Abstract
The quest for improved heterogeneous catalysts often leads to sophisticated solutions, which are expensive and tricky to scale up industrially. Herein, the effort to upgrade the existing inorganic nonmetallic materials has seldom been prioritized by the catalysis community, which could deliver cost-effective solutions to upgrade the industrial catalysts catalog. With this philosophy in mind, we demonstrate in this work that alloyed palladium-lead (Pd-Pb) deposited on novel precipitated calcium carbonate (PCC) supports could be considered an upgraded version of the industrial Lindlar catalyst for the semi-hydrogenation of phenylacetylene to styrene. By utilizing PCC supports of variable surface areas (up to 60 m2/g) and alloyed Pd-Pb loading, supported by material characterization tools, we showcase that achieving the “active-site isolation” feature could be the most pivotal criterion to maximize semi-hydrogenated alkenes selectivity at the expense of prohibiting the complete hydrogenation to alkanes. The calcite phase of our PCC supports governs the ultimate catalysis, via complexation with uniformly distributed alloyed Pb, which may facilitate the desired “active-site isolation” feature to boost the selectivity to the preferential product. Through this work, we also advocate increasing research efforts on mineral-based inorganic nonmetallic materials to deliver novel and improved cost-effective catalytic systems.
1. Introduction
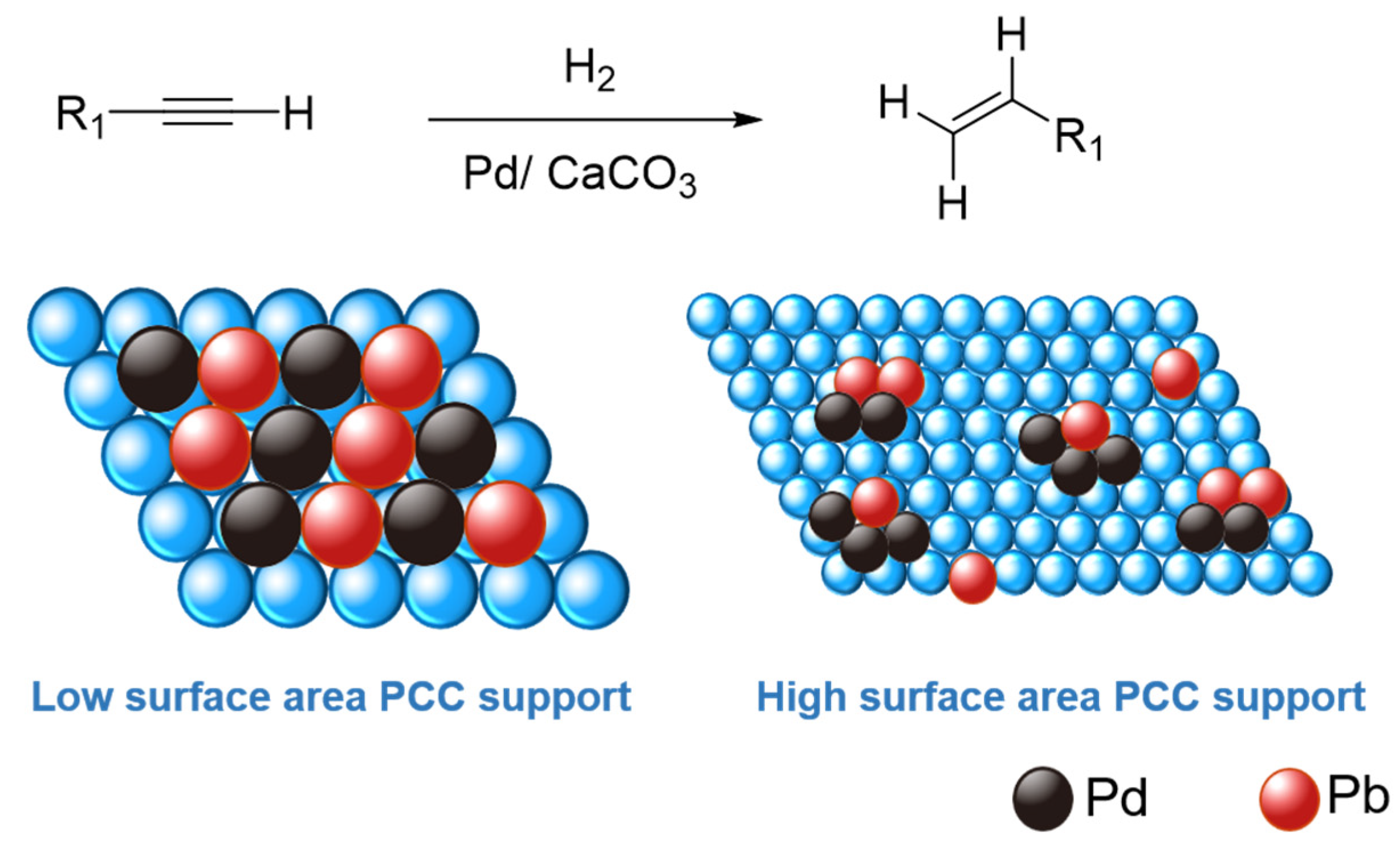
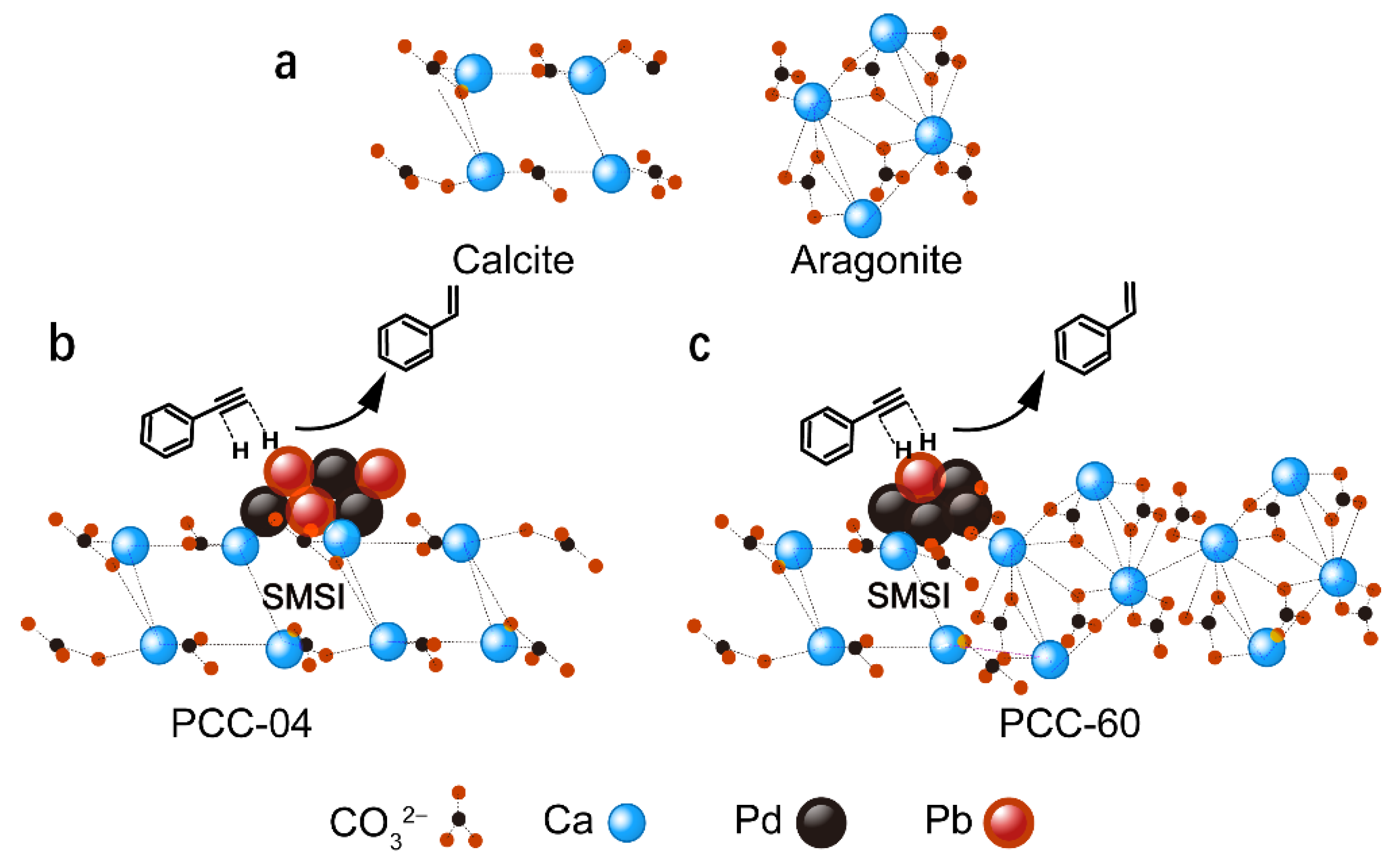
Semi-hydrogenation of carbon-carbon triple bond (C≡C) of alkyne to its corresponding alkene (C=C) is a reaction of high-industrial significance [1,2]. Among them, removing phenylacetylene from styrene feed, via such semi-hydrogenation, during styrene polymerization is essential because phenylacetylene, as an impurity above a concentration of 10 ppm, is poisonous to the polymerization catalysts and promotes quicker catalyst deactivation [3,4]. Therefore, the selective hydrogenation of phenylacetylene under benign conditions to the intermediate styrene is essential for the polymer industry. Styrene is a raw material for the polymer industry with an annual production of at least 30 million tons, including solid polystyrene (PS), expandable polystyrene (EPS), styrene-butadiene latex (SBL), acrylonitrile-butadiene-styrene/terpolymer (ABS), unsaturated polyester resins (UPR), and styrene-butadiene rubber (SBR) [5]. In addition, such alkynes semi-hydrogenation gives access to numerous target-oriented alkenes in organic synthesis, such as in fragrances, agrochemicals, and pharmaceuticals [6,7]. However, the major challenge here is, of course, to prohibit the complete hydrogenation to the corresponding alkane and maximize the semi-hydrogenated alkene product yields. Traditionally, the highly popular Lindlar catalyst, i.e., 5 wt% palladium (Pd) deposited on calcium carbonate (CaCO3) and partially poisoned/alloyed with external substance (e.g., lead (Pb), mercury (Hg), quinoline), is the most used catalytic material in the (petro-)chemical industry for semi-hydrogenation processes [2,8]. Since the surface of unmodified supported Pd catalyst is truly over-hydrogenated toward undesired alkanes, these active sites could be modified by “poisonous” alloyed Pb, via avoiding excessive Pd agglomeration and subsequent formation of “Pd-H” species in close proximity, which in turn pauses the reaction to deliver semi-hydrogenated alkenes (Figure 1) [9,10,11,12].
