BaCO3 Nanoparticles-Modified Composite Cathode with Improved Electrochemical Oxygen Reduction Kinetics for High-Performing Ceramic Fuel Cells
Abstract
:1. Introduction
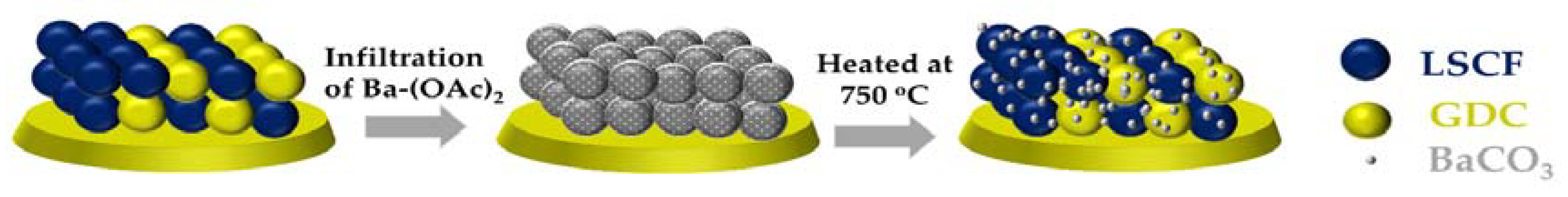
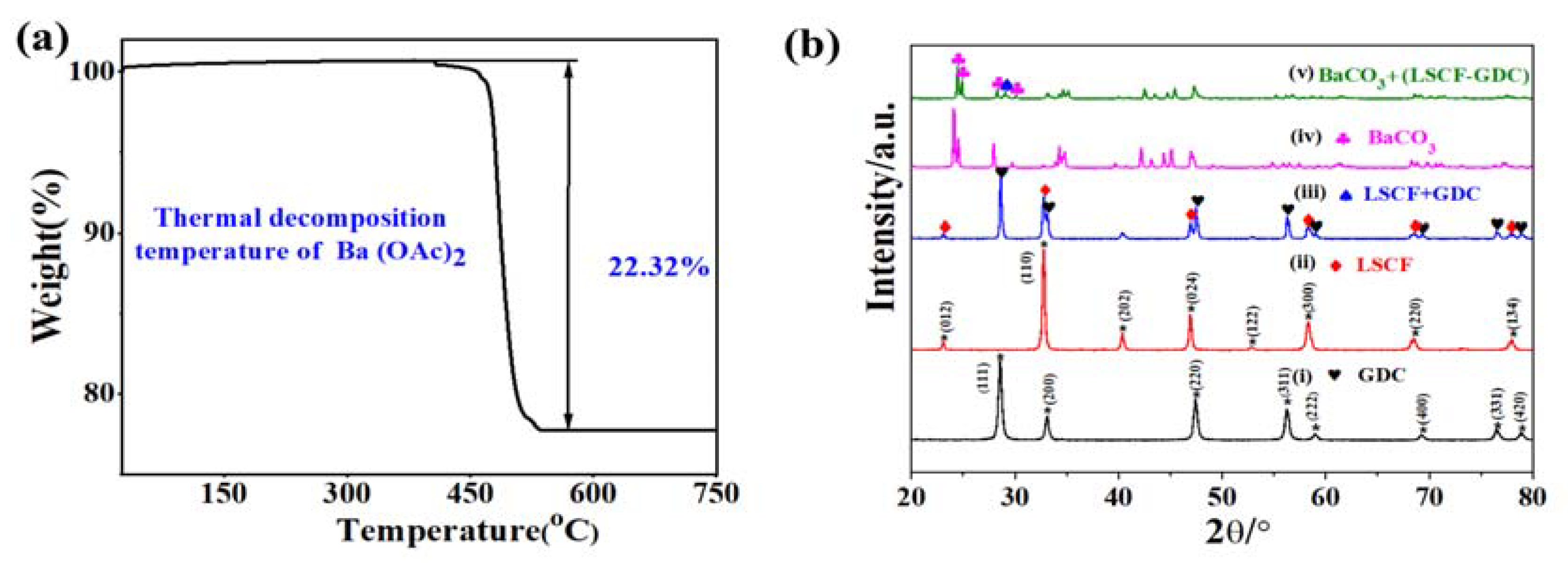
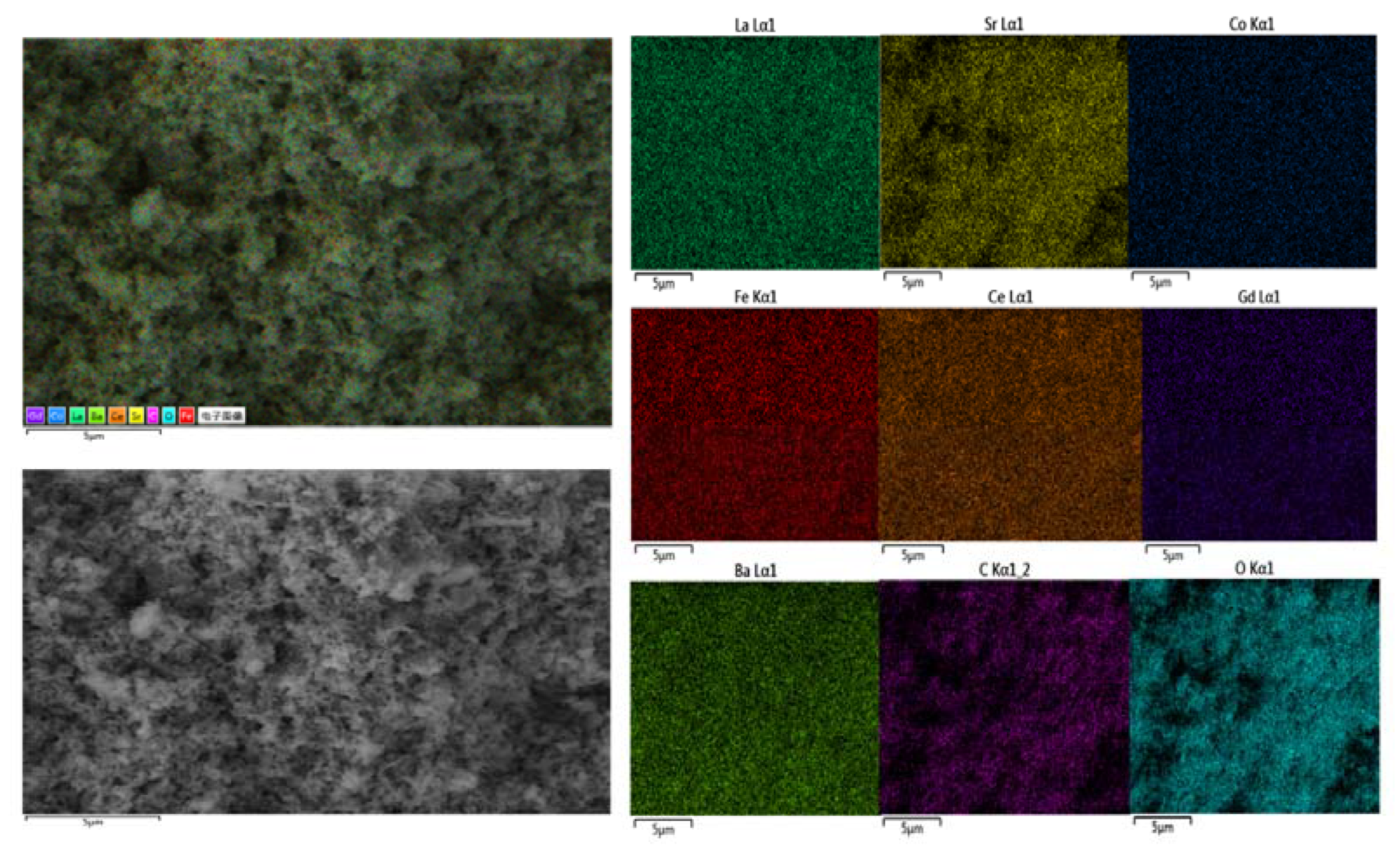
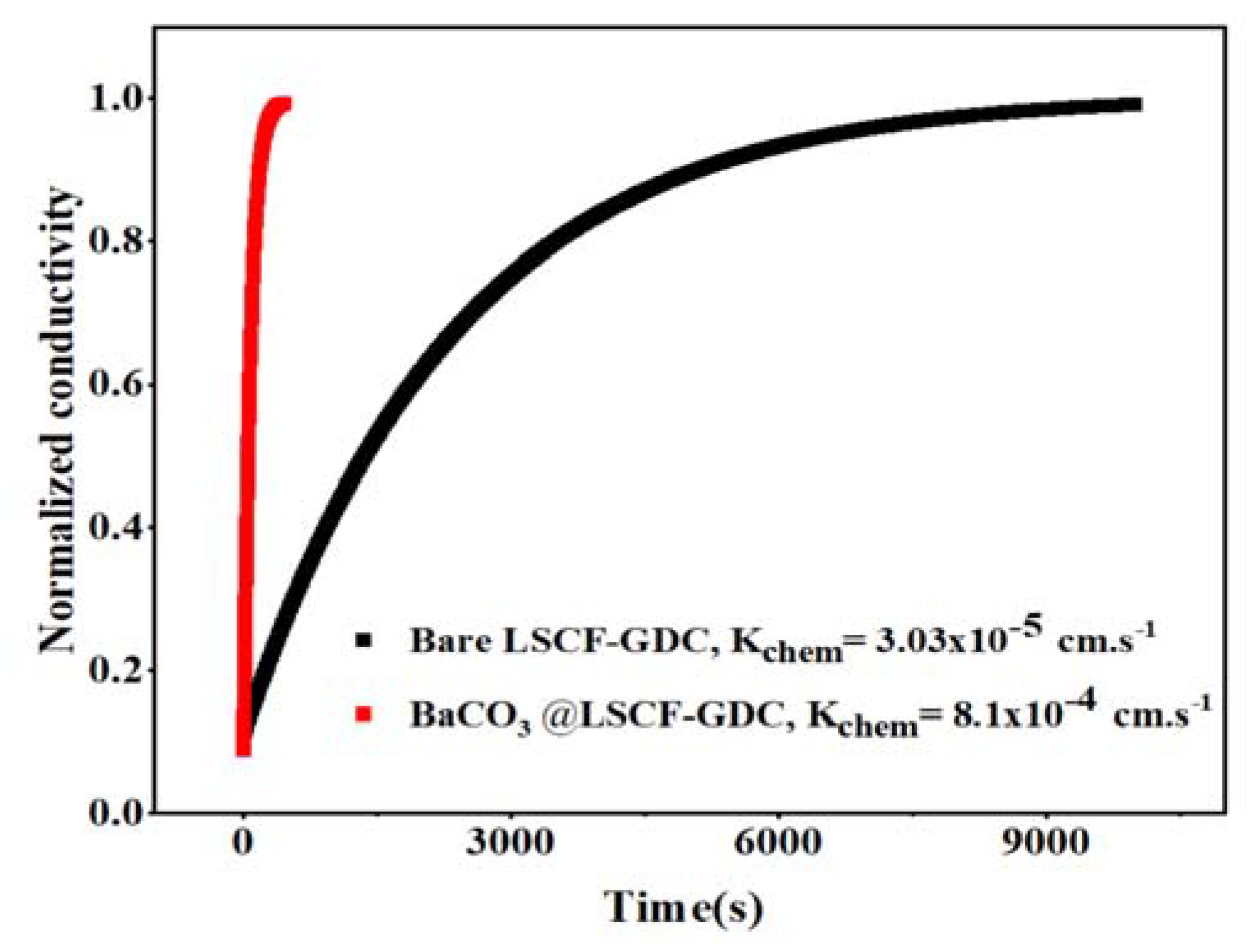
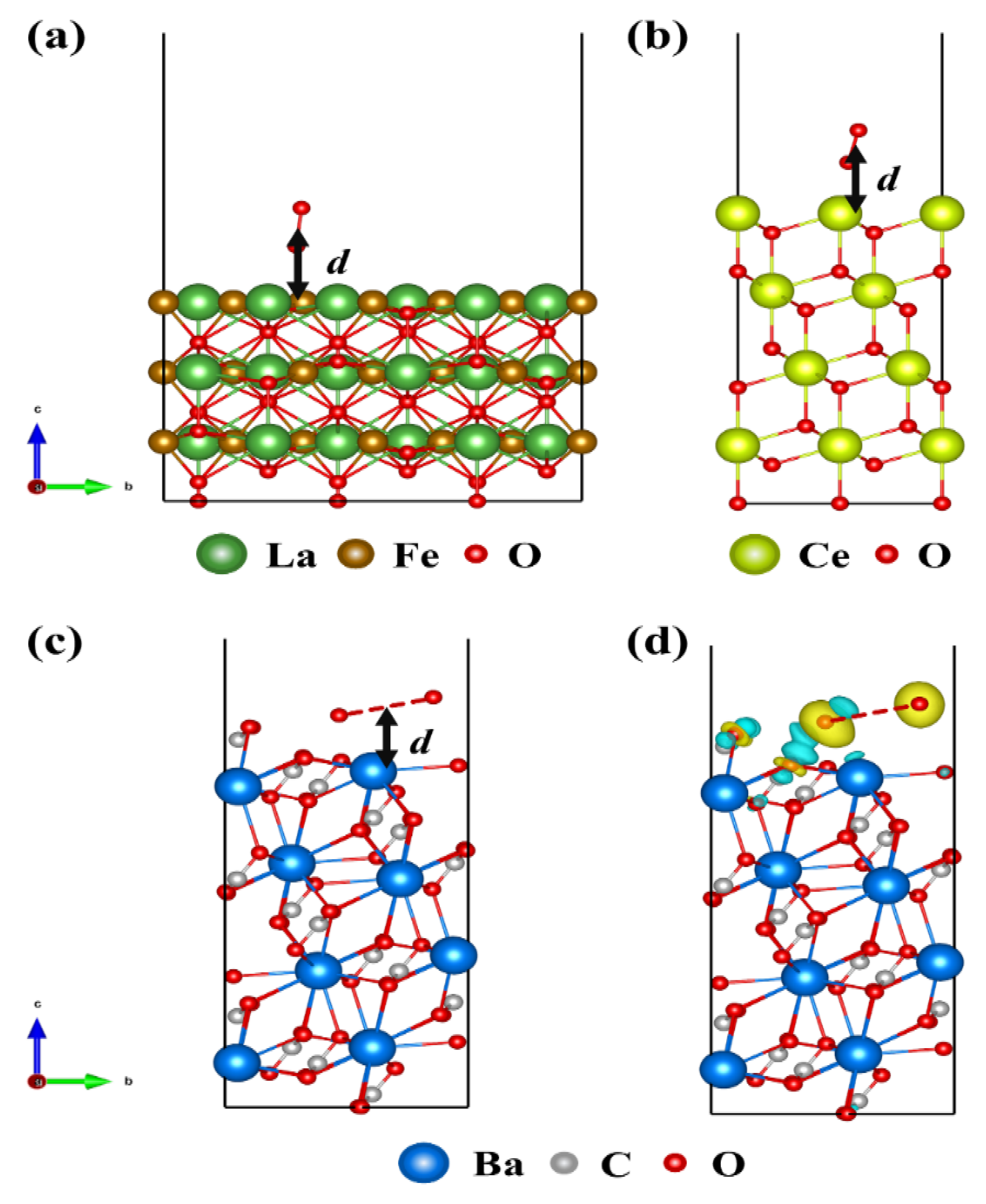
2. Results and Discussion
3. Experimental Section
3.1. Sample Preparation
3.2. Cell Fabrication and Electrochemical Testing
3.3. Characterization
3.4. Computational Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wachsman, E.D.; Marlowe, C.A.; Lee, K.T. Role of solid oxide fuel cells in a balanced energy strategy. Energy Environ. Sci. 2012, 5, 5498–5509. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Niu, Y.; Zhou, Y.; Zhang, W.; Zhang, Y.; Evans, C.; Luo, Z.; Kane, N.; Ding, Y.; Chen, Y.; Guo, X.; et al. Highly active and durable air electrodes for reversible protonic ceramic electrochemical cells enabled by an efficient bifunctional catalyst. Adv. Energy Mater. 2022, 12, 2103783. [Google Scholar] [CrossRef]
- Pirou, S.; Talic, B.; Brodersen, K.; Hauch, A.; Frandsen, H.L.; Skafte, T.L.; Persson, A.H.; Hogh, J.V.T.; Henriksen, H.; Navasa, M.; et al. Production of a monolithic fuel cell stack with high power density. Nat. Commun. 2022, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Liu, M. Novel cathodes for low-temperature solid oxide fuel cells. Adv. Mater. 2002, 14, 521–523. [Google Scholar] [CrossRef]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Fang, J.; Meng, Z.; Li, J.; Du, Y.; Qin, Y.; Jiang, Y.; Bai, W.; Chase, G.G. The effect of operating parameters on regeneration characteristics and particulate emission characteristics of diesel particulate filters. Appl. Therm. Eng. 2019, 148, 860–867. [Google Scholar] [CrossRef]
