Abstract
Photocatalysts based on g-C3N4 have been investigated in the CO2 reduction reaction under visible light irradiation (λ = 397, 427, 452 nm). Photocatalysts were prepared by melamine calcination at 500–600 °C with further platinum deposition (0.1–1.0 wt.%). The effect of the preparation conditions of g-C3N4 and the method of platinum deposition on the physicochemical properties and activity of photocatalysts was studied. The photocatalysts were investigated by X-ray photoelectron spectroscopy, X-ray absorption spectroscopy, X-ray diffraction, high resolution transmission electron microscopy, UV-Vis spectroscopy, and low temperature nitrogen adsorption techniques. It has been found that the efficiency of CO2 reduction is governed by the surface area of g-C3N4 and the presence of platinum in the metallic state, while the optimal content of platinum is 0.5 wt. %. The highest rate of CO2 reduction achieved over Pt/g-C3N4 photocatalyst is 13.2 µmol h−1 g−1 (397 nm), which exceeds the activity of pristine g-C3N4 by 7 times. The most active photocatalysts was prepared by calcining melamine in air at 600 °C, followed by modification with platinum (0.5 wt.%).
1. Introduction
Due to the growing concentration of greenhouse gases in the atmosphere, their utilization attracts a special attention [1,2]. The main component is CO2, which is produced by burning fossil fuels [3]. Other important issues are the growing energy consumption and the depletion of fossil fuels [4]. Thus, the conversion of CO2 to synthetic fuels would solve several problems at once. Such an opportunity is provided during the photocatalytic process of CO2 reduction, the products of which are CH4, CO, CH3OH, etc. [5,6]. Moreover, when using sunlight as the radiation source, photocatalytic CO2 conversion becomes an ideal green technology for solar-to-chemical conversion [7]. However, most of the described photocatalysts have a rather low efficiency when irradiated with visible light, which makes up a large part of the solar spectrum [8,9].
A quantity of compounds is used as photocatalysts, among which TiO2 has received tremendous attention [10,11,12,13,14,15,16]. However, TiO2-based photocatalysts are predominantly activated by irradiation of ultraviolet light only [17]. Compared to photocatalysts based on metal oxides, g-C3N4 has a fairly narrow band gap, which makes it a promising photocatalyst for processes under the visible light irradiation [18,19,20,21,22]. Additionally, a strongly negative conduction band potential (−1.1 V vs. NHE) is favorable for CO2 reduction [23]. Low cost, simplicity of preparation, and absence of toxicity make g-C3N4 an attractive photocatalyst from a practical point of view [24]. However, the photogenerated electrons and holes in a pristine g-C3N4 undergo rapid recombination, that leads to a decrease in photocatalytic activity [25]. To enhance the lifetime of charge carriers, metal deposition on the surface of g-C3N4 is shown to be one of the most effective techniques [26]. Platinum is widely used as a cocatalyst due to its high work function of electrons, which greatly improves charge separation [27]. This way, development of a composite material Pt/g-C3N4 has a positive effect on photocatalyst efficiency and CO2 reduction rate.
It is known that the synthesis conditions have a decisive influence on the properties and activity of the photocatalyst [28]. One of the most widely applied methods for synthesis g-C3N4 is thermal condensation of low-cost and non-toxic melamine at temperature around 400–600 °C [29,30]. Temperature variation leads to a significant modification in the morphology of the product, and, therefore, to a great extent alter the photocatalytic activity of g-C3N4. Additionally, the method of platinum deposition affects the photocatalytic activity. Depending on the synthesis procedure, Pt particles could be distributed differently on the surface. In case of aggregation of metal particles, the photocatalyst will demonstrate poor activity [31]. Thus, the relationship between preparation conditions, photocatalyst morphology, and photocatalytic activity is critical for determining approaches to the synthesis of highly active materials.
In the current work, we have shown the effect of preparation conditions on the morphology of g-C3N4 particles and the state of platinum, and, therefore, reveal the key factors that determine the activity of photocatalysts based on g-C3N4 in the photocatalytic reduction of CO2. The possibility of controlling the selectivity for CO2 reduction over Pt/g-C3N4 photocatalysts by changing the wavelength of the light source has been shown for the first time.
2. Results and Discussion
In this work, a series of g-C3N4-based photocatalyst modified with Pt particles was prepared. On the first stage, calcination temperature of melamine was varied in a range of 500–600 °C. After that, the Pt particles were deposited on the surface of g-C3N4 by two methods: the photochemical reduction or photodeposition method (PD) and by reduction with NaBH4 or chemical deposition (CD). The obtained photocatalysts were characterized by different techniques and studied in CO2 reduction reaction (CO2 RR) under visible light irradiation. Three LEDs were used as a radiation source: LED-397 (maximum intensity at λ = 397 nm), LED-427 (maximum intensity at λ = 427 nm), and LED-452 (maximum intensity at λ = 452 nm). The obtained photocatalysts were labeled as: X% Pt(PD or CD)/g-C3N4 T, where X–mass content of Pt, PD or CD – method of Pt deposition, T – calcination temperature of melamine.
2.1. Photocatalyst Characterization
To study the influence of the synthesis temperature on the textural characteristics of the samples obtained, g-C3N4 samples were tested by low temperature nitrogen adsorption method. The raise of synthesis temperature promotes the increase of surface area (Table 1). The growth of the specific surface area at a higher temperature is caused by an increase in the crystallinity of resulting g-C3N4. It is known that an increased surface area allows more active components to be deposited and also facilitates light absorption of the catalyst and adsorption of reagents.

Table 1.
Properties of synthesized g-C3N4 in dependence of calcination temperature of melamine.
