Acid-Catalyzed Etherification of Glycerol with Tert-Butanol: Reaction Monitoring through a Complete Identification of the Produced Alkyl Ethers
Abstract
:1. Introduction
2. Results and Discussion
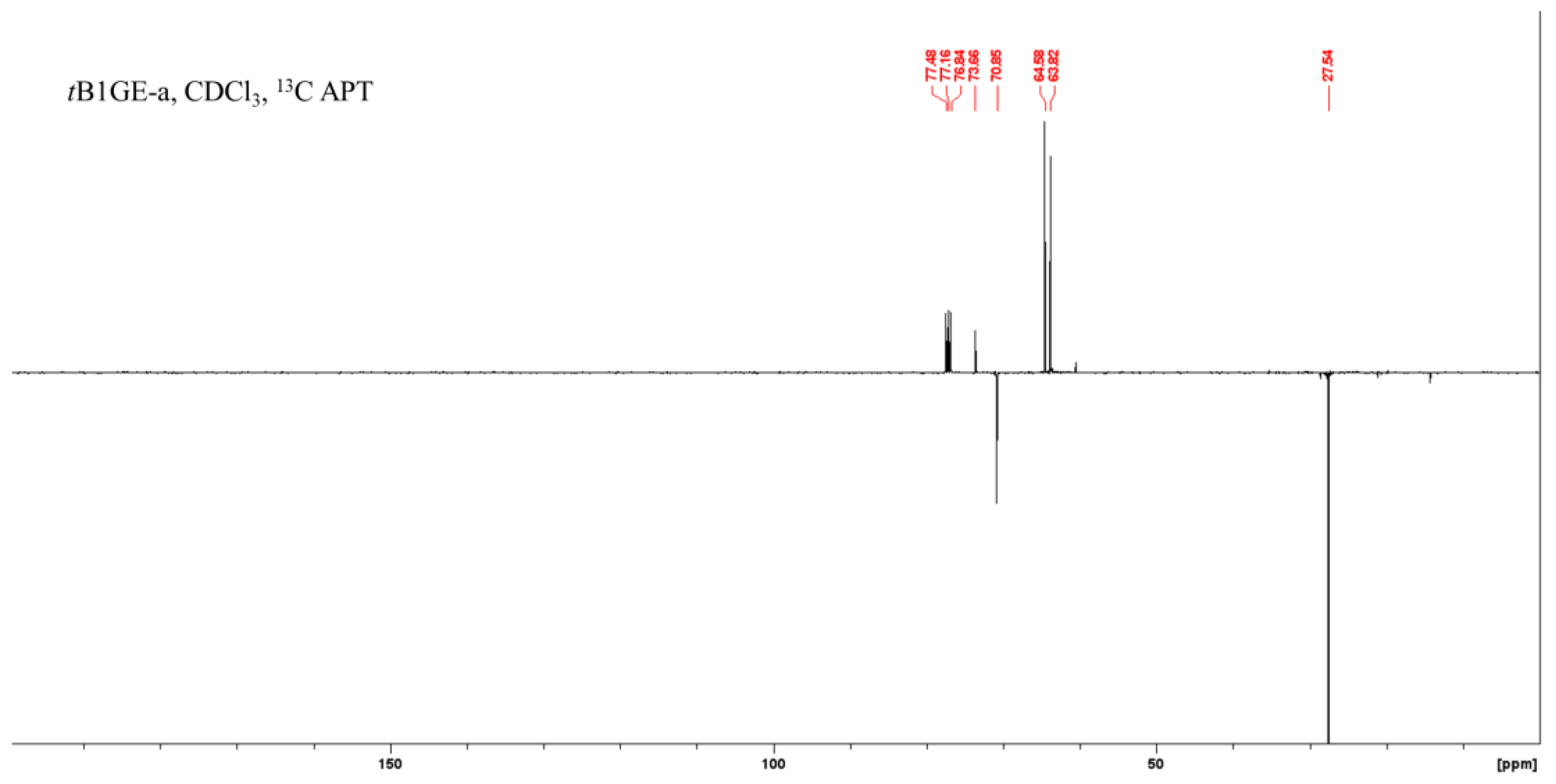
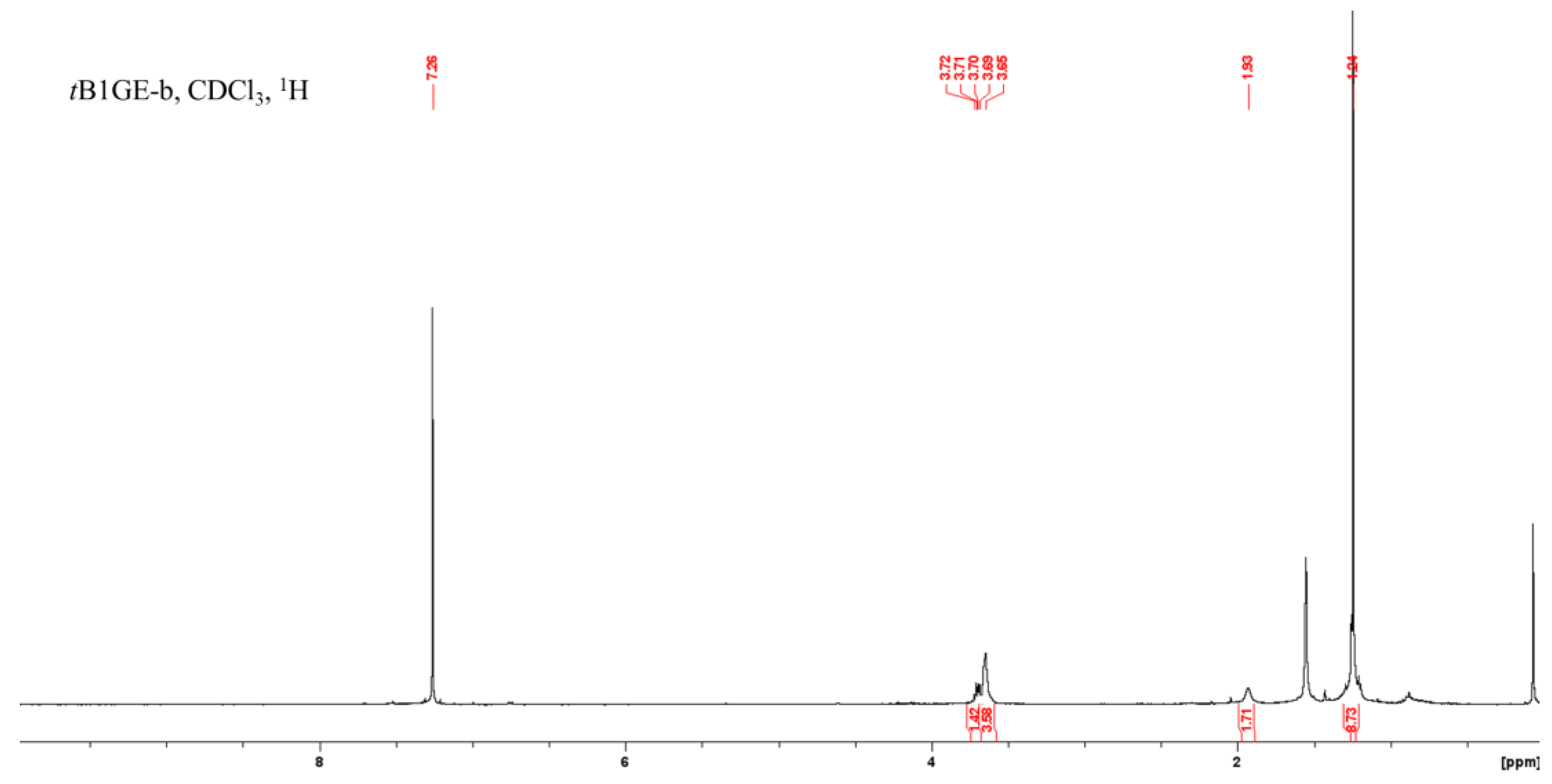
