Defect Engineering and Surface Polarization of TiO2 Nanorod Arrays toward Efficient Photoelectrochemical Oxygen Evolution
Abstract
:1. Introduction
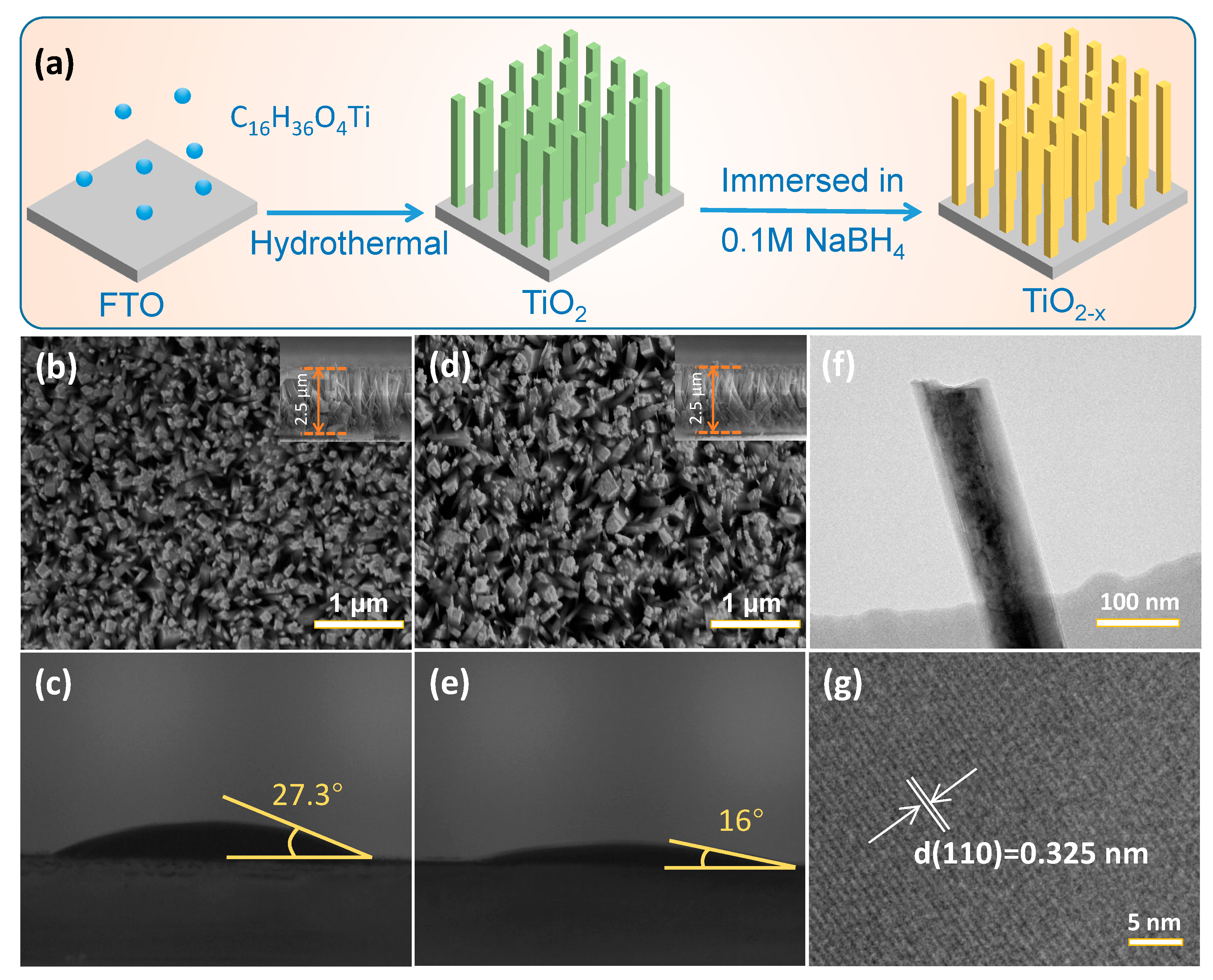
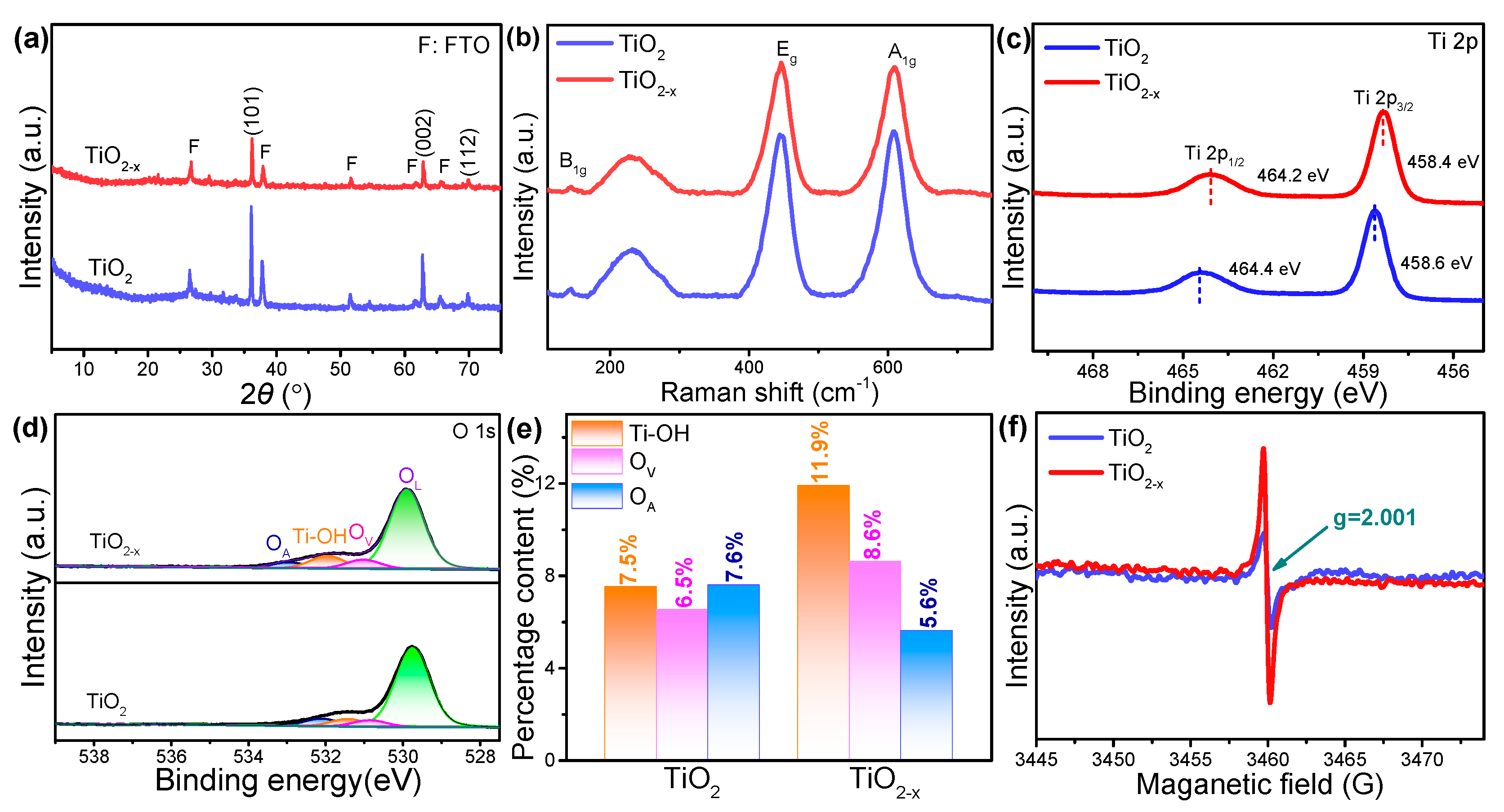
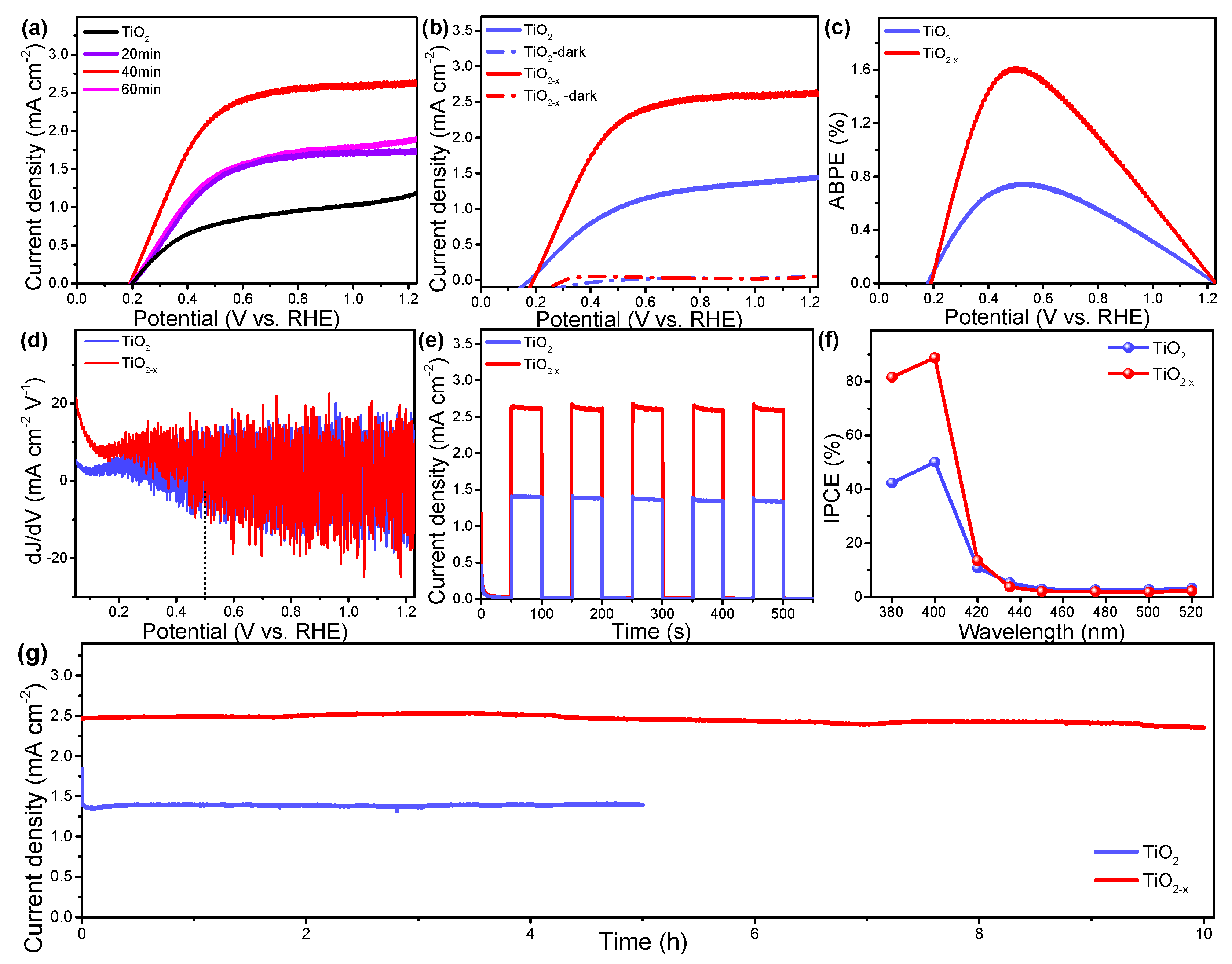
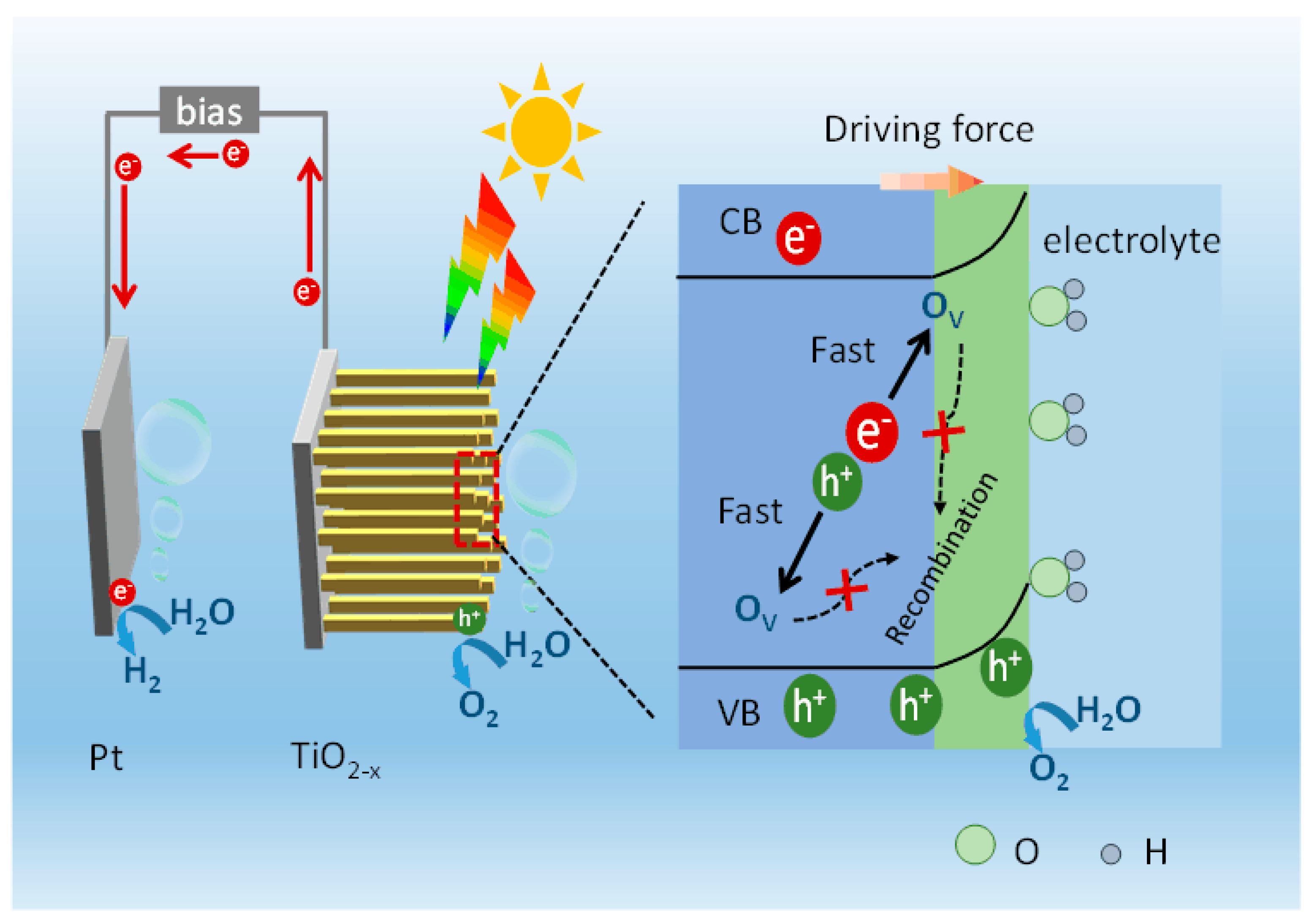
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials Synthesis
4.2. Material Characterization
4.3. Photoelectrochemical Measurements
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, J.; Li, C.; Wasielewski, M.R. Advances in solar energy conversion. Chem. Soc. Rev. 2019, 48, 1862–1864. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.S.; Shen, S. Catalysing artificial photosynthesis. Nat. Photonics 2013, 7, 944–946. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splittin. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.C.D.; Viswanathan, P.; Yadav, H.M.; Yoo, K.; Kang, H.C.; Lee, J.-J. Stand-Alone Photoelectrochemical Energy Conversions. Solar RRL 2021, 5, 2000517. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.-G.; Hua, W.; Ren, L.; Sun, H.; Hou, Z.; Huyan, Y.; Cao, Y.; Wei, C.; Kang, F. Navigating fast and uniform zinc deposition via a versatile metal-organic complex interphase. Energy Environ. Sci. 2022, 15, 1872–1881. [Google Scholar] [CrossRef]
- Yadav, H.M.; Thorat, N.D.; Yallapu, M.M.; Tofail, S.A.M.; Kim, J.S. Functional TiO2 nanocoral architecture for light-activated cancer chemotherapy. J. Mater. Chem. B 2017, 5, 1461–1470. [Google Scholar] [CrossRef]
- Khan Shahed, U.M.; Al-Shahry, M.; Ingler William, B. Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Butburee, T.; Bai, Y.; Wang, H.; Chen, H.; Wang, Z.; Liu, G.; Zou, J.; Khemthong, P.; Lu, G.Q.M.; Wang, L. 2D Porous TiO2 Single-Crystalline Nanostructure Demonstrating High Photo-Electrochemical Water Splitting Performance. Adv. Mater. 