The optical properties of the synthesized g-C3N4 samples were studied using UV-Vis spectroscopy (Figure 1a). Absorption edges slightly decrease from 2.82 to 2.75 eV with the increase in calcination temperature (Figure 1b), which corresponds to the band gap of g-C3N4 [28,32]. X-ray diffraction pattern (Figure S1) demonstrates a dominant peak at 2θ≈28°, that is attributed to the (002) plane of g-C3N4 [33].
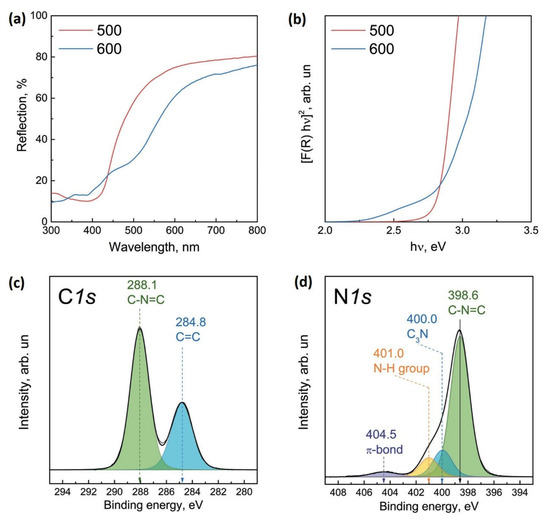
Figure 1.
Diffuse reflectance spectra of g-C3N4 (a) and Tauc’s plots depending on the calcination temperature of melamine (b); C1s (c) and N1s (d) core-level spectra of g-C3N4 obtained from melamine at 600 °C. The N1s spectrum is normalized to the integral intensity of the C1s peak corresponding to the spectrum of g-C3N4.
Additionally, the [N]/[C] ratio remains constant in the obtained catalysts. The C1s and N1s core-level spectra of g-C3N4 obtained at 600 °C are shown in Figure 1c,d. The C1s spectrum is well described by two peaks with binding energies at 284.8 and 288.1 eV. The first peak corresponds to carbon-containing impurities present on the surface of the photocatalyst under study (often used to calibrate the binding energy scale). The second peak is characteristic of C1s g-C3N4 and corresponds to carbon forming bonds with nitrogen atoms in the g-C3N4 structure [34,35]. In the case of the N1s spectrum, four peaks are observed with binding energies at 398.6, 400.0, 401.0, and 404.5 eV. According to the literature, the first peak refers to nitrogen atoms forming a C–N=C bond, the second one—to the N-(C)3 bond with three carbon atoms, and the third one—to the N-H terminal groups [34,35]. The fourth peak corresponds to an excited π-bond.
Therefore, we can conclude the synthesized photocatalysts are g-C3N4 with similar properties except surface area.
As mentioned above, two methods of platinum deposition on g-C3N4 were used: photodeposition (PD) and chemical reduction (CD). The key characteristics of catalysts affected the catalytic process are the oxidation state, surface concentration, and size distribution of nanoparticles of active components and could be varied depending on synthesis conditions. To determine these parameters, the catalysts Pt(CD)/g-C3N4 600 and Pt(PD)/g-C3N4 600 obtained by depositing platinum on g-C3N4 synthesized from melamine calcined at 600 °C were investigated by UV-Vis spectroscopy, X-ray photoelectron spectroscopy (XPS), high resolution transmission electron microscopy (HR TEM), and X-ray absorption spectroscopy.
Figure 2 represents the characterization results for platinized photocatalysts. Compared to unmodified samples of g-C3N4, the deposition of Pt promotes light absorption in region beyond 450 nm (Figure 2a,b), which is caused by localized surface plasmon resonance of platinum nanoparticles [36]. Broadening the absorption spectrum of photocatalysts favorably affects the rate of a photocatalytic reaction under the visible light irradiation.
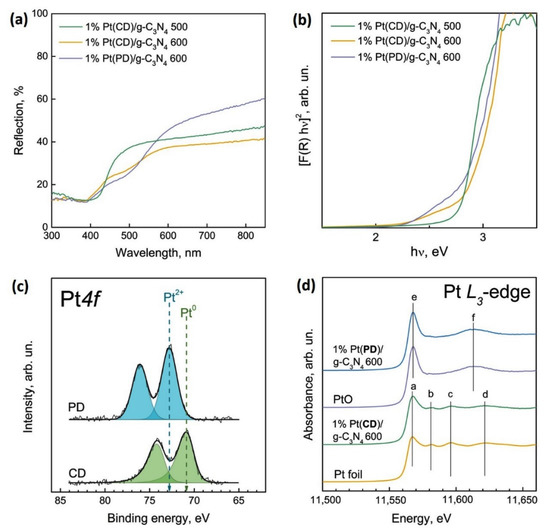
Figure 2.
Diffuse reflectance spectra of 1%Pt/g-C3N4 (a) and Tauc’s plots depending on the deposition method of Pt (b); Pt4f core-level spectra (c) and Pt L3-edge (d) spectra of Pt in 1%Pt/g-C3N4 catalyst obtained from melamine at 600 °C. The Pt4f core-level spectrum is normalized to the integral intensity of the C1s core-level peak corresponding to the spectrum of g-C3N4.
Figure 2c shows the Pt4f core-level spectra of 1% Pt/g-C3N4 photocatalyst obtained from melamine at 600 °C. In the case of chemical reduction, the Pt4f core-level spectrum is approximated by one Pt4f7/2-Pt4f7/2 doublet with a Pt4f7/2 binding energy at 70.9 eV. When Pt particles were deposited by photoreduction, a Pt4f7/2 binding energy is about 72.7 eV. In the literature, for bulk metallic platinum, the Pt4f7/2 binding energy is about 71.1–71.6 eV [37,38]. For bulk PtO, PtO2, and Pt(OH)4, the binding energies of Pt4f7/2 lie in the range of 72.3–73 eV, 74.0–74.1 eV, and 74.2–74.4 eV [39,40,41,42]. Thus, using chemical reduction deposition method platinum exist in the metallic state, while in the case of the photoreduction method, Pt is in the oxidized state Pt2+ in the form of PtO.