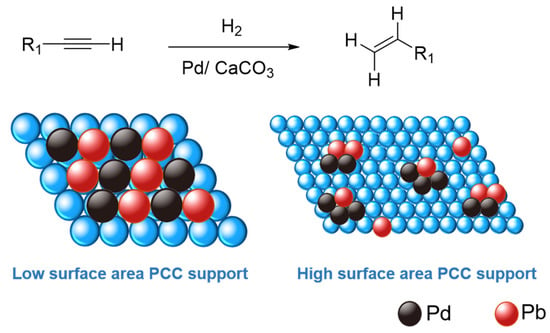
Figure 1.
Simplistic illustration of “active-site isolation” concept over low- and high-surface-area precipitated CaCO3 (PCC) supports.
Mechanistically, the key is to control the substrate adsorption on the metal active sites to promote selectivity toward alkene formation [13,14]. Herein, the catalysis community is seeking alternative pathways to deal with this challenge by developing environmentally friendly catalysts via emphasizing forming “isolated” metal sites to prevent agglomeration and, consequently, over-hydrogenation [13,14,15]. With this objective in mind, a wide variety of Pd/Pt-based advanced catalytic materials have been designed and screened for the semi-hydrogenation of alkynes: for example, (Lindlar-type) soluble or solid-supported Pd-(CaCO3)n clusters and supported on biogenic CaCO3 ([14,16]), as well as fabricated bimetallic catalysts (i.e., using non-Pb second elements: B, Cu, Zn, Mg, Cd, Ru, Fe, Ag [17,18,19,20,21,22,23,24,25]), carbonaceous-supported or confined catalysts (including carbides, carbon nanotubes and graphenes [13,26,27,28,29,30]), silica/alumina/TiO2/zeolite supported catalysts [13,31,32,33,34], colloidal catalysts [35], metal-organic framework (MOF)-derived catalysts [36], single-atom catalysts [37,38,39], and even solar-driven photocatalysts [40]. Although all these (non-poisoned) catalysts have delivered interesting reactivity and selectivity, these materials are unable to replace the traditional Lindlar catalysts in the industry, possibly because of their expensive nature that limits their potential industrial application, both in terms of cost and upscaling.
The major thrust of this research arena has been devoted to tuning catalytic metal sites, with a particular emphasis on providing “active-site isolation” to prevent over-hydrogenation [41,42]. In a typical hydrogenation process over heterogeneous catalysts, the impact of subsurface hydride species is often crucial in controlling the final product’s selectivity, which tends to promote over-hydrogenated products. Therefore, building an alloy via adding a second element (e.g., Pb in the traditional Lindlar catalyst) is a prominent way to deliver such “active-site isolation”. Such a strategy directly impacts the adsorption and activation of reactants, i.e., alkyne, which is also a typical π-ligand in organometallic chemistry [43]. The interaction between the alkyne reactant and the surface of a catalyst could proceed via either ethylidyne or π-complex modes [44]. To facilitate the semi-hydrogenation to alkene products from alkyne, the relatively weaker π-bonding adsorption mode should be preferred. That could be achieved by isolating active catalytic sites to avoid further hydrogenation [45]. Therefore, to upgrade the commercial Lindlar catalyst, an alternative strategy is required to deliver such an “active-site isolation”, to avoid any potential agglomeration of catalytically active metal sites.
With this objective in mind, we turned our attention toward upgrading the CaCO3 support of the Lindlar catalyst. Herein, our original hypothesis was to enhance the surface area of CaCO3 supports to facilitate such an “active-site isolation” philosophy. It is worth mentioning that commercial Lindlar catalysts or the analogous commercial Pd/Pt-supported CaCO3 catalysts have limited surface areas (typically < 10 m2/g). Hypothetically, it can be anticipated that an increased surface area of the catalyst support could be an alternate strategy to achieve the desired site isolation. Here in this work, we have screened numerous precipitated CaCO3 (PCC) supports with variable surface areas (up to 60 m2/g) and commercial Lindlar catalysts for the semi-hydrogenation of alkyne substrates. This work unexpectedly demonstrates the beneficial effect of low-surface area PCC supports. Additionally, our Pd-Pb/PCC catalyst delivered superior performance vis-à-vis its commercial counterpart, under lower catalyst loading and using optimized reaction conditions. For example, our catalyst (i.e., 5 wt% Pd on low-surface area PCC support) and commercial 5 wt% Pd-based Lindlar catalyst delivered 81% and 66% of selectivity toward styrene, respectively, after the semi-hydrogenation of phenylacetylene. Interestingly, an almost similar selectivity (~79%) was also obtained upon lowering the catalyst loading, i.e., using 1 wt% Pd on low-surface area PCC support, advocating the concept of “active-site isolation” over our PCC-based catalyst supports. These interesting catalytic results have also been well corroborated with an in-depth characterization using multi-modal spectroscopic and analytical techniques, including X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), scanning electron microscopy (SEM), scanning calorimetry (TGA-DSC), X-Ray photoelectron spectroscopy (XPS), and nitrogen physical adsorption and desorption (N2-BET). Such characterization has also been extended to the commercial Lindlar catalysts to establish their structure–reactivity relationships and provide a rationalization of the fundamental difference in the catalysis results reported in this work.
2. Results and Discussion
2.1. Catalyst Preparation and Characterization
This work introduces three different precipitated calcium carbonate (PCC) supports with variable surface areas: PCC-04, PCC-30, and PCC-60, where the number denotes their respective BET surface area in m2/g (see Table 1 for the physicochemical parameters’ comparison). In this work, we evaluated the performance of both Pb-modified and unmodified catalytic materials, representing Pd-Pb/PCC-xy and Pd/PCC-xy (xy: respective BET surface area in m2/g, see Table 1, Figures S1 and S2). For the sake of comparison with the commercial Lindlar catalysts, all catalytic materials were synthesized as originally reported by Lindlar and Dubuis [46].

Table 1.
The comparison of physical parameters between our PCC supports and catalysts vis-à-vis their commercial counterparts.
