Biochar from Lemon Stalks: A Highly Active and Selective Carbocatalyst for the Oxidation of Sulfamethoxazole with Persulfate
Abstract
1. Introduction
2. Results and Discussion
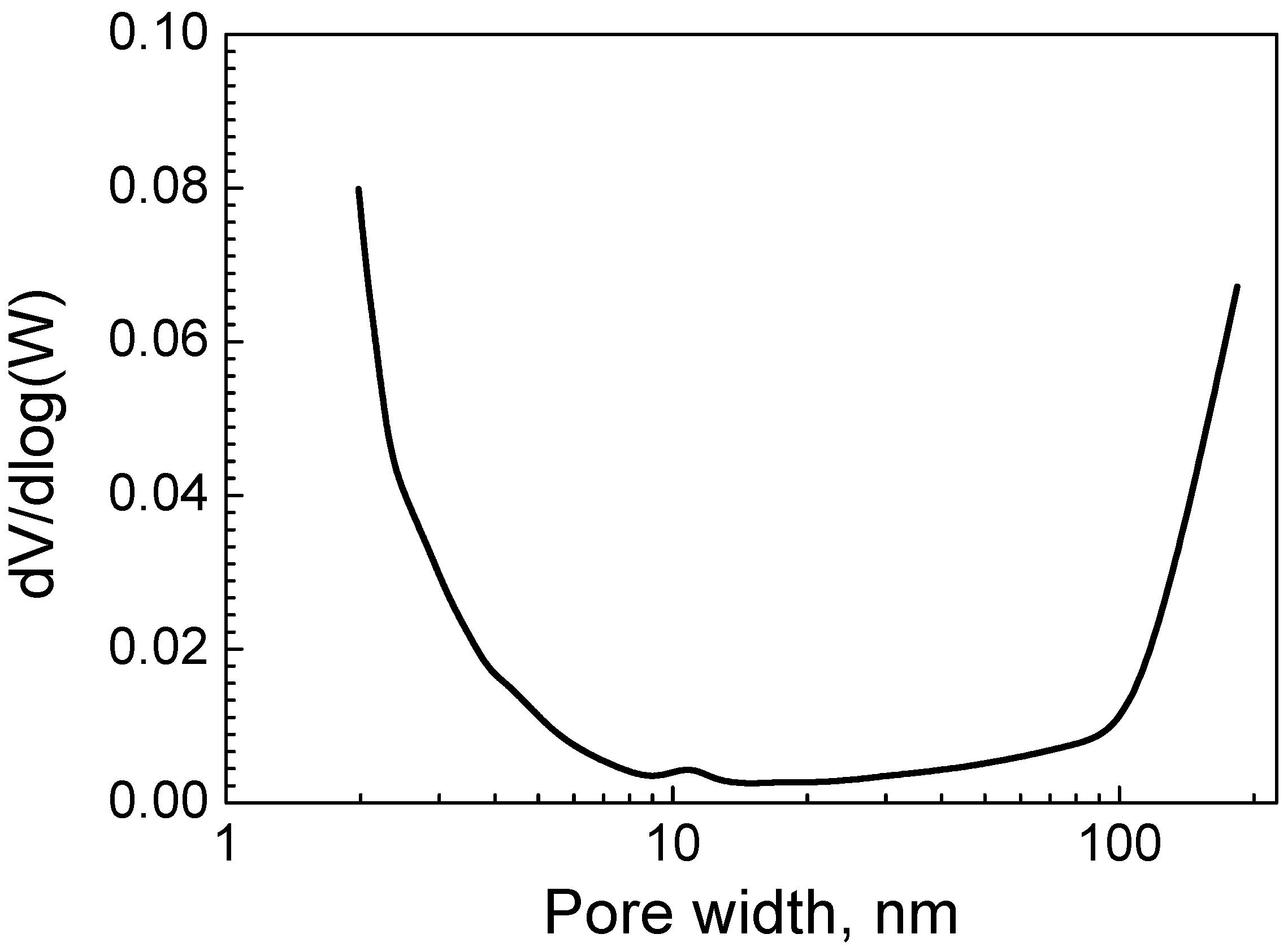
2.1. Biochar Characterization
2.2. Biochar Activity
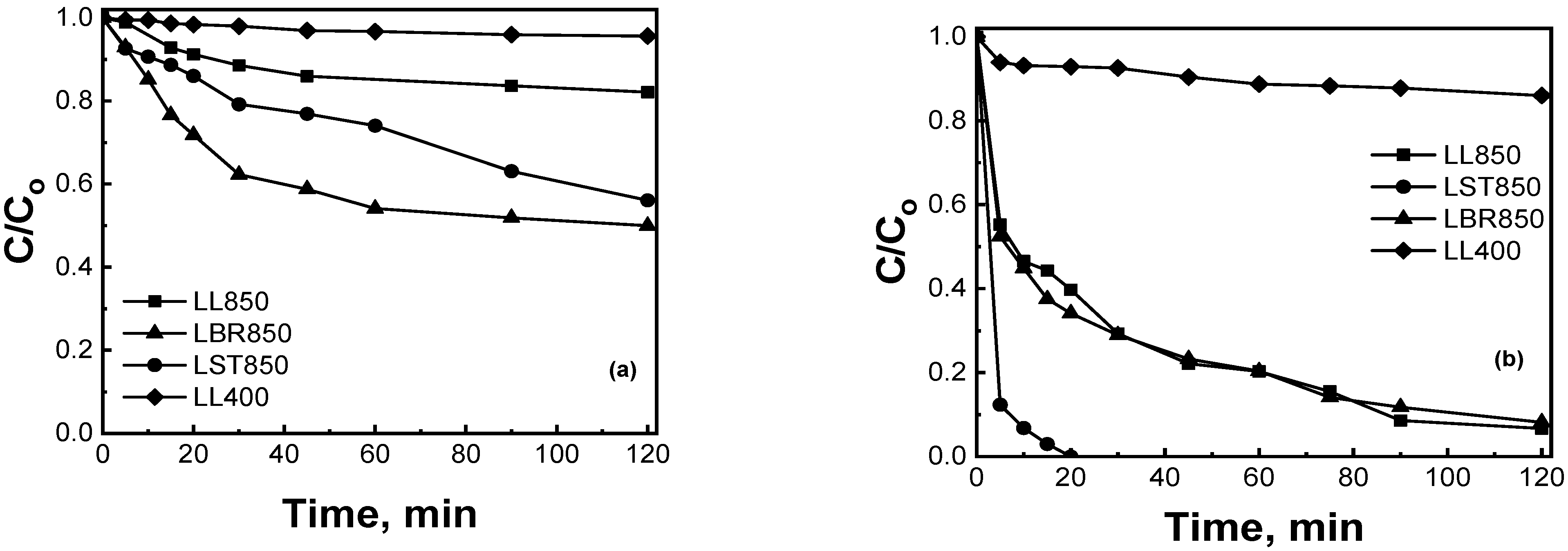
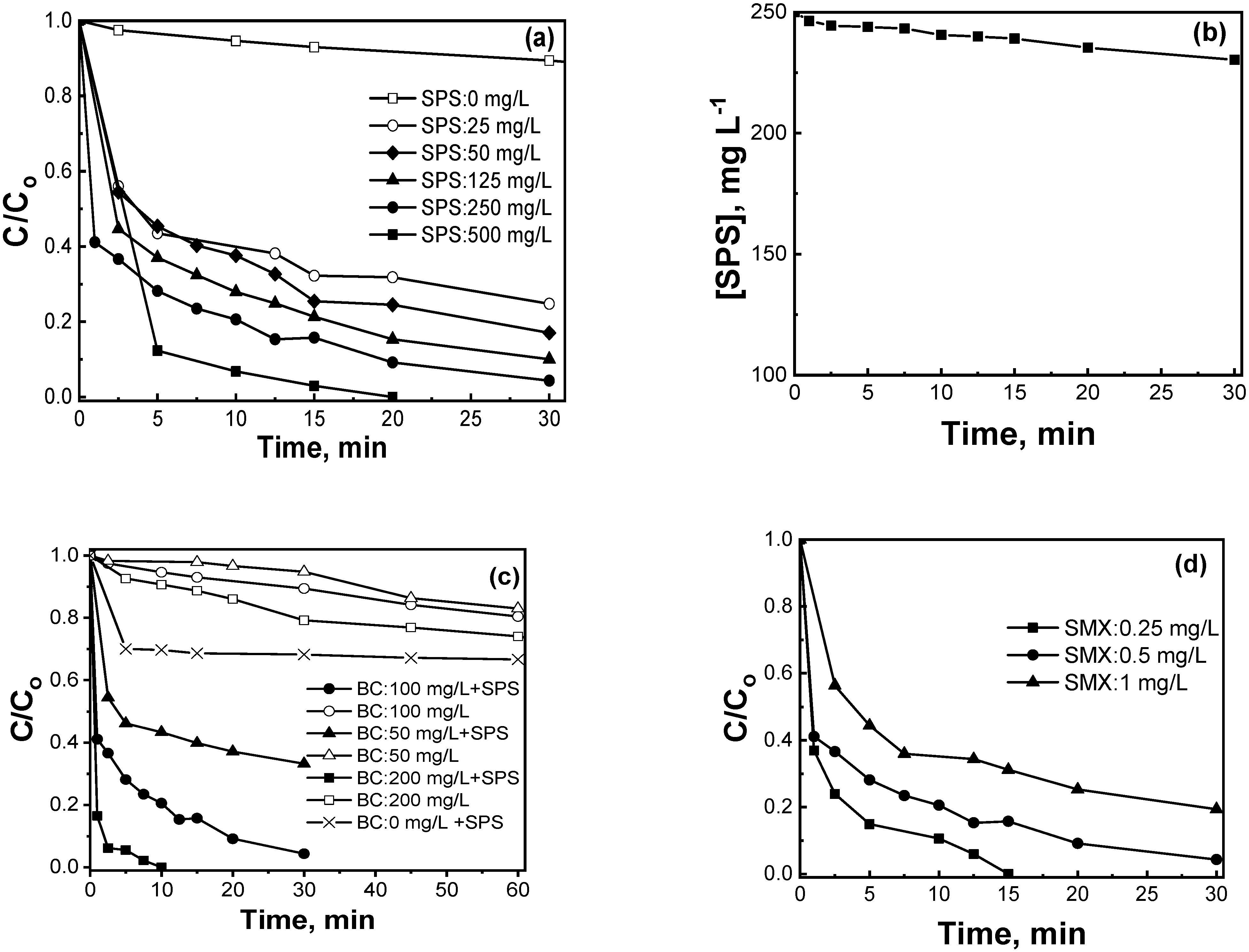
2.2.1. Preliminary Screening Experiments
2.2.2. Effect of pH
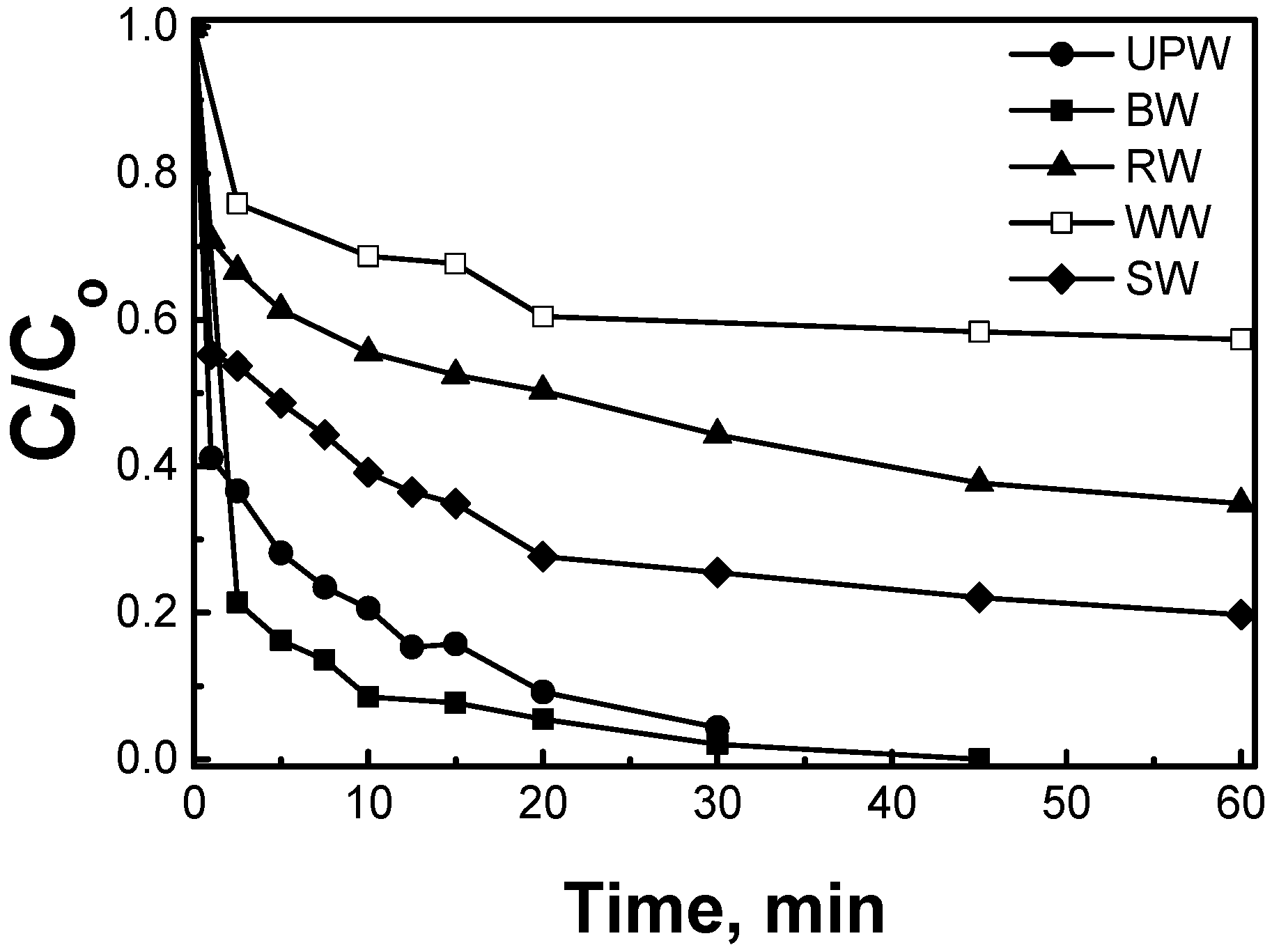
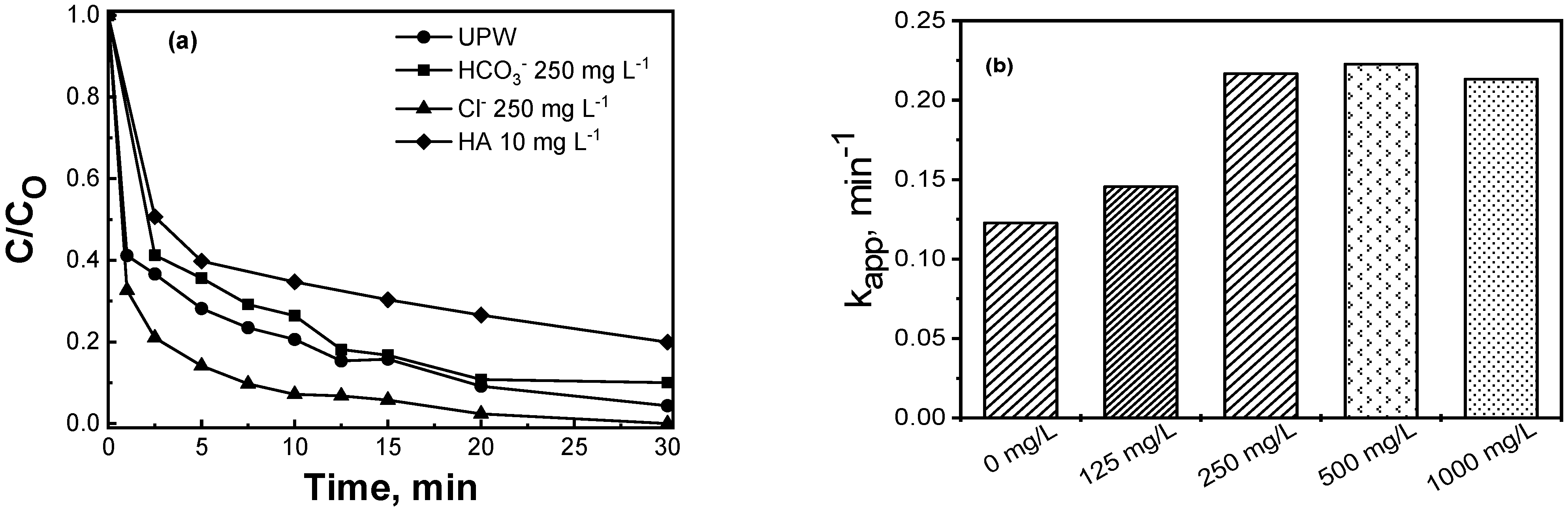
2.2.3. Effect of the Water Matrix
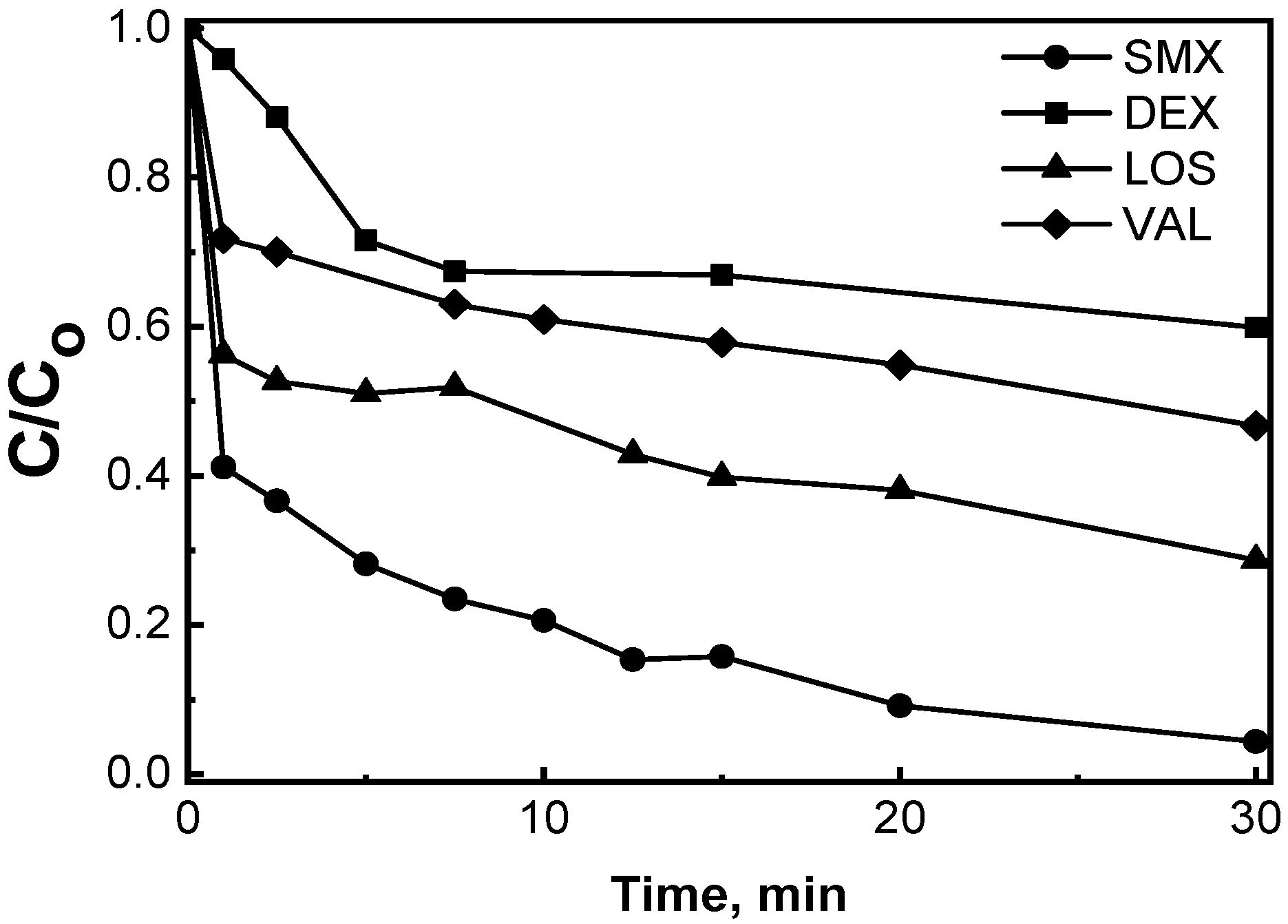
2.2.4. Effect of the Type of Pharmaceutical
2.3. Degradation Mechanism
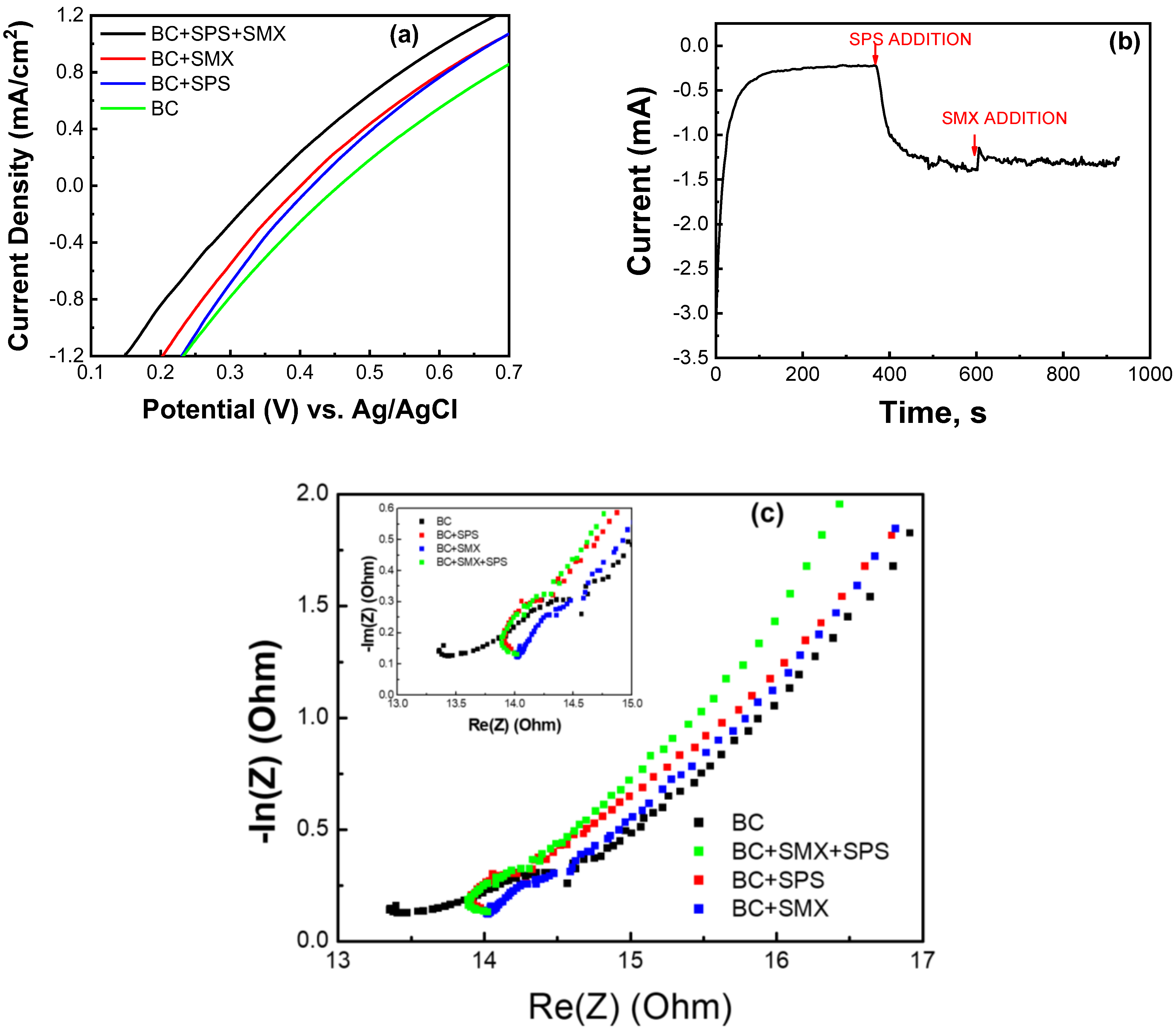
2.3.1. Electrochemical Characterization
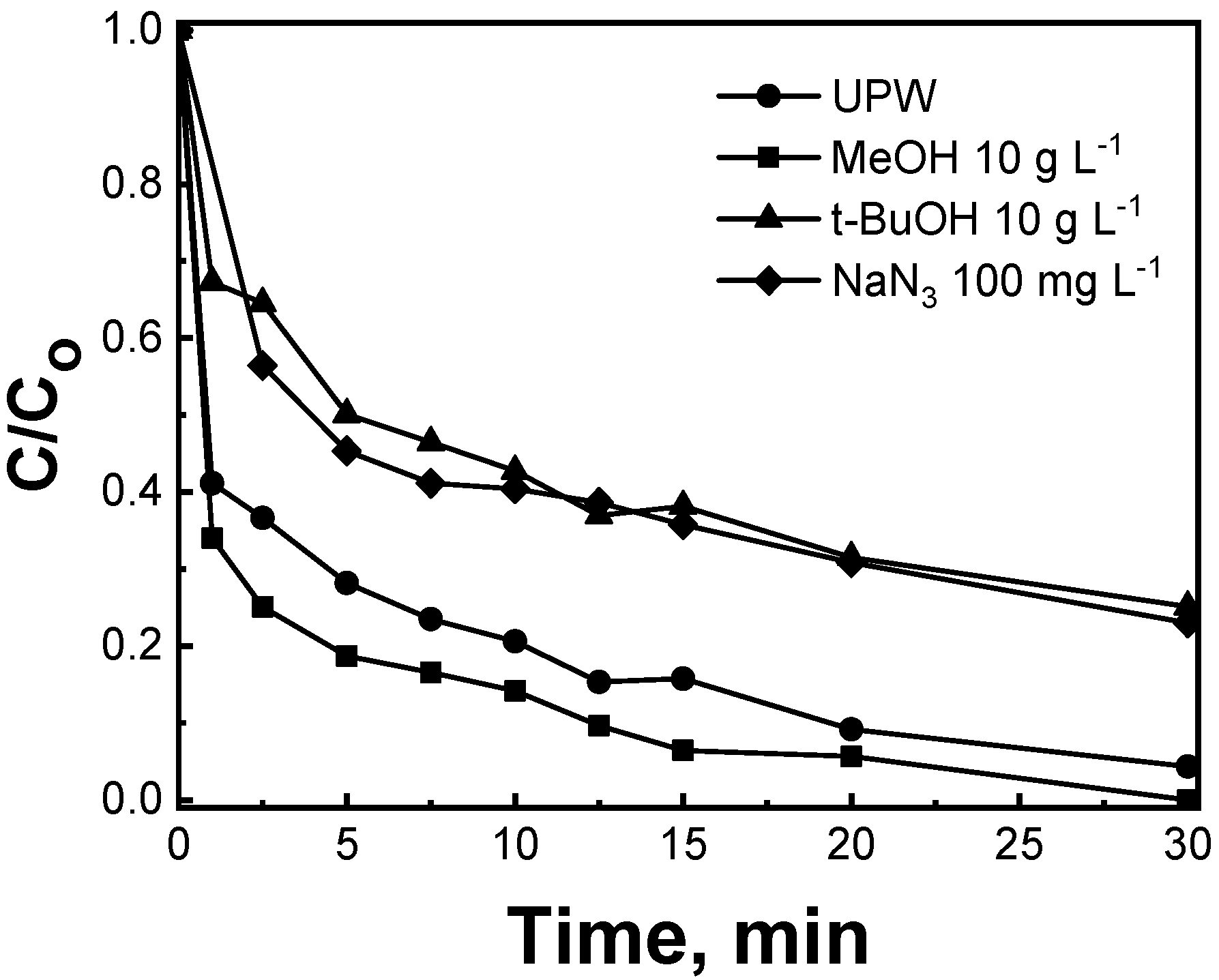
2.3.2. Effect of Scavengers
2.4. Biochar Reuse
3. Materials and Methods
3.1. Biochar Preparation
3.2. Biochar Characterization
3.3. Electrode Preparation
3.4. Chemicals and Aqueous Matrices
3.5. Experimental Procedure
3.6. Analytical Methods
4. Conclusions
- (1)
- Biochar characterization by various techniques gives useful information regarding its composition, as well as its structural and electronic properties; such information can be related to its activity and associated mechanisms (i.e., radical and electron transfer) for persulfate activation and subsequent SMX degradation. Specifically, the biochar has a significant amount of calcium carbonate, moderate SSA, and a pzc value 9.2. A total of 100% degradation of SMX can be achieved under pH = 3, 100 mg L−1 biochar, and 250 g L−1 SPS.
- (2)
- Process efficiency is dictated by the interactions between the properties of the biochar, the organic pollutant, the oxidant source, and the water matrix. This interplay eventually determines the dominant reaction pathways and kinetics.
