Effect of Platinum Precursor on the Properties of Pt/N-Graphene Catalysts in Formic Acid Decomposition
Abstract
:1. Introduction
2. Results and Discussion
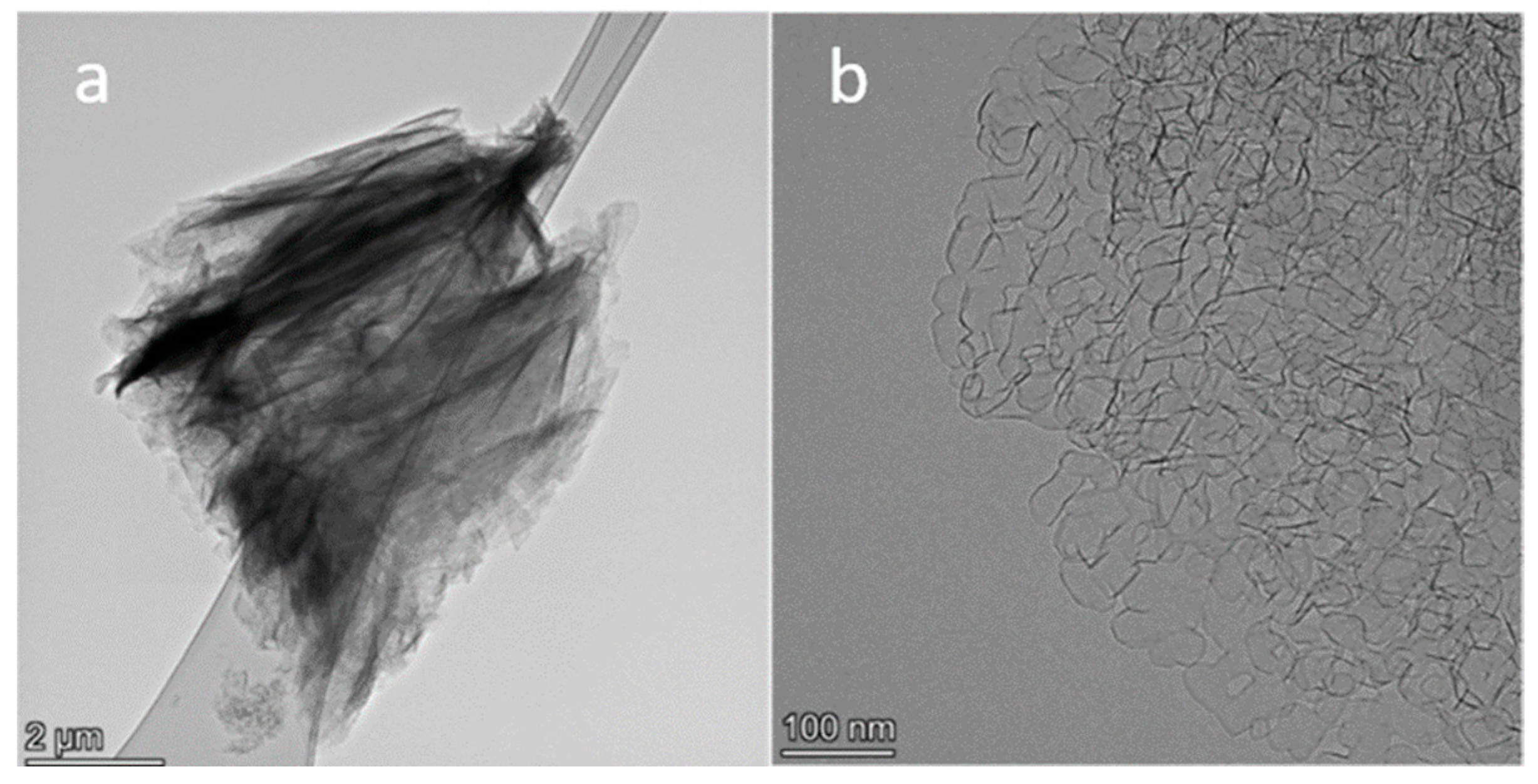
2.1. TEM Study of N-Graphene
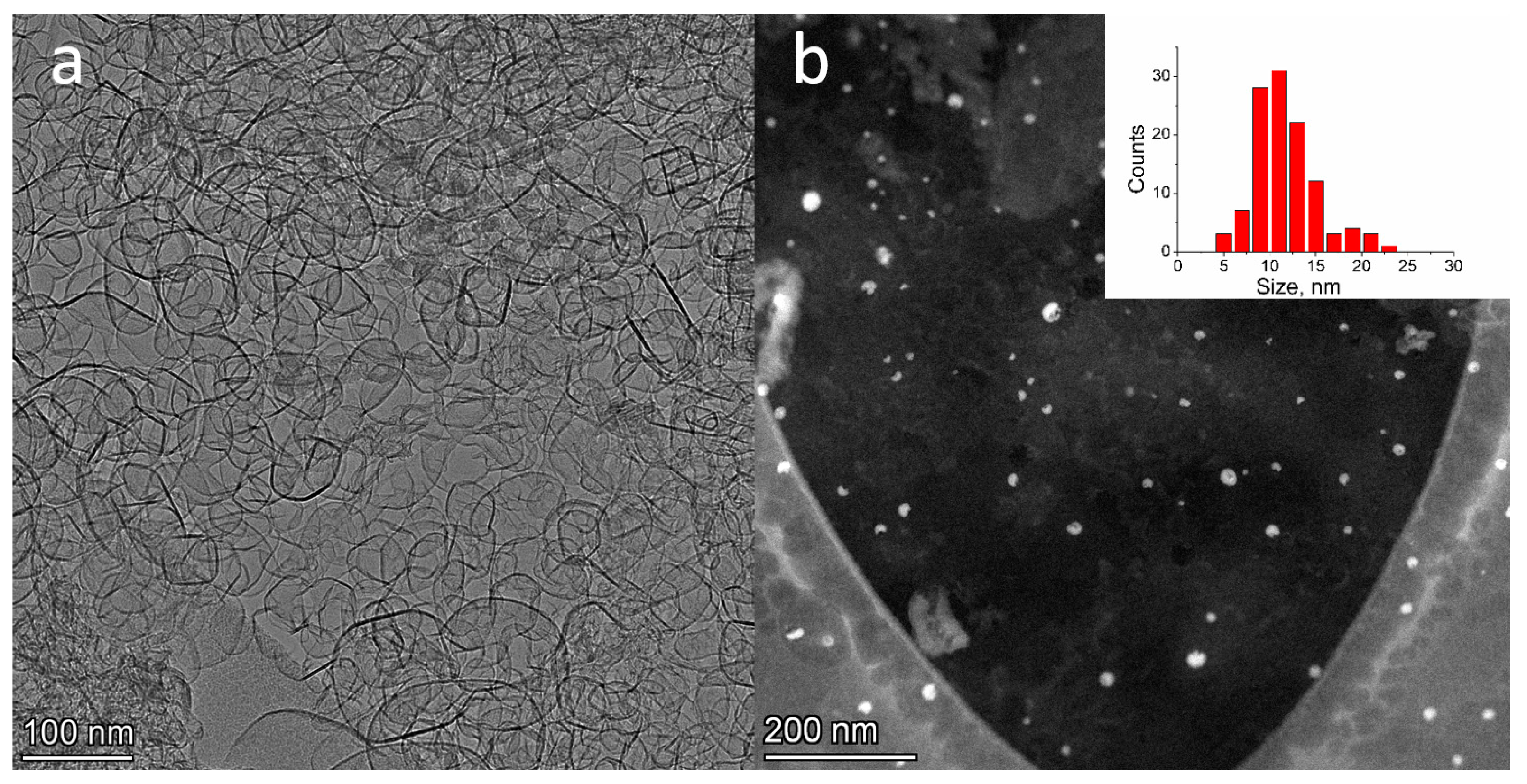
2.2. TEM Study of Pt/N-Graphene Catalysts Prepared Using Pt(NO3)4
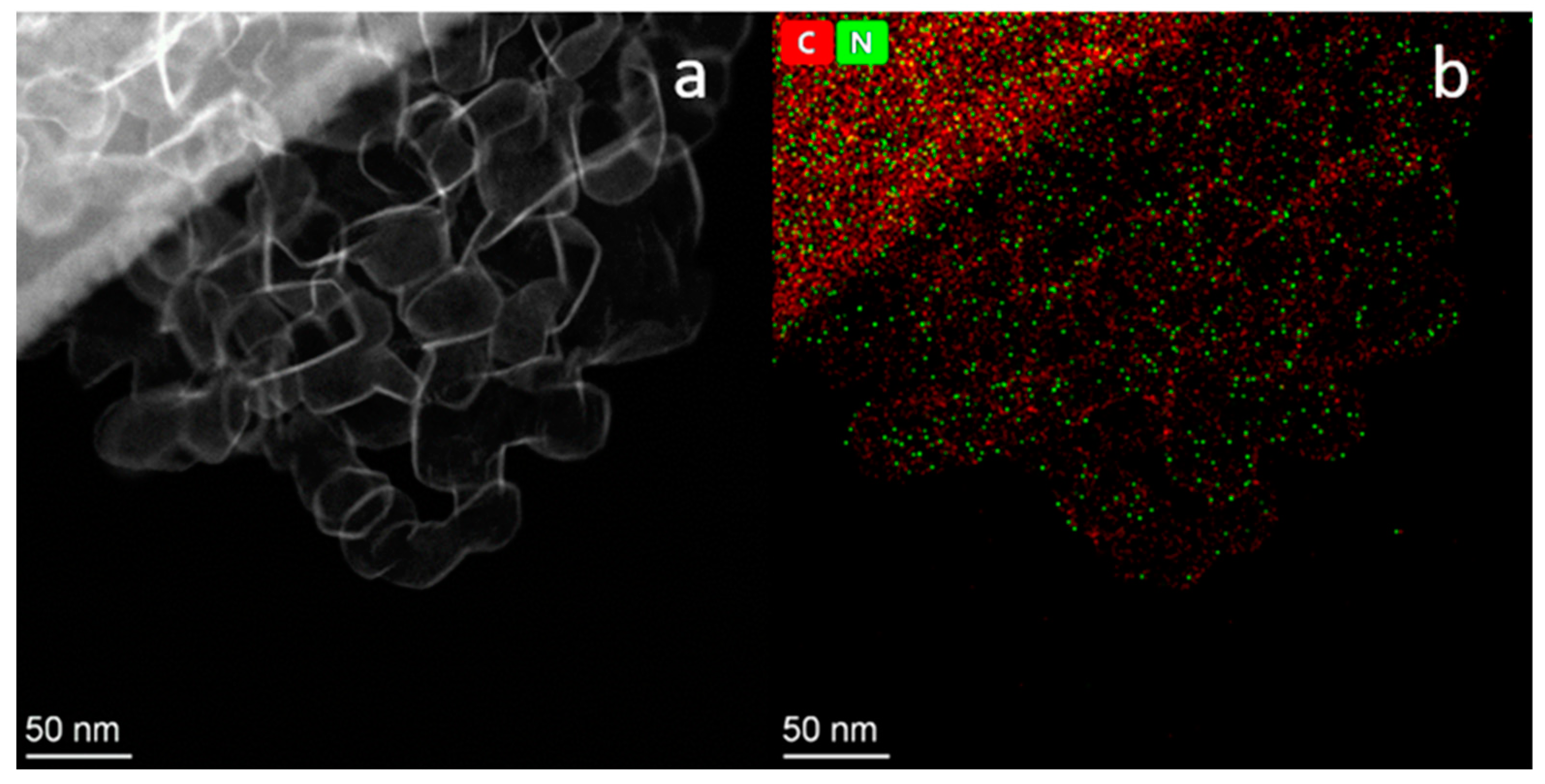
2.3. TEM Study of Pt/N-Graphene Catalysts Prepared Using H2PtCl6
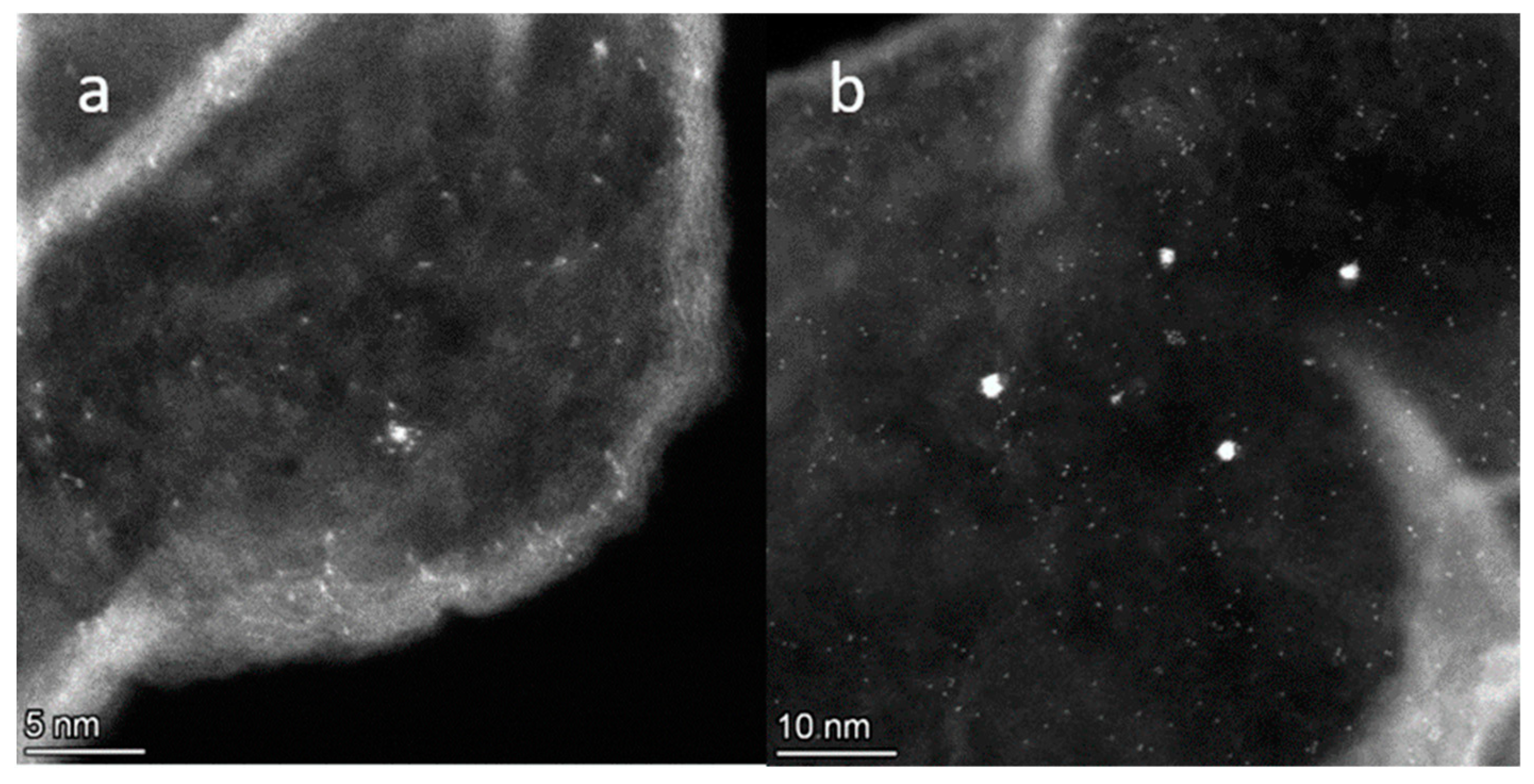
2.4. Determination of the Platinum Particle Size in Pt/N-Graphene Catalysts Prepared Using H2PtCl6
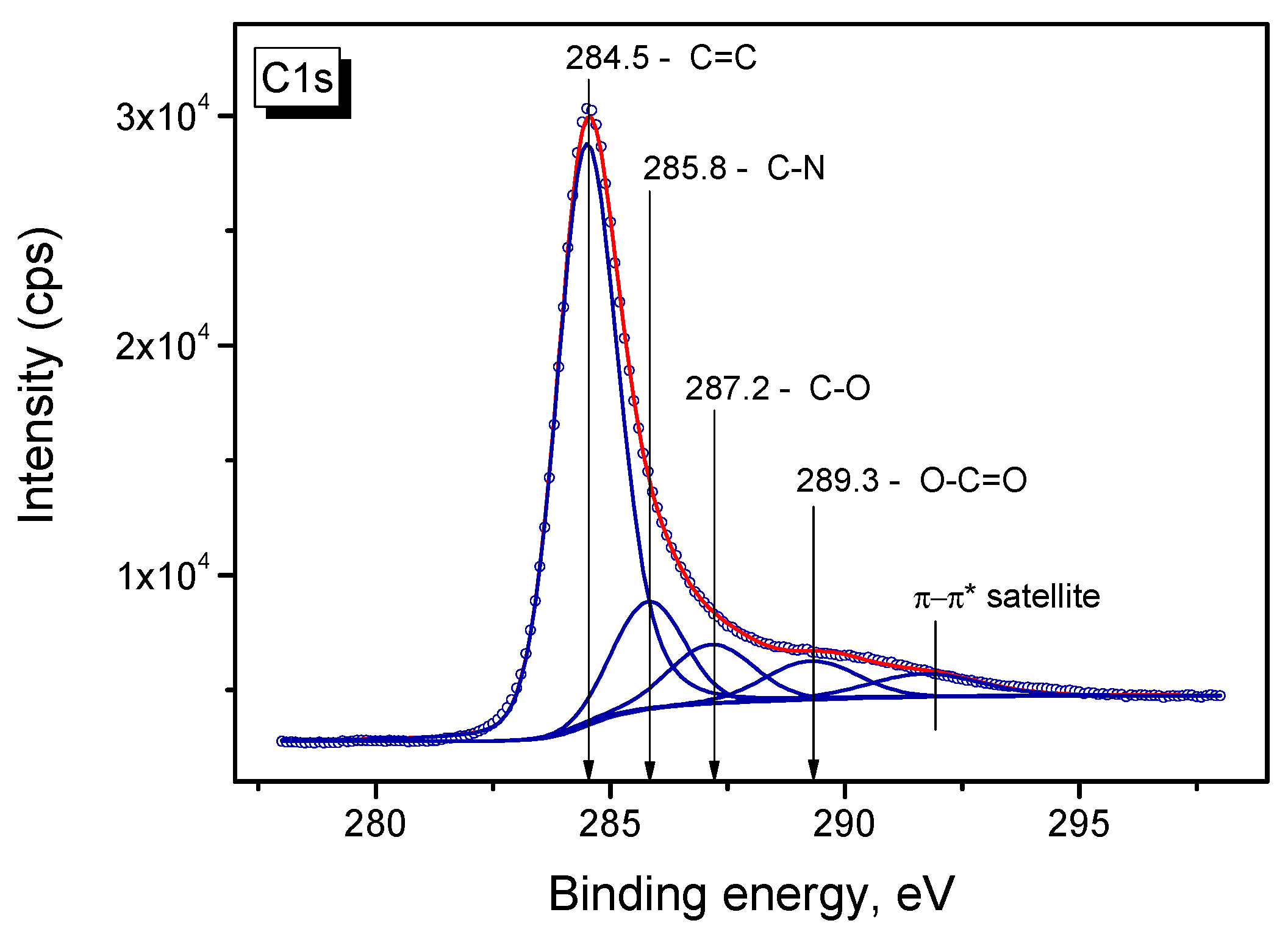
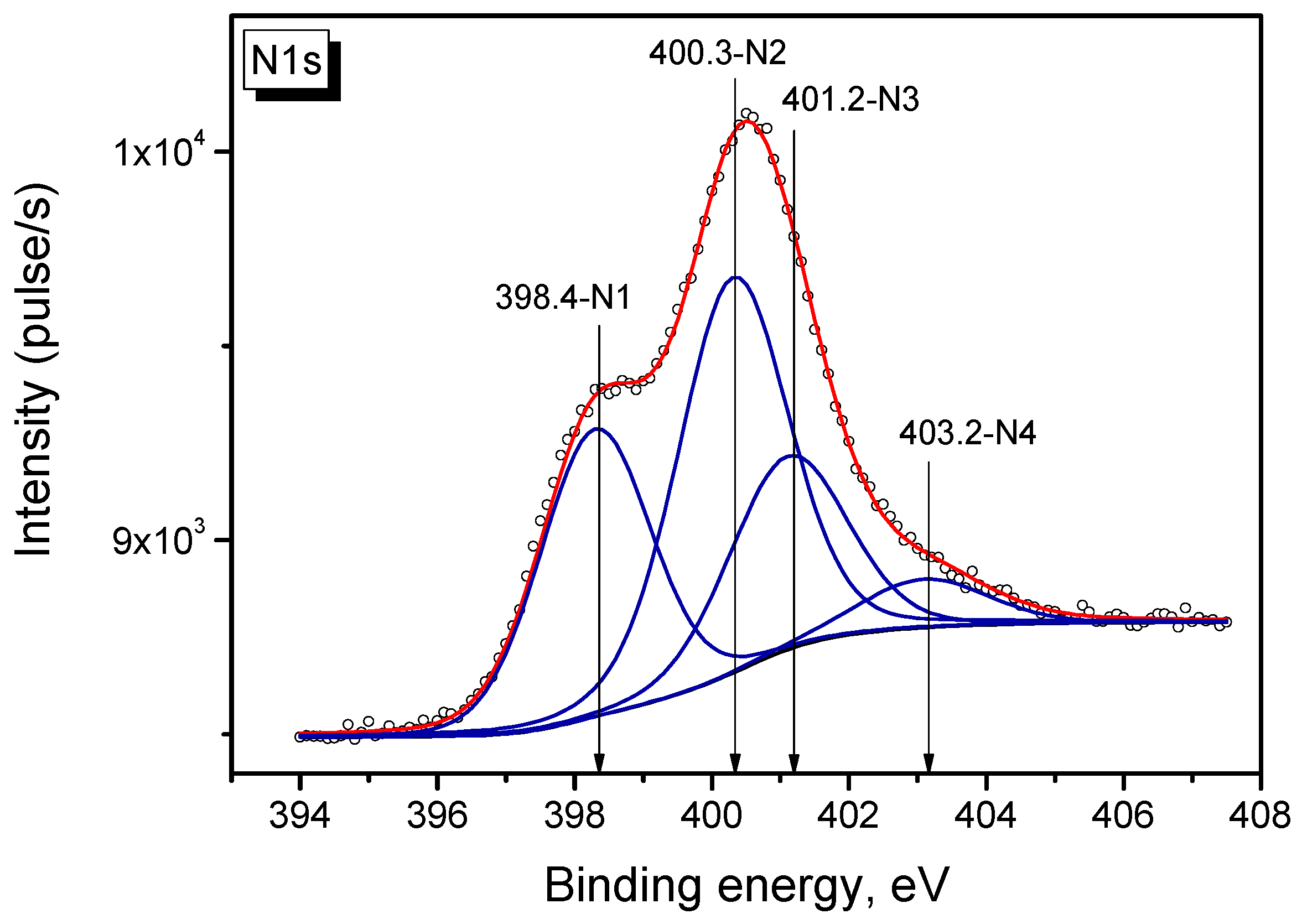
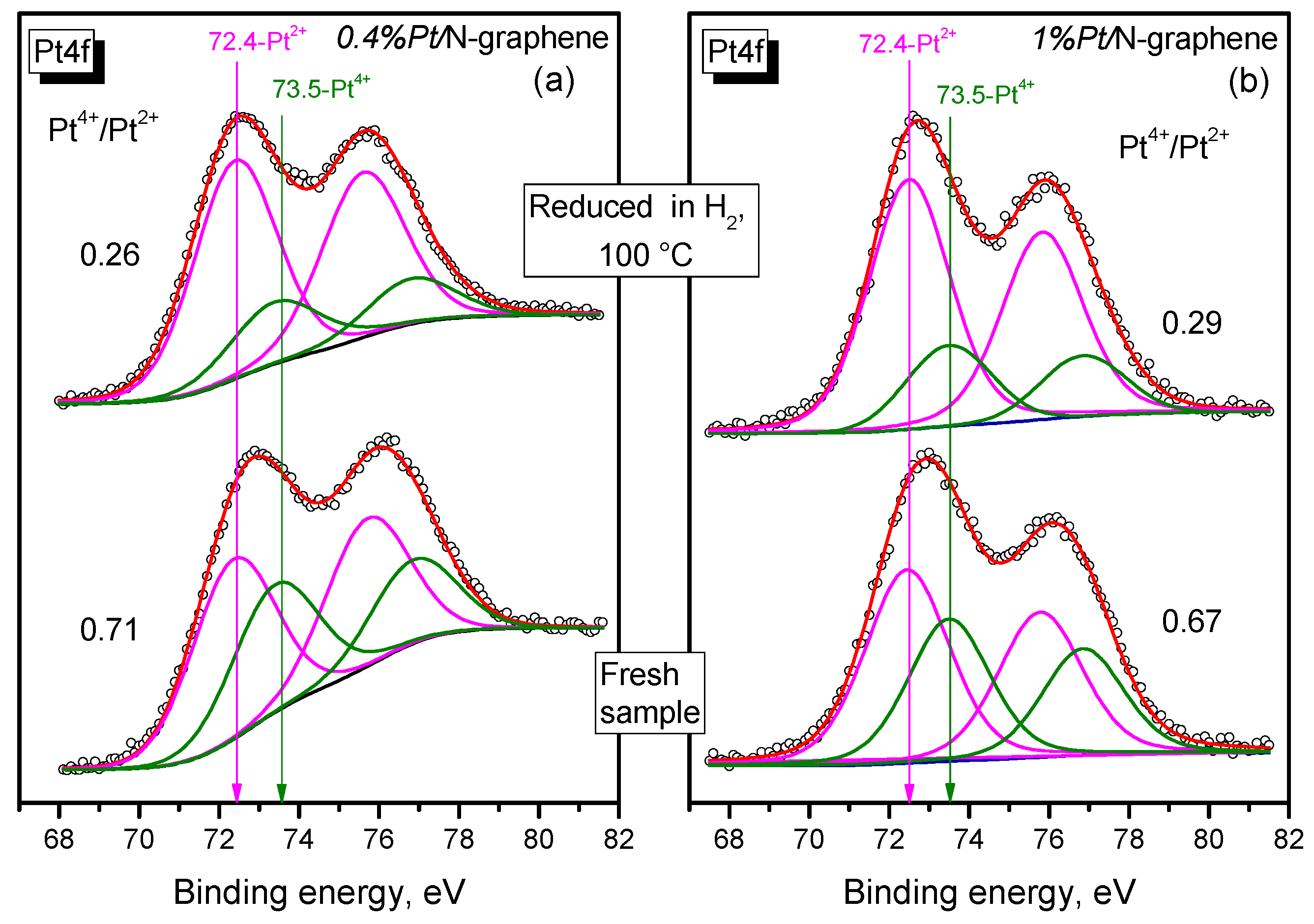
2.5. XPS Study of Pt/N-Graphene Catalysts Prepared Using H2PtCl6
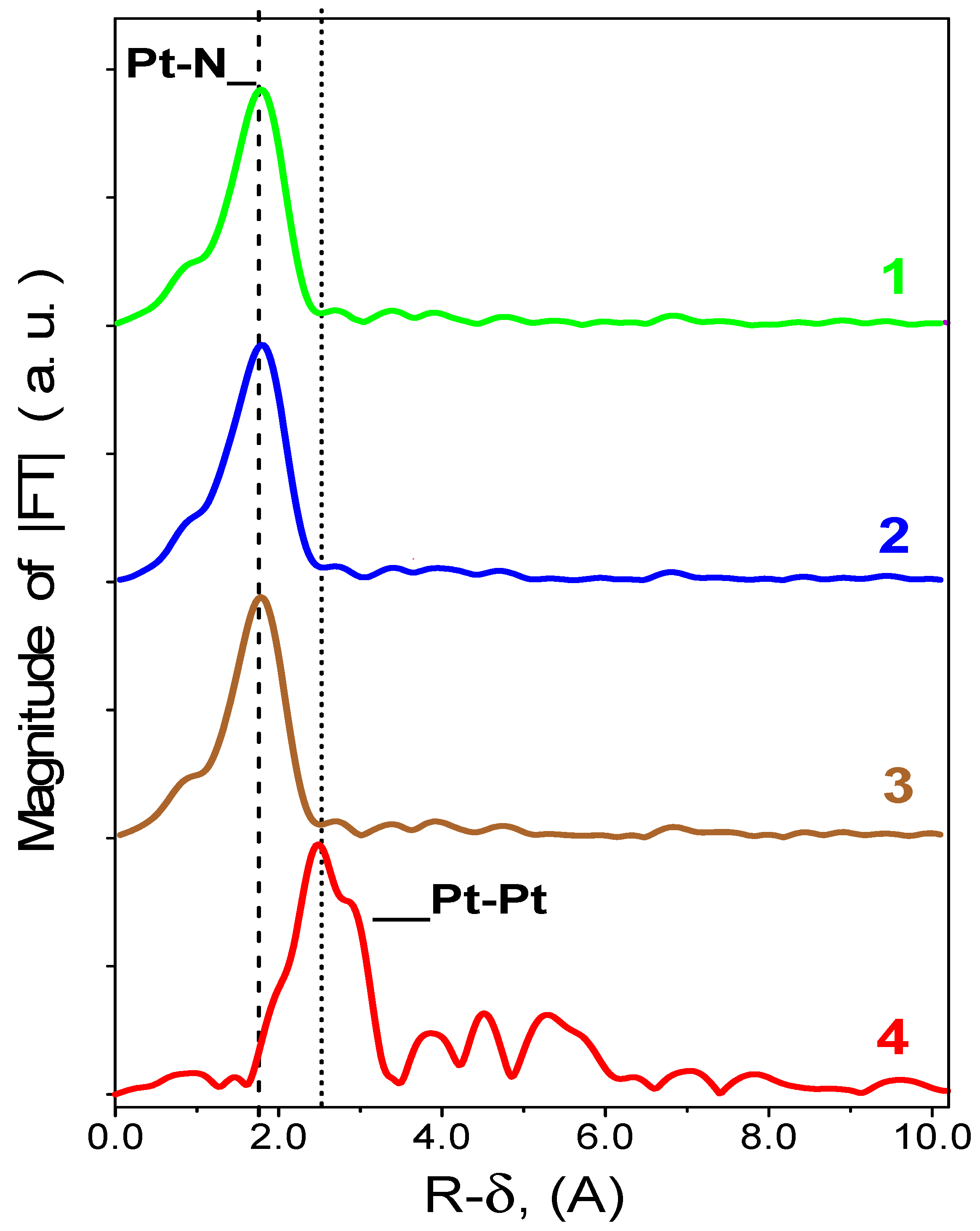
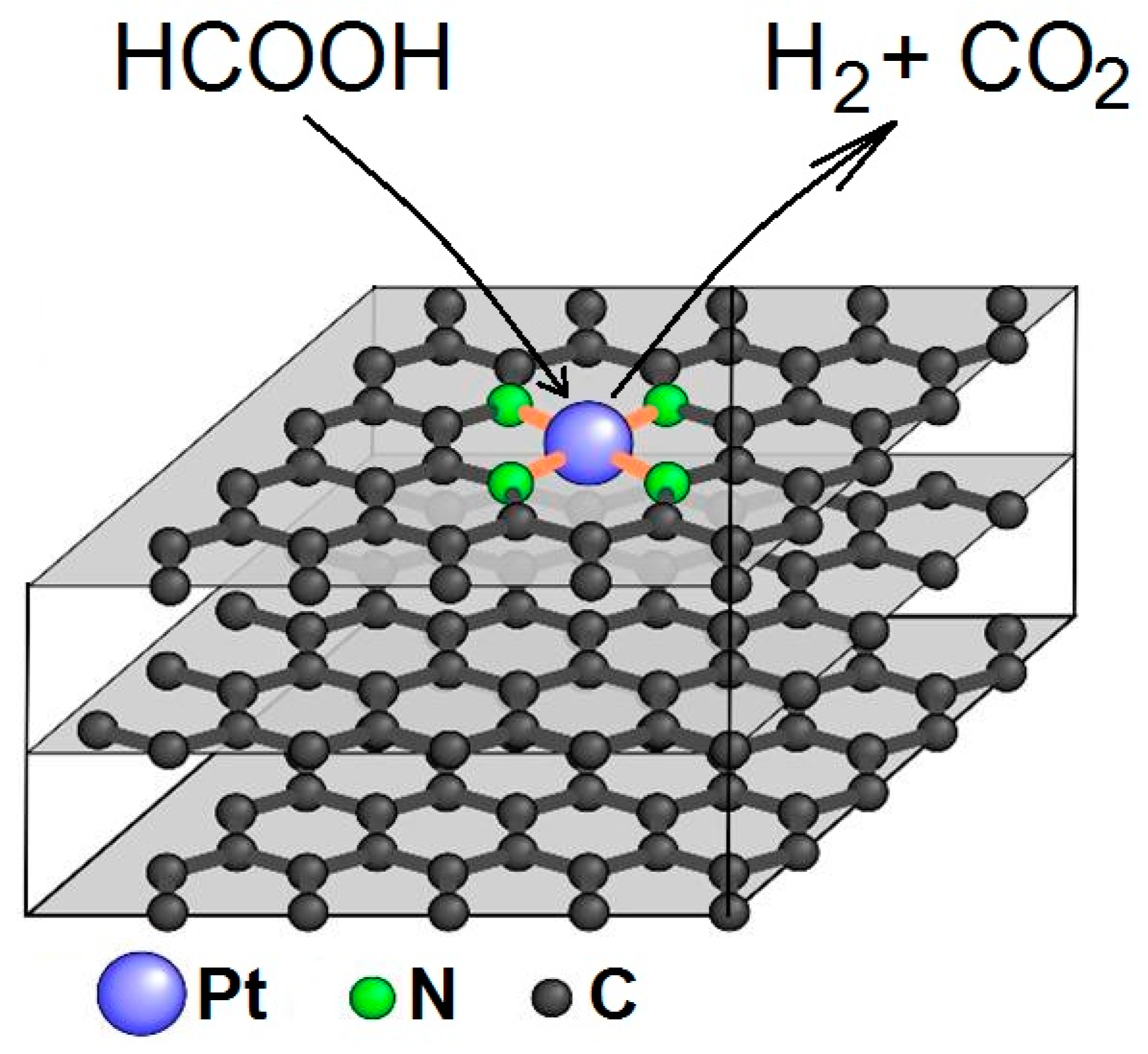
2.6. EXAFS Studies of Pt/N-Graphene Catalysts Prepared Using H2PtCl6
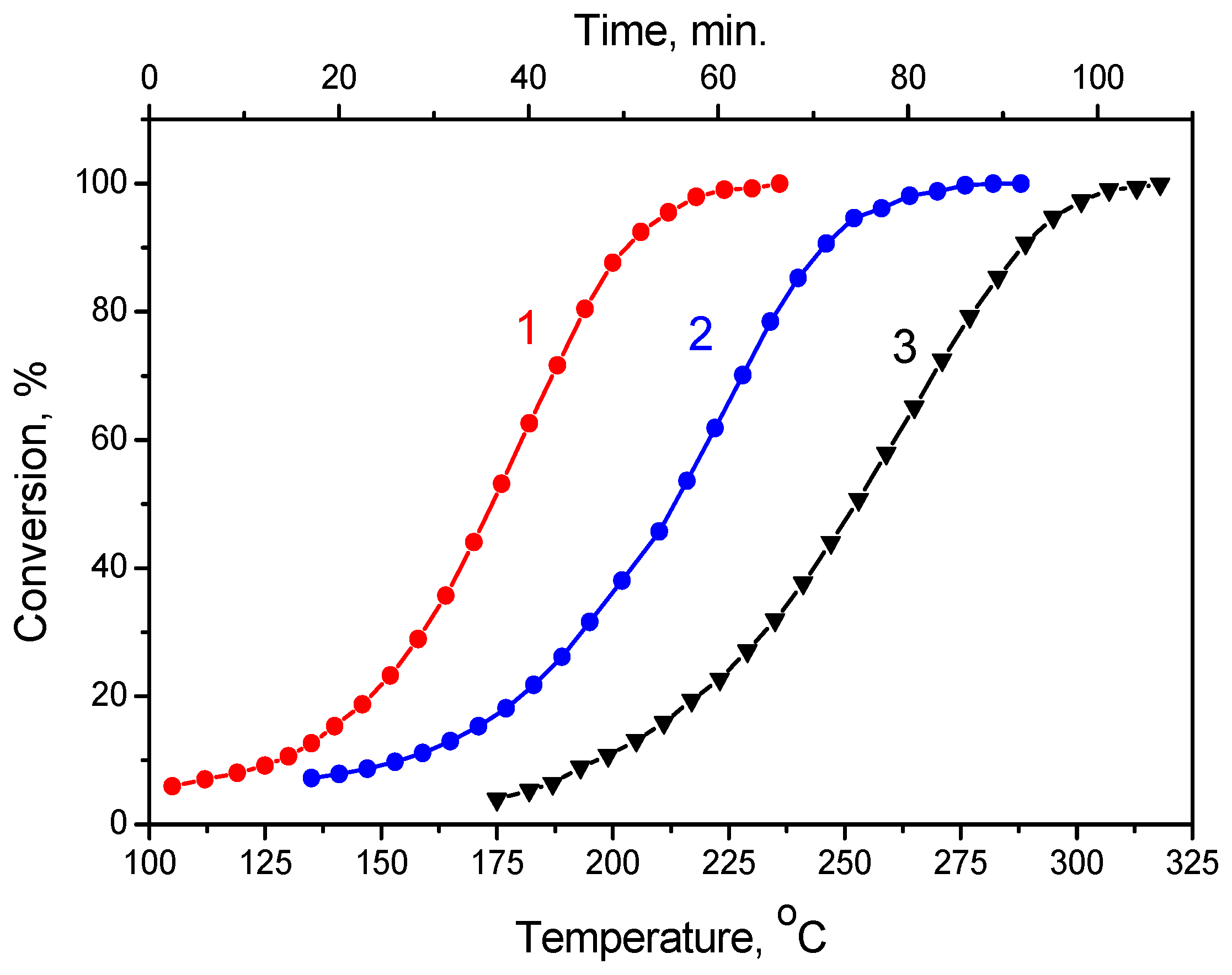
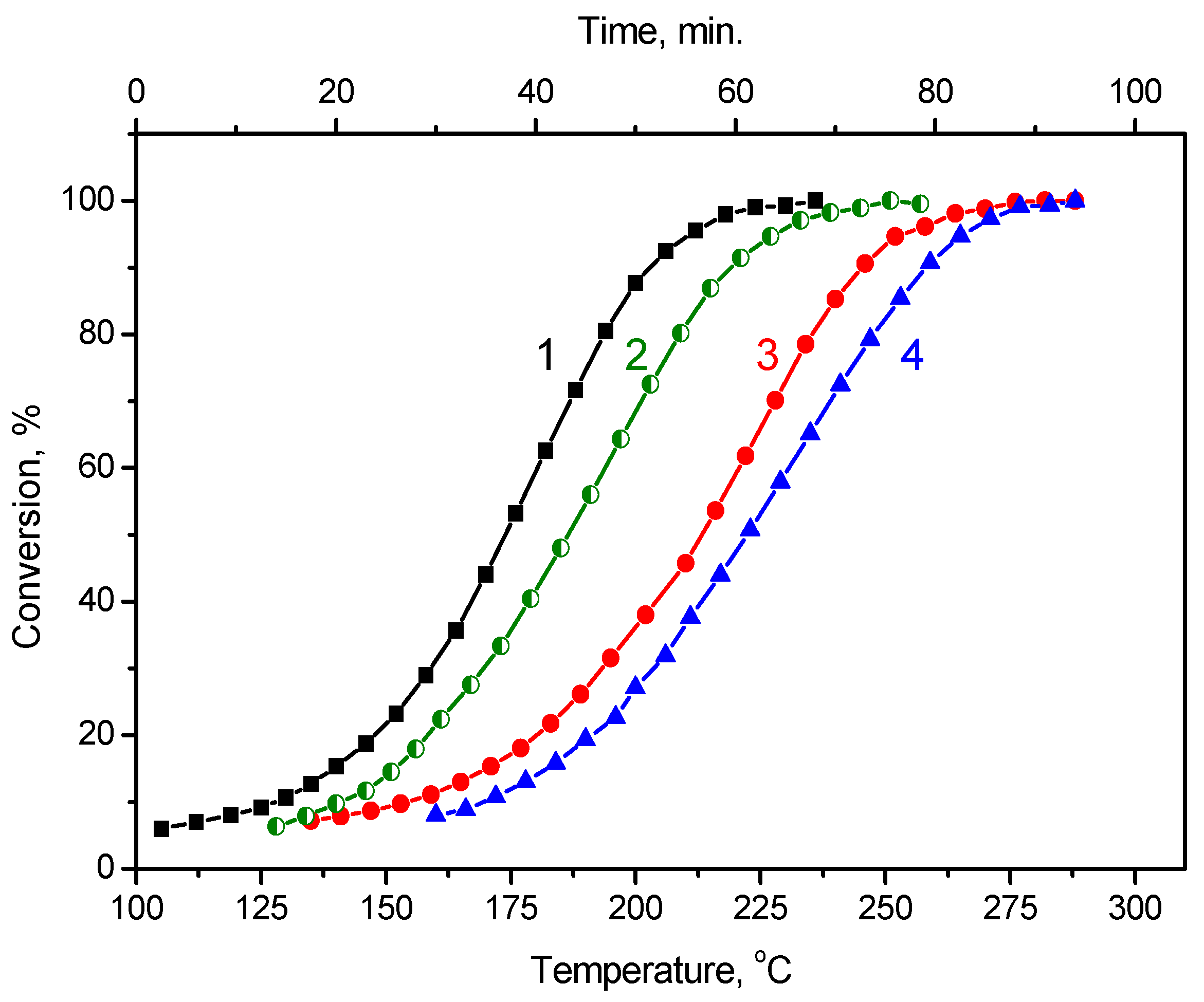
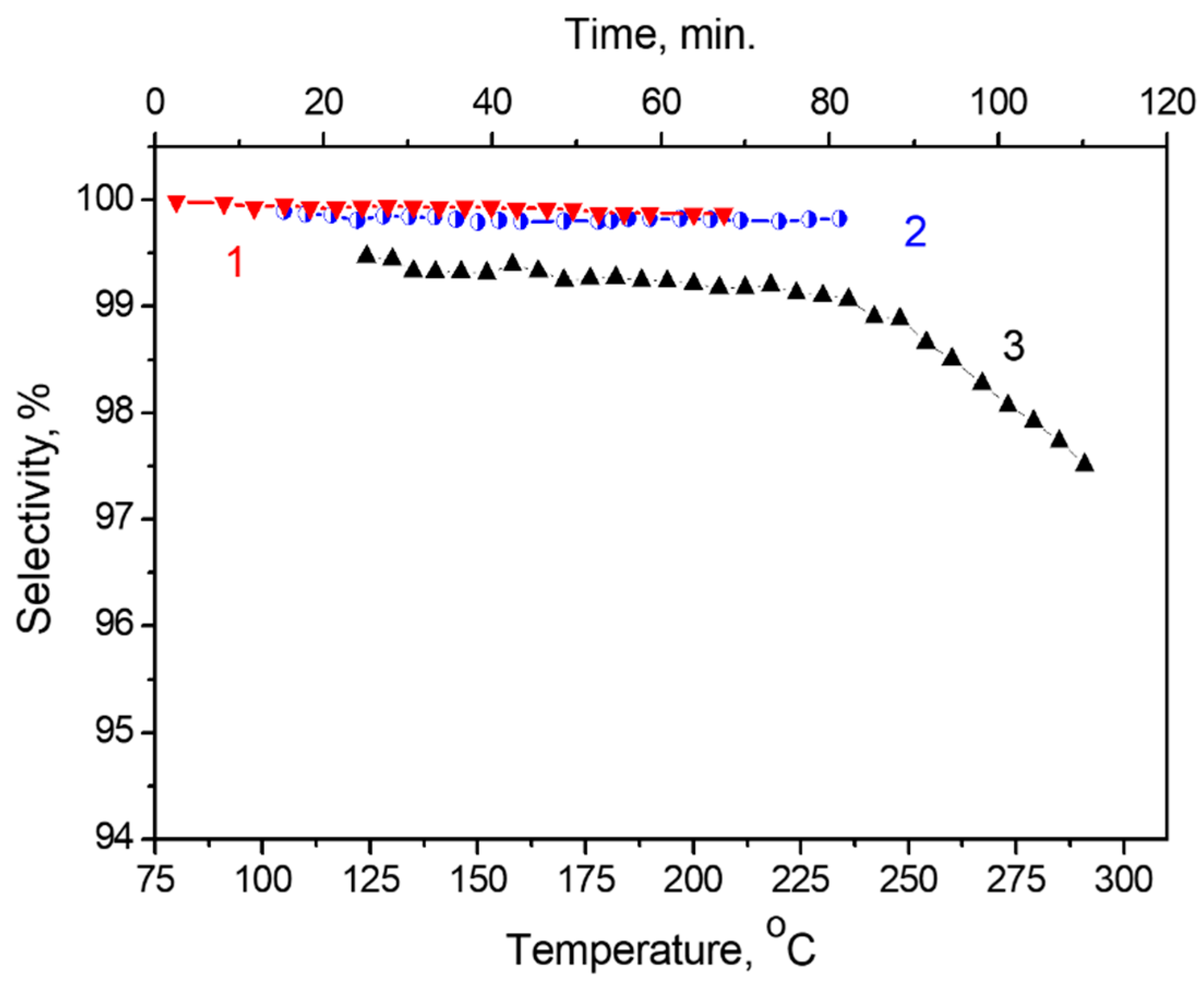
2.7. Formic Acid Decomposition over Pt/Graphene and Pt/N-Graphene Catalysts Synthesized Using Pt(NO3)4
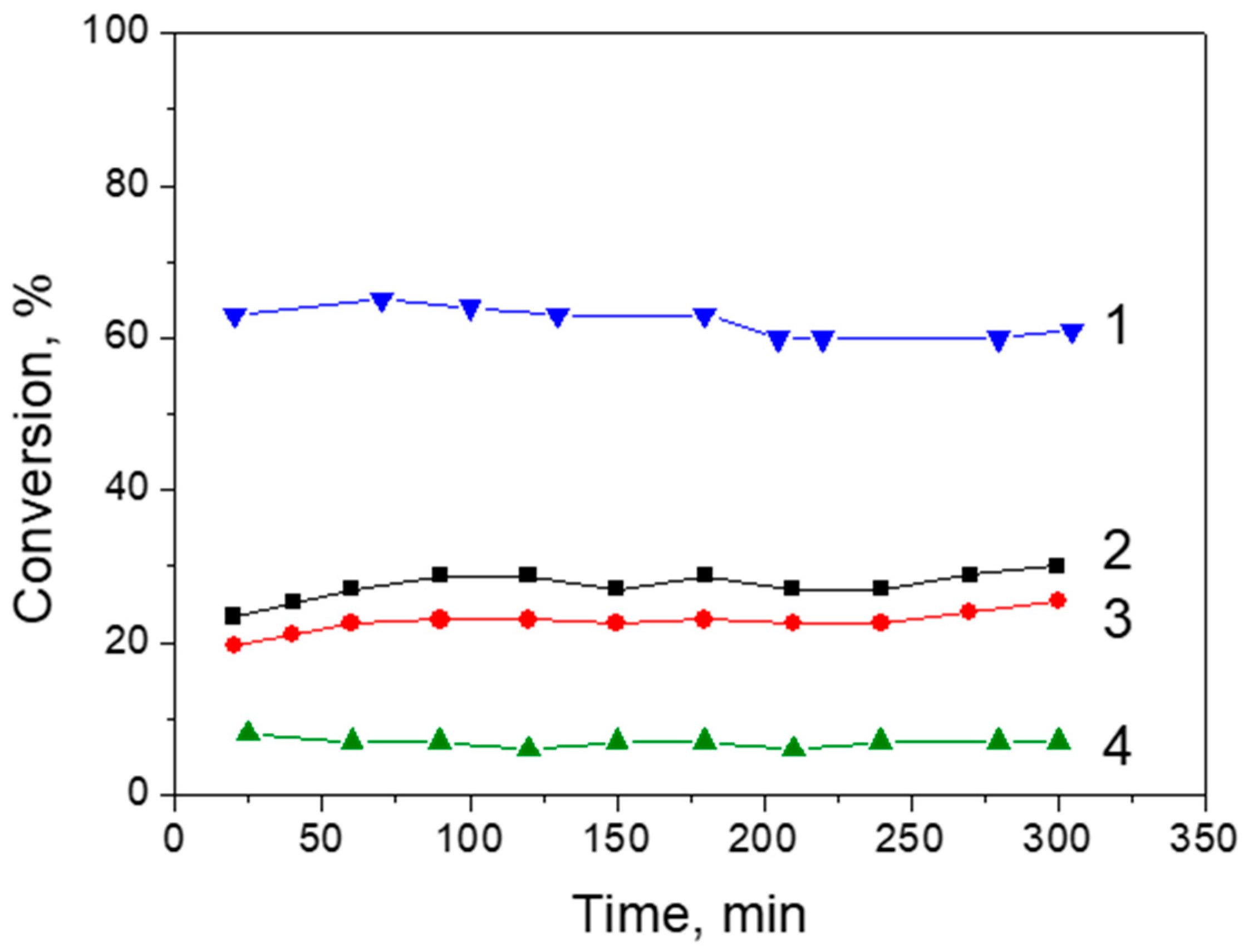
2.8. Formic Acid Decomposition over Pt/Graphene and Pt/N-Graphene Catalysts Synthesized Using H2PtCl6
3. Methods of Investigation
3.1. Synthesis of Graphene Doped with Nitrogen Atoms
3.2. Synthesis of Pt/N-Graphene Catalysts
3.3. Formic Acid Decomposition over Pt/N-graphene Catalysts
3.4. Physicochemical Methods for Investigation of Pt/N-Graphene Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gil-San-Millan, R.; Grau-Atienza, A.; Johnson, D.; Rico-Francés, S.; Serrano, E.; Linares, N.; García-Martínez, J. Improving hydrogen production from the hydrolysis of ammonia borane by using multifunctional catalysts. Int. J. Hydrogen Energy 2018, 43, 17100–17111. [Google Scholar] [CrossRef]