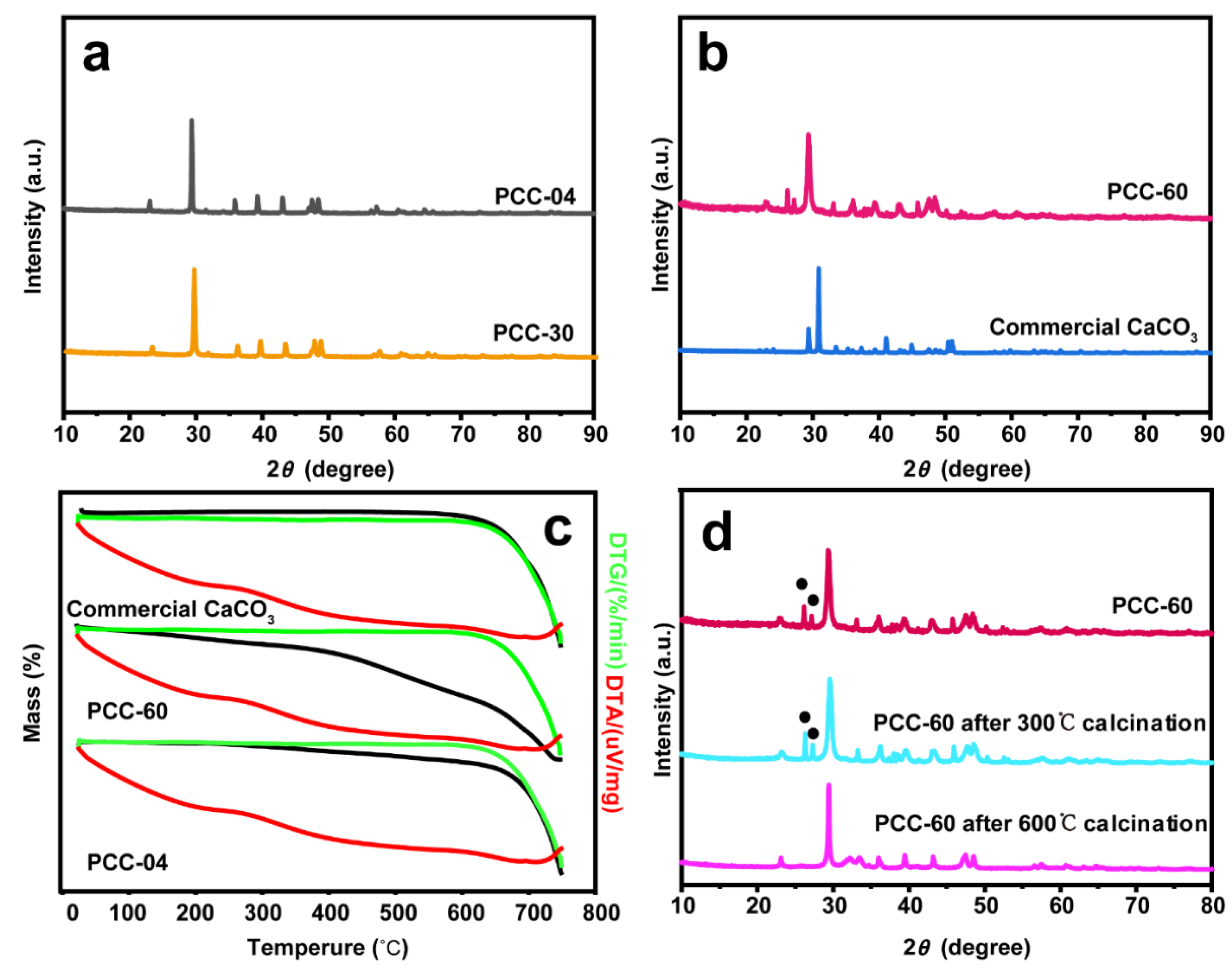
The physicochemical properties of our PCC supports and their commercial counterpart are illustrated in Figure 2. X-ray powder diffraction (XRD) has been performed to identify the phases of each material. PCC-04 and PCC-30 demonstrated responses at 2θ of 22.9°, 29.3°, 35.8°, 39.3°, 43.0°, 47.5°, 48.4°, and 57.2°, which belong to the diffraction peaks of (012), (104), (110), (113), (202), (018), (116), and (122) crystal planes, respectively (Figure 2a,b and Figure S3) [47], which corresponds to the calcite phase, the most stable polymorph of CaCO3. XRD of PCC-60 showed mixed phases of both calcite and aragonite CaCO3 (Figure 2b and Figure S3): diffraction peaks (i) at 23.2°, 29.44°, 36.13°, 43.3°, 47.7°, and 48.7 at 2θ could be attributed to (012), (014), (110), (202), (018), and (116) crystal planes, respectively (i.e., consistent with the standard card JCPDS: 85-0849 for calcite CaCO3) and (ii) at 26.35°, 27.21°, 33.12°, 37.88°, 45.85°, 48.44°, and 48.61° corresponds to (111), (021), (012), (112), (221), and (202), respectively (i.e., consistent with the standard card JCPDS: 41-1475 for aragonite CaCO3). Hence, PCC-60 comprises the trigonal stable calcite and orthorhombic (relatively) unstable aragonite phases [48]. Moreover, the lack of impurity within the XRD spectrum exhibits the high purity of our PCC supports.
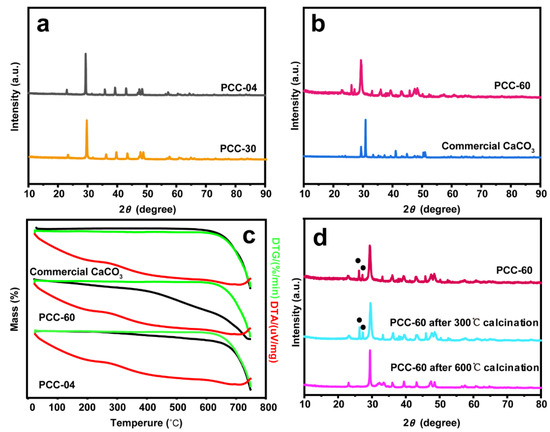
Figure 2.
X-ray powder diffraction (XRD) patterns of standalone (a) PCC-04 and PCC-30, as well as (b) PCC-60 and commercial CaCO3, indicate the existence of mixed phases (aragonite and calcite) in PCC-60. sample. (c) Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) of standalone PCC-04, PCC-60, and commercial CaCO3 supports, demonstrating higher mass loss in the PCC-60 sample only. (d) XRD pattern of standalone PCC-60 support after the calcination at 300 °C and 600 °C highlights the transformation from the relatively unstable aragonite phase to a more stable calcite phase (The black dots refer to the diffraction peak of the aragonite phase). We refer to Figures S3–S5 for additional characterization data.
Since PCC-60 is composed of an aragonite phase, thermogravimetric analysis (TGA) has been performed to verify the material’s phase transition during the gradual heating process (Figures S4 and S5). Figure 2c shows that the commercial CaCO3 began to decompose to CaO at > 600 °C, while PCC-60 and PCC-04 began to decompose continuously from > 200 °C and > 100 °C, respectively, probably attributed to the presence of Ca(OH)2 [49]. Herein, the extent of decomposition was significantly greater over PCC-60 (Figure S4c). At the temperature region between 300 °C and 450 °C, the aragonite phase in PCC-60 transformed into calcite, which could explain the higher mass loss [50]. To support this observation, XRD has been performed on the standalone PCC-60 support after calcining the sample at 300 °C, and 600 °C (Figure 2d). Both calcite and aragonite crystal phases were detected at 300 °C, but only calcite was observed when PCC-60 was thermally treated at 600 °C, which supports the mutual transformation between the two crystal forms of CaCO3 [51].
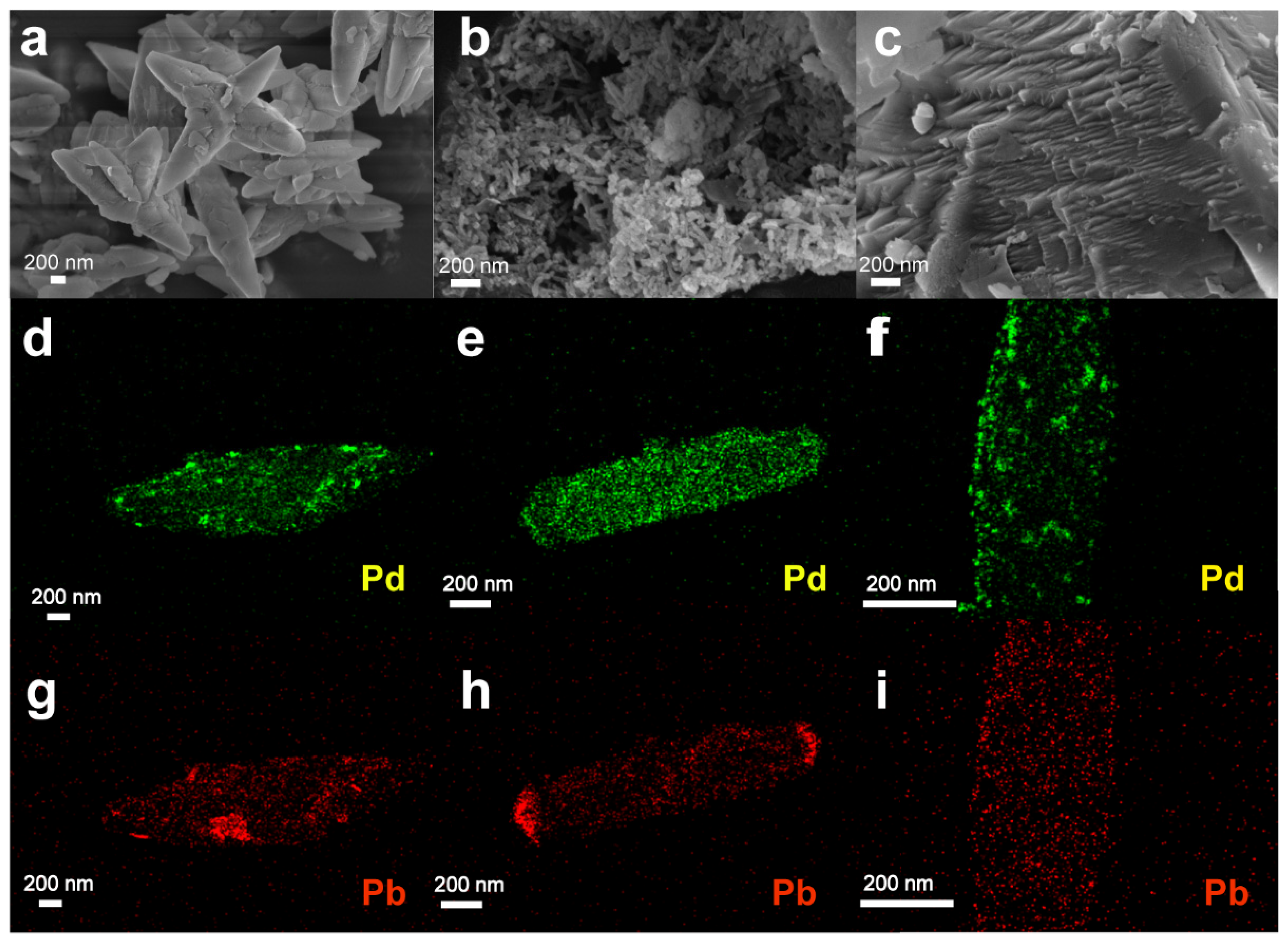
Next, we turned our attention to understanding the morphology of the CaCO3-based supports and their corresponding Pd catalysts, using both scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HRTEM) (Figure 3 and Figures S6–S12). Using SEM, the standalone PCC-04 support exhibited a distinct fusiform shape, with a length of about 2 μm, and displayed a crisscross structure (Figure 3a and Figure S6). The standalone PCC-30 showed a dense granular shape (Figure S7). In contrast, the PCC-60 support displayed a more organized network structure with a porous nature (Figure 3b and Figure S8). The commercial calcium carbonate exhibited a lamellar structure (Figure 3c and Figure S9).