- (3)
- Although several operating variables may determine efficiency to various degrees, particular emphasis must be given to the water matrix effect; this usually is underestimated since studies are mainly performed at conditions that are not environmentally realistic.
- (4)
- The biochar can be applied in more realistic applications, although higher amounts of biochar may be needed.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alduina, R. Antibiotics and Environment. Antibiotics 2020, 9, 202. [Google Scholar] [CrossRef]
- Baran, W.; Sochacka, J.; Wardas, W. Toxicity and Biodegradability of Sulfonamides and Products of Their Photocatalytic Degradation in Aqueous Solutions. Chemosphere 2006, 65, 1295–1299. [Google Scholar] [CrossRef]
- Deng, Y.; Li, B.; Zhang, T. Bacteria That Make a Meal of Sulfonamide Antibiotics: Blind Spots and Emerging Opportunities. Environ. Sci. Technol. 2018, 52, 3854–3868. [Google Scholar] [CrossRef]
- Manzetti, S.; Ghisi, R. The Environmental Release and Fate of Antibiotics. Mar. Pollut. Bull. 2014, 79, 7–15. [Google Scholar] [CrossRef]
- Thiele-Bruhn, D.S. Environmental Risks from Mixtures of Antibiotic Pharmaceuticals in Soils—A Literature Review; Umweltbundesamt: Dessau-Roßlau, Germany, 2019. [Google Scholar]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Bernot, M.J.; Smith, L.; Frey, J. Human and Veterinary Pharmaceutical Abundance and Transport in a Rural Central Indiana Stream Influenced by Confined Animal Feeding Operations (CAFOs). Sci. Total Environ. 2013, 445–446, 219–230. [Google Scholar] [CrossRef]