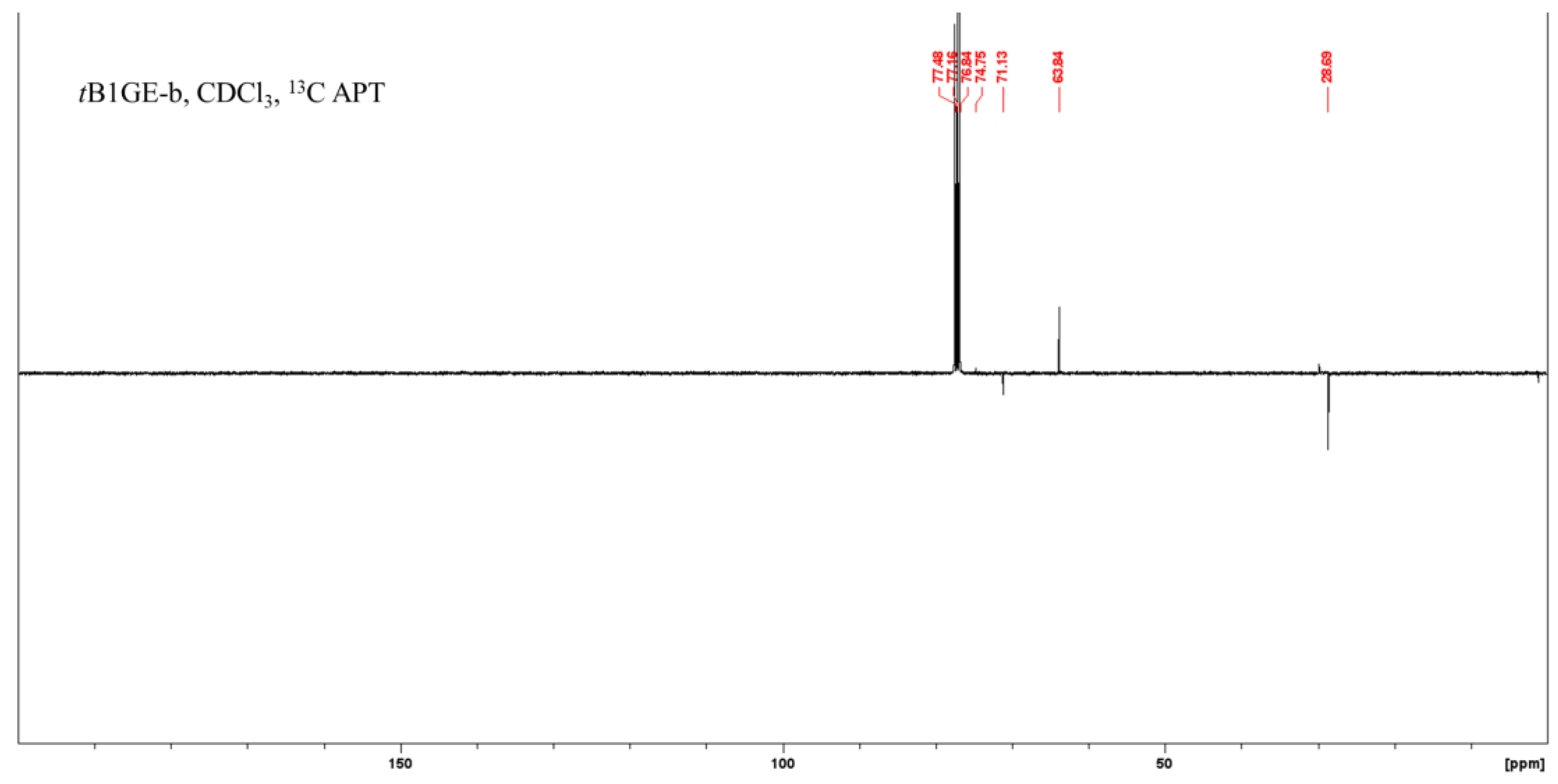
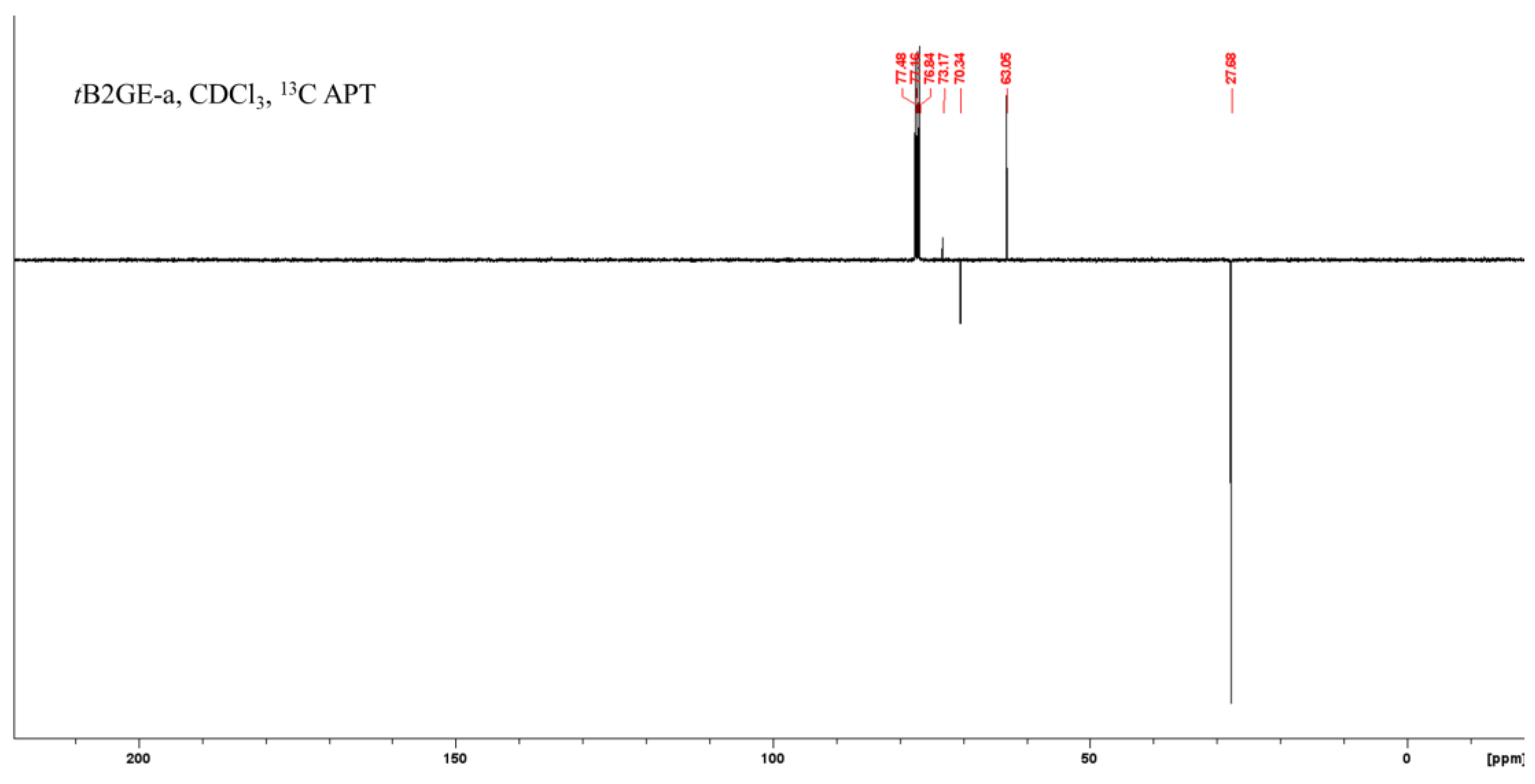
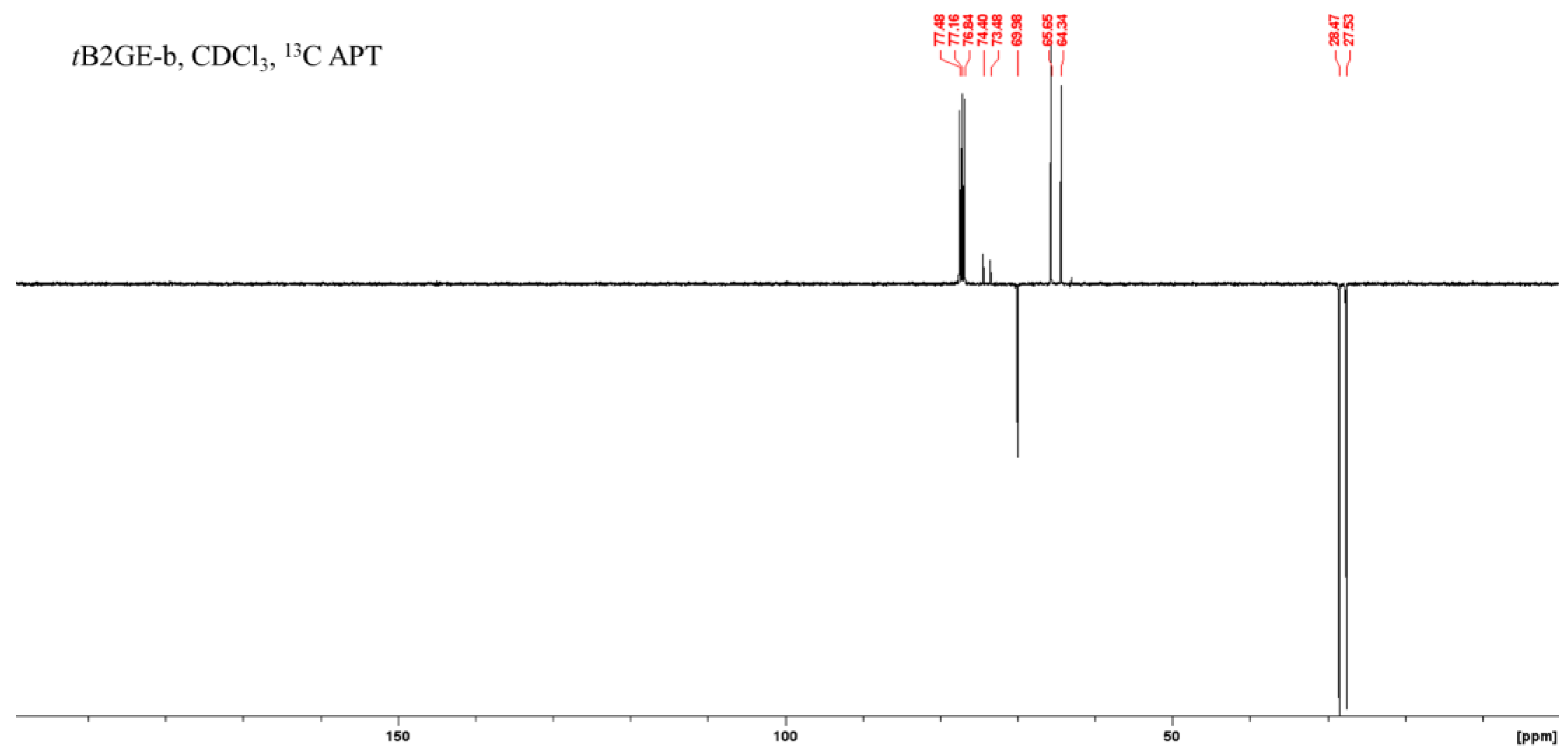
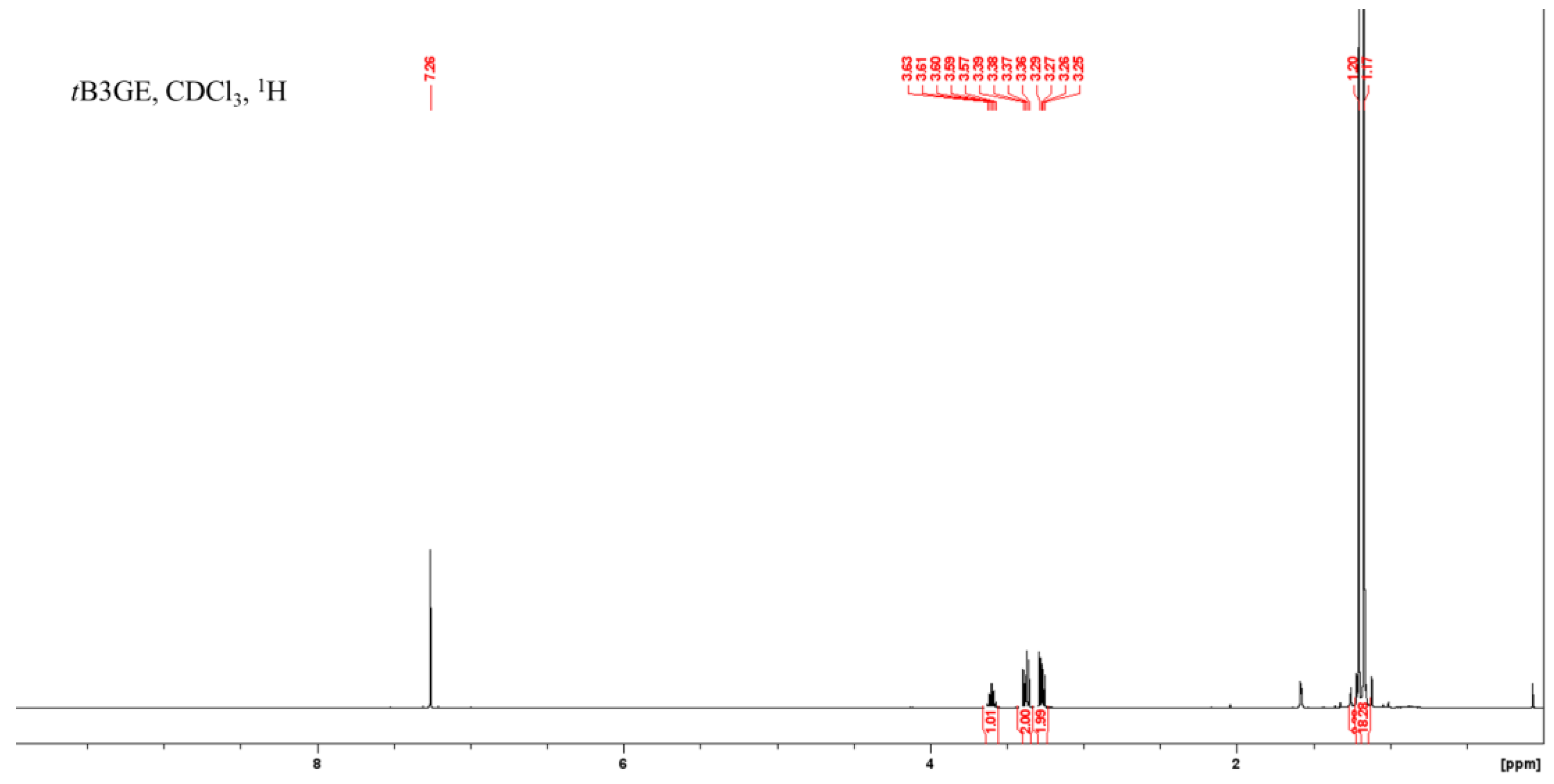
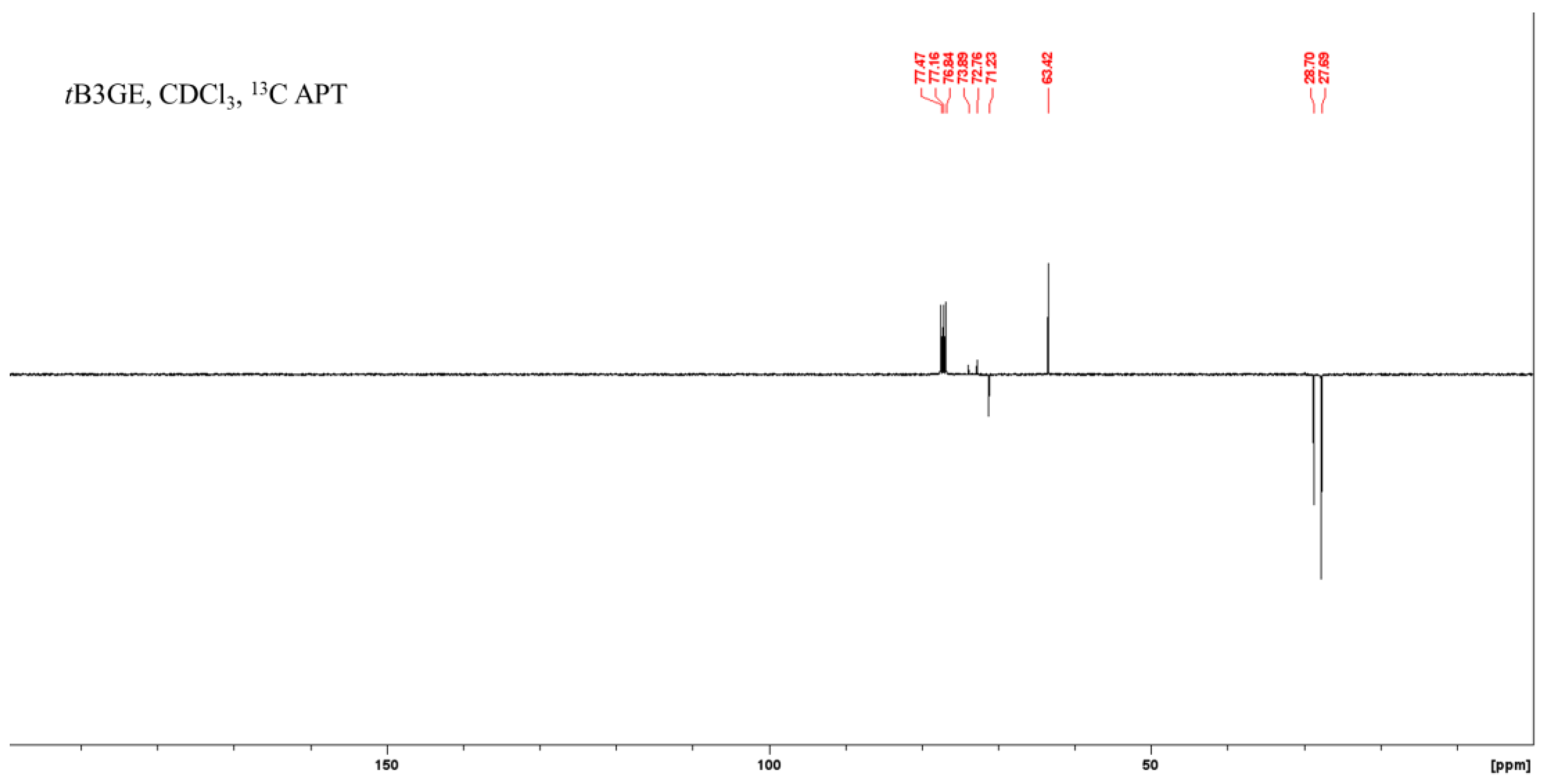
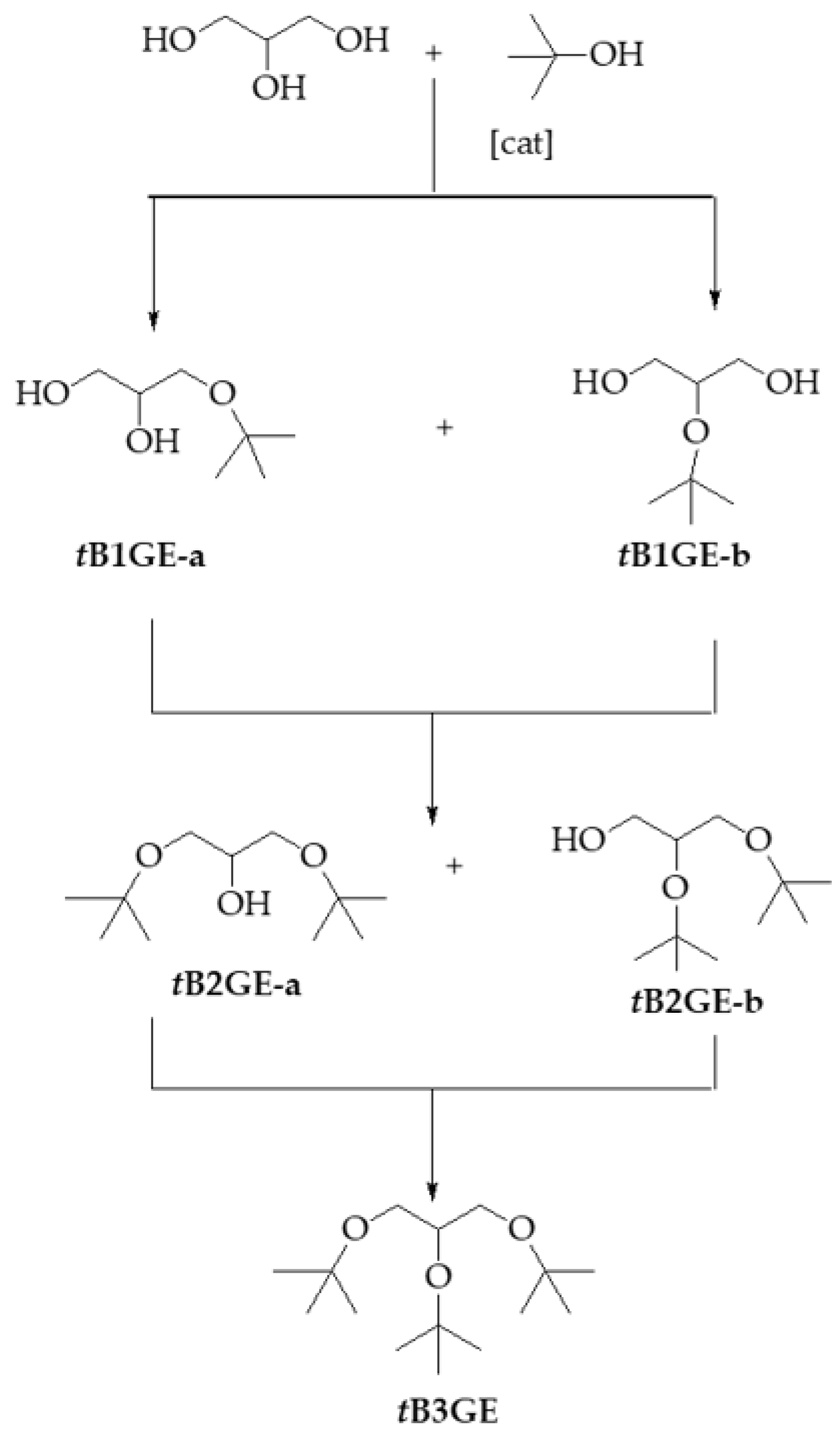
2.1. Characterization of Etherification Products
