Abstract
The control of stereo-, regio-, and chemo-selectivity in transition-metal-catalyzed coupling reactions is a key topic in organic synthesis. Several methods for controlling selectivity have been reported thus far. On the other hand, the reduction of catalyst loading during reactions is one of the most important issues in organic synthesis from the standpoint of green sustainable chemistry. As another advantage of reducing catalyst loading, the expression of reaction selectivity and the substituent effect caused by the reduction of catalyst loading to the parts-per-million (ppm) level in various catalytic reactions is presented herein.
1. Introduction
The control of stereo-, regio-, and chemo-selectivity in transition-metal-catalyzed coupling reactions is a key topic in organic synthesis. Several methods for controlling selectivity have been reported thus far. For example, stereoselectivity has been controlled using chiral ligands in most cases [,,,,]. Regarding the control in regioselectivity [,,,,,,,,], Yudin et al. reported the control of regioselectivity in allylic amination with and without the base []. Catalyst-dependent regioselective reactions have also been reported; Fairlamb et al. achieved site-selective cross-coupling of 2,4-dibromopyridine using specific types of catalysts, such as mononuclear, clusters, or nanoparticles []. Regioselective reactions of ligands have been the most extensively studied, and it has been reported that the existence or type of ligand affects regioselectivity [,,,,,]. With regard to chemoselectivity [,,,,,,,], Newman et al. achieved the selective cross-coupling reaction of phenyl esters with alkyl boranes by controlling decarbonylation with the ligand []. In addition, the selectivity control of the Ni-catalyzed Ullmann coupling reaction with and without Pd [], the control of Suzuki and Buchwald–Hartwig coupling reactions using aryl halides [], and the control of Hiyama and Ullmann coupling reactions using the valence of Pd nanoparticles [] have been reported to date. This study reports the expression of selectivity by reducing the Pd content to the parts-per-million (ppm) level.
Typically, catalytic activity increases with decreasing catalyst concentration in reactions that are catalyzed by molecular catalysts, in situ-generated metal nanoparticles, and metal nanoparticles, which act as a reservoir of an active form of the catalyst [,,,,,,,], probably because the aggregation of metal species is more likely to occur by increasing the metal content in the reaction solution. Therefore, it is possible to select the reaction field in the solution and on the surface of the nanoparticles by changing the metal content. That is, in the case of a high amount of Pd, Pd easily aggregates and the reaction occurs on the surface of the Pd aggregates. In contrast, the reaction occurs in the solution phase in the case of a low amount of Pd. Indeed, we observed the expression of substrate-dependent selectivity at the mol ppm level of Pd loading in allylic arylation (Scheme 1) []. In this paper, it is proposed that the reaction with the blanch-type substrate proceeds in solution phase, and the linear-type substrate reacts on the surface of the nanoparticle.
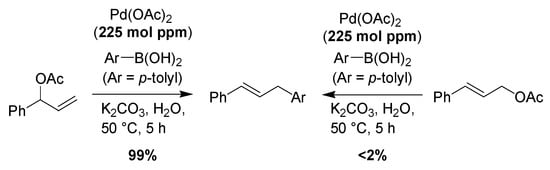
Scheme 1.
The substrate-dependent selective allylic arylation at the mol ppm level of Pd loading.
2. Results and Discussion
Based on the above-mentioned assumption, we first investigated chemoselectivity in the reaction of aryl halide. When 15 μmol of Pd(OAc)2 (3.0 mol% relative to aryl bromide) was heated in ethylene glycol in the presence of NaOH at 100 °C for 5 h, and subsequently 5-bromo-1,3-benzodioxole was added and heated at 100 °C for 5 h, the Ullmann coupling reaction occurred to give 5,5′-bi-1,3-benzodioxole (1) in an 83% yield. Even in the presence of dibenzo[a,e]cyclooctene (DCT, 6.0 mol% relative to aryl bromide), 1 was obtained in an 88% yield, indicating that the Ullmann coupling reaction proceeded on the surface of the Pd aggregates (Crabtree test). In contrast, hydrodebromination afforded 1,3-benzodioxole (2) after heating aryl bromide in the presence of 50 mol ppm of Pd(OAc)2 (Scheme 2). No 2 was obtained in the presence of DCT, indicating that hydrodebromination proceeded in the solution phase.
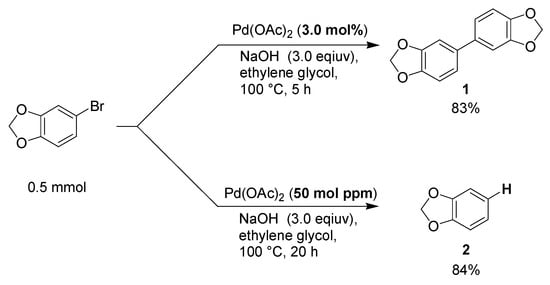
Scheme 2.
Chemoselectivity by catalyst loading.