- Iglesias, A.; Nebot, C.; Vázquez, B.I.; Miranda, J.M.; Abuín, C.M.F.; Cepeda, A. Detection of Veterinary Drug Residues in Surface Waters Collected Nearby Farming Areas in Galicia, North of Spain. Environ. Sci. Pollut. Res. 2014, 21, 2367–2377. [Google Scholar] [CrossRef]
- Veach, A.M.; Bernot, M.J. Temporal Variation of Pharmaceuticals in an Urban and Agriculturally Influenced Stream. Sci. Total Environ. 2011, 409, 4553–4563. [Google Scholar] [CrossRef]
- Hruska, K.; Franek, M. Sulfonamides in the Environment: A Review and a Case Report. Veterinární Medicína 2012, 57, 1–35. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological Effects of Antibiotics on Natural Ecosystems: A Review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Matongo, S.; Birungi, G.; Moodley, B.; Ndungu, P. Occurrence of Selected Pharmaceuticals in Water and Sediment of Umgeni River, KwaZulu-Natal, South Africa. Environ. Sci. Pollut. Res. 2015, 22, 10298–10308. [Google Scholar] [CrossRef]
- Peng, X.; Tan, J.; Tang, C.; Yu, Y.; Wang, Z. Multiresidue determination of fluoroquinolone, sulfonamide, trimethoprim, and chloramphenicol antibiotics in urban waters in china. Environ. Toxicol. Chem. 2008, 27, 73. [Google Scholar] [CrossRef]
- Yargeau, V.; Lopata, A.; Metcalfe, C. Pharmaceuticals in the Yamaska River, Quebec, Canada. Water Qual. Res. J. 2007, 42, 231–239. [Google Scholar] [CrossRef]
- Tamtam, F.; Mercier, F.; Le Bot, B.; Eurin, J.; Tuc Dinh, Q.; Clément, M.; Chevreuil, M. Occurrence and Fate of Antibiotics in the Seine River in Various Hydrological Conditions. Sci. Total Environ. 2008, 393, 84–95. [Google Scholar] [CrossRef]
- Barnes, K.K.; Kolpin, D.W.; Furlong, E.T.; Zaugg, S.D.; Meyer, M.T.; Barber, L.B. A National Reconnaissance of Pharmaceuticals and Other Organic Wastewater Contaminants in the United States—I) Groundwater. Sci. Total Environ. 2008, 402, 192–200. [Google Scholar] [CrossRef]
