Abstract
The widespread use of bisphenol A (BPA) in industry has resulted in BPA contamination of water bodies and even endocrine-disrupting effects on organisms and humans through water transmission. Advanced oxidation processes based on sulfate radicals have received increasing attention due to their ability to efficiently degrade endocrine disruptors (including BPA) in water. In this study, powdered iron (Fe(0)) and ferrous sulfate (Fe(II)) were used as activators to activate persulfate (PS) for the degradation of BPA. The effects of the dosage of the activator, the concentration of PS, the concentration of BPA, the initial solution pH, and the reaction temperature on the degradation efficiency of BPA in Fe(II)/PS and Fe(0)/PS systems were investigated, and the kinetics of BPA degradation under different reaction conditions were analyzed. The results showed that the optimal conditions were [Fe(II)] = 0.1 g/L, [PS] = 0.4 mM, [BPA] = 1 mg/L, T = 70 °C and pH = 5.0 for the Fe(II)/PS system and [Fe(0)] = 0.5 g/L, [PS] = 0.5 mM, [BPA] = 1 mg/L, T = 70 °C and pH = 5.0 for the Fe(0)/PS system; both systems were able to achieve equally good degradation of BPA. The degradation of BPA in the Fe(II)/PS system satisfied the pseudo-secondary kinetic equation under varying PS concentration conditions, otherwise the degradation of BPA in both systems conformed to the pseudo-first-order kinetic equation.
1. Introduction
Due to the rapid development of the chemical industry, many new chemical substances are constantly being synthesized for industrial, agricultural, and pharmaceutical use. Although these substances have brought benefits to the public, they have also caused serious environmental pollution issues. Bisphenol A (BPA) is an important organic chemical raw material used in the preparation of epoxy resins and polycarbonates which are widely used in production and life [1]. Many products contain BPA, such as plastic containers, thermal paper, coatings on cans, disposable paper cups, etc. Trace amounts of BPA are frequently detected in surface water due to the discharge of industrial wastewater or domestic sewage [2]. It is now clear that BPA is an environmental hormone that has a disruptive effect on the endocrine system of living organisms [3,4,5]. It is essential to reduce the concentration of BPA in drinking water sources to ensure the safety of drinking water. As the conventional treatment technologies (adsorption, filtration, and biochemical oxidation) are not completely effective in removing BPA [6,7,8,9], this poses a challenge to the treatment of BPA via advanced methods.
The removal of recalcitrant organic matter (including endocrine disruptor BPA) by advanced oxidation processes (AOPs) has become one of the hot spots in water treatment research. The AOPs mainly use highly oxidizing free radicals generated in situ to degrade organic matters, such as hydroxyl radicals (•OH, E0 = 1.9−2.7) and sulfate radicals (SO4•−, E0 = 2.5−3.1 V) [10,11]. Compared with hydroxyl radicals, sulfate radicals have a higher oxidation capacity for organic substances and are more adaptable to reaction conditions. Therefore, sulfate radical oxidation is more advantageous than the conventional hydroxyl radical oxidation in the treatment of recalcitrant organics [12]. Oxidants (hydrogen peroxide [13], ozone [14], persulfate (PS) [15], and peroxymonosulfate [16]) can be used as precursors to generate free radicals with the help of all kinds of energies or materials [17]. The means of activation can be ultraviolet light (UV) [18], heat [19], microwaves (MW) [20], ultrasound (US) [21], heavy metals (Fe [22], Co [23], Cu [24], Mn [25]), and carbon materials [26,27]. Among the four oxidants mentioned above, PS is favored by researchers because of its stable nature and ease of transport and storage [28]. PS alone (S2O82−, E0 = 2.01 V) has a weak ability to directly oxidize organic matters, while it can be activated to produce free radicals to acquire better performance toward organics degradation [29]. The molecule of PS contains an O—O bond (140 kJ/mol, 1.497 Å), which can be broken to form sulfate radicals at external energies (UV, US, MW, and heat) above the O—O bond energy [30]. In addition, PS can also undergo redox reaction with transition metals to form sulfate radicals [31]. As iron is a common transition metal that is abundant on earth, inexpensive, and non-toxic, it is considered as a benign catalyst used in AOPs [32]. For example, divalent iron (Fe(II)) could catalyze PS to generate sulfate radicals [33]. In addition, powdered iron (Fe(0)) has also been used to activate PS in removing organic matter [34].
Although the use of Fe(II) and Fe(0) for activating PS is relatively common in research, the economic and environmental friendliness of iron is very prominent. Iron-based PS systems have an advantage over other transition metals-based PS systems (Co/PS, Cu/PS, Mn/PS) in practical applications toward wastewater treatment. Fewer studies have been conducted using Fe(II)/PS and Fe(0)/PS systems to treat endocrine disruptor BPA; therefore, a comparative study of the two systems can provide favorable parameters for future applications of iron-based systems in actual wastewater treatment. In this study, Fe(0) and Fe(II) were used to remove BPA from an aqueous solution in the presence of PS. The abilities of two systems to remove BPA under different influencing factors were investigated and the corresponding reaction kinetic models were developed. At the same time, a profound analysis of the mechanism was carried out in terms of catalysis and degradation in Fe(0)/PS and Fe(II)/PS systems.