- Wang, S.F.; Lu, H.C.; Hsu, Y.F.; Jasinski, P. High-performance anode-supported solid oxide fuel cells with co-fired Sm0.2Ce0.8O2-δ|La0.8Sr0.2Ga0.8Mg0.2O-δ |Sm0.2Ce0.8O2-δ sandwiched electrolyte. Int. J. Hydrogen Energy 2022, 47, 5429–5438. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef]
- Kan, W.H.; Samson, A.J.; Thangadurai, V. Trends in electrode development for next-generation solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 17913–17932. [Google Scholar] [CrossRef] [Green Version]
- Ascolani-Yael, J.; Montenegro-Hernandez, A.; Baque, L.C.; Toscani, L.M.; Caneiro, A.; Mogni, L.V. Oxygen diffusion and surface exchange coefficients of porous La0.6Sr0.4Co0.2Fe0.8O3-δ decorated with Co3O4 nanoparticles. J. Electrochem. Soc. 2022, 169, 034514. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, Y.; Huang, K.; Xiong, X.; Wang, J.; Liu, M.; Ding, D.; Chen, Y.; Liu, T. Surface enhanced performance of La0.6Sr0.4Co0.2Fe0.8O3-δ cathodes by infiltration Pr-Ni-Mn-O progress. J. Alloys Compd. 2022, 902, 163337. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Shao, Z.; Zhou, W.; Zhu, Z. Advanced synthesis of materials for intermediate-temperature solid oxide fuel cells. Prog. Mater. Sci. 2012, 57, 804–874. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tanwar, K.; Suman, R.; Kumar, D.; Upadhyay, S.; Parkash, O. A brief review on ceria-based solid electrolytes for solid oxide fuel cells. J. Alloys Compd. 2019, 781, 984–1005. [Google Scholar] [CrossRef]
- Ni, N.; Xiao, W.; Zheng, C.; Jiang, J.; Yu, Y.; Hao, W.; Tang, M.; Shen, H.; Peng, D. Flash cosintering of a lanthanum strontium cobalt ferrite nanofibre/Gd-doped ceria bilayer structure. J. Eur. Ceram. Soc. 2022, 42, 2870–2878. [Google Scholar] [CrossRef]
- Zhang, S.L.; Shang, Y.B.; Li, C.X.; Li, C.J. Vacuum cold sprayed nanostructured La0.6Sr0.4Co0.2Fe0.8O3-δ as a high-performance cathode for porous metal-supported solid oxide fuel cells operating below 600 degrees C. Mater. Today Energy 2021, 21, 100815. [Google Scholar] [CrossRef]
