Abstract
Taxifolin is known to have multiple biological functions. It has been widely used as a multifunctional food additive, and consequently, the global demand for taxifolin is increasing. The main method for taxifolin production is an extraction from larch wood, but the global resources of larch are limited. Astilbin, taxifolin-3-o-rhamnoside, is abundant in many plants and much more readily available, meaning taxifolin can be obtained by deglycosylation of astilbin. In this study, a fungal strain, Aspergillus fumigatus SQH4, was isolated from an enrichment culture of Smilax glabra rhizome to achieve the deglycosylation reaction. A culture of SQH4, adjusted to pH 6.5, with 5 g/L astilbin achieved a yield of taxifolin of 91.3% after biotransformation for 14 h at 35 °C. These findings offer an alternative method for the production of taxifolin.
1. Introduction
Taxifolin (3, 5, 7, 3′, 4′-pentahydroxy flavanone), also known as dihydroquercetin and distylin, is a flavonoid found abundantly in many members of the Pinaceae family, such as Larix gmelinii [1], Larix sibirica [2], Pinus roxburghii and Cedrus deodara [3]. It is also found in plant foods, such as apples, red onions, tomatoes, white grapes, strawberries, mulberries, acai, peanuts, thyme, and citrus fruits [4]. Taxifolin has promising pharmacological activities for managing inflammation, cancerous tumors, microbial infections, oxidative stress, cardiovascular disease, and liver disorders [5,6,7]. The anti-cancer activity was the most potent of the pharmacological activities when evaluated using various in vitro and in vivo models [6]. Therefore, taxifolin can be a dietary supplement with demonstrated health benefits. As a food additive, the potent antioxidant properties of taxifolin can significantly extend the shelf life of lard, vegetable oils, milk powder, and confectionery. The European Food Safety Authority in February 2017 [4] and the U.S. Food and Drug Administration in September 2019 [8] both approved taxifolin for use as an ingredient in non-alcoholic beverages (up to 0.02 g/L), flavored fermented milk and dairy products (up to 0.02 g/kg), and chocolate products (up to 0.07 g/kg).
Although taxifolin can be chemically synthesized [9] or semi-synthesized from catechin [10], synthetic taxifolin is of lower purity and/or more costly than the natural product. Furthermore, since taxifolin is a food additive, consumers usually prefer “natural” products over “chemicals”. Currently, the main production process for natural taxifolin is by extraction from larch wood, mainly L. gmelinii [11,12,13,14,15,16], but the yield is relatively low, around 1–2%. Larch is mainly distributed in eastern Siberia, Russia, northeastern China, North Korea, and northeastern Mongolia [17]. Larch wood is widely used for furniture and buildings because of its excellent properties of rigidness, straight grain, and decay resistance. Only logging waste (stump, branch, root) and processing wastes (sawdust, crushed veneer, wood core, shavings) can be used for extracting taxifolin [14]. In addition, larch grows slowly, making large-scale larch plantation forestry uneconomic and impractical for industrial taxifolin production. Taxifolin is mainly produced in Russia, where there are relatively abundant larch resources; however, an insufficient supply of forestry and processing by-products has impeded the mass production of taxifolin.
Astilbin, taxifolin-3-o-rhamnoside, also occurs naturally in many herbaceous plants, such as Astilbe thunbergii [18], Smilax glabra [19,20,21,22], Vitis vinifera [23], and trees, such as Engelhardia roxburghiana [24], Heritiera littoralis [25] and Drimys brasiliensis [26]. For example, in the S. glabra rhizome (or Rhizoma Smilacis Glabrae), the astilbin content ranges from 1.15–4.76% [27]. Astilbin is, therefore, much more readily available and abundant than taxifolin, and when the rhamnosyl residue of astilbin is removed by deglycosylation, it is converted to taxifolin.