2. Results
2.1. Dosage of Activators
Figure 1a,b shows the effects of different dosages of Fe(II) and Fe(0) on activating PS in debating BPA, respectively. It can be seen in Figure 1a that PS was able to oxidize 19.86% of BPA without the addition of catalyst Fe(II), which meant that PS alone could oxidize organic matter at a slower rate. The addition of 0.1 g/L of Fe(II) was able to achieve an optimal BPA removal (C/C0 = 0.455) in the presence of PS. Subsequently, the dosage of Fe(II) was increased from 0.2 to 0.5 g/L, which brought about a gradual decrease in the BPA removal rate (0.5 g/L of Fe(II) corresponding to C/C0 = 0.706). This indicated that too much Fe(II) did not favor the catalytic reaction rate. The reaction of Fe(II) activating PS to generate SO4•− is shown in Equation (1) [35]. From this equation, Fe(II) is able to be oxidized to Fe(III) by PS, while PS is reduced to SO4•− and SO42−. However, the excess Fe(II) will react with the SO4•− formed in the water, converting SO4•− to SO42− (Equation (2)) [35,36] and thus losing the ability to degrade organic matter. Therefore, the optimization of the Fe(II) and PS dosing ratio is beneficial to the improvement of degradation efficiency. Figure 1b exhibits the trend of BPA degradation in Fe(0)/PS system. In contrast to the trend in Figure 1a, the residual concentration of BPA decreased gradually as the dosage of Fe(0) was increased from 0 to 0.5 g/L. The best BPA removal (C/C0 = 0.514) was achieved within 60 min with 0.5 g/L of Fe(0). While for the Fe(II)/PS system, the best BPA removal was acquired at Fe(II) of 0.1 g/L (C/C0 = 0.455). Since the activation of Fe(0) on PS is heterophasic, whereas the activation of Fe(II) on PS is homophasic, the performance on the optimal concentrations of the two catalysts is not consistent. In fact, the activation of PS by Fe(0) is an indirect process. Fe(0) should first be converted to Fe(II) before the reaction of Equation (1) occurs to activate PS. Fe(0) can be converted to Fe(II) under three conditions, see Equations (3)–(5) [37]. Since Fe(0) catalyzes PS in a heterogeneous manner, Fe(0) does not dissolve into solution quickly and is not easily overdosed compared to Fe(II), which becomes an advantage of Fe(0) in activating PS. Therefore, Fe(0) is able to avoid the problem of quenching SO4•− by generating too much Fe(II) in the reaction.
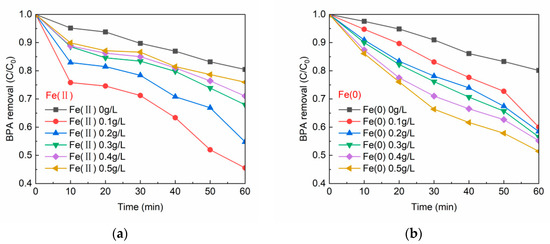
Figure 1.
(a) Fe(II) dosage vs. BPA removal; and (b) Fe(0) dosage vs. BPA removal. [BPA] = 5 mg/L, [PS] = 0.2 mM, T = 20 ± 1 °C, pH = 6.8 ± 0.2.
Figure 2a,b develops the kinetic models of BPA degradation by Fe(II)/PS and Fe(0)/PS, respectively. The degradation of BPA at varying dosages of Fe(II) and Fe(0) was in accordance with the pseudo first-order kinetic equations. The correlation coefficients (R2) of the established equations were all above 0.93. In Figure 2a, when Fe(II) was 0.1 g/L, the degradation of BPA obtained the maximum rate of 0.01171 min−1. In Figure 2b, the maximum BPA degradation rate occurred at 0.5 g/L of Fe(0).
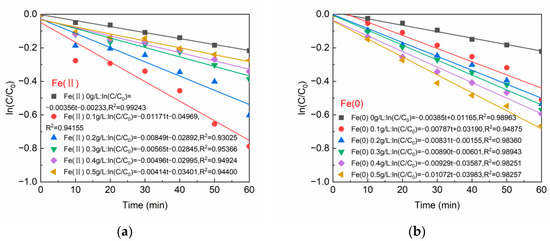
Figure 2.
(a) Reaction kinetics of Fe(II); and (b) reaction kinetics of Fe(0). [BPA] = 5 mg/L, [PS] = 0.2 mM, T=20 ± 1 °C, pH = 6.8 ± 0.2.
2.2. Concentrations of PS
Figure 3a depicts the changes in BPA removal at different PS concentrations with the addition of Fe(II). As can be seen from Figure 3a, Fe(II) had no ability to remove BPA at all when the PS concentration was 0 mM. Unlike Fe(II), Fe(0) exhibited limited removal of BPA (60 min, C/C0 = 0.801) in the absence of PS (Figure 3b), which should be attributed to the adsorption and reduction of BPA by nano-sized Fe(0) possessing a large specific surface area. In Figure 3a, as the PS concentration increased from 0.2 mM to 0.4 mM, the residual concentration of BPA in the solution continued to decrease. However, at a PS concentration of 0.5 mM, it was found that the change in BPA removal was not significant compared to that at a PS concentration of 0.4 mM. This indicated that the best treatment effect of BPA was achieved at PS of 0.4 mM. Similar results were obtained in a previous study which showed that a high concentration PS could inhibit the degradation of organics in Fe(II)/PS system [38]. Although PS as an oxidant could provide SO4•− through Fe(II) catalyzing, excess PS could react with the SO4•− (Equations (6)) [39,40] and reduce the amount of SO4•− in the solution, thus inhibiting the degradation of organics. However, it was found from Figure 3b that the increase in Fe(0) concentration from 0.1 mM to 0.5 mM did not bring about the inhibition of BPA degradation, and the best BPA removal was achieved at 0.5 mM of PS. Compared to Fe(II) for PS catalysis, 0.5 mM of PS was not excessive in the Fe(0) catalysis process. The reason may be that one part of the PS reacted with Fe(0) and oxidized it to Fe(II). Another part of PS reacted with the generated Fe(II) to produce SO4•−.