The surface atomic ratios of element measured by XPS are presented in Table 2. One can see that the deposition of platinum by chemical reduction leads to formation of metallic platinum nanoparticles. In contrast, the photoreduction of platinum leads to the formation of platinum oxide. Lowering the temperature calcination of melamine leads to an increase in [Pt]/[C] ratio, which is due to the different specific surface of the synthesized g-C3N4 samples (Table 2).

Table 2.
Relative atomic concentrations of elements in the surface layer of the catalysts, as well as the fraction of platinum in the metallic state.
X-ray absorption spectroscopy was additionally used to determine the bulk structure of platinum nanoparticles deposited on g-C3N4. The X-ray absorption near edge structure (XANES) spectra of the Pt L3-edge of the 1% Pt/g-C3N4 photocatalysts obtained from melamine upon calcination at 600 °C and with different methods of platinum deposition, as well as reference compounds of platinum—metal foil and PtO are shown in Figure 2d. The XANES spectra of the L3-edges of Pt catalysts have different shapes, which indicates a different phase composition of platinum nanoparticles. Thus, the spectrum of the L3-edge of the Pt in catalyst 1% Pt(CD)/g-C3N4 600 has the same structure as the spectrum of metal platinum (platinum foil). Indeed, the structure of the absorption spectra of 1% Pt(CD)/g-C3N4 600 and metallic platinum has a number of common features (peaks a-d), therefore, platinum nanoparticles are completely metallic and do not have a core–shell structure. At the same time, the spectrum of the L3-edge of the Pt catalyst 1% Pt(PD)/g-C3N4 600 has the same structure as the spectrum platinum oxide PtO. The structure of the absorption spectra of 1% Pt(PD)/g-C3N4 600 and platinum oxide has a number of common features (peaks e and f), which allows us to state that platinum nanoparticles in this photocatalyst are completely oxidized and also do not have the core–shell structure.
The HR TEM images showed that platinum is uniformly distributed over the g-C3N4 surface (Figure 3 and Figure S2). However, in the case of using chemical reduction, aggregates of several platinum nanoparticles are predominantly formed (Figure 3a). When Pt deposited using photoreduction, a larger number of single nanoparticles is observed (Figure 3b). The average size of platinum nanoparticles was about 7 nm and 4 nm for samples synthesized using chemical reduction and photoreduction, respectively.
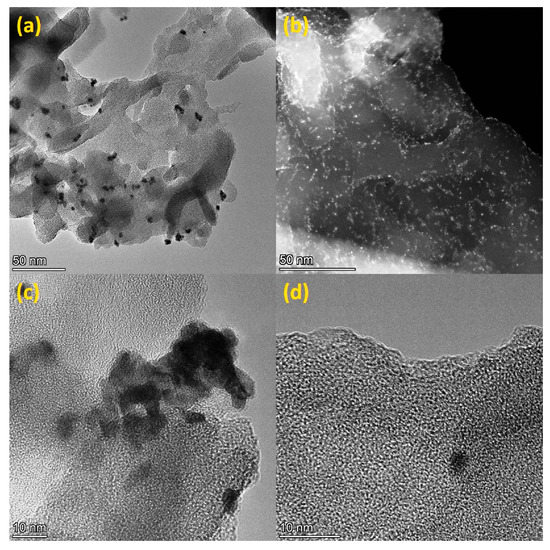
Figure 3.
HR TEM images of 0.5% Pt(CD)/g-C3N4 600 (a,c), HAADF STEM (b), and HR TEM (d) images of 0.5% Pt(PD)/g-C3N4 600.
Thus, applied methods allow us to study the structural and textural characteristics of the synthesized photocatalysts, that makes it possible to reveal the effect of these parameters on the activity and selectivity of photocatalysts in the photocatalytic CO2 RR.
2.2. Photocatalytic Activity
The activity of the synthesized photocatalysts was studied in the reaction of CO2 reduction under the irradiation of LED-397, LED-427, and LED-452 with the addition of 1 mL of water in the reactor. At the first stage, the influence of the melamine calcination temperature on the activity of the obtained photocatalysts 1% Pt/g-C3N4 was studied. The highest rates of CH4 formation and overall CO2 reduction rate are achieved over the samples obtained at 600 °C (Figure 4), which is explained by the largest specific surface of the samples (Table 1) and, as a result, a high number of adsorption sites.
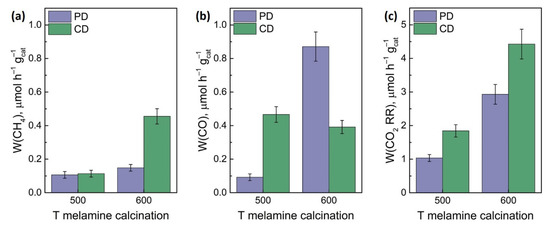
Figure 4.
The formation rate of CH4 (a), CO (b), and overall CO2 reduction rate (c) in dependence on melamine calcination temperature and platinum deposition technique. Photocatalysts: 1% Pt/g-C3N4 500, 1% Pt/g-C3N4 600. Conditions: m(cat.) = 30 mg, P0(CO2) = 1 atm, t(reaction) = 24 h, λ = 397 nm.
