Abstract
C−H methylation of sp2 and sp3 carbon centers is significant in many biological processes. Methylated drug candidates show unique properties due to the change in solubility, conformation and metabolic activities. Several photo-catalyzed, electrochemical, mechanochemical and metal-free techniques that are widely utilized strategies in medicinal chemistry for methylation of arenes and heteroarenes have been covered in this review.
1. Introduction
C−H functionalization has emerged as a potent tool in the field of medicinal chemistry [1,2]. Different strategies of C−H functionalization are being developed to incorporate various kinds of functional motifs in organic molecules [3,4,5,6]. Among them, C−H methylation is one of the major tools to introduce a methyl group in aliphatic or aromatic systems. The installation of a methyl group modulates the hydrophilicity, solubility and conformation of a molecule, which affects its physical properties and biological activities. In medicinal chemistry, it is known as the “Magic Methyl” effect [7,8,9,10]. Methylation governs the selectivity, improves the drug potency and decreases IC50 value. In 2018, retail sales surveyed Njardarson’s Top 200 pharmaceutical products that shows more than 73% of small molecules contain at least one methyl group [11,12]. Therefore, methylation has been the widely used strategy to modify bioactive compounds. Nitrogen heterocycles are among major classes of compounds involved in drug discovery. Extensive studies have shown that the installation of a methyl group adjacent to a heteroatom can modulate the conformation of a molecule, which can have great impact on its biological activities. Some amino acids, namely, alanine, valine, leucine, isoleucine differ only in the number and position of the methyl group. Direct methylation of small amino acids can help to get their higher analogues. So far, many protocols have been reported for installing methyl group on sp2 and sp3 carbon centers. Among all of them, C−H functionalization strategy has a profound effect in the direct installation of a methyl group into a molecule. Traditional transition metal catalyzed C−H functionalization requires harsh reaction conditions, expensive metal oxidant and also generates solvent waste [13,14]. Scientific communities have devised modern approaches such as the photo-redox strategy, which proved to be an effective alternative to thermal based radical reactions. Photo-redox C−H functionalization eliminates the need of radical initiators and proceeds under milder conditions [15,16]. Most of the time, it does not require any additional metal oxidants. Electrochemical C−H transformation uses electrical current as an oxidant has also become an alternative green approach [17]. Mechanochemical transformation (ball milling, hammering, etc.), which requires less solvent or no solvent is also a growing methodology in contrast to industrial scale synthesis [18,19,20]. Among most of these cases, a metal catalyst is required to perform the functionalization. Metal-free C−H methylation helps in eliminating the metal waste and making the product purification easier [21,22]. This review covers sustainable approaches (Photo-redox, electrochemical, mechanochemical and metal-free) for the C(sp2)−H and C(sp3)−H methylation.
2. Photo-Redox-Redox Catalyzed C(sp2)−H Methylation
Typically, the photo-redox C−H functionalization proceeds via single e− transfer in between the substrates and the photo-excited photocatalyst. Various types of light sources such as household CFL, white and blue LEDs, green lamps are being used [15,16]. Direct C–H alkylation of arenes and heteroarenes through photo-redox catalysis is an emerging area of interest since the light irradiation removes the necessity of external oxidants and also proceeds under milder condition. The overall process becomes cost effective and greener compared to traditional transition metal-catalyzed C–H functionalization. Various methyl sources were employed for C−H methylation of arenes and heteroarenes, some of which are discussed below.
2.1. Ir(III)-Catalysed Methylation Using Peroxides
Minisci reaction, originally discovered in 1971 by Francesco Minisci, is the introduction of an alkyl group in nitrogen containing heterocyles. However, this strategy was not suitable for complex bio-active molecules due to the involvement of high temperature and strong oxidant. Many groups around the globe tried to solve this problem and came up with modified Minisci type reactions, which increased the practicality of this reaction [23,24,25]. However, even after numerous efforts, small unstable alkyl radicals like methyl, ethyl and cyclopropyl were not compatible. In 2014, DiRocco and co-workers devised iridium catalyzed direct alkylation of nitrogen containing heterocycles using Me, Et, and c-Pr as alkylating reagent. The C−H methylation of various nitrogen containing drug molecules and agrochemicals were performed using tertiary butyl peracetate (t-BPA) as a methyl radical source in the presence of TFA and acetonitrile as a solvent (Scheme 1) [26]. The reaction proceeds at room temperature under the irradiation of 450 nm LEDs.
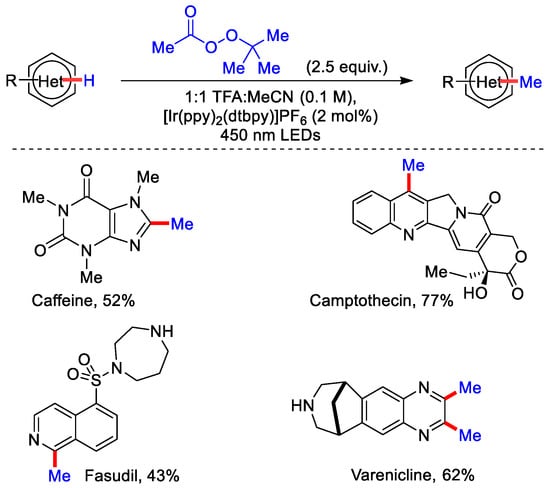
Scheme 1.
Photo-induced Ir(III)–catalyzed methylation of heterocycles using t-BPA.
As per the mechanism, decomposition of t-BPA take place through single e− transfer from Ir(III)∗ species. This reduction is only possible under an acidic reaction condition, which helps in reducing the energy barrier for reduction. This in turn generates an unstable α−peroxy radical, which further produces tert-butoxy radical. This radical undergoes β−scission to generate a methyl radical. Methyl radical then adds to C–2 position of the protonated heterocycle followed by the oxidation of amino radical cation by Ir(IV) to yield the desired product (Scheme 2).