On the other hand, the lower the catalyst concentration used, the slower the reaction rate, because the reaction rate depended on the collision frequency. That is, a subtle difference in the reaction rate was expected to become more observable by decreasing the catalyst concentration, thereby allowing the expression of selectivity in the reaction. To confirm the aforementioned scenario, the Suzuki coupling reaction, using bromobenzene or phenyl trifluoromethanesulfonate, was selected as a model reaction, because it is well known that the reactivity of these substrates are similar []. When the Suzuki coupling reaction was performed in the presence of 2.0 mol% of Pd(OAc)2 and PCy3 (1.2 equivalent relative to Pd) in THF at 50 °C for 24 h [], quantitative formations of the coupling product were achieved in both cases. In contrast, when Pd loading was decreased to 150 mol ppm, the coupling reaction occurred smoothly only in the case of bromobenzene, indicating that reaction selectivity can be controlled by changing the catalyst concentration (Scheme 3a). This result was derived from the difference in the rate of transmetalation step, because the oxidative addition of aryl triflate to Pd(0) species is faster than that of aryl bromide []. Indeed, the coupling reaction with 4-methylphenylboronic acid took place smoothly in the case of p-dibromobenzene to give 4,4″-dimethyl-1,1′:4′,1″-terphenyl (4) in an 85% yield. In contrast, only 4′-methylbiphenyl-4-yl triflate (3c) was obtained in an 11% yield in the case of 4-bromophenyl trifluoromethanesulfonate (Scheme 3b), suggesting that the transmetalation did not proceed after the oxidative addition in the C–OTf bond occurred preferentially. Furthermore, when the reaction of 4-bromophenyl trifluoromethanesulfonate with 4-methylphenylboronic acid was performed using 2.0 mol% of Pd(OAc)2 and PCy3 (1.2 equivalent relative to Pd) in THF at 50 °C for 24 h, 3c and 4 were obtained in 91% and 4% yields, respectively, whereas at 60 °C, the yields were 75% and 25%, respectively. These results are consistent with the assumption that the transmetalation of the Pd–OTf bond is difficult to facilitate.
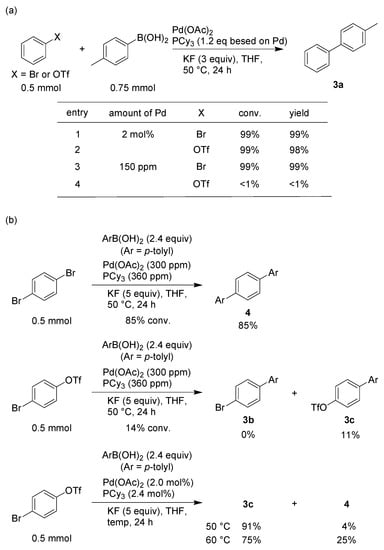
Scheme 3.
Expression of substrate-dependent selectivity at the ppm level of Pd. (a) Difference in the reactivity between aryl bromide and aryl triflate at ppm level of Pd. (b) Selective synthesis of terphenyl.
These data prompted us to consider that important information about the reaction mechanism could be obtained by decreasing the catalyst concentration. To confirm the above-mentioned assumption, the Suzuki coupling reaction in aqueous KOH solution was selected as a model reaction because this reaction occurs smoothly using mol ppm Pd content. When 1.0 mol% of Pd(OAc)2 was used in the Suzuki coupling reaction of several aryl bromides with arylboronic acids, quantitative yields of the products were obtained, even when a substituent was introduced to either the aryl bromide or the arylboronic acid (Table 1, entries 1–5). No substituent effect of aryl bromide was observed, even at 40 mol ppm of Pd(OAc)2 (entries 6–8). In contrast, at 40 mol ppm of Pd(OAc)2, the reaction did not proceed smoothly using the arylboronic acid with an electron-withdrawing group; however, the coupling product was obtained in a quantitative yield via reaction with arylboronic acids with electron-donating groups at 40 mol ppm (entries 6–10), which is consistent with the fact that transmetalation with arylboronic acid is the rate-determining step in the Suzuki coupling reaction of aryl bromides []. When the competitive reaction was performed using 4-methoxyphenylboronic acid and 4-(trifluoromethyl)phenylboronic acid, 4-methoxybiphenyl (3f) and 4-(trifluoromethyl)biphenyl (3g) were obtained in 99% and 46% yields, respectively. In the reaction of 4-bromotoluene with 4-(trifluoromethyl)phenylboronic acid to give 4-methyl-4′-(trifluoromethyl)biphenyl (3e) in a 15% yield, 85% of 4-bromotoluene was recovered and no other by-product was observed. These results are consistent with the assumption that transmetalation is the rate-determining step. Furthermore, little effect of steric hindrance was observed. Indeed, at 40 mol ppm of Pd(OAc)2, quantitative yields of the products were obtained even when a methyl group was introduced at ortho-position to either the aryl bromide or the arylboronic acid. Interestingly, when the Pd concentration was reduced to 10 mol ppm, the substituent effect of the aryl bromide was also observed (entries 11–13). In contrast, 4-methoxy-4′-methylbiphenyl (3d) and 3e were obtained in 44% and 94% yields, respectively, from the competitive reaction using 4-bromoanisole and 4-bromobenzotrifluoride as the substrate. Although the reason why the reaction of 4-bromobenzotrifluoride proceeded smoothly is unclear, the results of non-competitive and competitive experiments, as in Schmidt’s paper [], are thought to reflect the rate-determining step and the rate of oxidative addition (which is the first step in the reaction), respectively. 4-Methoxy-4′-(trifluoromethyl)biphenyl was obtained in a 29% yield from the reaction of 4-bromobenzotrifluoride with 4-methoxyphenylboronic acid using 10 mol ppm of Pd(OAc)2, suggesting that the impurities in 4-bromobenzotrifluoride did not retard the reaction. In addition, this result is consistent with the assumption that the non-competitive experiment reflects the rate-determining step. The observed substituent effect of aryl bromides at 10 mol ppm of Pd(OAc)2 may have originated from the rate of the transmetalation step or the reductive elimination step [,]. These results suggested that the change in the rate-determining step depending on the substrate combination could be confirmed by reducing catalyst loading.