- Liu, M.; Ding, D.; Blinn, K.; Li, X.; Nie, L.; Liu, M. Enhanced performance of LSCF cathode through surface modification. Int. J. Hydrogen Energy 2012, 37, 8613–8620. [Google Scholar] [CrossRef]
- Ding, D.; Li, X.; Lai, S.Y.; Gerdes, K.; Liu, M. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 2014, 7, 552–575. [Google Scholar] [CrossRef]
- Jiang, Z.; Xia, C.; Chen, F. Nano-structured composite cathodes for intermediate-temperature solid oxide fuel cells via an infiltration/impregnation technique. Electrochim. Acta 2010, 55, 3595–3605. [Google Scholar] [CrossRef]
- Jiang, S.P. Nanoscale and nano-structured electrodes of solid oxide fuel cells by infiltration: Advances and challenges. Int. J. Hydrogen Energy 2012, 37, 449–470. [Google Scholar] [CrossRef]
- dos Santos-Gómez, L.; Porras-Vázquez, J.; Losilla, E.; Martín, F.; Ramos-Barrado, J.; Marrero-López, D. Stability and performance of La0.6Sr0.4Co0.2Fe0.8O3-δ nanostructured cathodes with Ce0.8Gd0.2O1.9 surface coating. J. Power Source 2017, 347, 178–185. [Google Scholar] [CrossRef]
- Song, Y.-H.; Rehman, S.U.; Kim, H.-S.; Song, H.-S.; Song, R.-H.; Lim, T.-H.; Hong, J.-E.; Park, S.-J.; Huh, J.-Y.; Lee, S.-B. Facile surface modification of LSCF|GDC cathodes by epitaxial deposition of Sm0.5Sr0.5CoO3 via ultrasonic spray infiltration. J. Mater. Chem. A 2020, 8, 3967–3977. [Google Scholar] [CrossRef]
- DiGiuseppe, G.; Boddapati, V. Characterization of solid oxide fuel cells with LSCF-SDC composite cathodes. J. Energy 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Samreen, A.; Galvez-Sanchez, M.; Steinberger-Wilckens, R.; Arifin, N.A.; Saher, S.; Ali, S.; Qamar, A. Electrochemical performance of novel NGCO-LSCF composite cathode for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 21714–21721. [Google Scholar] [CrossRef]
- Liu, Y.; Bi, J.; Chi, B.; Pu, J.; Jian, L. Effects of impregnating palladium on catalytic performance of LSCF-GDC composite cathodes for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 6486–6492. [Google Scholar] [CrossRef]
