Abstract
Unsymmetrical 11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinoline-4,6-dione, incorporating a para-phenyl substituted pyridine unit fused by both 6- and 7-membered carbocyclic rings, has been prepared on the gram-scale via a multi-step procedure involving cyclization, hydrogenation and oxidation. Templating this diketone, in the presence of cobalt(II) chloride hexahydrate, with the corresponding aniline afforded in good yield five examples of doubly fused bis(arylimino)pyridine-cobalt(II) chlorides, Co1 (aryl = 2,6-dimethylphenyl), Co2 (2,6-diethylphenyl), Co3 (2,6-diisopropylphenyl), Co4 (2,4,6-trimethylphenyl) and Co5 (2,6-diethyl-4-methylphenyl). Structural characterization of Co1, Co2 and Co3 highlights the flexible nature of the inequivalent fused rings on the NNN’-ligand and the skewed disposition of the para-phenyl group. On activation with MAO, Co1–Co5 exhibited high activity for ethylene polymerization at 30 °C (up to 5.66 × 106 g (PE) mol−1 (Co) h−1) with the relative order being as follows: Co4 > Co1 > Co5 > Co3 > Co2. All polyethylenes were strictly linear, while their molecular weights and dispersities showed some notable variations. For Co1, Co2, Co4 and Co5, all polymerizations were well controlled as evidenced by the narrow dispersities of their polymers (Mw/Mn range: 1.8–2.7), while their molecular weights (Mw range: 2.9–10.9 kg mol−1) steadily increased in line with the greater steric properties of the N-aryl ortho-substituents. By contrast, the most hindered 2,6-diisopropyl counterpart Co3 displayed a broad distribution with bimodal characteristics (Mw/Mn = 10.3) and gave noticeably higher molecular weight polymer (Mw = 75.5 kg mol−1). By comparison, the MMAO-activated catalysts were generally less active, but showed similar trends in molecular weight and polymer dispersity. End group analysis of selected polymers via 13C and 1H NMR spectroscopy revealed the presence of both saturated and unsaturated polyethylenes in accordance with competing chain transfer pathways. Notably, when comparing Co3/MAO with its non-phenyl substituted analogue (E2,6-iPr2Ph)CoCl2/MAO, the former, though less controlled, displayed higher activity and molecular weight, a finding that points towards a role played by the remote para-phenyl group.
1. Introduction
Ever since the first communications of molecular iron and cobalt complexes as catalysts for ethylene polymerization towards the end of the 1990s [1], numerous efforts have been devoted to modifying the supporting 2,6-bis(imino)pyridine ligand framework (A, Scheme 1) [1,2,3]. Indeed, the importance of structural variations to A was noted early in their development with catalysts able to mediate both ethylene polymerization and oligomerization [1,2,3,4,5,6,7]. In the intervening years, a wide variety of alternative N,N,N chelating ligands related to A have been disclosed [8,9,10,11], including 2-imino-1,10-phenanthrolines (B, Scheme 1) [12,13,14,15,16], 2,8-bis(imino)quinolines [17,18] and various other pyridine-containing derivatives [19,20]. Significantly, B-type iron catalysts have reached the pilot-scale for the production of linear α-olefins [12], a disclosure that underscores their value for large scale industrialization.
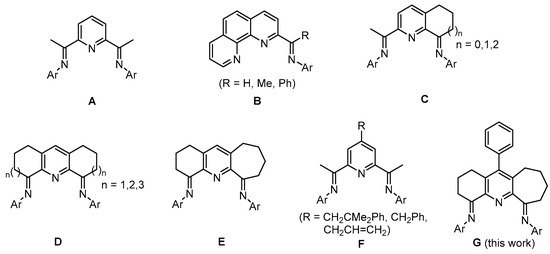
Scheme 1.
Parent bis(arylimino)pyridine A, its relatives B–F and the subject of this work, G.
With regard to specific changes to the N,N,N ligand framework, most efforts have focused on varying the steric and electronic properties of the N-aryl substituents belonging to A, with an aim to enhance the thermal stability and increase the activity of their cobalt and iron catalysts [21,22,23,24,25,26,27,28,29,30]. More recently, considerable efforts have been directed towards the development of carbocyclic-fused bis(imino)pyridine ligands, as the size of the fused ring can affect the catalyst performance and product type (e.g., C [17,18,31,32,33] and D [34,35,36], Scheme 1). For example, cobalt catalysts bearing the doubly fused six-membered ring D6,6 (n = 1, Scheme 1) [34] produced mixtures of linear α-olefins and polyethylene waxes in a good yield, while those involving the seven-membered ring counterpart D7,7 (n = 2, Scheme 1) [35] afforded linear polyethylene of low molecular weight with relatively narrow dispersity. On the other hand, for the larger and more flexible eight-membered ring D8,8 (n = 3, Scheme 1), their cobalt catalysts [36] generated high molecular weight linear polyethylene of narrow dispersity. On account of this marked effect of the fused ring size, the mixed ring cobalt complex E6,7 (Scheme 1) containing both six- and seven-membered carbocycles has also been explored [37]. Indeed, this class of unsymmetrical cobalt catalysts proved highly active, generating highly linear vinyl-terminated polyethylene with low molecular weights and extremely narrow dispersity when activated with MAO, while with MMAO, fully saturated polymers were detected.
Elsewhere, the functionalization of bis(imino)pyridine-cobalt or -iron complexes at the para-position of the central pyridine with alkyl or allyl groups have been reported (F, Scheme 1). Notably, this para-substitution had little effect on the activity of the catalyst but does have an effect on chain propagation with higher molecular weight polymers with broader dispersity generated for the allyl derivative. The origin of this behavior was uncertain but was tentatively attributed to a self-immobilization phenomenon [38].
Given the effect on catalyst performance and polymer properties caused by (i) ring size variations in D and E and (ii) the para-substituents to the central pyridine in F, we decided to target a carbocyclic-fused bis(imino)pyridine-cobalt catalyst para-functionalized with a phenyl group. We reasoned that the inclination of this aromatic group could potentially affect the flexibility of the neighboring carbocycles and, in turn, the steric protection of the metal center imparted by the N-aryl groups. Alternatively, the electronic properties of the para-phenyl group could influence the electropositivity of the active species as well as increase the solubility of the catalyst. To this end, we target five cobalt examples of G (Scheme 1) in which the steric and electronic properties of the two N-aryl groups are systematically varied. A thorough polymerization investigation is then undertaken using (G)CoCl2 that makes use of two different aluminoxane activators with the aim to identify trends in catalyst performance and to compare these findings with their non-phenyl substituted counterpart (E)CoCl2 [37]. Besides the polymerization study, full synthetic details for the cobalt complexes as well as the diketone precursor are presented.
2. Results
2.1. Synthesis and Characterization of Co1–Co5
The 4,6-bis(arylimino)-11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinoline-cobalt (II) chlorides, Co1 (aryl = 2,6-dimethylphenyl), Co2 (2,6-diethylphenyl), Co3 (2,6-diisopropylphenyl), Co4 (2,4,6-trimethylphenyl) and Co5 (2,6-diethyl-4-methylphenyl), were obtained in good yield (62–81%) via a one-pot route, whereby 11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinoline-4,6-dione (3) was treated with four equivalents of the corresponding aniline and CoCl2·6H2O in n-butanol at reflux with acetic acid utilized as catalyst ( Scheme 2). For Co1, a similar one-pot route was also conducted in acetic acid as solvent, though the yield of the isolated complex was lower (ca. 24%). All attempts to prepare the free 4,6-bis(arylimino)-11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinolines via the condensation of 3 with the corresponding aniline proved unsuccessful [24,26,39]. Complexes Co1–Co5 were characterized via microanalysis, FT-IR spectroscopy and, in the cases of Co1, Co2 and Co3, with single crystal X-ray diffraction.
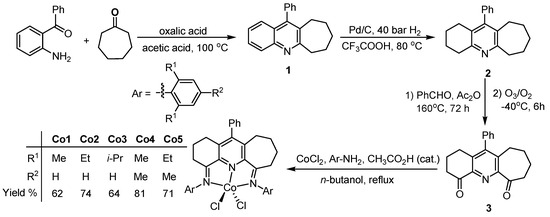
Scheme 2.
One-pot synthesis of Co1–Co5 including the stepwise route to diketone precursor 3.
The diketone itself, 3, was obtained using a multi-step procedure (Scheme 2). Initially, a Friedlander condensation reaction of 2-aminobenzophenone with cycloheptanone in acetic acid, in the presence of oxalic acid, gave 11-phenyl-6,7,8,9,10-pentahydrocyclohepta[b]quinoline (1) [40,41,42], which could then be hydrogenated with 10% Pd/C in trifluoroacetic acid to form 11-phenyl-1,2,3,4,6,7,8,9,10-nonahydrocyclohepta[b]quinoline (2) [43,44]. Compound 3 was then accessed in two steps, involving firstly a dehydration condensation reaction of 2 with benzaldehyde and then oxidation of the resulting intermediate with ozone/oxygen [43,45]. Compounds 1–3 were characterized using 1H NMR and 13C NMR spectroscopy (see experimental).
Single crystals of Co1, Co2 and Co3, suitable for the X-ray determinations, were grown via the slow diffusion of diethyl ether into dichloromethane solutions of the respective complex at ambient temperature. Views of Co1, Co2 and Co3 are depicted in Figure 1, Figure 2 and Figure 3, while selected bond distances and angles are given in Table 1.
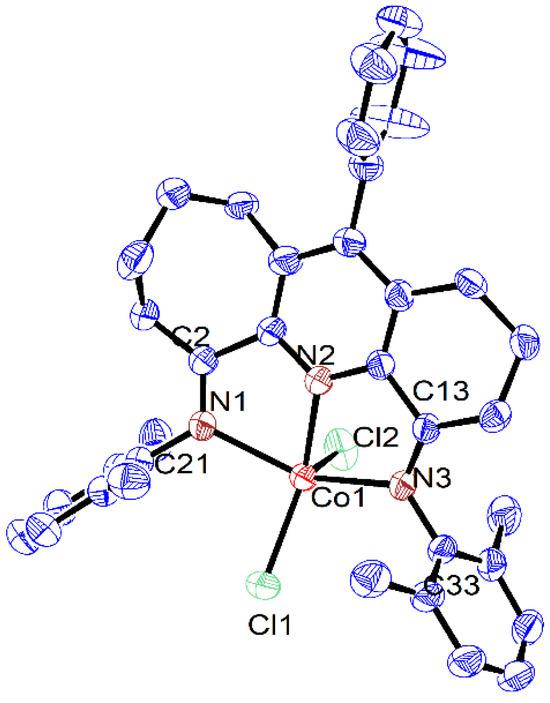
Figure 1.
