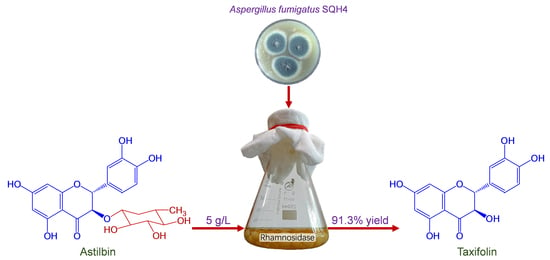
Production of Taxifolin from Astilbin by Fungal Biotransformation
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation of Fungal Strains for Microbial Transformation
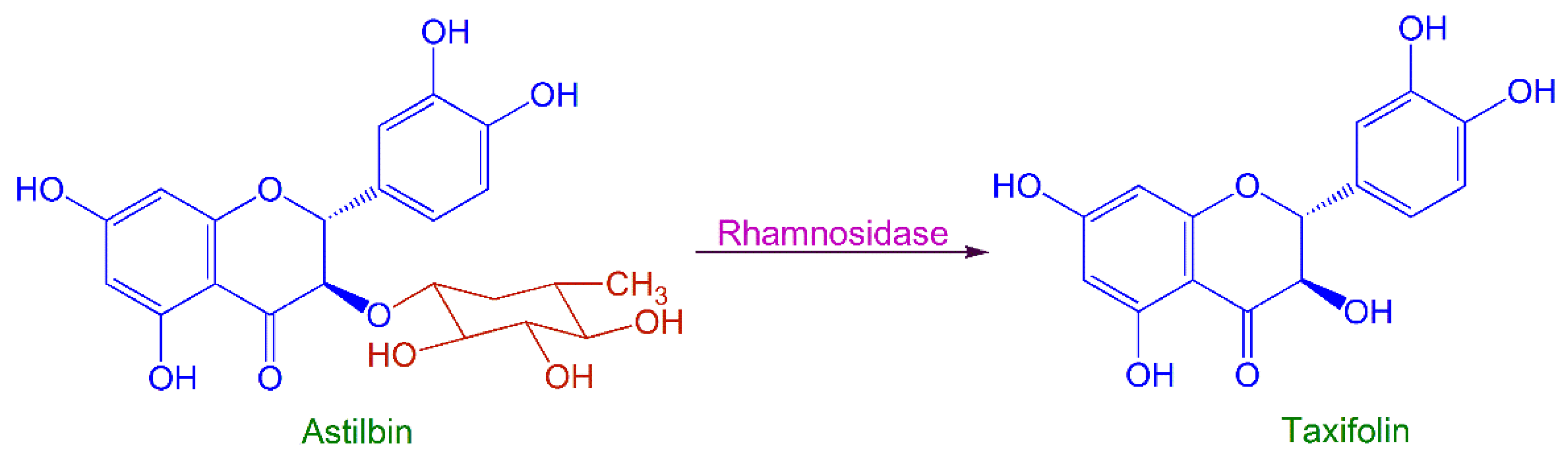
2.2. Process Optimization of SQH4 Biotransformation of Astilbin
2.2.1. pH
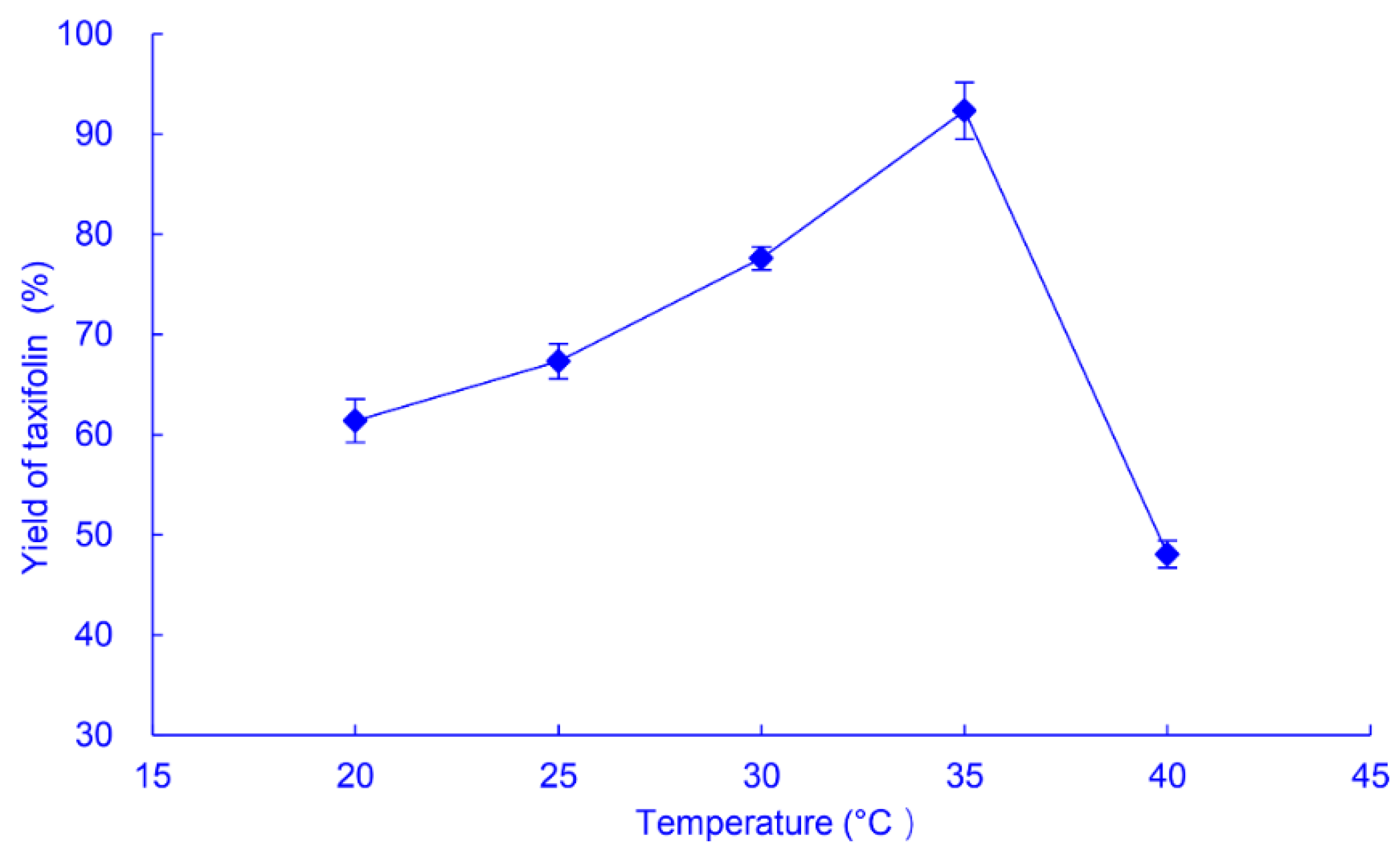
2.2.2. Temperature
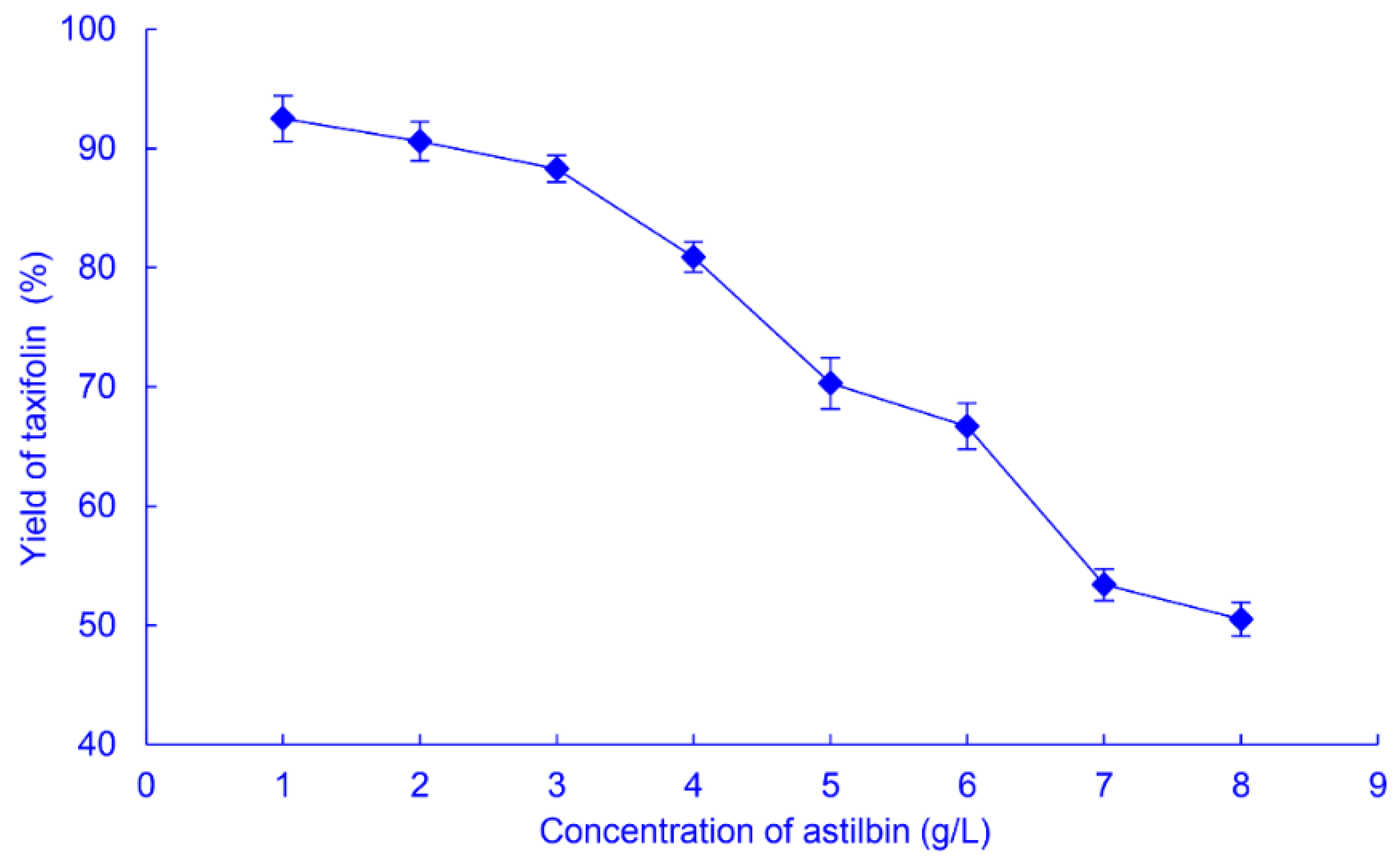
2.2.3. Astilbin Concentration
2.2.4. Biotransformation Time and Astilbin Concentration
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Isolation and Classification of Enzyme-Producing Strains
3.3. Screening of Fungal Strains for Ability to Biotransform Astilbin to Taxifolin