- Shen, J.; Yang, L.; Hu, K.; Luo, W.; Cheng, G. Rh nanoparticles supported on graphene as efficient catalyst for hydrolytic dehydrogenation of amine boranes for chemical hydrogen storage. Int. J. Hydrogen Energy 2015, 40, 1062–1070. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Wang, D.; Wu, S.; Cao, Z.; Zhang, K.; Liu, H.; Xin, S. Hydrogen generation from hydrolysis of sodium borohydride using nanostructured NiB catalysts. Int. J. Hydrogen Energy 2016, 41, 16077–16086. [Google Scholar] [CrossRef]
- Nielsen, M.; Alberico, E.; Baumann, W.; Drexler, H.-J.; Junge, H.; Gladiali, S.; Beller, M. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 2013, 495, 85–89. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; Wif, D.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Zhong, H.; Iguchi, M.; Chatterjee, M.; Himeda, Y.; Xu, Q.; Kawanami, H. Formic Acid-Based Liquid Organic Hydrogen Carrier System with Heterogeneous Catalysts. Adv. Sust. Syst. 2018, 2, 1700161. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.; Gao, L.; Jin, Z.; Ge, J.; Liu, C.; Xing, X. Recent progress in hydrogen production from formic acid decomposition. Int. J. Hydrogen Energy 2018, 43, 7055–7071. [Google Scholar] [CrossRef]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source—Recent developments and future trends. Energy Environ. Sci. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Eppinger, J.; Huang, K.-W. Formic acid as a hydrogen energy carrier. ACS Energy Lett. 2017, 2, 188–195. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R.H. Towards Sustainable Production of Formic Acid. ChemSusChem 2018, 11, 821–836. [Google Scholar] [CrossRef] [Green Version]
- Reichert, J.; Brunner, B.; Jess, A.; Wasserscheid, P.; Albert, J. Biomass Oxidation to Formic Acid in Aqueous Media Using Polyoxometalate Catalysts—Boosting Fa Selectivity by in-Situ Extraction. Energy Environ. Sci. 2015, 8, 2985–2990. [Google Scholar]
- Gromov, N.V.; Medvedeva, T.B.; Sorokina, K.N.; Samoylova, Y.V.; Rodikova, Y.A.; Parmon, V.N. Direct Conversion of Microalgae Biomass to Formic Acid under an Air Atmosphere with Soluble and Solid Mo-V-P Heteropoly Acid Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 18947–18956. [Google Scholar] [CrossRef]