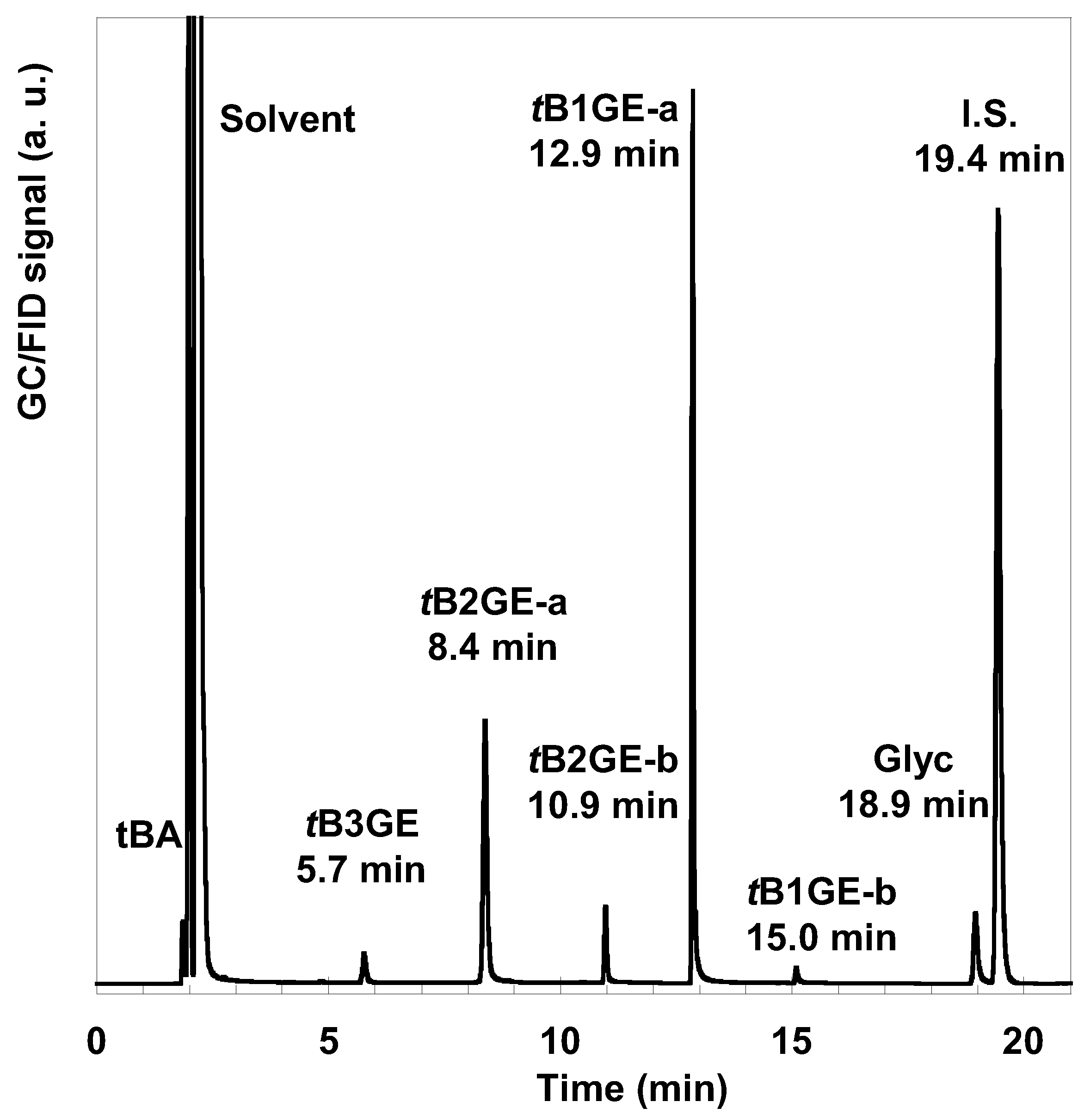
2.2. Glycerol Tert-Butylation Monitoring through GC Analyses
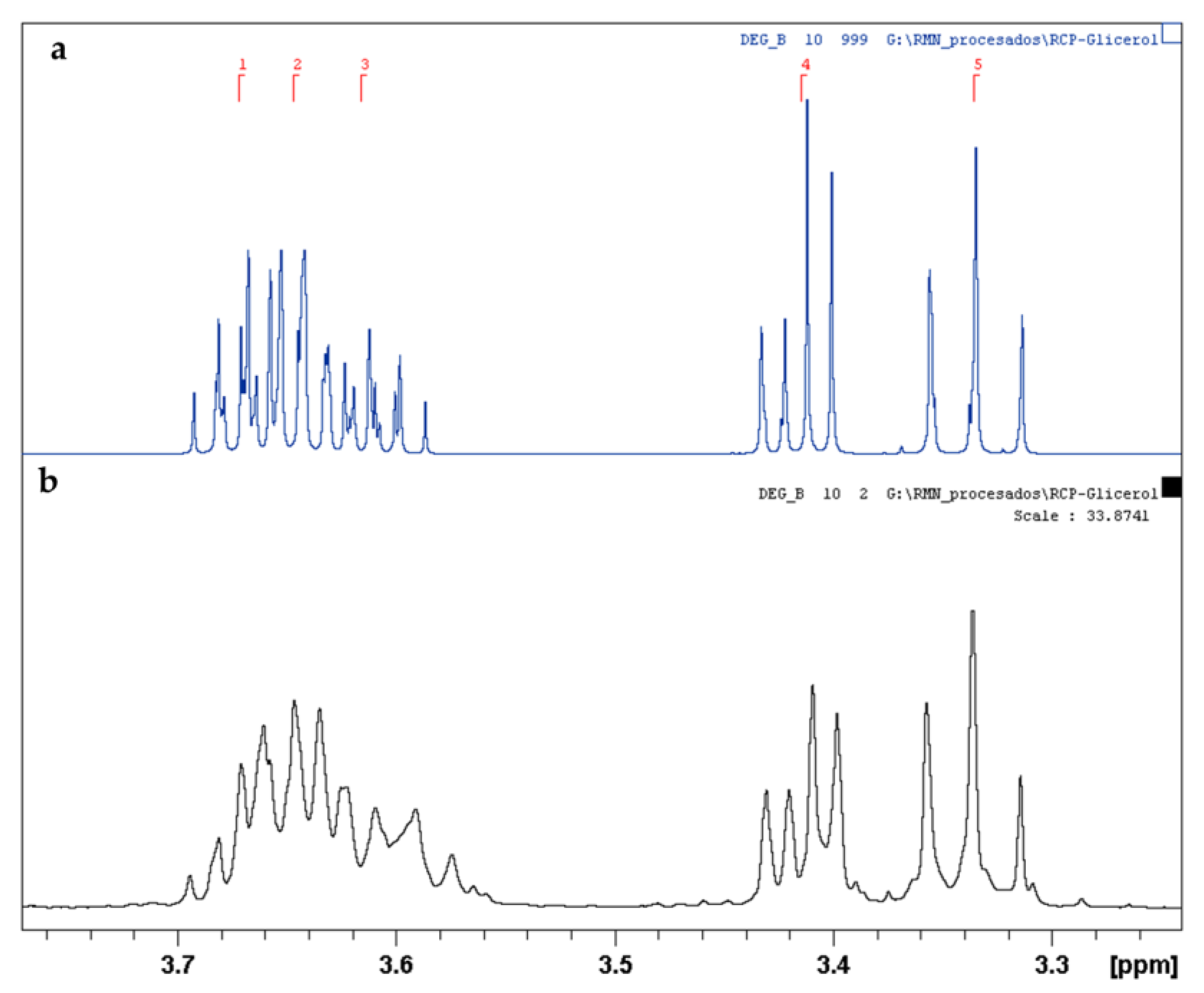
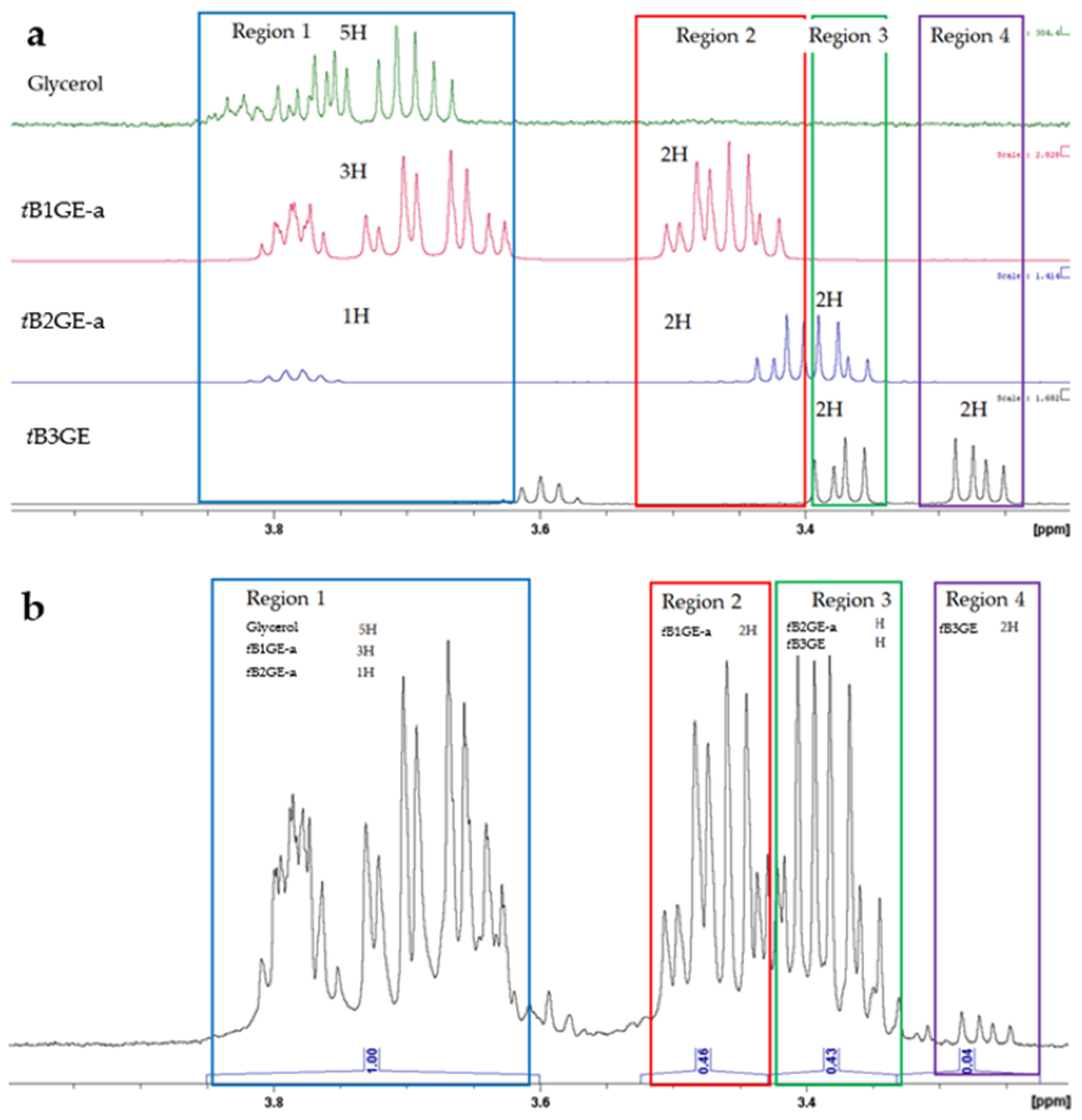
2.3. Glycerol Tert-Butylation Monitoring through 1H NMR Analyses