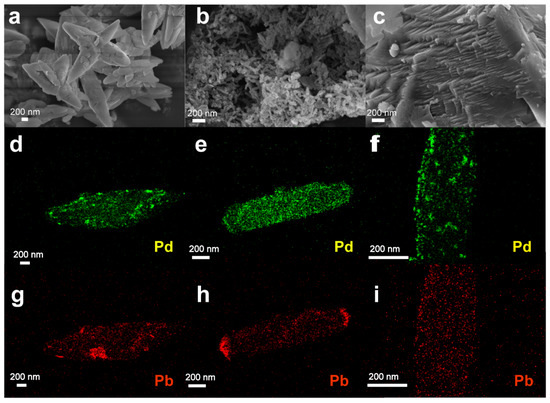
Figure 3.
(a–c) Scanning electron microscope (SEM) image of standalone (a) PCC-04, (b) PCC-60, and (c) commercial CaCO3 supports. (d–f) Pd and (g–i) Pb mapping by high-resolution transmission electron microscopy (HRTEM) on (d,g) 5 wt% Pd-Pb/PCC-04, (e,h) 5 wt% Pd-Pb/PCC-60, and (f,i) commercial 5 wt% Pd-Pb Lindlar catalysts. We refer to Figures S6–S12 for additional characterization data.
HRTEM analysis was performed on Pd-Pb loaded materials to analyze the dispersion of the Pd and Pb particles (Figure 3c and Figure S10). TEM-mapping on Pd-Pb/PCC-04 material presented a (relatively) uniform distribution of the Pb particles on the catalyst surface; in contrast, the Pd particles were situated primarily on the edges (Figure S10). In the case of the Pd-Pb/PCC-60 material, the Pd particles displayed “zonal clusters” throughout the material (Figure S11). The benchmark Lindlar catalyst reveals mixed characteristics: the uniform distribution of the Pb particles was visible (cf. similar to PCC-04 support), but with higher agglomeration/clustering of the Pd particles (cf. almost similar to PCC-60 support) (Figure 3f and Figure S12). The alloyed nature derived from Pd and Pb was visualized in all materials, including the commercial catalysts. However, the Pd-Pb alloyed nature and distribution are dissimilar and heterogeneous in nature (including the commercial catalyst).
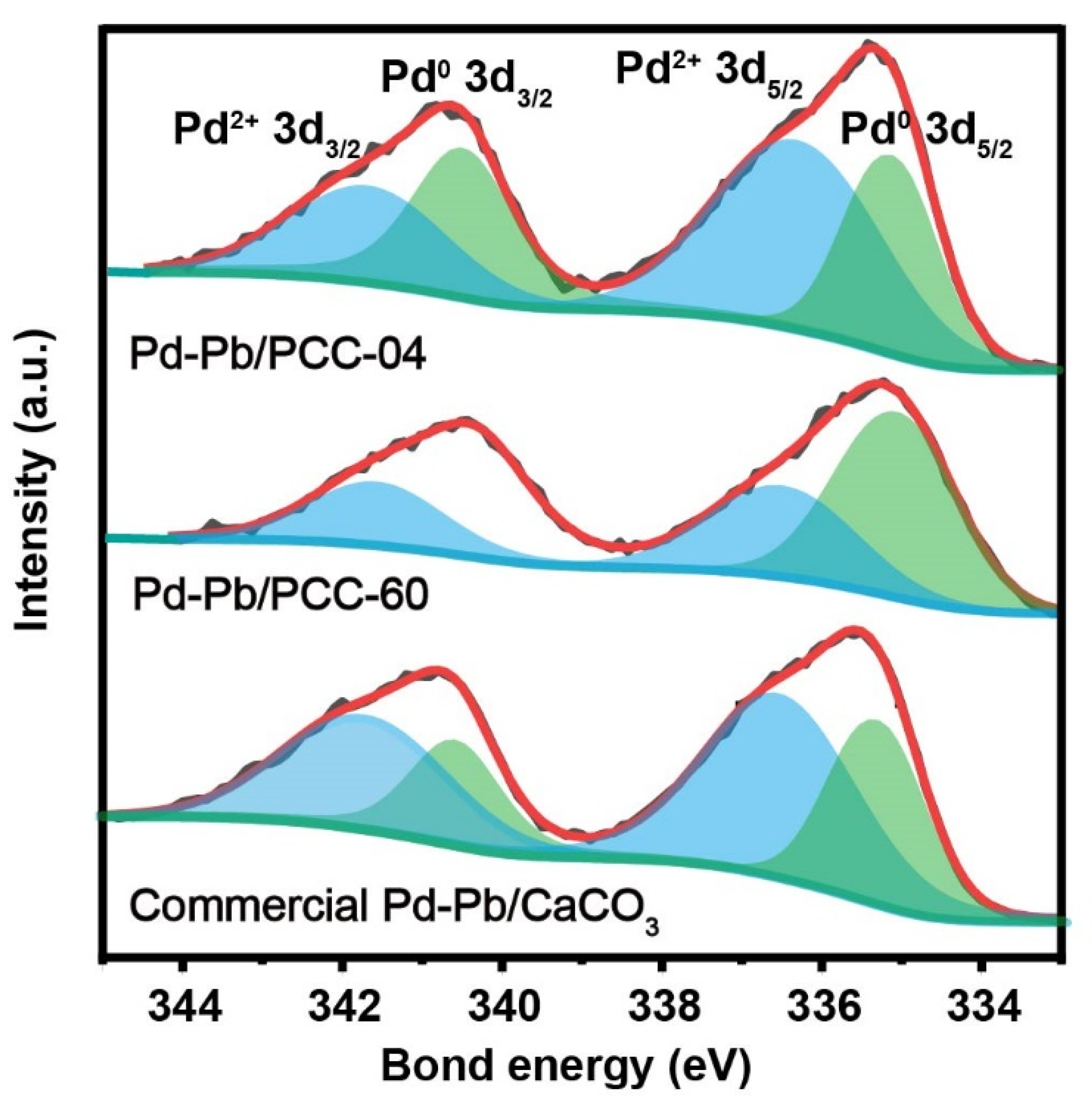
X-ray photoelectron spectroscopy (XPS) was performed to probe the catalyst’s surface, especially the local environment of palladium and oxygen (Figure 4 and Figures S13–S15). Herein, we restrict our discussion to the following three key materials: Pd-Pb/PCC-04 (i.e., Pd on low-surface area calcite CaCO3), Pd-Pb/PCC-60 (i.e., Pd on high-surface-area calcite and aragonite CaCO3), and commercial Lindlar catalyst (Pd-Pb/CaCO3) materials. Figure 4 demonstrates each material’s high-resolution XPS spectrum of the fitted Pd 3d peak. Regarding Pd, two species (Pd0 and Pd2+) were observed in all three catalysts, confirming the co-existence of Pd0 and Pd2+ on their surfaces, including the commercial samples. Herein, the Pd 3d spectrum consists of two main doublets after Gaussian fitting, indicating two distinct Pd states: Pd03d5/2 (335.2–335.4 eV), Pd2+3d5/2 (336.4–336.6 eV), Pd03d3/2 (340.5 eV) and Pd2+3d3/2 (341.7 eV). The oxidized Pd state is predominant on the low specific surface area supports (both PCC-04 and commercial catalysts), which could be attributed to the size effect or the strong chemical interaction between Pd and CO32− [52]. Over the high specific surface area support (PCC-60), the metallic feature of Pd is apparent due to its larger Pd particle size and lack of interaction with CO32−. As previously mentioned, it may eventually explain its “zonal clustering” feature (Figures S13–S15). Hence, it was speculated that the Pd particles formed on the low specific surface area CaCO3 could preferentially be in the vicinity of the (alloyed) Pb particles, which led to superior site-isolation features. On the contrary, the higher specific surface area PCC support contains fewer Pb atoms in the vicinity of the Pd atoms since the surface of the PCC support was “stretched”. The calculated Pd0/Pd2+ on the three carriers are 0.77 (PCC-04), 1.86 (PCC-60), and 0.56 (commercial catalyst), indicating that Pd-Pb/PCC-04 could have a moderate reducing ability and may have the best semi-hydrogenation effect along with the commercial catalyst. It can be seen that different CaCO3-based supports could significantly impact the ratio of the Pd0/Pd2+ species, especially on the high-surface area PCC supports.