Table 1.
The substituent effect in the Suzuki coupling reaction at different Pd amounts 1.
A similar trend was observed for the Hiyama coupling reaction in propylene glycol []. When 1.0 mol% of Pd(OAc)2 was used for the Hiyama coupling reaction of several aryl bromides with aryltrimethoxysilanes in propylene glycol, the reaction proceeded smoothly, although the yields varied slightly (Table 2, entries 1–5). In contrast, at 100 mol ppm of Pd(OAc)2, the reaction hardly proceeded using 4-fluorophenyltrimethoxysilane, although the coupling product was obtained in high yield by the reaction with 4-methoxyphenyltrimethoxysilane (entries 9 and 10). This result is consistent with the general trend that transmetalation with arylsilane is the rate-determining step in the Hiyama coupling reaction of aryl bromides [,]. At 10 mol ppm of Pd(OAc)2, the Hiyama coupling reaction of 4-bromobenzotrifluoride did not proceed smoothly (entries 11–13). In the competitive reaction using 4-bromotoluene and 4-bromobenzotrifluoride, 3a and 3g were obtained in 6% and 24% yields, respectively. As in the Suzuki coupling reaction, opposite tendencies were observed between competitive and non-competitive reactions. Because the transmetalation of arylsilanolate with arylpalladium species bearing electron-withdrawing groups is faster than that with arylpalladium species bearing electron-donating groups [], the observed difference may have originated from the rate of the reductive elimination step to produce the coupling product []. In allylic arylation, although similar yields were obtained at the 100 mol ppm level of Pd, the substituent effect of arylboronic acid became observable at 40 mol ppm of Pd loading (Table 3).

Table 2.
The substituent effect in the Hiyama coupling reaction in propylene glycol at different Pd amounts 1.

Table 3.
The substituent effect in allylic arylation at low Pd loading 1.
3. Materials and Methods
3.1. General Comments
All chemicals were commercially available and used without further purification unless otherwise mentioned. 1H NMR spectra in CDCl3 were recorded using a JEOL ECZ400s spectrometer (JEOL, Tokyo, Japan). Chemical shifts in 1H NMR are reported in δ ppm referenced to an internal tetramethylsilane standard (δ = 0).
3.2. Procedure for the Selective Reaction of 5-Bromo-1,3-Benzodioxole
5-Bromo-1,3-benzodioxole (100 mg, 0.5 mmol), NaOH (60 mg, 1.5 mmol), ethylene glycol (1 mL), and a THF solution of Pd(OAc)2 (1.0 mM, 25 μL, 50 mol ppm) were added to a screw-capped vial (No. 02, Maruemu Co., Osaka, Japan) with a stirring bar. After stirring at 100 °C for 20 h, the reaction mixture was extracted eight times using diethyl ether. The organic extract was dried over MgSO4 and concentrated under reduced pressure. The crude material was analyzed using 1H NMR, and the yield of 1,3-benzodioxole (2) was calculated to be 84% (Figure S2).
3.3. Procedure for the Substrate-Dependent Suzuki Coupling Reaction
Bromobenzene (87.5 mg, 0.5 mmol), 4-methylphenylboronic acid (102 mg, 0.75 mmol), KF (87.2 mg, 1.5 mmol), THF (1 mL), and a THF solution of Pd(OAc)2 and PCy3 (Pd: 1.0 mM, 75 μL, 150 mol ppm; phosphine: 1.2 mM, 75 μL, 180 mol ppm) were added to a screw-capped vial (No. 02, Maruemu Co., Osaka, Japan) with a stirring bar. After stirring at 50 °C for 24 h, the reaction mixture was extracted eight times using chloroform. The organic extract was dried over MgSO4 and concentrated under reduced pressure. The crude material was analyzed using 1H NMR, and the yield of 4,4″-dimethyl-1,1′:4′,1″-terphenyl (4) was calculated to be 85% (Figure S4).