3.4. Analytical Method
3.5. Optimization of Biotransformation Conditions
3.6. Data and Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kolhir, V.K.; Bykov, V.A.; Baginskaja, A.I.; Sokolov, S.Y.; Glazova, N.G.; Leskova, T.E.; Sakovich, G.S.; Tjukavkina, N.A.; Kolesnik, Y.A.; Rulenko, I.A. Antioxidant activity of a dihydroquercetin isolated from Larix gmelinii (Rupr.) Rupr. wood. Phytother. Res. 1996, 10, 478–482. [Google Scholar] [CrossRef]
- Kolesnik, Y.; Titova, E.; Chertkov, V.; Tashlitsky, V.; Tikhonov, V.; Shmatkov, D. Stereoisomeric composition of two bioflavonoids from Larix sibirica. Planta Med. 2011, 77, PA17. [Google Scholar] [CrossRef]
- Willför, S.; Ali, M.; Karonen, M.; Reunanen, M.; Arfan, M.; Harlamow, R. Extractives in bark of different conifer species growing in Pakistan. Holzforschung 2009, 63, 551–558. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific opinion on taxifolin-rich extract from Dahurian larch (Larix gmelinii). EFSA J. 2017, 15, e04682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef]
- Weidmann, A.E. Dihydroquercetin: More than just an impurity? Eur. J. Pharmacol. 2012, 684, 19–26. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. GRAS Notice, GRN No. 826-Dihydroquercetin. Available online: https://www.fda.gov/media/132030/download (accessed on 5 August 2022).