- Sakito, Y.; Hirano, A.; Imanishi, N.; Takeda, Y.; Yamamoto, O.; Liu, Y. Silver infiltrated La0.6Sr0.4Co0.2Fe0.8O3 cathodes for intermediate temperature solid oxide fuel cells. J. Power Source 2008, 182, 476–481. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Zhang, W.; Wei, Z.; Guan, K.; Meng, J.; Meng, F.; Meng, J.; Liu, X. Enhancing catalysis activity of La0.6Sr0.4Co0.8Fe0.2O3-δ cathode for solid oxide fuel cell by a facile and efficient impregnation process. Int. J. Hydrogen Energy 2019, 44, 13757–13767. [Google Scholar] [CrossRef]
- Paige, J.M.; Cheng, Y.; Pepin, P.A.; Curran, C.D.; Sun, D.; Chen, M.U.; McIntosh, S.; Vohs, J.M.; Gorte, R.J. Surface modification of SOFC cathodes by Co, Ni, and Pd oxides. Solid State Ion. 2019, 341, 115051. [Google Scholar] [CrossRef]
- Nadeem, M.; Hu, B.; Xia, C. Effect of NiO addition on oxygen reduction reaction at lanthanum strontium cobalt ferrite cathode for solid oxide fuel cell. Int. J. Hydrogen Energy 2018, 43, 8079–8087. [Google Scholar] [CrossRef]
- Hong, T.; Brinkman, K.; Xia, C. Copper oxide as a synergistic catalyst for the oxygen reduction reaction on La0.6Sr0.4Co0.2Fe0.8O3-δ perovskite structured electrocatalyst. J. Power Source 2016, 329, 281–289. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Ren, Y.; Li, Y.; Xia, C. Magnesium oxide as synergistic catalyst for oxygen reduction reaction on strontium doped lanthanum cobalt ferrite. Int. J. Hydrogen Energy 2018, 43, 3797–3802. [Google Scholar] [CrossRef]
- Li, M.; Sun, Z.; Yang, W.; Hong, T.; Zhu, Z.; Zhang, Y.; Wu, X.; Xia, C. Mechanism for the enhanced oxygen reduction reaction of La0.6Sr0.4Co0.2Fe0.8O3-δ by strontium carbonate. Phys. Chem. Chem. Phys. 2017, 19, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Lee, S.; Ohodnicki, P.; Brinkman, K. A highly scalable spray coating technique for electrode infiltration: Barium carbonate infiltrated La0.6Sr0.4Co0.2Fe0.8O3-δ perovskite structured electrocatalyst with demonstrated long term durability. Int. J. Hydrogen Energy 2017, 42, 24978–24988. [Google Scholar] [CrossRef]