Figure 3.
(a) PS dosage vs. BPA removal by Fe(II); and (b) PS dosage vs. BPA removal by Fe(0). [BPA] = 5 mg/L, [Fe(II)] = 0.1 g/L, [Fe(0)] = 0.1 g/L, T = 20 ± 1 °C, pH = 6.8 ± 0.2.
Figure 4a demonstrates the kinetic models of BPA degradation by Fe(II)/PS at varying PS concentrations. According to the curves in Figure 4a, 1/Ct exhibited a good linear fit to time in the presence of PS with a high linear fit coefficient (R2 ≥ 0.87132), indicating that the degradation of BPA followed the pseudo-secondary kinetic model well. The maximum reaction rate of 0.01484 (mg/L)−1min−1 was achieved when the PS concentration was 0.4 mM. The curves in Figure 4b revealed that the degradation of BPA at different PS concentrations in Fe(0)/PS conformed to the pseudo first-order kinetic equation. At a PS concentration of 0.5 mM, the degradation rate of BPA could reach 0.02366 (mg/L)−1min−1. BPA showed different degradation kinetics in Fe(II)/PS vs. Fe(0)/PS systems. This may be caused by the difference in the homogeneous catalysis of Fe(II)/PS and the heterogeneous catalysis of Fe(0)/PS.
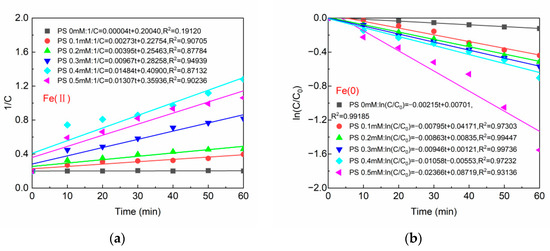
Figure 4.
(a) Reaction kinetics of Fe(II); and (b) reaction kinetics of Fe(0). [BPA] = 5 mg/L, [Fe(II)] = 0.1 g/L, [Fe(0)] = 0.1 g/L, T = 20 ± 1 °C, pH = 6.8 ± 0.2.
2.3. Concentrations of BPA
Figure 5a,b expresses the variation of residual BPA concentration in the solution after degradation of BPA by Fe(II)/PS and Fe(0)/PS at varying initial BPA concentrations, respectively. Figure 5a,b shows the same trend, i.e., the BPA removal effect gradually decreased as the initial BPA concentration increased from 1 mg/L to 5 mg/L. When the initial concentration of BPA was 1 mg/L, the removal rate of BPA was 82.1% in Fe(II)/PS system, and 83.4% in Fe(0)/PS system. When the initial concentration of BPA was increased to 5 mg/L, the removal rate of BPA decreased to 55.3% in Fe(II)/PS system and 45.6% in Fe(0)/PS system. This indicated that the increase in the concentration of the target pollutant (BPA) decreased the BPA degradation efficiency with a constant amount of PS and catalyst (Fe(II) or Fe(0)). Therefore, to obtain higher BPA removal, the amount of catalyst and oxidant needs to be increased proportionally while increasing the initial concentration of BPA.
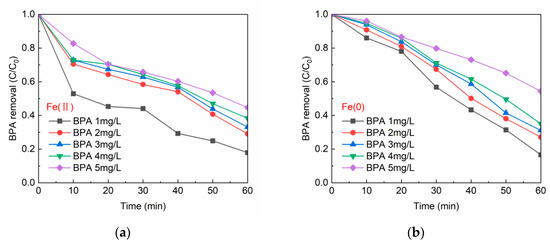
Figure 5.
(a) BPA concentration vs. BPA removal by Fe(II); and (b) BPA concentration vs. BPA removal by Fe(0). [PS] = 0.2 mM, [Fe(II)] = 0.1 g/L, [Fe(0)] = 0.1 g/L, T = 20 ± 1 °C, pH = 6.8 ± 0.2.
The kinetic models of BPA degradation were fitted in Figure 6a,b, and the BPA degradation in both systems Fe(0)/PS and Fe(II)/PS conformed to the pseudo first-order kinetic equation (R2 ≥ 0.93456). When the initial concentration of BPA was 1 mg/L, the degradation kinetic constant was 0.02537 (mg/L)−1min−1 in Fe(II)/PS system and 0.02854 (mg/L)−1min−1 in Fe(0)/PS system. When BPA was increased to 5 mg/L, the kinetic constant of BPA degradation decreased to 0.01232 (mg/L)−1min−1 in the Fe(II)/PS system, and 0.00994 (mg/L)−1min−1 in the Fe(0)/PS system, which was consistent with the trend of BPA residual concentration in aqueous solution in Figure 5a,b.
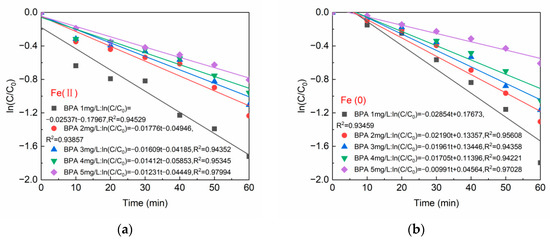
Figure 6.
(a) Reaction kinetics of Fe(II); and (b) reaction kinetics of Fe(0). [PS] = 0.2 mM, [Fe(II)] = 0.1 g/L, [Fe(0)] = 0.1 g/L, T = 20 ± 1 °C, pH = 6.8 ± 0.2.
