Explorative Sonophotocatalytic Study of C-H Arylation Reaction of Pyrazoles Utilizing a Novel Sonophotoreactor for Green and Sustainable Organic Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Developed Cleaning Bath Sonophotoreactor (CBSPR) and Its Characterization
Catalytic Activity Study
3. Experimental
3.1. General
3.2. General Procedures for Synthesized Arylated Pyrazole 3a-h Derivatives
3.2.1. Sonicated Reactions
3.2.2. Blue LED Reactions
3.2.3. Sonophotochemical Reaction
3.2.4. Reflux Condition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; p. 30. [Google Scholar]
- Wilson, K.R.; Sheldon, A.; Arends, I.; Hanefeld, U. Green Chemistry and Catalysis; Wiley-VCH: Hoboken, NJ, USA, 2007; 448p, ISBN 978-3-527-30715-9. (Hardcover). [Google Scholar]
- Smith, G.V.; Notheisz, F. Heterogeneous Catalysis in Organic Chemistry, 1st ed.; Academic Press: Cambridge, MA, USA, 1999; pp. 1–28. [Google Scholar]
- Morrell, D.G. Catalysis of Organic Reactions, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Sillanpää, M. Ultrasound Technology in Green Chemistry; SpringerBriefs in Molecular Science; Springer: Dordrecht, The Netherlands, 2011; ISBN 978-94-007-2408-2. [Google Scholar]
- Gharat, N.N.; Rathod, V.K. Ultrasound-Assisted Organic Synthesis. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Sonochemical Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–41. ISBN 9780128195406. [Google Scholar]
- Chatel, G. How Sonochemistry Contributes to Green Chemistry? Ultrason. Sonochemistry 2018, 40, 117–122. [Google Scholar] [CrossRef]
- Chatel, G. Sonochemistry—New Opportunities for Green Chemistry; Word Scientific Publishing Europe Ltd.: London, UK, 2017; p. 188. [Google Scholar]
- Cintas, P. Ultrasound and green chemistry – Further comments. Ultrason. Sonochemistry 2016, 28, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Ledari, R.; Rahimi, J.; Maleki, A. Synergistic Catalytic Effect between Ultrasound Waves and Pyrimidine-2,4-Diamine-Functionalized Magnetic Nanoparticles: Applied for Synthesis of 1,4-Dihydropyridine Pharmaceutical Derivatives. Ultrason. Sonochem. 2019, 59, 104737. [Google Scholar] [CrossRef] [PubMed]
- Mosslemin, M.H.; Nateghi, M.R. Rapid and Efficient Synthesis of Fused Heterocyclic Pyrimidines under Ultrasonic Irradiation. Ultrason. Sonochem. 2010, 17, 162–167. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z. A Highly Efficient Magnetic Solid Acid Catalyst for Synthesis of 2,4,5-Trisubstituted Imidazoles under Ultrasound Irradiation. Ultrason. Sonochem. 2013, 20, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Al-Bogami, A.S.; Saleh, T.S.; Zayed, E.M. Divergent Reaction Pathways for One-Pot, Three-Component Synthesis of Novel 4H-Pyrano[3,2-h]Quinolines under Ultrasound Irradiation. Ultrason. Sonochem. 2013, 20, 1194–1202. [Google Scholar] [CrossRef]
- Mokhtar, M.; Saleh, T.S.; Ahmed, N.S.; Al-Thabaiti, S.A.; Al-Shareef, R.A. An Eco-Friendly N-Sulfonylation of Amines Using Stable and Reusable Zn-Al-Hydrotalcite Solid Base Catalyst under Ultrasound Irradiation. Ultrason. Sonochem. 2011, 18, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, D.P.; Bareño, V.D.O.; Bosenbecker, J.; Drawanz, B.B.; Neuenfeldt, P.D.; Siqueira, G.M.; Cunico, W. Ultrasonics Promoted Synthesis of Thiazolidinones from 2-Aminopyridine and 2-Picolilamine. Ultrason. Sonochem. 2012, 19, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Hussein, E.M.; Khairou, K.S. Sonochemistry: Synthesis of Bioactive Heterocycles. Synth. Commun. 2014, 44, 2155–2191. [Google Scholar] [CrossRef]
- Chowdhury, P.; Viraraghavan, T. Sonochemical Degradation of Chlorinated Organic Compounds, Phenolic Compounds and Organic Dyes—A Review. Sci. Total Environ. 2009, 407, 2474–2492. [Google Scholar] [CrossRef]
- Mancuso, G.; Langone, M.; Andreottola, G.; Bruni, L. Effects of Hydrodynamic Cavitation, Low-Level Thermal and Low-Level Alkaline Pre-Treatments on Sludge Solubilisation. Ultrason. Sonochem. 2019, 59, 104750. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.H. The Effect of Frequency on Sonochemical Reactions III: Dissociation of Carbon Disulfide. Ultrason. Sonochem. 1997, 4, 49–54. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Power Ultrasound in Organic Synthesis: Moving Cavitational Chemistry from Academia to Innovative and Large-Scale Applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar] [CrossRef] [PubMed]
- According to ISO17025 Regulation: Instrument Calibration Services that Are Directly Traceable to the National Institute of Standards and Technology (NIST). Available online: https://acscalibration.com/calibration-services/instrument-calibration-services/ (accessed on 5 June 2022).