- Zhang, L.; Hong, T.; Li, Y.; Xia, C. CaO effect on the electrochemical performance of lanthanum strontium cobalt ferrite cathode for intermediate-temperature solid oxide fuel cell. Int. J. Hydrogen Energy 2017, 42, 17242–17250. [Google Scholar] [CrossRef]
- Hong, T.; Brinkman, K.S.; Xia, C. Barium carbonate nanoparticles as synergistic catalysts for the oxygen reduction reaction on La0.6Sr0.4Co0.2Fe0.8O3-δ solid-oxide fuel cell cathodes. ChemElectroChem 2016, 3, 805–813. [Google Scholar] [CrossRef]
- Desta, H.G.; Yang, Y.; Teketel, B.S.; Yang, Q.; Song, K.; Zhu, S.; Tian, D.; Chen, Y.; Luo, T.; Lin, B. Enhanced performance of La0.8Sr0.2FeO3-δ-Gd0.2Ce0.8O2-δ cathode for solid oxide fuel cells by surface modification with BaCO3 nanoparticles. Micromachines 2022, 13, 884. [Google Scholar] [CrossRef]
- Hwang, U.-Y.; Park, H.-S.; Koo, K.-K. Behavior of barium acetate and titanium isopropoxide during the formation of crystalline barium titanate. Ind. Eng. Chem. Res. 2004, 43, 728–734. [Google Scholar] [CrossRef]
- Joh, D.W.; Rath, M.K.; Park, J.W.; Park, J.H.; Cho, K.H.; Lee, S.; Yoon, K.J.; Lee, J.-H.; Lee, K.T. Sintering behavior and electrochemical performances of nano-sized gadolinium-doped ceria via ammonium carbonate assisted co-precipitation for solid oxide fuel cells. J. Alloys Compd. 2016, 682, 188–195. [Google Scholar] [CrossRef]
- Nie, L.; Liu, M.; Zhang, Y.; Liu, M. La0.6Sr0.4Co0.2Fe0.8O3-δ cathodes infiltrated with samarium-doped cerium oxide for solid oxide fuel cells. J. Power Source 2010, 195, 4704–4708. [Google Scholar] [CrossRef]
- Joh, D.W.; Cha, A.; Park, J.H.; Kim, K.J.; Bae, K.T.; Kim, D.; Choi, Y.K.; An, H.; Shin, J.S.; Yoon, K.J. In situ synthesized La0.6Sr0.4Co0.2Fe0.8O3-δ-Gd0.1Ce0.9O1.95 nanocomposite cathodes via a modified sol-gel process for intermediate temperature solid oxide fuel cells. ACS Appl. Nano Mater. 2018, 1, 2934–2942. [Google Scholar] [CrossRef]