A study of the activity of photocatalysts showed that the deposition of platinum (0.5 and 1 wt.%) leads to a significant increase in the rate of CH4 formation, while the rate of CO formation changes insignificantly (Figure 5a,b). Thus, by varying the content of platinum, it is possible to increase or decrease the selectivity of methane formation. Variation of the deposition method and platinum content showed that chemical reduction is more effective—the formation of metallic Pt is beneficial for methane formation and overall CO2 reduction (Figure 5c).
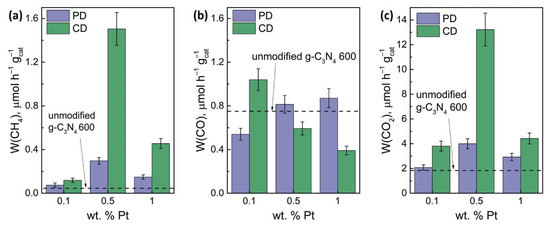
Figure 5.
The formation rate of CH4 (a), CO (b), and overall CO2 reduction rate (c) in dependence on platinum concentration and platinum deposition technique. Photocatalysts: (0.1–1)% Pt/g-C3N4 600. Conditions: m(cat.) = 30 mg, P0(CO2) = 1 atm, t(reaction) = 24 h, λ = 397 nm.
The highest rate of total reduction of CO2 was achieved over the 0.5% Pt(CD)/g-C3N4 600 photocatalyst and equal to 13.2 µmol h–1 g–1 under irradiation with λ = 397 nm, which exceeds the activity of unmodified g-C3N4 obtained by calcining melamine at 600 °C by a factor of 7.
The most active photocatalysts 0.5% Pt(CD)/g-C3N4 600 and 0.5% Pt(PD)/g-C3N4 600 were tested in the CO2 RR using LED-427 and LED-452 as sources of light irradiation (Figure 6). The highest activity was demonstrated by the sample obtained from melamine at 600 °C and modified with platinum by the chemical deposition method (0.5% Pt(CD)/g-C3N4 600). Under LED-427 irradiation, the overall CO2 reduction rate was 6.2 µmol h–1 g–1 (AQE = 0.13%).
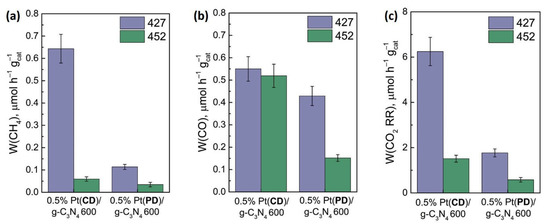
Figure 6.
The formation rate of CH4 (a), CO (b), and overall CO2 reduction rate (c). Conditions: m(cat.) = 30 mg, P0(CO2) = 1 atm, t(reaction) = 24 h, λ = 427 nm or 452 nm.
It should be noted in the presence of water vapor in the reactor, in addition to the photocatalytic CO2 reduction process (Equations (1) and (2)), a competing reaction of photocatalytic hydrogen formation occurs simultaneously (Equation (3)) [43,44]:
CO2 + 2e− + 2H+ = CO + H2O, E0 = −0.53 V vs. NHE at pH 7
CO2 + 8e− + 8H+ = CH4 + 2H2O, E0 = −0.24 V vs. NHE at pH 7
2H2O + 2e− = H2 + 2OH−, E0 = −0.41 V vs. NHE at pH 7
Since there are two competitive photocatalytic processes, we have evaluated the selectivity for CO2 reduction on an electron basis (Table 3).

Table 3.
Activity and selectivity of most active Pt/g-C3N4.
A lower selectivity of 0.5% Pt(CD)/g-C3N4 600 compared to 0.5% Pt(PD)/g-C3N4 600 is likely to be caused by presence of Pt in metallic state. On the surface of metallic Pt, H2 can release throw Tafel (Equation (4)) or Heyrovsky mechanism (Equation (5)) [45,46]:
2H· = H2,
H· + e− + H+ = H2
Thus, metallic platinum promotes both processes—CO2 reduction and H2 evolution. Moreover, the rate of CH4 formation is significantly increased compared to CO when using catalysts with platinum under LED-397 (Figure 5a). Despite the fact that the reduction of CO2 to CH4 is a thermodynamically favorable process, it requires 8e−/8H+ to proceed, which causes kinetic difficulties. When deposited on the surface of a semiconductor, platinum acts as an electron trap due to the formation of Schottky barrier at the metal/semiconductor contacts. Metallic Pt has one of the highest work functions of electron 5.65 eV, which allows efficient capture of electrons [27,47]. Thereby, the enrichment of electron density in Pt particles promotes the multielectron process of CH4 formation.
In the case of PtO/g-C3N4 photocatalysts obtained by platinum photodeposition (0.5% Pt(PD)/g-C3N4 600), charge separation is less efficient due to the lower electron work function of PtO compared to metallic Pt. Therefore, the total rate of CO2 reduction is lower than that for the photocatalyst 0.5% Pt(CD)/g-C3N4 600. In this case, a higher selectivity of CO2 reduction is achieved, because PtO particles are likely not suitable cocatalysts for the formation of H2 from water. Additionally, one can see (Table 3) that for all wavelengths, the rate of CO formation is higher than the rate of CH4 production.
It should be noted that the selectivity is also the key parameter of photocatalytic process as well as the activity of photocatalyst. As can be seen in Table 3, the decrease in energy of a photon with an increase in wavelength inhibits the H2 formation rate in a greater extent than the overall CO2 reduction reaction. A similar effect was previously shown for Ni3Mn-layered double hydroxide photocatalyst [48]. Due to a lower Gibbs free energy barrier for CO2 activation than for H2 evolution, H2 formation is suppressed when irradiated with low photon energy light. The processes of photocatalytic reduction of CO2 and H2 evolution on Pt/g-C3N4 photocatalysts likely have similar features.