- Weissler, A.; Anbar, M. The Strength of Chemical Bonds; Butterworths: Oxford, UK, 1962; Volume 11. [Google Scholar]
- Aerstin, F.G.P.; Timmerhaus, K.D.; Fogler, H.S. Effect of the n Resonance Parameter on Chemical Reaction Subjected to U I Trasonic Waves. AIChE J. 1967, 13, 453–456. [Google Scholar] [CrossRef]
- Luche, J.-L. Synthetic Organic Sonochemistry; Springer: New York, NY, USA, 1998. [Google Scholar]
- Ragaini, V.; Bianchi, C.L. Catalytic reaction. In Synthetic Organic Sonochemistry; Luche, J.-L., Ed.; Plenum Press: New York, NY, USA, 1998; pp. 232–235. [Google Scholar]
- Protti, S.; Manzini, S.; Fagnoni, M.; Albini, A. The contribution of photochemistry to green chemistry. In Eco-Friendly Synthesis of Fine Chemicals; Royal Society of Chemistry: London, UK, 2009; pp. 80–111. [Google Scholar]
- Oelgemöller, M.; Jung, C.; Mattay, J. Green Photochemistry: Production of Fine Chemicals with Sunlight. Pure Appl. Chem. 2007, 79, 1939–1947. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Maptue, N.; Bondock, S.; Oelgemöller, M. The Excimer Radiation System: A Powerful Tool for Preparative Organic Photochemistry. A Technical Note. Photochem. Photobiol. Sci. 2003, 2, 450–451. [Google Scholar] [CrossRef]
- Jung, C.; Funken, K.H.; Ortner, J. PROPHIS: Parabolic Trough-Facility for Organic Photochemical Syntheses in Sunlight. Photochem. Photobiol. Sci. 2005, 4, 409–411. [Google Scholar] [CrossRef]
- Watanabe, H.; Takemoto, M.; Adachi, K.; Okuda, Y.; Dakegata, A.; Fukuyama, T.; Ryu, I.; Wakamatsu, K.; Orita, A. Syntheses of Diarylethenes by Perylene-Catalyzed Photodesulfonylation from Ethenyl Sulfones. Chem. Lett. 2020, 49, 409–412. [Google Scholar] [CrossRef]
- Rauch, M.; Schmidt, S.; Arends, I.W.C.E.; Oppelt, K.; Kara, S.; Hollmann, F. Photobiocatalytic Alcohol Oxidation Using LED Light Sources. Green Chem. 2017, 19, 376–379. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, J.; Ravva, M.K.; Gremaud, L.; Sen, S. Blue LED Mediated Intramolecular C-H Functionalization and Cyclopropanation of Tryptamines: Synthesis of Azepino[4, 5-b]Indoles and Natural Product Inspired Polycyclic Indoles. Org. Lett. 2020, 22, 4537–4541. [Google Scholar] [CrossRef]
- Zand, Z.; Kazemi, F.; Partovi, A. Photocatalytic Synthesis of Anilides from Nitrobenzenes under Visible Light Irradiation: 2 in 1 Reaction. J. Photochem. Photobiol. B Biol. 2015, 152, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.I.; Sharma, A.; Sharma, S.; Sharma, A. Visible Light-Mediated Applications of Methylene Blue in Organic Synthesis. Org. Chem. Front. 2021, 8, 1694–1718. [Google Scholar] [CrossRef]
- Li, C.H.A.; Zhou, Z.; Vashishtha, P.; Halpert, J.E. The Future Is Blue (LEDs): Why Chemistry Is the Key to Perovskite Displays. Chem. Mater. 2019, 31, 6003–6032. [Google Scholar] [CrossRef]
- Kayahan, E.; Jacobs, M.; Braeken, L.; Thomassen, L.C.J.; Kuhn, S.; van Gerven, T.; Leblebici, M.E. Dawn of a New Era in Industrial Photochemistry: The Scale-up of Micro: The Mesostructured Photoreactors. Beilstein J. Org. Chem. 2020, 16, 2484–2504. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Łomot, D.; Colmenares, J.C. When Sonochemistry Meets Heterogeneous Photocatalysis: Designing a Sonophotoreactor towards Sustainable Selective Oxidation. Green Chem. 2020, 22, 4896–4905. [Google Scholar] [CrossRef]
- Saleh, T.S.; Al-Bogami, A.S.; Mekky, A.E.M.; Alkhathlan, H.Z. Sonochemical Synthesis of Novel Pyrano[3,4-e][1,3]Oxazines: A Green Protocol. Ultrason. Sonochem. 2017, 36, 474–480. [Google Scholar] [CrossRef]
- Ahmed, N.; Saleh, T.S.; El-Mossalamy, E.-S.H. An Efficiently Sonochemical Synthesis of Novel Pyrazoles, Bipyrazoles and Pyrazol-3- YlPyrazolo[3,4-d]Pyrimidines Incorporating 1H-Benzoimidazole Moiety. Curr. Org. Chem. 2013, 17, 194–202. [Google Scholar] [CrossRef]
- Saleh, T.S.; Eldebss, T.M.A.; Albishri, H.M. Ultrasound Assisted One-Pot, Three-Components Synthesis of Pyrimido[1,2-a]Benzimidazoles and Pyrazolo[3,4-b]Pyridines: A New Access via Phenylsulfone Synthon. Ultrason. Sonochem. 2012, 19, 49–55. [Google Scholar] [CrossRef]
- Saleh, T.S.; Abd El-Rahman, N.M.; Elkateb, A.A.; Shaker, N.O.; Mahmoud, N.A.; Gabal, S.A. Ultrasound Promoted Synthesis of Some Novel Fused Pyrans. Ultrason. Sonochem. 2012, 19, 491–497. [Google Scholar] [CrossRef]
