Performance and Stability of Doped Ceria–Zirconia Catalyst for a Multifuel Reforming
Abstract
1. Introduction
2. Results and Discussion
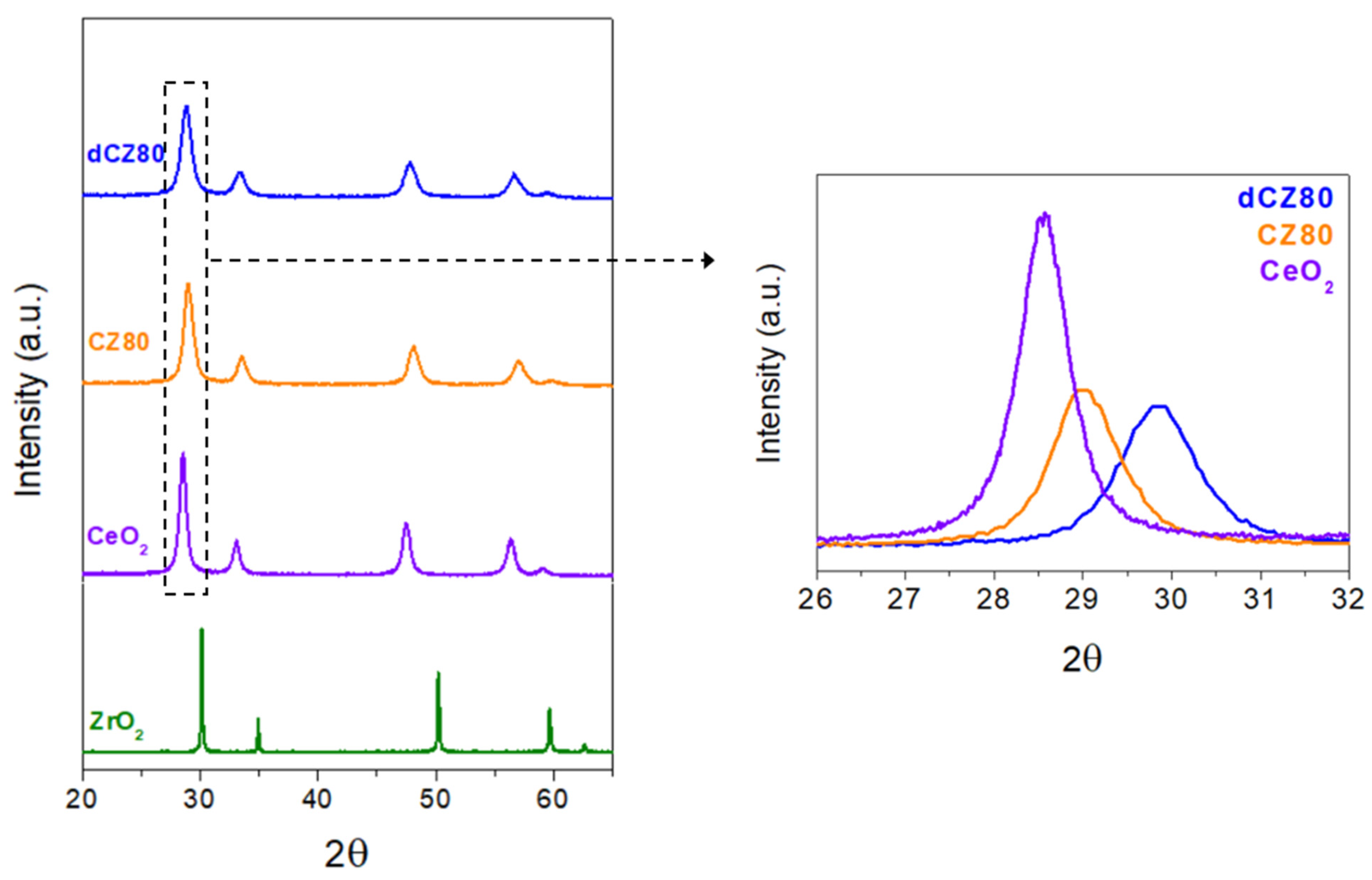
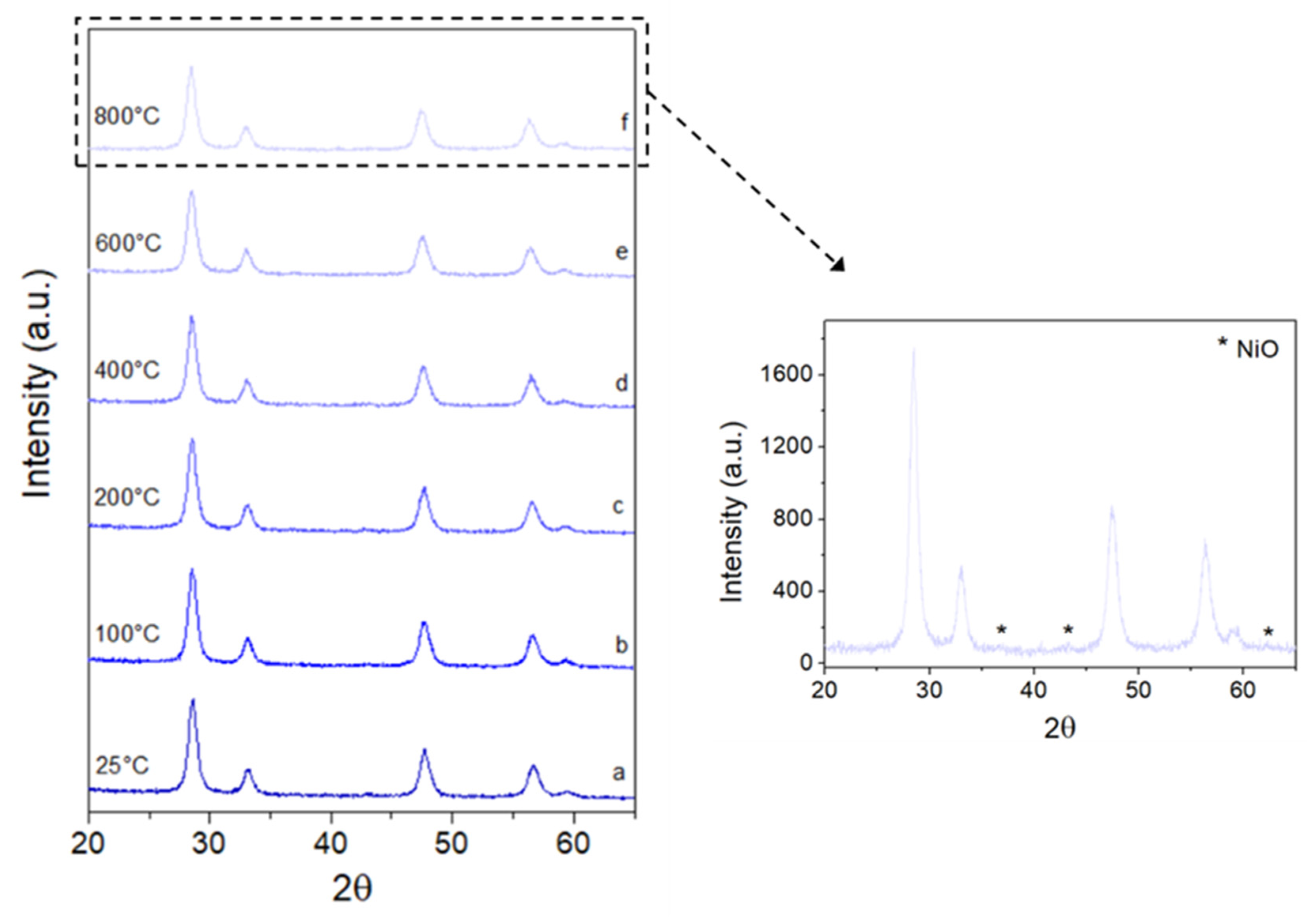
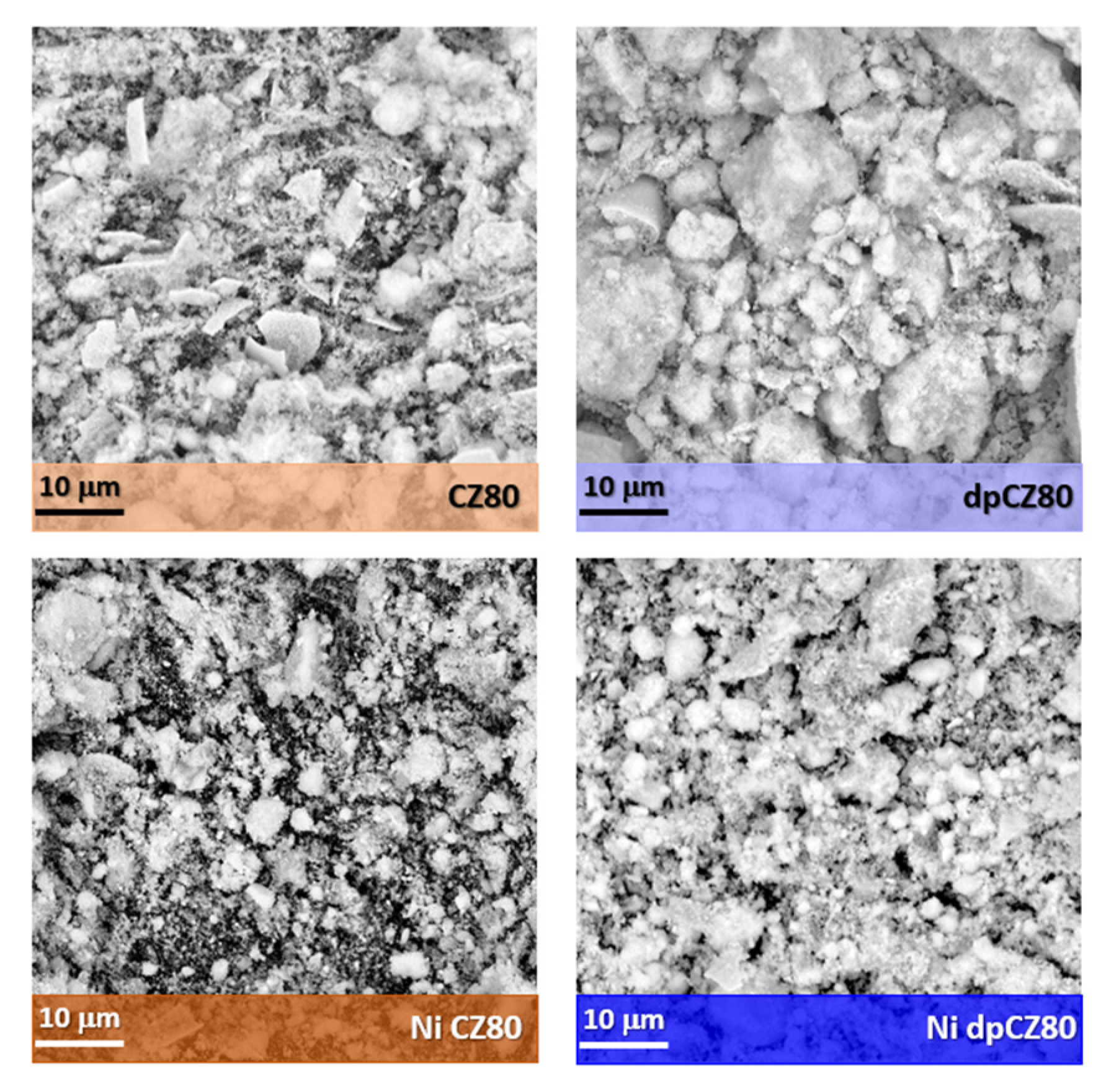
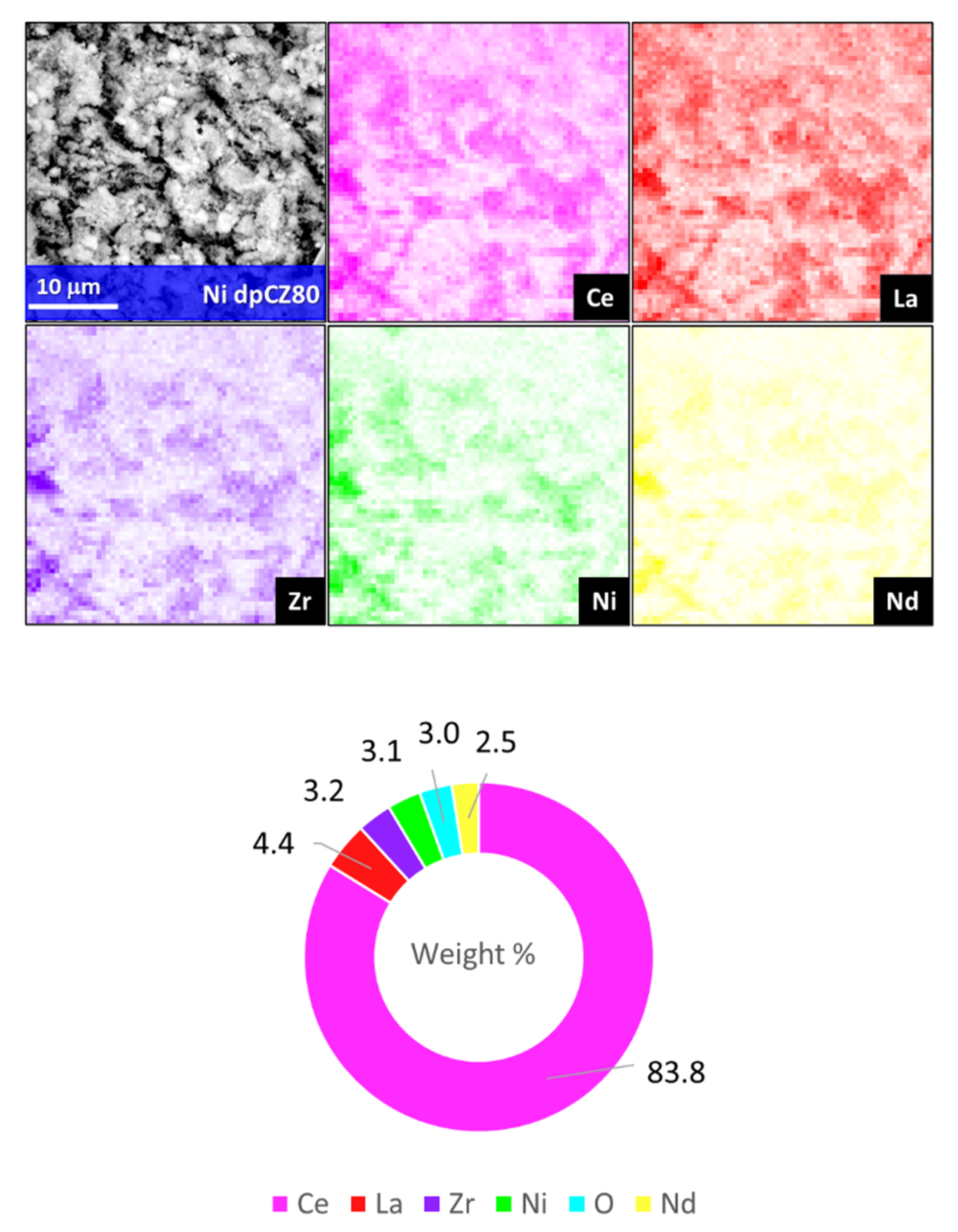
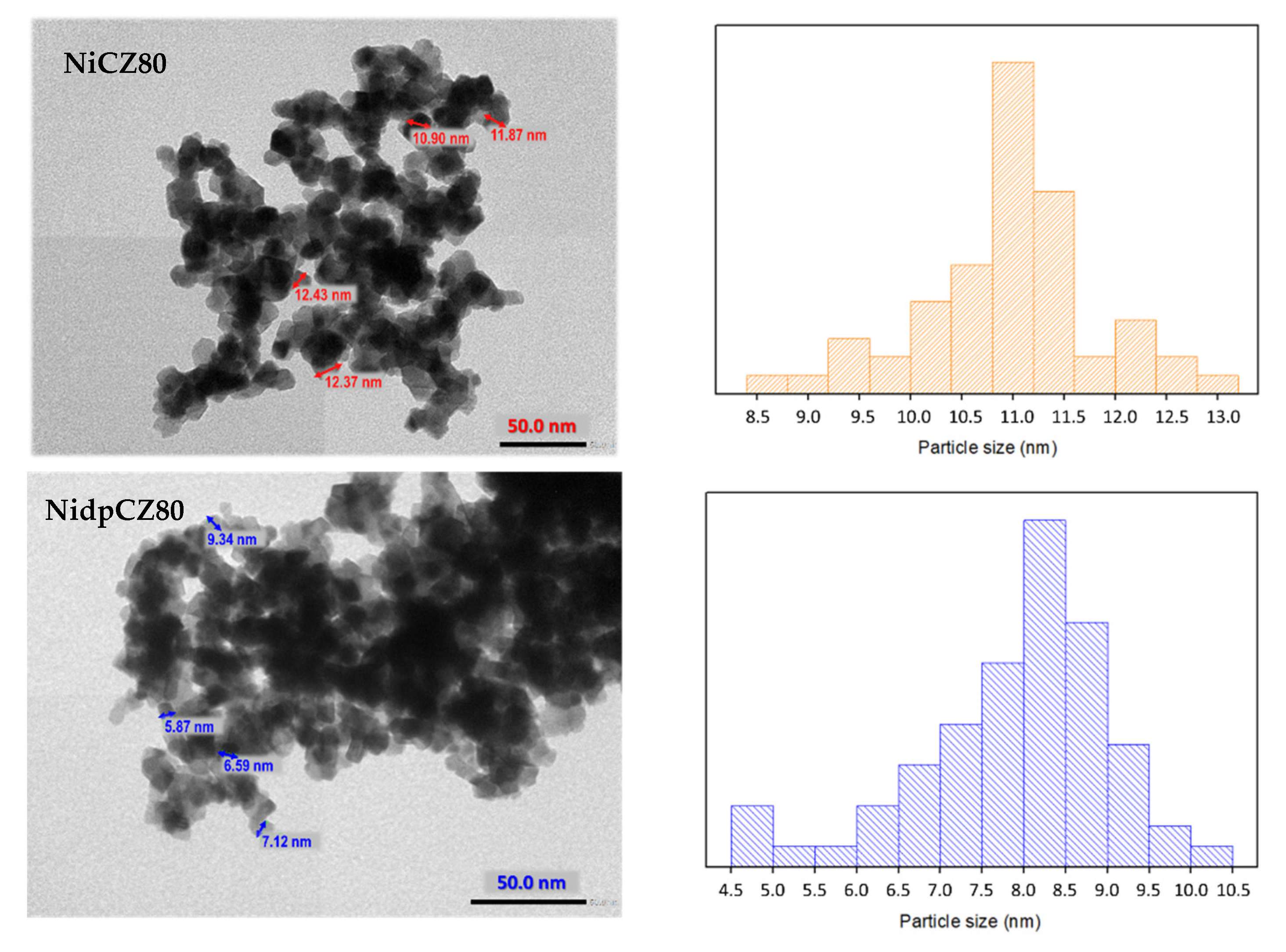
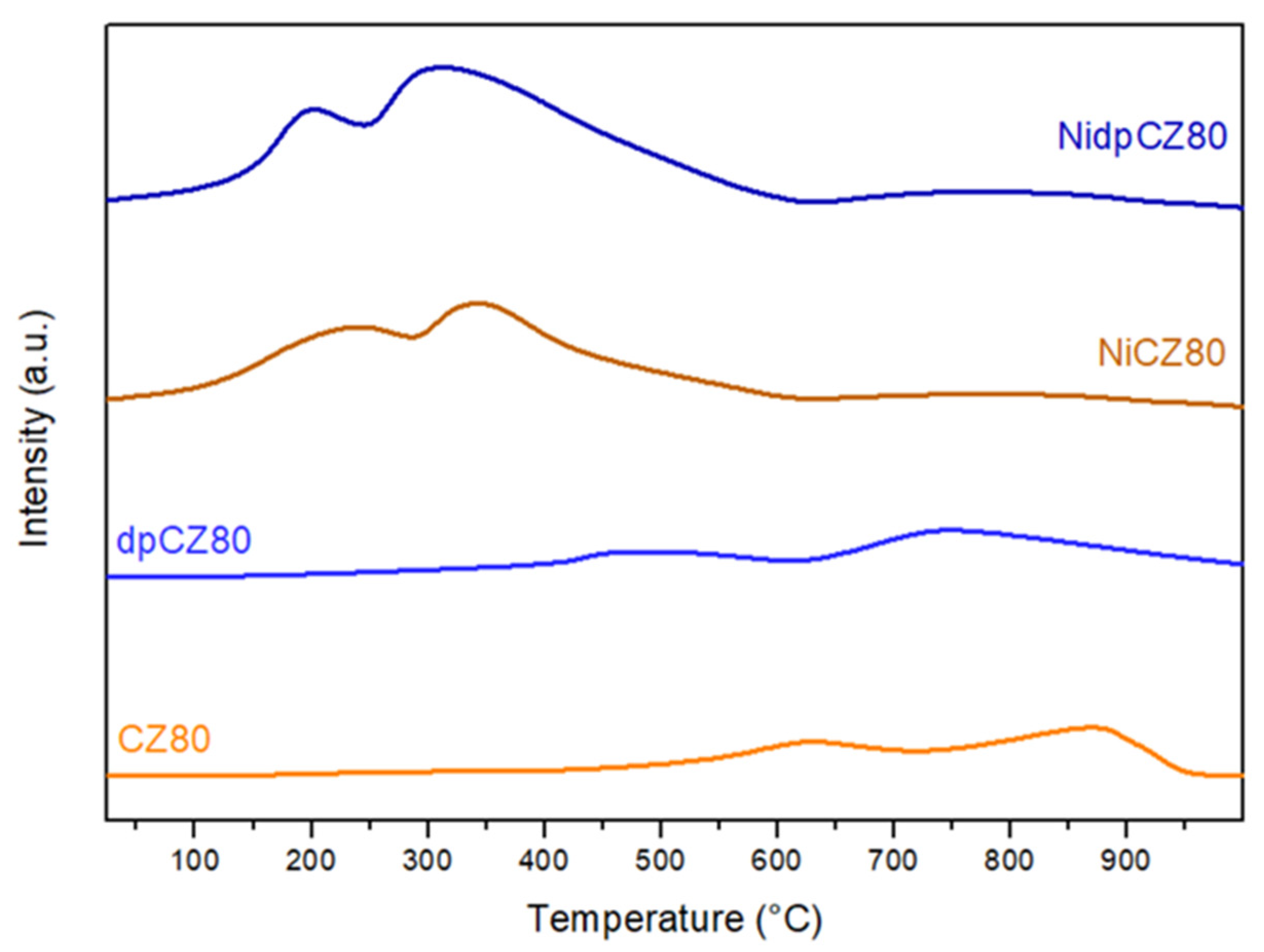
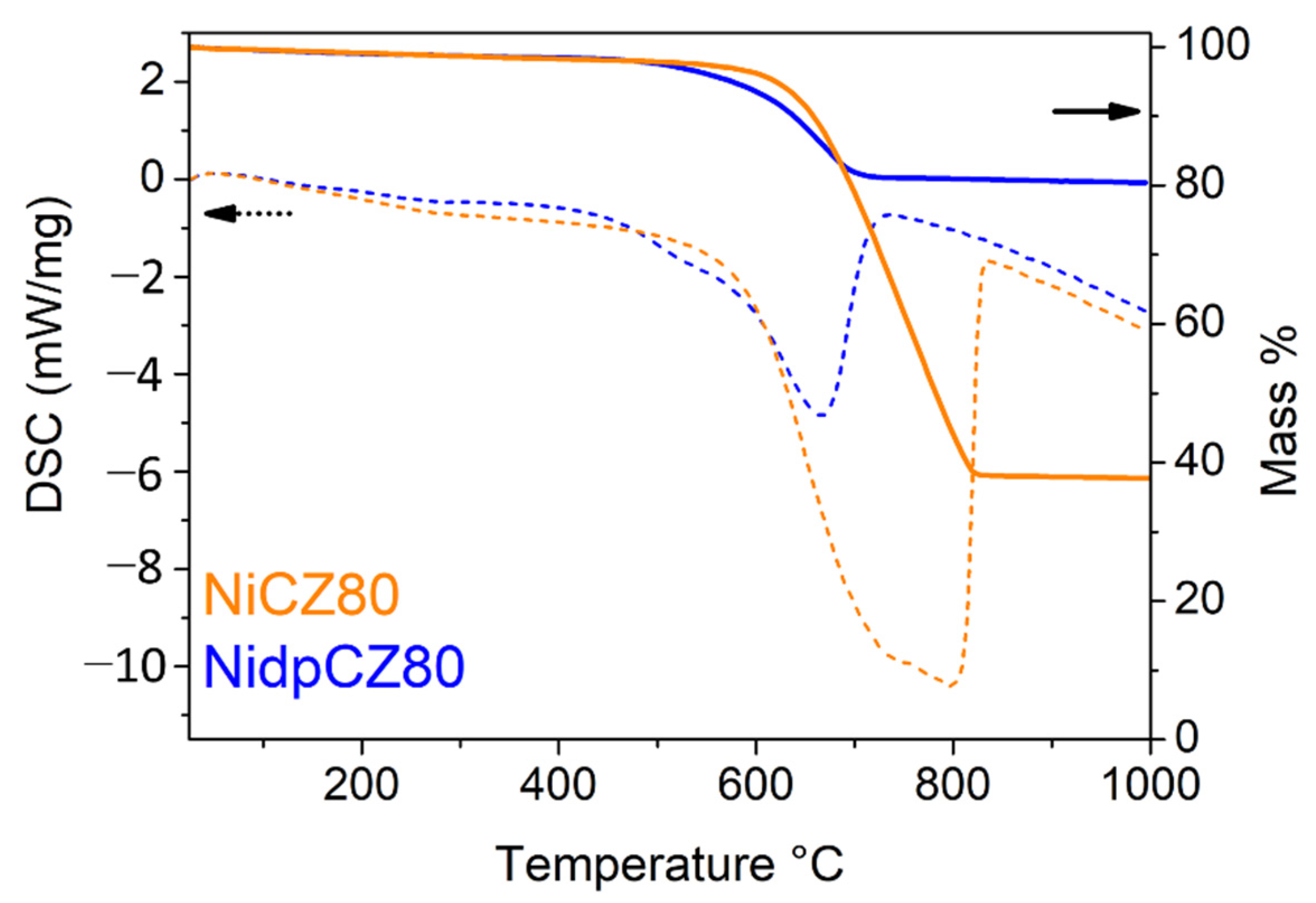
2.1. Characterization of Catalytic Systems
2.2. Catalytic Test Results
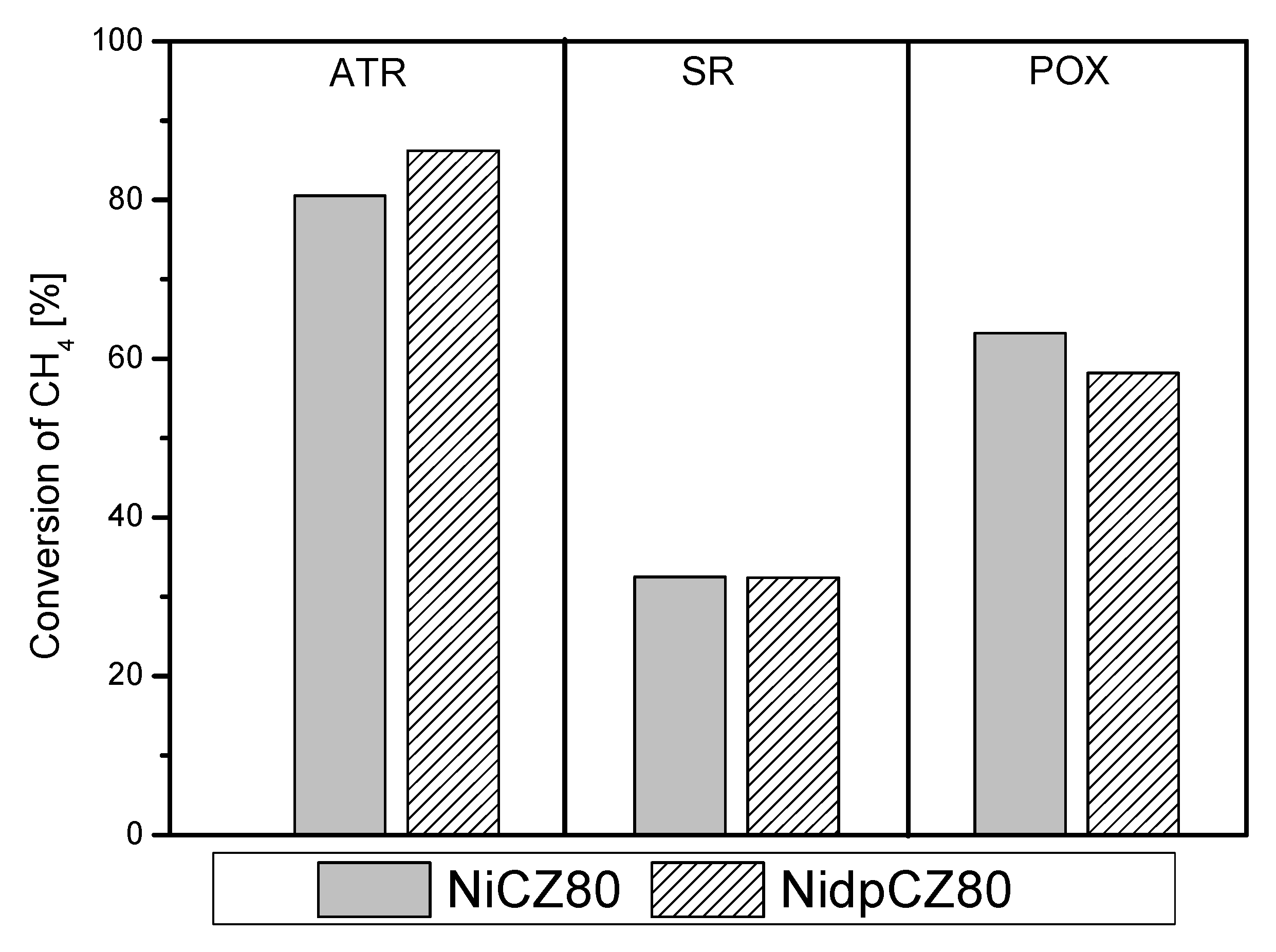
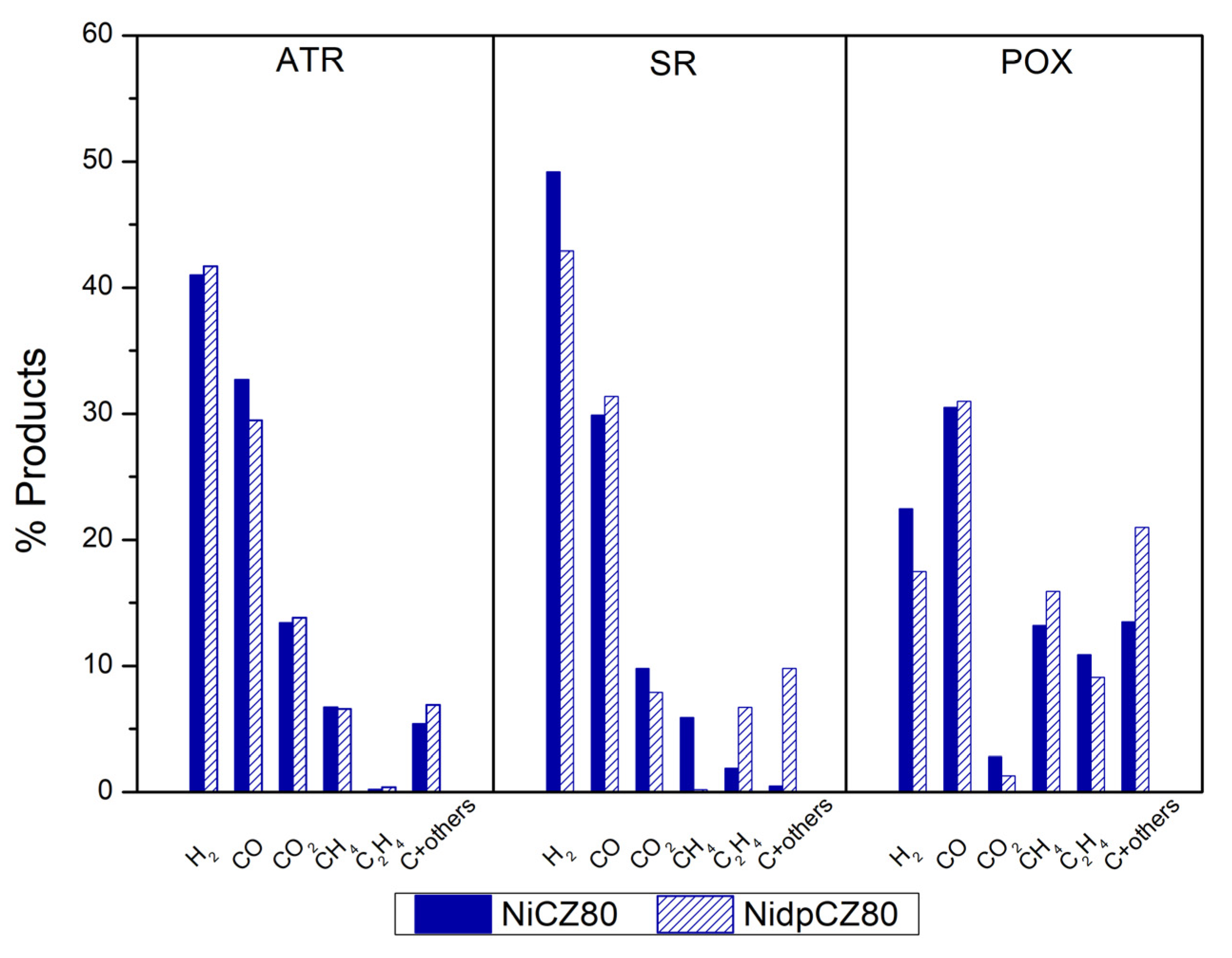
2.2.1. Methane as Fuel
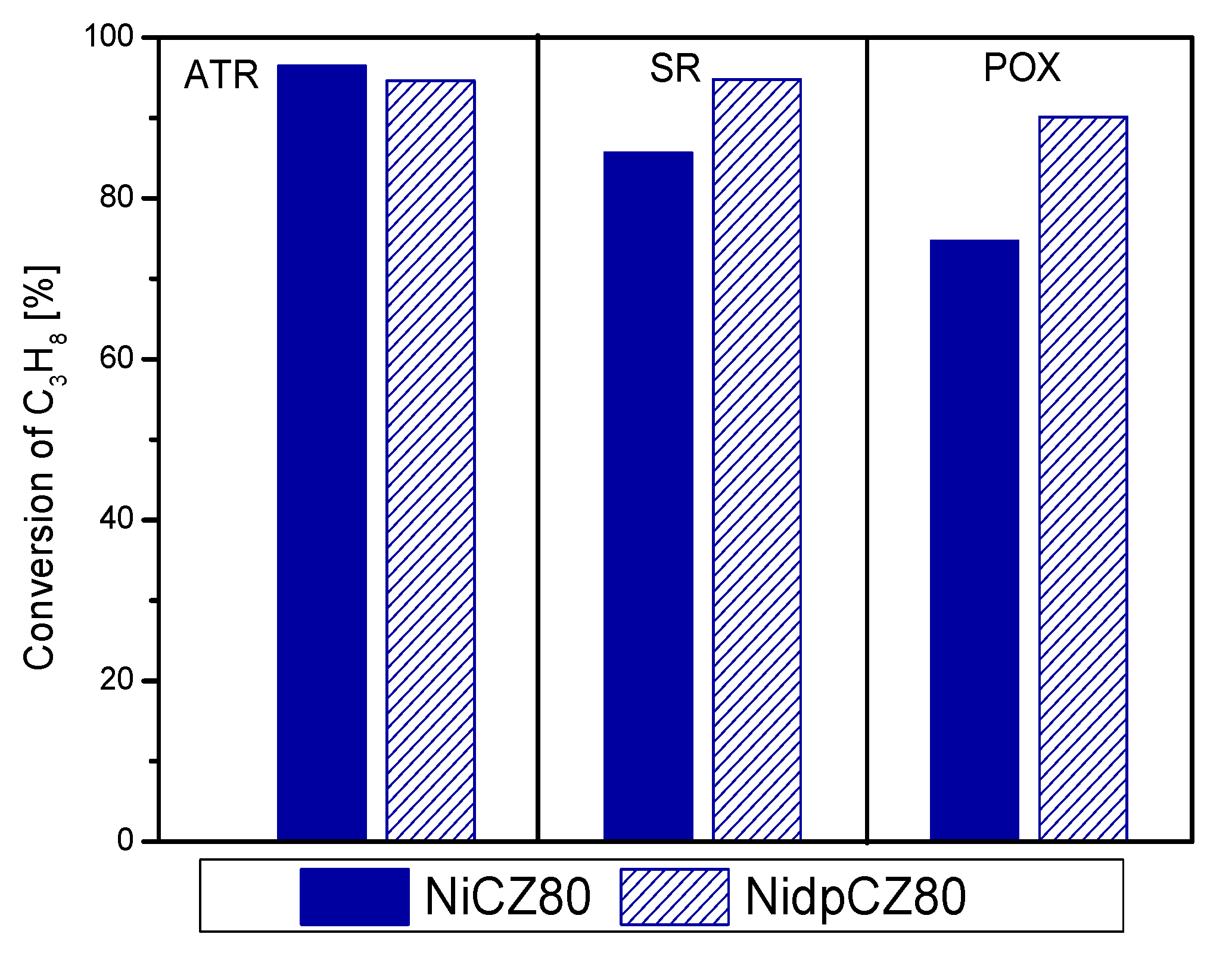
2.2.2. Propane as Fuel
2.2.3. Odorized Fuel with H2S
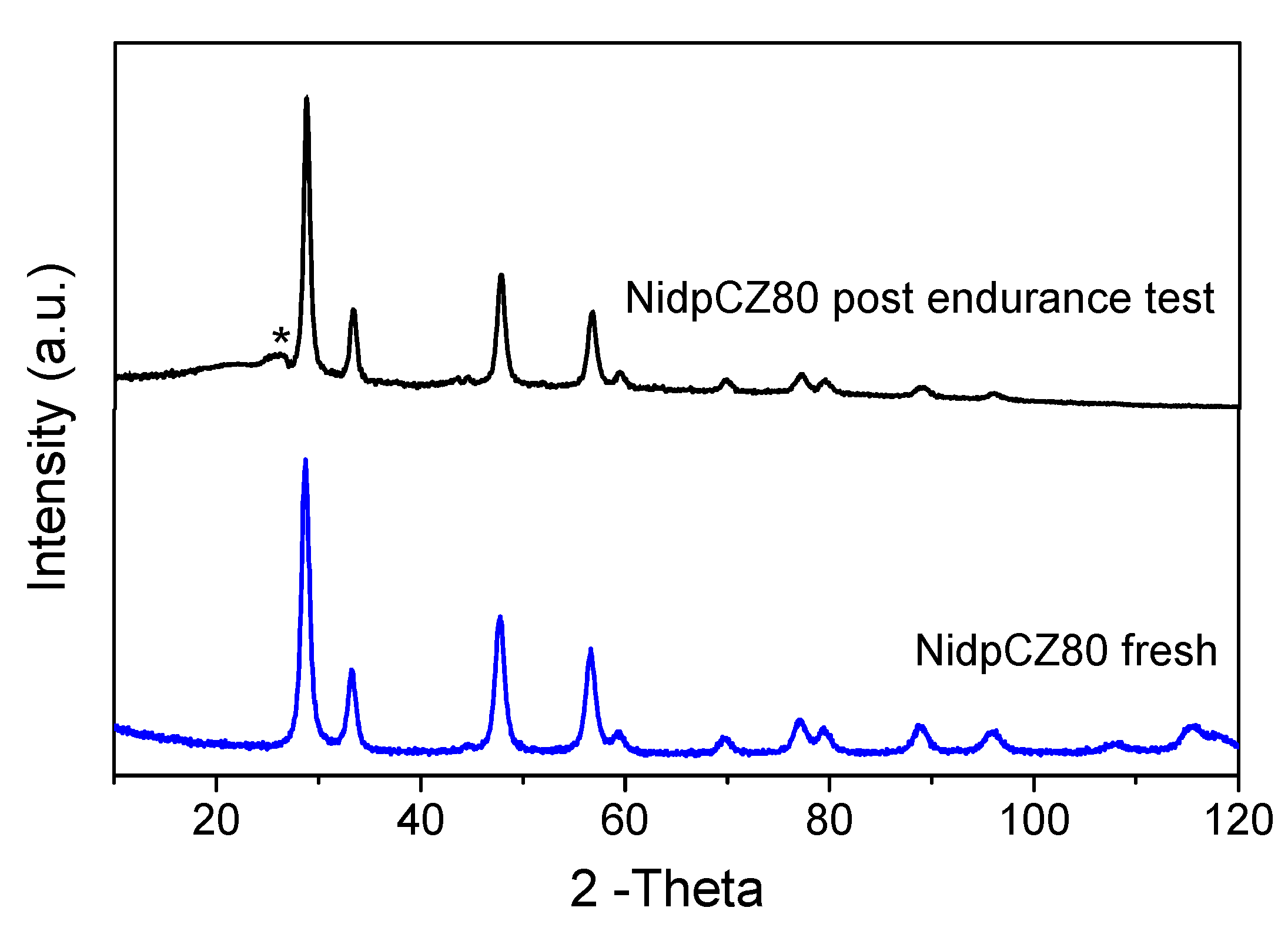
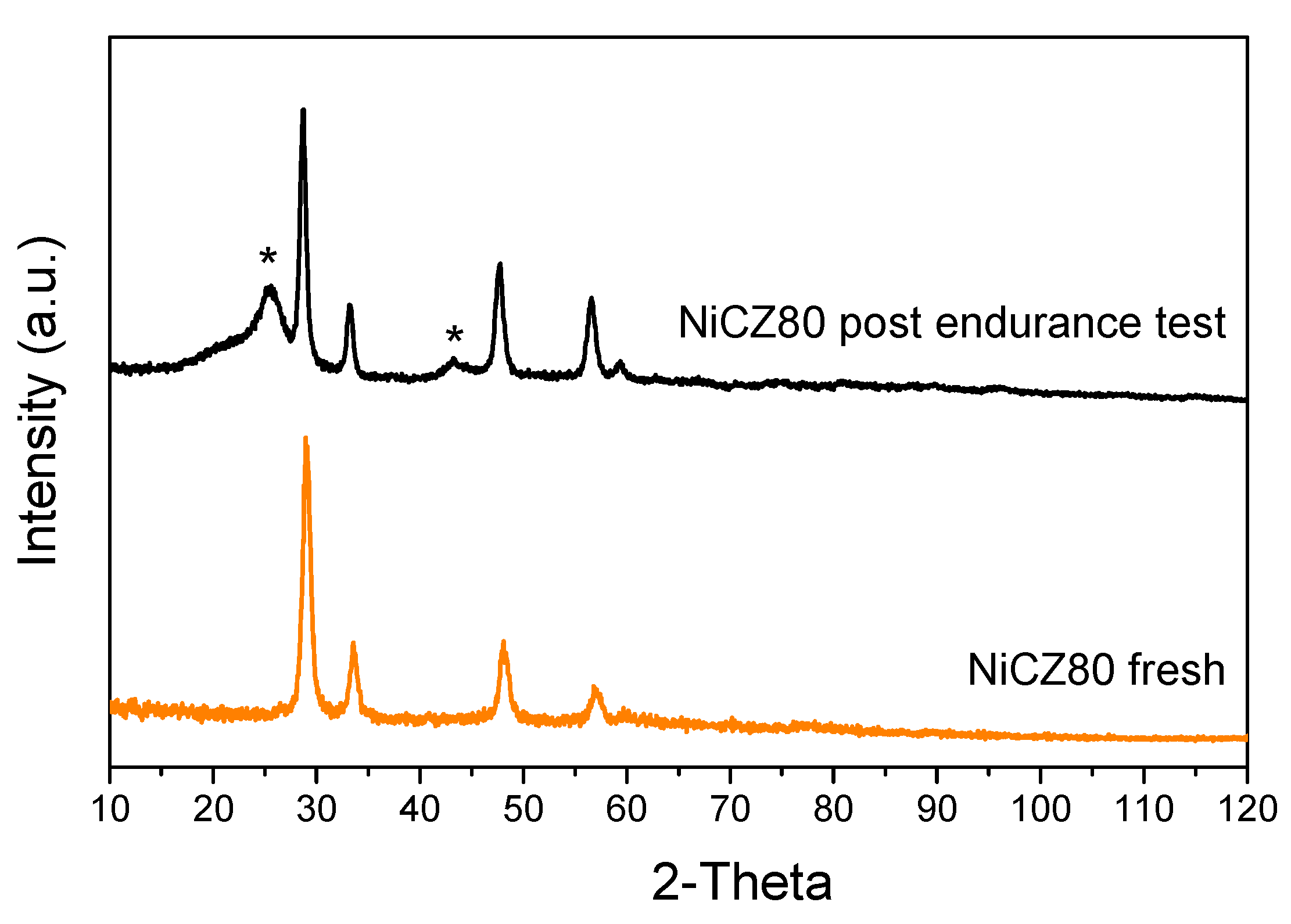
2.2.4. Endurance Test
3. Materials and Methods
3.1. Catalytic System
3.2. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Mitigation of Climate Change; Working Group III contribution to the Fifth Assessment Report; IPCC: Geneva, Switzerland, 2014; Volume 1454, p. 147. [Google Scholar]
- Asghar, M.I.; Jouttijärvi, S.; Jokiranta, R.; Lund, P.D. Remarkable ionic conductivity and catalytic activity in ceramic nanocomposite fuel cells. Int. J. Hydrogen Energy 2018, 43, 12892–12899. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Kilner, J.A.; Burriel, M. Materials for intermediate-temperature solid-oxide fuel cells. Annu. Rev. Mater. Res. 2014, 44, 365–393. [Google Scholar] [CrossRef]
- Park, S.; Vohs, J.M.; Gorte, R.J. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 2000, 404, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Siraj, K.; Raza, R.; Ali, A.; Tiwari, P.; Zhu, B.; Rafique, A.; Ali, A.; Kaleem Ullah, M.; Usman, A. A brief description of high temperature solid oxide fuel cell’s operation, materials, design, fabrication technologies and performance. Appl. Sci. 2016, 6, 75. [Google Scholar] [CrossRef]
- Frontera, P.; Modafferi, V.; Frusteri, F.; Bonura, G.; Bottari, M.; Siracusano, S.; Antonucci, P.L. Catalytic features of Ni/Ba–Ce0. 9–Y0. 1 catalyst to produce hydrogen for PCFCs by methane reforming. Int. J. Hydrog. Energy 2010, 35, 11661–11668. [Google Scholar] [CrossRef]
- Ettler, M.; Timmermann, H.; Malzbender, J.; Weber, A.; Menzler, N.H. Durability of Ni anodes during reoxidation cycles. J. Power Sources 2010, 195, 5452–5467. [Google Scholar] [CrossRef]
- Ru, Y.; Sang, J.; Xia, C.; Wei, W.-C.J.; Guan, W. Durability of direct internal reforming of methanol as fuel for solid oxide fuel cell with double-sided cathodes. Int. J. Hydrog. Energy 2020, 45, 7069–7076. [Google Scholar] [CrossRef]