2018, 30, 1705666. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Xu, Y.; Feng, Z.; Huang, J. Defect-assisted surface modification enhances the visible light photocatalytic performance of g-C3N4@C-TiO2 direct Z-scheme heterojunctions. Chin. J. Catal. 2019, 40, 424–433. [Google Scholar] [CrossRef]
- Pal, D.; Sarkar, A.; Ghosh, N.G.; Sanke, D.M.; Maity, D.; Karmakar, K.; Sarkar, D.; Zade, S.S.; Khan, G.G. Integration of LaCo(OH)x photo-electrocatalyst and plasmonic gold nanoparticles with Sb-doped TiO2 Nanorods for photoelectrochemical water oxidation. ACS Appl. Nano Mater. 2021, 4, 6111–6123. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Y.; Liu, X.; Wang, W.; Zhan, P.; Li, Z.; Zhang, Z. Visible Light Photoelectrochemical Properties of N-Doped TiO2 Nanorod Arrays from TiN. J. Nanomater. 2013, 2013, 1–8. [Google Scholar]
- Cheng, C.; Sun, Y. Carbon doped TiO2 nanowire arrays with improved photoelectrochemical water splitting performance. Appl. Surf. Sci. 2012, 263, 273–276. [Google Scholar] [CrossRef]
- Lu, R.; Wei, Y.; Chen, C.; Wu, T. Visible-light-driven photoelectrochemical water oxidation with Al doped TiO2 nanorod arrays. J. Alloy. Compd. 2019, 790, 99–108. [Google Scholar] [CrossRef]
- Ren, X.; Ji, Y.; Zhai, Y.; Yuan, N.; Ding, J.; Li, Y.; Yan, J.; Liu, S.F. Self-assembled CoOOH on TiO2 for enhanced photoelectrochemical water oxidation. J. Energy Chem. 2021, 60, 512–521. [Google Scholar] [CrossRef]
- Khnayzer, R.S.; Mara, M.W.; Huang, J.; Shelby, M.L.; Chen, L.X.; Castellano, F.N. Structure and Activity of Photochemically Deposited “CoPi” Oxygen Evolving Catalyst on Titania. ACS Catal. 2012, 2, 2150–2160. [Google Scholar] [CrossRef]
- Rahman, M.A.; Bazargan, S.; Srivastava, S.; Wang, X.; Abd-Ellah, M.; Thomas, J.P.; Heinig, N.F.; Pradhan, D.; Leung, K.T. Defect-rich decorated TiO2 nanowires for super-efficient photoelectrochemical water splitting driven by visible light. Energy Environ. Sci. 2015, 8, 3363–3373. [Google Scholar] [CrossRef]