The deglycosylation of a glycoside to form the aglycone can be performed by chemical or biological hydrolysis. However, chemical hydrolysis often degrades the aglycone, resulting in low yields. For example, Qiu et al. [28] found that astilbin could be hydrolyzed to taxifolin with 1 mol/L HCl at 80 °C, but a small proportion of the aglycone decomposed, reducing the yield. Biological hydrolysis has the advantages of high stereo- and regio-selectivity, mild reaction conditions, low cost, and less harmful waste products [29,30]. Biological hydrolysis can be carried out with isolated, purified enzymes or whole microbial cells containing the enzyme required. Hydrolysis by pure enzymes usually has the advantage of simple procedures and easy product isolation, but the cost of enzymes is usually high enough to make the process industrially uneconomical. Hydrolysis performed by microbial cells avoids the high cost of purified enzymes since the separation and purification steps for the enzymes are unnecessary. In addition, microbial cells may contain isoenzymes, which act synergistically to make hydrolysis more efficient.
To establish a novel method for taxifolin production, a process for fungal biotransformation of astilbin to taxifolin was developed. The reaction scheme is illustrated in Figure 1. A fungal strain, which could produce rhamnosidase to catalyze this reaction, was isolated from an enrichment culture of S. glabra rhizomes. When astilbin was added to a culture of this strain, it was enzymatically hydrolyzed to taxifolin in high yield.
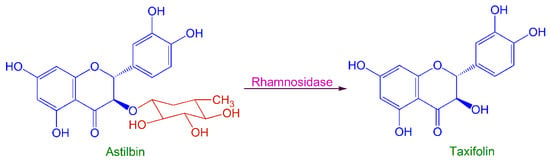
Figure 1.
Fungal biotransformation of astilbin to taxifolin.
2. Results and Discussion
2.1. Isolation of Fungal Strains for Microbial Transformation
The first task was to screen fungal strains to find those able to produce rhamnosidase to transform astilbin to taxifolin. Considering that the content of astilbin in Smilax glabra rhizomes is high, the rhizomes were used both as a source of fungal strains and as the screening medium for strains capable of transforming astilbin to taxifolin. Five fungal strains were isolated from the screening culture; then, their biotransformation ability was compared by adding astilbin (1 g/L) to the culture medium to determine the yield of taxifolin each could achieve (Table 1).

Table 1.
Yield of taxifolin obtained from biotransformation of astilbin by isolated fungal strains.
The yield of taxifolin varied widely between the five strains; SQH2 grew well but yielded no taxifolin, whereas SQH4 gave the highest yield of taxifolin, followed by SQH3 (Table 1, Figure 2).
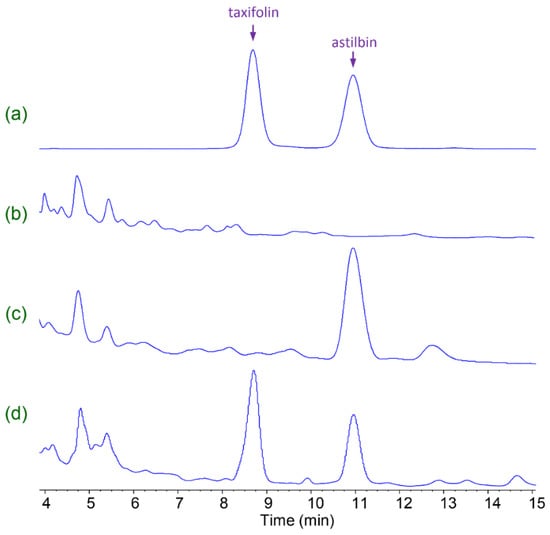
Figure 2.
High-performance liquid chromatography (HPLC) analysis of taxifolin and astilbin. (a) Standard taxifolin and astilbin dissolved in methanol; (b) Extract of SQH4 culture medium without added astilbin; (c) Extract of SQH2 culture medium, after incubation with 1 g/L astilbin; (d) Extract of SQH4 culture medium, after incubation with 1 g/L astilbin. Biotransformation was performed at 30 °C, pH 4.5, shaking at 200 r/min for 8 h.
Clearly, strain SQH2 was unable to biotransform astilbin to taxifolin (Figure 2c), indicating that this strain cannot produce rhamnosidase, the required deglycosylation enzyme. However, strain SQH4 transformed taxifolin to a 53.6% yield of astilbin, as determined by HPLC (Figure 2d), so this strain was selected for the scale-up of the biotransformation of astilbin to taxifolin. The final pH of the SQH4 culture medium was about 4.5, which raised the possibility of acid hydrolysis of astilbin to taxifolin instead of, or in addition to, enzymic hydrolysis. However, the pH of the SQH2 culture medium was also about 4.5, though no hydrolysis of astilbin to taxifolin was observed, indicating that astilbin hydrolysis is negligible in the fungal culture medium at pH 4.5.