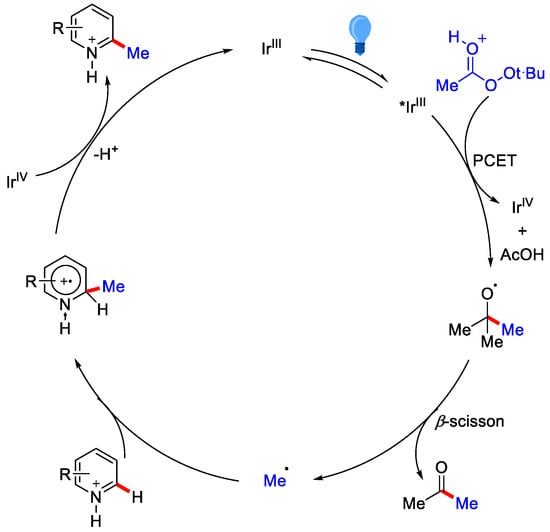
Scheme 2.
Mechanism of photo-induced Ir(III) catalyzed methylation of N-heterocycles.
This strategy can be widely applicable as it proceeds under milder conditions. It involves economic reagents and low molecular weight byproducts are generated, which can be distilled off easily. After several developments on radical based alkylation via peroxides, the idea to use methyl alcohol (CH3OH) as a methylating agent was introduced. CH3OH is more commonly available, cheap and safe compared to the energy intensive peroxides.
2.2. Methylation Using CH3OH
In 2015, MacMillan and co-workers demonstrated direct C−H methylation of heteroarenes using CH3OH by merging both photo-redox and hydrogen atom transfer (HAT) catalysis [27]. Alcohols were employed as alkylating reagents, which enabled the late-stage derivatization of pharmaceutically important molecules. Ir(ppy)2(dtbbpy)+ as a photocatalyst and thiol as a catalyst were used to get methylated heteroaromatic product under ambient temperature (Scheme 3). After the irradiation of blue LEDs, Ir(ppy)2(dtbbpy)+ excites to *Ir(III), which further gets oxidized to Ir(IV). In support of this pathway, they performed Stern–Volmer fluorescence quenching experiments, which showed that excited state *Ir(III) was quenched in the presence of the protonated heteroarene, not in the presence of the thiol catalyst. Then, Ir(IV) oxidizes the thiol catalyst to generate a thiyl radical that abstracts the hydrogen atom from the alcohol to form α−oxy radical. This radical reacts with heteroarene to generate radical cation that undergoes further deprotonation to form α−amino radical. Then, H2O gets eliminated by spin center shift (SCS) to form radical intermediate. SCS is based on the principle that a leaving group, which is adjacent to a radical, eliminates with the transferal of spin density to the adjacent atom.
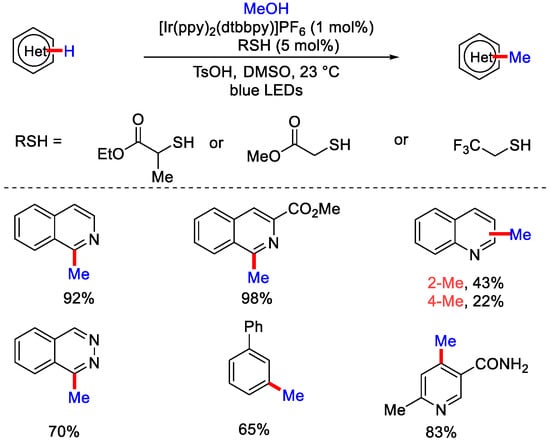
Scheme 3.
Photo-redox Ir(III) dual catalyzed methylation of heteroarenes.
The radical intermediate, which forms after SCS, gets protonated, followed by reduction by *Ir(III), to yield the desired product. In 2016, DiRocco, Krska and co-workers reported Ir catalyzed photo-redox hydroxymethylation of heteroaromatic bases using CH3OH in the presence of peroxides at room temperature. However, in some cases they observed the methylated product in a minor amount [28]. A major breakthrough was achieved by Li and co-workers, they demonstrated metal-free methylation of methylation of heteroarens at room temperature without using any external photo-sensitizers under the irradiation of ultra-violet light (Scheme 4) [29]. CH3OH and DCM were found to be the optimized set of co-solvents in the presence of TFA as an acid additive. TFA was playing the crucial role of increasing the electrophilicity of the heteroarenes by protonating it. However, under the optimized reaction condition, isoquinoline derivatives could not be methylated. Therefore, authors used two equivalents of benzophenone into TFA-acidified CH3OH. The addition of ketones into the reaction mixture increased the generation rate of hydroxymethyl radical (•CH2OH), since under light irradiation, triplet ketone will abstract the α–H of CH3OH efficiently. Furthermore, in pyridine cases, addition of H2O to the reaction system increased the yield significantly. Benzothiazole was also methylated via this method, and according to them, this was the only example where CH3OH was used as the methylating reagent for methylation of five-membered heterocycles. Moreover, they investigated the photophysical properties of the reactions by taking the 2-methyl quinoline as a model substrate, in which they observed photo-induced e− transfer process from CH3OH to heteroarene upon UV irradiation. As per the plausible mechanism, hydroxymethyl radical was generated by higher energy irradiation in the presence of DCM or benzophenones as an additive.
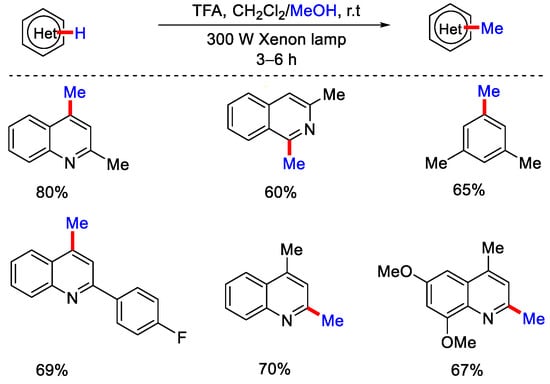
Scheme 4.