- Liang, J.; Wang, N.; Zhang, Q.; Liu, B.; Kong, X.; Wei, C.; Zhang, D.; Yan, B.; Zhao, Y.; Zhang, X. Exploring the mechanism of a pure and amorphous black-blue TiO2:H thin film as a photoanode in water splitting. Nano Energy 2017, 42, 151–156. [Google Scholar] [CrossRef]
- Chen, F.; Huang, H.; Guo, L.; Zhang, Y.; Ma, T. The Role of Polarization in Photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 10061–10073. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Kang, L.; Huang, H.; Ye, L.; Han, K.; Yang, S.; Yu, H.; Batmunkh, M.; Zhang, Y.; Ma, T. Surface-Halogenation-Induced Atomic-Site Activation and Local Charge Separation for Superb CO2 Photoreduction. Adv. Mater. 2019, 31, 1900546. [Google Scholar] [CrossRef]
- Ma, W.; Huang, K.; Wu, X.; Wang, M.; Feng, S. Surface polarization enables high charge separation in TiO2 nanorod photoanode. Nano Res. 2021, 14, 4056–4062. [Google Scholar] [CrossRef]
- Hosono, E.; Fujihara, S.; Kakiuchi, K.; Imai, H. Growth of Submicrometer-Scale Rectangular Parallelepiped Rutile TiO2 Films-in Aqueous TiCl3 Solutions under Hydrothermal Conditions. J. Am. Chem. Soc. 2004, 126, 7790–7791. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, X.; Li, Z.; Wang, J.H.; Zhu, L.; Yin, C.; Wang, Y.; Li, X.B.; Liu, Z.; Zhang, J.; et al. Superhydrophilic Graphdiyne Accelerates Interfacial Mass/Electron Transportation to Boost Electrocatalytic and Photoelectrocatalytic Water Oxidation Activity. Adv. Funct. Mater. 2019, 29, 1808079. [Google Scholar] [CrossRef]