2.4. Reaction Temperature
Temperature is an important factor affecting the rate of chemical reactions. The effect of varying temperatures on BPA removal in systems Fe(II)/PS and Fe(0)/PS are given in Figure 7a,b, respectively. With only PS in the system, the degradation rates of BPA in Figure 7a,b all increased with the temperature rising. The degradation of BPA via PS was close to 40% at the temperature of 70 °C in both Figures. According to previous studies [41,42,43], PS could be activated by heat to form SO4•− (Equation (7)), which in turn improved the degradation efficiency of organic matter. The elevation in temperature could increase the energy of the reaction system. As this energy exceeded the peroxide bond energy of PS, the peroxide bond broke and SO4•− was formed. When both Fe(II) (or Fe(0)) and PS were present in the BPA solution, Fe(II) (or Fe(0)) could activate PS together with the heat energy brought by the high temperature and produce more SO4•−. Thus, at 70 °C, the removal of BPA reached 96.7% by Fe(II)/PS and reached 94.5% by Fe(0)/PS.
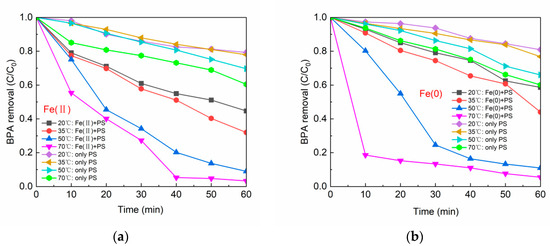
Figure 7.
(a) Reaction temperature vs. BPA removal by Fe(II); and (b) reaction temperature vs. BPA removal by Fe(0). [BPA] = 5 mg/L, [PS] = 0.2 mM, [Fe(II)] = 0.1 g/L, [Fe(0)] = 0.1 g/L, pH = 6.8 ± 0.2.
Figure 8a,b shows the kinetic models of BPA degradation at varying temperature conditions in the Fe(II)/PS system and Fe(0)/PS system, respectively. The pseudo first-order reaction kinetic equations (R2 ≥ 0.80025) were applied to the degradation of BPA at different temperatures in both Figure 8a,b. The degradation rates of BPA by the Fe(II)/PS system and Fe(0)/PS system at 70 °C reached 0.06130 (mg/L)−1min−1 and 0.03866 (mg/L)−1min−1, respectively.
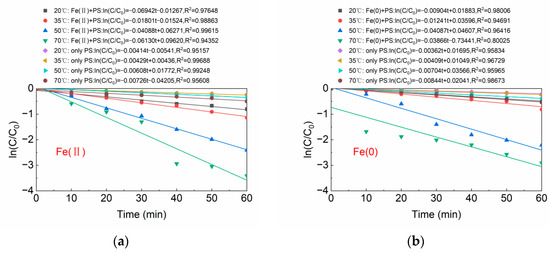
Figure 8.
(a) Reaction kinetics of Fe(II); and (b) reaction kinetics of Fe(0). [BPA] = 5 mg/L, [PS] = 0.2 mM, [Fe(II)] = 0.1 g/L, [Fe(0)] = 0.1 g/L, pH = 6.8 ± 0.2.
In order to assess the relationship between the reaction temperature and degradation rate of BPA, the Arrhenius equation (Equation (8)) [42] was used to fit the experimental data to investigate the correlation.
where kapp is the apparent rate constant (min−1), A is the pre-exponential factor (min−1), R is the gas constant (8.314 J/K·mol), T is the absolute temperature (K), and Ea is the activation energy (J/mol). The established thermodynamic equations are shown in Figure 9a,b. In the Fe(II)/PS system, R2, A and Ea were 0.94923, 1139.67 min−1, and 2.8 × 107 J/mol, respectively, which showed that the relationship between the BPA degradation rate and the temperature satisfied the Arrhenius equation, while in Fe(0)/PS system, R2 was 0.74757. Compared with the homogeneous system Fe(II)/PS, the heterogeneous activation system Fe(0)/PS acquired a low R2 when fitting into the Arrhenius equation. This may be because the process of heterogeneous activation involves the release of Fe(0) to Fe(II), which makes the thermodynamic laws difficult to obtain.
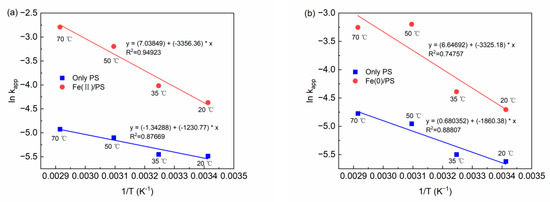
Figure 9.
(a) Thermodynamic equation of Fe(II); and (b) thermodynamic equation of Fe(0). [BPA] = 5 mg/L, [PS] = 0.2 mM, [Fe(II)] = 0.1 g/L, [Fe(0)] = 0.1 g/L, pH = 6.8 ± 0.2.
2.5. Initial pH
The initial pH value of solution is an important factor affecting the chemical reaction. Therefore, the degradation of BPA by two systems under different initial pH values was studied. Only the initial pH value of the solution was adjusted, and the pH value in the whole reaction process was not controlled. Figure 10a,b reflected the effect of changing pH values on the degradation of BPA by Fe(II)/PS and Fe(0)/PS. It was found that in Figure 10a,b, pH 5.0 was the optimal condition for both systems. The degradation of BPA was poor in both systems under alkaline conditions. On the one hand, the oxidation capacity of the systems was reduced due to the reaction of sulfate radicals converted to hydroxyl radicals in the presence of hydroxide (Equation (9)) [44]. On the other hand, the formation of Fe(OH)3 could lead to the inhibition of the catalytic reaction (Equation (10)). In the practical application of these two systems, if 5.0 is set as the best pH value, it needs to consume chemical regulators. In addition, the requirements for the corrosion resistance of pipelines have also been improved. One suggestion is that under the condition that partial removal of BPA is allowed in the pretreatment stage, it can be considered to degrade BPA at pH 6.5.