When a culture of strain SQH4 was filtered, and the mycelia were suspended in phosphate buffer (0.2 mol/L, pH 4.5) with a volume equal to that of the culture filtrate, and both were used to biotransform astilbin, the yields of taxifolin were 49.6% from the mycelia and 8.34% from the culture filtrate. This indicates that most of the rhamnosidase activity is retained in the mycelia of strain SQH4, and little is secreted into the medium.
HPLC analysis revealed that the biotransformation product, taxifolin, was mainly present in the culture medium, and almost no taxifolin was detected in mycelial methanol extracts of any fungal strain.
The ribosomal DNA internally transcribed spacer region (rDNA-ITS) sequence of strain SQH4 was compared by BLAST (Basic Local Alignment Search Tool) searching on NCBI (The National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov, accessed on 8 September 2017) and was found to be 100% homologous with Aspergillus fumigatus type strain ATCC1022, so SQH4 is a strain of A. fumigatus. SQH4 has been preserved as a patent microbial strain in the Guangdong Microbial Culture Center, China, with identifier number GDMCC 60239.
2.2. Process Optimization of SQH4 Biotransformation of Astilbin
2.2.1. pH
The initial pH of the culture medium for A. fumigatus SQH4 growth was 6.0, and the final pH was 4.5 after culture. The yield of taxifolin produced by SQH4 was determined at pHs between 4.5 and 8.0 (Figure 3), which determined that the optimal pH was 6.5, at which the taxifolin yield was 79.1% from 1 g/L astilbin. At pHs greater than 7.0, the yield decreased markedly; at pH 8.0, the taxifolin yield was about half (40.5%) of that at 6.5.
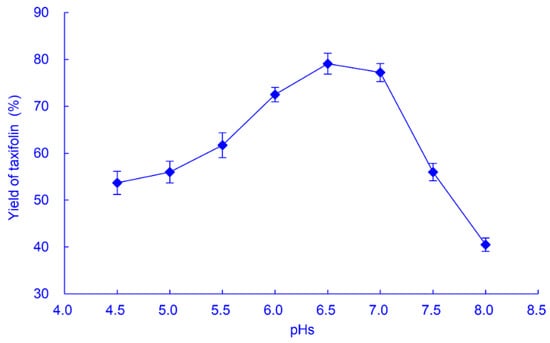
Figure 3.
Effect of pH on the yield of taxifolin from biotransformation of astilbin by A. fumigatus SQH4. Biotransformation was performed at 30 °C with shaking at 200 r/min for 8 h, with 1 g/L astilbin.
2.2.2. Temperature
Increasing the temperature can accelerate chemical reactions, but too high a temperature inactivates enzymes, both isolated and within microbial cells, so the effect of temperature on the yield of taxifolin produced by SQH4 from 1 g/L astilbin, after incubation for 8 h, was determined (Figure 4). The yield of taxifolin increased gradually with a temperature of 20 °C, reaching a maximum (92.3%) at 35 °C, then decreasing sharply at 40 °C, so the optimum temperature for biotransformation of astilbin to taxifolin by SQH4 is 35 °C.
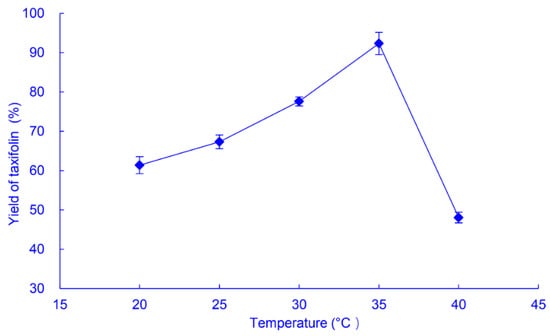
Figure 4.