ORTEP representation of Co1 with the thermal ellipsoids shown at the 50% probability level; all the hydrogen atoms have been omitted for clarity.
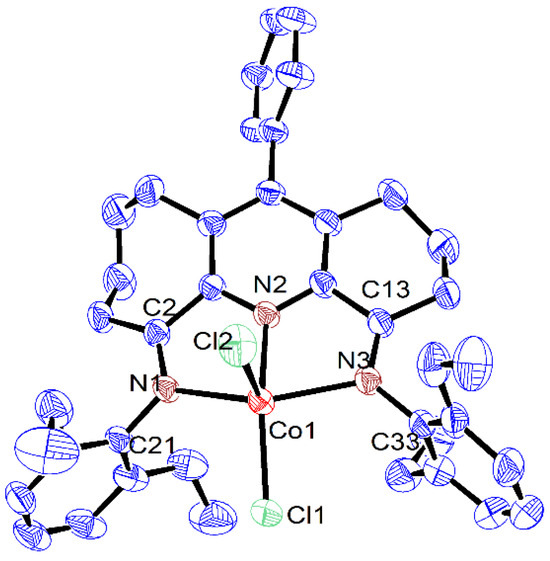
Figure 2.
ORTEP representation of Co2 with the thermal ellipsoids shown at the 50% probability level; all the hydrogen atoms and the dichloromethane have been omitted for clarity.
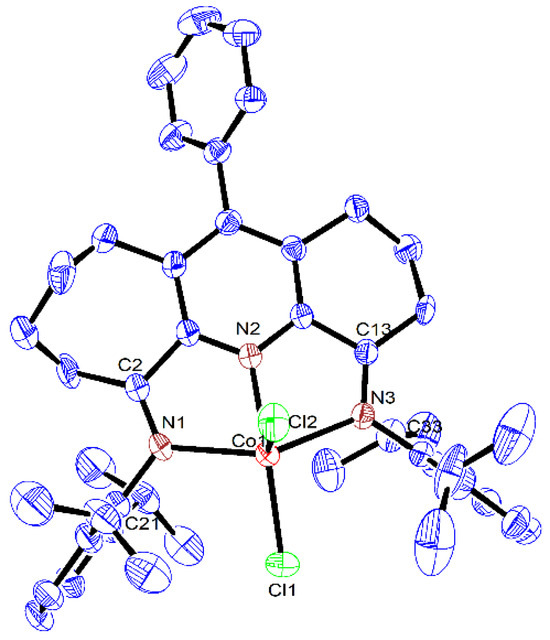
Figure 3.
ORTEP representation of Co3 with the thermal ellipsoids shown at the 50% probability level; all the hydrogen atoms have been omitted for clarity.

Table 1.
Selected bond lengths and angles for Co1, Co2 and Co3.
As has been noted previously [37], positional disorder can be a feature of mixed fused-ring complexes, and indeed in Co1 and Co3, the six- and seven-membered carbocyclic rings are disordered across both positions. Each structure was composed of a single cobalt center coordinated by a N,N,N’-coordinated 4,6-bis(arylimino)-11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinoline (aryl = 2,6-dimethylphenyl Co1, 2,6-diethylphenyl Co2, 2,6-diisopropylphenyl Co3) and two chloride ligands to complete a five-coordinate geometry that can be best viewed as distorted square-based pyramidal with three nitrogen atoms and Cl1 forming the base and Cl2 the apex. By using the tau value (where τ = 1 is an ideal trigonal bipyramid and τ = 0 an ideal square pyramidal), some degree of quantification of the distortion can be given [46]. For these three structures, τ = 0.10 (Co1), 0.11 (Co2) and 0.24 (Co3) (calculated using the equation (β-α)/60, where β and α are the two largest angles, β > α) which indicates some modest variation in the distortion from the idealized square-based pyramidal. The cobalt atom itself sits above the basal plane (defined by N1-N2-N3-Cl1) by 0.478 Å (Co1), 0.506 Å (Co2) and 0.593 Å (Co3), which resembles that seen in a number of their cobalt analogues [37]. Of the three cobalt–nitrogen distances, that involving the central pyridine (Co1-N2) displays the shortest bond length of 2.052(2) Å (Co1), 2.050(2) Å (Co2) and 2.062(2) Å (Co3) on account of the stronger coordination between Npy and the cobalt center. By comparison, the longer bond lengths involving the exterior nitrogen atoms show some dissimilarity with that involving N3 (6-membered ring) longer than for N1 (7-membered ring) for Co2 and Co3 (Co1-N3 2.221(2) Å (Co2), 2.221(2) Å (Co3) vs. Co1-N1 2.195(2) Å (Co2), 2.160(2) Å (Co3)), whereas the converse is true for Co1 (Co1-N3 2.183(2) Å vs. Co1-N1 2.202(2) Å (Co1). When the structure of Co3 is related to its non-phenyl substituted comparator (E2,6-iPr2Ph)CoCl2 (Scheme 1) [37], the variation in the Co-N3 and Co-N1 distances for the latter are considerably less pronounced, while the Co1-N2 distance is slightly shorter (2.040(1) vs. 2.062(2) Å Co3). Hence, it is evident the para-phenyl group on the central pyridine has some effect on the binding properties of the N,N,N’ chelating ligand in Co3. In terms of the N,N,N’ coordination plane in Co1–Co3, some minor deviation from coplanarity is observed between the pyridine and imine groups as is evidenced by the torsion angles of N1-C2-C1-N2 (4.604° Co1, 9.950° Co2, 4.616° Co3) and N2-C14-C13-N3 (8.022° Co1, 2.906° Co2, 5.692° Co3). As expected, the saturated sections of the six- and seven-membered carbocycles adopt puckered arrangements, with the latter showing the most flexibility. With respect to the para-phenyl group, there is some dissimilarity in the inclination of the phenyl group with respect to the adjacent pyridine plane (torsion angle: 79.8° Co1, 66.1° Co2, 54.8° Co3), which highlights the rotational flexibility of this unit. As is common, the N-aryl rings are inclined close to perpendicular with respect to the N,N,N coordination planes in all three cobalt structures [37]. There were no obvious intermolecular interactions in each structure.
In the IR spectra of Co1–Co5, characteristic absorption peaks for the imine double bonds are seen at around 1620 cm−1, which correspond to values of wavenumber typically seen for coordinated imines in related cobalt complexes [35,36,37,39]. In addition, their microanalytical data for all five complexes support compositions of the type LCoCl2.
2.2. Ethylene Polymerization Studies
In previous studies involving N,N,N-cobalt(II) halide complexes such as A–F (Scheme 1), high productivity for ethylene polymerization has been achieved when these precatalysts were pretreated with either MAO (methylaluminoxane) or MMAO (modified MAO) [8,9,18,34,35,36,37,38,47,48,49]. From a previous study [2], the Co3 precatalyst was the most steric hindered one in the set, which was thought of as a highly soluble and thermostable precatalyst that could obtain a higher molecular weight polymer. To magnify the advantage of the catalytic system in this work, Co3 was chosen to screen the polymerization conditions. Consequently, this work employs both of these aluminoxanes as part of two parallel studies concerned with exploring both their effectiveness as activators for Co1–Co5 and also their influence on the properties of the polymers produced. All runs were conducted in toluene in a stainless-steel reactor (PC2H4 = 5 or 10 atm) or Schlenk vessel (PC2H4 = 1 atm) with the ethylene pressure initially set at 10 atm.
2.2.1. Ethylene Polymerization Evaluation Using Co1–Co5 with MAO as Activator
With MAO initially employed as the aluminoxane, we set about determining an effective set of operating conditions for the polymerizations by using Co3 as our test precatalyst. As part of this preliminary optimization, we systematically examine the effects of the activator to precatalyst ratio (i.e., Al:Co molar ratio), run temperature, reaction time and the pressure of ethylene; the complete set of results are given in Table 2.

Table 2.
Ethylene polymerization evaluation using Co1–Co5 with MAO as activator a.
In view of the key role played by the aluminoxane activator, we first looked at determining the optimal Al:Co molar ratio using Co3/MAO. Accordingly, this was adjusted from 2500:1 to 5000:1 with the reaction temperature kept at 30 °C and the resulting level of catalytic activity monitored (entries 1–6, Table 2). The highest value of 4.18 × 106 g (PE) mol−1 (Co) h−1 was obtained with the ratio at 4000:1 (Table 2). Nonetheless, good activity was also realized with the Al:Co molar ratio at 2500:1 (3.57 × 106 g (PE) mol−1 (Co) h−1) (entry 1, Table 2) or 5000:1 (3.65 × 106 g (PE) mol−1 (Co) h−1) (entry 6, Table 2), reflecting the broad range in Al:Co molar ratios that can sustain high activity.
With regard to the polyethylenes generated using the different molar ratios, broad dispersities (Mw/Mn range: 10.3–11.6) were a feature with clear evidence of bimodality seen in their GPC traces (Figure 4). In terms of their molecular weights, these fell in the range 65.6 to 82.6 kg mol−1, with a ratio of 4500:1 yielding the highest molecular weight polymer. Interestingly, at ratios in excess of 4500:1, the molecular weight starts to decrease as chain transfer from the active cobalt species to MAO (or AlMe3) likely becomes more prominent [35].
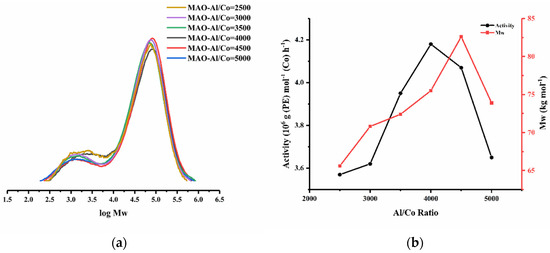
Figure 4.