- Xi, X.; Abe, H.; Naito, M. Effect of composition on microstructure and polarization resistance of solid oxide fuel cell anode Ni-YSZ composites made by co-precipitation. Ceram. Int. 2014, 40, 16549–16555. [Google Scholar] [CrossRef]
- Mehran, M.T.; Khan, M.Z.; Lee, S.-B.; Lim, T.-H.; Park, S.; Song, R.-H. Improving sulfur tolerance of Ni-YSZ anodes of solid oxide fuel cells by optimization of microstructure and operating conditions. Int. J. Hydrog. Energy 2018, 43, 11202–11213. [Google Scholar] [CrossRef]
- Costa-Nunes, O.; Gorte, R.J.; Vohs, J.M. Comparison of the performance of Cu–CeO2–YSZ and Ni–YSZ composite SOFC anodes with H2, CO, and syngas. J. Power Sources 2005, 141, 241–249. [Google Scholar] [CrossRef]
- Escudero, M.J.; Valero, C.; Cauqui, M.Á.; Goma, D.; Yeste, M.P. Ni-Ce-ZrO2 system as anode material for direct internal reforming biogas solid oxide fuel cells. Fuel 2022, 322, 124247. [Google Scholar] [CrossRef]
- Osazuwa, O.U.; Abidin, S.Z. An overview on the role of lanthanide series (rare earth metals) in H2 and syngas production from CH4 reforming processes. Chem. Eng. Sci. 2020, 227, 115863. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Wu, H.; Yang, W.; He, D. Effects of Nd, Ce, and La modification on catalytic performance of Ni/SBA-15 catalyst in CO2 reforming of CH4. Chin. J. Catal. 2014, 35, 1520–1528. [Google Scholar] [CrossRef]
- Dedov, A.G.; Loktev, A.S.; Komissarenko, D.A.; Mazo, G.N.; Shlyakhtin, O.A.; Parkhomenko, K.V.; Kiennemann, A.A.; Roger, A.-C.; Ishmurzin, A.V.; Moiseev, I.I. Partial oxidation of methane to produce syngas over a neodymium–calcium cobaltate-based catalyst. Appl. Catal. A Gen. 2015, 489, 140–146. [Google Scholar] [CrossRef]
- Sartoretti, E.; Novara, C.; Chiodoni, A.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts doped with La and Nd: How acid-base sites and redox properties determine the oxidation mechanisms. Catal. Today 2022, 390, 117–134. [Google Scholar] [CrossRef]
- Pappacena, A.; Graziutti, R.; Boaro, M.; Trovarelli, A. CeO2-ZrO2 catalysts for the use of biogas in IT-SOFC. ECS Trans. 2015, 68, 2789–2795. [Google Scholar] [CrossRef]
- Nguyen, T.G.H.; Sakamoto, M.; Uchida, T.; Doan, D.C.T.; Dang, M.C.; Tu, P.H.; Sasaki, K.; Shiratori, Y. Development of paper-structured catalyst for application to direct internal reforming solid oxide fuel cell fueled by biogas. Int. J. Hydrog. Energy 2019, 44, 10484–10497. [Google Scholar] [CrossRef]