3.4. General Procedure for Suzuki Coupling Reaction at Mol Ppm Level of Pd Loading
Bromobenzene (78 mg, 0.5 mmol), 4-methylphenylboronic acid (102 mg, 0.75 mmol), TBAB (161 mg, 0.5 mmol), aqueous KOH solution (1.5 M, 1 mL), and a THF solution of Pd(OAc)2 (0.5 mM, 20 μL, 20 mol ppm) were added to a screw-capped vial (No. 02, Maruemu Co., Osaka, Japan) with a stirring bar. After stirring at 90 °C for 1 h, the reaction mixture was extracted eight times using diethyl ether. The organic extract was dried over MgSO4 and concentrated under reduced pressure. The crude material was analyzed using 1H NMR, and the yield of 4-methylbiphenyl (3a) was calculated to be 90% (Figure S3).
3.5. General Procedure for Hiyama Coupling Reaction at the Mol Ppm Level of Pd Loading
4-Bromotoluene (85 mg, 0.5 mmol), trimethoxyphenylsilane (149 mg, 0.75 mmol), KF (87 mg, 1.5 mmol), propylene glycol (1 mL), and a THF solution of Pd(OAc)2 (0.5 mM, 20 μL, 20 mol ppm) were added to a screw-capped vial (No. 02, Maruemu Co., Osaka, Japan) with a stirring bar. After stirring at 100 °C for 1 h, the reaction mixture was extracted eight times using diethyl ether. The organic extract was dried over MgSO4 and concentrated under reduced pressure. The crude material was analyzed using 1H NMR, and the yield of 4-methylbiphenyl (3a) was calculated to be 88%.
3.6. General Procedure for Allylic Arylaton at the Mol Ppm Level of Pd Loading
α-Vinylbenzyl acetate (0.088 g, 0.5 mmol), 4-methylphenylboronic acid (102 mg, 0.75 mmol), aqueous K2CO3 solution (1.5 M, 1 mL), and a THF solution of Pd(OAc)2 (1.0 mM, 50 μL, 100 mol ppm) were added to a screw-capped vial (No. 02, Maruemu Co., Osaka, Japan) with a stirring bar. After stirring at 70 °C for 5 h, the reaction mixture was extracted eight times using diethyl ether. The organic extract was dried over MgSO4 and concentrated under reduced pressure. The crude material was analyzed using 1H NMR, and the yield of (E)-3-(4-methylphenyl)-1-phenylpropene (4a) was calculated to be 66% (Figure S10).
3.7. Compounds Data
5,5′-bi-1,3-benzodioxole (1) [CAS: 4791-89-3]: 1H NMR (CDCl3) δ = 6.96–6.99 (m, 4 H), 6.85 (d, J = 7.6 Hz, 2 H), 5.99 (s, 4 H); 13C NMR (CDCl3) δ 148.1, 146.8, 135.4, 120.4, 108.6, 107.6, 101.2.
1,3-benzodioxole (2) [CAS: 274-09-9]: 1H NMR (CDCl3) δ 6.86–6.81 (m, 4 H), 5.95 (s, 2 H); 13C NMR (CDCl3) δ 147.5, 121.7, 108.8, 100.7, 31.1.
4-Methylbiphenyl (3a) [CAS: 644-08-6]: 1H NMR (CDCl3) δ 7.60–7.55 (m, 2 H), 7.50–7.47 (m, 2 H), 7.43–7.39 (m, 2 H), 7.33–7.29 (m, 1 H), 7.25–7.20 (m, 2 H), 2.38 (s, 3 H); 13C NMR (CDCl3) δ 141.1, 138.3, 136.9, 129.4, 128.7, 128.7, 126.9, 21.1.
4,4″-dimethyl-1,1′:4′,1″-terphenyl (4) [CAS: 97295-31-3]: 1H NMR (CDCl3) δ 7.64 (s, 2 H), 7.53 (d, J = 7.6 Hz, 2 H), 7.26 (d, J = 8.8 Hz, 3 H), 2.39 (s, 3 H); 13C NMR (CDCl3) δ 137.9, 137.2, 134.0, 129.6, 127.4, 127.0, 21.3.
4-Methoxybiphenyl (3f) [CAS: 613-37-6]: 1H NMR (CDCl3) δ 7.58-7.51 (m, 4 H), 7.42 (t, J = 7.6 Hz, 2 H), 7.31 (t, J = 7.6 Hz, 1 H), 6.98 (d, J = 8.7 Hz, 2 H), 3.85 (s, 3 H); 13C NMR (CDCl3) δ 159.1, 140.7, 133.7, 128.6, 128.0, 126.6, 126.6, 114.2, 55.3.
4-Trifluoromethylbiphenyl (3g) [CAS: 398-36-7]: 1H NMR (CDCl3) δ 7.76–7.68 (m, 4 H), 7.68–7.58 (m, 2 H), 7.51–7.38 (m, 3 H); 13C NMR (CDCl3) δ 144.7, 139.7, 129.3 (q, J = 32.3 Hz), 129.0, 128.2, 127.6, 127.4, 127.3, 125.6 (q, J = 3.2 Hz), 124.3 (q, J = 272.1 Hz).