(a) GPC traces for the polymers obtained using Co3/MAO at various Al:Co molar ratios and (b) plots of catalytic activity and polymer Mw as a function of the Al:Co molar ratio.
Next, with the Al:Co molar ratio maintained at 4000:1, the run temperature using Co3/MAO was varied between 20 °C and 60 °C (entries 4, and 7–10, Table 2). With the temperature at 30 °C, the highest activity of 4.18 × 106 g (PE) mol−1 (Co) h−1 was recorded, which compares to 40 °C seen for its non-phenyl containing comparator (E2,6-iPr2Ph)CoCl2 (Scheme 1). As the temperature increased beyond 30 °C, the activity steadily lowered, reaching a minimum of 1.75 × 106 g (PE) mol−1 (Co) h−1 at 60 °C. Such a decline in performance may be due to the lower solubility of ethylene at higher temperatures [50], while partial deactivation of the active species may provide another possible contributing factor [2]. As for the molecular weight of the polymer, this gradually descended as the temperature increased from 85.4 kg mol−1 at 20 °C to 32.7 kg mol−1 at 60 °C (Figure 5) on account of the greater rate of chain transfer occurring [35]. Moreover, the dispersity narrowed (Mw/Mn: from 12.3 to 5.7) as the temperature was raised, while the bimodal feature became less pronounced. Although uncertain, the broad dispersity could plausibly originate from the multiple active centers of Co3 [27], whereby the phenyl group could adopt either a more coplanar or a more orthogonal conformation with respect to the pyridine unit, leading to variations in the flexibility of the neighboring fused carbocycles and the inclination of the N-2,6-diisopropylphenyl group. In turn these structural differences could impact on the number of active species and/or chain transfer pathways (e.g., chain transfer to aluminum or β-H elimination to metal or monomer [51,52,53,54,55,56]). Based on this proposal, the higher temperature would conceivably drive the favored conformation of the phenyl group to give to a more uniform active species or promote a single chain transfer pathway.
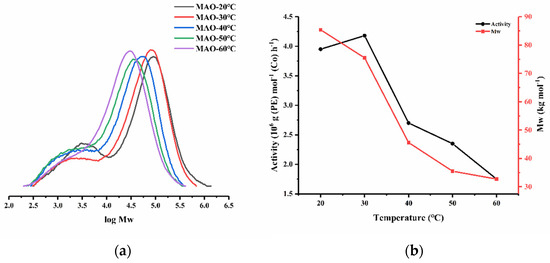
Figure 5.
(a) GPC traces for the polymers acquired using Co3/MAO at various temperatures and (b) plots of catalytic activity and polymer Mw as a function of reaction temperature.
To look into the performance of Co3/MAO over time, the polymerizations were performed at set times of 5, 15, 30, 45 and 60 min with the Al:Co molar ratio at 4000:1 and the temperature fixed at 30 °C (entries 4 and 11–14, Table 2). The maximum activity of 8.10 × 106 g (PE) mol−1 (Co) h−1 was achieved after a 5 min run time (entry 11, Table 2), which then reduced by more than three times after 1 h, culminating in a level of 2.44 × 106 g (PE) mol−1 (Co) h−1 (Figure 6). Evidently, the active species was rapidly generated following the addition of MAO and then was able to sustain a reasonably constant level of performance as time elapsed, a finding that reflects its good lifetime [35]. On the other hand, the molecular weight of the polymer remained essentially constant over time [Mw range: 75.5–78.4 kg mol−1].
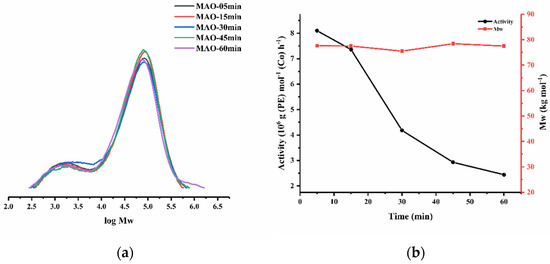
Figure 6.
(a) GPC traces for the polymers acquired using Co3/MAO at various reaction times and (b) plots of its catalytic activity and polymer Mw as a function of the reaction time.
To explore the influence of ethylene pressure on the performance of Co3/MAO, this parameter was initially lowered to 5 atm and then 1 atm with the conditions otherwise identical. At 5 atm, the catalytic activity decreased by approximately half while the molecular weight was slightly lowered (entry 16, Table 2). On the other hand, at 1 atm of ethylene pressure, the polymerization activity dropped more dramatically to 0.60 × 106 g (PE) mol−1 (Co) h−1 while the molecular weight was lowered to 56.3 kg mol−1 (entry 15, Table 2). Hence, it is apparent that higher ethylene pressure not only promotes higher molecular weight polymer but also higher activity, which can be attributed to the higher concentration of ethylene gas in the reaction system [37].
Finally, the remaining cobalt complexes, Co1, Co2, Co4 and Co5, were screened in combination with MAO using the optimized conditions established for Co1 (Al:Co molar ratio, 4000:1; temp., 30 °C; PC2H4, 10 atm) (entries 17–20, Table 2). Examination of the data reveals that all these precatalysts, including Co3, displayed high activity in the range 3.50 to 5.66 × 106 g (PE) mol−1 (Co) h−1, with the relative level being Co4 (2,4,6-trimethyl) > Co5 (2,6-diethyl-4-methyl) > Co1 (2,6-dimethyl) > Co3 (2,6-diisopropyl) > Co2 (2,6-diethyl) (Figure 7). Analysis of this order would suggest that both steric and electronic properties are influential. Notably, para-methyl Co4 and Co5 showed a higher activity than their para-H comparators, implying that the electron-donating properties of this substituent were beneficial; the improved solubility could also be a supporting factor [35,36,37]. Moreover, Co4 displayed a superior activity to Co5, indicating that the bulkier ortho groups have an unfavorable effect on catalytic activity [37].
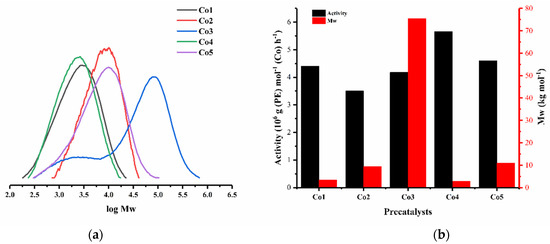
Figure 7.
(a) GPC traces for the polymers produced using Co1–Co5 with MAO as activator and (b) a bar chart showing their relative catalytic activities and polymer molecular weight.
With respect to the polymer properties, narrow dispersities were a feature of all the polymers generated using Co1, Co2, Co4 and Co5 (Mw/Mn range: 1.8–2.7), which implies good control. Moreover, the molecular weights of their polymers (Mw range: 2.9–10.9 kg mol−1) steadily increased in line with the greater steric properties of the N-aryl ortho-substituents. Indeed, the lower molecular weight polymers generated using Co1 and Co4 fall in the range expected for commercially important polyethylene waxes [37]. By contrast, the most hindered 2,6-diisopropyl counterpart, Co3, as mentioned earlier, displayed quite different behavior by producing a polymer exhibiting a broad distribution with bimodal characteristics (Mw/Mn = 10.3) and noticeably higher molecular weight (Mw, 75.5 kg mol−1). Certainly, the latter suggests that the bulky ortho-isopropyl groups more effectively restrict chain transfer and termination, leading to more effective chain propagation. Notably, when compared to its non-phenyl substituted analogue (E2,6-iPr2Ph)CoCl2 (Scheme 1, Mw, 22.75 kg mol−1; Mw/Mn, 1.9) [37], the molecular weight of the polymer produced using Co3 was significantly higher and the dispersity distinctly broader (Table 2). Though uncertain, when comparing the catalytic behavior of Co3 with the other cobalt precatalysts (Co1, Co2, Co4 and Co5), it appears that Co3 containing the most sterically hindered ortho-isopropyl groups is more significantly affected by the presence of potential rotamers [27,38].
To enable a comparison of the current cobalt catalysts with previously reported examples of carbocyclic-fused (D)CoCl2 and (E)CoCl2 (Scheme 1), Figure 8 collects together their activity, molecular weight and dispersity data and presents this alongside that obtained using (G)CoCl2 (Co3); the N-aryl group in each case is 2,6-diisopropylphenyl [34,35,36,37]. All polymerization catalysts were performed under comparable conditions using MAO as activator at PC2H4 as 10 atm.
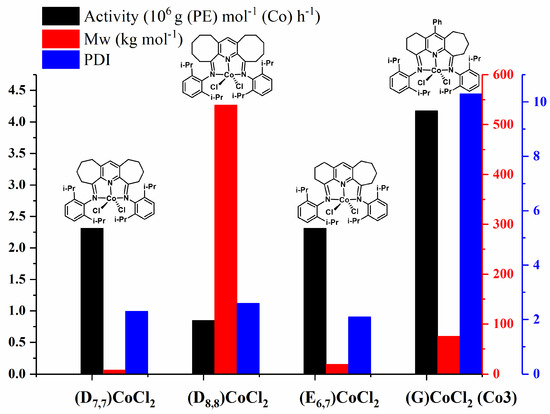
Figure 8.
Bar chart comparing catalytic activity and polymer molecular weight/dispersity found for (G)CoCl2 (Co3) with carbocyclic-fused (D7,7)CoCl2, (D8,8)CoCl2 and (E6,7)CoCl2 (Scheme 1, N-aryl = 2,6-i-Pr2C6H3); all runs were conducted with MAO, PC2H4 as 10 atm at 30 or 40 °C.