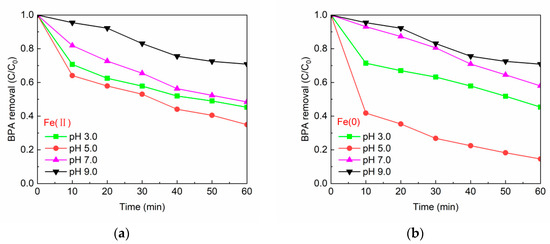
Figure 10.
(a) Reaction temperature vs. BPA removal by Fe(II); and (b) reaction temperature vs. BPA removal by Fe(0). [BPA] = 5 mg/L, [PS] = 0.2 mM, [Fe(II)] = 0.1 g/L, [Fe(0)] = 0.1 g/L, T = 20 ± 1 °C.
The kinetic equations for the degradation of BPA by the Fe(II)/PS and Fe(0)/PS systems at different pH values are given in Figure 11a,b, respectively. The BPA degradation curves satisfied the pseudo first-order kinetic equation (R2 ≥ 0.89857) except for the degradation of BPA by the Fe(0)/PS system at pH 9. The maximum kinetic constant (0.02818 (mg/L)−1min−1) appeared in the Fe(0)/PS system at pH 5.
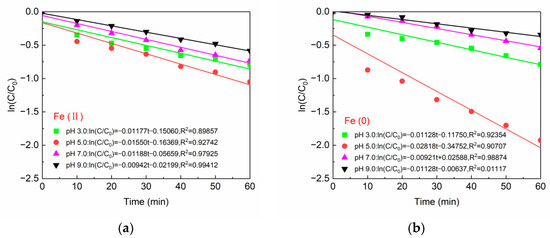
Figure 11.
(a) Reaction kinetics of Fe(II); and (b) reaction kinetics of Fe(0). [BPA] = 5 mg/L, [PS] = 0.2 mM, [Fe(II)] = 0.1 g/L, [Fe(0)] = 0.1 g/L, T = 20 ± 1 °C.
2.6. Quenching Experiments and Mechanism Analysis
In order to identify which type of free radicals appeared in the reaction, methanol (Me(OH)) and tert-Butyl alcohol (TBA) were used as extinguishing agents to investigate the degree of inhibition of the chemical reaction. MeOH is an effective scavenger for both •OH (k = 9.7 × 108 M−1 s−1) and SO4•− (k = 1.1 × 107 M−1 s−1), while TBA is an effective scavenger for •OH (k = 6.0 × 108 M−1 s−1), but not for SO4•− (k = 9.1 × 105 M−1 s−1) [45]. Figure 12a,b exhibits BPA degradation in the presence of either MeOH or TBA in the Fe(II)/PS and Fe(0)/PS systems, respectively. Both MeOH and TBA were found to inhibit BPA degradation in both systems. The inhibitory effect of Me(OH) on the two systems was greater than that of TBA. It suggested that both SO4•− and •OH played roles in the oxidation of BPA in the two systems.
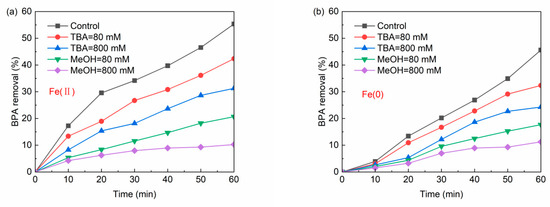
Figure 12.
(a) Quenching experiment by Fe(II) system; and (b) quenching experiment by Fe(II) system. [BPA] = 5 mg/L, [PS] = 0.2 mM, [Fe(II)] = 0.1 g/L, [Fe(0)] = 0.1 g/L, T = 20 ± 1 °C.
Figure 13 depicts the mechanism of PS activation and BPA degradation in the presence of Fe(II) and [Fe(0)] catalysts. In the Fe(0)/PS system, Fe(0) is first transformed into Fe(II) through three pathways (Equations (3)–(5)), thus playing the role of activating PS. In the Fe(II)/PS system, the dissolved Fe(II) directly undergoes a redox reaction with PS to generate SO4•− and Fe(III) (Equation (2)). Fe(III) can be transformed into Fe(II) with the gain of an electron. Therefore, Fe(II) and Fe(III) in solution can stably activate PS if they are in a sustainable equilibrium. At the same time, when the OH− concentration in the solution is high, it can react with SO4•− to generate hydroxyl radicals, which also has a good oxidation effect on organic matter. Therefore, under the combined action of SO4•− and •OH, BPA can be oxidized and the final products will be H2O and CO2.
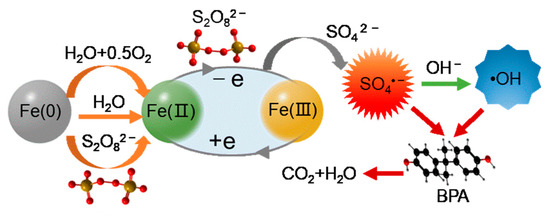
Figure 13.
Schematic diagram of the mechanism of the Fe system activating PS to degrade BPA.