- Saleh, T.S.; Abd EL-Rahman, N.M. Ultrasound Promoted Synthesis of Substituted Pyrazoles and Isoxazoles Containing Sulphone Moiety. Ultrason. Sonochem. 2009, 16, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Abd EL-Rahman, N.M.; Saleh, T.S.; Mady, M.F. Ultrasound Assisted Synthesis of Some New 1,3,4-Thiadiazole and Bi(1,3,4-Thiadiazole) Derivatives Incorporating Pyrazolone Moiety. Ultrason. Sonochem. 2009, 16, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Gahloth, D.; Dunstan, M.S.; Quaglia, D.; Klumbys, E.; Lockhart-Cairns, M.P.; Hill, A.M.; Derrington, S.R.; Scrutton, N.S.; Turner, N.J.; Leys, D. Structures of Carboxylic Acid Reductase Reveal Domain Dynamics Underlying Catalysis. Nat. Chem. Biol. 2017, 13, 975–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budiman, Y.P.; Friedrich, A.; Radius, U.; Marder, T.B. Copper-Catalysed Suzuki-Miyaura Cross-Coupling of Highly Fluorinated Aryl Boronate Esters with Aryl Iodides and Bromides and Fluoroarene−Arene π-Stacking Interactions in the Products. ChemCatChem 2019, 11, 5387–5396. [Google Scholar] [CrossRef]
- Margulis, M.A.; Margulis, I.M. Mechanism of Sonochemical Reactions and Sonoluminescence. High Energy Chem. 2004, 38, 285–294. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Neppiras, E.A. Acoustic cavitation. Phys. Rep. 1980, 61, 159–251. [Google Scholar] [CrossRef]
- Anton, I. Cavitatian; Romanian Academy Publisher Calea: Bucharest, Romania, 1985; Volume 2. [Google Scholar]
- Zhou, Y.; Wu, J.; Lemmon, E.W. Thermodynamic Properties of Dimethyl Carbonate. J. Phys. Chem. Ref. Data 2011, 40, 043106. [Google Scholar] [CrossRef]
- Rodríguez, A.; Canosa, J.; Domínguez, A.; Tojo, J. Viscosities of Dimethyl Carbonate or Diethyl Carbonate with Alkanes at Four Temperatures. New UNIFAC-VISCO Parameters. J. Chem. Eng. Data 2003, 48, 146–151. [Google Scholar] [CrossRef]
- Mason, T.J. The design of ultrasonic reactors for environmental remediation. In Ultrasound in Environmental Protection; Elsevier: Amsterdam, The Netherlands, 2001; pp. 247–268. [Google Scholar]
- X-Ray Crystallography A Single Crystal of Compound 3c Was Obtained by Slow Evaporation from Ethanol. The Crystal Structure Was Solved and Refined Using Using CrysAlisPro Software at 296 K under the Mo Ka Radiation. The Structure Solution Was Performed Using SHELXSe2013 Method and Refined by Fullematrix Leastesquares Methods on F2 Using SHELXLe2013 Method. The Structure in This Paper Has Been Deposited with the Cambridge Crystallographic Data Centre as Supplementary Publication Number CCDC 2160317 Copies of the Data Can Be Obtained, Free of Charge. Available online: https://www.ccdc.cam.ac.uk (accessed on 2 June 2022).
- X-Ray Crystallography A Single Crystal of Compound 3e Was Obtained by Slow Evaporation from Ethanol. The Crystal Structure Was Solved and Refined Using CrysAlisPro Software at 296 K under the Mo Ka Radiation. The Structure Solution Was Performed Using SHELXSe2013 Method and Refined by Fullematrix Leastesquares Methods on F2 Using SHELXLe2013 Method. Crystallographic Data (Excluding Structure Factors) for the Structure in this Paper Has Been Deposited with the Cambridge Crystallographic Data Centre as Supplementary Publication Number CCDC 2160318 Copies of the Data Can Be Obtained, Free of Charge. Available online: https://www.ccdc.cam.ac.uk (accessed on 2 June 2022).
- El-bendary, M.M.; Saleh, T.S.; Al-Bogami, A.S. Ultrasound Assisted High-Throughput Synthesis of 1,2,3-Triazoles Libraries: A New Strategy for “Click” Copper-Catalyzed Azide-Alkyne Cycloaddition Using Copper(I/II) as a Catalyst. Catal. Lett. 2018, 148, 3797–3810. [Google Scholar] [CrossRef]
- Do, H.Q.; Khan, R.M.K.; Daugulis, O. A General Method for Copper-Catalyzed Arylation of Arene C-H Bonds. J. Am. Chem. Soc. 2008, 130, 15185–15192. [Google Scholar] [CrossRef] [Green Version]
- Gandeepan, P.; Koeller, J.; Korvorapun, K.; Mohr, J.; Ackermann, L. Sichtbares Licht Ermöglicht Ruthenium-katalysierte Meta -C-H-Alkylierung Bei Raumtemperatur. Angew. Chem. 2019, 131, 9925–9930. [Google Scholar] [CrossRef]
- Margulis, M.A. Cavitation-diffusion model of the spatial distribution of radicals in an ultrasonic field. Russ. J. Phys. Chem. 1976, 50, 534–537. [Google Scholar]
- Riyadh, S.M.; Farghaly, T.A.; Abdallah, M.A.; Abdalla, M.M.; Abd El-Aziz, M.R. New Pyrazoles Incorporating Pyrazolylpyrazole Moiety: Synthesis, Anti-HCV and Antitumor Activity. Eur. J. Med. Chem. 2010, 45, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.S.; Narasimharao, K.; Ahmed, N.S.; Basahel, S.N.; Al-Thabaiti, S.A.; Mokhtar, M. Mg-Al Hydrotalcite as an Efficient Catalyst for Microwave Assisted Regioselective 1,3-Dipolar Cycloaddition of Nitrilimines with the Enaminone Derivatives: A Green Protocol. J. Mol. Catal. A Chem. 2013, 367, 12–22. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Abdel, E.; Hafez, A. 1,3-Dipolar Cycloadditions of Some Nitrilimines and Nitrile Oxides to 3-N,N-Dimethylamino-1-Oxopropene Derivatives. J. Chem. Res. Synop. 1999, 360–361. [Google Scholar] [CrossRef]
- Agilent, C.P. Agilent Technologies. Yarnton, England. 2012. Available online: https://www.agilent.com/cs/library/usermanuals/Public/Autochem_User_Manual.pdf (accessed on 5 June 2022).