- Wu, F.; Yu, Y.; Yang, H.; German, L.N.; Li, Z.; Chen, J.; Yang, W.; Huang, L.; Shi, W.; Wang, L.; et al. Simultaneous Enhancement of Charge Separation and Hole Transportation in a TiO2-SrTiO3 Core-Shell Nanowire Photoelectrochemical System. Adv. Mater. 2017, 29, 1701432. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Peng, S.; Bao, Y.; Wang, Y.; Lei, B.; Wang, Z.; Huang, Z.; Gao, Y. Control of interface between anatase TiO2 nanoparticles and rutile TiO2 nanorods for efficient photocatalytic H2 generation. J. Power Sources 2018, 376, 11–17. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Raman spectra of titanium dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Choi, H.C.; Jung, Y.M.; Kim, S.B. Size effects in the Raman spectra of TiO2 nanoparticles. Vib. Spectrosc. 2005, 37, 33–38. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Lu, X.; Qian, F.; Tong, Y.; Zhang, J.Z.; Lordi, V.; Rocha Leao, C.; Li, Y. Computational and Photoelectrochemical Study of Hydrogenated Bismuth Vanadate. J. Phys. Chem. C 2013, 117, 10957–10964. [Google Scholar] [CrossRef]
- Wang, S.; Chen, P.; Yun, J.H.; Hu, Y.; Wang, L. An Electrochemically Treated BiVO4 Photoanode for Efficient Photoelectrochemical Water Splitting. Angew. Chem. Int. 2017, 56, 8500–8504. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, J.G.; Sun, H.H.; Wei, B. Heterostructured TiO2/NiTiO3 nanorod arrays for inorganic sensitized solar cells with significantly enhanced photovoltaic performance and stability. ACS Appl. Mater. Interfaces 2018, 10, 11580–11586. [Google Scholar] [CrossRef]
- Zheng, L.; Ye, X.; Deng, X.; Wang, Y.; Zhao, Y.; Shi, X.; Zheng, H. Black Phosphorus Quantum Dot-Sensitized TiO2 Nanotube Arrays with Enriched Oxygen Vacancies for Efficient Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 15906–15914. [Google Scholar] [CrossRef]
