Response Surface Methodology for Optimization of Bisphenol A Degradation Using Fe3O4-Activated Persulfate
Abstract
1. Introduction
2. Results and Discussion
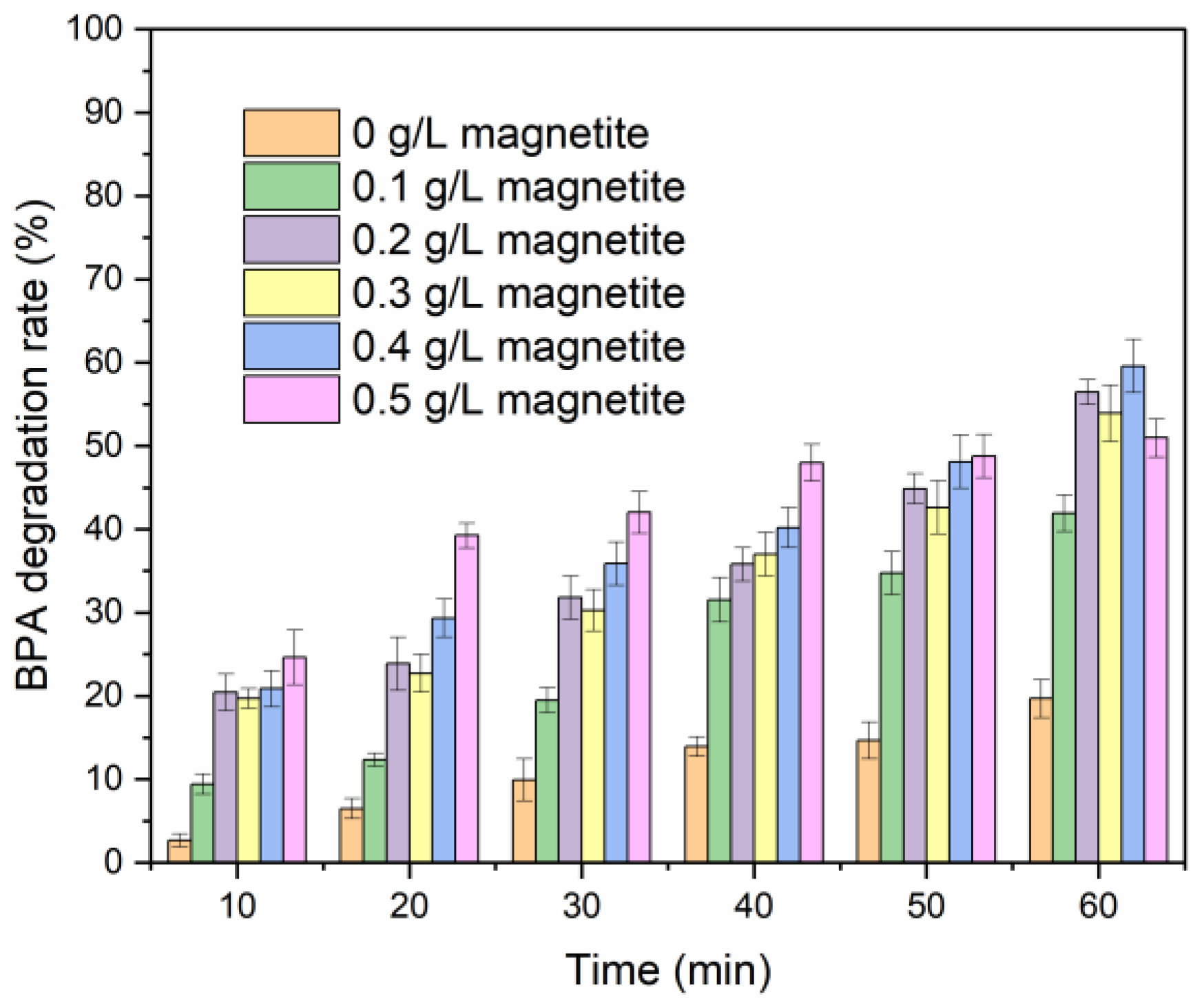
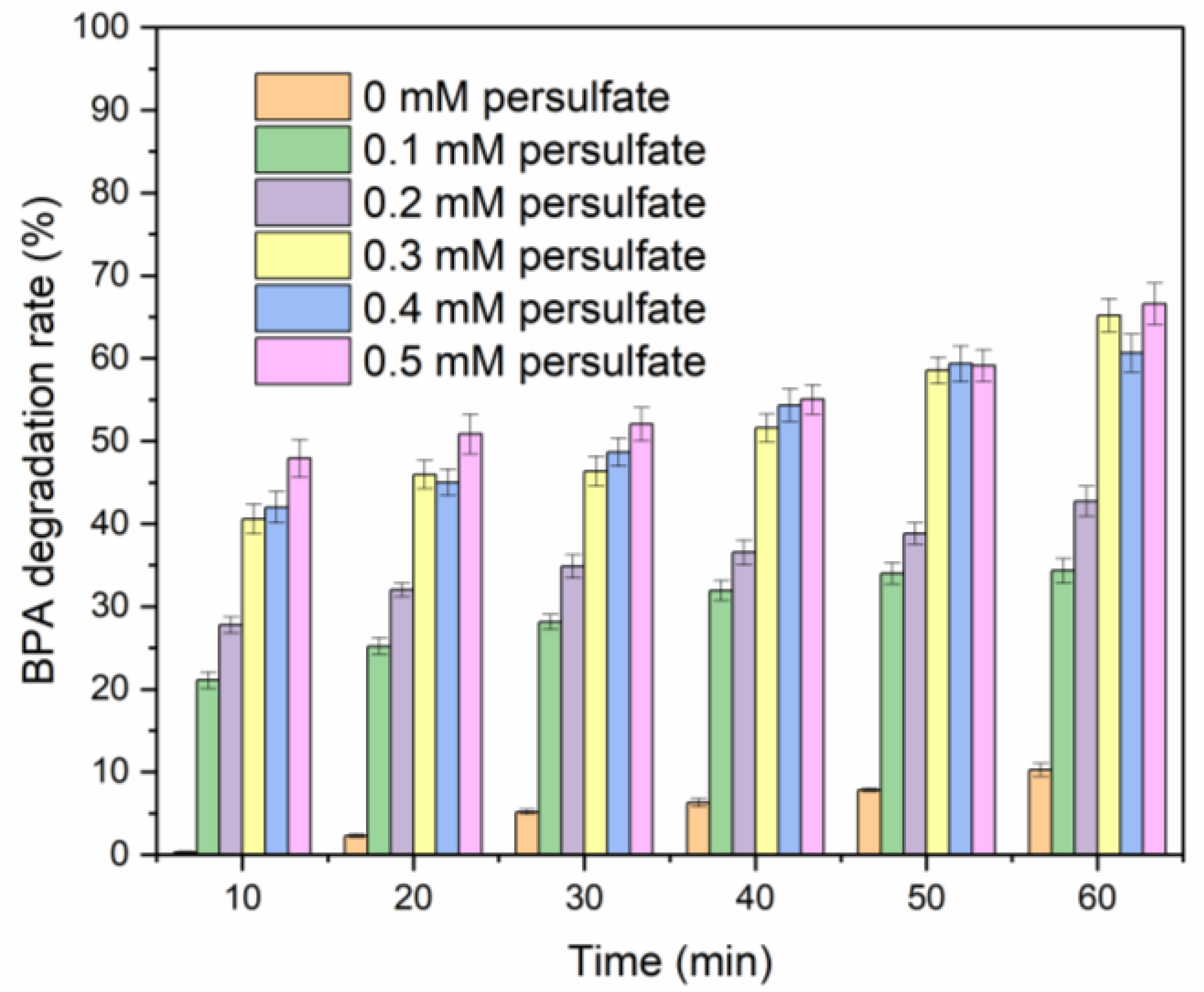
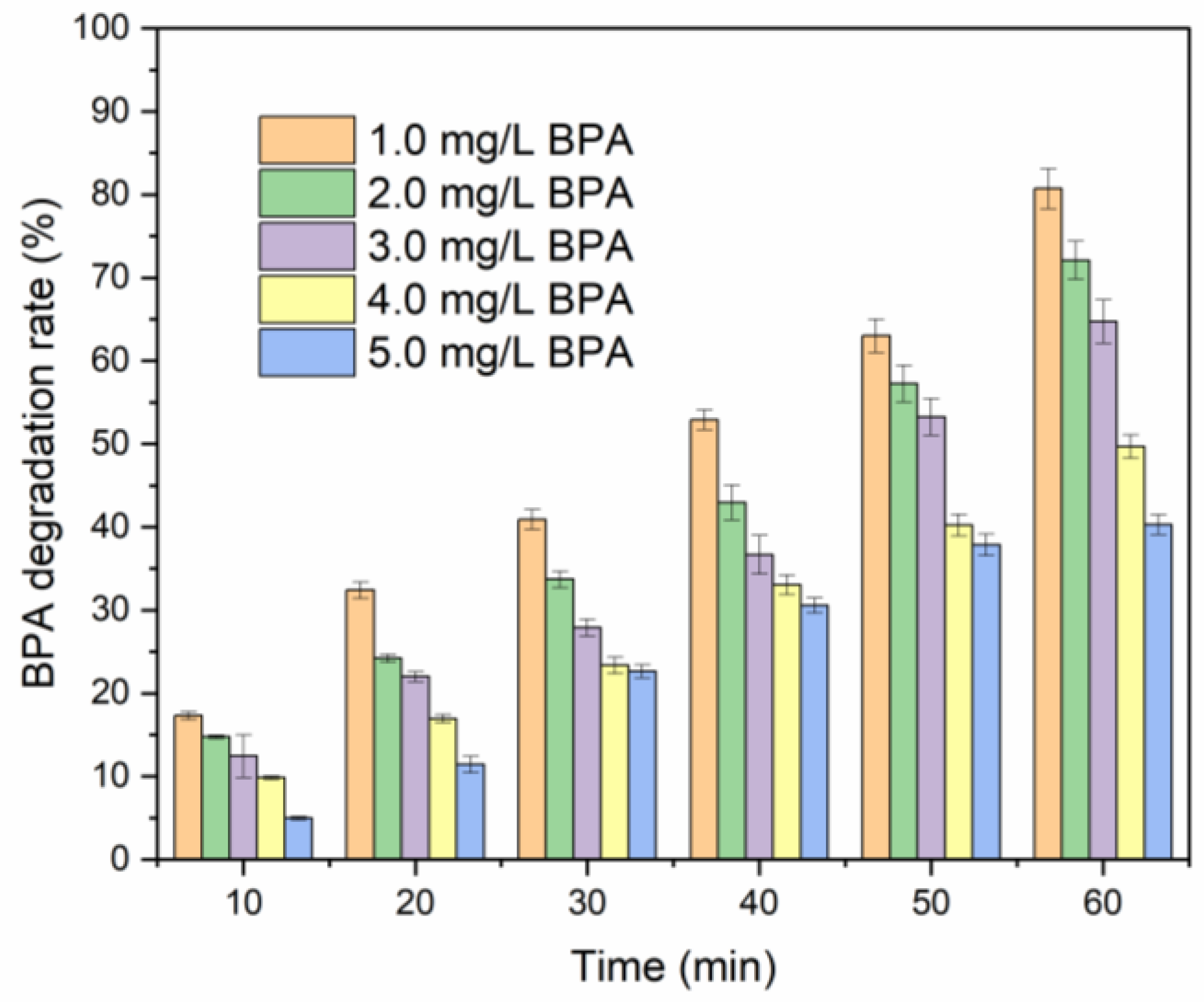
2.1. Single-Factor Experiments
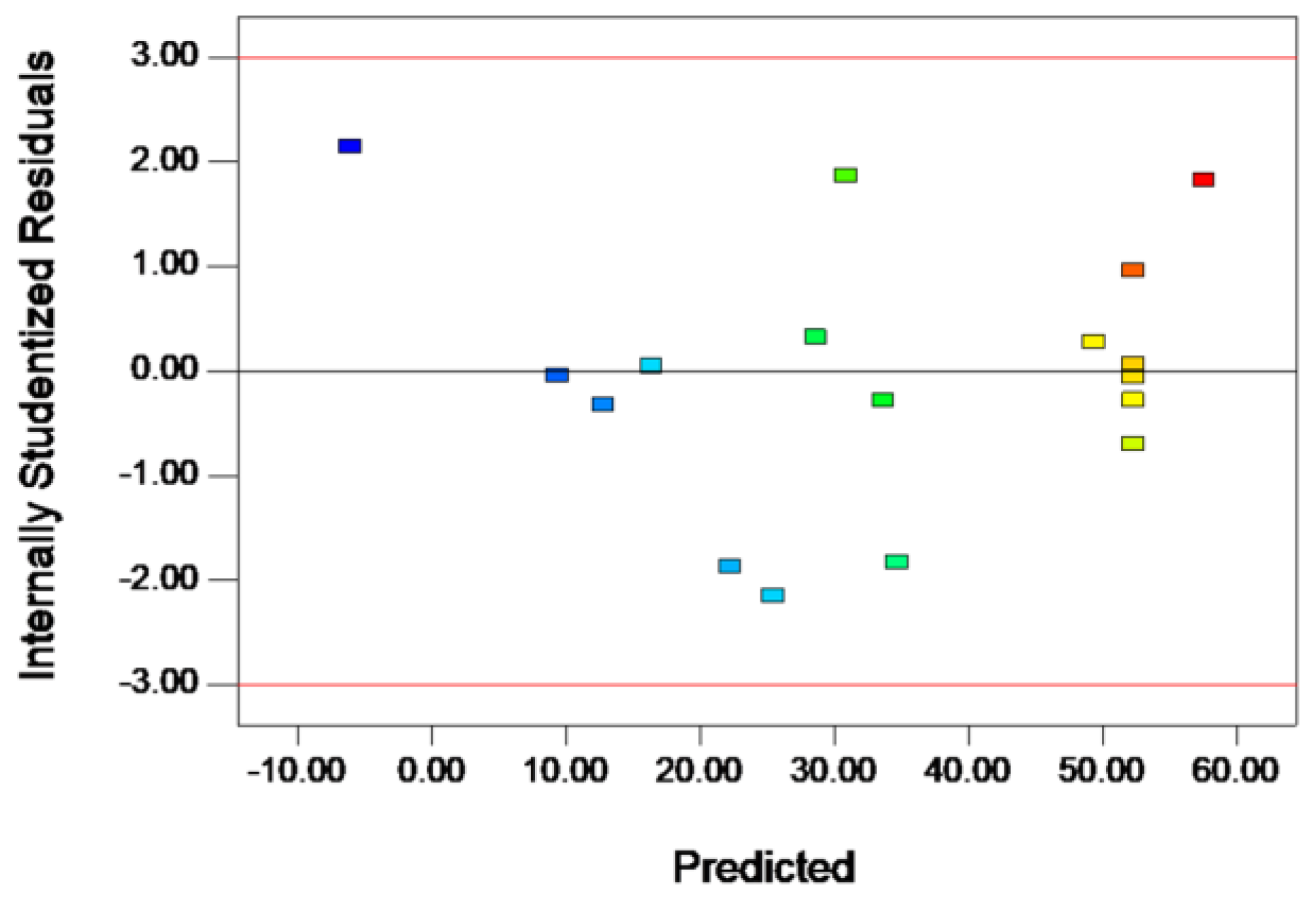
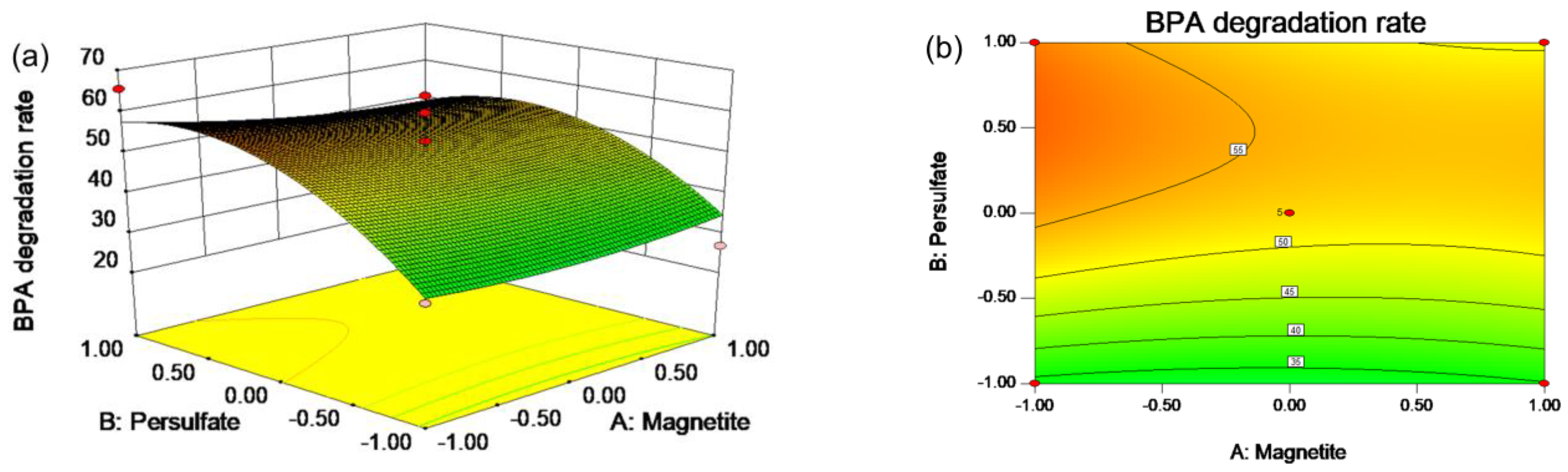
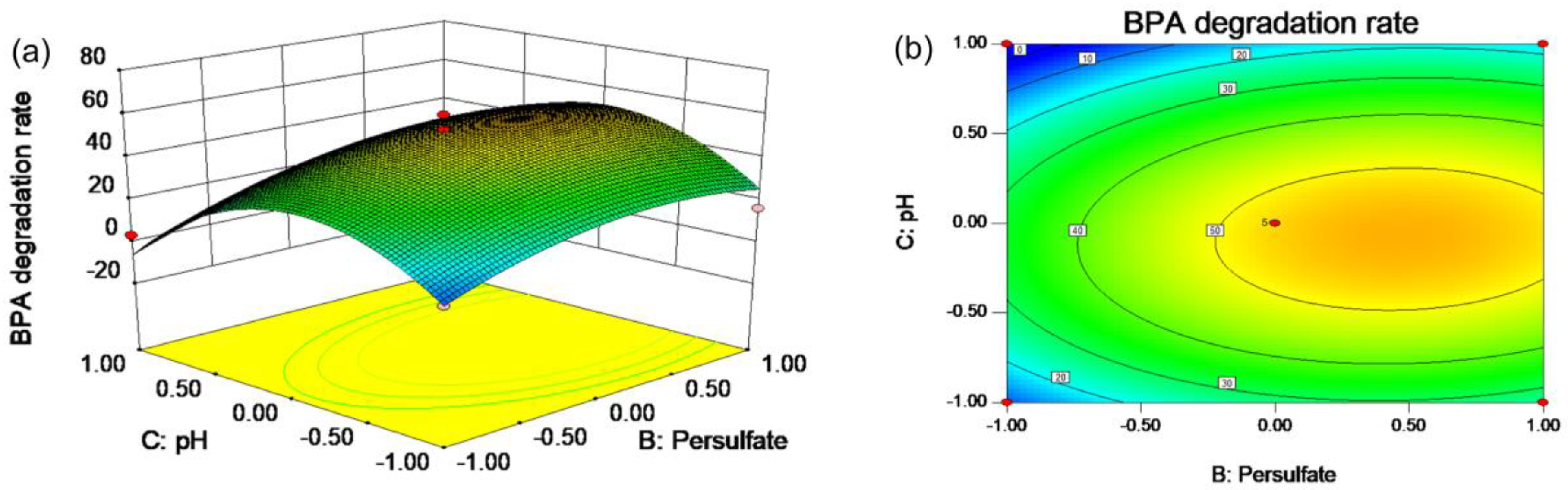
2.2. RSM Experiments
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Procedures
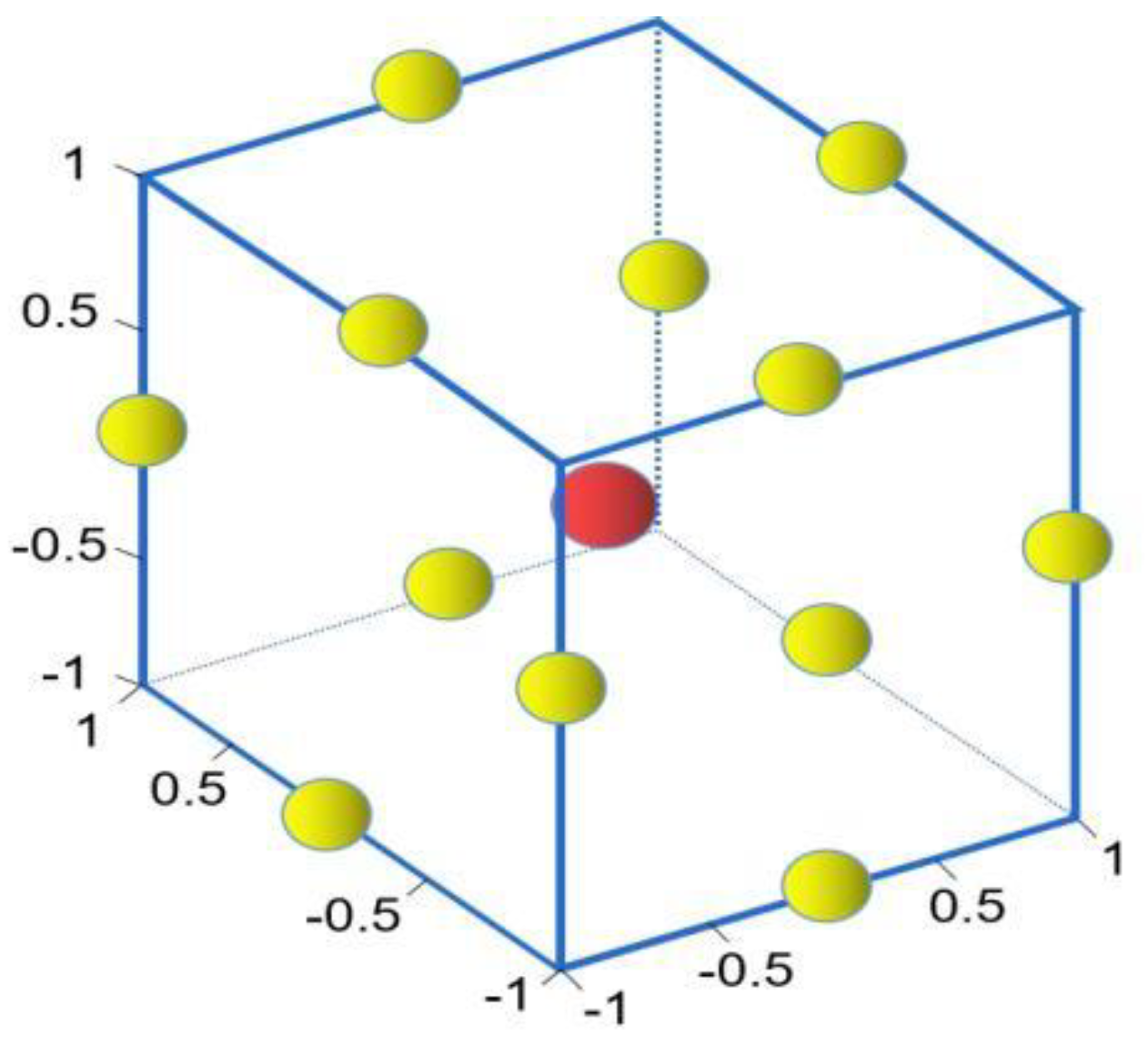
3.3. RSM Design
3.4. Analysis Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godswill, A.C.; Godspel, A.C. Physiological effects of plastic wastes on the endocrine system (Bisphenol A, Phthalates, Bisphenol S, PBDEs, TBBPA). Int. J. Bioinform. Comput. Biol. 2019, 4, 11–29. [Google Scholar]
- Wnuczek, K.; Puszka, A.; Podkościelna, B. Synthesis and spectroscopic snalyses of new polycarbonates based on bisphenol A-free components. Polymers 2021, 13, 4437. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Petrik, L.F. A review of pharmaceuticals and endocrine-disrupting compounds: Sources, effects, removal, and detections. Water Air Soil Pollut. 2013, 224, 1170. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Roddick, F.; Fan, L.; Aziz, H.A. Application of ozone for the removal of bisphenol A from water and wastewater—A review. Chemosphere 2013, 90, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.