Methylation of heterocycles without using a transition metal catalyst and external photo–sensitizers.
The nucleophilic attack of methyl radical on the protonated heteroarene leads to the formation of radical cation intermediate, which undergo further reaction. This results in the formation of enamine structure, which after tautomerization gives the desired methylated product. This strategy was well applied on medicinally relevant pyridines, quinolines and isoquinolines. In the same year, Barriault, Scaiano and co-workers also developed metal-free photochemical methylation of quinolines, pyridine and phenanthridines using CH3OH in the presence of UVA irradiation (Scheme 5) [30]. The reaction proceeds without the use of any external photocatalyst. Control experiments suggested that acid and UV LEDs are essential for the reaction. The reaction yield was not affected, even in the presence of air, which indicates that the reaction proceeds via singlet excited state.
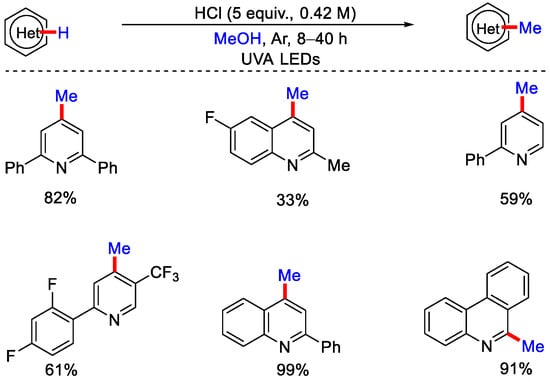
Scheme 5.
UV irradiated methylation of heteroarenes by photo–activation of CH3OH.
The authors performed Kinetic isotopic effect (KIE) studies and observed a KIE of 1.86; based on this they proposed that the quenching of substrate lepidine occurs in the reaction by CH3OH through proton coupled electron transfer (PCET), which implies e− transfer occurring first followed by proton transfer.
Under UV light irradiation, protonated heteroarene first excites to a singlet state. This radical gets quenched by CH3OH to generate •CH2OH and protonated radical intermediate. •CH2OH radical reacts with electrophilic protonated heteroarene to give radical cation intermediate. This forms another intermediate via PCET, followed by the elimination of H2O and sequential tautomerisation to give the methylated product (Scheme 6).
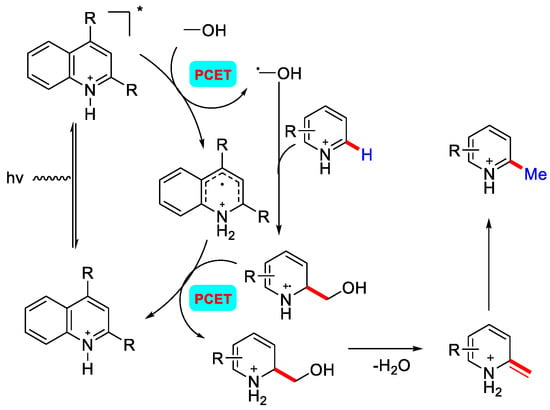
Scheme 6.
Mechanism of photo–activation of CH3OH under UV irradiation.
The same group came up with a more general procedure, in which they negate the UV-mediated decomposition pathway by using visible light. Ir photocatalysed conditions allowed the use of lower energy irradiation. The authors reported that excited Ir(III) undergoes reductive quench to oxidize chloride into a chlorine atom, which further participate in HAT process and abstracts a hydrogen atom from CH3OH to form a radical, then this radical further attacks the heteroarene to generate the radical cation intermediate, which gets reduced by Ir(II) to form another intermediate. Then, H2O elimination followed by tautomerisation gives the desired methylated product (Scheme 7) [31]. Surprisingly, when isopropanol was used as the alkylating reagent, reduced heteroarenes were observed as major product instead of alkylated heteroarenes.
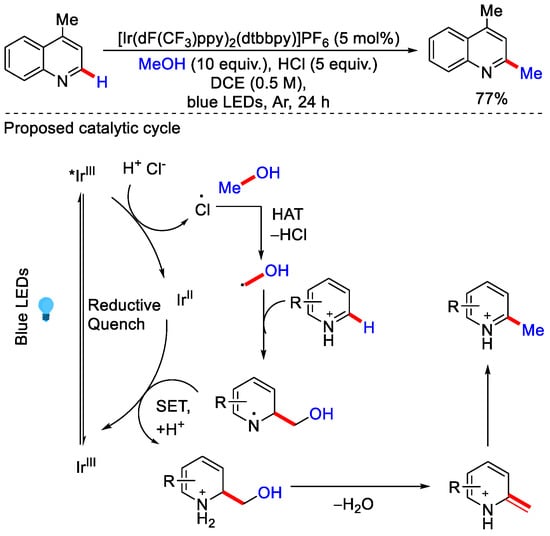
Scheme 7.
Methylation of heteroarene by activation of CH3OH under blue LED.
2.3. Methylation Using Acetic Acid and Derivatives
Acetic acid is an inexpensive and commonly available methyl source. It is the byproduct in many of the industrial processes. So, the usage of acetic acid and its derivatives as methylating reagent can offer several advantages. Earlier, Minisci and co-workers also used the decarboxylative concept by incorporating strong oxidant and high temperature for the generation of alkyl radical, however, the use of strong oxidants resulted in the formation of radical addition derived byproducts and also made the procedure inapplicable for compounds containing sensitive functional groups. Over time, this strategy was further developed by Shang, Fu and coworkers, where they prepared the N-(acyloxy)pthalimide and N-(hydroxyl)pthalimide, that generates the alkyl radical by decarboxylation via photo-redox catalysis in absence of any external oxidants. The authors used two mol % of iridium photocatalyst in the presence of indium(III) triflate as Lewis acid co-catalyst for selective methylation at α-position of phenanthridine (Scheme 8) [15]. They proposed Ir(III)/Ir(II) cycle as it was thermodynamically favorable to oxidize Ir(II)to Ir(III) by N-(acyloxy) pthalimide. Ir(II) reduces N-(acyloxy) pthalimide (NAP) to form alkyl radical, which further attacks the protonated heteroarene to generate radical cation intermediate. This was followed by oxidation/rearomatization by *Ir(III) to regenerate Ir(II) and give the methylated product with 32% yield (Scheme 9) [32].