- Lo Faro, M.; Modafferi, V.; Frontera, P.; Antonucci, P.; Aricò, A.S. Catalytic behavior of Ni-modified perovskite and doped ceria composite catalyst for the conversion of odorized propane to syngas. Fuel Process. Technol. 2013, 113, 28–33. [Google Scholar] [CrossRef]
- Dantas, S.C.; Escritori, J.C.; Soares, R.R.; Hori, C.E. Effect of different promoters on Ni/CeZrO2 catalyst for autothermal reforming and partial oxidation of methane. Chem. Eng. J. 2010, 156, 380–387. [Google Scholar] [CrossRef]
- Escritori, J.C.; Dantas, S.C.; Soares, R.R.; Hori, C.E. Methane autothermal reforming on nickel–ceria–zirconia based catalysts. Catal. Commun. 2009, 10, 1090–1094. [Google Scholar] [CrossRef]
- Ismagilov, I.Z.; Matus, E.V.; Kuznetsov, V.V.; Kerzhentsev, M.A.; Yashnik, S.A.; Prosvirin, I.P.; Mota, N.; Navarro, R.M.; Fierro, J.L.G.; Ismagilov, Z.R. Hydrogen production by autothermal reforming of methane over NiPd catalysts: Effect of support composition and preparation mode. Int. J. Hydrog. Energy 2014, 39, 20992–21006. [Google Scholar] [CrossRef]
- Lim, S.-S.; Lee, H.-J.; Moon, D.-J.; Kim, J.-H.; Park, N.-C.; Shin, J.-S.; Kim, Y.-C. Autothermal reforming of propane over Ce modified Ni/LaAlO3 perovskite-type catalysts. Chem. Eng. J. 2009, 152, 220–226. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lim, Y.-S.; Park, N.-C.; Kim, Y.-C. Catalytic autothermal reforming of propane over the noble metal-doped hydrotalcite-type catalysts. Chem. Eng. J. 2009, 146, 295–301. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Rane, V.H.; Rajput, A.M. Simultaneous thermal cracking and oxidation of propane to propylene and ethylene. AIChE J. 1998, 44, 2293–2301. [Google Scholar] [CrossRef]
- Frontera, P.; Antonucci, P.L.; Macario, A. Focus on Materials for Sulfur-Resistant Catalysts in the Reforming of Biofuels. Catalysts 2021, 11, 1029. [Google Scholar] [CrossRef]
- Lo Faro, M.; Antonucci, V.; Antonucci, P.L.; Aricò, A.S. Fuel flexibility: A key challenge for SOFC technology. Fuel 2012, 102, 554–559. [Google Scholar] [CrossRef]
- Ruiz, J.A.C.; Passos, F.B.; Bueno, J.M.C.; Souza-Aguiar, E.F.; Mattos, L.V.; Noronha, F.B. Syngas production by autothermal reforming of methane on supported platinum catalysts. Appl. Catal. A Gen. 2008, 334, 259–267. [Google Scholar] [CrossRef]
- Pino, L.; Vita, A.; Cipitì, F.; Lagana, M.; Recupero, V. Performance of Pt/CeO2 catalyst for propane oxidative steam reforming. Appl. Catal. A Gen. 2006, 306, 68–77. [Google Scholar] [CrossRef]