-H.; Dong, F.-X.; Yan, L.; Chen, Z.-L.; Qian, W.; Kong, L.-J.; Zhang, Z.-W.; Zhang, T.; Tao, X.-Q.; Du, J.-J.; et al. Synergistic oxidation of Bisphenol A in a heterogeneous ultrasound-enhanced sludge biochar catalyst/persulfate process: Reactivity and mechanism. J. Hazard. Mater. 2020, 384, 121385. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Fang, G.; Chen, Y.; Xie, M.; Zhu, F.; Ma, L.; Zhou, D.; Zhan, J. Efficient activation of persulfate decomposition by Cu2FeSnS4 nanomaterial for bisphenol A degradation: Kinetics, performance and mechanism studies. Appl. Catal. B Environ. 2019, 253, 278–285. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Akbari, S.; Ghanbari, F.; Moradi, M. Bisphenol A degradation in aqueous solutions by electrogenerated ferrous ion activated ozone, hydrogen peroxide and persulfate: Applying low current density for oxidation mechanism. Chem. Eng. J. 2016, 294, 298–307. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, M. Microwave-assisted persulfate/peroxymonosulfate process for environmental remediation. Curr. Opin. Chem. Eng. 2022, 36, 100826. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Fedorov, K.; Plata-Gryl, M.; Khan, J.A.; Boczkaj, G. Ultrasound-assisted heterogeneous activation of persulfate and peroxymonosulfate by asphaltenes for the degradation of BTEX in water. J. Hazard. Mater. 2020, 397, 122804. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Ghanbari, F.; Moradi, M. Photocatalysis assisted by peroxymonosulfate and persulfate for benzotriazole degradation: Effect of pH on sulfate and hydroxyl radicals. Water Sci. Technol. 2015, 72, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Forouzesh, M.; Ebadi, A.; Aghaeinejad-Meybodi, A. Degradation of metronidazole antibiotic in aqueous medium using activated carbon as a persulfate activator. Sep. Purif. Technol. 2018, 210, 145–151. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Yang, S.; Zhang, S.; Xu, Q.; Chen, P.; Du, Y.; Xing, Y. Cobalt-loaded cherry core biochar composite as an effective heterogeneous persulfate catalyst for bisphenol A degradation. RSC Adv. 2022, 12, 7284–7294. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zhang, Y.; Teng, Y.; Fan, M. Sulfate radical and its application in decontamination technologies. Crit. Rev. Environ. Sci. Technol. 2014, 45, 1756–1800. [Google Scholar] [CrossRef]
- Gao, Y.; Champagne, P.; Blair, D.; He, O.; Song, T. Activated persulfate by iron-based materials used for refractory organics degradation: A review. Water Sci. Technol. 2020, 81, 853–875. [Google Scholar] [CrossRef]
- Gao, F.; Li, Y.; Xiang, B. Degradation of bisphenol A through transition metals activating persulfate process. Ecotoxicol. Environ. Saf. 2018, 158, 239–247. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, Y.; Wang, P.; Li, H.; Dong, W. Degradation of bisphenol A in aqueous solution by persulfate activated with ferrous ion. Environ. Sci. Pollut. Res. 2013, 20, 4947–4953. [Google Scholar] [CrossRef]