2.4. Etherification of the Tert-Butyl Glycerol Monoether
3. Materials and Methods
3.1. Materials and Analytical Techniques
3.2. Tert-Butylation Reactions
3.3. Isolation of the Tert-Butyl Ethers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
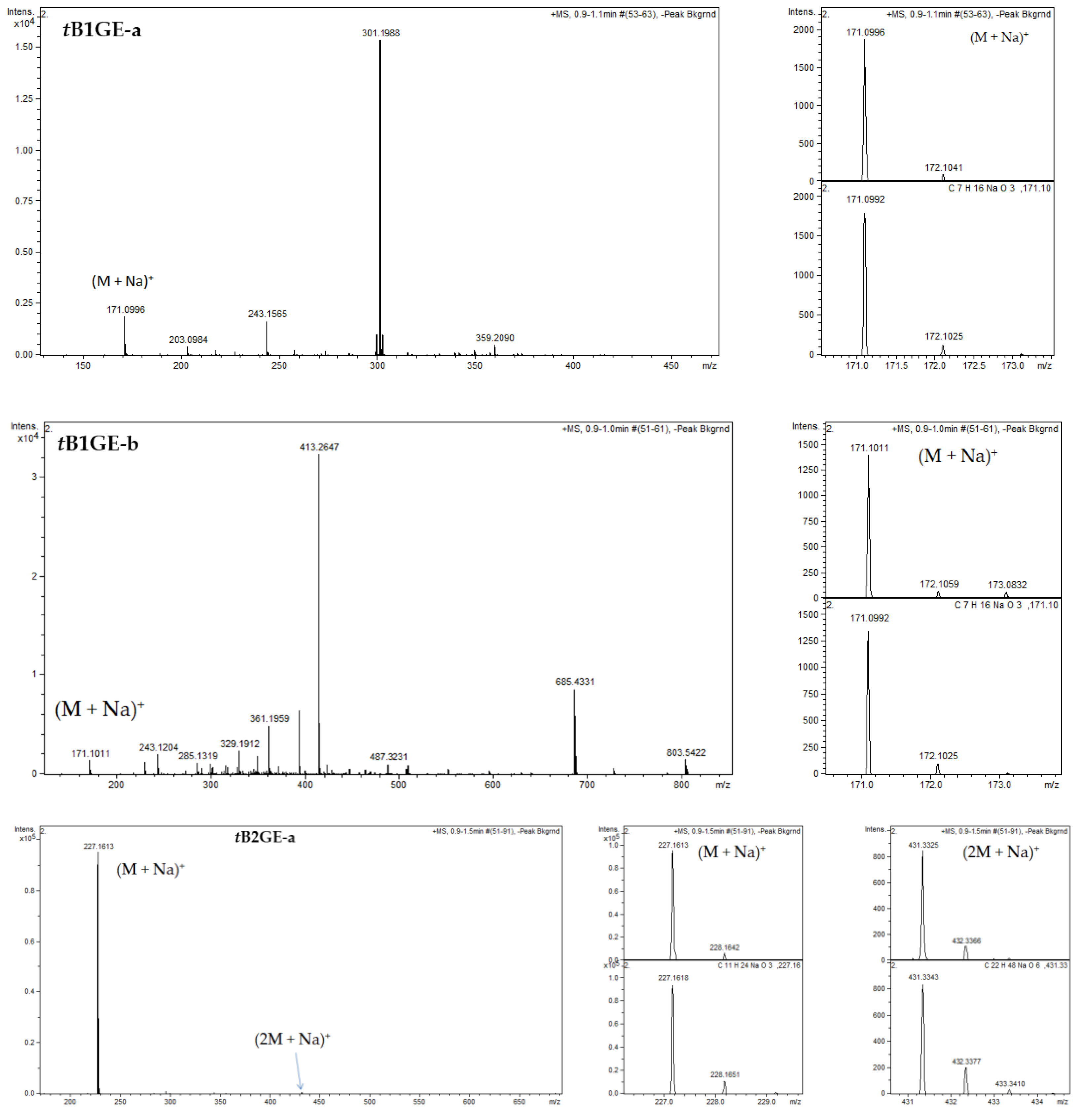