- Zhou, B.X.; Ding, S.S.; Wang, Y.; Wang, X.R.; Huang, W.Q.; Li, K.; Huang, G.F. Type-II/type-II band alignment to boost spatial charge separation: A case study of g-C3N4 quantum dots/a-TiO2/r-TiO2 for highly efficient photocatalytic hydrogen and oxygen evolution. Nanoscale 2020, 12, 6037–6046. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. J. Mater. Chem. A 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Wang, B.; Yang, S. Constructing the Fe/Cr double (oxy)hydroxides on Fe3O4 for boosting the electrochemical oxygen evolution in alkaline seawater and domestic sewage. Appl. Catal. B Environ. 2022, 302, 120847. [Google Scholar] [CrossRef]
- Liu, Q.-Y.; Wang, H.-D.; Tang, R.; Cheng, Q.; Yuan, Y.-J. Rutile TiO2 nanoparticles with oxygen vacancy for photocatalytic nitrogen fixation. ACS Appl. Nano Mater. 2021, 4, 8674–8679. [Google Scholar] [CrossRef]
- Zhang, R.; Bi, L.; Wang, D.; Lin, Y.; Zou, X.; Xie, T. An effective method to understand photo-generated charge transfer processes of Z-scheme Ti/α-Fe2O3/g-C3N4 photocatalysts for hydrogen evolution. Catal. Commun. 2020, 142, 106028. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, J.; Wang, Y.; Wu, M.; Meng, M.; Tian, Y.; Li, X.; Zhang, J.; Zheng, L.; Jiang, Z.; et al. Insights into the effects of surface/bulk defects on photocatalytic hydrogen evolution over TiO2 with exposed {001} facets. Appl. Catal. B Environ. 2018, 220, 126–136. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, J.-H.; Jo, Y.-M.; Lee, J.-H. Heterojunction between bimetallic metal-organic framework and TiO2: Band-structure engineering for effective photoelectrochemical water splitting. Nano Research 2022, 15, 8502–8509. [Google Scholar] [CrossRef]
- Dong, G.; Cheng, X.; Bi, Y. Hierarchical TiO2 photoanodes with spatial charge separation for efficient oxygen evolution reaction. Solar RRL 2020, 5, 2000449. [Google Scholar] [CrossRef]
- Cheng, X.; Dong, G.; Zhang, Y.; Feng, C.; Bi, Y. Dual-bonding interactions between MnO2 cocatalyst and TiO2 photoanodes for efficient solar water splitting. Appl. Catal. B Environ. 2020, 267, 118723. [Google Scholar] [CrossRef]