- dos Santos, A.J.; Sirés, I.; Brillas, E. Removal of bisphenol A from acidic sulfate medium and urban wastewater using persulfate activated with electroregenerated Fe2+. Chemosphere 2021, 263, 128271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q.; Yin, X. Simultaneous removal of bisphenol A and phosphate in zero-valent iron activated persulfate oxidation process. Chem. Eng. J. 2016, 303, 458–466. [Google Scholar] [CrossRef]
- Girit, B.; Dursun, D.; Olmez-Hanci, T.; Arslan-Alaton, I. Treatment of aqueous bisphenol A using nano-sized zero-valent iron in the presence of hydrogen peroxide and persulfate oxidants. Water Sci. Technol. 2015, 71, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhang, Q.; Chen, B.Y.; Hong, J. Oxidation of bisphenol A by persulfate via Fe3O4-α-MnO2 nanoflower-like catalyst: Mechanism and efficiency. Chem. Eng. J. 2019, 357, 337–347. [Google Scholar] [CrossRef]
- Ioffe, M.; Long, M.; Radian, A. Systematic evaluation of activated carbon-Fe3O4 composites for removing and degrading emerging organic pollutants. Environ. Res. 2021, 198, 111187. [Google Scholar] [CrossRef]
- Zhu, Y.; Yue, M.; Natarajan, V.; Kong, L.; Ma, L.; Zhang, Y.; Zhao, Q.; Zhan, J. Efficient activation of persulfate by Fe3O4@β-cyclodextrin nanocomposite for removal of bisphenol A. RSC Adv. 2018, 8, 14879–14887. [Google Scholar] [CrossRef]
- Safabakhsh, T.; Daraei, H.; Saha, N.; Khodakarim, S. Degradation of Bisphenol A from aqueous solutions using Fe3O4 as a persulfate activator. Desalin. Water Treat. 2019, 166, 115–121. [Google Scholar] [CrossRef]
- Rao, Y.; Qu, L.; Yang, H.; Chu, W. Degradation of carbamazepine by Fe(II)-activated persulfate process. J. Hazard. Mater. 2014, 268, 23–32. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Sun, C.; Sun, J.Q.; Zhou, R. Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe3O4 nanoparticles process. Sep. Purif. Technol. 2015, 142, 182–188. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 Nanoparticles and their Magnetic Properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Zhu, C.; Fang, G.; Dionysiou, D.D.; Liu, C.; Gao, J.; Qin, W.; Zhou, D. Efficient transformation of DDTs with persulfate activation by zero-valent iron nanoparticles: A mechanistic study. J. Hazard. Mater. 2016, 316, 232–241. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Du, X.Z.; Huang, W.L. Temperature effect on the kinetics of persulfate oxidation of p-chloroaniline. Chin. Chem. Lett. 2011, 22, 358–361. [Google Scholar] [CrossRef]
- He, W.; Zhu, Y.; Zeng, G.; Zhang, Y.; Wang, Y.; Zhang, M.; Long, H.; Tang, W. Efficient removal of perfluorooctanoic acid by persulfate advanced oxidative degradation: Inherent roles of iron-porphyrin and persistent free radicals. Chem. Eng. J. 2019, 392, 123640. [Google Scholar] [CrossRef]