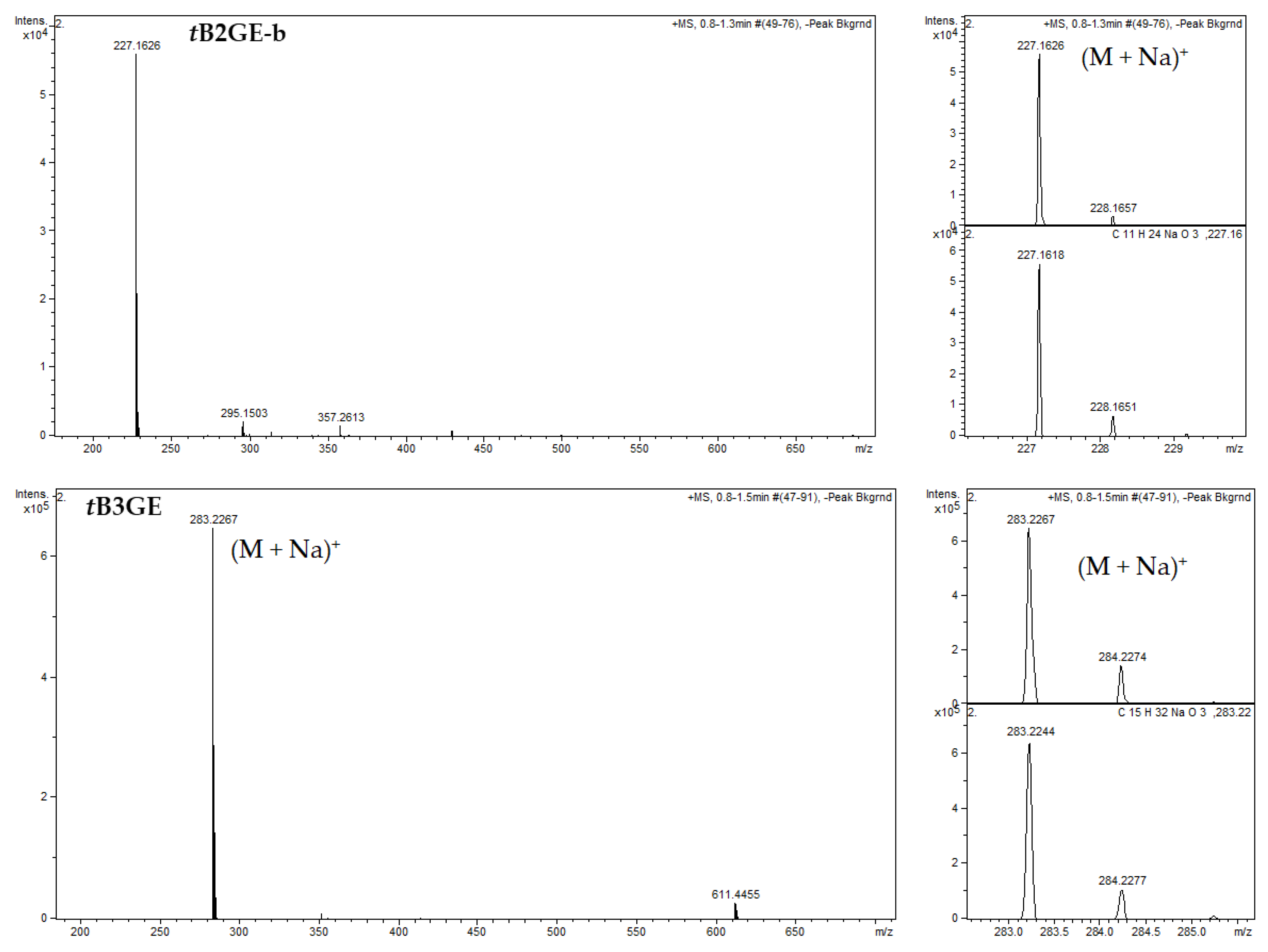
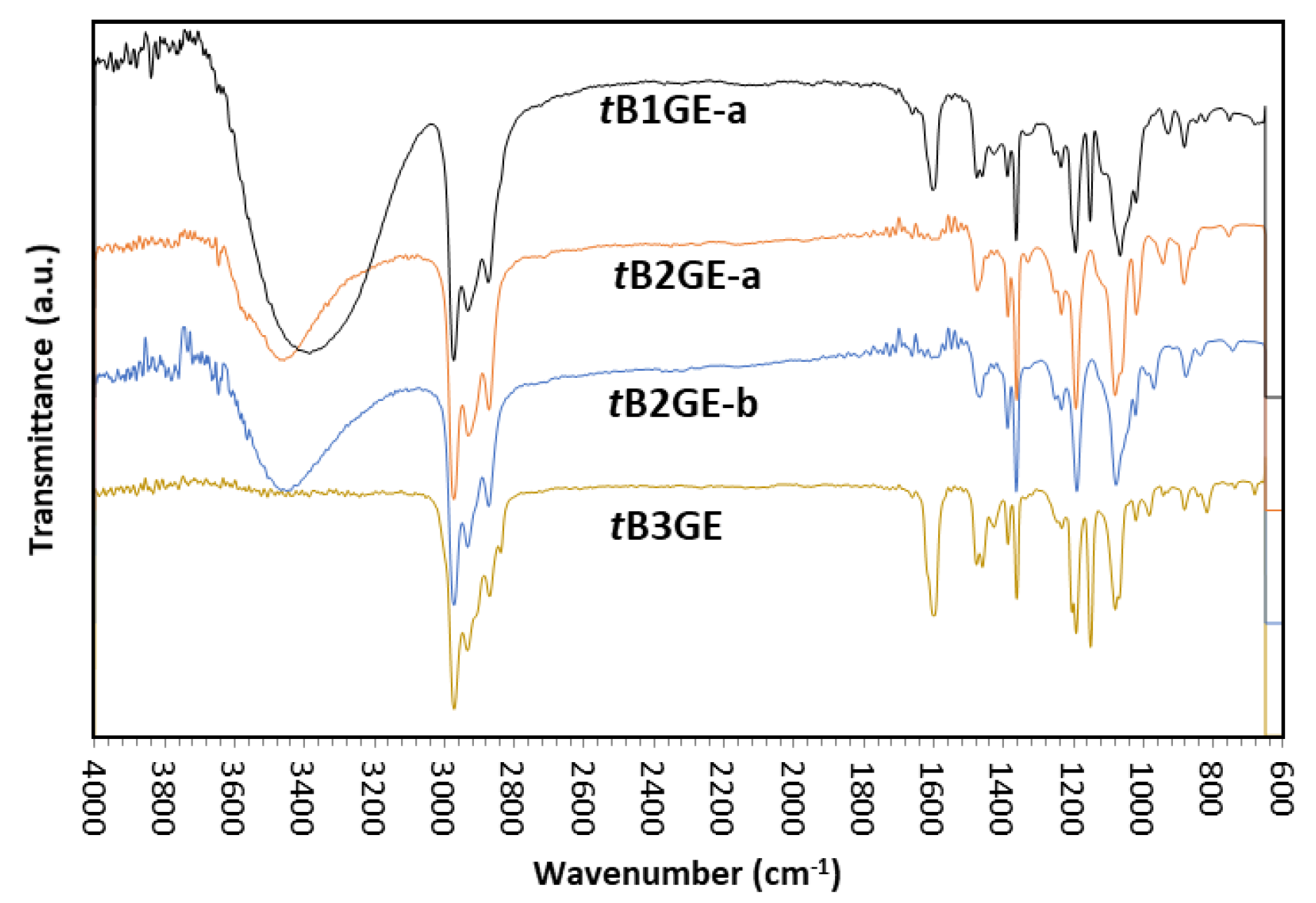
Appendix B
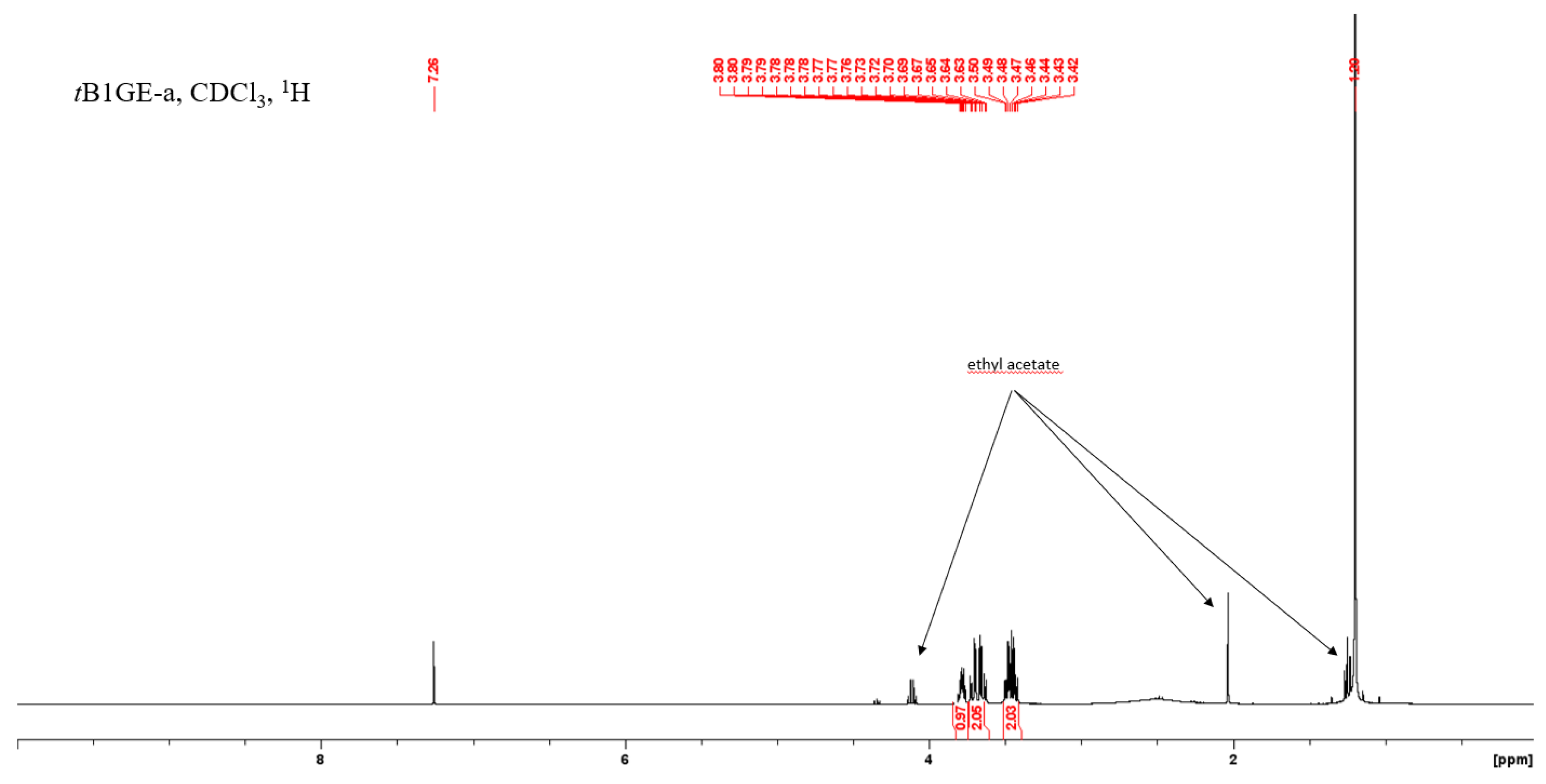


References
- ChemAnalyst. Glycerine Market Analysis. Available online: https://www.chemanalyst.com/industry-report/glycerine-market-635 (accessed on 7 August 2023).