- Jew, S.; Kim, H.; Bae, S.; Kim, J.; Park, H. Enantioselective synthetic method for 3-hydroxyflavanones: An approach to (2R, 3R)-3′,4′-O-dimethyltaxifolin. Tetrahedron Lett. 2000, 41, 7925–7928. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Ghidouche, S.; Ducrot, P.H. Flavonoids: Hemisynthesis, reactivity, characterization and free radical scavenging activity. Molecules 2007, 12, 2228–2258. [Google Scholar] [CrossRef]
- Liu, Z.; Gu, H.; Yang, L. A novel approach for the simultaneous extraction of dihydroquercetin and arabinogalactan from Larix gmelinii by homogenate-ultrasound-synergistic technique using the ionic liquid. J. Mol. Liq. 2018, 261, 41–49. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, J.; Chen, F.; Yang, F.; Zu, Y.; Yang, L. Development of an ionic liquid-based microwave-assisted method for the extraction and determination of taxifolin in different parts of Larix gmelinii. Molecules 2014, 19, 19471–19490. [Google Scholar] [CrossRef]
- Ma, C.; Yang, L.; Wang, W.; Yang, F.; Zhao, C.; Zu, Y. Extraction of dihydroquercetin from Larix gmelinii with ultrasound-assisted and microwave-assisted alternant digestion. Int. J. Mol. Sci. 2012, 13, 8789–8804. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zu, Y.; Long, J.; Fu, Y.; Li, S.; Zhang, D.; Li, J.; Wink, M.; Efferth, T. Enzymatic water extraction of taxifolin from wood sawdust of Larix gmelini (Rupr.) Rupr. and evaluation of its antioxidant activity. Food Chem. 2011, 126, 1178–1185. [Google Scholar] [CrossRef]
- Wiesbeck, F. Process for the Manufacture of Taxifolin from Wood. US 9073889B2, 7 July 2015. [Google Scholar]
- Xia, Y.; Wang, Y.; Li, W.; Ma, C.; Liu, S. Homogenization-assisted cavitation hybrid rotation extraction and macroporous resin enrichment of dihydroquercetin from Larix gmelinii. J. Chromatogr. B 2017, 1070, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-J.; Jun, Z.; Han, A.-H.; Wang, X.-J.; Wang, X.