At first glance, Figure 8 shows that both the ring size of the fused carbocycle and the introduction of a phenyl group to the central pyridine unit can affect the catalytic performance of these cobalt catalysts and their polymer properties. A deeper analysis reveals several key points. First, the molecular weight of the resulting polymers with respect to the cobalt precatalyst declines in the following order: (D8,8)CoCl2 (n = 3, Scheme 1) > (G)CoCl2 (Co3) > (E6,7)CoCl2 > (D7,7)CoCl2 (n = 2, Scheme 1). This downward trend reflects the steric and flexibility variations of the fused carbocycles and the impact of the para-phenyl group incorporation on the relative rates of chain propagation and chain termination [39]. Second, the catalytic activity of this series follows the sequence (G)CoCl2 (Co3) > (E6,7)CoCl2 ~ (D7,7)CoCl2 (n = 2, Scheme 1) > (D8,8)CoCl2 (n = 3, Scheme 1), suggesting that the combination of the fused six- and seven-membered carbocycles in (G)CoCl2 (Co3) and (E6,7)CoCl2 provide the most suitable coordination environment to allow effective chain propagation. Furthermore, the presence of the para-phenyl group also positively affects the catalytic activity. It is worth mentioning that six-membered ring (D6,6)CoCl2 (n = 1, Scheme 1), though not displayed in the figure, was less selective and forms mixtures of low molecular weight polyethylene waxes and α-olefins [34]. In most cases, these fused cobalt precatalysts tend to deliver polymers with narrow polydispersity, with the exception of (G)CoCl2 (Co3) which produces unusually broad dispersity; an observation that we have tentatively attributed to the rotational isomerism of the para-phenyl group and knock-on effect on the number of active species (vide supra) [27,38]. Evidently, this observation caused by the appended para-phenyl warrants further investigation as it provides an unusual long-range means to influence polymer properties.
2.2.2. Ethylene Polymerization Evaluation Using Co1–Co5 with MMAO as Activator
With MMAO now employed in place of MAO, Co3 was again utilized as the test precatalyst so as to determine an optimized set of conditions that could be used to screen the remaining cobalt precatalysts. Once again, the optimization process involved varying the Al:Co molar ratio, run temperature, reaction time and ethylene pressure (Table 3).

Table 3.
Ethylene polymerization evaluation using Co1–Co5 with MMAO as activator a.
By running Co3/MMAO at 30 °C, the optimal Al:Co ratio was identified as 2500:1, leading to a highpoint in activity of 3.24 × 106 g (PE) mol−1 (Co) h−1 (entry 3 vs. entries 1,2,4,5, Table 3 and Figure S5), which was noticeably less than that seen with the Co3/MAO system (Al:Co as 4000:1), highlighting a benefit in terms of cost effectiveness of the reduced amount of activator. By comparison, Co3/MAO exhibited a maximum activity of 4.18 × 106 g (PE) mol−1 (Co) h−1 at a ratio of 4000:1, an observation that reflects the differences in the function of the particular activator. Similarly, at 2500:1, the highest molecular weight polymer of 72.9 kg mol−1 was achieved which compares favorably with that seen with Co3/MAO. Furthermore, as the ratio is further increased, chain transfer to aluminum starts to become more important, resulting in a perceptible drop in molecular weight. As seen with the Co3/MAO runs, the dispersity of the polymers was broad across all molar ratios examined (Mw/Mn range: 8.9–10.4), with a bimodal feature evident in all cases (Figure S5). As noted earlier, we suggest that the origin of this behavior stems from the potential multiple active centers of the catalyst.
Secondly, the temperature of the polymerization using Co3/MMAO was progressively increased from 20 °C to 60 °C with the Al:Co molar ratio fixed at 2500:1 (entries 3 and 6–9, Table 3). From the tabulated data, the optimal temperature was 20 °C, with the activity reaching its peak of 3.56 × 106 g (PE) mol−1 (Co) h−1, which compares to 30 °C for Co3/MAO, reflecting how the nature of the activator can impact on the thermal stability of the catalyst. On the other hand, at 60 °C, the level had declined to only 0.8 × 106 g (PE) mol−1 (Co) h−1 (Figure S6), as deactivation of the active species had become more important. As for the molecular weight of the polymer, this gradually decreased as temperature increased in accordance with the rate of chain termination becoming more prominent [35,37]. Furthermore, the dispersity narrowed, and the bimodality became less visible in a manner that resembles that seen with Co3/MAO. As mentioned earlier, we propose that the higher temperature promotes the favored conformation of the phenyl group, leading to a more uniform active species or to a single chain transfer pathway.
Variation in the run time from 5 min to 60 min, with the temperature kept at 20 °C and the Al:Co molar ratio at 2500:1 (entries 3 and 10–13, Table 3), identified the highest activity of 7.02 × 106 g (PE) mol−1 (Co) h−1 after 5 min. This activity seen after 5 min is notably less than that for Co3/MAO, highlighting how variations in the aluminoxane activator can impact on the generation of the active species. By 60 min, the activity had dropped to 1.85 × 106 g (PE) mol−1 (Co) h−1, in line with gradual deactivation of the active species following a its rapid generation (Figure S7). Inspection of the molecular weight of the polyethylene revealed this to steadily increase over time (from 66.5 kg mol−1 to 97.6 kg mol−1), while the dispersity remained broad with bimodal characteristics throughout (Mw/Mn range: 5.0–15.9).
The response of Co3/MMAO to variations in pressure was comparable with that seen for Co3/MAO, with a reduction in the ethylene pressure from 10 to 5 atm resulting in a slight decrease in both the molecular weight and activity (Mw from 89.3 kg mol−1 to 79.5 kg mol−1 and activity from 3.56 × 106 g (PE) mol−1 (Co) h−1 to 2.96 × 106 g (PE) mol−1 (Co) h−1). By comparison, when the pressure was reduced to 1 atm, the decline in activity was more dramatic (Mw to 66.2 kg mol−1 and activity to 0.53 × 106 g (PE) mol−1 (Co) h−1), highlighting the critical pressure required to provide good catalytic performance.
With an operative set of conditions identified using Co3/MMAO, these were then utilized to evaluate Co1, Co2, Co4 and Co5 (entries 16–19, Table 3). Examination of the resulting data reveals their values of activity to fall between 2.20 and 3.56 × 106 g (PE) mol−1 (Co) h−1), which is generally less than that for Co3/MAO, and indeed in a narrower range. Nonetheless, the relative level of performance is as follows: Co3 (2,6-diisopropyl) > Co5 (2,6-diethyl-4-methyl) > Co1 (2,6-dimethyl) > Co2 (2,6-diethyl) > Co4 (2,4,6-trimethyl) (Figure S8). Compared to the runs performed using MAO, the order was noticeably different, which we presume can be credited to differences in the aluminoxane activator, though the relatively narrow range in values of activity may be a contributing reason. Moreover, this sequence is distinct from what has been reported for related cobalt complexes, in which less steric hindrance generally drives higher activity [35,36,37].
Nevertheless, the most sterically hindered Co3/MMAO affords the highest molecular weight polyethylene (Mw = 89.3 kg mol−1) with the broadest dispersity, which agrees with that observed for Co3/MAO and supports the fact that the bulky isopropyl ortho-substituents inhibit chain transfer [34,37,57]. On the other hand, Co1, Co2, Co4 and Co5 display better control as is borne out by their narrow dispersities (Mw/Mn range: 2.5–3.3), while their molecular weights are considerably lower (Mw range: = 3.7–13.6 kg mol−1), with the lowest being for para-methyl containing Co1 and Co4 (Mw = 3.7–3.8 kg mol−1). Overall, the correlation between the polymer type and the precatalyst for the runs using MMAO mirrors that seen with MAO. Furthermore, it highlights the unexpected role played by the para-phenyl group, especially with bulkier N-aryl ortho-substituents whereby multi-site behavior becomes operational, leading to broad dispersities. Notably, the non-phenyl-containing analogue (E2,6-iPr2Ph)CoCl2 (Scheme 1) under MMAO activation forms lower molecular weight polyethylene (Mw with 19.38 × 106 g (PE) mol−1 (Co) h−1) and much narrower dispersity (Mw/Mn < 2.1) [37].
2.2.3. Microstructural Analysis of the Polyethylene
On scrutiny of Table 2 and Table 3, it is apparent that all polymers display melting points of 122 °C or above, which supports the formation of highly linear polyethylene [35]. To verify this claim and gather some information as to end-group composition, two representative samples prepared using Co4/MAO (Mw = 5.66 kg mol−1; entry 19, Table 2) and Co3/MMAO (Mw = 89.3 kg mol−1; entry 6, Table 3) were characterized with 1H and 13C NMR spectroscopy in 1,1,2,2-tetrachloroethane-d2 at 100 °C.
For the lower molecular weight sample generated using Co4/MAO (entry 19, Table 2), an intense single peak around δ 29.44 in the 13C NMR spectrum corresponds to the –(CH2CH2)n– repeat unit that confirms the linear nature of the polymer (Figure 9) [35,36,37]. The lower intensity peaks at δ 31.67 (d), 22.38 (e) and 13.74 (f) can be assigned to an n-propyl group, while the weak downfield peaks at δ 139.00 (b) and 113.85 (a) were characteristic of a terminal vinyl group [2,37,39].
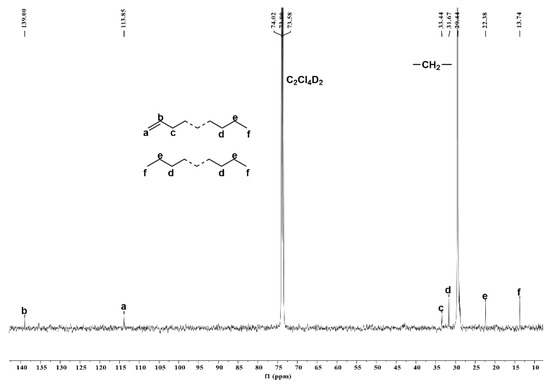
Figure 9.
13C NMR spectrum of the polyethylene obtained using Co4/MAO at a run temperature of 30 °C (entry 19, Table 2); recorded in 1,1,2,2-tetrachloroethane-d2 at 100 °C.
More support for the presence of a vinyl group is provided in the 1H NMR spectrum of this sample (Figure 10), which displays characteristic downfield multiplets around δ 5.86 (Hb) and 5.02 (Ha). Other key signals include the chain end methyl (Hf) that is visible most upfield at δ 0.98. Notably, from the integral ratio of Hb:Hf (1/7.95) (Figure 10), the molar ratio of unsaturated to fully saturated polyethylene can be calculated as 6/5 (see Supporting Information) [30,39,58]. On this basis, β-H transfer to the metal or monomer presents the main termination pathway when compared to chain transfer to aluminum (Scheme 3) [2,51,54].