- International Energy Agency. Renewables 2022. Analysis and Forecast to 2027; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Morais Lima, P.J.; da Silva, R.M.; Chaves Girão Neto, C.A.; Câmara Gomes e Silva, N.; da Silva Souza, J.E.; Nunes, Y.L.; Sousa dos Santos, J.C. An overview on the conversion of glycerol to value-added products via chemical and biochemical routes. Biotechnol. Appl. Biochem. 2022, 69, 2794–2818. [Google Scholar] [CrossRef] [PubMed]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279. [Google Scholar] [CrossRef]
- Pirzadi, Z.; Meshkani, F. From glycerol production to its value-added uses: A critical review. Fuel 2022, 329, 125044. [Google Scholar] [CrossRef]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of crude glycerol to value-added products: Perspectives of process technology, economics and environmental issues. Biotechnol. Rep. 2020, 27, e00487. [Google Scholar] [CrossRef] [PubMed]
- Gujar, J.P.; Modhera, B. A review on catalytic conversion of biodiesel derivative glycerol to bio-olefins. Mater. Today Proc. 2023, 72, 2723–2730. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Muldoon, V.L.; Deng, S. Crude Glycerol and glycerol as fuel and fuel additives in combustion applications. Renew. Sust. Energ. Rev. 2022, 159, 112206. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, B.; Lawal, A. Recovery and utilization of crude glycerol, a biodiesel byproduct. RSC Adv. 2022, 12, 27997. [Google Scholar] [CrossRef]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renew. Sust. Energ. Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Olson, A.L.; Tunér, M.; Verhelst, S. A concise review of glycerol derivatives for use as fuel additives. Heliyon 2023, 9, e13041. [Google Scholar] [CrossRef]
- Queste, S.; Boudin, P.; Touraud, D.; Kunz, W.; Aubry, J.-M. Short chain glycerol 1-monoethers—A new class of solvo-surfactants. Green Chem. 2006, 8, 822–830. [Google Scholar] [CrossRef]
- Gu, Y.; Azzouzi, A.; Pouilloux, Y.; Jérôme, F.; Barrault, J. Hetrogeneously catalyzed etherification of glycerol: New pathways for transformation of glycerol to more valuable chemicals. Green Chem. 2008, 10, 164–167. [Google Scholar] [CrossRef]
- Sutter, M.; Da Silva, E.; Duguet, N.; Raoul, Y.; Métay, E.; Lemaire, M. Glycerol Ether Synthesis: A Bench Test for Green Chemistry Concepts and Technologies. Chem. Rev. 2015, 115, 8609–8651. [Google Scholar] [CrossRef] [PubMed]
- Palanychamy, P.; Lim, S.; Yap, Y.H.; Leong, L.K. Critical Review of the Various Reaction Mechanisms for Glycerol Etherification. Catalysts 2022, 12, 1487. [Google Scholar] [CrossRef]
- Behr, A.; Obendorf, L. Development of a Process for the Acid-Catalyzed Etherification of Glycerine and Isobutane Forming Glycerine Tertiary Butyl Ethers. Eng. Life Sci. 2002, 2, 179–208. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B. Liquid–Liquid–Solid Mass Transfer and Phase Behavior of Heterogeneous Etherification of Glycerol with Isobutene. AIChE J. 2018, 64, 2526–2535. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Romero, A.; Santos, A. Improved Etherification of Glycerol with Tert-Butyl Alcohol by the Addition of Dibutyl Ether as Solvent. Catalysts 2019, 9, 378. [Google Scholar] [CrossRef]
- Klepáčová, K.; Mravec, D.; Bajus, M. tert-Butylation of glycerol catalysed by ion-exchange resins. Appl. Catal. A Gen. 2005, 294, 141–147. [Google Scholar] [CrossRef]
- Melero, J.A.; Vicente, G.; Morales, G.; Paniagua, M.; Moreno, J.M.; Roldán, R.; Ezquerro, A.; Pérez, C. Acid-catalyzed etherification of bio-glycerol and isobutylene over sulfonic mesostructured silicas. Appl. Catal. A Gen. 2008, 346, 44–51. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, B.; Yi, C.; Lei, Z.; Xu, J. Etherification of Glycerol with Isobutylene to Produce Oxygenate Additive Using Sulfonated Peanut Shell Catalyst. Ind. Eng. Chem. Res. 2010, 49, 12399–12404. [Google Scholar] [CrossRef]