4-Methoxy-4′-methylbiphenyl (3d) [CAS: 53040-92-9]: 1H NMR (CDCl3) δ 7.51 (d, J = 9.0 Hz, 2 H), 7.45 (d, J = 8.1 Hz, 2 H), 7.22 (d, J = 8.1 Hz, 2 H), 6.96 (d, J = 9.0 Hz, 2 H), 3.84 (s, 3 H), 2.38 (s, 3 H); 13C NMR (CDCl3) δ 158.9, 137.9, 136.3, 133.7, 129.4, 127.9, 126.6, 114.2, 55.3, 21.0.
4-Fluoro-4′-methylbiphenyl (3h) [CAS: 72093-43-7]: 1H NMR (CDCl3) δ 7.62–7.53 (m, 4 H), 7.51–7.42 (m, 2H), 7.37–7.33 (m, 1 H), 7.16–7.10(m, 2 H); 13C NMR (CDCl3) δ 163.8, 161.3, 140.3, 137.4 (d, J = 3.9 Hz), 128.9, 128.8 (d, J = 8.7 Hz), 127.4, 127.1, 115.9, 115.7 (d, J = 28.8 Hz).
4-Methyl-4′-(trifluoromethyl)biphenyl (3e) [CAS: 97067-18-0]: 1H NMR (CDCl3) δ 7.67 (m, 4 H), 7.50 (d, J = 8.4 Hz, 2 H), 7.28 (d, J = 8.4 Hz, 2 H), 2.41 (s, 3 H); 13C NMR (CDCl3) δ 144.6, 138.1, 136.8, 129.7, 129.2 (q, J = 32.3 Hz), 127.2, 127.1, 125.6 (q, J = 4.2 Hz), 124.3 (q, J = 271.7 Hz), 21.1.
(E)-3-(4-methylphenyl)-1-phenylpropene (4a) [CAS: 134539-86-9]: 1H NMR (CDCl3) δ 7.13–7.37 (m, 9 H), 6.45 (dt, J = 15.2 Hz, 1 H), 6.34 (dt, J = 16 Hz, 1 H) 3.51 (d, J = 6.4 Hz, 2 H), 2.33 (s, 3 H); 13C NMR (CDCl3) δ 137.6, 137.1, 135.8, 130.9, 129.6, 129.3, 128.7, 128.6, 127.2, 126.2, 39.1, 21.2.
(E)-3-(4-methoxyphenyl)-1-phenylpropene (4d) [CAS: 35856-80-5]: 1H NMR (CDCl3) δ 7.35 (d, J = 7.6 Hz, 2 H), 7.29 (t, J = 12.8 Hz, 2 H), 7.22–7.19 (m, 1 H), 7.16 (d, J = 8.8 Hz, 2 H), 6.86 (d, J = 8.8 Hz, 2 H), 6.43 (d, J = 15.6 Hz, 1 H), 6.34 (ddt, J = 6.4, 9.2, 12.8 Hz, 1 H), 3.80 (s, 3 H), 3.49 (d, J = 6.4 Hz, 2 H); 13C NMR (CDCl3) δ 158.1, 137.6, 132.3, 130.8, 129.8, 129.7, 128.6, 127.2, 126.2, 114.0, 55.4, 38.6.
(E)-1-phenyl-3-(4-trifluoromethylphenyl)propene (4e) [CAS: 723341-12-6]: 1H NMR (CDCl3) δ 7.56 (d, J = 8.4 Hz, 2 H), 7.36 (d, J = 8.4 Hz, 2 H), 7.31 (d, J = 7.2 Hz, 2 H), 7.31 (t, J = 6.8 Hz, 2 H), (t, J = 6.8 Hz, 2 H), 6.45 (d, J = 16 Hz, 1 H), 6.32 (tt, J = 13.6 Hz, 1H), 3.60 (d, J = 6.8 Hz, 2 H); 13C NMR (CDCl3) δ 144.4, 137.2, 132.0, 129.1, 128.7, 128.5, 128.0, 127.5, 126.3, 125.8, 125.5 (q, J = 15.2 Hz), 39.2.
(E)-1-(4-methoxyphenyl)-3-(4-methylphenyl)propene (4b) [CAS:183621-28-5]: 1H NMR (CDCl3) δ 7.28 (d, J = 8.8 Hz, 2 H), 7.13 (s, 4 H), 6.83 (d, J = 8.8 Hz, 2 H), 6.34 (d, J = 15.6 Hz, 1 H), 6.23–6.16 (m, 1 H), 3.79 (s, 3 H), 3.48 (d, J = 6.8 Hz, 2 H), 2.33 (s, 3 H); 13C NMR (CDCl3) δ 158.8, 137.4, 135.7, 130.4, 130.2, 129.3, 128.7, 127.4, 127.3, 114.0, 55.4, 39.0, 21.2.