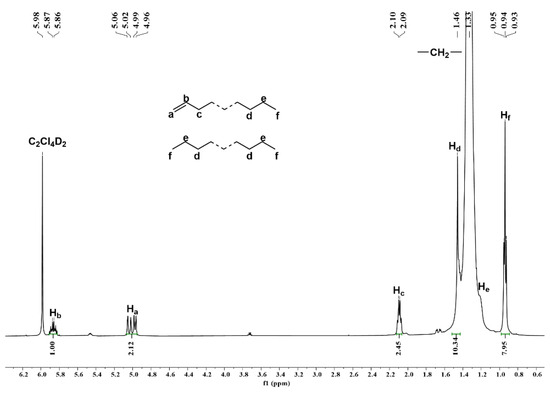
Figure 10.
1H NMR spectrum of the polyethylene obtained using Co4/MAO at a run temperature of 30 °C (entry 19, Table 2); recorded in 1,1,2,2-tetrachloroethane-d2 at 100 °C.
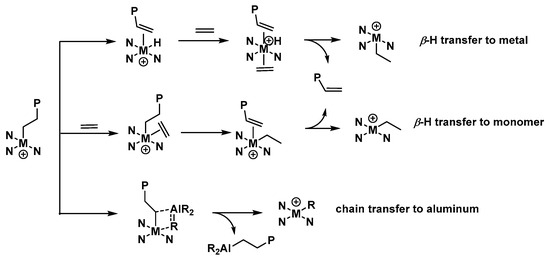
Scheme 3.
Potential pathways for the β-H transfer to metal or monomer and chain transfer to aluminum.
By comparison, for the higher molecular weight sample generated using Co3/MMAO (entry 6, Table 3), the 1H NMR spectrum similarly showed evidence for unsaturated and fully saturated polymers (Figure S10). However, the molar ratio of unsaturated (vinyl-end) to saturated polyethylene (propyl-end) was quite different, with a value of 4/5 determined (see Supporting Information). According to this finding, chain transfer to aluminum now forms the main pathway rather than the β-H transfer to the metal or the monomer. This finding once again reflects the significant function of an activator on chain transfer pathways.
3. Experimental Section
3.1. General Procedures
Manipulations that required air- and moisture- sensitive compounds were employed under an atmosphere of nitrogen using standard Schlenk techniques or using a nitrogen atmosphere glovebox. Toluene was heated to reflux for more than 12 h over sodium and distilled under a nitrogen atmosphere before use. Isobutyl-modified methylaluminoxane (MMAO, 2.46 M in n-heptane, containing 20–25% Al(i-Bu)3) and methylaluminoxane (MAO, 1.42 M in toluene) were purchased from Anhui Botai Electronic Materials Co., Chuzhou, China, while high purity ethylene was purchased from Beijing Yansan Petrochemical Co. (Beijing, China) and used as received. Other compounds were purchased from Acros (Geel, Belgium), Aldrich (Saint Louis, MO, USA) or Beijing Chemical Industry Group Co. (Beijing, China). The FT-IR spectra were recorded on a Perkin Elmer System 2000 FT-IR spectrometer (PerkinElmer Scientific, Waltham, MA, USA) at ambient temperature. Elemental analyses were performed on a Flash EA 1112 microanalyzer (Thermo Fisher Scientific, Waltham, MA, USA). Molecular weight (Mw) and dispersity (Mw/Mn) of the polyethylenes were recorded with an Agilent PLGPC 220GPC system (Agilent Technologies Inc., Santa Clara, CA, USA) operating at 150 °C using 1,2,4-trichlorobenzene as eluting solvent. Sample preparation involved dissolving the polyethylenes in 1,2,4-trichlorobenzene at ca. 170 °C for more than 8 h. A Perkin Elmer TA-Q2000 DSC (PerkinElmer Scientific, Waltham, MA, USA) was used to measure the melting points of the polymers under a nitrogen atmosphere. The general procedure was based on 5.0 mg of sample being heated to 160 °C at the rate of 20 °C min−1, then kept for 3 min at 160 °C and cooled to −20 °C at the rate of 20 °C min−1; this was again heated to 160 °C at the rate of 10 °C min−1. The 1H and 13C NMR spectra of the polyethylenes were recorded at 100 °C using a Bruker AVANVE 500 MHz instrument (Bruker Corporation, Billerica, MA, USA). Sample preparation: the polyethylene (about 30 mg) selected for high temperature 1H and 13C NMR spectroscopic measurements was dissolved in 1,1,2,2-tetrachloroethane-d2 (about 0.6 mL), containing TMS as the internal standard, at 120 °C for more than 2 h before the spectrum was recorded. 11-Phenyl-6,7,8,9,10-pentahydrocyclohepta[b]quinoline was prepared according to related procedures [40,41,42,59].
3.2. Synthesis of 11-phenyl-1,2,3,4,6,7,8,9,10-nonahydrocyclohepta[b]quinoline
This procedure for hydrogenation is based on that reported in the literature [37,43,44]. A stainless steel 250 mL autoclave, equipped with a magnetic stir bar, was charged with 11-phenyl-6,7,8,9,10-penthydrocyclohepta[b]quinoline (27.3 g, 0.10 mol), 10% Pd/C (2.7 g) and trifluoroacetic acid (100 mL). The autoclave was evacuated and refilled with hydrogen three times and then maintained at a hydrogen pressure of 40 bar while the reaction was stirred at 80 °C for 16 h. After cooling to ambient temperature, the hydrogen pressure was carefully released, and the reaction mixture filtered. The trifluoroacetic acid was removed using distillation under reduced pressure to give a light green oil. This oil was poured into a saturated aqueous sodium bicarbonate solution (500 mL) which, on vigorous stirring, produced a large amount of white precipitate. Solid sodium bicarbonate was then added, and the pH adjusted to 7. The mixture was then stirred for 1 h and filtered to afford the title compound as a white solid (26.8 g, 96%). 1H NMR (400 MHz, CDCl3): δ 7.42 (dd, J = 12.1, 7.3 Hz, 2H), 7.34 (t, J = 7.3 Hz, 1H), 7.09–7.02 (m, 2H), 3.06–3.01 (m, 1H), 2.92 (dd, J = 10.9, 6.0 Hz, 3H), 2.44–2.39 (m, 1H), 2.33–2.19 (m, 4H), 1.82 (td, J = 11.6, 6.1 Hz, 4H), 1.74–1.61 (m, 4H), 1.50–1.43 (m, 1H). 13C NMR (101 MHz, CDCl3): δ 160.32, 153.98, 148.76, 139.17, 133.05, 128.70, 128.50, 128.36, 128.02, 127.35, 127.07, 39.25, 32.96, 32.40, 30.16, 27.88, 27.80, 27.36, 26.79, 23.09.
3.3. Synthesis of 11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinoline-4,6-dione
Based on that previously reported [37,43,45], the title compound was prepared in two steps. In step 1, a mixture of 11-phenyl-1,2,3,4,6,7,8,9,10-nonahydrocyclohepta[b]quinoline (25.0 g, 0.0396 mol) and benzaldehyde (83.0 g, 0.792 mol) in acetic anhydride (71.0 g, 0.693 mol) was stirred at reflux under a nitrogen atmosphere for 72 h (monitored by TLC). After cooling to room temperature, residual acetic anhydride was removed via rotary evaporation. The benzaldehyde was then removed under reduced pressure following the addition of distilled water (100 mL) to give a viscous brown oil. Subsequently, methanol (250 mL) was added, and the mixture was heated to reflux for 0.5 h and then stirred at −30 °C overnight to afford, following filtration, a viscous solid. This crude product was taken up in methanol (100 mL) for 2 h and then filtered to give a yellow powder. Further treatment of this powder with methanol (200 mL) for 2 h followed by filtration gave the pure intermediate compound, 4,6-bis(benzylidene)-11-phenyl-1,2,3,4,6,7,8,9,10-nonahydrocyclohepta[b]quinoline, as a yellow powder (31.15 g, 85%).
In the second step, a quantity of 4,6-bis(benzylidene)-11-phenyl-1,2,3,4,6,7,8,9,10-nonahydrocyclohepta[b]quinoline (18.0 g, 39.6 mmol) was dissolved in dichloromethane (200 mL) and methanol (600 mL). The solution was treated with a mixture of dry ozone and oxygen at a temperature below −40 °C for 12 h until the solution became totally clear (monitored with TLC). Nitrogen was then bubbled through the mixture for 10 min to remove any residual ozone. Dimethyl sulfide (16 mL) was added, and the temperature of the mixture was slowly raised to room temperature and then left to stir overnight. All volatiles were removed under reduced pressure, affording a dark red oil. To remove the residual benzaldehyde, water (200 mL) was added, and then an azeotropic distillation using rotary evaporation performed and repeated (×8). The resulting yellow suspension was filtered to give the crude product which was recrystallized with ethyl acetate to form pure 11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinoline-4,6-dione as a pale-yellow solid (5.8 g, 48%). 1H NMR (400 MHz, CDCl3): δ 7.63–7.40 (m, 3H, aryl-H), 7.19–7.05 (m, 2H, aryl-H), 2.74 (d, J = 12.1 Hz, 4H), 2.64 (s, 2H), 2.58 (s, 2H), 2.04 (s,3H, methylene but ethyl acetate as impurity), 1.91–1.79 (m, 2H), 1.77–167 (m, 2H). 13C NMR (101 MHz, CDCl3): δ 205.78 (C=O), 196.01 (C=O), 156.13 (pyridine-C), 150.58 (pyridine-C), 147.10 (pyridine-C), 140.81 (pyridine-C), 136.11 (pyridine-C), 135.69 (Aryl-C), 129.20 (Aryl-C), 128.15 (Aryl-C), 40.14, 39.41, 28.16, 26.97, 25.16, 22.43, 21.10. FT-IR (cm−1): 2937 (m), 1739 (w), 1698 (s, νC=O), 1548 (m), 1492 (m), 1442 (m), 1403 (m), 1326 (m), 1218 (m), 1175 (m), 1118(m), 1046 (m), 972 (m), 931 (m), 848 (m), 779 (s), 724 (s), 705 (s), 669 (m).