- Gao, Z.; Barnett, S.A. Reduced-temperature firing of anode-supported solid oxide fuel cells. ECS Trans. 2013, 58, 231. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Guan, K.; Wei, Z.; Zhang, X.; Meng, J.; Liu, X.; Meng, J. La0.6Sr0.4Co0.2Fe0.8O3-δ/CeO2 Heterostructured composite nanofibers as a highly active and robust cathode catalyst for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2019, 11, 26830–26841. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Guan, K.; Wei, Z.; Meng, J.; Meng, J.; Liu, X. Enhancing activity and durability of A-Site-deficient (La0.6Sr0.4)0.95Co0.2Fe0.8O3-δ cathode by surface modification with PrO2-δ nanoparticles. ACS Sustain. Chem. Eng. 2020, 8, 3367–3380. [Google Scholar] [CrossRef]
- Huang, Y.L.; Hussain, A.M.; Wachsman, E.D. Nanoscale cathode modification for high performance and stable low-temperature solid oxide fuel cells (SOFCs). Nano Energy 2018, 49, 186–192. [Google Scholar] [CrossRef]
- Xia, C.; Liu, M. Microstructures, conductivities, and electrochemical properties of Ce0.9Gd0.1O2 and GDC-Ni anodes for low-temperature SOFCs. Solid State Ion. 2002, 152, 423–430. [Google Scholar] [CrossRef]
- Van Herle, J.; Ihringer, R.; Cavieres, R.V.; Constantin, L.; Bucheli, O. Anode supported solid oxide fuel cells with screen-printed cathodes. J. Eur. Ceram. Soc. 2001, 21, 1855–1859. [Google Scholar] [CrossRef]
- Ling, Y.; Lu, X.; Niu, J.; Chen, H.; Ding, Y.; Ou, X.; Zhao, L. Antimony doped barium strontium ferrite perovskites as novel cathodes for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2016, 666, 23–29. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Wu, Y.; Chu, Y.; Tian, D.; Lu, X.; Guo, L.; Lin, B.; Feng, P.; Ling, Y. Highly active self-assembled hybrid catalyst with multiphase heterointerfaces to accelerate cathodic oxygen reduction of intermediate-temperature solid oxide fuel cells. Ceram. Int. 2020, 46, 9661–9668. [Google Scholar] [CrossRef]