(E)-1-(4-methoxyphenyl)-3-(4-methylphenyl)propene (4c) [CAS: 1669332-26-6]: 1H NMR (CDCl3) δ 7.54 (d, J = 8.0 Hz, 1 H), 7.44 (d, J = 8.0 Hz, 1 H), 7.14 (s, 2 H), 6.46 (t, J = 3.6 Hz, 1 H), 3.54 (d, J = 4.4 Hz, 1 H), 2.34 (s, 2 H); 13C NMR (CDCl3) δ 141.0, 136.5, 136.0, 132.5, 129.6, 129.4, 128.7, 126.9, 126.3, 125.5 (q, J = 15.2 Hz), 39.0, 21.2.
4. Conclusions
In summary, the expressions of several selectivities in various catalytic reactions were confirmed by reducing catalyst concentrations. In the reaction catalyzed by in situ-generated Pd nanoparticles, the catalytic reaction on the Pd surface and in the solution phase could be controlled by changing catalyst loading. A slight difference in the reaction rate was confirmed by the decrease in catalyst concentration. Moreover, the substituent effect was confirmed using the Pd catalyst at the mol ppm level, and the rate-determining step in the catalytic reaction could be predicted. That is, this study’s results indicate that control of reaction selectivity or confirmation of the reaction mechanism could be achieved simply by reducing the catalyst concentration. Therefore, reducing the catalyst concentration is crucial not only in terms of environmental aspects and industrial applications, but also for synthetic chemistry [,]. This investigation regarding other catalytic reactions is now in progress to extend the versatility of this concept.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13071115/s1, Figures S1–14: 1H and 13C spectra of products.
Author Contributions
Conceptualization, M.K. and A.O.; investigation, M.K., T.S., S.M. and M.Y.; writing—original draft preparation, M.K.; writing—review and editing, T.S., S.M., M.Y., Y.M., O.S. and A.O.; supervision, A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI (No. 22K05201).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, H.-J.; Liu, K.; Yu, J.; Zhang, L.; Gong, L.-Z. Switchable Stereoselectivity in Bromoaminocyclization of Olefins Catalyzed by Brønsted Acids of Anionic Chiral CoIII Complexes. Angew. Chem. Int. Ed. 2017, 56, 11931–11935. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Sakaguchi, S. Inversions in Asymmetric Conjugate Addition Reaction of Cyclic Enones Catalyzed by the Cu/NHC-AgX System: Factors Affecting the Stereoselective Formation of Both Enantiomers. J. Organomet. Chem. 2017, 846, 407–416. [Google Scholar] [CrossRef]
- Fu, S.; Chen, N.-Y.; Liu, X.; Shao, Z.; Luo, S.-P.; Liu, Q. Ligand-Controlled Cobalt-Catalyzed Transfer Hydrogenation of Alkynes: Stereodivergent Synthesis of Z- and E-Alkenes. J. Am. Chem. Soc. 2016, 138, 8588–8594. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xie, F.; Ma, Z.; Liu, Y.; Zhang, W. Switchable Stereoselectivity: The Effects of Substituents on the D2-Symmetric Biphenyl Backbone of Phosphoramidites in Copper-Catalyzed Asymmetric Conjugate Addition Reactions with Triethylaluminum. Adv. Synth. Catal. 2012, 354, 1941–1947. [Google Scholar] [CrossRef]
- Chen, G.; Gui, J.; Li, L.; Liao, J. Chiral Sulfoxide-Olefin Ligands: Completely Switchable Stereoselectivity in Rhodium-Catalyzed Asymmetric Conjugate Additions. Angew. Chem. Int. Ed. 2011, 50, 7681–7685. [Google Scholar] [CrossRef]
- Scott, N.W.J.; Ford, M.J.; Jeddi, N.; Eyles, A.; Simon, L.; Whitwood, A.C.; Tanner, T.; Willans, C.E.; Fairlamb, I.J.S. A Dichotomy in Cross-Coupling Site Selectivity in a Dihalogenated Heteroarene: Influence of Mononuclear Pd, Pd Clusters, and Pd Nanoparticles-the Case for Exploiting Pd Catalyst Speciation. J. Am. Chem. Soc. 2021, 143, 9682–9693. [Google Scholar] [CrossRef]
- Wu, F.; Lu, S.; Zhu, S. Regioselectivity-Switchable Intramolecular Hydroarylation of Ynone. Adv. Synth. Catal. 2020, 362, 5632–5636. [Google Scholar] [CrossRef]