- Feret, F.R. Determination of the crystallinity of calcined and graphitic cokes by X-ray diffraction. Analyst 1998, 123, 595–600. [Google Scholar] [CrossRef]
- Menon, P.G. Coke on catalysts-harmful, harmless, invisible and beneficial types. J. Mol. Catal. 1990, 59, 207–220. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]



| Catalyst | Ni Content wt/wt% | Composition of Inlet Feed | Duration of Test | Space Velocity | Methane Conversion % | H2 Yield | Ref. |
|---|---|---|---|---|---|---|---|
| Ni/CeZrO2 | 10% | 2CH4/1H2O/0.5O2 | 25 h | 12 mgcatalyst/180 mL/min | 50 at 800 °C | 40% | [21] |
| Ni/Ce0.5Zr0.5O2 | 10% | 2CH4/1H2O/0.5O2 | 24 h | 12 mgcatalyst/180 mL/min | 50 at 800 °C | 90% | [22] |
| NiPd/20La2O3/10Ce0.5Zr0.5O2/Al2O3 | 10%(Ni) 0.5%(Pd) | 1CH4/1H2O/0.75O2 | 27 h | -- | 97 at 850 °C | 69% | [23] |
| Ni/Cu0.05Ce0.2Zr0.1Al0.65Ox | 10% | 1CH4/0.5O2/2.5H2O | -- | 4800 h−1 | 83 at 650 °C | -- | [24] |
| Ni-modified LSFCO/CGO | 10% | C3H8/0.5O2/2.5H2O | 100 h | 120,000 h−1 | 95 at 800 °C | 50% (mean value) | [20] |
| Ni/MgAl | 15% | C3H8/0.37O2/3H2O | -- | 9600 mL/gcat h | 100 at 700 °C | 55% | [25] |
| NidpCZ80 | 3% | 1CH4/0.5O2/2.5 H2O | 10 h | 120,000 h−1 | 86.4 at 800 °C | 52% | This work |