2.7. Comparison of This Study with Other Studies
There are also some previous studies on the degradation of pollutants by Fe(II)/PS and Fe(0)/PS systems. Some examples of representative literature were selected and are listed in Table 1, and they were compared with the research results of this paper. From the perspective of the removal rate of target pollutants, the removal rates obtained in these studies are all concentrated at about 70%. The degradation efficiency of the two systems for BPA in this paper is also comparable to other systems. These target pollutants are recalcitrant organic substances. Thus, to achieve 100% degradation, it is necessary to increase the amount of oxidant and catalyst and control the temperature and pH of the reaction within an appropriate range. During the pretreatment of source water, it can also be considered to control the BPA removal rate at around 70%.

Table 1.
Comparison with related process treatment effects in the relevant literature.
3. Materials and Methods
3.1. Materials
The chemicals used in the experiment are summarized in Table 2. The purity of the chemicals is at least analytically pure. The solutions used in the experiments were prepared using ultrapure water.

Table 2.
Chemicals used in this study.
3.2. Methods
A BPA stock solution of 1000 mg/L was prepared with ultrapure water and then diluted according to the concentration needed for the experiment. A beaker with a certain concentration of BPA solution was placed on a magnetic stirrer (200 r/min) and stirred for 20 min (to allow the solution to reach the desired initial temperature), and then a certain amount of Fe(II) (or Fe(0)) and PS was added to the beaker to initiate the reaction. At this point, the time was started and samples were taken every 10 min. The sample was filtered through a 0.22 μm filter membrane and injected into a sample vial, to which 0.5 mL of methanol was added to terminate the reaction. The sample vials were put into high performance liquid chromatography (HPLC, G7121A, Agilent Technologies Inc., Beijing, China) to detect the concentration of BPA. Sulfuric acid and sodium hydroxide were used to adjust pH of BPA solution, and different temperatures were achieved by activating the heating button on the magnetic stirrer.
4. Conclusions
In this study, PS activated by the homogeneous catalyst Fe(II) and heterogeneous catalyst Fe(0) were used to degrade the representative endocrine disruptor BPA in water, and the following conclusions were drawn.
- The degradation effect of BPA was similar for both systems (Fe(II)/PS and Fe(0)/PS), and the Fe(0)/PS system had an advantage in the slow release of Fe(II), which was less likely to cause Fe(II) overload resulting in the consumption of free radicals.
- The concentrations of oxidant, catalyst, and substrate had a large influence on the reaction, and the concentration ratio among them was very important for the degradation of BPA.
- Temperature and pH were important factors affecting the reaction. A lower pH (5.0) was very suitable for the degradation of BPA in both systems. High temperature could enhance the degradation of BPA due to the synergistic activation of PS by an iron catalyst and thermal energy.
- Most of the kinetic equations for BPA degradation under different conditions followed pseudo first-order kinetic equations and were highly correlated.
Author Contributions
Conceptualization, T.S. and X.Y.; methodology, H.D.; software, H.W.; validation, B.Y. and H.W.; formal analysis, L.C.; investigation, H.L.; data curation, H.L.; writing—original draft preparation, T.S.; writing—review and editing, H.D.; visualization, L.C.; supervision, B.Y.; project administration, T.S.; funding acquisition, T.S. and X.Y.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly funded by the Natural Science Foundation of Jilin Province of China (grant number YDZJ202201ZYTS681) and the Natural Science Foundation of Jilin Province of China (grant number YDZJ202201ZYTS630).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
All authors would like to thank Jilin Jianzhu University for the support of this work.
Conflicts of Interest
The authors declare no conflict with any personal circumstances.
References
- Bousoumah, R.; Leso, V.; Iavicoli, I.; Huuskonen, P.; Viegas, S.; Porras, S.P.; Santonen, T.; Frery, N.; Robert, A.; Ndaw, S. Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: A systematic review. Sci. Total Environ. 2021, 783, 146905. [Google Scholar] [CrossRef] [PubMed]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Kwack, S.J.; Kim, H.S.; Lee, B.M. Estrogenic endocrine-disrupting chemicals: Molecular mechanisms of actions on putative human diseases. J. Toxicol. Environ. Health B 2014, 17, 127–174. [Google Scholar] [CrossRef]
- Tarafdar, A.; Sirohi, R.; Balakumaran, P.A.; Reshmy, R.; Madhavan, A.; Sindhu, R.; Binod, P.; Kumar, Y.; Kumar, D.; Sim, S.J. The hazardous threat of Bisphenol A: Toxicity, detection and remediation. J. Hazard. Mater. 2022, 423, 127097. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bermejo, M.; Mas-Pérez, I.; Murillo-Llorente, M.T. The role of the bisphenol A in diabetes and obesity. Biomedicines 2021, 9, 666. [Google Scholar] [CrossRef] [PubMed]