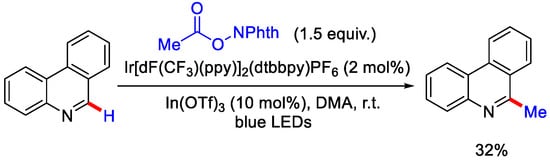
Scheme 8.
Ir(III) catalyzed α–C−H methylation of phenanthridine via decarboxylation of NAP.
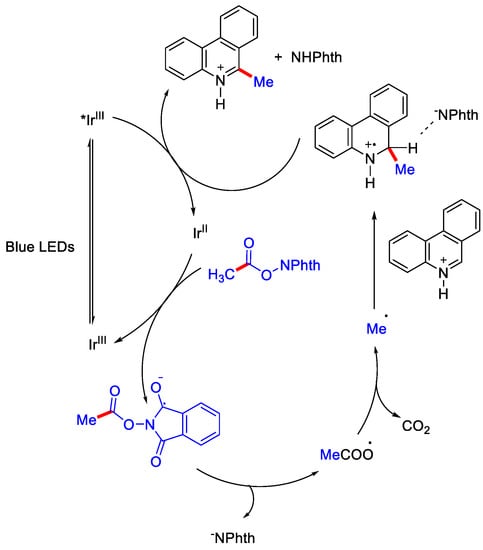
Scheme 9.
Mechanism involved in Ir(III) catalyzed α– methylation of phenanthridine with N-(acyloxy) phthalimides.
This protocol suffered with low yield and the use of expensive metal catalysts, which were later avoided by Genovino, Frenette and co-workers [33]. They developed metal-free methylation of lipidines from readily available acetic acid using an MesAcr(9-mesityl-10-methyl acridinium) as a photocatalyst and hypervalent iodine reagent under the irradiation of blue LEDs.
The authors believed that inefficient oxidation of neutral heteroaromatic or trace carboxylate may be responsible for the formation of MesAcr radical in a small amount as a part of initiation step. MesAcr radical transfers an e− to hypervalent iodine adduct, which undergo in radical anion collapse to form a methyl radical. Theis methyl radical attacks the protonated heteroarene to form an intermediate. After deprotonation and single e− transfer, the desired methylated product forms.
In 2018, Murlidhar and co-workers reported the one-pot Minisci protocol with carboxylic acid, N-(acyloxy)phthalimides (NAPs) and 4-CzIPN as photocatalyst (PC) (Scheme 10) [34].
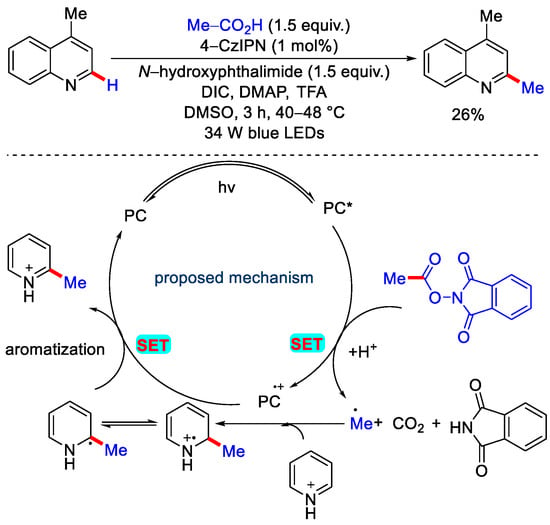
Scheme 10.
One pot minisci protocol for methylation of quinoline via decarboxylation of acetic acid.
This reaction occurred under mild conditions and showed a high degree of functional group tolerance. This protocol was also applied to drug like scaffolds that contains sensitive functional groups. As per mechanism, excited photocatalyst quenched by NAP via single e− transfer (SET) and forms alkyl radical by reductive fermentation. Alkyl radical attacks the protonated heteroarene to generate adduct, which undergoes deprotonation to form α−amino radical. Finally, aromatization leads to corresponding methylated product (Scheme 10). These above discussed strategies of decarboxylative methylation were not widely applicable for methylation of different classes of heterocycles due to their low yield. Hu and co-workers reported the decarboxylative alkylation of quinoxalin-2(1H)-ones at C-3 position under visible light irradiation. Ru(II) as catalyst, phenyliodine(III) dicarboxylate and aliphatic acids as alkylating reagent and DMSO as solvent were employed [35]. Under visible light irradiation, Ru(II) excited to *Ru(II) that undergoes single e− transfer (SET) with PhI(OOCMe)2 to generate methyl radical and Ru(III). This methyl radical attacks on quinoxalin-2(1H)-ones and forms another radical species, which is then oxidized by Ru(III) to produce a nitrogen cation. Finally, deprotonation gives the desired methylated product (Scheme 11).
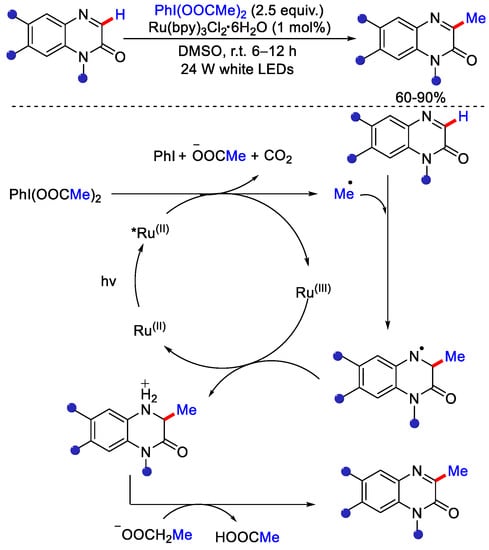
Scheme 11.