- Gromov, N.V.; Taran, O.P.; Delidovich, I.V.; Pestunov, A.V.; Rodikova, Y.A.; Yatsenko, D.A.; Zhizhina, E.G.; Parmon, V.N. Hydrolytic Oxidation of Cellulose to Formic Acid in the Presence of Mo-V-P Heteropoly Acid Catalysts. Catal. Today 2016, 278, 74–81. [Google Scholar] [CrossRef]
- Jia, L.; Bulushev, D.A.; Podyacheva, O.Y.; Boronin, A.I.; Kibis, L.S.; Gerasimov, E.Y.; Beloshapkin, S.; Seryak, I.A.; Ismagilov, Z.R.; Ross, J.R.H. Pt nanoclusters stabilized by N-doped carbon nanofibers for hydrogen production from formic acid. J. Catal. 2013, 307, 94–102. [Google Scholar] [CrossRef]
- Ayusheev, A.B.; Taran, O.P.; Seryak, I.A.; Podyacheva, O.Y.; Descorme, C.; Besson, M.; Kibis, L.S.; Boronin, A.I.; Romanenko, A.I.; Ismagilov, Z.R.; et al. Ruthenium nanoparticles supported on nitrogen-doped carbon nanofibers for the catalytic wet air oxidation of phenol. Appl. Catal. B 2014, 146, 177–185. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Shalagina, A.E.; Podyacheva, O.Y.; Barnakov, C.N.; Kozlov, A.P.; Kvon, R.I.; Ismagilov, I.Z.; Kerzhentsev, M.A. Synthesis of nitrogen-containing carbon materials for solid polymer fuel cell cathodes. Kinet. Catal. 2007, 48, 581–588. [Google Scholar] [CrossRef]
- Podyacheva, O.Y.; Ismagilov, Z.R. Nitrogen-Doped Carbon Nanomaterials: To the Mechanism of Growth, Electrical Conductivity and Application in Catalysis. Catal. Today 2015, 249, 12–22. [Google Scholar] [CrossRef]
- Zacharska, M.; Bulusheva, L.G.; Lisitsyn, A.S.; Beloshapkin, S.; Guo, Y.; Chuvilin, A.L.; Shlyakhova, E.V.; Podyacheva, O.Y.; Leahy, J.J.; Okotrub, A.V.; et al. Factors Influencing the Performance of Pd/C Catalysts in the Green Production of Hydrogen from Formic Acid. ChemSusChem 2017, 10, 720–730. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Kriventsov, V.V.; Malykhin, S.E.; Chichkan, A.S.; Podyacheva, O.Y. Stabilization of Palladium Atoms in Nitrogen-Doped Porphyrin-Like Fragments of Carbon Nanofibers. J. Struct. Chem. 2018, 59, 839–846. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Kriventsov, V.V.; Malykhin, S.E.; Svintsitskiy, D.A.; Podyacheva, O.Y.; Lisitsyn, A.S.; Richards, R.M. Nature of Active Palladium Sites on Nitrogen Doped Carbon Nanofibers in Selective Hydrogenation of Acetylene. Diam. Relat. Mater. 2018, 89, 67–73. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Zacharska, M.; Lisitsyn, A.S.; Podyacheva, O.Y.; Hage, F.S.; Ramasse, Q.M.; Bangert, U.; Bulusheva, L.G. Single Atoms of Pt-Group Metals Stabilized by N-Doped Carbon Nanofibers for Efficient Hydrogen Production from Formic Acid. ACS Catal. 2016, 6, 3442–3451. [Google Scholar] [CrossRef]
- Zacharska, M.; Podyacheva, O.Y.; Kibis, L.S.; Boronin, A.I.; Senkovskiy, B.V.; Gerasimov, E.Y.; Taran, O.P.; Ayusheev, A.B.; Parmon, V.N.; Leahy, J.J.; et al. Ruthenium Clusters on Carbon Nanofibers for Formic Acid Decomposition: Effect of Doping the Support with Nitrogen. ChemCatChem 2015, 7, 2910–2917. [Google Scholar] [CrossRef]
- Golub, F.S.; Beloshapkin, S.; Gusel’nikov, A.V.; Bolotov, V.A.; Parmon, V.N.; Bulushev, D.A. Boosting hydrogen production from formic acid over Pd catalysts by deposition of N-containing precursors on the carbon support. Energies 2019, 12, 3885. [Google Scholar] [CrossRef]
- Ning, X.; Li, Y.; Dong, B.; Wang, H.; Yu, H.; Peng, F.; Yang, Y. Electron transfer dependent catalysis of Pt on N-doped carbon nanotubes: Effects of synthesis method on metal-support interaction. J. Catal. 2017, 348, 100–109. [Google Scholar] [CrossRef]
- Huang, C.; Li, C.; Shi, G. Graphene based catalysts. Energy Environ. Sci. 2012, 5, 8848–8868. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, W.; Fu, C.F.; Yang, J. Single Pd atom and Pd dimer embedded graphene catalyzed formic acid dehydrogenation: A first-principles study. Int. J. Hydrogen Energy 2018, 43, 6997–7006. [Google Scholar] [CrossRef]
- Jeyakumar, T.C.; Sivasankar, C. Dehydrogenation of formic acid catalysed by M-embedded nitrogen-doped graphene (M = Fe, Ru, Os): A DFT study. New J. Chem. 2019, 43, 1440–1448. [Google Scholar] [CrossRef]