Effects of temperature on the yield of taxifolin from biotransformation of astilbin by A. fumigatus SQH4. Biotransformation was performed at pH 6.5 with shaking at 200 r/min for 8 h, with 1 g/L astilbin.
2.2.3. Astilbin Concentration
Higher productivity from a biochemical reaction can be obtained by optimizing the substrate concentration. The yields of taxifolin obtained from astilbin concentrations of 1−8 g/L, after biotransformation by SQH4 for 8 h were determined (Figure 5).
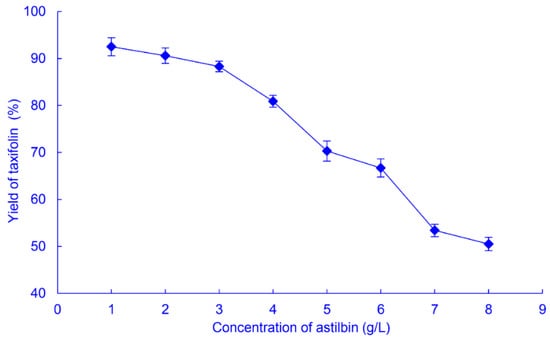
Figure 5.
The yield of taxifolin from biotransformation of astilbin at different concentrations by A. fumigatus SQH4. Biotransformation was performed at 35 °C, pH 6.5, with shaking at 200 r/min for 8 h.
The taxifolin yield initially decreased only slightly with increasing astilbin concentration, from 92.5% at 1 g/L to 88.3% at 3 g/L (Figure 5), corresponding to mass yields of 0.625 and 1.79 g/L, respectively. Increasing the astilbin concentration further resulted in marked decreases in taxifolin conversion yield; however, the mass yield could be improved by prolonging the biotransformation time at astilbin concentrations over 3 g/L (see below).
2.2.4. Biotransformation Time and Astilbin Concentration
Prolonging the biotransformation time can improve the yield of products. However, excessive time may result in bacterial spoilage and degradation of the reaction products, so the time must be optimized to maximize the yield. The effect of biotransformation time on the taxifolin yield was determined with 4, 5, and 6 g/L astilbin (Figure 6).
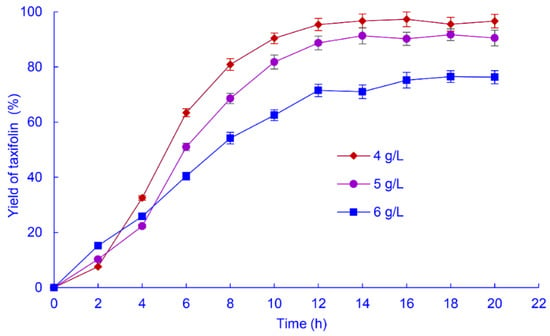
Figure 6.
Time course of the yield of taxifolin from biotransformation of astilbin at different concentrations by A. fumigatus SQH4. Biotransformation was performed at 35 °C, pH 6.5, with shaking at 200 r/min.
At 6 g/L astilbin, hydrolysis for 20 h produced a 76.7% taxifolin yield, corresponding to a mass yield of 3.11 g/L. At 5 g/L astilbin, the taxifolin yield was 91.3% after 14 h (mass yield 3.08 g/L). At 4 g/L astilbin, the taxifolin yield was 96.7% after 14 h (mass yield 2.61 g/L). Although the mass yield of taxifolin was slightly higher at 6 g/L than 5 g/L astilbin, the much shorter time (14 h) required and less substrate consumption at 5 g/L makes this the optimum astilbin concentration.
3. Materials and Methods
3.1. Materials and Chemicals
Standard taxifolin and astilbin (both of 98% purity) purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Smilax glabra rhizomes purchased from Bozhou Chinese Herbal Medicine Management (Bozhou, Anhui, China). HPLC-grade methanol purchased from Merck (Shanghai, China). All other chemicals were of analytical or biological grade from local suppliers.
3.2. Isolation and Classification of Enzyme-Producing Strains
Smilax glabra rhizome powder (20 g) and sterile water (20 mL) were combined in a sterilized 250-mL conical flask, stirred until homogeneous, then incubated for 3 days at 30 °C to enrich endogenous fungi. The fungi were isolated using the spread-plate technique on potato dextrose agar (PDA). Colonies with different colors or shapes were selected, transferred to fresh PDA plates, and cultured for 3 days at 30 °C to obtain a pure culture of each fungal strain.