| NiCZ80 | 3% | 1CH4/0.5O2/2.5 H2O | 10 h | 120,000 h−1 | 80 at 800 °C | 48% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frontera, P.; Malara, A.; Macario, A.; Miceli, M.; Bonaccorsi, L.; Boaro, M.; Pappacena, A.; Trovarelli, A.; Antonucci, P.L. Performance and Stability of Doped Ceria–Zirconia Catalyst for a Multifuel Reforming. Catalysts 2023, 13, 165. https://doi.org/10.3390/catal13010165
Frontera P, Malara A, Macario A, Miceli M, Bonaccorsi L, Boaro M, Pappacena A, Trovarelli A, Antonucci PL. Performance and Stability of Doped Ceria–Zirconia Catalyst for a Multifuel Reforming. Catalysts. 2023; 13(1):165. https://doi.org/10.3390/catal13010165
Chicago/Turabian StyleFrontera, Patrizia, Angela Malara, Anastasia Macario, Mariachiara Miceli, Lucio Bonaccorsi, Marta Boaro, Alfonsina Pappacena, Alessandro Trovarelli, and Pier Luigi Antonucci. 2023. "Performance and Stability of Doped Ceria–Zirconia Catalyst for a Multifuel Reforming" Catalysts 13, no. 1: 165. https://doi.org/10.3390/catal13010165
APA StyleFrontera, P., Malara, A., Macario, A., Miceli, M., Bonaccorsi, L., Boaro, M., Pappacena, A., Trovarelli, A., & Antonucci, P. L. (2023). Performance and Stability of Doped Ceria–Zirconia Catalyst for a Multifuel Reforming. Catalysts, 13(1), 165. https://doi.org/10.3390/catal13010165