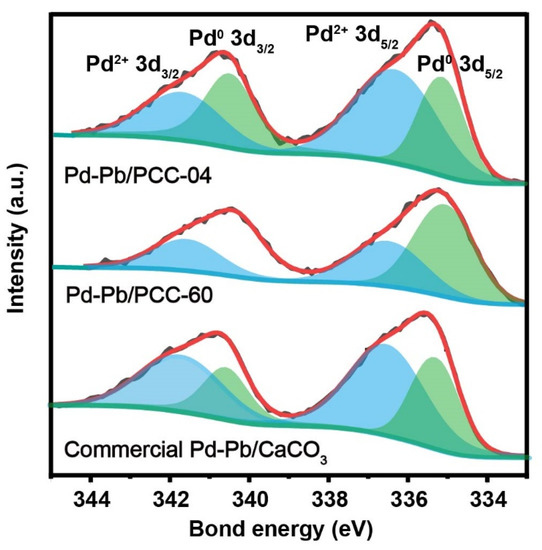
Figure 4.
X-ray photoelectron spectroscopy (XPS) profile of Pd 3d on 5 wt% Pd-Pb/PCC-04, 5 wt% Pd-Pb/PCC-60, and commercial 5 wt% Pd-Pb/CaCO3. We refer to Figures S13–S15 for additional characterization data.
2.2. Catalysis: Study on Semi-Hydrogenation of Phenylacetylene
All materials were screened in the semi-hydrogenation of phenylacetylene, as a model substrate to rationalize the understanding of our material characterization (Table 2). Generally, all non-Pb alloyed catalysts lead to the fully hydrogenated ethyl benzene as a sole product, indicating the over-hydrogenated nature of all catalysts in the absence of Pb. Upon Pb-alloying, all materials delivered a complete conversion with semi-hydrogenated styrene as a major product. Moreover, 5 wt% Pd-Pb catalysts on PCC-04 and PCC-30 delivered a similar selectivity (~80%), while 5 wt% Pd-Pb catalysts on PCC-60 and the commercial Lindlar catalyst delivered similar styrene selectivity (~67%) under our reaction conditions. However, over the 1 wt% Pd-Pb catalysts, complete conversion was also achieved, where the higher selectivity to styrene could be maintained over all the PCC-supported catalysts (Table S1). Upon lowering the Pd loading, the superior “site-isolation” feature could be obtained over the PCC supports, which might explain the similar performance achieved at lower Pd loading.

Table 2.
Catalysts screening with phenylacetylene as a model substrate a.
Although complete conversions were achieved within 30 min of reaction, longer reaction times were also carried out to test the over-hydrogenated nature of our optimized catalysts (i.e., 5 wt% Pd-Pb/PCC-04 and commercial 5 wt% Pd-Pb Lindlar catalyst). As seen in Figure S16, the selectivity toward over-hydrogenated ethylbenzene has not been increased over time using our 5 wt% Pd-Pb/PCC-04 catalytic material. Herein, the selectivity toward the desired styrene products remained almost constant after 6 h of reaction. However, a different reaction profile was obtained over the commercial Lindlar catalyst, using our optimized reaction conditions: the gradual decrease in (semi-hydrogenated) styrene selectivity at the expense of the increasing selectivity to (over-hydrogenated) ethylbenzenes over time. Such a sharp contrast of the time-monitored reaction profile unequivocally supports our PCC-derived catalytic material’s superior, stable, and robust performance compared to its commercial counterpart (Table S2).
2.3. Mechanistic Hypothesis and Post-Reacted Catalyst Characterization
Based on the above-mentioned material characterization and their catalytic performance, we argue that the nature and type of the CaCO3 support hold the key to delivering superior “active-site isolation”, which promotes the formation of the semi-hydrogenated products. Both the PCC-04 supported and the commercial catalysts provided excellent selectivity, while the higher surface area of PCC-60 has surprisingly limited added value. Although it was initially anticipated that a higher surface area could facilitate catalytic active-site isolation, such a phenomenon has not been reflected in the materials’ characterization or catalytic performance. Contrarily, it indicates that a strong metal-support interaction (SMSI) might play a decisive role in governing catalysis [53]. The SMSI phenomenon is typically a function of numerous physicochemical properties, including charge transfer and the adsorption between nanoparticles and carriers [53,54]. Depending on the catalyst and the reaction involved, some phenomena will dominate, as demonstrated in this case. Changes in the oxidation state of the metal atoms of the nanoparticles or carrier metal ions may accompany it. XPS shows that in the same 5 wt% Pb catalysts, Pd electron transfer occurs when the carrier changes. It indirectly justifies in favor of the existence of SMSI in the present case. Herein, XRD analysis revealed that PCC-60 comprises an almost equal volume of aragonite and calcite crystal forms (Figure S3c). Hence, we hypothesize that the bonding of the Pd particles to the aragonite crystals might be unstable or absent. Since the stronger interaction between the Pd particles and calcite is well established (i.e., strong SMSI) and influential for the catalysis (see Figure 5), we argue that the presence of the aragonite phase is detrimental to the catalysis (or has limited impact). Alternatively, our PCC-04-supported Pd-based catalyst delivered superior product selectivity and robustness compared to the commercial Lindlar catalyst. Next, we performed TEM studies over post-reacted catalytic materials: 5 wt% Pd-Pb/PCC-04 and Commercial 5 wt% Pd-Pb/CaCO3 (Figure 6). Interestingly, both materials delivered similar features after the reaction: the uniform distribution of the Pb and Pd clustering or agglomeration is noticed in both cases. Such an agglomeration of Pd particles after the reaction is not uncommon [55]; therefore, the catalytically relevant “active-site-isolation” feature is well suited for PCC-04 support compared to the other catalysts used in this study. It implies that Pb might also be preferentially combined with calcite forms (possibly through the complexation as confirmed by its homogeneous distribution before or after the reaction) (Figure 5 and Figure 6), inhibiting the interaction between the alkyne substrate and the catalytically active sites. However, the similar Pb loading spread across the lower-surface area samples may significantly enhance the desired site-isolation features, leading to improved alkene selectivity.
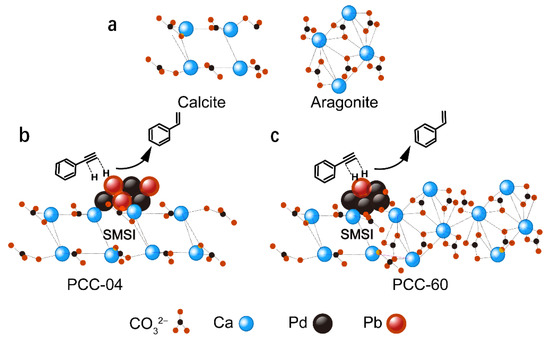
Figure 5.
Simplified illustration of our PCC-derived catalysts’ working hypothesis: (a) crystal model diagram of calcite and aragonite. A model reaction diagram of the semi-hydrogenation of phenylacetylene over (b) PCC-04 (i.e., consists of calcite phase only) and (c) PCC-60 (i.e., contains both calcite and aragonite phases) support. Only the calcite phase is catalytically relevant and delivers a strong metal-support interaction (SMSI), which allowed uniform distribution of Pd/Pb, presumably through complexation, key criteria to provide the “active-site isolation”, a feature necessary for the catalysis presented in this study.

Figure 6.
Transmission electron microscopic (TEM) images of post-reacted (a) Pd-Pb/PCC-04 and (b) commercial Pd-Pb/CaCO3 catalytic material. (c,d) Pd and (e,f) Pb mapping on (c,e) Pd-Pb/PCC-04 and (d,f) commercial Pd-Pb Lindlar catalysts.
3. Materials and Methods
All chemicals and materials were purchased in the highest purity grade available and used without further purification: Phenylacetylene (Alfa Aesar, Haverhill, MA, USA, 98+%), diphenylacetylene (Energy Chemical, ZeSheng, Anhui, China, 98%), 1-heptene (Alfa Aesar, Haverhill, MA, USA, 98+%), 3-phenyl-2-propyn-1-ol (HEOWNS, TianJin, China, 96%), biphenylacetylene (Energy Chemical, ZeSheng, Anhui, China, 98%), 4-ethynyltoluene (HEOWNS, TianJin, China, 98%), 4-ethynylanisole (Bide, ShangHai, China, 98%), 4-fluorophenylacetylene (Energy Chemical, ZeSheng, Anhui, China, 98%), 4-ethynylbenzaldehyde (Bide, ShangHai, China, 98%), cyclohexane (Innochem, BeiJing, China, 99.5%), ethanol (Innochem, BeiJing, China, AR, 95%), tetrahydrofuran (Innochem, BeiJing, China, 99%, AR), mesitylene (Alfa Aesar, Haverhill, MA, USA, 98+%), ethyl acetate (Sinopharm, ShangHai, China, AR), methanol (Innochem, BeiJing, China, AR, 99.5%), deuterated chloroform (Innochem, BeiJing, China, 99.8 atom % D), palladium chloride (Bide, ShangHai, China, 99%), hydrochloric acid (Sinopharm, ShangHai, China, 37%), sodium formate (Macklin, ShangHai, China, 99.5%), lead acetate (Aladdin, ShangHai, China, 99%), 5 wt% Pd/CaCO3 Lindlar catalyst (Strem Chemicals Inc, North of Boston, MA, USA), commercial CaCO3 (Macklin, ShangHai, China, 99%). All precipitated CaCO3 (PCC) supports were obtained from Omya International.