- Yang, X.-H.; Davison, R.T.; Nie, S.-Z.; Cruz, F.A.; McGinnis, T.M.; Dong, V.M. Catalytic Hydrothiolation: Counterion-Controlled Regioselectivity. J. Am. Chem. Soc. 2019, 141, 3006–3013. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, H.; Fu, Y. Mechanistic Study on Ligand-Controlled Cobalt-Catalyzed Regioselectivity-Switchable Hydroarylation of Styrenes. Chem. Eur. J. 2013, 19, 12093–12103. [Google Scholar] [CrossRef]
- Bedford, R.B.; Durrant, S.J.; Montgomery, M. Catalyst-Switchable Regiocontrol in the Direct Arylation of Remote C-H Groups in Pyrazolo [1,5-a]pyrimidines. Angew. Chem. Int. Ed. 2015, 54, 8787–8790. [Google Scholar] [CrossRef]
- Dai, X.; Chen, Y.; Garrell, S.; Liu, H.; Zhang, L.-K.; Palani, A.; Hughes, G.; Nargund, R. Ligand-Dependent Site-Selective Suzuki Cross-Coupling of 3,5-Dichloropyridazines. J. Org. Chem. 2013, 78, 7758–7763. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Yoshikai, N. Regioselectivity-Switchable Hydroarylation of Styrenes. J. Am. Chem. Soc. 2011, 133, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Dubovyk, I.; Watson, I.D.G.; Yudin, A.K. Chasing the Proton Culprit from Palladium-Catalyzed Allylic Amination. J. Am. Chem. Soc. 2007, 129, 14172–14173. [Google Scholar] [CrossRef]
- Miller, K.M.; Jamison, T.F. Ligand-Switchable Directing Effects of Tethered Alkenes in Nickel-Catalyzed Additions to Alkynes. J. Am. Chem. Soc. 2004, 126, 15342–15343. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.; Xu, S.; Ding, K. Switchable Cobalt-Catalyzed α-Olefination and α-Alkylation of Nitriles with Primary Alcohols. Org. Lett. 2021, 23, 5028–5032. [Google Scholar] [CrossRef]
- Masson-Makdissi, J.; Vandavasi, J.K.; Newman, S.G. Switchable Selectivity in the Pd-Catalyzed Alkylative Cross-Coupling of Esters. Org. Lett. 2018, 20, 4094–4098. [Google Scholar] [CrossRef]
- Zhu, B.; Yan, L.-K.; Yao, L.-S.; Ren, H.; Li, R.-H.; Guan, W.; Su, Z.-M. Orthogonal Reactivity of Ni(I)/Pd(0) Dual Catalysts for Ullmann C-C Cross-Coupling: Theoretical Insight. Chem. Commun. 2018, 54, 7959–7962. [Google Scholar] [CrossRef]
- Dhital, R.N.; Sen, A.; Sato, T.; Hu, H.; Ishii, R.; Hashizume, D.; Takaya, H.; Uozumi, Y.; Yamada, Y.M.A. Activator-Promoted Aryl Halide-Dependent Chemoselective Buchwald-Hartwig and Suzuki-Miyaura Type Cross-Coupling Reactions. Org. Lett. 2020, 22, 4797–4801. [Google Scholar] [CrossRef]
- Ohtaka, A.; Kotera, T.; Sakon, A.; Ueda, K.; Hamasaka, G.; Uozumi, Y.; Shinagawa, T.; Shimomura, O.; Nomura, R. Fluoride-Free Hiyama Coupling Reaction Catalyzed by Linear Polystyrene-Stabilized PdO Nanoparticles in Water: Specific Reactivity of PdO Nanoparticles over Pd Nanoparticles. Synlett 2016, 27, 1202–1206. [Google Scholar] [CrossRef]
- Hong, X.; Liang, Y.; Houk, K.N. Mechanisms and Origins of Switchable Chemoselectivity of Ni-Catalyzed C(aryl)-O and C(acyl)-O Activation of Aryl Esters with Phosphine Ligands. J. Am. Chem. Soc. 2014, 136, 2017–2025. [Google Scholar] [CrossRef]
- So, C.M.; Yuen, O.Y.; Ng, S.S.; Chen, Z. General Chemoselective Suzuki-Miyaura Coupling of Polyhalogenated Aryl Triflates Enabled by an Alkyl-Heteroaryl-Based Phosphine Ligand. ACS Catal. 2021, 11, 7820–7827. [Google Scholar] [CrossRef]
- Fleige, M.; Janssen-Müller, D.; Daniliuc, C.G.; Glorius, F. Switchable Selectivity in an NHC-Catalysed Dearomatizing Annulation Reaction. Nat. Chem. 2015, 7, 842–847. [Google Scholar] [CrossRef]
- Ishida, J.; Nakatsuji, M.; Nagata, T.; Kawasaki, H.; Suzuki, T.; Obora, Y. Synthesis and Characterization of N,N-Dimethylformamide-Protected Palladium Nanoparticles and Their Use in the Suzuki-Miyaura Cross-Coupling Reaction. ACS Omega 2020, 5, 9598–9604. [Google Scholar] [CrossRef]
- Lee, A.F.; Ellis, P.J.; Fairlamb, I.J.S.; Wilson, K. Surface Catalysed Suzuki-Miyaura Cross-Coupling by Pd Nanoparticles: An Operando XAS Study. Dalton Trans. 2010, 39, 10473–10482. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.K.; Ornelas, C.; Salmon, L.; Aranzaes, J.R.; Astruc, D. “Homeopathic” Catalytic Activity and Atom-Leaching Mechanism in Miyaura-Suzuki Reactions under Ambient Conditions with Precise Dendrimer-Stabilized Pd Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 8644–8648. [Google Scholar] [CrossRef] [PubMed]