- Qu, J.; Fan, M. The Current State of Water Quality and Technology Development for Water Pollution Control in China. Crit. Rev. Environ. Sci. Technol. 2010, 40, 519–560. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, B.-T.; Teng, Y. Distribution of Phthalate Acid Esters in Lakes of Beijing and Its Relationship with Anthropogenic Activities. Sci. Total Environ. 2014, 476–477, 107–113. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zhao, L.-X.; Lin, J.-M. Study on Superoxide and Hydroxyl Radicals Generated in Indirect Electrochemical Oxidation by Chemiluminescence and UV-Visible Spectra. J. Environ. Sci. 2008, 20, 1006–1011. [Google Scholar] [CrossRef]
- Shukla, P.; Sun, H.; Wang, S.; Ang, H.M.; Tadé, M.O. Co-SBA-15 for Heterogeneous Oxidation of Phenol with Sulfate Radical for Wastewater Treatment. Catal. Today 2011, 175, 380–385. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of Organic Contaminants in Water with Sulfate Radicals Generated by the Conjunction of Peroxymonosulfate with Cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zhao, L.; Lin, J.-M. Determination of Folic Acid by Chemiluminescence Based on Peroxomonosulfate-Cobalt(II) System. Talanta 2008, 74, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-T.; Lin, J.-M. Chemiluminescence and Energy Transfer Mechanism of Lanthanide Ions in Different Media Based on Peroxomonosulfate System. Luminescence 2010, 25, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.J.; Bruell, C.J.; Marley, M.C.; Sperry, K.L. Thermally Activated Persulfate Oxidation of Trichloroethylene (T.C.E.) and 1,1,1-Trichloroethane (T.C.A.) in Aqueous Systems and Soil Slurries. Soil Sediment Contam. Int. J. 2003, 12, 207–228. [Google Scholar] [CrossRef]