- Tunç, M.S. Decolorization of azo dye Everdirect Supra Red BWS in an aqueous solution by heat-activated persulfate: Optimization using response surface methodology. Desalin. Water Treat. 2019, 158, 372–384. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, C.; Tang, S.; Sun, M.; Zhang, Y.; Rao, Y.; Wang, Z.; Ke, J. Fe3+-sulfite complexation enhanced persulfate Fenton-like process for antibiotic degradation based on response surface optimization. Sci. Total Environ. 2020, 727, 138773. [Google Scholar] [CrossRef] [PubMed]
- Kusic, H.; Peternel, I.; Koprivanac, N.; Bozic, A.L. Iron-activated persulfate oxidation of an azo dye in model wastewater: Influence of iron activator type on process optimization. J. Environ. Eng. 2011, 137, 454–463. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, X.; Lei, L.; Sun, H.; Niu, Z.; Zhou, Z.; Xu, Z.; Hou, H. Efficient activation of persulfate by calcium sulfate whisker supported nanoscale zero-valent iron for methyl orange removal. RSC Adv. 2021, 11, 452–461. [Google Scholar] [CrossRef]
- Silveira, J.E.; Barreto-Rodrigues, M.; Cardoso, T.O.; Pliego, G.; Munoz, M.; Zazo, J.A.; Casas, J.A. Nanoscale Fe/Ag particles activated persulfate: Optimization using response surface methodology. Water Sci. Technol. 2017, 75, 2216–2224. [Google Scholar] [CrossRef]