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2018, A64, 112. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
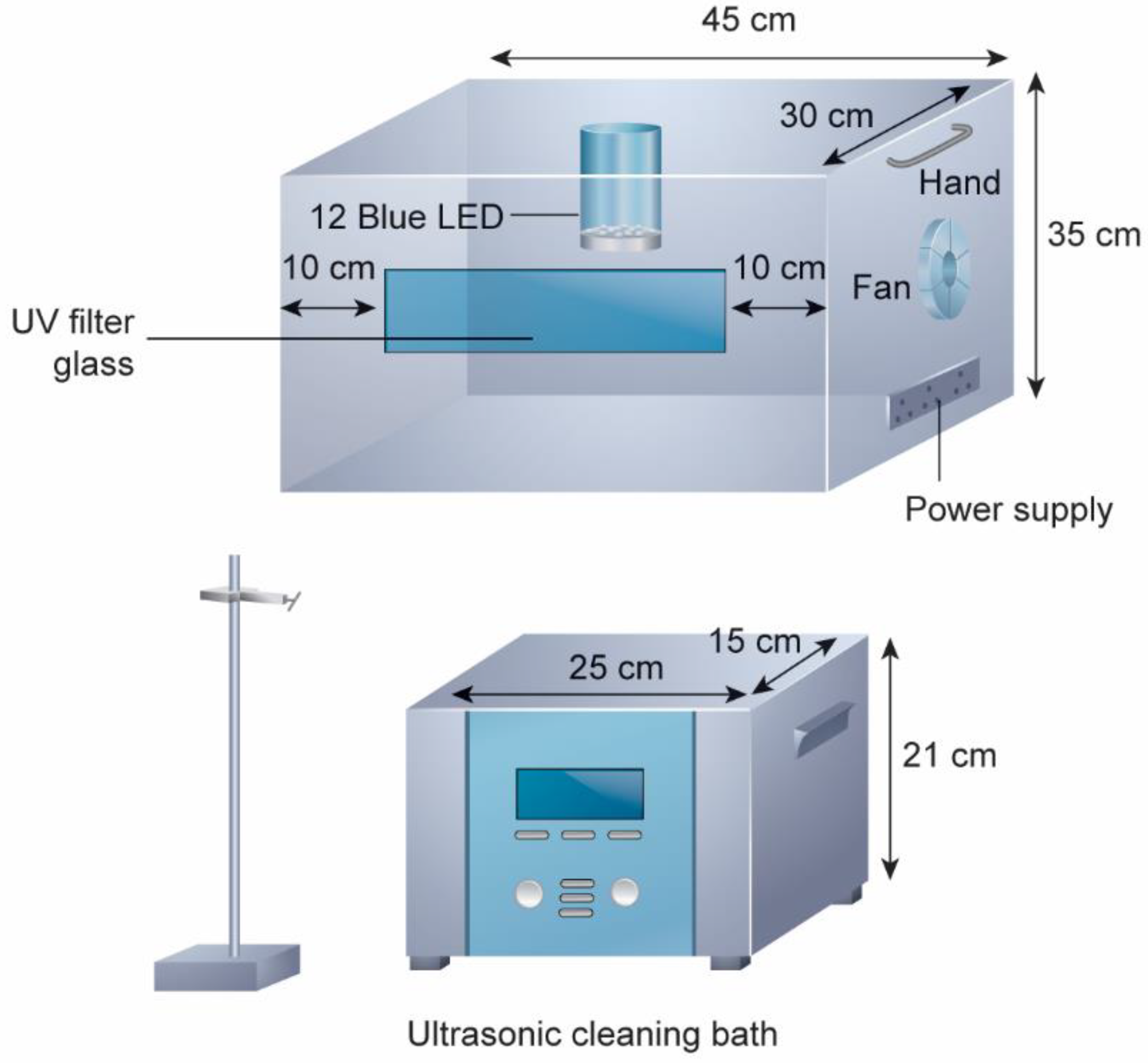
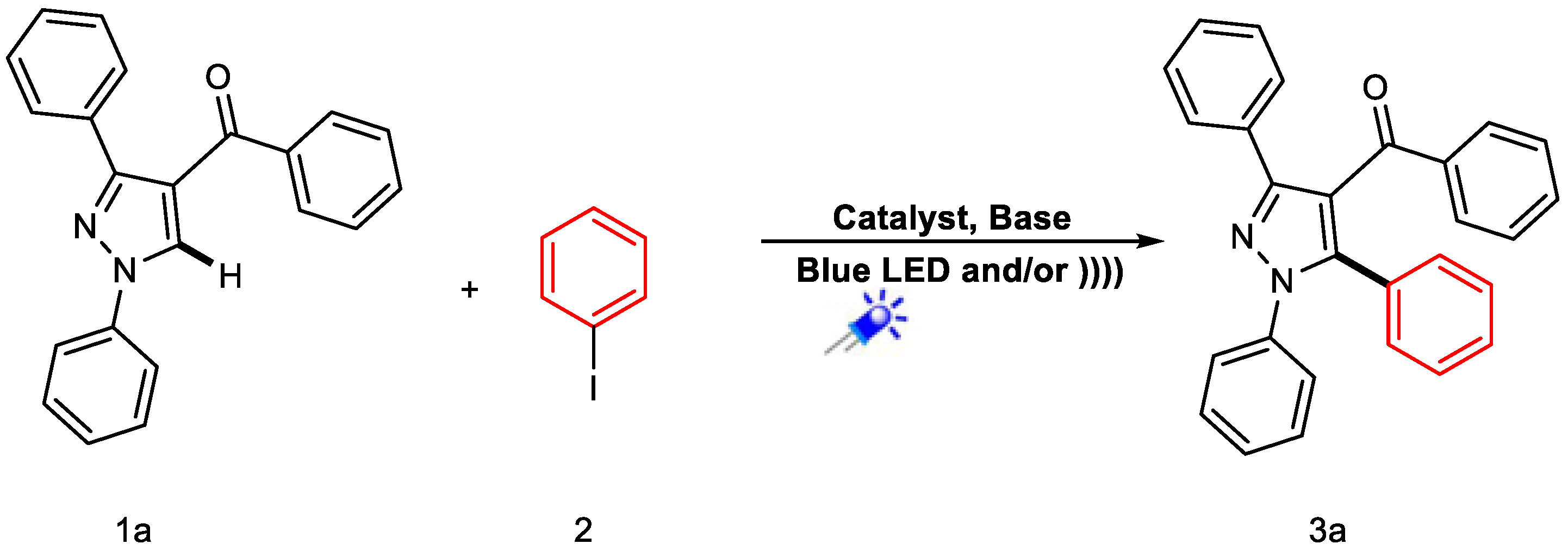
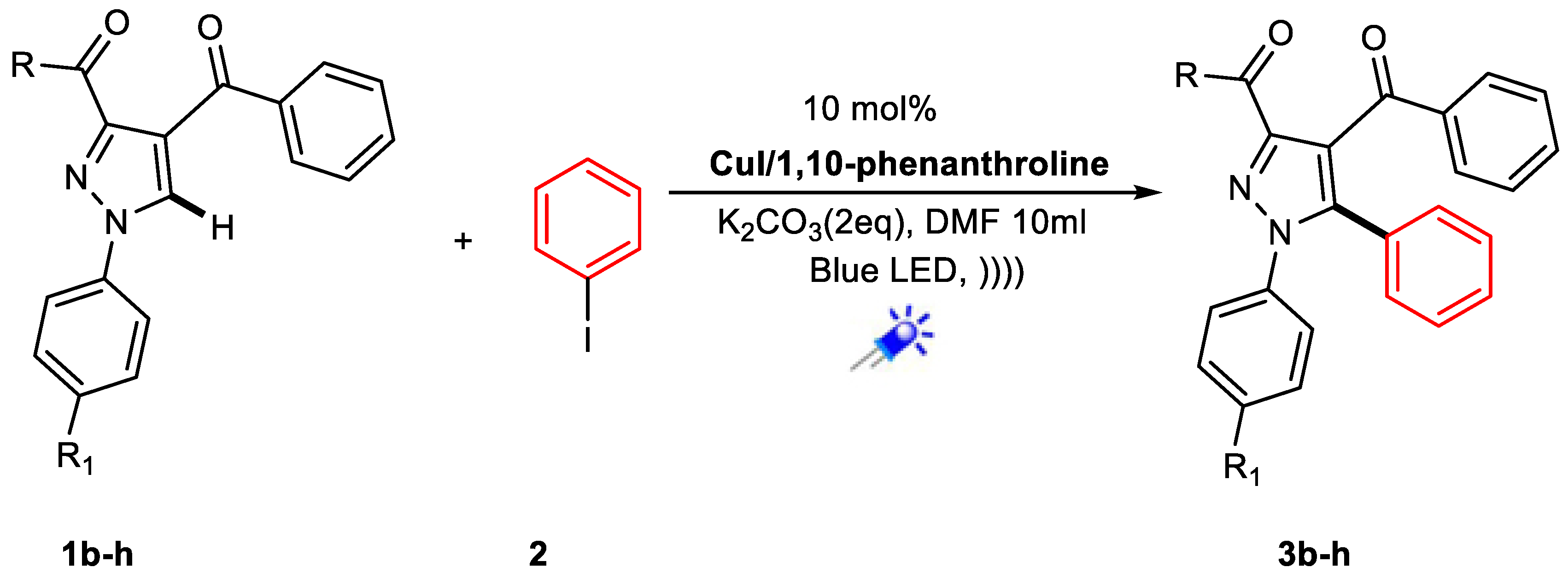
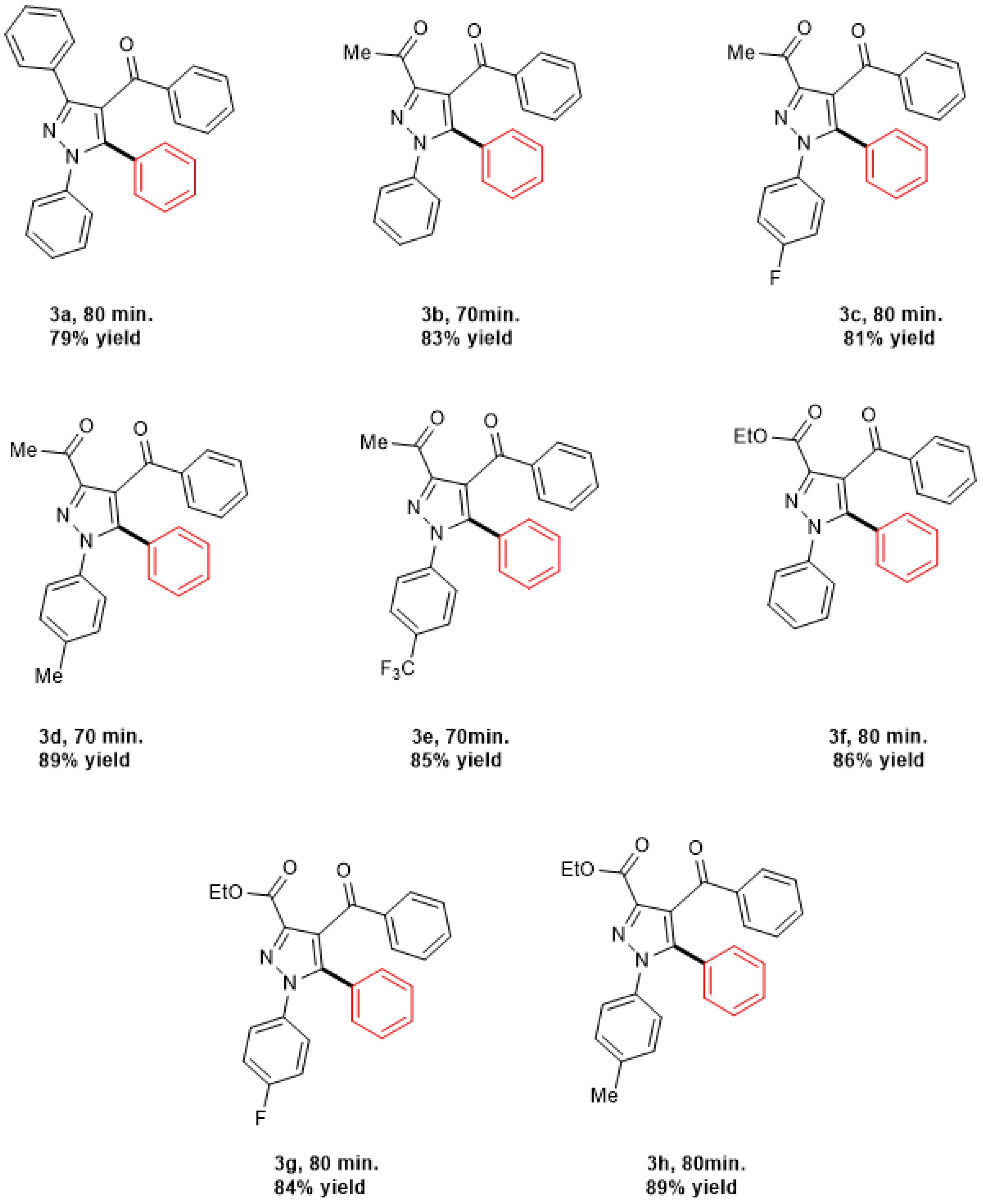
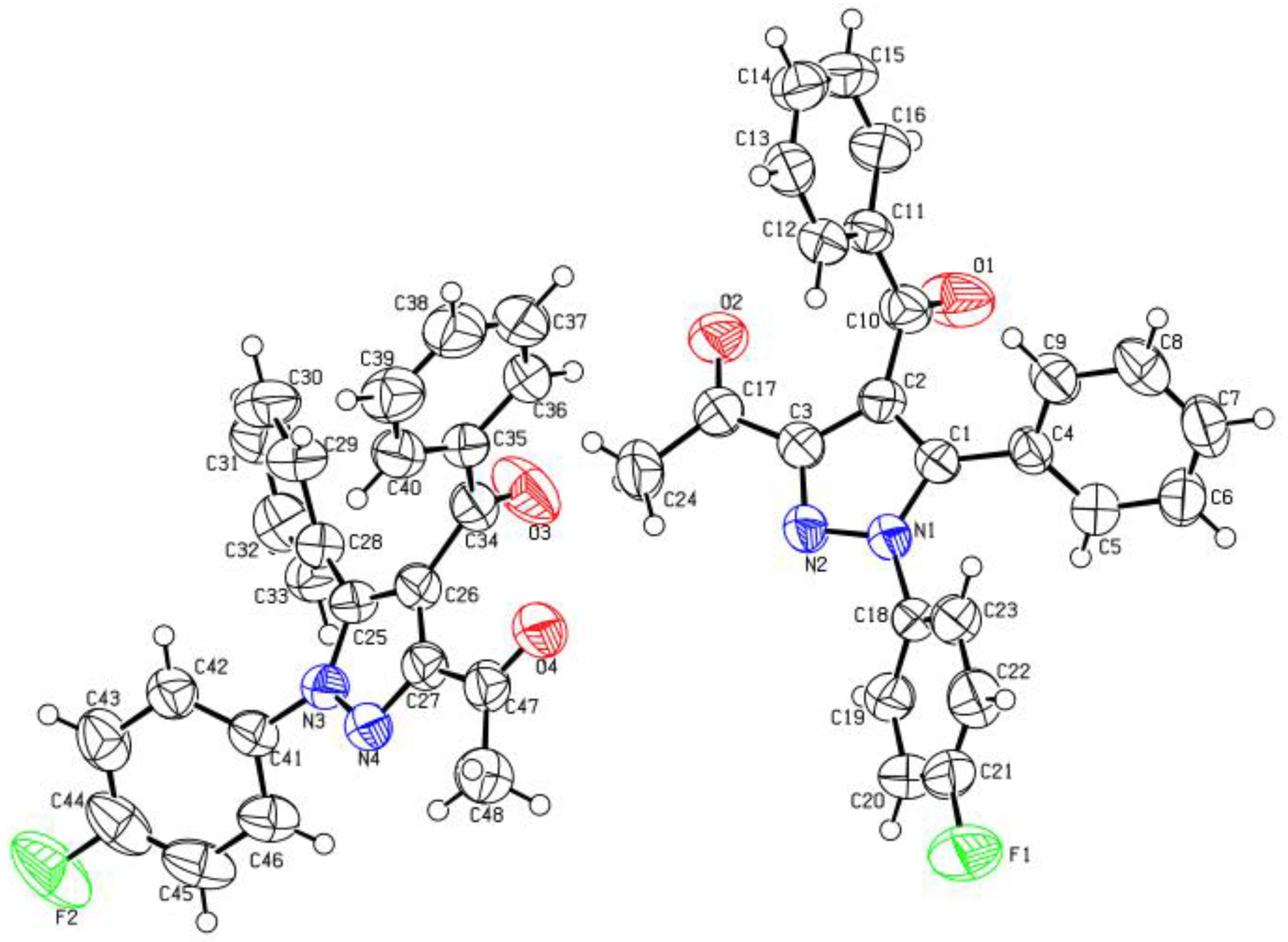
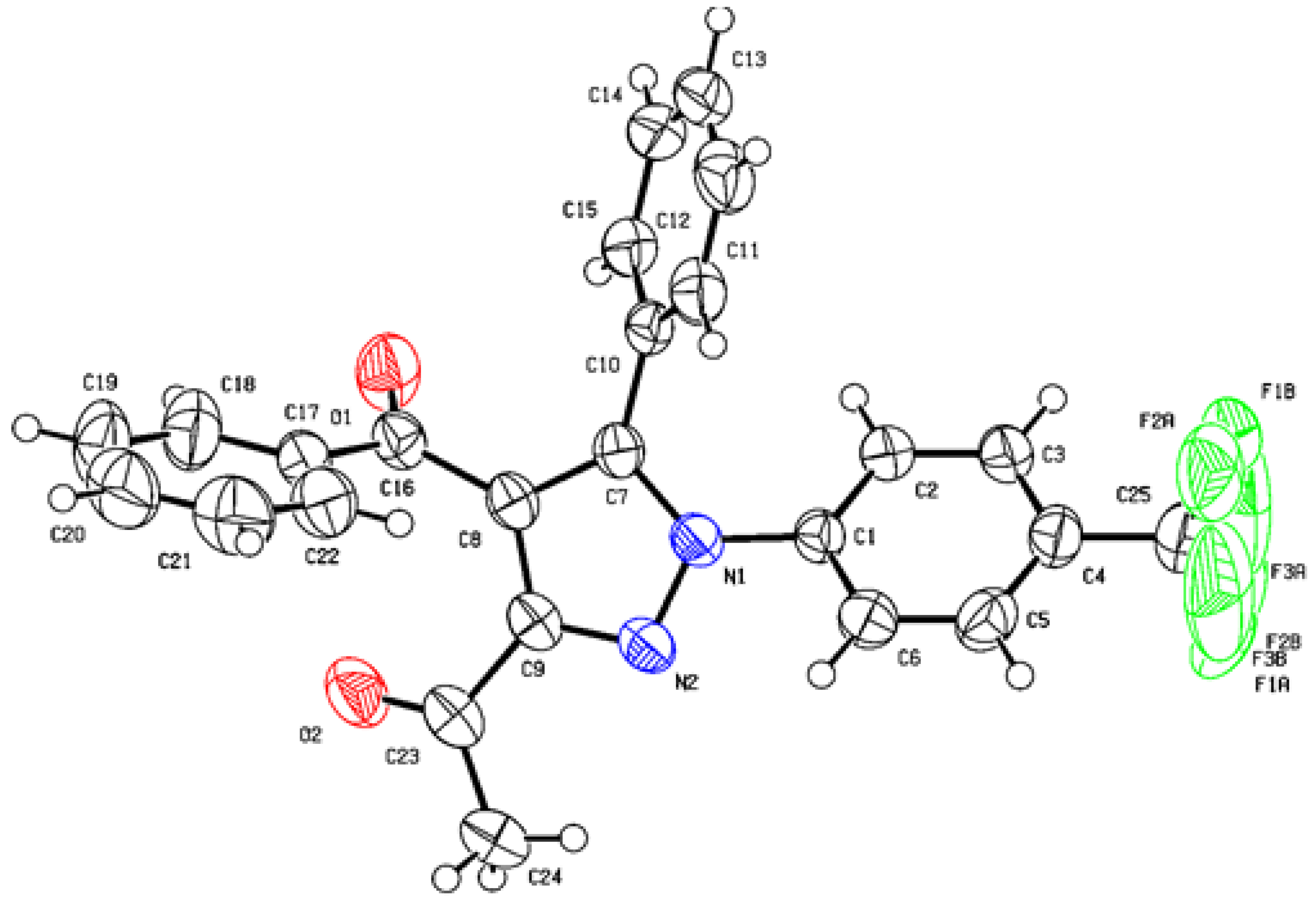
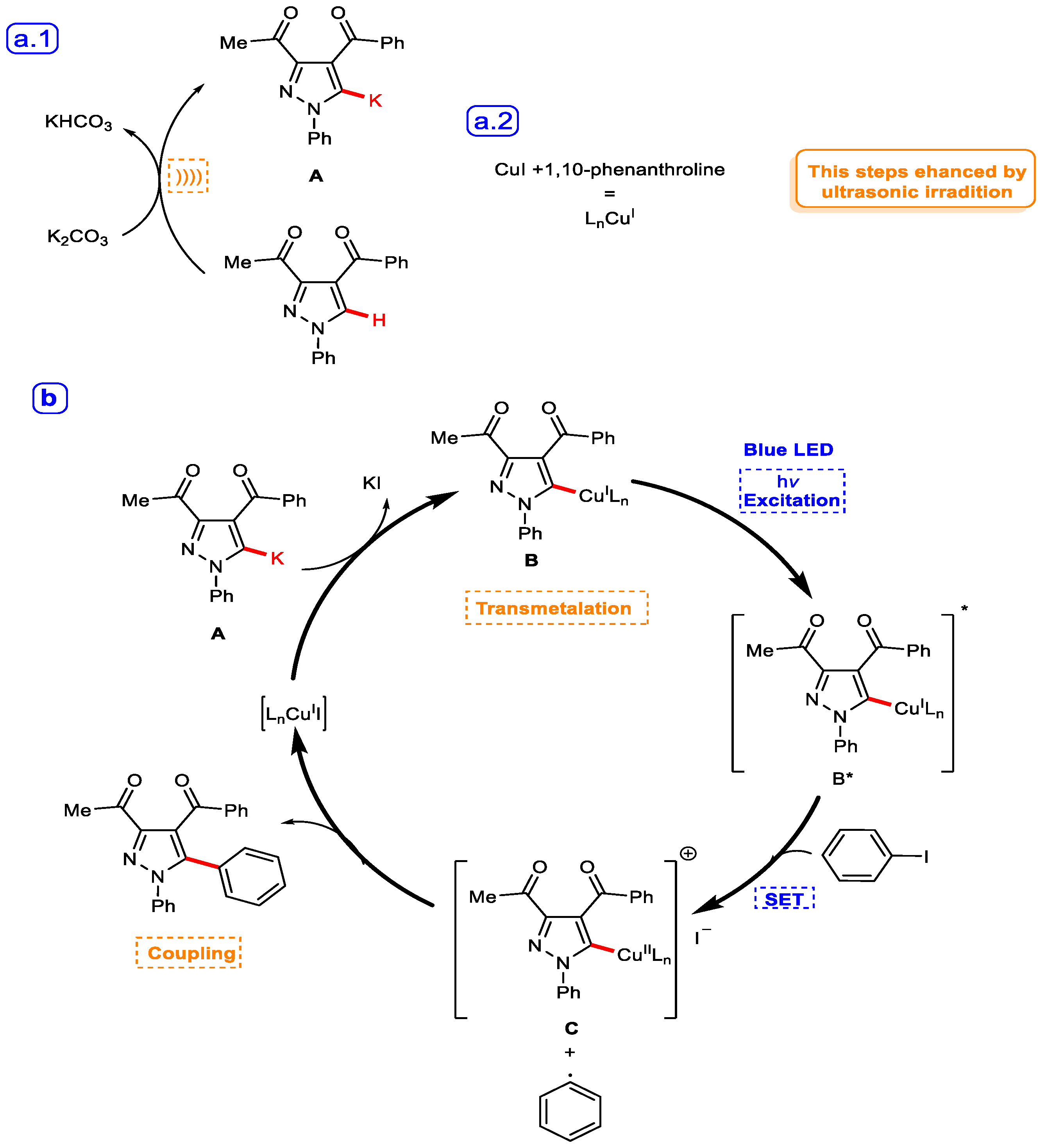
| Entry | Conditions | Catalyst | Solvent | Base (Equiv.) | Temp. | Time of Reaction | % Yield |
|---|---|---|---|---|---|---|---|
| 1 | Ultrasound (US) | No catalyst | DMF | CsF (2) | 80 °C | 4 h | 0 |
| 2 | Blue LED | No catalyst | DMF | CsF (2) | 80 °C | 10 h | 0 |
| 3 | US | 10 mol% 1,10-phenanthroline | DMF | CsF (2) | 80 °C | 8 h | 0 |