Thus, by changing the wavelength of the light source, it is possible to control the selectivity of the photocatalytic reduction of carbon dioxide. Despite the activity of photocatalysts not reaching the highest values presented in the literature, even selectivity of 0.5% Pt(CD)/g-C3N4 600 photocatalysts under LED-397 exceeds some values described in the literature for CO2 reduction over semiconductor-based photocatalysts, modified with Pt particles (Table 4).

Table 4.
Selectivity and activity of some semiconductor-based photocatalysts presented in the literature.
Thereby, Pt/g-C3N4 photocatalysts prepared with a simple two-step synthesis method demonstrate the high selectivity in the CO2 reduction reaction.
3. Materials and Methods
3.1. Photocatalyst Synthesis
Synthesis of g-C3N4 was carried out from melamine by calcination at different temperature. A ceramic crucible with 2 g of melamine (Sigma-Aldrich, St. Louis, USA, 99%), covered with a lid, was placed in a muffle furnace (Smolenskoye SKTB SPU, Smolensk, Russia) and kept at a certain temperature (500, 600 °C) in air for 2 h. The heating rate to the set temperature was 10 degrees per minute. The resulting sample of g-C3N4 was ground in a mortar.
After that, platinum was deposited on g-C3N4 surface in two ways: by chemical reduction (CD) and by photodeposition (PD).
In the first case, platinum was deposited on a g-C3N4 sample according to the following procedure: a sample of g-C3N4 (500 mg) was dispersed using ultrasound, impregnated with an 0.02 M H2PtCl6 (Reakhim, Moscow, Russia, 98%) solution, followed by reduction with an excess of NaBH4 (Acros Organics, Geel, Belgium, 99%) solution. The resulting photocatalyst was washed in a centrifuge and dried in air at 50 °C for 5 h.
In the second case, a suspension was prepared consisting of a sample of g-C3N4 (500 mg) and 40 mL of a 20% solution of ethanol in water and placed into a photocatalytic reactor. The mixture was purged with argon for 30 min and then illuminated with a 380 nm LED (30 V, 1 A) for 3 h. The resulting photocatalyst was washed in a centrifuge and dried in air at 50 °C for 5 h.
The amount of deposited platinum on g-C3N4 was 0.1, 0.5, and 1 wt. %.
3.2. Photocatalyst Characterization
The photocatalysts were characterized by UV-Vis optical absorption spectroscopy, X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy, high-resolution transmission electron microscopy (HR TEM), including the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) technique, N2 low-temperature adsorption.
The chemical composition of the catalyst surface was studied by XPS with a photoelectron spectrometer (SPECS Surface Nano Analysis GmbH, Berlin, Germany) using non-monochromatized Al Kα radiation (hυ = 1486.6 eV). The spectrometer was equipped with a PHOIBOS-150 hemispherical analyzer (SPECS Surface Nano Analysis GmbH, Berlin, Germany) and an XR-50 X-ray source with a double Al/Mg anode.
The chemical state of platinum in the bulk of the catalysts was studied using X-ray absorption spectroscopy at the station of the Kurchatov Synchrotron Radiation Source (Moscow, Russia). The electron energy in the storage ring was 2.5 GeV at a beam current of 50–150 mA. To monochromatize synchrotron radiation, we used a silicon single crystal with (111) orientation in the form of a cut-out monoblock mounted on a goniometric head. The energy resolution achieved was ΔE/E = 2 × 10−4. The X-ray absorption spectra of the catalysts were obtained in transmission geometry. The X-ray beam intensity before and after passing through the sample was measured using ionization chambers equipped with Keithley 6487 digital picoammeters (Keithley Instruments, Ohio, USA).
The microstructure of the photocatalysts was studied by HR TEM using a ThemisZ electron microscope (Thermo Fisher Scientific, Waltham, MA, USA) at an accelerating voltage of 200 kV. The microscope was equipped with a SuperX energy-dispersive spectrometer and a spherical aberration corrector. The maximum resolution of the microscope was 0.06 nm. For the HR TEM analysis, the samples were ultrasonically dispersed onto perforated carbon substrates attached to aluminum grids.
The diffuse reflectance UV-Vis spectra were measured using a UV-2501 PC spectrophotometer with an ISR-240A diffuse reflectance unit (Shimadzu, Kyoto, Japan).
The specific surface area was calculated by the Brunauer-Emmett-Teller method using nitrogen adsorption isotherms measured at liquid nitrogen temperatures with an automatic Micromeritics ASAP 2400 sorptometer (Micromeritics, Norcross, USA).
X-ray diffraction pattern was recorded on a D8 Advance diffractometer equipped with a Lynxeye linear detector (Bruker AXS GmbH, Karlsruhe, Germany). The diffraction patterns were obtained in the 2θ range from 10 to 80° with a step of 0.05° using Cu Kα radiation (λ = 1.5418 Å).
3.3. Photocatalytic Activity Test
The activity of photocatalysts was tested in CO2 reduction reaction in a batch reactor (170 mL). A photocatalyst (30 mg) was deposited on a glass support and placed in the reactor. After that, the reactor containing 1 mL of water was purged with CO2 for 1 h, and the light source was turned on. Details are described elsewhere [31]. In this work, 3 light emitting diodes (LEDs) were used as light sources (Figure 7): LED-397 with maximum intensity at 397 nm (power density 60 mWcm−2), LED-427 with maximum intensity at 427 nm (power density 56 mWcm−2), and LED-452 with maximum intensity at 452 nm (power density 42 mWcm−2).