3.4. Synthesis of 4,6-di(arylimino)-11-phenyl-1,2,3,7,8,9,10-heptahydrocycloghept[b]quinoline-cobalt(II) Chloride (Co1–Co5)
3.4.1. Aryl = 2,6-dimethylphenyl (Co1)
A suspension of 11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinoline-4,6-dione (0.153 g, 0.5 mmol), 2,4-dimethylaniline (0.242 g, 2.0 mmol) and CoCl2·6H2O (0.095 g, 0.4 mmol) in n-butanol (10 mL), containing several drops of glacial acetic acid (ca. 0.5 mL) as catalyst, was stirred and heated to reflux for about 6 h. On cooling to room temperature, the majority of the acetic acid and n-butanol were removed under reduced pressure. Dichloromethane (2 mL) was added to dissolve the residue, and then diethyl ether (30 mL) was introduced to induce precipitation. Following stirring for 10 min, the suspension was allowed to stand, and the supernatant solution was discarded. This process of diethyl ether (30 mL) addition, stirring and discarding of the solution was repeated two more times. The resulting solid was filtered and washed with diethyl ether (3 × 15 mL). The solid was dried at 60 °C to give Co1 as a brown powder (0.159 g, 62%). FT-IR (cm−1): 2941 (w), 2862 (w), 1671 (w), 1620 (m, νC=N), 1595 (w), 1564 (m), 1466 (s), 1442 (s), 1374 (m), 1326 (m), 1214 (s), 1164 (m), 1126 (m), 1093 (m), 950 (m), 916 (m), 847 (m), 768 (s), 705 (s). Elemental Analysis: Calcd for C36H37Cl2N3Co (641.55) C, 67.40, H, 5.81, N, 6.55. Found: C, 67.13, H, 6.11, N, 6.42%.
3.4.2. Aryl = 2,6-diethylphenyl (Co2)
By using the same method as described for Co1 but with 2,6-diethylaniline as the arylamine, Co2 was obtained as a brown powder (0.207 g, 74%). FT-IR (cm−1): 2964 (m), 2931 (m), 2872 (m), 1616 (m, νC=N), 1566 (m), 1447 (s), 1373 (m), 1330 (m), 1215 (s), 1074 (s), 951 (m), 855 (m), 808 (m), 774 (s), 701 (s). Elemental Analysis: Calcd for C40H45Cl2N3Co (697.65) C, 68.87, H, 6.50, N, 6.02. Found: C, 68.64, H, 6.71, N, 6.17%.
3.4.3. Aryl = 2,6-diisopropylphenyl (Co3)
By using the same method as described for Co1 but with 2,6-diisopropylaniline as the arylamine, Co3 was obtained as a brown powder (0.192 g, 64%). FT-IR (cm−1): 2962 (s), 2867 (m), 1616 (m, νC=N), 1561 (m), 1441 (s), 1383 (m), 1361 (m), 1325 (m), 1250 (s), 1214 (s), 1185 (m), 1051 (s), 943 (m), 853 (m), 804 (m), 773 (s), 707 (s). Elemental Analysis: Calcd for C44H53Cl2N3Co (753.76) C, 70.11, H, 7.09, N, 5.57. Found: C, 69.89, H, 7.31, N, 5.38%.
3.4.4. Aryl = 2,4,6-trimethylphenyl (Co4)
By using the same method as described for Co1 but with 2,4,6-trimethylaniline as the arylamine, Co4 was obtained as a brown powder (0.218 g, 81%). FT-IR (cm−1): 2916 (m), 2863 (m), 1620 (m, νC=N), 1565 (m), 1476 (s), 1442 (s), 1376 (m), 1325 (m), 1218 (s), 1152 (m), 1075 (m), 1031 (m), 952 (m), 852 (s), 779 (m), 705 (s). Elemental Analysis: Calcd for C38H41Cl2N3Co (669.60) C, 68.16, H, 6.17, N, 6.28. Found: C, 67.90, H, 6.52, N, 6.03%.
3.4.5. Aryl = 2,6-diethyl-4-methylphenyl (Co5)
By using the same method as described for Co1 but with 2,6-diethyl-4-methylaniline as the arylamine, Co5 was obtained as a brown powder (0.207 g, 71%). FT-IR (cm−1): 2964 (m), 2928 (m), 2870 (m), 1614 (m, νC=N), 1566 (m), 1457 (s), 1374 (m), 1332 (m), 1216 (s), 1150 (m), 1073 (m), 951 (m), 857 (s), 775 (m), 704 (s). Elemental Analysis: Calcd for C42H49Cl2N3Co (725.71) C, 69.51, H, 6.81, N, 5.79. Found: C, 69.27, H, 6.98, N, 5.40%.
3.5. Polymerization Study
3.5.1. Ethylene Polymerization at 5 or 10 atm Ethylene Pressure
The higher-pressure polymerization runs were carried out in a stainless-steel autoclave (0.25 L) equipped with a pressure control system, a mechanical stirrer and a temperature controller. The autoclave was first heated to remove any trace amounts of water. Then, a pre-determined amount of cobalt precatalyst was added and the vessel purged using two cycles of pressurization/venting with nitrogen and then pressurized to 3 atm of ethylene to check air tightness. After the required temperature was reached, the ethylene pressure was decreased to 1 atm and distilled toluene (2 × 25 mL) added with a syringe. The contents were then stirred for 5 min at 400 rpm to ensure dissolution of the precatalyst. The calculated amount of activator (MAO or MMAO) was then added via syringe, followed by additional toluene (2 × 25 mL) to take the total volume of solvent to 100 mL. The ethylene pressure was immediately pressurized to 5 or 10 atm and the mechanical stirring commenced. After the required reaction time, the autoclave was allowed to cool to room temperature and the pressure was slowly released. The contents were quenched with 10% hydrochloric acid in ethanol. The polymer was washed with ethanol, filtered under reduced pressure, dried at 100 °C and then finally weighed.
3.5.2. Ethylene Polymerization at 1 atm Ethylene Pressure
The polymerizations at 1 atm were carried out in a Schlenk vessel. The cobalt complex was added into the vessel and three cycles of pressurization/venting with nitrogen were applied before the vessel was left under an ethylene pressure of 1 atm. Toluene (30 mL) was injected to dissolve the precatalyst and the mixture was stirred and then warmed to the required temperature. The pre-determined amount of activator (MAO or MMAO) was immediately injected with a syringe and the run was commenced. After the required time, the supply of ethylene was stopped, and the vessel was allowed to cool to room temperature. Once the ethylene pressure was released, the mixture was quenched with 10% hydrochloric acid in ethanol. The polyethylene was collected by filtration and then dried at 100 °C before the weight of polymer was recorded.
3.6. X-ray Structure Determinations
Single-crystal X-ray diffraction studies of Co1, Co2 and Co3 were conducted using an XtaLAB SynergyR single-crystal diffractometer. Cell parameters were obtained by performing the global refinement of the positions of all collected reflections with monochromatic Cu-Kα radiation (λ = 1.54184 Å) at 170(2) K. The intensities were corrected for Lorentz and polarization effects and empirical absorption. The structures were solved using direct methods and refined with full-matrix least-squares on F2. Non-hydrogen atoms were refined anisotropically. All hydrogen atoms were placed in their calculated positions. Structure solution and refinement were carried out by using the Olex2 1.5 package and SHELXTL [60]. The SQUEEZE option of the crystallographic program PLATON was applied to remove free solvents from the structure of Co1 [61].
4. Conclusions
In summary, the novel diketone, 11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinoline-4,6-dione (3), was successfully synthesized in a multi-step procedure, and then employed in a one-pot method to form cobalt complexes, Co1–Co5. Besides their characterization via microanalysis and FT-IR spectroscopy, Co1, Co2 and Co3 were additionally the subject of single X-ray determinations. Upon activation with either MAO or MMAO, all cobalt complexes exhibited high activity (MAO > MMAO) for ethylene polymerization, generating strictly linear polymers, with Co4/MAO displaying the highest productivity of 5.66 × 106 g (PE) mol−1 (Co) h−1 at 30 °C. In terms of the polymer molecular weight, Co1, Co2, Co4 and Co5 had a predilection towards forming low molecular weight polyethylene with narrow dispersity, underlining the good control in place. Conversely, ortho-isopropyl Co3, under activation with either MAO or MMAO, displayed a preference for forming polyethylenes of not only higher molecular weight but also broad bimodal distributions. Moreover, this distinct behavior for Co3 is quite different from its non-phenyl substituted comparator (E2,6-Pr2Ph)CoCl2 (Scheme 1), where the molecular weight is lower and the dispersity significantly narrower [37]. Although uncertain, we believe this multi-site-like behavior displayed by Co3 derives from the para-phenyl group, which can affect the flexibility of the neighboring carbocycles and, in turn, the steric protection of the metal center imparted by the ortho-isopropyl substituents. However, it would appear that, for this effect to be operational, the steric properties of the ortho-substituents need to exceed that provided by methyl (Co1, Co4) and ethyl (Co2, Co5). This unexpected role of the remote para-phenyl group on influencing polymer properties will be the subject of a future investigation. As a final point, end-group analysis of representative polymer samples using 1H and 13C NMR spectroscopy highlights the competition of β-H transfer to the metal or the monomer with chain transfer to aluminum as termination pathways in these polymerizations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13101387/s1, Figure S1: 1H NMR spectrum of 11-phenyl-1,2,3,4,6,7,8,9,10-nonahydrocyclohepta[b]quinoline (2); recorded in CDCl3 (400 MHz); Figure S2: 13C NMR spectrum of 11-phenyl-1,2,3,4,6,7,8,9,10-nonahydrocyclohepta[b]quinoline (2); recorded in CDCl3 (400 MHz); Figure S3: 1H NMR spectrum of 11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinoline-4,6-dione (3); recorded in CDCl3 (400 MHz); Figure S4: 13C NMR spectrum of 11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinoline-4,6-dione (3); recorded in CDCl3 (400 MHz); Figure S5: (a) GPC traces for the polymers produced using Co3/MMAO at various Al:Co molar ratios and (b) plots of catalytic activity and Mw of the polymers as a function of the Al:Co molar ratio; Figure S6: (a) GPC traces for the polymers produced using Co3/MMAO at various temperatures and (b) plots of catalytic activity and Mw of the polymers as a function of reaction temperature; Figure S7: (a) GPC traces for the polymers produced using Co3/MMAO at various reaction times and (b) plots of catalytic activity and Mw of the polymers as a function of the reaction time; Figure S8: (a) GPC traces for the polymers produced using Co1–Co5 with MMAO as activator and (b) a bar chart comparing their catalytic activities and polyethylene molecular weight; Figure S9: 1H NMR spectrum of the polyethylene produced using Co3/MMAO at a run temperature of 20 °C (entry 6, Table 3); recorded in 1,1,2,2-tetrachloroethane-d2 at 100 °C; Table S1: Crystal data and structure refinement for Co1, Co2 and Co3; CheckCIF/PLATON report for Co1, Co2 and Co3.