- Feng, J.R.; Wang, G.C. Theoretical insight into the role of nitrogen in the formic acid decomposition over Pt13/N-GNS. Appl. Surf. Sci. 2021, 539, 148192. [Google Scholar] [CrossRef]
- Grad, O.; Mihet, M.; Dan, M.; Blanita, G.; Radu, T.; Berghian-Grosan, C.; Lazar, M.D. Au/reduced graphene oxide composites: Eco-friendly preparation method and catalytic applications for formic acid dehydrogenation. J. Mater. Sci. 2019, 54, 6991–7004. [Google Scholar] [CrossRef]
- Li, S.J.; Ping, Y.; Yan, J.M.; Wang, H.L.; Wu, M.; Jiang, Q. Facile synthesis of AgAuPd/graphene with high performance for hydrogen generation from formic acid. J. Mater. Chem. A 2015, 3, 14535–14538. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, P.; Xu, D.; Li, Z.; Guo, Q. Ultrafine palladium nanoparticles anchored on NH2-functionalized reduced graphene oxide as efficient catalyst towards formic acid dehydrogenation. Int. J. Hydrogen Energy 2020, 45, 30396–30403. [Google Scholar] [CrossRef]
- Yan, J.M.; Li, S.J.; Yi, S.S.; Wulan, B.R.; Zheng, W.T.; Jiang, Q. Anchoring and upgrading ultrafine NiPd on room-temperature-synthesized bifunctional NH2-N-rGO toward low-cost and highly efficient catalysts for selective formic acid dehydrogenation. Adv. Mater. 2018, 30, 1703038. [Google Scholar] [CrossRef]
- Moroz, E.M.; Kriventsov, V.V.; Kochubei, D.I. EuroPt-1 catalyst: Radial distribution of electron density X-ray diffraction and EXAFS studies. J. Struct. Chem. 2009, 50, 1082–1087. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Stoyanovskii, V.O.; Rogov, V.A.; Kriventsov, V.V.; Mishakov, I.V. The Role of Chemisorbed Water in Formation and Stabilization of Active Sites on Pd/Alumina Oxidation Catalysts. Catal. Today 2018, 307, 102–110. [Google Scholar] [CrossRef]
- Baklanova, N.I.; Morozova, N.B.; Kriventsov, V.V.; Titov, A.T. Synthesis and microstructure of Iridium coatings on carbon fibers. Carbon 2013, 56, 243–254. [Google Scholar] [CrossRef]