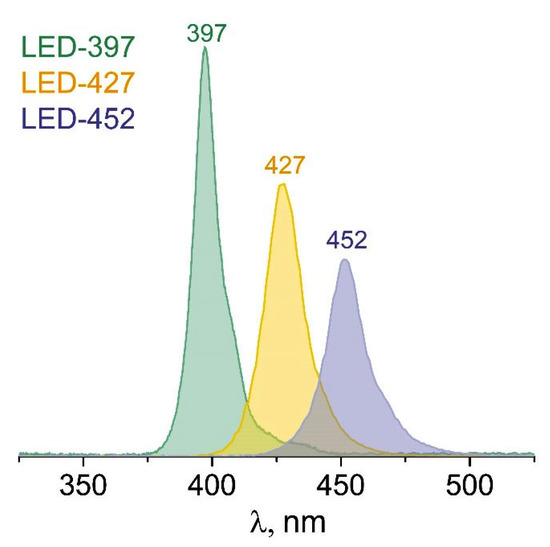
Figure 7.
Spectra of LEDs with the wavelength at maximum intensity.
Gas probe was analyzed with a gas chromatograph “GH-1000” (Chromos, Moscow, Russia) equipped with the flame ionization detector and thermal conductivity detector to identify the products of CO2 reduction and H2, respectively. The overall rate of CO2 reduction was calculated according to the following equation:
where n(CH4) and n(CO) are the amounts of CH4 and CO (µmol), 2 and 8 are the coefficients for electron balance, t is the time of reaction (h), and m is the weight of photocatalyst (g).
The selectivity of CO2 reduction was defined as:
The apparent quantum efficiency (AQE) was calculated according to the following equation:
where Nphot is the calculated photon flux equal to 5.8 mmol h−1 for LED-427.
4. Conclusions
In this work, the synthesis conditions were studied for the photocatalytic activity and selectivity of photocatalysts based on g-C3N4 modified with Pt or PtO particles. It is shown that the determining factors for reaction efficiency are the surface area of g-C3N4 and the oxidation state of the metal. Platinum in the metallic state contributes to an increase in the activity of the photocatalyst, but high selectivity is achieved only at a wavelength of 427 nm and higher. Platinum oxide is a more selective cocatalyst with respect to the photocatalytic reduction of CO2, but the reaction rate in this case is low. The highest rate of CO2 reduction achieved over 0.5% Pt(CD)/g-C3N4 600 photocatalyst is 13.2 µmol h−1 g−1 (λ = 397 nm) with selectivity for CO2 reduction equal to 51.5%. The maximum selectivity is 94.8% and achieved over the same 0.5% Pt(CD)/g-C3N4 600 photocatalyst under irradiation with a wavelength of 450 nm.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13020273/s1, Figure S1: XRD patterns of the g-C3N4 600; Figure S2: HR TEM image of 1% Pt(CD)/g-C3N4 600 (a) and HAADF STEM image of 1% Pt(PD)/g-C3N4 600 (b).
Author Contributions
Investigation, data curation, visualization, writing—original draft preparation, funding acquisition, project administration, A.A.S.; investigation, data curation, visualization, writing—original draft preparation, A.Y.K.; investigation, data curation, A.V.Z.; investigation, data curation, E.Y.G.; writing—review and editing, supervision, conceptualization, project administration, E.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Russian Science Foundation (Grant #21-73-10235).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The XPS and HR TEM experiments were performed using the facilities of the shared research center “National Center of Investigation of Catalysts” at Boreskov Institute of Catalysis. The authors thank Alexander Trigub for the support during the beamtime at the Kurchatov Synchrotron Radiation Source (Kurchatov Institute, Moscow, Russia) and Svetlana Cherepanova for XRD study (Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Centi, G.; Perathoner, S. The chemical engineering aspects of CO2 capture, combined with its utilisation. Curr. Opin. Chem. Eng. 2023, 39, 100879. [Google Scholar] [CrossRef]
- Sreedhar, A.; Ta, Q.T.H.; Noh, J.S. Advancements in the photocatalytic activity of various bismuth-based semiconductor/Ti3C2 MXene interfaces for sustainable environmental management: A review. J. Ind. Eng. Chem. 2022, 115, 26–47. [Google Scholar] [CrossRef]
- Hanifa, M.; Agarwal, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. A review on CO2 capture and sequestration in the construction industry: Emerging approaches and commercialised technologies. J. CO2 Util. 2023, 67, 102292. [Google Scholar] [CrossRef]
- Kouser, S.; Hezam, A.; Ara Khanum, S. Final Rational Design and Engineering of Efficient Metal Organic Framework for Visible Light-driven Photocatalytic carbon-di-oxide Reduction. Inorg. Chim. Acta 2022, 546, 121287. [Google Scholar] [CrossRef]
- Ghosh, U.; Majumdar, A.; Pal, A. Photocatalytic CO2 reduction over g-C3N4 based heterostructures: Recent progress and prospects. J. Environ. Chem. Eng. 2021, 9, 104631. [Google Scholar] [CrossRef]
- Francis Kurisingal, J.; Kim, H.; Hyeak Choe, J.; Seop Hong, C. Covalent organic framework-based catalysts for efficient CO2 utilization reactions. Coord. Chem. Rev. 2022, 473, 214835. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Long, X.; Zou, J.; Hu, J.; Jiao, F.P. Design of hollow nanostructured photocatalysts for clean energy production. Coord. Chem. Rev. 2023, 477, 214953. [Google Scholar] [CrossRef]
- Tahir, M.B.; Asiri, A.M.; Nawaz, T. A perspective on the fabrication of heterogeneous photocatalysts for enhanced hydrogen production. Int. J. Hydrogen Energy 2020, 45, 24544–24557. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Yamauchi, M.; Saito, H.; Sugimoto, T.; Mori, S.; Saito, S. Sustainable organic synthesis promoted on titanium dioxide using coordinated water and renewable energies/resources. Coord. Chem. Rev. 2022, 472, 214773. [Google Scholar] [CrossRef]