Author Contributions
Conceptualization, W.-H.S.; methodology, Y.W. and Z.W.; software, Y.W., Y.M. and W.Z.; validation, Y.W., S.Z. and Q.Z; formal analysis, Y.W.; investigation, Y.W. and Z.W G.A.S.; resources, W.-H.S.; data curation, Y.W.; writing—original draft preparation, Y.W.; writing—review and editing, Y.W., G.A.S., Q.Z. and W.-H.S.; visualization, W.Z. and W.-H.S.; supervision, W.-H.S.; project administration, W.-H.S.; funding acquisition, W.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21871275).
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
Z.W. thanks the support from the Nature Science Foundation of Hebei Province (B2022204020) and Talent Introduction Foundation of Hebei Agricultural University (YJ201931). Research Project of Fundamental Scientific Research Business Expenses of Provincial Colleges and Universities in Hebei Province (KY2021028); Opening Fund of CAS Key Laboratory of Engineering Plastics, Institute of Chemistry. G.A.S. thanks the Chinese Academy of Sciences for a President's International Fellowship for Visiting Scientists.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bianchini, C.; Giambastiani, G.; Luconi, L.; Meli, A. Olefin oligomerization, homopolymerization and copolymerization by late transition metals supported by (imino)pyridine ligands. Coord. Chem. Rev. 2010, 254, 431–455. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Zhang, W.; Sun, W.-H. Carbocyclic-fused N,N,N-pincer ligands as ring-strain adjustable supports for iron and cobalt catalysts in ethylene oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef]
- Wang, Z.; Mahmood, Q.; Zhang, W.; Sun, W.-H. Recent progress on the tridentate iron complex catalysts for ethylene oligo-/polymerization. In Advances in Organometallic Chemistry; Pérez, P.J., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 79, pp. 41–86. [Google Scholar]
- Chirik, P.J. Carbon-Carbon Bond Formation in a Weak Ligand Field: Leveraging Open-Shell First-Row Transition-Metal Catalysts. Angew. Chem. Int. Ed. 2017, 56, 5170–5181. [Google Scholar] [CrossRef]
- Humphries, M.J.; Tellmann, K.P.; Gibson, V.C.; White, A.J.P.; Williams, D.J. Investigations into the Mechanism of Activation and Initiation of Ethylene Polymerization by Bis(imino)pyridine Cobalt Catalysts: Synthesis, Structures, and Deuterium Labeling Studies. Organometallics 2005, 24, 2039–2050. [Google Scholar] [CrossRef]
- Sun, W.-H. Novel Polyethylenes via Late Transition Metal Complex Pre-catalysts. In Polyolefins: 50 years after Ziegler and Natta II: Polyolefins by Metallocenes and Other Single-Site Catalysts; Kaminsky, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 163–178. [Google Scholar]
- Champouret, Y.; Hashmi, O.H.; Visseaux, M. Discrete iron-based complexes: Applications in homogeneous coordination-insertion polymerization catalysis. Coord. Chem. Rev. 2019, 390, 127–170. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly Active Iron and Cobalt Catalysts for the Polymerization of Ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; McTavish, S.J.; Solan, G.A.; White, A.J.P.; Williams, D.J.; Britovsek, G.J.P.; Kimberley, B.S.; Maddox, P.J. Novel olefin polymerization catalysts based on iron and cobalt. Chem. Commun. 1998, 849–850. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly Reactive Ligands and a Gateway to New Families of Catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef]
- Ma, J.; Feng, C.; Wang, S.L.; Zhao, K.Q.; Sun, W.-H.; Redshaw, C.; Solan, G.A. Bi- and tri-dentate imino-based iron and cobalt pre-catalysts for ethylene oligo-/polymerization. Inorg. Chem. Front. 2014, 1, 14–34. [Google Scholar] [CrossRef]
- Sun, W.-H.; Jie, S.; Zhang, S.; Zhang, W.; Song, Y.; Ma, H. Iron Complexes Bearing 2-Imino-1,10-phenanthrolinyl Ligands as Highly Active Catalysts for Ethylene Oligomerization. Organometallics 2006, 25, 666–677. [Google Scholar] [CrossRef]
- Jie, S.; Zhang, S.; Wedeking, K.; Zhang, W.; Ma, H.; Lu, X.; Deng, Y.; Sun, W.-H. Cobalt(II) complexes bearing 2-imino-1,10-phenanthroline ligands: Synthesis, characterization and ethylene oligomerization. C. R. Chim. 2006, 9, 1500–1509. [Google Scholar] [CrossRef]
- Pelletier, J.D.A.; Champouret, Y.D.M.; Cadarso, J.; Clowes, L.; Gañete, M.; Singh, K.; Thanarajasingham, V.; Solan, G.A. Electronically variable imino-phenanthrolinyl-cobalt complexes; synthesis, structures and ethylene oligomerisation studies. J. Organomet. Chem. 2006, 691, 4114–4123. [Google Scholar] [CrossRef]
- Jie, S.; Zhang, S.; Sun, W.-H.; Kuang, X.; Liu, T.; Guo, J. Iron(II) complexes ligated by 2-imino-1,10-phenanthrolines: Preparation and catalytic behavior toward ethylene oligomerization. J. Mol. Catal. A Chem. 2007, 269, 85–96. [Google Scholar] [CrossRef]
- Jie, S.; Zhang, S.; Sun, W.-H. 2-Arylimino-9-phenyl-1,10-phenanthrolinyl-iron, -cobalt and -nickel Complexes: Synthesis, Characterization and Ethylene Oligomerization Behavior. Eur. J. Inorg. Chem. 2007, 2007, 5584–5598. [Google Scholar] [CrossRef]
- Sun, W.-H.; Kong, S.; Chai, W.; Shiono, T.; Redshaw, C.; Hu, X.; Guo, C.; Hao, X. 2-(1-(Arylimino)ethyl)-8-arylimino-5,6,7-trihydroquinolylcobalt dichloride: Synthesis and polyethylene wax formation. Appl. Catal. A-Gen. 2012, 447–448, 67–73. [Google Scholar] [CrossRef]
- Zhang, W.; Chai, W.; Sun, W.-H.; Hu, X.; Redshaw, C.; Hao, X. 2-(1-(Arylimino)ethyl)-8-arylimino-5,6,7-trihydroquinoline Iron(II) Chloride Complexes: Synthesis, Characterization, and Ethylene Polymerization Behavior. Organometallics 2012, 31, 5039–5048. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Baugh, S.P.D.; Hoarau, O.; Gibson, V.C.; Wass, D.F.; White, A.J.P.; Williams, D.J. The role of bulky substituents in the polymerization of ethylene using late transition metal catalysts: A comparative study of nickel and iron catalyst systems. Inorg. Chim. Acta 2003, 345, 279–291. [Google Scholar] [CrossRef]
- Zabel, D.; Schubert, A.; Wolmershäuser, G.; Jones, R.L.; Thiel, W.R. Iron and Cobalt Complexes of Tridentate N-Donor Ligands in Ethylene Polymerization: Efficient Shielding of the Active Sites by Simple Phenyl Groups. Eur. J. Inorg. Chem. 2008, 2008, 3648–3654. [Google Scholar] [CrossRef]
- Abu-Surrah, A.S.; Lappalainen, K.; Repo, T.; Piironen, U.; Leskela, M. New bis(imino)pyridine-iron(II)- and cobalt(II)-based catalysts: Synthesis, characterization and activity towards polymerization of ethylene. J. Organomet. Chem. 2002, 648, 55–61. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Liang, T.; Redshaw, C.; Li, Y.; Sun, W.-H. Synthesis, characterization and catalytic behavior toward ethylene of 2-[1-(4,6-dimethyl-2-benzhydrylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridylmetal (iron or cobalt) chlorides. Dalton Trans. 2013, 42, 9188–9197. [Google Scholar] [CrossRef]
- Yu, J.; Huang, W.; Wang, L.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-Dibenzhydryl-4-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridylcobalt(II) dichlorides: Synthesis, characterization and ethylene polymerization behavior. Dalton Trans. 2011, 40, 10209–10214. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhao, W.; Cao, X.-P.; Liang, T.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-dibenzhydryl-4-chlorophenylimino)ethyl]-6-[1-aryliminoethyl]pyridyl cobalt dichlorides: Synthesis, characterization and ethylene polymerization behavior. J. Organomet. Chem. 2012, 713, 209–216. [Google Scholar] [CrossRef]
- Mahmood, Q.; Ma, Y.; Hao, X.; Sun, W.-H. Substantially enhancing the catalytic performance of bis(imino)pyridylcobaltous chloride pre-catalysts adorned with benzhydryl and nitro groups for ethylene polymerization. Appl. Organomet. Chem. 2019, 33, e4857. [Google Scholar] [CrossRef]
- Bariashir, C.; Zhang, R.; Vignesh, A.; Ma, Y.; Liang, T.; Sun, W.-H. Enhancing Ethylene Polymerization of NNN-Cobalt(II) Precatalysts Adorned with a Fluoro-substituent. ACS Omega 2021, 6, 4448–4460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Z.; Han, M.; Xiang, J.; Solan, G.A.; Ma, Y.; Liang, T.; Sun, W.-H. Fluorinated cobalt catalysts and their use in forming narrowly dispersed polyethylene waxes of high linearity and incorporating vinyl functionality. Catal. Sci. Technol. 2021, 11, 656–670. [Google Scholar] [CrossRef]