| Name | CAS No. | Molecular Formula | Molecular Weight | Color | Structure |
|---|---|---|---|---|---|
| Bisphenol A | 80-05-7 | C15H16O2 | 228.29 | White | 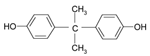 |
| Independent Variables | Symbols | Range and Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Magnetite dosage (g/L) | A | 0.3 | 0.4 | 0.5 |
| PS concentration (mM) | B | 0.1 | 0.2 | 0.3 |
| Initial pH | C | 3.0 | 5.0 | 7.0 |
| Run | A: Magnetite(g/L) | B: PS(mM) | C: Initial pH | BPA Removal (%) | |
|---|---|---|---|---|---|
| Actual Value | Predicted Value | ||||
| 1 | 0 | 1 | −1 | 16.14 | 25.46 |
| 2 | 0 | −1 | 1 | 3.24 | −6.08 |
| 3 | 0 | −1 | −1 | 9.16 | 9.36 |
| 4 | 0 | 0 | 0 | 50.20 | 52.34 |
| 5 | −1 | 0 | 1 | 14.12 | 22.23 |
| 6 | −1 | 0 | −1 | 30.04 | 28.64 |
| 7 | −1 | 1 | 0 | 65.52 | 57.60 |
| 8 | 0 | 0 | 0 | 59.80 | 52.34 |
| 9 | 0 | 0 | 0 | 46.90 | 52.34 |
| 10 | −1 | −1 | 0 | 32.48 | 33.69 |
| 11 | 1 | 0 | −1 | 39.02 | 30.91 |
| 12 | 1 | 1 | 0 | 50.60 | 49.39 |
| 13 | 0 | 0 | 0 | 51.90 | 52.34 |
| 14 | 1 | −1 | 0 | 26.80 | 34.72 |
| 15 | 1 | 0 | 1 | 11.38 | 12.79 |
| 16 | 0 | 1 | 1 | 16.58 | 16.38 |
| 17 | 0 | 0 | 0 | 52.90 | 52.34 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 5700.29 | 9 | 633.37 | 8.39 | 0.0052 | significant |
| A-Magnetite | 25.78 | 1 | 25.78 | 0.34 | 0.5773 | |
| B-Persulfate | 744.21 | 1 | 744.21 | 9.86 | 0.0164 | |