The rDNA-ITS sequence analysis was used for species identification of the selected fungal strain. Extraction of genomic DNA, polymerase chain reaction (PCR) amplification of the rDNA-ITS, and sequencing of the purified PCR products were completed by Sangon Biotech Co., Ltd. (Shanghai, China). The sequences were compared with the rDNA-ITS sequences in the NCBI (http://www.ncbi.nlm.nih.gov, accessed on 8 September 2017) using the BLAST (Basic Local Alignment Search Tool) program.
3.3. Screening of Fungal Strains for Ability to Biotransform Astilbin to Taxifolin
Each fungal strain was cultured on PDA for 3 days, then physiological saline (10 mL) was added to suspend the spores. Spore suspension (5 mL) was inoculated into an enzyme-producing medium (50 mL; 20 g/L sucrose, 8 g/L peptone, 5 g/L yeast extract, 5 g/L KH2PO4, 5 g/L NaCl, 1 g/L MgSO4·7H2O and 1 g/L MnSO4·H2O, pH 6.0) and cultured for 3 days on an orbital shaker at 30 °C and 200 r/min. Methanolic astilbin (50 mg in 1 mL) was added to the culture medium to a final concentration of 1 g/L, then incubation continued for 8 h under the same conditions. After incubation, each fungal culture was filtered. The culture filtrate and mycelia were each extracted twice with an equivalent volume of ethyl acetate or methanol, respectively, and then the solvents were removed by rotary evaporation and the residues dissolved in methanol (5 mL). The solution was diluted and filtered through a 0.45 μm organic filter membrane, and the taxifolin concentration was determined by HPLC.
3.4. Analytical Method
HPLC analysis was performed with a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) equipped with two LC-20AT pumps and an SPD-20A UV/VIS detector. Chromatographic separation was performed on an Alltima C18 reversed-phase column (5 µm, 4.6 mm × 250 mm, (Alltech, Deerfield, IL, USA). The mobile phase was methanol-water (43:57, v/v) at a flow rate of 1.0 mL/min. The absorbance was measured at a wavelength of 290 nm. Peak area was used to calculate the quantity of taxifolin based on a standard curve of authentic standard.
3.5. Optimization of Biotransformation Conditions
The conditions used in Section 3.3 were systematically varied, and taxifolin yields were determined as in Section 3.4. The pH was varied from 4.5–8.0, at 30 °C, for 8 h, with 1 g/L astilbin. The temperature was varied from 20–40 °C, at pH 6.5 for 8 h, with 1 g/L astilbin. The substrate concentration was varied from 1–8 g/L astilbin, at 35 °C and pH 6.5, for 8 h. The biotransformation time was optimized by following the time course for 20 h, with 4, 5, or 6 g/L astilbin, at 35 °C and pH 6.5.
3.6. Data and Statistical Analysis
Mean values of all data were obtained from triplicate experiments. Differences were considered to be statistically significant at p < 0.05. The yield of taxifolin transformed from astilbin was calculated as follows:
4. Conclusions
A process was developed for fungal enzymic biotransformation of astilbin to taxifolin, which avoids the yield loss from aglycone degradation during acid hydrolysis. A fungal strain, A. fumigatus SQH4, capable of biotransforming astilbin to taxifolin, was isolated from an enrichment culture of S. glabra rhizomes. SQH4 culture, after pH adjustment to 6.5, was used as a crude enzyme preparation. Astilbin added to the crude enzyme preparation at 5 g/L was converted to a 91.3% yield of taxifolin after 14 h at 35 °C. The biotransformation could be achieved by adding astilbin directly to the SQH4 culture medium or by suspending the isolated fungal mycelia in a pH 6.5 buffer. The advantage of using a buffer is relative freedom from contaminating substances compared with a culture medium, which facilitates the isolation and purification of taxifolin but requires filtration or centrifugation to isolate the fungal mycelia. The use of isolated mycelia in a buffer has clear operational advantages in industrial applications.