- Ioannidi, A.; Arvaniti, O.S.; Nika, M.-C.; Aalizadeh, R.; Thomaidis, N.S.; Mantzavinos, D.; Frontistis, Z. Removal of Drug Losartan in Environmental Aquatic Matrices by Heat-Activated Persulfate: Kinetics, Transformation Products and Synergistic Effects. Chemosphere 2022, 287, 131952. [Google Scholar] [CrossRef]
- Darsinou, B.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D. Sono-Activated Persulfate Oxidation of Bisphenol A: Kinetics, Pathways and the Controversial Role of Temperature. Chem. Eng. J. 2015, 280, 623–633. [Google Scholar] [CrossRef]
- Giannakopoulos, S.; Frontistis, Z.; Vakros, J.; Poulopoulos, S.G.; Manariotis, I.D.; Mantzavinos, D. Combined Activation of Persulfate by Biochars and Artificial Light for the Degradation of Sulfamethoxazole in Aqueous Matrices. J. Taiwan Inst. Chem. Eng. 2022, 136, 104440. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, K.; Zhai, Z.; Zhao, T.; Yuan, D.; Jiao, T.; Zhang, Q.; Tang, S. Molybdenum co-catalytic promotion for Fe3+/peroxydisulfate process: Performance, mechanism, and immobilization. Chem. Eng. J. 2022, 438, 135656. [Google Scholar] [CrossRef]
- Pan, S.; Zhai, Z.; Yang, K.; Xiang, Y.; Tang, S.; Zhang, Y.; Jiao, T.; Zhang, Q.; Yuan, D. β-Lactoglobulin amyloid fibrils supported Fe(III) to activate peroxydisulfate for organic pollutants elimination. Sep. Purif. Technol. 2022, 289, 120806. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, H.; Zhu, E.; Yang, K.; Yuan, D.; Jiao, T.; Zhang, Q.; Tang, S. Application of inorganic materials as heterogeneous cocatalyst in Fenton/Fenton-like processes for wastewater treatment. Sep. Purif. Technol. 2022, 295, 121293. [Google Scholar] [CrossRef]