Ru catalyzed decarboxylative methylation of quinoxalin–2(1H)–ones under visible light irradiation.
It was experimentally concluded that the methyl source was PhI(COOMe)2 not DMSO. They used deuterated DMSO and observed only 3-methylquinoxalin-2(1H)-one as product without any deuterium incorporation. This strategy proved to be good for the synthesis of bioactive nonpeptideangiotensin II. In 2020, Xu, Song and co-workers reported the electrophotocatalysis methylation of lepidine [36] by merging the electrochemistry and photocatalysis methodologies, which allowed the efficient decarboxylative C−H methylation. They reported the methylation reaction via anodic oxidation of Ce(III) to Ce(IV), which combines with the carboxylic acid to form a complex. This complex undergoes photo-induced ligand metal charge transfer to give back Ce(III) and the carboxyl radical. Decarboxylation of carboxyl radical generates a methyl radical that attacks the protonated lepidine to generate a radical cation. This radical cation forms another radical after deprotonation. Highly exothermic oxidation of this radical by Ce(IV), leads to the formation of methylated product in good yield.
2.4. Methylation Using Dimethylsulfoxide (DMSO)
Besides peroxides, alcohols, and carboxylic acids, DMSO can also be used as methylating reagent. It is cheap, non-toxic and commercially available. Activation of DMSO with electrophilic reagents has widely been used in the transformation like oxidation and chlorination of alcohols and the formation of new bonds by Pummerer-type reaction. Despite this utility, a single e− transfer (SET) process using DMSO remained a challenge. Initially, only a few research groups worked on the activation of DMSO using peroxide and iron salts (Fenton’s reagent) for the methylation of heteroarenes. In 2018, the Glorius group demonstrated iridium catalyzed photo-induced radical based methylation and trideutero-methylation of quinolines and isoquinolines using dimethyl sulfoxide. DMSO with phosphorus-based activator (PhPOCl2) generates the chloro(dimethyl)sulfonium ion, which undergo a reduction followed by the homolytic cleavage of the C−S bond to generate the methyl radical (Scheme 12) [37].
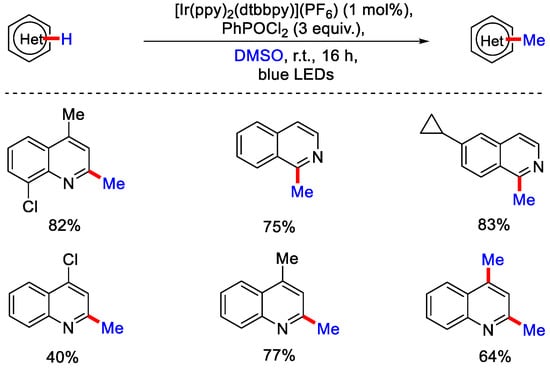
Scheme 12.
Ir catalyzed methylation of of quinolines and isoquinolines using DMSO.
Authors performed the Stern–Volmer luminescence studies and revealed that lepidinium was formed by the partial hydrolysis of PhPOCl2 or TMSCl in DMSO, which was responsible for the observed quenching in the reaction mechanism. Under blue LED irradiation, this lepidinium ion undergo single e− transfer by excited iridium(III) photocatalyst and forms an intermediate. This acts as an e− shuttle and reduced chloro(dimethyl)sulfonium generates a methyl radical. Then, this radical attacks on the most e− deficient position of lepidinium followed by deprotonation and SET with Ir(IV) to give methylated product. By the moderate alteration in the conditions methylthiomethylation was also possible using TMSCl, with DMSO/Acetone or DMSO/Chloroform as solvent mixtures. New methods were developed via a metal-free manner due to their prime importance. In 2019, the Chen group applied a transition metal-free α−methylation of 1,8-naphthyridines by using DMSO as methyl source, as well as solvent (Scheme 13) [38].
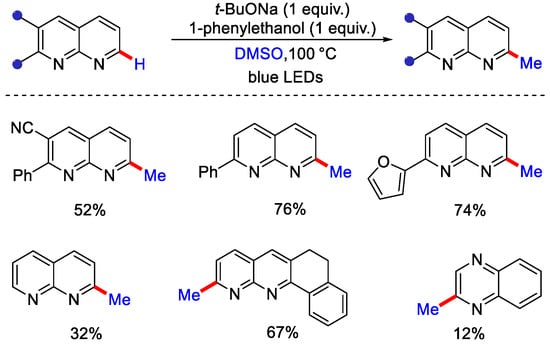
Scheme 13.
C−H methylation of 1,8-naphthyridines using DMSO as methyl source.
According to the mechanism, 1,8 naphthyridines substrate on treating with 1-phenyl ethanol and base undergo Meerwein–Ponndorf–Verley type reduction to give sodium alkoxide, which forms a transition state by interacting with its imine unit. Methyl radical was generated by DMSO via SET under light and t-BuONa, which later interacts with tautomerised imine to form the corresponding adduct. This interconverts to imine intermediate followed by dehydro-aromatization to yield the desired methylated product.
2.5. Ru-Catalysed Methylation Using Methyl Boronic Acids
Alkyl boronic acids are also a prime source of alkyl group. They are readily available, moisture insensitive, highly efficient and show excellent functional group tolerance, and have broad applicability. Under the mild reaction conditions, they can be used for late stage functionalization of complex substrates. In 2016, Chen and co-workers showcased the incorporation of 1° and 2° alkyl groups into various N-heteroarenes utilizing alkyl boronic acid as alkylating reagent [39]. Incorporation of 1° alkyl radicals was the challenging task in the traditional Minisci reaction, and was solved by this method. The authors used acetoxybenziodoxole (BI-OAc) as an oxidant, [Ru(bpy)3]Cl2 as a photocatalyst in the HFIP solvent at 30 °C. As per the reaction mechanism, the BI-OAc oxidant was reduced via SET from photoexcited Ru(II)* to form a radical-anion intermediate, which further forms a radical by iodine–oxygen bond cleavage. This radical reacts with boronic acid to form a methyl radical, which then attacks the protonated N-heteroarenes to form σ complex. Then this intermediate gets oxidized by Ru(III), and after deprotonation, methylated product forms (Scheme 14). The major focus of this work was alkylation of heteroarenes, and only one example of methylation of 4–chloroquinoline was reported with 46% yield.