In a previous report [28], S. glabra rhizomes containing astilbin were hydrolyzed with 1 mol/L HCl, then the resulting taxifolin was extracted. However, only 3.96 g of taxifolin was obtained from 1000 g of rhizomes. This method has the advantage of avoiding the extraction of astilbin, but the yield of taxifolin is lower (0.39%0), even compared with that from larch wood (1–2%). It is, therefore, unlikely that this method can be applied industrially.
In this study, by comparison, astilbin was biotransformed to taxifolin in >90% yield. Future research will focus on optimization of the culture conditions for fungal growth to increase the biomass yield, which should further improve the taxifolin yield. The method developed here provides a potential alternative method for large-scale taxifolin production, with potential applications in the food industry.
5. Patents
Chinese Patent ZL201711178892.4 resulted from the work reported in this manuscript.
Author Contributions
Conceptualization, X.C. and J.M.; methodology, J.M. and Y.W.; validation, Y.Y. and G.Y.; formal analysis and investigation, J.M. and Y.Y.; resources and visualization, P.W. and Y.W.; writing—original draft preparation, J.M.; writing—review and editing, G.Y.; supervision, P.W. and X.C.; project administration and funding acquisition, X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhejiang Food and Drug Administration, China (Grant No. 2018014) and the Key Research and Development Program of Zhejiang Province, China (Grant No. 2021C03088).
Data Availability Statement
The data in the article are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the corresponding author.
References
- Kolhir, V.K.; Bykov, V.A.; Baginskaja, A.I.; Sokolov, S.Y.; Glazova, N.G.; Leskova, T.E.; Sakovich, G.S.; Tjukavkina, N.A.; Kolesnik, Y.A.; Rulenko, I.A. Antioxidant activity of a dihydroquercetin isolated from Larix gmelinii (Rupr.) Rupr. wood. Phytother. Res. 1996, 10, 478–482. [Google Scholar] [CrossRef]
- Kolesnik, Y.; Titova, E.; Chertkov, V.; Tashlitsky, V.; Tikhonov, V.; Shmatkov, D. Stereoisomeric composition of two bioflavonoids from Larix sibirica. Planta Med. 2011, 77, PA17. [Google Scholar] [CrossRef]
- Willför, S.; Ali, M.; Karonen, M.; Reunanen, M.; Arfan, M.; Harlamow, R. Extractives in bark of different conifer species growing in Pakistan. Holzforschung 2009, 63, 551–558. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific opinion on taxifolin-rich extract from Dahurian larch (Larix gmelinii). EFSA J. 2017, 15, e04682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef]
- Weidmann, A.E. Dihydroquercetin: More than just an impurity? Eur. J. Pharmacol. 2012, 684, 19–26. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. GRAS Notice, GRN No. 826-Dihydroquercetin. Available online: https://www.fda.gov/media/132030/download (accessed on 5 August 2022).
- Jew, S.; Kim, H.; Bae, S.; Kim, J.; Park, H. Enantioselective synthetic method for 3-hydroxyflavanones: An approach to (2R, 3R)-3′,4′-O-dimethyltaxifolin. Tetrahedron Lett. 2000, 41, 7925–7928. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Ghidouche, S.; Ducrot, P.H. Flavonoids: Hemisynthesis, reactivity, characterization and free radical scavenging activity. Molecules 2007, 12, 2228–2258. [Google Scholar] [CrossRef]
- Liu, Z.; Gu, H.; Yang, L. A novel approach for the simultaneous extraction of dihydroquercetin and arabinogalactan from Larix gmelinii by homogenate-ultrasound-synergistic technique using the ionic liquid. J. Mol. Liq. 2018, 261, 41–49. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, J.; Chen, F.; Yang, F.; Zu, Y.; Yang, L. Development of an ionic liquid-based microwave-assisted method for the extraction and determination of taxifolin in different parts of Larix gmelinii. Molecules 2014, 19, 19471–19490. [Google Scholar] [CrossRef]