| 4 | Blue LED | 10 mol% 1,10-phenanthroline | DMF | CsF (2) | 80 °C | 8 h | 0 |
| 5 | US and blue LED | 10 mol% 1,10-phenanthroline | DMF | CsF (2) | 80 °C | 8 h | 0 |
| 6 | US | 10 mol% CuI | DMF | CsF (2) | 80 °C | 3.5 h | 39 |
| 7 | Blue LED | 10 mol% CuI | DMF | CsF (2) | 80 °C | 9 h | 31 |
| 8 | US and blue LED | 10 mol% CuI | DMF | CsF (2) | 80 °C | 2.5 h | 48 |
| 9 | US | 10 mol% CuI, 10 mol% phenanthroline | DMF | CsF (2) | 80 °C | 2.5 h | 63 |
| 10 | Blue LED | 10 mol% CuI, 10 mol% phenanthroline | DMF | CsF (2) | 80 °C | 7 h | 55 |
| 11 | US and blue LED | 10 mol% CuI, 10 mol% phenanthroline | DMF | CsF (2) | 80 °C | 2 h | 81 |
| 12 | US and blue LED | 10 mol% CuI, 10 mol% phenanthroline | DMF | K2CO3(2) | 80 °C | 1.5 h | 82 |
| 13 | US and blue LED | 10 mol% CuI, 10 mol% phenanthroline | DMF | K2CO3 (1) | 80 °C | 1.5 h | 80 |