- Zhao, W.; Yi, C.; Yang, B.; Hu, J.; Huang, X. Etherification of glycerol and isobutylene catalyzed over rare earth modified Hβ-zeolite. Fuel Process. Technol. 2013, 112, 70–75. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Taboada, E.; Llorca, J.; Cesteros, Y. Microwave-assisted synthesis of sulfonic acid-functionalized microporous materials for the catalytic etherification of glycerol with isobutene. Green Chem. 2013, 15, 2230–2239. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Mokaya, R.; Cesteros, Y. Tuning the acidic and textural properties of ordered mesoporous silicas for their application as catalysts in the etherification of glycerol with isobutene. Catal. Today 2014, 227, 171–178. [Google Scholar] [CrossRef]
- Bozkurt, Ö.D.; Bağlar, N.; Çelebi, S.; Uzun, A. Screening of solid acid catalysts for etherification of glycerol with isobutene under identical conditions. Catal. Today 2020, 357, 483–494. [Google Scholar] [CrossRef]
- Klepáčová, K.; Mravec, D.; Bajus, M. Etherification of Glycerol with tert-Butyl Alcohol Catalysed by Ion-Exchange Resins. Chem. Pap. 2006, 60, 224–230. [Google Scholar] [CrossRef]
- Frusteri, F.; Arena, F.; Bonura, G.; Cannilla, C.; Spadaro, L.; Di Blasi, O. Catalytic etherification of glycerol by tert-butyl alcohol to produce oxygenated additives for diesel fuel. Appl. Catal. A Gen. 2009, 367, 77–83. [Google Scholar] [CrossRef]
- Pico, M.P.; Romero, A.; Rodríguez, S.; Santos, A. Etherification of Glycerol by tert-Butyl Alcohol: Kinetic Model. Ind. Eng. Chem. Res. 2012, 51, 9500–9509. [Google Scholar] [CrossRef]
- Celdeira, P.A.; Gonçalves, M.; Figueiredo, F.C.A.; Dal Bosco, S.M.; Mandelli, D.; Carvalho, W.A. Sulfonated niobia and pillared clay as catalysts in etherification reaction of glycerol. Appl. Catal. A Gen. 2014, 478, 98–106. [Google Scholar] [CrossRef]
- Gonçalves, M.; Soler, F.C.; Isoda, N.; Carvalho, W.A.; Mandelli, D.; Sepúlveda, J. Glycerol conversion into value-added products in presence of a green recyclable catalyst: Acid black carbon obtained from coffee ground wastes. J. Taiwan Inst. Chem. Eng. 2016, 60, 294–301. [Google Scholar] [CrossRef]
- Srinivas, M.; Raveendra, G.; Parameswaram, G.; Sai Prasad, P.S.; Lingaiah, N. Cesium exchanged tungstophosphoric acid supported on tin oxide: An efficient solid acid catalyst for etherification of glycerol with tert-butanol to synthesize biofuel additives. J. Mol. Catal. A Chem. 2016, 413, 7–14. [Google Scholar] [CrossRef]
- Estevez, R.; López, M.I.; Jiménez-Sanchidrián, C.; Luna, D.; Romero-Salguero, F.J.; Bautista, F.M. Etherification of glycerol with tert-butyl alcohol over sulfonated hybrid silicas. Appl. Catal. A Gen. 2016, 526, 155–163. [Google Scholar] [CrossRef]
- Melero, J.A.; Vicente, G.; Morales, G.; Paniagua, M.; Bustamante, J. Oxygenated Compounds Derived from Glycerol for Biodiesel Formulation: Influence on EN 14214 Quality Parameters. Fuel 2010, 89, 2011–2018. [Google Scholar] [CrossRef]
- Lee, H.J.; Seung, D.; Jung, K.S.; Kim, H.; Filimonov, I.N. Etherification of glycerol by isobutylene: Tuning the product composition. Appl. Catal. A Gen. 2010, 390, 235–244. [Google Scholar] [CrossRef]
- Altaner, C.M.; Saake, B. Quantification of the chemical composition of lignocellulosics by solution 1H NMR spectroscopy of acid hydrolysates. Cellulose 2016, 23, 1003–1010. [Google Scholar] [CrossRef]
- De Souza, A.C.; Rietkerk, T.; Selin, C.G.M.; Lankhorst, P.P. A robust and universal NMR method for the compositional analysis of polysaccharides. Carbohydr. Polym. 2013, 95, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Scott, G.M.; Amidon, T.E.; Kiemle, D.J.; Stipanovic, A.J. Quantitative analysis of sugars in wood hydrolyzates with 1H NMR during the autohydrolysis of hardwoods. Bioresour. Technol. 2009, 100, 6398–6406. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, A.; Alegria-Dallo, I.; García-Yoldi, Í.; Sarobe, Í.; Sánchez, D.; Otazu, E.; Funcia, I.; Gil, M.J.; Martínez-Merino, V. Pretreatment and enzymatic hydrolysis for the efficient production of glucose and furfural from wheat straw, pine and poplar chips. Bioresour. Technol. 2019, 288, 121583. [Google Scholar] [CrossRef]
- Cornejo, A.; Bimbela, F.; Moreira, R.; Hablich, K.; García-Yoldi, Í.; Maisterra, M.; Portugal, A.; Gandía, L.M.; Martínez-Merino, V. Production of Aromatic Compounds by Catalytic Depolymerization of Technical and Downstream Biorefinery Lignins. Biomolecules 2020, 10, 1338. [Google Scholar] [CrossRef]