Powder X-ray diffraction (XRD) analysis was performed using a Bruker D8 ADVANCE X diffractometer (Bruker, Karlsruhe, Germany) using a Cu Kα radiation source (λ = 0.154 nm). Diffraction patterns are collected in the 2θ range between 10° and 80° with a step size of 4°/min. The catalysts’ lattice constant and average grain size is analyzed using JADE software to analyze the recorded data. Scanning electron microscopy (SEM) images were acquired on a thermal field emission scanning electron microscope model Zeiss SIGMA (Carl Zeiss AG, Oberkochen, Germany). High-resolution transmission electron microscope (HRTEM) images were obtained using a Tecnai G3 F30 S-TWIN (FEI, Hillsboro, OR, USA). Thermal gravimetric analysis (TGA-DSC/TGA-DTA) was performed using a NETZSCH 449 F5/F3 Jupiter (NETZSCH, Selb, Germany) synchronous thermal analyzer. Under the N2 atmosphere, the temperature increased from room to 800 °C at 10 °C/min: temperature stability 0.1 °C, balance sensitivity 0.1 µL. The Brunauer–Emmett–Teller (BET) is measured by a nitrogen physical adsorption (ASAP 2460 Micrometrics) (Micromeritics, Norcross, GA, USA). Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) was performed on an Agilent ICPOES730 (Agilent, Palo Alto, CA, USA) instrument. X-ray photoelectron spectroscopy (XPS) was performed using a non-monochromatic Thermo ESCALAB 250Xi X spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) to generate Al Kα radiation (hν = 1486.6 eV) (10−10 Torr) under ultra-high vacuum. The charging effect is corrected relative to the carbon peak C 1s (284.8 eV). The deconvolution of the peak spectrum is performed by XPSPEAK41 software [56], using Gaussian–Lorentzian linear and Shirley background subtraction.
3.1. Synthesis of Pd/PCC and Pd-Pb/PCC-Based Catalytic Materials
The synthesis protocol of catalytic materials is similar to the originally reported by Lindlar et al. [46,57]. In a typical synthesis, 0.46 mmol of PdCl2 was taken into a 10 mL Erlenmeyer flask to achieve 5 wt% loadings and followed by 0.2 mL of 37% hydrochloric acid. The flask was stirred at approximately 30 °C until the PdCl2 was completely dissolved. Next, the resultant acidic solution was transferred to a 10 mL beaker filled with 2.5 mL of distilled water, where 3 mol/L aqueous sodium hydroxide solution was slowly added to bring the pH of the solution to 4.0–4.5. Next, the above solution was immersed in a water bath and heated at 85 °C. Next, 1 g of CaCO3 support (PCC-04, PCC-30, PCC-60, commercial CaCO3) was added, and the temperature was held until all the Pd precipitated, which took approximately 15 min. While keeping the mixture at 85 °C, 330 μL HCOONa solution (0.47 g of HCOONa was added to 10 mL water to form a 0.7 mol/L solution) was added and stirred for 40 min, followed by the addition of 250 μL HCOONa solution and stirring for 40 min. Then, it was washed by centrifugation with the addition of deionized water and dried in an oven at 60 °C. The final catalytic materials were labeled as Pd/PPC-04, Pd/PPC-30, Pd/PPC-60, and commercial Pd/CaCO3. In order to obtain the products Pd-Pb/PPC-04, Pd-Pb/PPC-30, Pd-Pb/PPC-60, and commercial Pd-Pb/CaCO3, the above non-centrifugally dried products were retained. Next, 3.3 mL of deionized water and 1 mL of 7.7% (CH3COO)2Pb solution (0.45 g of (CH3COO)2Pb was added to 5 mL water to form a solution) were added to the above mixture and stirred for 40 min. Finally, it was washed by centrifugation with the addition of deionized water and dried in an oven at 60 °C.
3.2. Catalytic Testing
All catalytic hydrogenations were performed in a 250 mL Wattcas autoclave (WP-MSAR-250A). In a typical experiment, 1 mmol of alkyne substrate, 5 wt% catalyst, and 3 mL of cyclohexane (as solvent) were added to the reactor. After purging three times with H2, H2 was filled to 20 bar at ambient temperature. The reaction temperature and time were kept according to the need under strong magnetic stirring. After the reaction, the batch reactor was cooled to room temperature, and the product was separated from the catalyst by filtration through a microporous membrane. Next, mesitylene was added as an internal standard and then diluted with ethyl acetate. Finally, the collected sample solution was identified by a gas chromatograph−mass spectrometer combination (GCMS-QP2010 SE) and quantified by a gas chromatograph (GC-2014) with HPINNOWAX capillary column (30 m × 0.250 mm × 0.25 μm) or NMR spectroscopy. The GC detecting conditions were as follows: nitrogen was used as carrier gas; injection port temperature: 350 °C; detector (FID) temperature: 300 °C; column temperature: 50 °C, heating to 250 °C with a heating rate of 6 °C/min. 1H NMR data were recorded with Bruker Advance III (400 MHz) spectrometer with internal standard (mesitylene). The purified product was added with 1 mmol of mesitylene as the internal standard and mixed thoroughly. Moreover, 50 μL of internal standard containing reaction solution was added into 0.75 mL deuterium chloroform (CDCl3) for analysis.
4. Conclusions
With an aim to upgrade the industrial Lindlar catalyst for the semi-hydrogenation of phenyl acetylene to styrene, we demonstrated that newly derived precipitated CaCO3 (PCC) holds the critical criteria to deliver superior catalytic performance compared to the commercial Lindlar catalyst. Herein, our low-surface area PCC-derived Pd-Pb catalytic material provided superior catalytic performance, stability, and robustness compared to its two counterpart materials: i.e., Pd-Pb over both high-surface area PCC materials and the commercial Lindlar catalyst. Our low-surface area Pd-Pb/PCC could deliver similar reactivity and selectivity even after lowering the metallic loading, indicating that the “active-site isolation” and “strong metal-support interaction” (SMSI) features are possibly among the contributing factors that may impact the catalysis. A diverse characterization technique has been employed to understand the catalytic performance from the material perspective. The uniform metallic distribution was accomplished through complexation between the alloyed Pb and calcite phase, delivering the desired active-site isolation necessary for the catalysis. Owing to the superior catalytic performance, stability, and robustness, we represent our PCC-derived catalyst as a promising candidate to upgrade the current state of Lindlar catalysts in the chemical industry. Through this work, we also advocate to actively research cost-effective inorganic supports or minerals instead of providing (mainly) elegant and expensive solutions. We certainly believe that this alternative research approach could eventually deliver better performance and also be easier to scale up for a potential industrial application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13010050/s1. Figure S1: The physical adsorption spectrum of the (a) PCC-04, (b) PCC-30, (c) PCC-60, and (d) commercial CaCO3; Figure S2: The physical adsorption spectrum of 5 wt% (a) Pd-Pb/PCC-04, (b) Pd-Pb/PCC-30, (c) Pd-Pb/PCC-60, and (d) commercial Pd-Pb/CaCO3 (Lindlar catalyst). Figure S3: The XRD spectrum of the (a) PCC-04, (b) PCC-30, (c) PCC-60, and (d) commercial CaCO3; Figure S4: Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) of (a) PCC-04, (b) PCC-30, (c) PCC-60, and (d) commercial CaCO3; Figure S5: Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) of the commercial 5 wt% Pd-Pb/CaCO3 material (Lindlar catalyst); Figure S6: (a–d) Scanning electron microscope (SEM) images of standalone PCC-04 support, including mapping of (e) oxygen and (f) calcium; Figure S7. (a–d) Scanning electron microscope (SEM) images of standalone PCC-30 support, including mapping of (e) oxygen and (f) calcium; Figure S8. (a–d) Scanning electron microscope (SEM) images of standalone PCC-60 support, including mapping of (e) oxygen and (f) calcium; Figure S9. (a–d) Scanning electron microscope (SEM) images of standalone commercial CaCO3 support, including mapping of (e) oxygen and (f) calcium; Figure S10. (a–d) Transmission Electron Microscope (TEM) images of standalone 5 wt% Pd-Pb/PCC-04, including (e) electronic image, (f) overlapping mapping of key elements, as well as individual mapping of (g) carbon, (h) oxygen, (i) calcium (j) palladium, and (k) lead. Figure S11. (a–d) Transmission Electron Microscope (TEM) images of standalone 5 wt% Pd-Pb/PCC-60, including (e) electronic image, (f) overlapping mapping of key elements, as well as individual mapping of (g) carbon, (h) oxygen, (i) calcium (j) palladium, and (k) lead; Figure S12. (a–d) Transmission Electron Microscope (TEM) images of commercial 5 wt% Pd-Pb/CaCO3 (i.e., Lindlar), including (e) electronic image, (f) overlapping of key elements, as well as individual mapping of (g) carbon, (h) oxygen, (i) calcium (j) palladium, and (k) lead; Figure S13: X-ray photoelectron spectroscopy (XPS) profiles of 5 wt% Pd-Pb/PCC-04 material, including (a) full spectrum, (b) C 1s, (c) Ca 2p, (d) O 1s, and (e) Pd 3d; Figure S14: X-ray photoelectron spectroscopy (XPS) profiles of 5 wt% Pd-Pb/PCC-60, including (a) full spectrum, (b) C 1s, (c) Ca 2p, (d) O 1s, and (e) Pd 3d; Figure S15: X-ray photoelectron spectroscopy (XPS) profiles of commercial Pd-Pb/CaCO3 (i.e., Lindlar catalyst), including (a) full spectrum, (b) C 1s, (c) Ca 2p, (d) O 1s, and (e) Pd 3d; Figure S16: Time-monitored data of (a) 5 wt% Pd-Pb/PCC-04, (b) 5 wt% Commercial 5 wt% Pd-Pb/CaCO3; Table S1: The catalyst screening with phenyl acetylene as a model substrate; Table S2: Comparison of semi-hydrogenation performance of alkyne over Pd-based catalysts. References [10,16,18,22,23,24,25,26,29,36,37,38] are cited in Supplementary Materials.