- Alimardanov, A.; Schmieder-van de Vondervoot, L.; de Vries, A.H.M.; de Vries, J.G. Use of “Homeopathic” Ligand-Free Palladium as Catalyst for Aryl-Aryl Coupling Reactions. Adv. Synth. Catal. 2004, 346, 1812–1817. [Google Scholar] [CrossRef]
- de Vries, A.H.M.; Mulders, J.M.C.A.; Mommers, J.H.M.; Henderickx, H.J.W.; de Vries, J.G. Homeopathic Ligand-Free Palladium as a Catalyst in the Heck Reaction. A Comparison with a Palladacycle. Org. Lett. 2003, 5, 3285–3288. [Google Scholar] [CrossRef] [PubMed]
- Deraedt, C.; Astruc, D. “Homeopathic” Palladium Nanoparticles Catalysis of Cross Carbon-Carbon Coupling Reactions. Acc. Chem. Res. 2014, 47, 494–503. [Google Scholar] [CrossRef]
- Tubaro, C.; Biffis, A.; Gonzato, C.; Zecca, M.; Basato, M. Reactivity of Chelating Dicarbene Metal Complex Catalysts, I: An Investigation on the Heck Reaction. J. Mol. Catal. A Chem. 2006, 248, 93–98. [Google Scholar] [CrossRef]
- Astruc, D.; Ornelas, C.; Diallo, A.K.; Ruiz, J. Extremely Efficient Catalysis of Carbon-Carbon Bond Formation Using “Click” Dendrimer-Stabilized Palladium Nanoparticles. Molecules 2010, 15, 4947–4960. [Google Scholar] [CrossRef]
- Ohtaka, A.; Kawase, M.; Usami, A.; Fukui, S.; Yamashita, M.; Yamaguchi, K.; Sakon, A.; Shiraki, T.; Ishida, T.; Nagata, S.; et al. Mechanistic Study on Allylic Arylation in Water with Linear Polystyrene-Stabilized Pd and PdO Nanoparticles. ACS Omega 2019, 4, 15764–15770. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Jutand, A.; Mosleh, A. Rate and Mechanism of Oxidative Addition of Aryl Triflates to Zerovalent Palladium Complexes. Evidence for the Formation of Cationic (σ-Aryl)palladium Complexes. Organometallics 1995, 14, 1810–1817. [Google Scholar] [CrossRef]
- Hirakawa, T.; Uramoto, Y.; Yanagisawa, S.; Ikeda, T.; Inagaki, K.; Morikawa, Y. First-Principles Molecular Dynamics Analysis of Ligand-Free Suzuki-Miyaura Cross-Coupling in Water: Transmetalation and Reductive Elimination. J. Phys. Chem. C 2017, 121, 19904–19914. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Al-Halaiqa, A.; Smirnov, V.V. Heck Reactions of Alkenes with Aryl Iodides and Aryl Bromides: Rate-Determining Steps Deduced from a Comparative Kinetic Study of Competing and Noncompeting Reactions. Kinet. Catal. 2007, 48, 716–727. [Google Scholar] [CrossRef]
- Witte, F.; Zucker, S.P.; Paulus, B.; Tzschucke, C.C. Unexpected Substituent Effects in Aryl-Aryl Negishi Cross-Coupling Reactions Rationalized by Density Functional Theory and Natural Charges. Organometallics 2021, 40, 591–599. [Google Scholar] [CrossRef]
- Shekhar, S.; Hartwig, J.F. Distinct Electronic Effects on Reductive Eliminations of Symmetrical and Unsymmetrical Bis-Aryl Platinum Complexes. J. Am. Chem. Soc. 2004, 126, 13016–13027. [Google Scholar] [CrossRef]
- Ichii, S.; Hamasaka, G.; Uozumi, Y. The Hiyama Cross-Coupling Reaction at Parts Per Million Levels of Pd: In Situ Formation of Highly Active Spirosilicates in Glycol Solvents. Chem. Asian J. 2019, 14, 3850–3854. [Google Scholar] [CrossRef]
- Amatore, C.; Grimaud, L.; Le Duc, G.; Jutand, A. Three Roles for the Fluoride Ion in Palladium-Catalyzed Hiyama Reactions: Transmetalation of [ArPdFL2] by Ar’Si(OR)3. Angew. Chem. Int. Ed. 2014, 53, 6982–6985. [Google Scholar] [CrossRef]
- Denmark, S.E.; Smith, R.C.; Chang, W.-T.T. Probing the Electronic Demands of Transmetalation in the Palladium-Catalyzed Cross-Coupling of Arylsilanolates. Tetrahedron 2011, 67, 4391–4396. [Google Scholar] [CrossRef]
- Hübner, S.; de Vries, J.G.; Farina, V. Why Does Industry Not Use Immobilized Transition Metal Complexes as Catalysts? Adv. Synth. Catal. 2016, 358, 3–25. [Google Scholar] [CrossRef]
- Horbaczewskyj, C.S.; Fairlamb, I.J.S. Pd-Catalyzed Cross-Couplings: On the Importance of the Catalyst Quantity Descriptors, Mol % and ppm. Org. Process Res. Dev. 2022, 26, 2240–2269. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