- Ali, T.; Qiao, W.; Zhang, D.; Liu, W.; Sajjad, S.; Yan, C.; Su, R. Surface Sulfur Vacancy Engineering of Metal Sulfides Promoted Desorption of Hydrogen Atoms for Enhanced Electrocatalytic Hydrogen Evolution. J. Phys. Chem. C 2021, 125, 12707–12712. [Google Scholar] [CrossRef]
- Ali, T.; Wang, X.; Tang, K.; Li, Q.; Sajjad, S.; Khan, S.; Farooqi, S.A.; Yan, C. SnS2 Quantum Dots Growth on MoS2: Atomic-Level Heterostructure for Electrocatalytic Hydrogen Evolution. Electrochim. Acta 2019, 300, 45–52. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. Google Patents No.US3330697A, 26 September 1967. [Google Scholar]
- Tian, D.; Lin, B.; Yang, Y.; Chen, Y.; Lu, X.; Wang, Z.; Liu, W.; Traversa, E. Enhanced performance of symmetrical solid oxide fuel cells using a doped ceria buffer layer. Electrochim. Acta 2016, 208, 318–324. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Liechtenstein, A.I.; Anisimov, V.I.; Zaanen, J. Density-functional theory, and strong interactions: Orbital ordering in Mott-Hubbard insulators. Phys. Rev. B 1995, 52, R5467–R5470. [Google Scholar] [CrossRef]
- Neuhauser, D.; Baer, R.; Rabani, E. Communication: Embedded fragment stochastic density functional theory. J. Chem. Phys. 2014, 141, 041102. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]


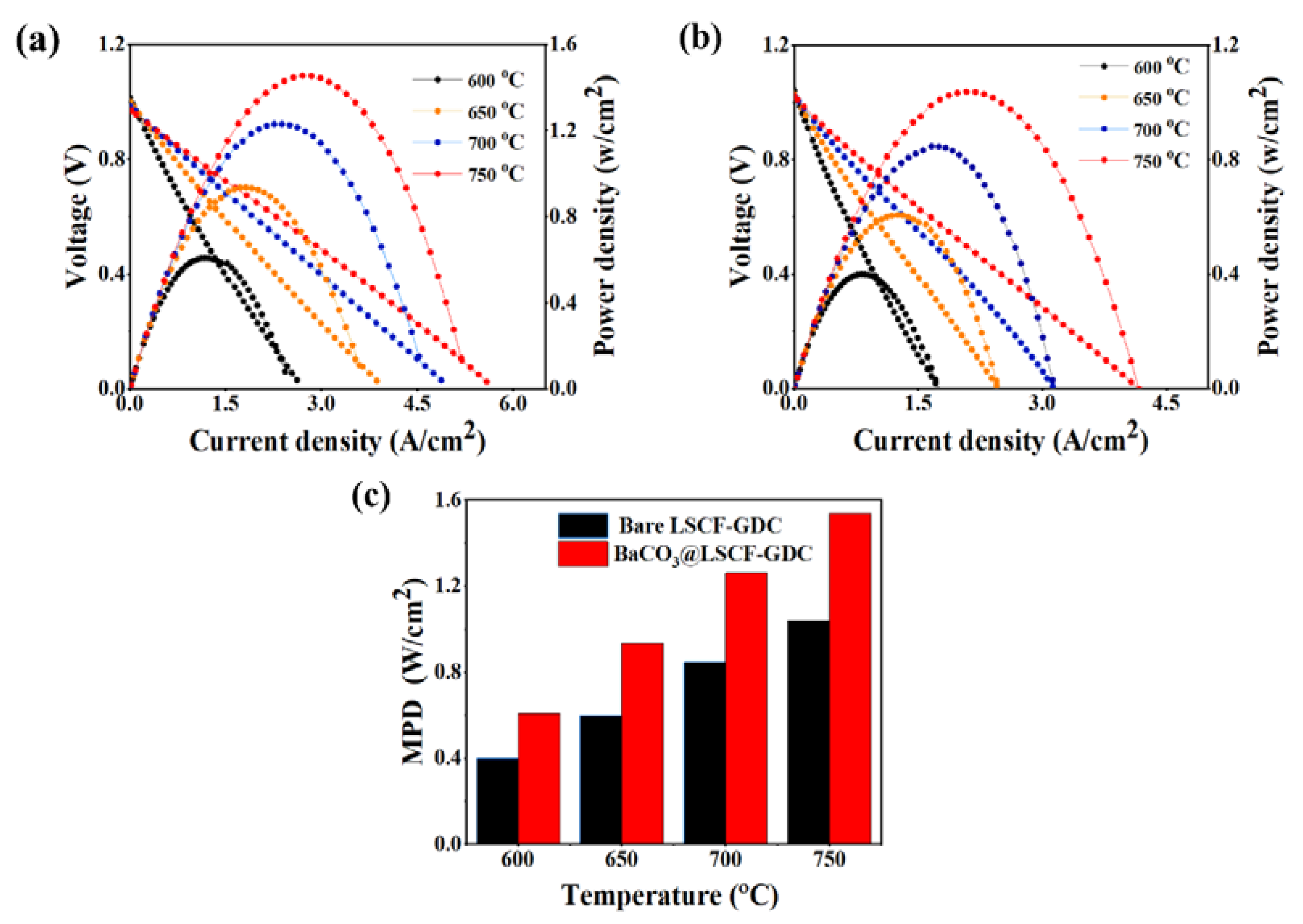
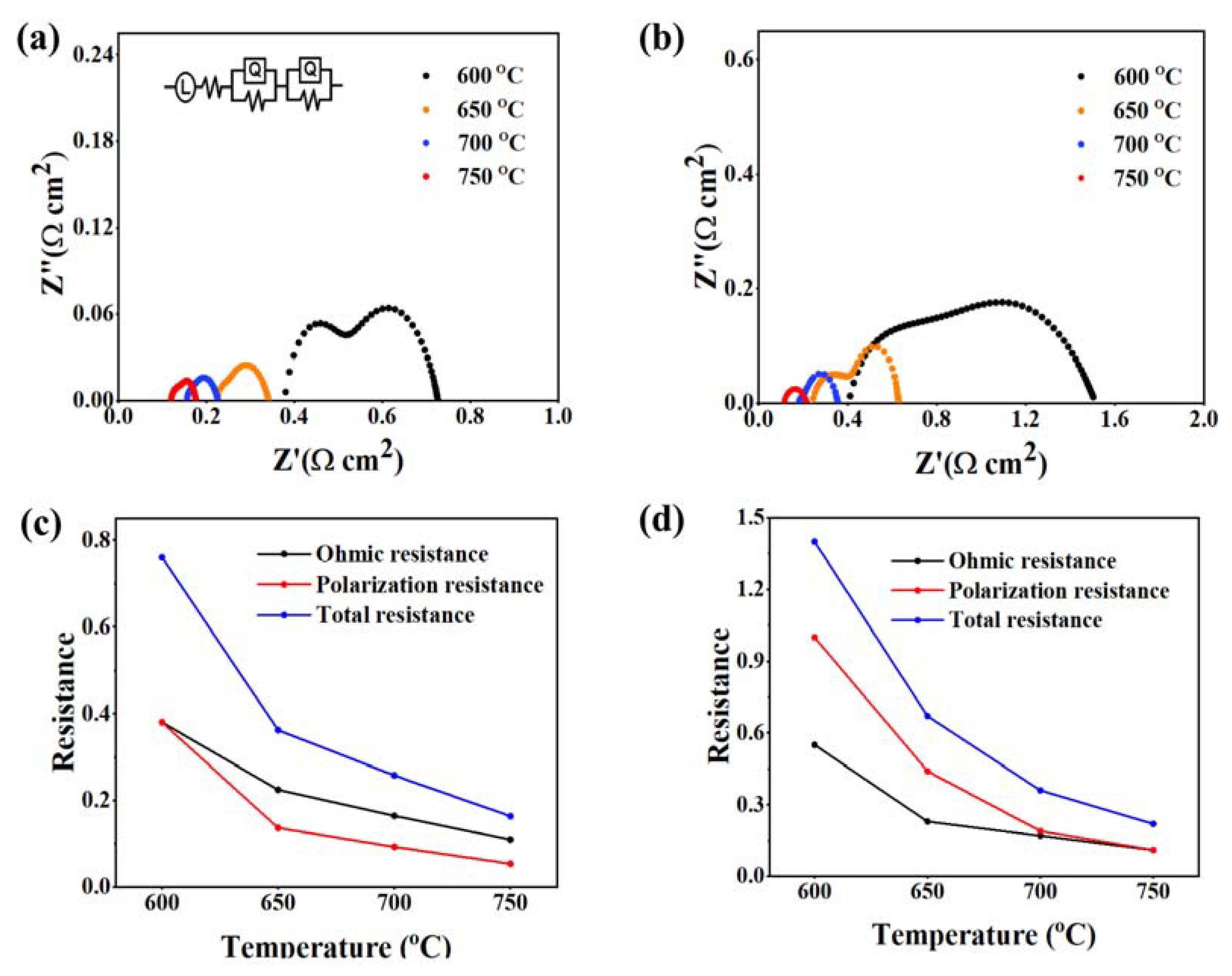
| Half Cell | Cathode + Infiltrated Material | MPD (W/cm2) at 650 °C | Improving Factor (%) | Ref. |
|---|---|---|---|---|
| NiO-YSZ|YSZ|GDC | LSCF–GDC + BaCO3 | Bare: 0.6 Infiltrated: 0.94 | 56.6 | This work |
| NiO-SDC|SDC | LSCF + CuO | Bare: 0.54 Infiltrated: 0.72 | 25 | [31] |
| NiO-SDC|SDC | LSCF-SDC + CaO | Bare: 0.62 Infiltrated: 0.83 | 34.24 | [35] |
| NiO-SDC|SDC | LSCF + CaO | Bare: 0.6 Infiltrated: 0.80 | 33.3 | [35] |