- Xiao, J.; Zhao, F.; Zhong, J.; Huang, Z.; Fan, L.; Peng, L.; Zhou, S.-F.; Zhan, G. Performance enhancement of hematite photoanode with oxygen defects for water splitting. Chem. Eng. J. 2020, 402, 126163. [Google Scholar] [CrossRef]
- Sun, H.; Hua, W.; Li, Y.; Wang, J.-G. Conformal coating of superhydrophilic metal-organic complex toward substantially improved photoelectrochemical water oxidation. Chem. Eng. J. 2022, 427, 131004. [Google Scholar] [CrossRef]
- Kim Tae, W.; Choi, K.-S. Nanoporous BiVO4 photoanodes with Dual-Layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, L.; Ming, J.; Liu, B.; Zhao, Y.; Hou, Y.; Ding, Z.; Xu, C.; Zhang, Z.; Long, J. Amorphous Ta2OxNy-enwrapped TiO2 rutile nanorods for enhanced solar photoelectrochemical water splitting. Appl. Catal. B Environ. 2019, 243, 481–489. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Dong, G.; Zhang, Y.; Lu, G.; Bi, Y. Homostructural Ta3N5 nanotube/nanoparticle photoanodes for highly efficient solar-driven water splitting. Appl. Catal. B Environ. 2020, 277, 119217. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.-G.; Fan, Y.; Sun, H.; Hua, W.; Liu, H.; Wei, B. Plasmonic TiN boosting nitrogen-doped TiO2 for ultrahigh efficient photoelectrochemical oxygen evolution. Appl. Catal. B Environ. 2019, 246, 21–29. [Google Scholar] [CrossRef]
- Zhou, B.X.; Ding, S.S.; Zhang, B.J.; Xu, L.; Chen, R.S.; Luo, L.; Huang, W.Q.; Xie, Z.; Pan, A.; Huang, G.F. Dimensional transformation and morphological control of graphitic carbon nitride from water-based supramolecular assembly for photocatalytic hydrogen evolution: From 3D to 2D and 1D nanostructures. Appl. Catal. B Environ. 2019, 254, 321–328. [Google Scholar] [CrossRef]
- Bae, S.; Kim, D.; Kim, H.; Gu, M.; Ryu, J.; Kim, B.S. Modulating Charge Separation Efficiency of Water Oxidation Photoanodes with Polyelectrolyte-Assembled Interfacial Dipole Layers. Adv. Funct. Mater. 2019, 30, 1908492. [Google Scholar] [CrossRef]