Author Contributions
Conceptualization, project administration, A.D.C. and J.F.; investigation, methodology, visualization, Y.Z., L.G. and Y.L.; writing—original draft preparation, Y.Z. and A.D.C.; writing—review and editing, Y.Z., L.G., Y.L., J.F. and A.D.C.; supervision, A.D.C. Herein, Y.Z., L.G. and Y.L. have contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank Craig DePorter, Nicole Russ and Sarah Gysin, from Omya International, for their support. Omya International is kindly acknowledged for financial support.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript and its Supplementary Material Files.
Conflicts of Interest
The authors declare the following financial interests/personal relationships that may be considered as potential competing interests: Jamal Ftouni reports a relationship with Omya International AG via employment.
References
- Pálinkó, I. Shiego Nishimura: Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis: Wiley, New York, Chichester, Weinheim, Brisbane, Singapore, Toronto, 2001. Appl. Catal. A Gen. 2002, 232, 289–290. [Google Scholar] [CrossRef]
- By, H.C.; Rase, H.F. Handbook of Commercial Catalysts. Heterogeneous Catalysts. By Howard F. Rase. Angew. Chem. Int. Ed. 2004, 43, 2324–2325. [Google Scholar]
- Vergunst, T.; Kapteijn, F.; Moulijn, J.A. Optimization of Geometric Properties of a Monolithic Catalyst for the Selective Hydrogenation of Phenylacetylene. Ind. Eng. Chem. Res. 2001, 40, 2801–2809. [Google Scholar] [CrossRef]
- Huang, X.; Wilhite, B.; McCready, M.J.; Varma, A. Phenylacetylene Hydrogenation in a Three-Phase Catalytic Packed-Bed Reactor: Experiments and Model. Chem. Eng. Sci. 2003, 58, 3465–3471. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, Y.; Wang, X.; Haribal, V.; Liu, J.; Neal, L.M.; Bao, Z.; Wu, Z.; Wang, H.; Li, F. A Tailored Multi-Functional Catalyst for Ultra-Efficient Styrene Production under a Cyclic Redox Scheme. Nat. Commun. 2021, 12, 1329. [Google Scholar] [CrossRef]
- Shi Shun, A.L.K.; Tykwinski, R.R. Synthese von Polyin-Naturstoffen. Angew. Chem. Int. Ed. 2006, 118, 1050–1073. [Google Scholar] [CrossRef]
- Bonrath, W.; Medlock, J.; Schutz, J.; Wustenberg, B.; Netscher, T. Hydrogenation in the Vitamins and Fine Chemicals Industry—An Overview. In Hydrogenation; InTech: Leeds, UK, 2012. [Google Scholar]
- Stachurski, J.; Thomas, J.M. Structural Aspects of the Lindlar Catalyst for Selective Hydrogenation. Catal. Lett. 1988, 1, 67–72. [Google Scholar] [CrossRef]
- García-Mota, M.; Bridier, B.; Pérez-Ramírez, J.; López, N. Interplay between Carbon Monoxide, Hydrides, and Carbides in Selective Alkyne Hydrogenation on Palladium. J. Catal. 2010, 273, 92–102. [Google Scholar] [CrossRef]
- Chaparro, S.; Martinez, J. Selective Hydrogenation of Functionalized Alkynes to (E)-Alkenes, Using Ordered Alloys as Catalysts. ACS Catal. 2016, 6, 2121–2125. [Google Scholar]
- Tew, M.W.; Janousch, M.; Huthwelker, T.; Van Bokhoven, J.A. The Roles of Carbide and Hydride in Oxide-Supported Palladium Nanoparticles for Alkyne Hydrogenation. J. Catal. 2011, 283, 45–54. [Google Scholar] [CrossRef]
- Bugaev, A.L.; Usoltsev, O.A.; Guda, A.A.; Lomachenko, K.A.; Pankin, I.A.; Rusalev, Y.V.; Emerich, H.; Groppo, E.; Pellegrini, R.; Soldatov, A.V.; et al. Palladium Carbide and Hydride Formation in the Bulk and at the Surface of Palladium Nanoparticles. J. Phys. Chem. C 2018, 122, 12029–12037. [Google Scholar] [CrossRef]
- Vilé, G.; Almora-Barrios, N.; Mitchell, S.; Lõpez, N.; Pérez-Ramírez, J. From the Lindlar Catalyst to Supported Ligand-Modified Palladium Nanoparticles: Selectivity Patterns and Accessibility Constraints in the Continuous-Flow Three-Phase Hydrogenation of Acetylenic Compounds. Chem. Eur. J. 2014, 20, 5926–5937. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Soberanas, J.; Hernández-Garrido, J.C.; Cerón-Carrasco, J.P.; Leyva-Pérez, A. Selective Semi-Hydrogenation of Internal Alkynes Catalyzed by Pd–CaCO3 Clusters. J. Catal. 2022, 408, 43–55. [Google Scholar] [CrossRef]
- Tejeda-Serrano, M.; Mon, M.; Ross, B.; Gonell, F.; Ferrando-Soria, J.; Corma, A.; Leyva-Pérez, A.; Armentano, D.; Pardo, E. Isolated Fe(III)-O Sites Catalyze the Hydrogenation of Acetylene in Ethylene Flows under Front-End Industrial Conditions. J. Am. Chem. Soc. 2018, 140, 8827–8832. [Google Scholar] [CrossRef]
- Chaparro, S.; Martinez, J.J.; Rojas, H.A.; Pineda, A.; Luque, R. Selective Continuous Flow Phenylacetylene Hydrogenation over Pd-Biogenic Calcium Carbonate. Catal. Today 2021, 368, 181–186. [Google Scholar] [CrossRef]
- Chan, C.W.A.; Mahadi, A.H.; Li, M.M.J.; Corbos, E.C.; Tang, C.; Jones, G.; Kuo, W.C.H.; Cookson, J.; Brown, C.M.; Bishop, P.T.; et al. Interstitial Modification of Palladium Nanoparticles with Boron Atoms as a Green Catalyst for Selective Hydrogenation. Nat. Commun. 2014, 5, 5787. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, X.; Li, L.; Liu, X.; Huang, Y.; Pan, X.; Wang, A.; Li, J.; Zhang, T. PdZn Intermetallic Nanostructure with Pd-Zn-Pd Ensembles for Highly Active and Chemoselective Semi-Hydrogenation of Acetylene. ACS Catal. 2016, 6, 1054–1061. [Google Scholar] [CrossRef]
- Pei, G.; Liu, X.; Chai, M.; Wang, A.; Zhang, T. Isolation of Pd Atoms by Cu for Semi-Hydrogenation of Acetylene: Effects of Cu Loading. Chin. J. Catal. 2017, 38, 1540–1548. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Shi, Y.; Liu, C.; Wu, L. CuPd Alloy Decorated SnNb2O6 Nanosheets as a Multifunctional Photocatalyst for Semihydrogenation of Phenylacetylene under Visible Light. Chem. Eng. J. 2022, 429, 132018. [Google Scholar] [CrossRef]
- Li, C.; Shao, Z.; Pang, M.; Williams, C.T.; Zhang, X.; Liang, C. Carbon Nanotubes Supported Mono- and Bimetallic Pt and Ru Catalysts for Selective Hydrogenation of Phenylacetylene. Ind. Eng. Chem. Res. 2012, 51, 4934–4941. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, P.; Mao, X.; Liu, S.; Li, L.; Wang, L.; Shao, Q.; Xu, Y.; Huang, X. A Top-Down Strategy to Realize Surface Reconstruction of Small-Sized Platinum-Based Nanoparticles for Selective Hydrogenation. Angew. Chem. Int. Ed. 2021, 60, 17430–17434. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Domínguez, S.; Berenguer-Murcia, Á.; Cazorla-Amorós, D.; Linares-Solano, Á. Semihydrogenation of Phenylacetylene Catalyzed by Metallic Nanoparticles Containing Noble Metals. J. Catal. 2006, 243, 74–81. [Google Scholar] [CrossRef]
- Gao, R.; Xu, J.; Wang, J.; Lim, J.; Peng, C.; Pan, L.; Zhang, X.; Yang, H.; Zou, J.J. Pd/Fe2O3 with Electronic Coupling Single-Site Pd-Fe Pair Sites for Low-Temperature Semihydrogenation of Alkynes. J. Am. Chem. Soc. 2022, 144, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Shao, F.; Zhao, Z.; Li, X.; Wei, Z.; Wang, J. Single-Atom Ni-Modified Al2O3-Supported Pd for Mild-Temperature Semi-Hydrogenation of Alkynes. ACS Catal. 2022, 12, 14846–14855. [Google Scholar] [CrossRef]
- Xia, L.; Li, D.; Long, J.; Huang, F.; Yang, L.; Guo, Y.; Jia, Z.; Xiao, J.; Liu, H. N-Doped Graphene Confined Pt Nanoparticles for Efficient Semi-Hydrogenation of Phenylacetylene. Carbonarbon 2019, 145, 47–52. [Google Scholar] [CrossRef]
- Huang, F.; Jia, Z.; Diao, J.; Yuan, H.; Su, D.; Liu, H. Palladium Nanoclusters Immobilized on Defective Nanodiamond-Graphene Core-Shell Supports for Semihydrogenation of Phenylacetylene. J. Energy Chem. 2019, 33, 31–36. [Google Scholar] [CrossRef]