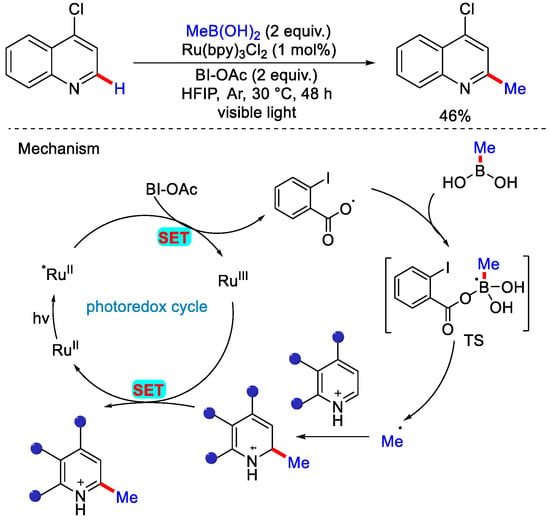
Scheme 14.
Ru catalyzed photocatalytic methylation of 4–chloroquinoline using methyl boronic acid.
2.6. Methylation Using Methane Gas
Methane and other lighter hydrocarbons are widely used for the soaring production of natural gas, which makes it an important raw material. In 2018, Zuo and co-workers used methane gas as methylating reagent for its properties like low-cost fuel gas and abundancy. The authors reported the synergistic merger of hydrogen atom transfer (HAT) catalysis and ligand to metal charge transfer (LMCT) using abundant and inexpensive cerium salt and alcohol catalyst. Ce(IV) salt and alcohol generates the Ce(IV)-alkoxy complex, which undergo photo-induced LMCT to form alkoxy radical, which abstracts the hydrogen from methane to produce the methyl radical. Then, under high pressure (5000 kPa~50 bar) of methane, methyl free radical performs methylation of isoquinoline (Scheme 15) [40]. Further, photocatalytic amination of methane, ethane and other hydrocarbons using ditertbutyl azodicarboxylate (DBAB) were also demonstrated in a continuous flow reactor. However, with methane gas being flammable, storage and handling challenges were associated with this protocol.
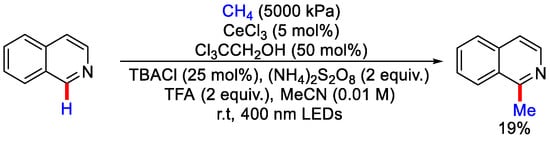
Scheme 15.
Photo-induced Ce catalyzed C−H methylation of isoquinoline using CH4.
3. Photo-Redox Catalysed C(sp3)−H Methylation
In medicinal chemistry, methylation of C(sp3)−H bonds plays a vital role in increasing the efficiency of drug molecules. However, it is energetically more challenging to activate the aliphatic C−H bond compared to the aromatic C−H bond due to the rotatable nature of hybridized sp3 bonds and the formation of weaker M−C(sp3) bonds [41]. The need of C(sp3)−H methylation, alpha to heteroatom is in demand for cyclic as well as acyclic systems, which can proceed without using any directing group. However, due to the low acidity of α− protons and the possibility of several C−H oxidation pathways, this sort of methodology faces several problems. Despite this, scientists came up with different approaches for the methylation of C(sp3)−H bond via a photo-redox technique that is discussed below.
3.1. Oxidative C(sp3)−H Methylation
In 2017, the MacMillan group developed methylation of C(sp3)−H by synergistic merger of Photo-redox, nickel and hydrogen atom transfer (HAT) catalysis. For the alkylation, the authors were using quinuclidine for HAT catalysis while a modification was carried out for methylation, as quinuclidine was proved to be harmful for the efficiency of the reaction due to its undesired reactivity with MeOTs. MeOTs and CsBr were used to prepare MeBr in situ. In the tentative mechanism proposed by the authors, bromide anion undergoes in the catalytic cycle to form bromide radical via single-electron transfer (SET) by the triplet excited state of Ir(III), which gets reduced to Ir(II) as shown in Scheme 16 [42].
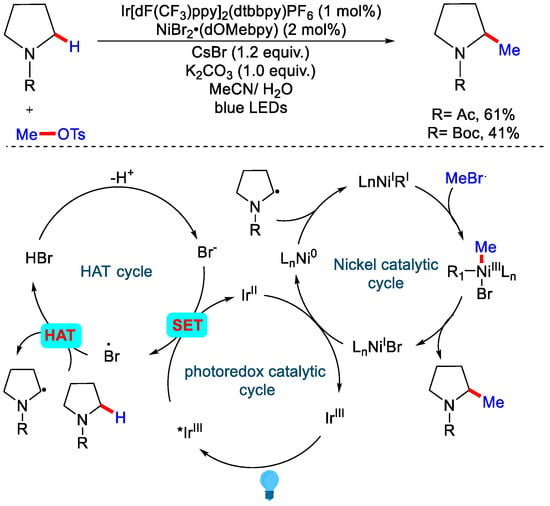
Scheme 16.
Ir catalyzed α–C(sp3)−H methylation via polarity-match based approach.