- Zhang, B.; Chou, L.; Bi, Y. Tuning surface electronegativity of BiVO4 photoanodes toward high-performance water splitting. Appl. Catal. B Environ. 2020, 262, 118267. [Google Scholar] [CrossRef]
- Zhou, T.; Li, L.; Li, J.; Wang, J.; Bai, J.; Xia, L.; Xu, Q.; Zhou, B. Electrochemically reduced TiO2 photoanode coupled with oxygen vacancy-rich carbon quantum dots for synergistically improving photoelectrochemical performance. Chem. Eng. J. 2021, 425, 131770. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Tian, Y.; Dang, K.; Zhang, Q.; Shen, Z.; Zhan, S. Conjugated pi electrons of MOFs drive charge separation at heterostructures interface for enhanced photoelectrochemical water oxidation. Small 2021, 17, 2100367. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zang, W.; Kou, Z.; Zhang, L.; Xu, G.; Lv, J.; Gao, X.; Pan, Z.; Wang, J.; Wu, Y. Assembling of Bi atoms on TiO2 nanorods boosts photoelectrochemical water splitting of semiconductors. Nanoscale 2020, 12, 4302–4308. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhu, J.; Yuan, Y.; Li, M.; Yang, Y.; Liu, Y.; Chen, M.; Cao, D.; Yan, X. Dual-regulation charge separation strategy with the synergistic effect of 1D/0D heterostructure and inserted ferroelectric layer for boosting photoelectrochemical water oxidation. J. Mater. Chem. A 2021, 9, 7594–7605. [Google Scholar] [CrossRef]
- Cui, W.; Bai, H.; Shang, J.; Wang, F.; Xu, D.; Ding, J.; Fan, W.; Shi, W. Organic-inorganic hybrid-photoanode built from NiFe-MOF and TiO2 for efficient PEC water splitting. Electrochim. Acta 2020, 349, 136383. [Google Scholar] [CrossRef]