- Liu, T.; Liu, M.; Ma, Y.; Solan, G.A.; Liang, T.; Sun, W.-H. Cobalt Catalysts Bearing ortho-(4,4′-Dichlorobenzhydryl) Substituents and Their Use in Generating Narrowly Dispersed Polyethylene of High Linearity. Eur. J. Inorg. Chem. 2022, 2022, e202200396. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, S.; Ma, Y.; Solan, G.A.; Sun, Y.; Sun, W.-H. CF3O-Functionalized Bis(arylimino)pyridine–Cobalt Ethylene Polymerization Catalysts: Harnessing Solvent Effects on Performance and Polymer Properties. Organometallics 2022, 41, 3237–3248. [Google Scholar] [CrossRef]
- Han, M.; Oleynik, I.I.; Liu, M.; Ma, Y.; Oleynik, I.V.; Solan, G.A.; Liang, T.; Sun, W.-H. Ring size enlargement in an ortho -cycloalkyl-substituted bis(imino)pyridine-cobalt ethylene polymerization catalyst and its impact on performance and polymer properties. Appl. Organomet. Chem. 2021, 36, e6529. [Google Scholar] [CrossRef]
- Ba, J.; Du, S.; Yue, E.; Hu, X.; Flisak, Z.; Sun, W.-H. Constrained formation of 2-(1-(arylimino)ethyl)-7-arylimino-6,6-dimethylcyclopentapyridines and their cobalt(II) chloride complexes: Synthesis, characterization and ethylene polymerization. RSC Adv. 2015, 5, 32720–32729. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Yue, E.; Liang, T.; Hu, X.; Sun, W.-H. Controlling the molecular weights of polyethylene waxes using the highly active precatalysts of 2-(1-aryliminoethyl)-9-arylimino-5,6,7,8-tetrahydrocycloheptapyridylcobalt chlorides: Synthesis, characterization, and catalytic behavior. Dalton Trans. 2016, 45, 657–666. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Zhang, W.; Oleynik, I.I.; Vignesh, A.; Oleynik, I.V.; Hu, X.; Sun, Y.; Sun, W.-H. Highly Linear Polyethylenes Achieved Using Thermo-Stable and Efficient Cobalt Precatalysts Bearing Carbocyclic-Fused NNN-Pincer Ligand. Molecules 2019, 24, 1176. [Google Scholar] [CrossRef] [PubMed]
- Appukuttan, V.K.; Liu, Y.; Son, B.C.; Ha, C.-S.; Suh, H.; Kim, I. Iron and Cobalt Complexes of 2,3,7,8-Tetrahydroacridine-4,5(1H,6H)-diimine Sterically Modulated by Substituted Aryl Rings for the Selective Oligomerization to Polymerization of Ethylene. Organometallics 2011, 30, 2285–2294. [Google Scholar] [CrossRef]
- Du, S.; Zhang, W.; Yue, E.; Huang, F.; Liang, T.; Sun, W.-H. α,α′-Bis(arylimino)-2,3:5,6-bis(pentamethylene)pyridylcobalt Chlorides: Synthesis, Characterization, and Ethylene Polymerization Behavior. Eur. J. Inorg. Chem. 2016, 2016, 1748–1755. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Mahmood, Q.; Liu, Q.; Ma, Y.; Hao, X.; Sun, W.-H. Bis(imino)pyridines Incorporating Doubly Fused Eight-Membered Rings as Conformationally Flexible Supports for Cobalt Ethylene Polymerization Catalysts. Organometallics 2018, 37, 380–389. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Guo, J.; Liu, Q.; Solan, G.A.; Liang, T.; Sun, W.-H. Bis(imino)pyridines fused with 6- and 7-membered carbocylic rings as N,N,N-scaffolds for cobalt ethylene polymerization catalysts. Dalton Trans. 2019, 48, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Cámpora, J.; Naz, A.M.; Palma, P.; Rodríguez-Delgado, A.; Álvarez, E.; Tritto, I.; Boggioni, L. Iron and Cobalt Complexes of 4-Alkyl-2,6-diiminopyridine Ligands: Synthesis and Ethylene Polymerization Catalysis. Eur. J. Inorg. Chem. 2008, 2008, 1871–1879. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, N.; Xiang, J.; Solan, G.A.; Suo, H.; Ma, Y.; Liang, T.; Sun, W.-H. Bis-cycloheptyl-fused bis(imino)pyridine-cobalt catalysts for PE wax formation: Positive effects of fluoride substitution on catalytic performance and thermal stability. Dalton Trans. 2020, 49, 9425–9437. [Google Scholar] [CrossRef]
- Rajendran, S.; Sivalingam, K.; Karnam Jayarampillai, R.P.; Wang, W.L.; Salas, C.O. Friedländer's synthesis of quinolines as a pivotal step in the development of bioactive heterocyclic derivatives in the current era of medicinal chemistry. Chem. Biol. Drug. Des. 2022, 100, 1042–1085. [Google Scholar] [CrossRef]
- Dabiri, M.; Baghbanzadeh, M.; Nikcheh, M.S. Oxalic Acid: An Efficient and Cost-Effective Organic Catalyst for the Friedländer Quinoline Synthesis under Solvent-Free Conditions. Monatsh. Chem. 2007, 138, 1249–1252. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Q.; Ma, N.; Liu, S.; Han, M.; Yan, X.; Liu, Q.; Solan, G.A.; Sun, W.-H. Direct synthesis of ring-fused quinolines and pyridines catalyzed by NNHY-ligated manganese complexes (Y = NR2 or SR). Catal. Sci. Technol. 2021, 11, 8026–8036. [Google Scholar] [CrossRef]
- Bell, T.W.; Khasanov, A.B.; Drew, M.G.B. Role of Pyridine Hydrogen-Bonding Sites in Recognition of Basic Amino Acid Side Chains. J. Am. Chem. Soc. 2002, 124, 14092–14103. [Google Scholar] [CrossRef] [PubMed]
- Vierhapper, F.W.; Eliel, E.L. Selective Hydrogenation of Quinoline and Its Homologs, Isoquinoline, and Phenyl-Substituted Pyridines in the Benzene Ring. J. Org. Chem. 1975, 40, 2729–2734. [Google Scholar] [CrossRef]
- Thummel, R.P.; Lefoulon, F.; Cantu, D.; Mahadevan, R. Polyaza Cavity-Shaped Molecules. Annelated Derivatives of 2- (2'-Py ridyl)-1,8-naphthyridine and 2,2'-Bi-1,8-naphthyridine. J. Org. Chem. 1984, 49, 2208–2212. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Seitz, M.; Milius, W.; Alt, H.G. Iron(II) coordination compounds with ω-alkenyl substituted bis(imino)pyridine ligands: Self-immobilizing catalysts for the polymerization of ethylene. J. Mol. Catal. A Chem. 2007, 261, 246–253. [Google Scholar] [CrossRef]
- Pelascini, F.; Wesolek, M.; Peruch, F.; Lutz, P.J. Modified Pyridine-Bis(imine) Iron and Cobalt Complexes: Synthesis, Structure, and Ethylene Polymerization Study. Eur. J. Inorg. Chem. 2006, 2006, 4309–4316. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Sun, Y.; Hu, X.; Solan, G.A.; Sun, W.-H. Thermally stable and highly active cobalt precatalysts for vinyl-polyethylenes with narrow polydispersities: Integrating fused-ring and imino-carbon protection into ligand design. New J. Chem. 2016, 40, 8012–8023. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Q.; Rempel, G.L. Solubility of ethylene in toluene and toluene/styrene–butadiene rubber solutions. J. Appl. Polym. Sci. 2005, 96, 645–649. [Google Scholar] [CrossRef]
- Gibson, V.C.; Solan, G.A. Olefin Oligomerizations and Polymerizations Catalyzed by Iron and Cobalt Complexes Bearing Bis(imino)pyridine Ligands. In Catalysis without Precious Metals; Bullock, R.M., Ed.; Wiley-VCH: Weinheim, Germany, 2010; pp. 111–141. [Google Scholar]
- Gibson, V.C.; Solan, G.A. Iron-Based and Cobalt-Based Olefin Polymerisation Catalysts. In Metal Catalysts in Olefin Polymerization; Guan, Z., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 107–158. [Google Scholar]
- Small, B.L.; Brookhart, M. Iron-Based Catalysts with Exceptionally High Activities and Selectivities for Oligomerization of Ethylene to Linear α-Olefins. J. Am. Chem. Soc. 1998, 120, 7143–7144. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Mastroianni, S.; Solan, G.A.; Baugh, S.P.D.; Redshaw, C.; Gibson, V.C.; White, A.J.P.; Williams, D.J.; Elsegood, M.R.J. Oligomerisation of Ethylene by Bis(imino)pyridyliron and -cobalt Complexes. Chem. Eur. J. 2000, 6, 2221–2231. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.; Jordan, R.F. (α-Diimine)nickel Complexes That Contain Menthyl Substituents: Synthesis, Conformational Behavior, and Olefin Polymerization Catalysis. Organometallics 2017, 36, 2784–2799. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Bruce, M.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; Mastroianni, S.; McTavish, S.J.; Redshaw, C.; Solan, G.A.; Stromberg, S.; et al. Iron and Cobalt Ethylene Polymerization Catalysts Bearing 2,6-Bis(Imino)Pyridyl Ligands: Synthesis, Structures, and Polymerization Studies. J. Am. Chem. Soc. 1999, 121, 8728–8740. [Google Scholar] [CrossRef]
- Zhang, Q.; Zuo, Z.; Ma, Y.; Liang, T.; Yang, X.; Sun, W.-H. Fluorinated 2,6-bis(arylimino)pyridyl iron complexes targeting bimodal dispersive polyethylenes: Probing chain termination pathways via a combined experimental and DFT study. Dalton Trans. 2022, 51, 8290–8302. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Liu, B.; Yue, E.; Liu, Q.; Yang, X.; Wang, Z.; Sun, W.-H. A Ruthenium Catalyst with Unprecedented Effectiveness for the Coupling Cyclization of γ-Amino Alcohols and Secondary Alcohols. ACS Catal. 2016, 6, 1247–1253. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).