Then, this bromide radical undergoes a HAT cycle and abstracts the hydrogen atom from the N-protected cyclic molecule to yield the corresponding radical of heterocycle. The reactive carbon center of this radical intermediate reacts with Ni(0) species to form Ni(I) complex, which undergoes an oxidative addition with MeBr and forms Ni(III) adduct, and after reductive elimination, the desired methylated product was obtained. Although various strategies for C−H functionalization methods have developed, it remains challenging for direct functionalization of unactivated C−H bonds of hydrocarbons, particularly those used in petroleum compounds. In 2020, Mi, Li and co-workers reported the methylation of unactivated sp3 and sp2 C−H bonds by using a p-type doped gallium nitride nanowires (GaN–NW) surface as a robust catalyst and CH3OH as a methylating reagent (Scheme 17) [43]. CH3OH is considered to be the most promising sustainable resource as it can be produced from CO2 reduction. Earlier, the same group showed that methyl carbene (:CH2) can be generated by the photocatalytic activation of CH3OH on the surface of gallium nitride nanowires (GaN–NW). Selective C−H insertion of highly reactive methyl carbenes was governed by its strong physical adsorption on GaN surface. This makes the GaN surface more selective towards more substituted carbon centers. However, when the temperature was raised, selectivity for 1° C−H enhanced due to the desorption of carbenes from GaN surface. This phenomenon was also consistent with classical carbene chemistry. This strategy proved to be very useful as methylation of simple alkanes and arenes were performed with high efficiency and can have a wide range of applications.
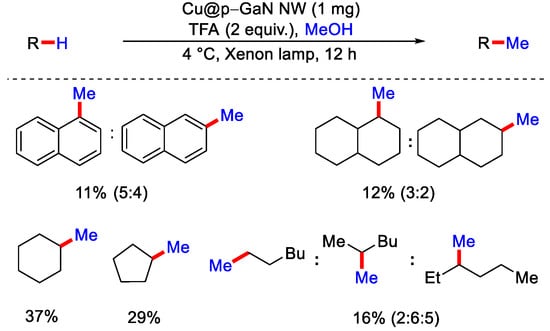
Scheme 17.
GaN mediated C−H methylation of unactivated sp3 and sp2 C−H bonds.
3.2. Formation of Radical via Peroxides
In 2021, the Stahl group demonstrated C(sp3)−H methylation of pharmaceutically important molecules using Ir-Ni dual catalysis. Ir[dF(CF3)ppy]2tBubpyPF6 as photosensitizer, NiCl2•dimethoxyethane (NiCl2•dme) to promote C−C coupling, DTBP or DCP as the peroxide source and TFAH or B(OH)3 as acid additive were used in the presence of trifluoroethanol or acetonitrile as solvent depending upon the substrate (Scheme 18) [44]. Upon blue LED lamp irradiation, alkoxy radical was generated by the homolytic cleavage of O−O bond of peroxide. Generated alkoxy radical then undergoes HAT and β−methyl scission in parallel. This generates a substrate radical via HAT with tertiary alcohol as a by-product and methyl radical via β−methyl scission with ketone as a by-product. It was observed that β−methyl scission favors a high temperature, while HAT favors a room temperature.
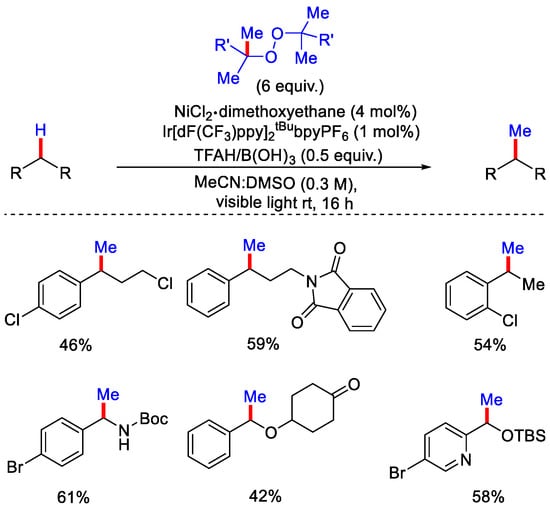
Scheme 18.
Ir–Ni dual photocatalytic C(sp3)−H methylation using peroxide.
Ni catalyst was found to be essential for the reaction since without NiCl2•dme, methyl radical gives methane gas by promoting HAT, while in the presence of NiCl2•dme, substrate was methylated (Scheme 19). This strategy greatly enhanced the magic methyl effect, which promoted drug discovery and development.
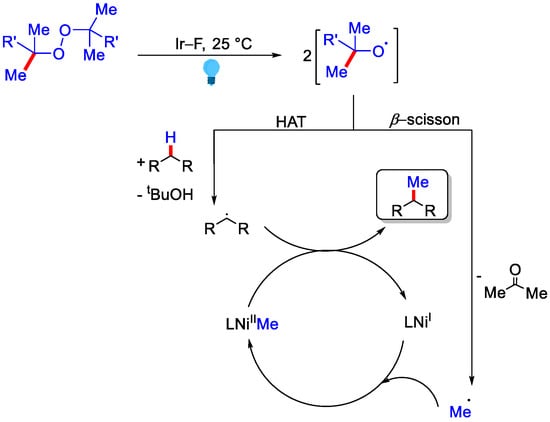
Scheme 19.
The mechanism involved for photocatalytic methylation using peroxide that undergoes HAT and β−methyl scission in parallel.
4. C−H Methylation by Electrochemical Oxidation
Electrochemical C–H functionalization became an advanced and efficient tool to achieve C−C bond formation in a cleaner and sustainable fashion. In electrochemical transformation, the electric current serves as an oxidant that eliminates the need of a stoichiometric amount of traditional metal based oxidants [45,46]. This also diminishes the additional side reactions. A merger of transition metal-catalyzed and electrochemical C−H methylation can help to explore the magic methyl effect in greener and sustainable fashion. In 2017, Mei and co-workers reported the first Pd catalyzed C(sp2)–H methylation of ketoximes with MeBF3K in the presence of Pd(OAc)2 under electrochemical conditions, in which electric current was used as an oxidant instead of metal oxidants (Scheme 20) [47]. Kinetic isotopic effect experiments concluded that C–H bond cleavage is not involved in the rate determining step of the catalytic cycle.