- Wang, L.; Si, W.; Ye, Y.; Wang, S.; Hou, F.; Hou, X.; Cai, H.; Dou, S.X.; Liang, J. Cu-Ion-Implanted and Polymeric Carbon Nitride-Decorated TiO2 Nanotube Array for Unassisted Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 44184–44194. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Chen, D.; Guo, Z.; Ruan, M. Oxygen vacancies engineering in TiO2 homojunction/ZnFe-LDH for enhanced photoelectrochemical water oxidation. Chem. Eng. J. 2020, 395, 125101. [Google Scholar] [CrossRef]
- Huang, C.; Bian, J.; Guo, Y.; Huang, M.; Zhang, R.-Q. Thermal vacuum de-oxygenation and post oxidation of TiO2 nanorod arrays for enhanced photoelectrochemical properties. J. Mater. Chem. A 2019, 7, 5434–5441. [Google Scholar] [CrossRef]
- Dong, Z.; Cai, Y.; Zhang, K.; Chu, Z.; Han, S.; Li, Z. Electrochemical reduction induced self-doping of oxygen vacancies into Ti–Si–O nanotubes as efficient photoanode for boosted photoelectrochemical water splitting. Int. J. Hydrogen Energy 2021, 46, 3554–3564. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liang, S.; Sun, H.; Hua, W.; Wang, J.-G. Defect Engineering and Surface Polarization of TiO2 Nanorod Arrays toward Efficient Photoelectrochemical Oxygen Evolution. Catalysts 2022, 12, 1021. https://doi.org/10.3390/catal12091021
Li Y, Liang S, Sun H, Hua W, Wang J-G. Defect Engineering and Surface Polarization of TiO2 Nanorod Arrays toward Efficient Photoelectrochemical Oxygen Evolution. Catalysts. 2022; 12(9):1021. https://doi.org/10.3390/catal12091021
Chicago/Turabian StyleLi, Yueying, Shiyu Liang, Huanhuan Sun, Wei Hua, and Jian-Gan Wang. 2022. "Defect Engineering and Surface Polarization of TiO2 Nanorod Arrays toward Efficient Photoelectrochemical Oxygen Evolution" Catalysts 12, no. 9: 1021. https://doi.org/10.3390/catal12091021
APA StyleLi, Y., Liang, S., Sun, H., Hua, W., & Wang, J.-G. (2022). Defect Engineering and Surface Polarization of TiO2 Nanorod Arrays toward Efficient Photoelectrochemical Oxygen Evolution. Catalysts, 12(9), 1021. https://doi.org/10.3390/catal12091021