- Orudzhev, F.F.; Gasanova, F.G.; Aliev, Z.M.; Isaev, A.B. Photoelectrocatalytic oxidation of phenol on platinum-modified TiO2 nanotubes. Nanotechnol. Russ. 2012, 7, 482–485. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalysis for Hydrogen Production and CO2 Reduction: The Case of Copper-Catalysts. ChemCatChem 2019, 11, 368–382. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Elseman, A.M.; Mohamed, M.M.; Ren, Y.; Salama, T.M.; Mohamed, M.B.I. Magnetic TiO2/CoFe2O4 Photocatalysts for Degradation of Organic Dyes and Pharmaceuticals without Oxidants. Nanomaterials 2022, 12, 3290. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Falara, P.P.; Ibrahim, I.; Kontos, A.G. Magnetic Metal Oxide-Based Photocatalysts with Integrated Silver for Water Treatment. Materials 2022, 15, 4629. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Lyulyukin, M.N.; Markovskaya, D.V.; Selishchev, D.S.; Cherepanova, S.V.; Kozlov, D.V. Synthesis of Cd1-: XZnxS photocatalysts for gas-phase CO2 reduction under visible light. Photochem. Photobiol. Sci. 2019, 18, 871–877. [Google Scholar] [CrossRef]
- Cheng, S.P.; Wei, L.W.; Wang, H.P. Photocatalytic reduction of CO2 to methanol by Cu2O/TiO2 heterojunctions. Sustainability 2022, 14, 374. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, M.; Zhuzhang, H.; Zhang, G.; Anpo, M.; Wang, X. Gradient Zn-doped poly heptazine imides integrated with a van der Waals homojunction boosting visible light-driven water oxidation activities. ACS Catal. 2021, 11, 13463–13471. [Google Scholar] [CrossRef]
- Zheng, D.; Zhou, J.; Fang, Z.; Heil, T.; Savateev, A.; Zhang, Y.; Antonietti, M.; Zhang, G.; Wang, X. H2 and CH4 production from bio-alcohols using condensed poly(heptazine imide) with visible light. J. Mater. Chem. A 2021, 9, 27370–27379. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Antoniadou, M.; Kaltzoglou, A.; Sakellis, E.; Katsaros, F.; Sygellou, L.; Arfanis, M.K.; Salama, T.M.; Falaras, P. Silver decorated TiO2/g-C3N4 bifunctional nanocomposites for photocatalytic elimination of water pollutants under UV and artificial solar light. Results Eng. 2022, 14, 100470. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, X.; Zhang, C.; Lin, L.; Xing, W.; Yu, Z.; Zhang, G.; Wang, X. Improved Charge Separation in Poly(heptazine-triazine) Imides with Semi-coherent Interfaces for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202210849. [Google Scholar] [CrossRef]
- Durmus, Z.; Maijenburg, A.W. A review on graphitic carbon nitride (g-C3N4)—Metal organic framework (MOF) heterostructured photocatalyst materials for photo(electro)chemical hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 36784–36813. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, H. Coupled adsorption and photocatalysis of g-C3N4 based composites: Material synthesis, mechanism, and environmental applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Llatance-Guevara, L.; Flores, N.E.; Barrionuevo, G.O.; Mullo Casillas, J.L. Waste Biomass Selective and Sustainable Photooxidation to High-Added-Value Products: A Review. Catalysts 2022, 12, 1091. [Google Scholar] [CrossRef]
- Das, S.; Das, S.; Nair, R.G.; Chowdhury, A. Magnetically separable ZnFe2O4 grafted g-C3N4/rGO ternary nanocomposites for enhanced photo-Fenton catalytic activity under visible-light. Mater. Today Sustain. 2022, 21, 100263. [Google Scholar] [CrossRef]
- Ran, J.; Jaroniec, M.; Qiao, S.Z. Cocatalysts in Semiconductor-based Photocatalytic CO2 Reduction: Achievements, Challenges, and Opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef]
- Lin, H.; Wu, J.; Zhou, F.; Zhao, X.; Lu, P.; Sun, G.; Song, Y.; Li, Y.; Liu, X.; Dai, H. Graphitic carbon nitride-based photocatalysts in the applications of environmental catalysis. J. Environ. Sci. 2023, 124, 570–590. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Fan, M.; Wang, X.; Walbridge, M.L.; Nong, Q.; Wu, Y.; Zhao, L. Z-scheme SnO2-x/g-C3N4 composite as an efficient photocatalyst for dye degradation and photocatalytic CO2 reduction. Sol. Energy Mater. Sol. Cells 2015, 137, 175–184. [Google Scholar] [CrossRef]
- Saraev, A.A.; Kurenkova, A.Y.; Gerasimov, E.Y.; Kozlova, E.A. Broadening the Action Spectrum of TiO2-Based Photocatalysts to Visible Region by Substituting Platinum with Copper. Nanomaterials 2022, 12, 1584. [Google Scholar] [CrossRef]
- Lorenz, F.; Moustakas, N.G.; Peppel, T.; Strunk, J. Comparative Studies of Oxygen-Free Semiconductors in Photocatalytic CO2 Reduction and Alcohol Degradation. Chem. Ing. Tech. 2022, 94, 1776–1783. [Google Scholar] [CrossRef]