- Bucur, S.; Diacon, A.; Mangalagiu, I.; Mocanu, A.; Rizea, F.; Dinescu, A.; Ghebaur, A.; Boscornea, A.C.; Voicu, G.; Rusen, E. Bisphenol A adsorption on silica particles modified with beta-cyclodextrins. Nanomaterials 2021, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Z.H.; Zhang, J.; Huang, R.P.; Yin, H.; Dang, Z.; Wu, P.-X.; Liu, Y. Insights into removal mechanisms of bisphenol A and its analogues in municipal wastewater treatment plants. Sci. Total Environ. 2019, 692, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Cydzik-Kwiatkowska, A.; Zielińska, M.; Bernat, K.; Bułkowska, K.; Wojnowska-Baryła, I. Insights into mechanisms of bisphenol A biodegradation in aerobic granular sludge. Bioresour. Technol. 2020, 315, 123806. [Google Scholar] [CrossRef]
- Zielińska, M.; Bułkowska, K.; Cydzik-Kwiatkowska, A.; Bernat, K.; Wojnowska-Baryła, I. Removal of bisphenol A (BPA) from biologically treated wastewater by microfiltration and nanofiltration. Int. J. Environ. Sci. Technol. 2016, 13, 2239–2248. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Madihi-Bidgoli, S.; Asadnezhad, S.; Yaghoot-Nezhad, A.; Hassani, A. Azurobine degradation using Fe2O3@ multi-walled carbon nanotube activated peroxymonosulfate (PMS) under UVA-LED irradiation: Performance, mechanism and environmental application. J. Environ. Chem. Eng. 2021, 9, 106660. [Google Scholar] [CrossRef]
- Govindan, K.; Raja, M.; Noel, M.; James, E.J. Degradation of pentachlorophenol by hydroxyl radicals and sulfate radicals using electrochemical activation of peroxomonosulfate, peroxodisulfate and hydrogen peroxide. J. Hazard. Mater. 2014, 272, 42–51. [Google Scholar] [CrossRef]
- Li, R.; Speed, D.; Siriwardena, D.; Fernando, S.; Thagard, S.M.; Holsen, T.M. Comparison of hydrogen peroxide-based advanced oxidation processes for the treatment of azole-containing industrial wastewater. Chem. Eng. J. 2021, 425, 131785. [Google Scholar] [CrossRef]
- Lu, H.; Li, Q.; Feng, W. Application Progress of O3/UV Advanced Oxidation Technology in the Treatment of Organic Pollutants in Water. Sustainability 2022, 14, 1556. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Cai, Y.; Yuan, R.; Wang, F.; Qian, Y.; Chen, Z.; Zhou, B.; Chen, H. Efficient degradation and defluorination of perfluorobutyric acid under UV irradiation in the presence of persulfate. J. Clean. Prod. 2021, 327, 129472. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbali, P.; Mahdipour, F.; Wacławek, S.; Lin, K.Y.A.; Ghanbari, F. Insights into the synergistic role of photocatalytic activation of peroxymonosulfate by UVA-LED irradiation over CoFe2O4-rGO nanocomposite towards effective Bisphenol A degradation: Performance, mineralization, and activation mechanism. Chem. Eng. J. 2023, 453, 139556. [Google Scholar] [CrossRef]
- Rad, T.S.; Khataee, A.; Pouran, S.R.; Joo, S.W. The key role of free radicals generated from activation of H2O2, S2O82− and ozone over chromium/cerium co-doped magnetite nanoparticles. Sep. Purif. Technol. 2020, 239, 116538. [Google Scholar]
- Ghanbari, F.; Khatebasreh, M.; Mahdavianpour, M.; Mashayekh-Salehi, A.; Aghayani, E.; Lin, K.Y.A.; Noredinvand, B.K. Evaluation of peroxymonosulfate/O3/UV process on a real polluted water with landfill leachate: Feasibility and comparative study. Korean J. Chem. Eng. 2021, 38, 1416–1424. [Google Scholar] [CrossRef]
- Yin, L.; Wei, J.; Qi, Y.; Tu, Z.; Qu, R.; Yan, C.; Wang, Z.; Zhu, F. Degradation of pentachlorophenol in peroxymonosulfate/heat system: Kinetics, mechanism, and theoretical calculations. Chem. Eng. J. 2022, 434, 134736. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, M. Microwave-assisted persulfate/peroxymonosulfate process for environmental remediation. Curr. Opin. Chem. Eng. 2022, 36, 100826. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Mengelizadeh, N.; Khodadadi Saloot, M.; Mohebi, S.; Balarak, D. Sonochemical degradation of ciprofloxacin by hydrogen peroxide and persulfate activated by ultrasound and ferrous ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128627. [Google Scholar] [CrossRef]
- Kim, C.; Ahn, J.Y.; Kim, T.Y.; Shin, W.S.; Hwang, I. Activation of persulfate by nanosized zero-valent iron (NZVI): Mechanisms and transformation products of NZVI. Environ. Sci. Technol. 2018, 52, 3625–3633. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.F.; Zhang, L.; Xu, H.Y. Enhancement strategies for efficient activation of persulfate by heterogeneous cobalt-containing catalysts: A review. Chemosphere 2021, 291, 132954. [Google Scholar] [CrossRef]
- Ding, Y.; Fu, L.; Peng, X.; Lei, M.; Wang, C.; Jiang, J. Copper catalysts for radical and nonradical persulfate based advanced oxidation processes: Certainties and uncertainties. Chem. Eng. J. 2022, 427, 131776. [Google Scholar] [CrossRef]