- Chistyakov, A.V.; Tsodikov, M.V.; Murzin, V.Y.; Yandieva, F.A.; Zubavichus, Y.V.; Kozitsyna, N.Y.; Gekhman, A.E.; Kriventsov, V.V.; Moiseev, I.I. Cocatalytic effect of Palladium and Zinc in the condensation of alcohol carbon backbones into hydrocarbons. Kinet. Catal. 2011, 52, 258–272. [Google Scholar] [CrossRef]
- Loponov, K.N.; Kriventsov, V.V.; Nagabhushana, K.S.; Boennemann, H.; Kochubey, D.I.; Savinova, E.R. Combined in situ EXAFS and Electrochemical Investigation of the Oxygen Reduction Reaction on Unmodified and Se-Modified Ru/C. Catal. Today 2009, 147, 260–269. [Google Scholar] [CrossRef]
- Beck, I.E.; Kriventsov, V.V.; Ivanov, D.P.; Zaikovsky, V.I.; Bukhtiyarov, V.I. XAFS Study of Pt/Al2O3 Nanosystem with Metal-Oxide Active Component. Nucl. Instrum. Methods Phys. Res. Sect. A 2009, 603, 108–110. [Google Scholar] [CrossRef]
- Beck, I.E.; Kriventsov, V.V.; Fedotov, M.A.; Bukhtiyarov, V.I. Unique Stability of μ-Hydroxo Ligands in Pt(IV) Complexes Towards Alkaline Hydrolysis. Nucl. Instrum. Methods Phys. Res. Sect. A 2009, 603, 182–184. [Google Scholar] [CrossRef]
- Beck, I.E.; Kriventsov, V.V.; Ivanov, D.P.; Yakimchuk, E.P.; Novgorodov, B.N.; Zaikovskii, V.I.; Bukhtiyarov, V.I. Study of the Local Structure of Supported Nanostructural Platinum Catalysts. J. Struct. Chem. 2010, 51 (Suppl. S1), S11–S19. [Google Scholar] [CrossRef]
- Buyanova, N.E.; Ibragimova, N.B.; Karnaukhov, A.P.; Koroleva, N.G. Separate determination of the surface of complex catalysts by chromatographic methods. III. Palladium on aluminum oxide. Kinet. Catal. 1972, 13, 1295–1302. (In Russian) [Google Scholar]
- Gupta, S.; Qiao, L.; Zhao, S.; Xu, H.; Lin, Y.; Devaguptapu, S.V.; Wang, X.; Swihart, M.T.; Wu, G. Highly Active and Stable Graphene Tubes Decorated with FeCoNi Alloy Nanoparticles via a Template-Free Graphitization for Bifunctional Oxygen Reduction and Evolution. Adv. Energy Mater. 2016, 6, 1601198. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, X.; Chu, Y.; Wang, L.; Kang, W.; Wei, D.; Li, H.; Xiong, S. Nitrogen/oxygen co-doped monolithic carbon electrodes derived from melamine foam for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 17730. [Google Scholar] [CrossRef]