- Topchiyan, P.; Vasilchenko, D.; Tkachev, S.; Sheven, D.; Eltsov, I.; Asanov, I.; Sidorenko, N.; Saraev, A.; Gerasimov, E.; Kurenkova, A.; et al. Highly Active Visible Light-Promoted Ir/g-C3N4 Photocatalysts for the Water Oxidation Reaction Prepared from a Halogen-Free Iridium Precursor. ACS Appl. Mater. Interfaces 2022, 14, 35600–35612. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.K. In situ construction of g-C3N4/g-C3N 4 metal-free heterojunction for enhanced visible-light photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef]
- Liu, H.; Chen, D.; Wang, Z.; Jing, H.; Zhang, R. Microwave-assisted molten-salt rapid synthesis of isotype triazine-/heptazine based g-C3N4 heterojunctions with highly enhanced photocatalytic hydrogen evolution performance. Appl. Catal. B Environ. 2017, 203, 300–313. [Google Scholar] [CrossRef]
- Kunwar, S.; Sui, M.; Pandey, P.; Gu, Z.; Pandit, S.; Lee, J. Improved Configuration and LSPR Response of Platinum Nanoparticles via Enhanced Solid State Dewetting of In-Pt Bilayers. Sci. Rep. 2019, 9, 1329. [Google Scholar] [CrossRef]
- Tenney, S.A.; He, W.; Ratliff, J.S.; Mullins, D.R.; Chen, D.A. Characterization of Pt-Au and Ni-Au clusters on TiO2(110). Top. Catal. 2011, 54, 42–55. [Google Scholar] [CrossRef]
- Steinrück, H.P.; Pesty, F.; Zhang, L.; Madey, T.E. Ultrathin films of Pt on TiO2(110): Growth and chemisorption-induced surfactant effects. Phys. Rev. B 1995, 51, 2427–2439. [Google Scholar] [CrossRef]
- Barr, T.L. An ESCA study of the termination of the passivation of elemental metals. J. Phys. Chem. 1978, 82, 1801–1810. [Google Scholar] [CrossRef]
- Bernsmeier, D.; Sachse, R.; Bernicke, M.; Schmack, R.; Kettemann, F.; Polte, J.; Kraehnert, R. Outstanding hydrogen evolution performance of supported Pt nanoparticles: Incorporation of preformed colloids into mesoporous carbon films. J. Catal. 2019, 369, 181–189. [Google Scholar] [CrossRef]
- Gołąbiewska, A.; Lisowski, W.; Jarek, M.; Nowaczyk, G.; Zielińska-Jurek, A.; Zaleska, A. Visible light photoactivity of TiO2 loaded with monometallic (Au or Pt) and bimetallic (Au/Pt) nanoparticles. Appl. Surf. Sci. 2014, 317, 1131–1142. [Google Scholar] [CrossRef]
- Smirnov, M.Y.; Vovk, E.I.; Nartova, A.V.; Kalinkin, A.V.; Bukhtiyarov, V.I. An XPS and STM Study of Oxidized Platinum Particles Formed by the Interaction between Pt/HOPG with NO2. Kinet. Catal. 2018, 59, 653–662. [Google Scholar] [CrossRef]
- Gulati, S.; Vijayan, S.; Mansi; Kumar, S.; Harikumar, B.; Trivedi, M.; Varma, R.S. Recent advances in the application of metal-organic frameworks (MOFs)-based nanocatalysts for direct conversion of carbon dioxide (CO2) to value-added chemicals. Coord. Chem. Rev. 2023, 474, 214853. [Google Scholar] [CrossRef]
- Domínguez-Espíndola, R.B.; Arias, D.M.; Rodríguez-González, C.; Sebastian, P.J. A critical review on advances in TiO2-based photocatalytic systems for CO2 reduction. Appl. Therm. Eng. 2022, 216, 119009. [Google Scholar] [CrossRef]
- Sun, N.; Zhu, Y.; Li, M.; Zhang, J.; Qin, J.; Li, Y.; Wang, C. Thermal coupled photocatalysis over Pt/g-C3N4 for selectively reducing CO2 to CH4 via cooperation of the electronic metal-support interaction effect and the oxidation state of Pt. Appl. Catal. B Environ. 2021, 298, 120565. [Google Scholar] [CrossRef]
- Lasia, A. Mechanism and kinetics of the hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 19484–19518. [Google Scholar] [CrossRef]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef]
- Tan, L.; Xu, S.M.; Wang, Z.; Hao, X.; Li, T.; Yan, H.; Zhang, W.; Zhao, Y.; Song, Y.F. 600 nm induced nearly 99% selectivity of CH4 from CO2 photoreduction using defect-rich monolayer structures. Cell Rep. Phys. Sci. 2021, 2, 100322. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Y.; Zhang, Q.; Deng, W.; Wang, Y. MgO- and Pt-promoted TiO2 as an efficient photocatalyst for the preferential reduction of carbon dioxide in the presence of water. ACS Catal. 2014, 4, 3644–3653. [Google Scholar] [CrossRef]
- Zhai, Q.; Xie, S.; Fan, W.; Zhang, Q.; Wang, Y.; Deng, W.; Wang, Y. Photocatalytic conversion of carbon dioxide with water into methane: Platinum and Copper(I) oxide co-catalysts with a core-shell structure. Angew. Chem. 2013, 52, 5776–5779. [Google Scholar] [CrossRef]
- Guo, Q.; Fu, L.; Yan, T.; Tian, W.; Ma, D.; Li, J.; Jiang, Y.; Wang, X. Improved photocatalytic activity of porous ZnO nanosheets by thermal deposition graphene-like g-C3N4 for CO2 reduction with H2O vapor. Appl. Surf. Sci. 2020, 509, 144773. [Google Scholar] [CrossRef]
- Peng, H.; Guo, R.T.; Lin, H.; Liu, X.Y. Synthesis of Bi2O3/g-C3N4 for enhanced photocatalytic CO2 reduction with a Z-scheme mechanism. RSC Adv. 2019, 9, 37162–37170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).