| NiO-YSZ|YSZ|GDC | LSCF + CeO2 (nanofiber) | Bare: 0.75 Infiltrated: 0.981 | 30 | [43] |
| NiO-YSZ|YSZ|GDC | (La0.6Sr0.4)0.95Co0.2Fe0.8O3-δ +Pr0.8Ce0.2O2-δ | Bare: 0.75 Infiltrated: 1.07 | 42.47 | [44] |
| NiO-YSZ|YSZ|GDC | (La0.6Sr0.4)0.95Co0.2Fe0.8O3-δ + PrO2-δ | Bare: 0.751 Infiltrated: 1.108 | 47.53 | [44] |
| Ni-GDC|GDC | (LSCF–GDC) + La0.6Sr0.4CoO3-δ | Bare: 0.7 Infiltrated: 1.3 | 85.7 | [45] |
| d (Å) | RO-O (Å) | O1 (e) | O2 (e) | Sum (e) | |
|---|---|---|---|---|---|
| LaFeO3 | 3.04 | 1.56 | −0.08 | −0.47 | −0.55 |
| CeO2 | 2.48 | 1.36 | −0.14 | −0.40 | −0.54 |
| BaCO3 | 2.61 | 4.05 | −0.41 | −0.46 | −0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desta, H.G.; Yang, Q.; Tian, D.; Zhu, S.; Lu, X.; Song, K.; Yang, Y.; Chen, Y.; Li, B.; Lin, B. BaCO3 Nanoparticles-Modified Composite Cathode with Improved Electrochemical Oxygen Reduction Kinetics for High-Performing Ceramic Fuel Cells. Catalysts 2022, 12, 1046. https://doi.org/10.3390/catal12091046
Desta HG, Yang Q, Tian D, Zhu S, Lu X, Song K, Yang Y, Chen Y, Li B, Lin B. BaCO3 Nanoparticles-Modified Composite Cathode with Improved Electrochemical Oxygen Reduction Kinetics for High-Performing Ceramic Fuel Cells. Catalysts. 2022; 12(9):1046. https://doi.org/10.3390/catal12091046
Chicago/Turabian StyleDesta, Halefom G., Quan Yang, Dong Tian, Shiyue Zhu, Xiaoyong Lu, Kai Song, Yang Yang, Yonghong Chen, Baihai Li, and Bin Lin. 2022. "BaCO3 Nanoparticles-Modified Composite Cathode with Improved Electrochemical Oxygen Reduction Kinetics for High-Performing Ceramic Fuel Cells" Catalysts 12, no. 9: 1046. https://doi.org/10.3390/catal12091046
APA StyleDesta, H. G., Yang, Q., Tian, D., Zhu, S., Lu, X., Song, K., Yang, Y., Chen, Y., Li, B., & Lin, B. (2022). BaCO3 Nanoparticles-Modified Composite Cathode with Improved Electrochemical Oxygen Reduction Kinetics for High-Performing Ceramic Fuel Cells. Catalysts, 12(9), 1046. https://doi.org/10.3390/catal12091046