- Wu, P.; Qian, Y.; Du, P.; Zhang, H.; Cai, C. Facile synthesis of nitrogen-doped graphene for measuring the releasing process of hydrogen peroxide from living cells. J. Mater. Chem. 2012, 22, 6402. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S.; Paukshtis, E.A.; Svintsitskiy, D.A.; Ismagilov, Z.R.; Parmon, V.N. Surface Modification of Single-Walled Carbon Nanotubes by Functional Nitrogen-Containing Groups and Study of Their Properties. Dokl. Phys. Chem. 2017, 476, 186–189. [Google Scholar] [CrossRef]
- Arrigo, R.; Schuster, M.E.; Xie, Z.; Yi, Y.; Wowsnick, G.; Sun, L.L.; Hermann, K.E.; Friedrich, M.; Kast, P.; Hävecker, M.; et al. Nature of the N–Pd Interaction in Nitrogen-Doped Carbon Nanotube Catalysts. ACS Catal. 2015, 5, 2740. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-doped graphene and its electrochemical applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Li, D.; Yu, C.; Wang, M.; Zhang, Y.; Pan, C. Synthesis of nitrogen doped graphene from graphene oxide within an ammonia flame for high performance supercapacitors. RSC Adv. 2014, 4, 55394. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S.; Svintsitskiy, D.A.; Gerasimov, E.Y.; Parmon, V.N. A Method for Synthesis of Nitrogen-Doped Graphene with High Specific Surface Area. Dokl. Phys. Chem. 2020, 495, 159–165. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S.; Bedilo, A.F.; Shuvarakova, E.I. Synthesis of Carbon-Mineral Composites and Graphene, Fullerenes. Nanotub. Carbon Nanostruct. 2020, 28, 402–406. [Google Scholar] [CrossRef]
- Santiago, S.R.M.; Wong, Y.-A.; Lin, T.-N.; Chang, C.-H.; Yuan, C.-T.; Shen, J.-L. Effect of nitrogen doping on the photoluminescence intensity of graphene quantum dots. Optics Lett. 2017, 42, 3642–3645. [Google Scholar] [CrossRef] [PubMed]
- NIST Crystallographic Data Center. ICSD Code: 76153 for Bulk Metal Pt0; NIST Crystallographic Data Center: Gaithersburg, MD, USA, 2008. Available online: https://www.nist.gov/programs-projects/crystallographic-databases (accessed on 7 August 2022).