- Talavera-Prieto, N.M.C.; Ferreira, A.G.M.; Moreira, R.J.; Portugal, A.T.G. Monitoring of the Transesterification Reaction by Continuous Off-Line Density Measurements. Fuel 2020, 264, 116877. [Google Scholar] [CrossRef]
- Kundu, R.; De, S. Characterization and analysis of the triglyceride transesterification process. Biomass Convers. Biorefinery 2023, 13, 4933–4948. [Google Scholar] [CrossRef]
- González, M.D.; Cesteros, Y.; Llorca, J.; Salagre, P. Boosted selectivity toward high glycerol tertiary butyl ethers by microwave-assisted sulfonic acid-functionalization of SBA-15 and beta zeolite. J. Catal. 2012, 290, 202–209. [Google Scholar] [CrossRef]
- Veiga, P.M.; Dias, A.G.; Henriques, C.A. Identification of Ethyl and t-Butyl Glyceryl Ethers Using Gas Chromatography Coupled with Mass Spectrometry. J. Braz. Chem. Soc. 2018, 29, 1328–1335. [Google Scholar] [CrossRef]
- Jamróz, M.E.; Jarosz, M.; Witowska-Jarosz, J.; Bednarek, E.; Tecza, W.; Jamróz, M.H.; Dobrowolski, J.C.; Kijeński, J. Mono-, Di-, and Tri-Tert-Butyl Ethers of Glycerol: A Molecular Spectroscopic Study. Spectroc. Acta Part A Mol. Biomol. Spectr. 2007, 67, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.J. WinDNMR:Dynamic NMR Spectra for Windows. J. Chem. Educ. 1995, 72, 1086. [Google Scholar] [CrossRef]
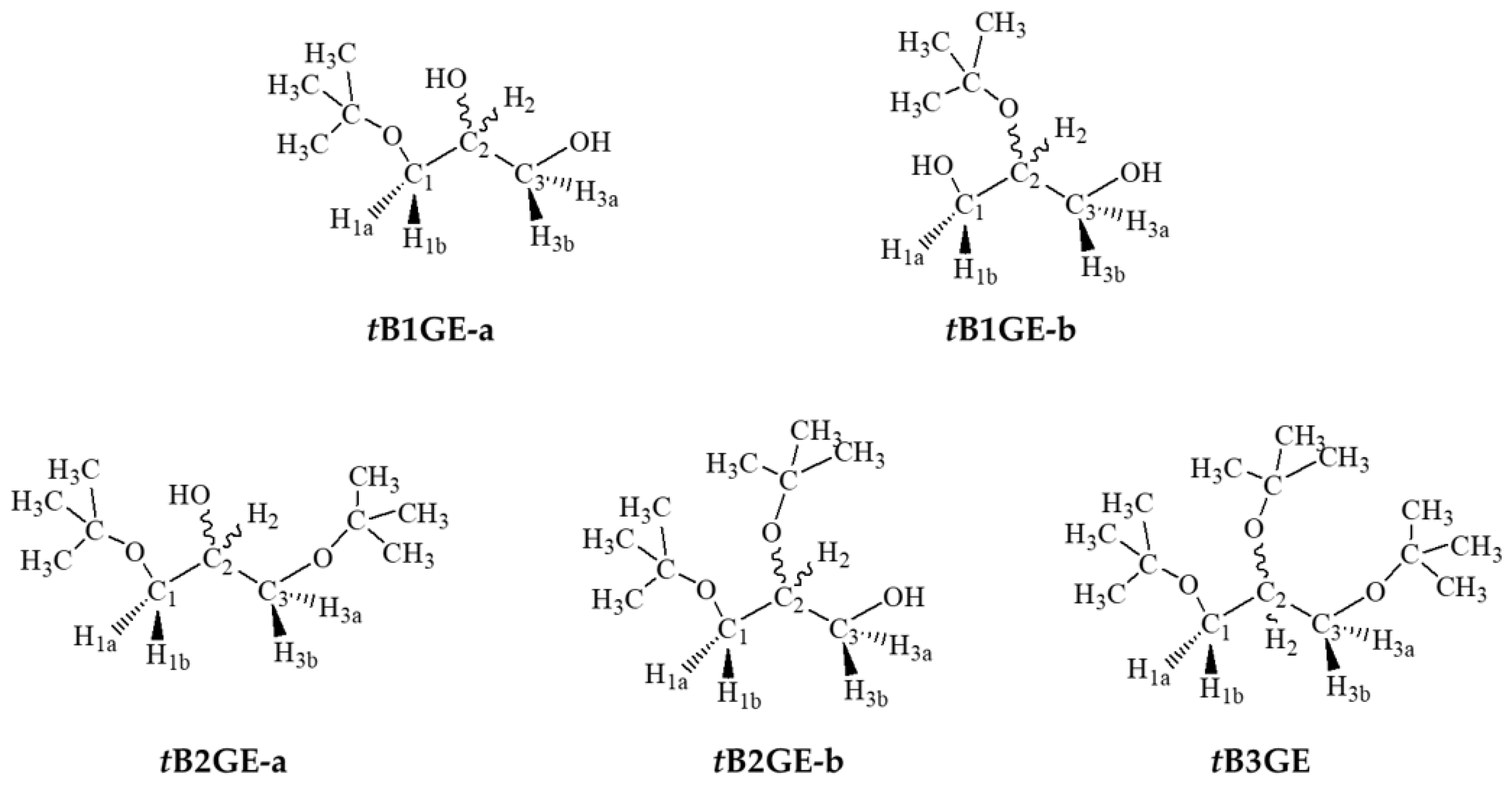

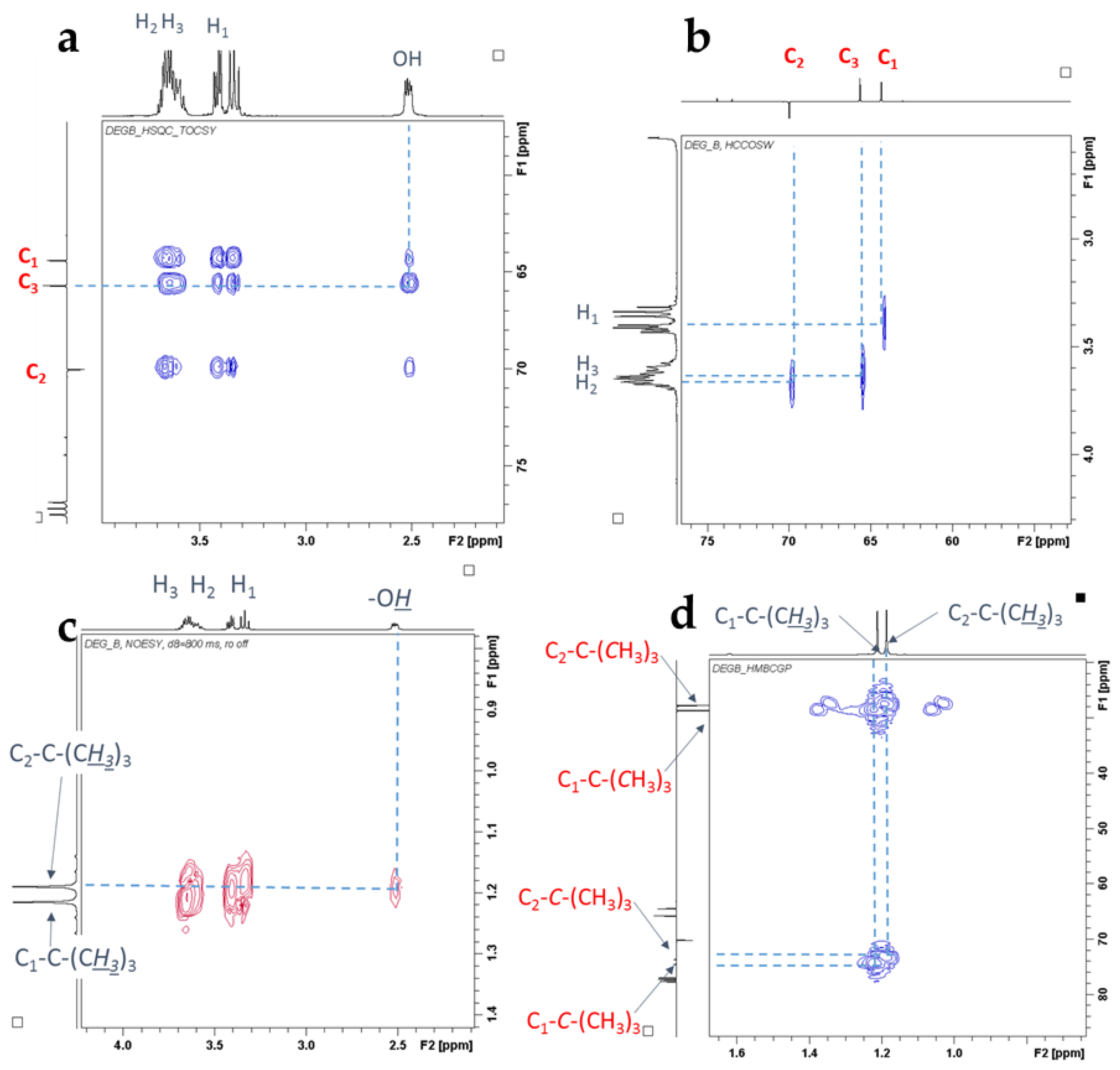
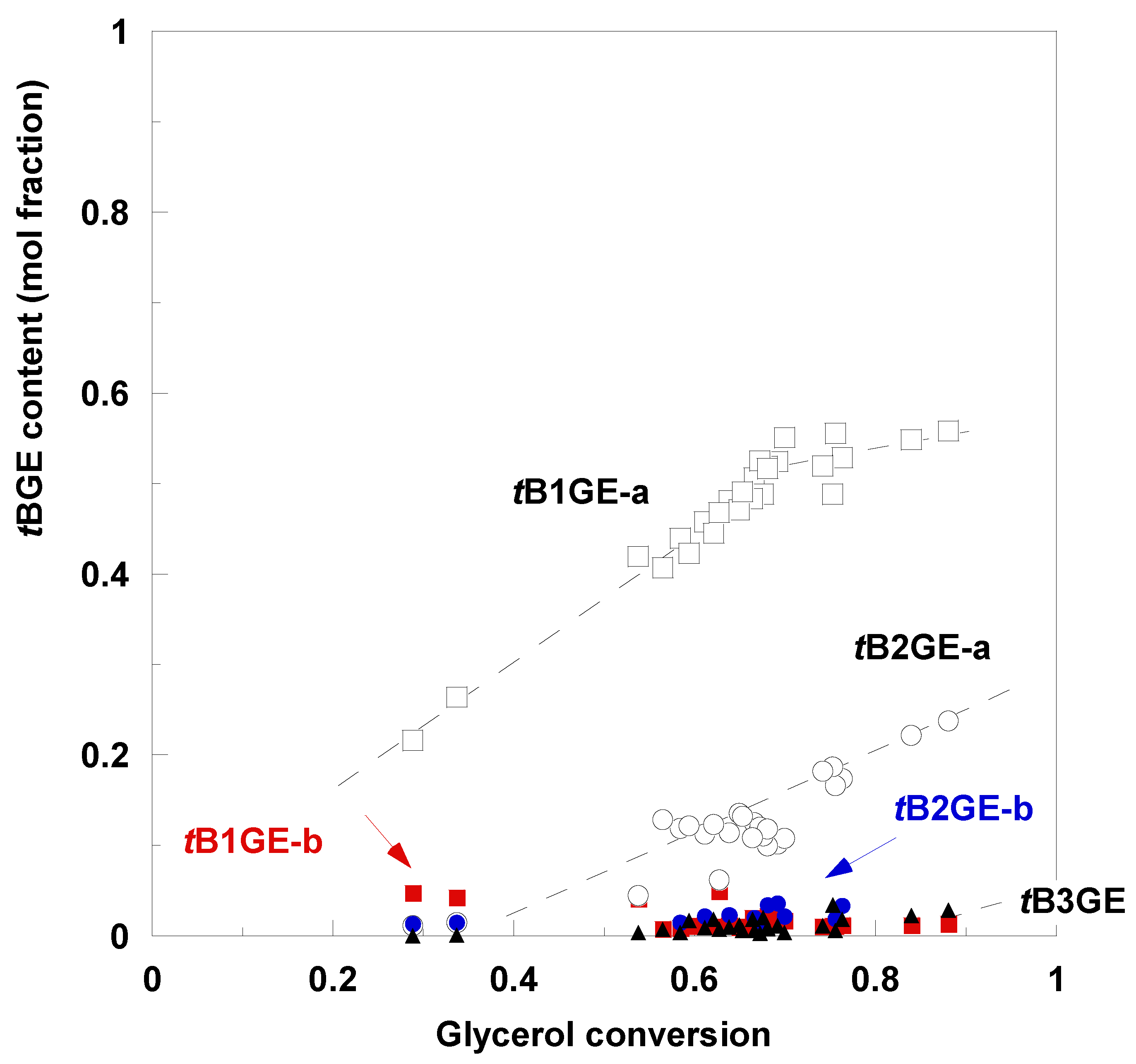


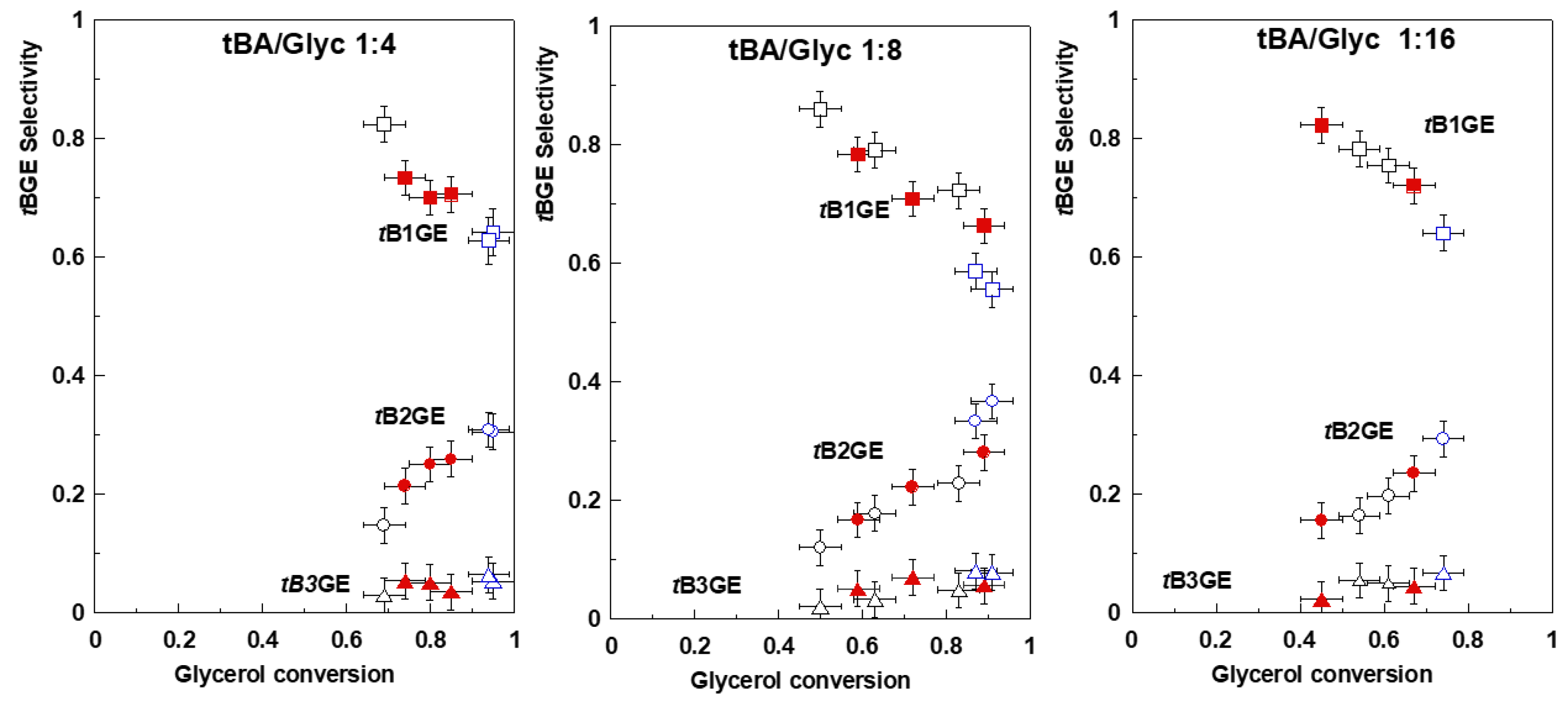

| Ether | H1a (J1a,2) | H1b (J1b,2) | (J1a,1b) | H2 | H3a (J3a,2) | H3b (J3b,2) | (J3a,3b) | C1–OC [CH3]3 | C2–OC [CH3]3 | C3–OC [CH3]3 |
|---|---|---|---|---|---|---|---|---|---|---|
| tB1GE-a | 3.43 (5.88) | 3.49 (3.92) | (9.10) | 3.78 | 3.65 (4.95) | 3.70 (3.91) | (11.4) | 1.20 | - | - |
| tB1GE-b | 3.65 | - | - | 3.71 | 3.65 a | - | - | 1.24 | - | - |
| tB2GE-a | 3.37 (5.92) | 3.42 (5.06) | (8.97) | 3.78 | 3.37 a (5.92) a | 3.42 b (5.06) b | (8.98) | 1.19 | - | 1.19 |
| tB2GE-b | 3.34 (8.58) | 3.41 (4.30) | (8.62) | 3.65 | 3.61 | 3.61 | - | 1.21 | 1.19 | - |
| tB3GE | 3.27 (5.32) | 3.37 (5.92) | (9.22) | 3.60 | 3.27 a (5.32) a | 3.37 b (5.92) b | (9.22) a,b | 1.17 | 1.20 | 1.17 |
| Ether | C1 | C1–O–C–(CH3)3 a | C1–O–C–(CH3)3 a | C2 | C2–O–C–(CH3)3 a | C2–O–C–(CH3)3 a | C3 |
|---|---|---|---|---|---|---|---|
| tB1GE-a | 63.92 | 27.59 | 73.69 | 70.80 | - | - | 64.69 |
| tB1GE-b | 63.83 | - | - | 71.12 | 28.68 | 74.75 | 63.83 |
| tB2GE-a | 63.09 | 27.70 | 73.17 | 70.38 | - | - | 63.09 |
| tB2GE-b | 64.19 | 28.31 | 74.24 | 69.82 | 27.37 | 73.32 | 65.49 |
| tB3GE | 63.53 | 27.72 | 72.75 | 71.34 | 28.57 | 73.87 | 63.53 |
| Region, Rj | δ (ppm) | Glycerol | tB1GE-a | tB2GE-a | tB3GE |
|---|---|---|---|---|---|
| 1 | 3.850–3.604 | 5 | 3 | 1 | - |
| 2 | 3.524–3.429 | - | 2 | - | - |
| 3 | 3.429–3.333 | - | - | 4 | 2 |
| 4 | 3.333–3.225 | - | - | - | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornejo, A.; Reyero, I.; Campo, I.; Arzamendi, G.; Gandía, L.M. Acid-Catalyzed Etherification of Glycerol with Tert-Butanol: Reaction Monitoring through a Complete Identification of the Produced Alkyl Ethers. Catalysts 2023, 13, 1386. https://doi.org/10.3390/catal13101386
Cornejo A, Reyero I, Campo I, Arzamendi G, Gandía LM. Acid-Catalyzed Etherification of Glycerol with Tert-Butanol: Reaction Monitoring through a Complete Identification of the Produced Alkyl Ethers. Catalysts. 2023; 13(10):1386. https://doi.org/10.3390/catal13101386
Chicago/Turabian StyleCornejo, Alfonso, Inés Reyero, Idoia Campo, Gurutze Arzamendi, and Luis M. Gandía. 2023. "Acid-Catalyzed Etherification of Glycerol with Tert-Butanol: Reaction Monitoring through a Complete Identification of the Produced Alkyl Ethers" Catalysts 13, no. 10: 1386. https://doi.org/10.3390/catal13101386
APA StyleCornejo, A., Reyero, I., Campo, I., Arzamendi, G., & Gandía, L. M. (2023). Acid-Catalyzed Etherification of Glycerol with Tert-Butanol: Reaction Monitoring through a Complete Identification of the Produced Alkyl Ethers. Catalysts, 13(10), 1386. https://doi.org/10.3390/catal13101386