| 14 | US and blue LED | 10 mol% CuI, 10 mol% phenanthroline | DMF | K2CO3 (3) | 80 °C | 1.5 h | 82 |
| 15 | US and blue LED | 10 mol% CuI, 10 mol% phenanthroline | EtOH | K2CO3 (2) | 80 °C | 1.5 h | 80 |
| 16 | US and blue LED | 10 mol% CuI, 10 mol% phenanthroline | DCM | K2CO3 (2) | 80 °C | 1.5 h | 65 |
| 17 | US and blue LED | 10 mol% CuI, 10 mol% phenanthroline | 1,4-Dioxan | K2CO3 (2) | 80 °C | 1.5 h | 68 |
| 18 | US and blue LED | 10 mol% CuI, 10 mol% phenanthroline | DMF | Pot. acetate (2) | 80 °C | 2.5 h | 75 |
| 19 | US and blue LED | 10 mol% CuI, 10 mol% phenanthroline | DMF | Pot. phosphate (2) | 80 °C | 3 h | 70 |
| Entry | Catalyst mol% | Yield | Time | Blue LED Distance (cm) |
|---|---|---|---|---|
| 1 | 5 mol% CuI, 5 mol% phenanthroline | 78% | 2 h | 2 |
| 2 | 10 mol% CuI, 10 mol% phenanthroline | 82% | 1.5 h | 2 |
| 3 | 15 mol% CuI, 15 mol% phenanthroline | 82% | 1.5 h | 2 |
| 4 | 10 mol% CuI, 10 mol% phenanthroline | 68% | 3 h | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, T.S.; Al-Bogami, A.S.; Narasimharao, K.; Khan, Z.A.; Amenabar, I.; Mokhtar, M. Explorative Sonophotocatalytic Study of C-H Arylation Reaction of Pyrazoles Utilizing a Novel Sonophotoreactor for Green and Sustainable Organic Synthesis. Catalysts 2022, 12, 868. https://doi.org/10.3390/catal12080868
Saleh TS, Al-Bogami AS, Narasimharao K, Khan ZA, Amenabar I, Mokhtar M. Explorative Sonophotocatalytic Study of C-H Arylation Reaction of Pyrazoles Utilizing a Novel Sonophotoreactor for Green and Sustainable Organic Synthesis. Catalysts. 2022; 12(8):868. https://doi.org/10.3390/catal12080868
Chicago/Turabian StyleSaleh, Tamer S., Abdullah S. Al-Bogami, Katabathini Narasimharao, Ziya A. Khan, Iban Amenabar, and Mohamed Mokhtar. 2022. "Explorative Sonophotocatalytic Study of C-H Arylation Reaction of Pyrazoles Utilizing a Novel Sonophotoreactor for Green and Sustainable Organic Synthesis" Catalysts 12, no. 8: 868. https://doi.org/10.3390/catal12080868
APA StyleSaleh, T. S., Al-Bogami, A. S., Narasimharao, K., Khan, Z. A., Amenabar, I., & Mokhtar, M. (2022). Explorative Sonophotocatalytic Study of C-H Arylation Reaction of Pyrazoles Utilizing a Novel Sonophotoreactor for Green and Sustainable Organic Synthesis. Catalysts, 12(8), 868. https://doi.org/10.3390/catal12080868