- Giannakopoulos, S.; Vakros, J.; Dracopoulos, V.; Manariotis, I.D.; Mantzavinos, D.; Lianos, P. Enhancement of the Photoelectrocatalytic Degradation Rate of a Pollutant in the Presence of a Supercapacitor. J. Clean. Prod. 2022, 377, 134456. [Google Scholar] [CrossRef]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an Eco-Friendly and Economical Adsorbent for the Removal of Colorants (Dyes) from Aqueous Environment: A Review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Ntaflou, M.; Vakros, J. Transesterification Activity of Modified Biochars from Spent Malt Rootlets Using Triacetin. J. Clean. Prod. 2020, 259, 120931. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and K.O.H. Activation of Biochar: Experimental and Modeling Studies. Microporous Mesoporous Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Kong, S.-H.; Loh, S.-K.; Bachmann, R.T.; Rahim, S.A.; Salimon, J. Biochar from Oil Palm Biomass: A Review of Its Potential and Challenges. Renew. Sustain. Energy Rev. 2014, 39, 729–739. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass Pyrolysis—A Review of Modelling, Process Parameters and Catalytic Studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Renewable Energy Sources: Engineering, Technology, Innovation: I.C.O.R.E.S. 2017; Mudryk, K., Werle, S., Eds.; Springer Proceedings in Energy; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-72370-9. [Google Scholar]
- Fresh Lemons and Limes: Leading Producers Worldwide 2021. Statista. Available online: https://www.statista.com/statistics/1045016/world-lemons-and-limes-major-producers/ (accessed on 24 August 2021).