-J. Biomass and carbon pool of Larix gmelini young and middle age forest in Xing’an Mountains Inner Mongolia. Acta Ecol. Sin. 2007, 27, 1756–1762. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Sakanaka, M. Effects of Astilbe thunbergii rhizomes on wound healing. Part 1. Isolation of promotional effectors from Astilbe thunbergii rhizomes on burn wound healing. J. Ethnopharmacol. 2007, 109, 72–77. [Google Scholar] [CrossRef]
- Lu, C.-L.; Zhu, Y.-F.; Hu, M.-M.; Wang, D.-M.; Xu, X.-J.; Lu, C.-J.; Zhu, W. Optimization of astilbin extraction from the rhizome of Smilax glabra, and evaluation of its anti-inflammatory effect and probable underlying mechanism in lipopolysaccharide-induced RAW264.7 macrophages. Molecules 2015, 20, 625–644. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Cheung, H. Antioxidant activity of Rhizoma Smilacis Glabrae extracts and its key constituent-astilbin. Food Chem. 2009, 115, 297–303. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, R.; Shi, Y.; Zhang, X.; Tian, C.; Xia, D. Antioxidant and anti-inflammatory activities of six flavonoids from Smilax glabra Roxb. Molecules 2020, 25, 5295. [Google Scholar] [CrossRef]
- Chen, L.; Yin, Y.; Yi, H.; Xu, Q.; Chen, T. Simultaneous quantification of five major bioactive flavonoids in Rhizoma Smilacis Glabrae by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2007, 43, 1715–1720. [Google Scholar] [CrossRef]
- Souquet, J.; Labarbe, B.; Guernevé, C.; Cheynier, V.; Moutounet, M. Phenolic composition of grape stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef]
- Huang, H.; Cheng, Z.; Shi, H.; Xin, W.; Wang, T.; Yu, L. Isolation and characterization of two flavonoids, engeletin and astilbin, from the leaves of Engelhardia roxburghiana and their potential anti-inflammatory properties. J. Agric. Food Chem. 2011, 59, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Christopher, R.; Nyandoro, S.S.; Chacha, M.; de Koning, C.B. A new cinnamoylglycoflavonoid, antimycobacterial and antioxidant constituents from Heritiera littoralis leaf extracts. Nat. Prod. Res. 2014, 28, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Mecchi, M.C.; Lago, J.H.G. Chemical constituents derived from Drimys brasiliensis Miers (Winteraceae). Nat. Prod. Res. 2013, 27, 1927–1929. [Google Scholar] [CrossRef]