- Wang, Z.; Garg, A.; Wang, L.; He, H.; Dasgupta, A.; Zanchet, D.; Janik, M.J.; Rioux, R.M.; Román-Leshkov, Y. Enhancement of Alkyne Semi-Hydrogenation Selectivity by Electronic Modification of Platinum. ACS Catal. 2020, 10, 6763–6770. [Google Scholar] [CrossRef]
- Yu, W.; Xin, Z.; Niu, S.; Lin, T.W.; Guo, W.; Xie, Y.; Wu, Y.; Ji, X.; Shao, L. Nanosized Palladium on Phosphorus-Incorporated Porous Carbon Frameworks for Enhanced Selective Phenylacetylene Hydrogenation. Catal. Sci. Technol. 2017, 7, 4934–4939. [Google Scholar] [CrossRef]
- Huang, F.; Deng, Y.; Chen, Y.; Cai, X.; Peng, M.; Jia, Z.; Xie, J.; Xiao, D.; Wen, X.; Wang, N.; et al. Anchoring Cu1 Species over Nanodiamond-Graphene for Semi-Hydrogenation of Acetylene. Nat. Commun. 2019, 10, 4431. [Google Scholar] [CrossRef]
- Wu, H.; Yang, X.; Zhao, S.; Zhai, L.; Wang, G.; Zhang, B.; Qin, Y. Encapsulation of Atomically Dispersed Pt Clusters in Porous TiO2 for Semi-Hydrogenation of Phenylacetylene. Chem. Commun. 2022, 58, 1191–1194. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, B.; Ge, H.; Gu, X.; Zhang, S.; Qin, Y. Porous TiO2 /Pt/TiO2 Sandwich Catalyst for Highly Selective Semihydrogenation of Alkyne to Olefin. ACS Catal. 2017, 7, 6567–6572. [Google Scholar] [CrossRef]
- Markov, P.V.; Mashkovsky, I.S.; Bragina, G.O.; Wärnå, J.; Gerasimov, E.Y.; Bukhtiyarov, V.I.; Stakheev, A.Y.; Murzin, D.Y. Particle Size Effect in Liquid-Phase Hydrogenation of Phenylacetylene over Pd Catalysts: Experimental Data and Theoretical Analysis. Chem. Eng. J. 2019, 358, 520–530. [Google Scholar] [CrossRef]
- Domínguez-Domínguez, S.; Berenguer-Murcia, Á.; Linares-Solano, Á.; Cazorla-Amorós, D. Inorganic Materials as Supports for Palladium Nanoparticles: Application in the Semi-Hydrogenation of Phenylacetylene. J. Catal. 2008, 257, 87–95. [Google Scholar] [CrossRef]
- Delgado, J.A.; Benkirane, O.; Claver, C.; Curulla-Ferré, D.; Godard, C. Advances in the Preparation of Highly Selective Nanocatalysts for the Semi-Hydrogenation of Alkynes Using Colloidal Approaches. Dalt. Trans. 2017, 46, 12381–12403. [Google Scholar] [CrossRef] [PubMed]
- Choe, K.; Zheng, F.; Wang, H.; Yuan, Y.; Zhao, W.; Xue, G.; Qiu, X.; Ri, M.; Shi, X.; Wang, Y.; et al. Fast and Selective Semihydrogenation of Alkynes by Palladium Nanoparticles Sandwiched in Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2020, 59, 3650–3657. [Google Scholar] [CrossRef]
- Yin, X.P.; Tang, S.F.; Zhang, C.; Wang, H.J.; Si, R.; Lu, X.L.; Lu, T.B. Graphdiyne-Based Pd Single-Atom Catalyst for Semihydrogenation of Alkynes to Alkenes with High Selectivity and Conversion under Mild Conditions. J. Mater. Chem. A 2020, 8, 20925–20930. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, S.; Wang, Y.; Dong, J.; Chen, W.; He, D.; Wang, D.; Yang, J.; Zhu, Y.; Zhu, H.; et al. Isolated Single-Atom Pd Sites in Intermetallic Nanostructures: High Catalytic Selectivity for Semihydrogenation of Alkynes. J. Am. Chem. Soc. 2017, 139, 7294–7301. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Q.; Wang, X.; Chen, W.; Leng, L.; Zhang, M.; Horton, J.H.; Liu, B.; Xu, Q.; Wu, W.; et al. Highly Active and Stable Palladium Single-Atom Catalyst Achieved by a Thermal Atomization Strategy on an SBA-15 Molecular Sieve for Semi-Hydrogenation Reactions. ACS Appl. Mater. Interfaces 2021, 13, 2530–2537. [Google Scholar] [CrossRef]
- Lian, J.; Chai, Y.; Qi, Y.; Guo, X.; Guan, N.; Li, L.; Zhang, F. Unexpectedly Selective Hydrogenation of Phenylacetylene to Styrene on Titania Supported Platinum Photocatalyst under 385 Nm Monochromatic Light Irradiation. Chin. J. Catal. 2020, 41, 598–603. [Google Scholar] [CrossRef]
- Prins, R. Hydrogen Spillover. Facts and Fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Wang, A.; Zhang, T. Selective Hydrogenation over Supported Metal Catalysts: From Nanoparticles to Single Atoms. Chem. Rev. 2020, 120, 683–733. [Google Scholar] [CrossRef] [PubMed]
- Studt, F.; Abild-Pedersen, F.; Bligaard, T.; Sørensen, R.Z.; Christensen, C.H.; Nørskov, J.K. Identification of Non-Precious Metal Alloy Catalysts for Selective Hydrogenation of Acetylene. Science 2008, 320, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Shamsiev, R.S.; Finkelshtein, E.I. Adsorption of Phenylacetylene and Styrene on Palladium Surface: A DFT Study. J. Mol. Model. 2018, 24, 143. [Google Scholar] [CrossRef] [PubMed]
- Vilé, G.; Albani, D.; Nachtegaal, M.; Chen, Z.; Dontsova, D.; Antonietti, M.; López, N.; Pérez-Ramírez, J. A Stable Single-Site Palladium Catalyst for Hydrogenations. Angew. Chem. Int. Ed. 2015, 54, 11265–11269. [Google Scholar] [CrossRef]
- Lindlar, H.; Dubuis, R. Palladium Catalyst for Partial Reduction of Acetylenes. Org. Synth. 2003, 46, 89–92. [Google Scholar]
- Yaseen, S.A.; Yiseen, G.A.; Li, Z. Elucidation of Calcite Structure of Calcium Carbonate Formation Based on Hydrated Cement Mixed with Graphene Oxide and Reduced Graphene Oxide. ACS Omega 2019, 4, 10160–10170. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Lv, Y.; Yin, P.; Lei, T. Porous Calcite CaCO3 Microspheres: Preparation, Characterization and Release Behavior as Doxorubicin Carrier. Colloids Surf. B Biointerfaces 2020, 186, 110720. [Google Scholar] [CrossRef]
- Giammaria, G.; Lefferts, L. Catalytic Effect of Water on Calcium Carbonate Decomposition. J. CO2 Util. 2019, 33, 341–356. [Google Scholar] [CrossRef]
- Yoshioka, S.; Kitano, Y. Transformation of Aragonite to Calcite through Heating. Geochem. J. 1985, 19, 245–249. [Google Scholar] [CrossRef]
- Gopi, S.; Subramanian, V.K.; Palanisamy, K. Aragonite–Calcite–Vaterite: A Temperature Influenced Sequential Polymorphic Transformation of CaCO3 in the Presence of DTPA. Mater. Res. Bull. 2013, 48, 1906–1912. [Google Scholar] [CrossRef]
- Wang, L.; Yin, P.; Zhang, L.L.; Shen, S.C.; Xu, S.L.; Chen, P.; Liang, H.W. Nitrogen-Fixing of Ultrasmall Pd-Based Bimetallic Nanoclusters on Carbon Supports. J. Catal. 2020, 389, 297–304. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of Metal-Support Interactions in Heterogeneous Catalysts to Enhance Activity and Selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, B.; Lin, Y.; Wang, Q.; Zhang, Q.; Su, D.S. Enhanced Chemoselective Hydrogenation through Tuning the Interaction between Pt Nanoparticles and Carbon Supports: Insights from Identical Location Transmission Electron Microscopy and X-ray Photoelectron Spectroscopy. ACS Catal. 2016, 6, 7844–7854. [Google Scholar] [CrossRef]
- Ji, G.; Duan, Y.; Zhang, S.; Fei, B.; Chen, X.; Yang, Y. Selective Semihydrogenation of Alkynes Catalyzed by Pd Nanoparticles Immobilized on Heteroatom-Doped Hierarchical Porous Carbon Derived from Bamboo Shoots. ChemSusChem 2017, 10, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Z.; Cullen, D.A.; Hu, W.; Huang, J.; Yao, L.; Peng, Z.; Liao, P.; Wang, R. Distribution and Valence State of Ru Species on CeO2 Supports: Support Shape Effect and Its Influence on CO Oxidation. ACS Catal. 2019, 9, 11088–11103. [Google Scholar] [CrossRef]
- Lindlar, H. Ein Neuer Katalysator Für Selektive Hydrierungen. Helv. Chim. Acta 1952, 35, 446–450. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).