 | 73.30 | 42.90 | 27.63 | 3.85 | 0.03 | 4.14 | 4.89 |
| 4 | 333.15 | 5 | 100.00 | 83.00 | 74.00 | 43.00 | 27.39 | 3.85 | 0.03 | 4.03 | 4.99 |
| 5 | 333.15 | 10 | 100.00 | 144.60 | 44.50 | 11.00 | 29.95 | 3.85 | 0.03 | 7.01 | 3.00 |
| 6 | 333.15 | 10 | 100.00 | 61.80 | 103.71 | 34.35 | 21.06 | 3.85 | 0.00 | 3.00 | 7.00 |
| 7 | 353.15 | 5 | 100.00 | 83.00 | 74.00 | 43.00 | 27.39 | 3.85 | 0.03 | 4.03 | 4.99 |
| 8 | 323.15 | 15 | 100.10 | 86.30 | 73.30 | 42.90 | 27.63 | 3.85 | 0.03 | 4.14 | 4.89 |
| 9 | 353.15 | 2 | 100.00 | 83.00 | 74.00 | 43.00 | 27.39 | 3.85 | 0.03 | 4.03 | 4.99 |
| 10 | 343.15 | 10 | 100.00 | 83.00 | 74.00 | 43.10 | 27.63 | 3.33 | 0.53 | 4.14 | 4.89 |
| Initial Mass (g) | Initial Concentration (mol/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | T (K) | Catalyst | CSO | 33% HP | PA | H2O | [H2O]aq | [DB]org | [Ep]org | [HP]aq | [PA]aq |
| 1V | 328.15 | 4.00 | 100.04 | 103.00 | 89.00 | 8.00 | 21.39 | 3.85 | 0.03 | 5.00 | 6.01 |
| 2V | 338.15 | 8.00 | 100.04 | 103.00 | 59.00 | 38.00 | 29.73 | 3.85 | 0.03 | 5.00 | 3.98 |
| Run | Dried Amberlite IR-120 (g) | T (K) | Initial [H2O] (mol/L) | Initial [HP] (mol/L) | Initial [PA] (mol/L) | Initial [PPA] (mol/L) |
|---|---|---|---|---|---|---|
| 1 | 9.80 | 343.15 | 27.51 | 4.06 | 4.95 | 0.00 |
| 2 | 9.80 | 333.15 | 27.82 | 4.09 | 4.86 | 0.00 |
| 3 | 9.80 | 353.15 | 27.51 | 4.06 | 4.95 | 0.00 |
| 4 | 7.00 | 333.15 | 30.31 | 7.04 | 2.90 | 0.00 |
| 5 | 7.00 | 343.15 | 29.95 | 7.01 | 3.00 | 0.00 |
| 6 | 5.00 | 333.15 | 21.08 | 3.00 | 7.00 | 0.00 |
| 7 | 5.00 | 343.15 | 21.09 | 3.00 | 7.00 | 0.00 |
| Units | Estimates | HPD (%) | |
|---|---|---|---|
| L/mol/g of dried cat/min | −8.57 | 0.63 | |
| - | 16.87 | 13.38 |
| Units | Estimates | HPD (%) | |
|---|---|---|---|
| L/mol/min | −1.43 | 16.14 | |
| - | 12.76 | 30.59 | |
| 1/min | −8.06 | 0.81 | |
| - | 13.86 | 18.58 | |
| L/min | 0.50 | >100 | |
| A | L2/mol2 | 0.24 | 10.21 |
| B | L/mol | 0.92 | 6.92 |
| C | L/mol | 0.45 | 19.40 |
| A | B | C | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | ||||||||
| 0.70 | 1 | |||||||
| 0.18 | 0.07 | 1 | ||||||
| 0.02 | 0.12 | −0.03 | 1 | |||||
| −0.80 | −0.41 | −0.13 | 0.01 | 1 | ||||
| A | 0.36 | 0.17 | 0.21 | −0.17 | −0.34 | 1 | ||
| B | −0.07 | −0.16 | 0.00 | −0.03 | 0.26 | −0.32 | 1 | |
| C | −0.13 | −0.04 | 0.06 | 0.11 | 0.13 | −0.65 | 0.13 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Kebir, N.; Cai, X.; Leveneur, S. In-Depth Kinetic Modeling and Chemical Analysis for the Epoxidation of Vegetable Oils in a Liquid–Liquid–Solid System. Catalysts 2023, 13, 274. https://doi.org/10.3390/catal13020274
Meng Y, Kebir N, Cai X, Leveneur S. In-Depth Kinetic Modeling and Chemical Analysis for the Epoxidation of Vegetable Oils in a Liquid–Liquid–Solid System. Catalysts. 2023; 13(2):274. https://doi.org/10.3390/catal13020274
Chicago/Turabian StyleMeng, Yudong, Nasreddine Kebir, Xiaoshuang Cai, and Sebastien Leveneur. 2023. "In-Depth Kinetic Modeling and Chemical Analysis for the Epoxidation of Vegetable Oils in a Liquid–Liquid–Solid System" Catalysts 13, no. 2: 274. https://doi.org/10.3390/catal13020274
APA StyleMeng, Y., Kebir, N., Cai, X., & Leveneur, S. (2023). In-Depth Kinetic Modeling and Chemical Analysis for the Epoxidation of Vegetable Oils in a Liquid–Liquid–Solid System. Catalysts, 13(2), 274. https://doi.org/10.3390/catal13020274