- Dong, G.; Chen, B.; Liu, B.; Stoyanov, S.R.; Cao, Y.; Yang, M.; Zhang, B. UV stimulated manganese dioxide for the persulfate catalytic degradation of bisphenol A. Catalysts 2021, 11, 502. [Google Scholar] [CrossRef]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. Oxidation of sulfamethoxazole by rice husk biochar-activated persulfate. Catalysts 2021, 11, 850. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Fernandes, A.; Makoś, P.; Boczkaj, G. Treatment of bitumen post oxidative effluents by sulfate radicals based advanced oxidation processes (S-AOPs) under alkaline pH conditions. J. Clean. Prod. 2018, 195, 374–384. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Qiu, J.; Zhou, L. Inactivation of microorganisms in drinking water using combined treatment with ultraviolet and sodium persulfate. Desalination Water Treatement 2022, 249, 103–108. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Zhu, L. Activation of persulfate by Co3O4 nanoparticles for orange G degradation. Rsc Adv. 2016, 6, 758–768. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Mehralipour, J.; Azarin, G.; Godini, K.; Shabanlo, A. Decomposition of sodium dodecylbenzene sulfonate surfactant by electro/Fe2+-activated persulfate process from aqueous solutions. Glob. NEST J. 2017, 19, 115–121. [Google Scholar]
- Li, X.; Zhou, M.; Pan, Y.; Xu, L. Pre-magnetized Fe°/persulfate for notably enhanced degradation and dechlorination of 2, 4-dichlorophenol. Chem. Eng. J. 2017, 307, 1092–1104. [Google Scholar] [CrossRef]
- Rodriguez, S.; Vasquez, L.; Costa, D.; Romero, A.; Santos, A. Oxidation of Orange G by persulfate activated by Fe (II), Fe (III) and zero valent iron (ZVI). Chemosphere 2014, 101, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Xie, X.F.; Huang, S.B.; Liang, H.Y. Effect of chelating agent on oxidation rate of aniline in ferrous ion activated persulfate system at neutral pH. J. Cent. South Univ. 2014, 21, 1441–1447. [Google Scholar] [CrossRef]
- Zou, X.; Zhou, T.; Mao, J.; Wu, X. Synergistic degradation of antibiotic sulfadiazine in a heterogeneous ultrasound-enhanced Fe°/persulfate Fenton-like system. Chem. Eng. J. 2014, 257, 36–44. [Google Scholar] [CrossRef]
- Bu, L.; Shi, Z.; Zhou, S. Modeling of Fe (II)-activated persulfate oxidation using atrazine as a target contaminant. Sep. Purif. Technol. 2016, 169, 59–65. [Google Scholar] [CrossRef]
- Gao, F.; Li, Y.; Xiang, B. Degradation of bisphenol A through transition metals activating persulfate process. Ecotoxicol. Environ. Saf. 2018, 158, 239–247. [Google Scholar] [CrossRef]
- Ghanbari, F.; Wang, Q.; Hassani, A.; Wacławek, S.; Rodríguez-Chueca, J.; Lin, K.Y.A. Electrochemical activation of peroxides for treatment of contaminated water with landfill leachate: Efficacy, toxicity and biodegradability evaluation. Chemosphere 2021, 279, 130610. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I.; Genc, B. Bisphenol A treatment by the hot persulfate process: Oxidation products and acute toxicity. J. Hazard. Mater. 2013, 263, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Potakis, N.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D. Oxidation of bisphenol A in water by heat-activated persulfate. J. Environ. Manag. 2017, 195, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Gao, N.; Deng, Y.; An, N.; Deng, J. Heat-activated persulfate oxidation of diuron in water. Chem. Eng. J. 2012, 203, 294–300. [Google Scholar] [CrossRef]
- Zhao, L.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q.; Yin, X. Simultaneous removal of bisphenol A and phosphate in zero-valent iron activated persulfate oxidation process. Chem. Eng. J. 2016, 303, 458–466. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Lu, X.; Xu, W.; Gong, Q.; Ding, J.; Dan, B.; Xie, P. Removal of acetaminophen in the Fe2+/persulfate system: Kinetic model and degradation pathways. Chem. Eng. J. 2019, 358, 1091–1100. [Google Scholar] [CrossRef]
- Rao, Y.F.; Qu, L.; Yang, H.; Chu, W. Degradation of carbamazepine by Fe (II)-activated persulfate process. J. Hazard. Mater. 2014, 268, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Dong, Z.; Li, M.; Song, X.; Zhang, M.; Jiang, C.; Feiyun, S. Degradation of diatrizoate in water by Fe (II)-activated persulfate oxidation. Chem. Eng. J. 2019, 361, 1333–1344. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Trimethoprim degradation by Fenton and Fe (II)-activated persulfate processes. Chemosphere 2018, 191, 97–105. [Google Scholar] [CrossRef]
- Liu, C.S.; Shih, K.; Sun, C.X.; Wang, F. Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Sci. Total Environ. 2012, 416, 507–512. [Google Scholar] [CrossRef]
- Hussain, I.; Zhang, Y.; Huang, S.; Du, X. Degradation of p-chloroaniline by persulfate activated with zero-valent iron. Chem. Eng. J. 2012, 203, 269–276. [Google Scholar] [CrossRef]
- Hussain, I.; Zhang, Y.; Huang, S. Degradation of aniline with zero-valent iron as an activator of persulfate in aqueous solution. Rsc Adv. 2014, 4, 3502–3511. [Google Scholar] [CrossRef]
- Hayat, W.; Zhang, Y.; Hussain, I.; Du, X.; Du, M.; Yao, C.; Huang, S.; Si, F. Efficient degradation of imidacloprid in water through iron activated sodium persulfate. Chem. Eng. J. 2019, 370, 1169–1180. [Google Scholar] [CrossRef]
- Nie, M.; Yan, C.; Li, M.; Wang, X.; Bi, W.; Dong, W. Degradation of chloramphenicol by persulfate activated by Fe2+ and zerovalent iron. Chem. Eng. J. 2015, 279, 507–515. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Li, J.; Qiu, J.; Cai, C.; Zhang, H. Degradation of Acid Orange 7 by persulfate activated with zero valent iron in the presence of ultrasonic irradiation. Sep. Purif. Technol. 2014, 122, 41–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).