| C-pH | 300.62 | 1 | 300.62 | 3.98 | 0.0862 | |
| AB | 21.34 | 1 | 21.34 | 0.28 | 0.6113 | |
| AC | 34.34 | 1 | 34.34 | 0.45 | 0.5216 | |
| BC | 10.11 | 1 | 10.11 | 0.13 | 0.7252 | |
| A2 | 15.77 | 1 | 15.77 | 0.21 | 0.6615 | |
| B2 | 457.60 | 1 | 457.60 | 6.06 | 0.0433 | |
| C2 | 3951.59 | 1 | 3951.59 | 52.35 | 0.0002 | |
| Residual | 528.35 | 7 | 75.48 | |||
| Lack of Fit | 438.02 | 3 | 146.01 | 6.47 | 0.0516 | not significant |
| Pure Error | 90.33 | 4 | ||||
| Cor Total | 6228.64 | 16 | 22.58 | |||
| R2 = 0.9152 | ||||||
| Adjusted R2 = 0.8061 | ||||||
| C.V.% = 25.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Yu, Y. Response Surface Methodology for Optimization of Bisphenol A Degradation Using Fe3O4-Activated Persulfate. Catalysts 2023, 13, 128. https://doi.org/10.3390/catal13010128
Chen S, Yu Y. Response Surface Methodology for Optimization of Bisphenol A Degradation Using Fe3O4-Activated Persulfate. Catalysts. 2023; 13(1):128. https://doi.org/10.3390/catal13010128
Chicago/Turabian StyleChen, Shufang, and Yan Yu. 2023. "Response Surface Methodology for Optimization of Bisphenol A Degradation Using Fe3O4-Activated Persulfate" Catalysts 13, no. 1: 128. https://doi.org/10.3390/catal13010128
APA StyleChen, S., & Yu, Y. (2023). Response Surface Methodology for Optimization of Bisphenol A Degradation Using Fe3O4-Activated Persulfate. Catalysts, 13(1), 128. https://doi.org/10.3390/catal13010128