- Lykoudi, A.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of Sulfamethoxazole with Persulfate Using Spent Coffee Grounds Biochar as Activator. J. Environ. Manage. 2020, 271, 111022. [Google Scholar] [CrossRef]
- Magioglou, E.; Frontistis, Z.; Vakros, J.; Manariotis, I.; Mantzavinos, D. Activation of Persulfate by Biochars from Valorized Olive Stones for the Degradation of Sulfamethoxazole. Catalysts 2019, 9, 419. [Google Scholar] [CrossRef]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of Antibiotic Sulfamethoxazole by Biochar-Activated Persulfate: Factors Affecting the Activation and Degradation Processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. Oxidation of Sulfamethoxazole by Rice Husk Biochar-Activated Persulfate. Catalysts 2021, 11, 850. [Google Scholar] [CrossRef]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar Obtained by Carbonization of Spent Coffee Grounds and Its Application in the Construction of an Energy Storage Device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Gao, G.; Cheong, L.-Z.; Wang, D.; Shen, C. Pyrolytic Carbon Derived from Spent Coffee Grounds as Anode for Sodium-Ion Batteries. Carbon Resour. Convers. 2018, 1, 104–108. [Google Scholar] [CrossRef]
- Bourikas, K.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Potentiometric Mass Titrations: Experimental and Theoretical Establishment of a New Technique for Determining the Point of Zero Charge (P.Z.C.) of Metal (Hydr)Oxides. J. Phys. Chem. B 2003, 107, 9441–9451. [Google Scholar] [CrossRef]
- Mrozik, W.; Minofar, B.; Thongsamer, T.; Wiriyaphong, N.; Khawkomol, S.; Plaimart, J.; Vakros, J.; Karapanagioti, H.; Vinitnantharat, S.; Werner, D. Valorisation of agricultural waste derived biochars in aquaculture to remove organic micropollutants from water—Experimental study and molecular dynamics simulations. J. Environ. Manag. 2021, 300, 113717. [Google Scholar] [CrossRef] [PubMed]
- Grilla, E.; Vakros, J.; Konstantinou, I.; Manariotis, I.D.; Mantzavinos, D. Activation of persulfate by biochar from spent malt rootlets for the degradation of trimethoprim in the presence of inorganic ions. J. Chem. Technol. Biotechnol. 2020, 95, 2348–2358. [Google Scholar] [CrossRef]
- Fang, G.; Wu, W.; Liu, C.; Dionysiou, D.D.; Deng, Y.; Zhou, D. Activation of Persulfate with Vanadium Species for P.C.B.s Degradation: A Mechanistic Study. Appl. Catal. B Environ. 2017, 202, 1–11. [Google Scholar] [CrossRef]
- Avisar, D.; Primor, O.; Gozlan, I.; Mamane, H. Sorption of Sulfonamides and Tetracyclines to Montmorillonite Clay. Water. Air. Soil Pollut. 2010, 209, 439–450. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Frontistis, Z.; Mantzavinos, D.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Screening of Heterogeneous Catalysts for the Activated Persulfate Oxidation of Sulfamethoxazole in Aqueous Matrices. Does the Matrix Affect the Selection of Catalyst? J. Chem. Technol. Biotechnol. 2019, 8, 2425–2432. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, D.; Zhao, X. Heterogeneous Degradation of Refractory Pollutants by Peroxymonosulfate Activated by CoOx-Doped Ordered Mesoporous Carbon. Chem. Eng. J. 2017, 328, 1112–1121. [Google Scholar] [CrossRef]
- Chen, L.; Zuo, X.; Yang, S.; Cai, T.; Ding, D. Rational Design and Synthesis of Hollow Co3O4@Fe2O3 Core-Shell Nanostructure for the Catalytic Degradation of Norfloxacin by Coupling with Peroxymonosulfate. Chem. Eng. J. 2019, 359, 373–384. [Google Scholar] [CrossRef]
- Lai, L.; Yan, J.; Li, J.; Lai, B. Co/Al2O3-EPM as Peroxymonosulfate Activator for Sulfamethoxazole Removal: Performance, Biotoxicity, Degradation Pathways and Mechanism. Chem. Eng. J. 2018, 343, 676–688. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Jojoa-Sierra, S.D.; Berrio-Perlaza, K.E.; Ferraro, F.; Torres-Palma, R.A. Structure-Reactivity Relationship in the Degradation of Three Representative Fluoroquinolone Antibiotics in Water by Electrogenerated Active Chlorine. Chem. Eng. J. 2017, 315, 552–561. [Google Scholar] [CrossRef]
- Ao, X.; Liu, W.; Sun, W.; Cai, M.; Ye, Z.; Yang, C.; Lu, Z.; Li, C. Medium Pressure UV-Activated Peroxymonosulfate for Ciprofloxacin Degradation: Kinetics, Mechanism, and Genotoxicity. Chem. Eng. J. 2018, 345, 87–97. [Google Scholar] [CrossRef]
- Sichel, C.; Garcia, C.; Andre, K. Feasibility Studies: U.V./Chlorine Advanced Oxidation Treatment for the Removal of Emerging Contaminants. Water Res. 2011, 45, 6371–6380. [Google Scholar] [CrossRef]
- Li, A.; Wu, Z.; Wang, T.; Hou, S.; Huang, B.; Kong, X.; Li, X.; Guan, Y.; Qiu, R.; Fang, J. Kinetics and Mechanisms of the Degradation of P.P.C.P.s by Zero-Valent Iron (Fe°) Activated Peroxydisulfate (P.D.S.) System in Groundwater. J. Hazard. Mater. 2018, 357, 207–216. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-Mediated Activation of Persulfate and Peroxymonosulfate in Both Homogeneous and Heterogeneous Ways: A Review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Ashfaq, M.; Li, Y.; Wang, Y.; Chen, W.; Wang, H.; Chen, X.; Wu, W.; Huang, Z.; Yu, C.-P.; Sun, Q. Occurrence, Fate, and Mass Balance of Different Classes of Pharmaceuticals and Personal Care Products in an Anaerobic-Anoxic-Oxic Wastewater Treatment Plant in Xiamen, China. Water Res. 2017, 123, 655–667. [Google Scholar] [CrossRef]
- Botero-Coy, A.M.; Martínez-Pachón, D.; Boix, C.; Rincón, R.J.; Castillo, N.; Arias-Marín, L.P.; Manrique-Losada, L.; Torres-Palma, R.; Moncayo-Lasso, A.; Hernández, F. An Investigation into the Occurrence and Removal of Pharmaceuticals in Colombian Wastewater. Sci. Total Environ. 2018, 642, 842–853. [Google Scholar] [CrossRef]
- Casado, J.; Rodríguez, I.; Ramil, M.; Cela, R. Selective Determination of Antimycotic Drugs in Environmental Water Samples by Mixed-Mode Solid-Phase Extraction and Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2014, 1339, 42–49. [Google Scholar] [CrossRef]
- Azuma, T.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Ishida, M.; Hisamatsu, K.; Yunoki, A.; Mino, Y.; et al. Environmental Fate of Pharmaceutical Compounds and Antimicrobial-Resistant Bacteria in Hospital Effluents, and Contributions to Pollutant Loads in the Surface Waters in Japan. Sci. Total Environ. 2019, 657, 476–484. [Google Scholar] [CrossRef]