- Lu, B.; Liu, Q.; Nichols, F.; Mercado, R.; Morris, D.; Li, N.; Zhang, P.; Gao, P.; Ping, Y.; Chen, S. Oxygen Reduction Reaction Catalyzed by Carbon-Supported Platinum Few-Atom Clusters: Significant Enhancement by Doping of Atomic Cobalt. Research 2020, 2020, 9167829. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, M.A.; Selyutin, A.G.; Ishchenko, A.V.; Lisitsyn, A.S.; Koltunov, K.Y.; Sobolev, V.I. Co/multi-walled carbon nanotubes as highly efficient catalytic nanoreactor for hydrogen production from formic acid. Int. J. Hydrogen Energy 2020, 45, 19420–19430. [Google Scholar] [CrossRef]
- Tang, C.; Surkus, A.E.; Pohl, M.M.; Agostini, G.; Schneider, M.; Junge, H.; Beller, M. A stable nanocobalt catalyst with highly dispersed CoNx active sites for the selective dehydrogenation of formic acid. Angew. Chem. Int. Ed. 2017, 56, 16616–16620. [Google Scholar] [CrossRef] [PubMed]
- Bulushev, D.A.; Bulusheva, L.G. Catalysts with single metal atoms for the hydrogen production from formic acid. Catal. Rev. 2021, 1–40. [Google Scholar] [CrossRef]
- Podyacheva, O.; Bulushev, D.; Suboch, A.; Svintsitskiy, D.; Lisitsyn, A.; Modin, E.; Chuvilin, A.; Gerasimov, E.; Sobolev, V.; Parmon, V. Highly Stable Single-atom Catalyst with Ion ic Pd Active Sites Supported on N-doped Carbon Nanotubes for Formic Acid Decomposition. ChemSusChem 2018, 11, 3724–3727. [Google Scholar] [CrossRef]
- Podyacheva, O.; Lisitsyn, A.; Kibis, L.; Boronin, A.; Stonkus, O.; Zaikovskii, V.; Suboch, A.; Sobolev, V.; Parmon, V. Nitrogen Doped Carbon Nanotubes and Nanofibers for Green Hydrogen Production: Similarities in the Nature of Nitrogen Species, Metal–Nitrogen Interaction, and Catalytic Properties. Energies 2019, 12, 3976. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S.; Bedilo, A.F.; Shuvarakova, E.I.; Parmon, V.N. Template Synthesis of Graphene. Dokl. Phys. Chem. 2019, 488, 154–157. [Google Scholar] [CrossRef]
- Buyanov, R.A. Zakoksovanie katalizatorov (Catalyst Coking); Nauka, M., Ed.; Nauka: Novosibirsk, Russia, 1983; p. 207. (In Russian) [Google Scholar]
- Available online: http://xpspeak.software.informer.com/4.1/ (accessed on 7 August 2022).
- Levichev, E.B. Status and perspectives of VEPP-4 complex. In Particles and Nuclei, Letters; Nauka: Moscow, Russia, 2016; p. 13. (In Russian) [Google Scholar]
- Kochubey, D.I. EXAFS Spectroscopy of Catalysts; Nauka: Novosibirsk, Russia, 1992. (In Russian) [Google Scholar]
- Klementev, K. Extraction of the fine structure from x-ray absorption spectra. J. Phys. D Appl. Phys. 2001, 34, 209–217. [Google Scholar] [CrossRef]
- Binsted, N.; Campbell, J.V.; Gurman, S.J.; Stephenson, P.C. SERC Daresbury Laboratory EXCURV92 Program; Daresbury Laboratory: Warrington, UK, 1991. [Google Scholar]
- Kaprielova, K.M.; Yakovina, O.A.; Ovchinnikov, I.I.; Koscheev, S.V.; Lisitsyn, A.S. Preparation of platinum-on-carbon catalysts via hydrolytic deposition: Factors influencing the deposition and catalytic properties. Appl. Catal. A 2012, 449, 203–214. [Google Scholar] [CrossRef]
- Gor, G.Y.; Thommes, M.; Cychosz, K.A.; Neimark, A.V. Quenched Solid Density Functional Theory Method for Characterization of Mesoporous Carbons by Nitrogen Adsorption. Carbon 2012, 50, 1583–1590. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]

| Sample | The Average Pt Particle Size, nm | |
|---|---|---|
| From CO Chemisorption | From STEM HAADF | |
| 0.2% Pt/N-graphene (Pt(NO3)4) | 10 | 12 |
| 1% Pt/N-graphene (Pt(NO3)4) | 15 | 16 |
| Sample | CO/Pt, % | The Average Pt Particle Size, nm | |
|---|---|---|---|
| From CO Chemisorption | From STEM | ||
| 0.2% Pt/N-graphene (H2PtCl6) | 2.0 | 50 | 0.2 |
| 0.4% Pt/N-graphene (H2PtCl6) | 3.0 | 33 | 0.2 |
| 1% Pt/N-graphene (H2PtCl6) | 5.9 | 17 | 0.2 |
| 1% Pt/graphene (H2PtCl6) | 24.4 | 2.5 | 3 |
| Catalyst | T50%, °C | ||
|---|---|---|---|
| Precursor | |||
| Without Pt | Pt (NO3)4 | H2PtCl6 | |
| 1% Pt/N-graphene | — | 170 | 151 |
| 0.4% Pt/N-graphene | — | — | 178 |
| 0.2% Pt/N-graphene | — | 213 | 200 |
| N-graphene | 245 | — | — |
| Catalysts | TOF, s−1 |
|---|---|
| 0.2% Pd/N-CNTs | 0.07 |
| 1% Pd/N-CNTs | 0.21 |
| 1% Pd/N-C | 0.28 |
| 0.2% Pt/N-CNTs pr | 0.21 |
| 0.2% Pt/N-CNTs imp | 0.26 |
| 0.3% Pt/N-CNFs | 0.25 |
| 1% Pt/N-CNFs | 0.24 |
| 1% Pt/N-graphene | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chesnokov, V.V.; Kriventsov, V.V.; Prosvirin, I.P.; Gerasimov, E.Y. Effect of Platinum Precursor on the Properties of Pt/N-Graphene Catalysts in Formic Acid Decomposition. Catalysts 2022, 12, 1022. https://doi.org/10.3390/catal12091022
Chesnokov VV, Kriventsov VV, Prosvirin IP, Gerasimov EY. Effect of Platinum Precursor on the Properties of Pt/N-Graphene Catalysts in Formic Acid Decomposition. Catalysts. 2022; 12(9):1022. https://doi.org/10.3390/catal12091022
Chicago/Turabian StyleChesnokov, Vladimir V., Vladimir V. Kriventsov, Igor P. Prosvirin, and Evgeny Yu. Gerasimov. 2022. "Effect of Platinum Precursor on the Properties of Pt/N-Graphene Catalysts in Formic Acid Decomposition" Catalysts 12, no. 9: 1022. https://doi.org/10.3390/catal12091022
APA StyleChesnokov, V. V., Kriventsov, V. V., Prosvirin, I. P., & Gerasimov, E. Y. (2022). Effect of Platinum Precursor on the Properties of Pt/N-Graphene Catalysts in Formic Acid Decomposition. Catalysts, 12(9), 1022. https://doi.org/10.3390/catal12091022