- Ma, C.; Yang, L.; Wang, W.; Yang, F.; Zhao, C.; Zu, Y. Extraction of dihydroquercetin from Larix gmelinii with ultrasound-assisted and microwave-assisted alternant digestion. Int. J. Mol. Sci. 2012, 13, 8789–8804. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zu, Y.; Long, J.; Fu, Y.; Li, S.; Zhang, D.; Li, J.; Wink, M.; Efferth, T. Enzymatic water extraction of taxifolin from wood sawdust of Larix gmelini (Rupr.) Rupr. and evaluation of its antioxidant activity. Food Chem. 2011, 126, 1178–1185. [Google Scholar] [CrossRef]
- Wiesbeck, F. Process for the Manufacture of Taxifolin from Wood. US 9073889B2, 7 July 2015. [Google Scholar]
- Xia, Y.; Wang, Y.; Li, W.; Ma, C.; Liu, S. Homogenization-assisted cavitation hybrid rotation extraction and macroporous resin enrichment of dihydroquercetin from Larix gmelinii. J. Chromatogr. B 2017, 1070, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-J.; Jun, Z.; Han, A.-H.; Wang, X.-J.; Wang, X.-J. Biomass and carbon pool of Larix gmelini young and middle age forest in Xing’an Mountains Inner Mongolia. Acta Ecol. Sin. 2007, 27, 1756–1762. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Sakanaka, M. Effects of Astilbe thunbergii rhizomes on wound healing. Part 1. Isolation of promotional effectors from Astilbe thunbergii rhizomes on burn wound healing. J. Ethnopharmacol. 2007, 109, 72–77. [Google Scholar] [CrossRef]
- Lu, C.-L.; Zhu, Y.-F.; Hu, M.-M.; Wang, D.-M.; Xu, X.-J.; Lu, C.-J.; Zhu, W. Optimization of astilbin extraction from the rhizome of Smilax glabra, and evaluation of its anti-inflammatory effect and probable underlying mechanism in lipopolysaccharide-induced RAW264.7 macrophages. Molecules 2015, 20, 625–644. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Cheung, H. Antioxidant activity of Rhizoma Smilacis Glabrae extracts and its key constituent-astilbin. Food Chem. 2009, 115, 297–303. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, R.; Shi, Y.; Zhang, X.; Tian, C.; Xia, D. Antioxidant and anti-inflammatory activities of six flavonoids from Smilax glabra Roxb. Molecules 2020, 25, 5295. [Google Scholar] [CrossRef]
- Chen, L.; Yin, Y.; Yi, H.; Xu, Q.; Chen, T. Simultaneous quantification of five major bioactive flavonoids in Rhizoma Smilacis Glabrae by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2007, 43, 1715–1720. [Google Scholar] [CrossRef]
- Souquet, J.; Labarbe, B.; Guernevé, C.; Cheynier, V.; Moutounet, M. Phenolic composition of grape stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef]
- Huang, H.; Cheng, Z.; Shi, H.; Xin, W.; Wang, T.; Yu, L. Isolation and characterization of two flavonoids, engeletin and astilbin, from the leaves of Engelhardia roxburghiana and their potential anti-inflammatory properties. J. Agric. Food Chem. 2011, 59, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Christopher, R.; Nyandoro, S.S.; Chacha, M.; de Koning, C.B. A new cinnamoylglycoflavonoid, antimycobacterial and antioxidant constituents from Heritiera littoralis leaf extracts. Nat. Prod. Res. 2014, 28, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Mecchi, M.C.; Lago, J.H.G. Chemical constituents derived from Drimys brasiliensis Miers (Winteraceae). Nat. Prod. Res. 2013, 27, 1927–1929. [Google Scholar] [CrossRef]
- Zhang, Q.-F.; Li, S.-C.; Lai, W.-I.; Cheung, H.-Y. β-Cyclodextrin facilitates simultaneous analysis of six bioactive components in Rhizoma Smilacis Glabrae by capillary zone electrophoresis. Food Chem. 2009, 113, 684–691. [Google Scholar] [CrossRef]
- Qiu, X.-L.; Zhang, Q.-F. Acidic hydrolysis of astilbin and its application for the preparation of taxifolin from Rhizoma Smilacis Glabrae. J. Chem. Res. 2021, 45, 290–294. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Riva, S. Biocatalytic modification of natural products. Curr. Opin. Chem. Biol. 2001, 5, 106–111. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).