- Mandaric, L.; Diamantini, E.; Stella, E.; Cano-Paoli, K.; Valle-Sistac, J.; Molins-Delgado, D.; Bellin, A.; Chiogna, G.; Majone, B.; Diaz-Cruz, M.S.; et al. Contamination Sources and Distribution Patterns of Pharmaceuticals and Personal Care Products in Alpine Rivers Strongly Affected by Tourism. Sci. Total Environ. 2017, 590–591, 484–494. [Google Scholar] [CrossRef]
- Cortez, F.S.; Souza, L.D.S.; Guimarães, L.L.; Almeida, J.E.; Pusceddu, F.H.; Maranho, L.A.; Mota, L.G.; Nobre, C.R.; Moreno, B.B.; Abessa, D.M.D.S. et al. Ecotoxicological Effects of Losartan on the Brown Mussel Perna Perna and Its Occurrence in Seawater from Santos Bay (Brazil). Sci. Total Environ. 2018, 637–638, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, O.S.; Bairamis, F.; Konstantinou, I.; Mantzavinos, D.; Frontistis, Z. Degradation of Antihypertensive Drug Valsartan in Water Matrices by Heat and Heat/Ultrasound Activated Persulfate: Kinetics, Synergy Effect and Transformation Products. Chem. Eng. J. Adv. 2020, 4, 100062. [Google Scholar] [CrossRef]
- Martínez-Pachón, D.; Ibáñez, M.; Hernández, F.; Torres-Palma, R.A.; Moncayo-Lasso, A. Photo-Electro-Fenton Process Applied to the Degradation of Valsartan: Effect of Parameters, Identification of Degradation Routes and Mineralization in Combination with a Biological System. J. Environ. Chem. Eng. 2018, 6, 7302–7311. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Multianalyte Method for the Determination of Pharmaceuticals in Wastewater Samples Using Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 4229–4245. [Google Scholar] [CrossRef]
- Mohseni, S.N.; Amooey, A.A.; Tashakkorian, H.; Amouei, A.I. Removal of Dexamethasone from Aqueous Solutions Using Modified Clinoptilolite Zeolite (Equilibrium and Kinetic). Int. J. Environ. Sci. Technol. 2016, 13, 2261–2268. [Google Scholar] [CrossRef]
- Ioannidi, A.A.; Vakros, J.; Frontistis, Z.; Mantzavinos, D. Tailoring the Biochar Physicochemical Properties Using a Friendly Eco-Method and Its Application on the Oxidation of the Drug Losartan through Persulfate Activation. Catalysts 2022, 12, 1245. [Google Scholar] [CrossRef]
- Li, M.; Bi, G.Y.; Xiang, L.; Chen, X.T.; Qin, Y.J.; Mo, C.H.; Zhou, S.Q. Improved cathodic oxygen reduction and bioelectricity generation of electrochemical reactor based on reduced graphene oxide decorated with titanium-based composites. Bioresour. Techn. 2020, 296, 122319. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Z.; Liang, G.; Zhang, X.; Xie, X. Catalyst Bridging-Mediated Electron Transfer for Nonradical Degradation of Bisphenol A via Natural Manganese Ore-Cornstalk Biochar Composite Activated Peroxymonosulfate. Chem. Eng. J. 2021, 426, 131777. [Google Scholar] [CrossRef]
- Yun, E.-T.; Yoo, H.-Y.; Bae, H.; Kim, H.-I.; Lee, J. Exploring the Role of Persulfate in the Activation Process: Radical Precursor Versus Electron Acceptor. Environ. Sci. Technol. 2017, 51, 10090–10099. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Yu, X.; Wu, Y.; Mo, C.; Luo, M.; Li, L.; Zhou, S.; Liu, Q.; Wang, N.; et al. FeN4-doped carbon nanotubes derived from metal organic frameworks for effective degradation of organic dyes by peroxymonosulfate: Impacts of FeN4 spin states. Chem. Eng. J. 2022, 431, 133339. [Google Scholar] [CrossRef]
- Li, M.; Mo, C.H.; Luo, X.; He, K.Y.; Yan, J.F.; Wu, Q.; Yu, P.F.; Han, W.; Feng, N.X.; Yeung, K.L.; et al. Exploring key reaction sites and deep degradation mechanism of perfluorooctane sulfonate via peroxymonosulfate activation under electrocoagulation process. Water Res. 2021, 207, 117849. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.-S.; Ren, N. Singlet Oxygen-Dominated Peroxydisulfate Activation by Sludge-Derived Biochar for Sulfamethoxazole Degradation through a Nonradical Oxidation Pathway: Performance and Mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Wang, J.; Deng, Y.; Liu, Y.; Feng, H.; Chen, S.; Ren, X. Magnetic Nitrogen-Doped Sludge-Derived Biochar Catalysts for Persulfate Activation: Internal Electron Transfer Mechanism. Chem. Eng. J. 2019, 364, 146–159. [Google Scholar] [CrossRef]
- Ntzoufra, P.; Vakros, J.; Frontistis, Z.; Tsatsos, S.; Kyriakou, G.; Kennou, S.; Manariotis, I.D.; Mantzavinos, D. Effect of Sodium Persulfate Treatment on the Physicochemical Properties and Catalytic Activity of Biochar Prepared from Spent Malt Rootlets. J. Environ. Chem. Eng. 2021, 9, 105071. [Google Scholar] [CrossRef]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. On the Performance of a Sustainable Rice Husk Biochar for the Activation of Persulfate and the Degradation of Antibiotics. Catalysts 2021, 11, 1303. [Google Scholar] [CrossRef]





| Biomass | BC (mg L−1) | SPS (mg L−1) | Removal (%) | Time (min) | Reference |
|---|---|---|---|---|---|
| Coffee grounds | 200 | 1000 | 97 | 75 | [40] |
| Malt rootlets | 90 | 250 500 * | 94 74 * | 90 120 * | [42] [48] |
| Olive stones | 200 | 1000 | 65 | 75 | [41] |
| Rice husks | 100 | 500 | 96 | 120 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakopoulos, S.; Vakros, J.; Frontistis, Z.; Manariotis, I.D.; Venieri, D.; Poulopoulos, S.G.; Mantzavinos, D. Biochar from Lemon Stalks: A Highly Active and Selective Carbocatalyst for the Oxidation of Sulfamethoxazole with Persulfate. Catalysts 2023, 13, 233. https://doi.org/10.3390/catal13020233
Giannakopoulos S, Vakros J, Frontistis Z, Manariotis ID, Venieri D, Poulopoulos SG, Mantzavinos D. Biochar from Lemon Stalks: A Highly Active and Selective Carbocatalyst for the Oxidation of Sulfamethoxazole with Persulfate. Catalysts. 2023; 13(2):233. https://doi.org/10.3390/catal13020233
Chicago/Turabian StyleGiannakopoulos, Spyridon, John Vakros, Zacharias Frontistis, Ioannis D. Manariotis, Danae Venieri, Stavros G. Poulopoulos, and Dionissios Mantzavinos. 2023. "Biochar from Lemon Stalks: A Highly Active and Selective Carbocatalyst for the Oxidation of Sulfamethoxazole with Persulfate" Catalysts 13, no. 2: 233. https://doi.org/10.3390/catal13020233
APA StyleGiannakopoulos, S., Vakros, J., Frontistis, Z., Manariotis, I. D., Venieri, D., Poulopoulos, S. G., & Mantzavinos, D. (2023). Biochar from Lemon Stalks: A Highly Active and Selective Carbocatalyst for the Oxidation of Sulfamethoxazole with Persulfate. Catalysts, 13(2), 233. https://doi.org/10.3390/catal13020233