- Zhang, Q.-F.; Li, S.-C.; Lai, W.-I.; Cheung, H.-Y. β-Cyclodextrin facilitates simultaneous analysis of six bioactive components in Rhizoma Smilacis Glabrae by capillary zone electrophoresis. Food Chem. 2009, 113, 684–691. [Google Scholar] [CrossRef]
- Qiu, X.-L.; Zhang, Q.-F. Acidic hydrolysis of astilbin and its application for the preparation of taxifolin from Rhizoma Smilacis Glabrae. J. Chem. Res. 2021, 45, 290–294. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Riva, S. Biocatalytic modification of natural products. Curr. Opin. Chem. Biol. 2001, 5, 106–111. [Google Scholar] [CrossRef]
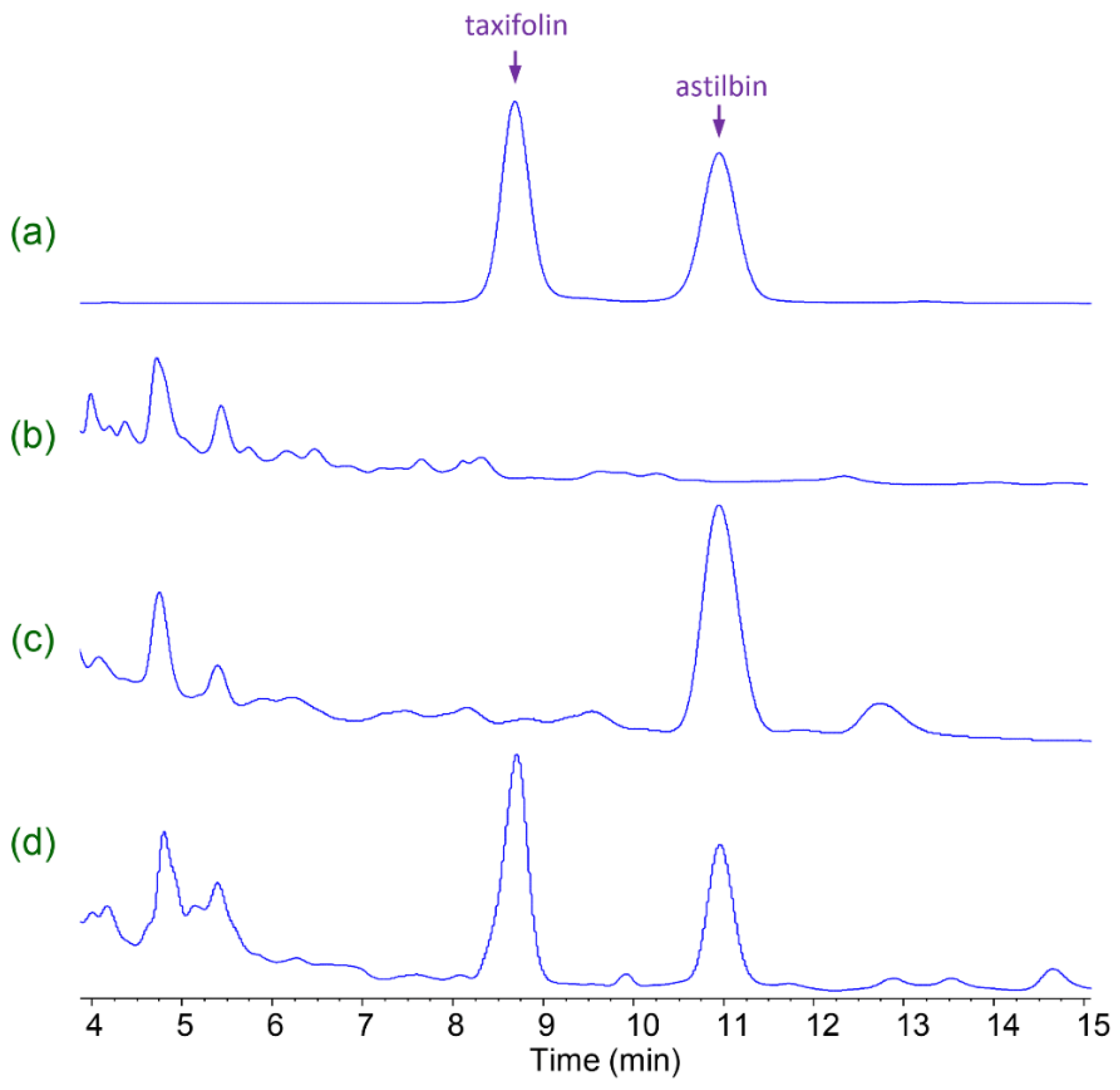


| Strains | Dry Cell Yield (g/L) | Mass Yield of Taxifolin (g/L) | Yield of Taxifolin (%) |
|---|---|---|---|
| SQH1 | 3.24 | 0.127 | 18.8 |
| SQH2 | 3.71 | 0 | 0 |
| SQH3 | 4.37 | 0.165 | 24.4 |
| SQH4 | 3.88 | 0.362 | 53.6 |
| SQH5 | 2.65 | 0.0978 | 14.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, J.; Chen, X.; Wang, P.; Wu, Y.; Yi, Y.; Ying, G. Production of Taxifolin from Astilbin by Fungal Biotransformation. Catalysts 2022, 12, 1037. https://doi.org/10.3390/catal12091037
Mei J, Chen X, Wang P, Wu Y, Yi Y, Ying G. Production of Taxifolin from Astilbin by Fungal Biotransformation. Catalysts. 2022; 12(9):1037. https://doi.org/10.3390/catal12091037
Chicago/Turabian StyleMei, Jianfeng, Xiang Chen, Pingya Wang, Yichun Wu, Yu Yi, and Guoqing Ying. 2022. "Production of Taxifolin from Astilbin by Fungal Biotransformation" Catalysts 12, no. 9: 1037. https://doi.org/10.3390/catal12091037
APA StyleMei, J., Chen, X., Wang, P., Wu, Y., Yi, Y., & Ying, G. (2022). Production of Taxifolin from Astilbin by Fungal Biotransformation. Catalysts, 12(9), 1037. https://doi.org/10.3390/catal12091037