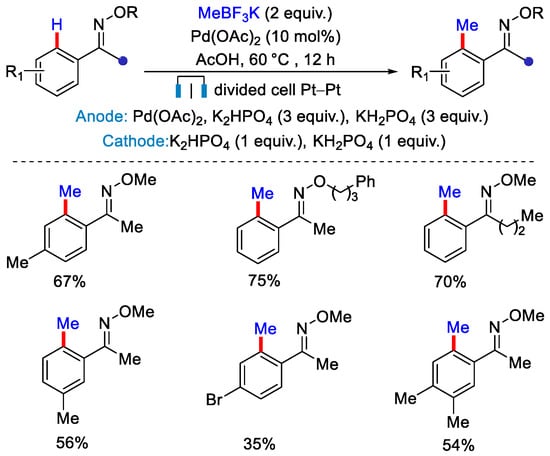
Scheme 20.
Pd catalyzed ortho-C−H methylation in electrochemically divided cell.
As per the plausible mechanism, first the Pd catalyst coordinates with the nitrogen atom of the substrate and then the ortho-C−H activation takes place followed by the formation of five-membered palladacycle. Then, in situ generated methyl radical (from MeBF3K) reacts with it, to produce a Pd(III) or Pd(IV) intermediate, followed by the reductive elimination of this intermediate to deliver ortho methylated product and regenerates the palladium catalyst. Pd(I) could be oxidized to Pd(II) by anodic oxidation (Scheme 21).
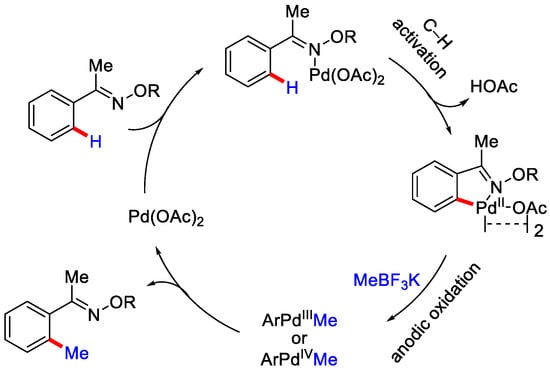
Scheme 21.
The mechanism involved for the electrochemical methylation of heterocycles in a divided cell.
A divided cell was used in this protocol, which typically complicates the overall reaction set up. In 2019, the same group modified this strategy using undivided Pt-Pt electrochemical cells with substrates containing pyridyl directing group (Scheme 22) [48].
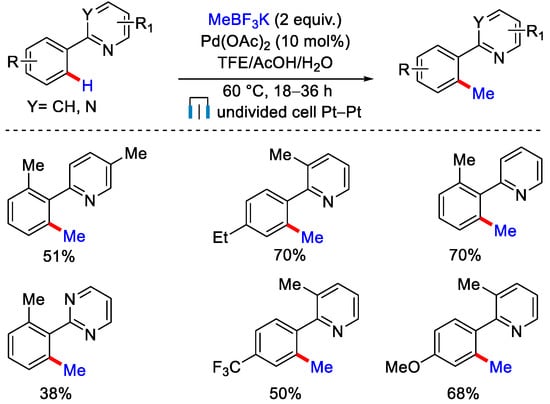
Scheme 22.
Pd catalyzed ortho–C−H methylation of heterocycles in an undivided electrochemical cell.
Pd(OAc)2 as a catalyst, MeBF3K as a methyl coupling partner in the presence of TFE/AcOH/H2O as a co-solvent were found to be the optimized set of reagents. Substrates containing e− rich substituents gave a good yield compared to e− withdrawing substituents. After performing some kinetic isotopic effect experiments, they concluded that C−H bond cleavage was involved in the rate determining step. This reaction mechanism was somewhat similar to their previous methodology. Very recently, Lin, Terrett and co-workers performed electrochemically driven late stage C(sp3)−H methylation of complex molecules (Scheme 23) [49]. The protocol was based on C−H activation followed by organozinc(Me2Zn)-mediated C−C bond formation. The authors employed a modified Shono-oxidation technique, which has a broad reaction scope and a high functional group tolerance. This protocol was applicable to gram scale synthesis. Drug molecules and natural products like celecoxib derivative, acetyl-nor-nicotine, and nootropic drug pramiracetam were methylated under this protocol.
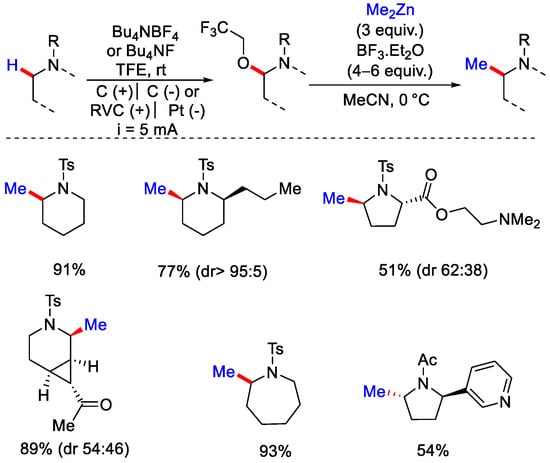
Scheme 23.
Electrochemical C(sp3)−H oxidation for the late–Stage methylation.
5. Rh-Catalyzed Mechanochemical C−H Methylation
Scientists always really strived for the development of robust and cleaner synthetic protocols for the synthesis of organic molecules. In the search of new and cleaner transformations, a solvent free approach has been achieved by the chemists towards organic synthesis, popularly known as mechanochemistry. In mechanochemistry, chemical transformation is achieved by the means of mechanical stress, where the reaction takes place due to the influence of mechanical energy [50,51].
Since it avoids the use of solvents, no solvent waste is generated after the completion of the reaction, unlike any other batch processes that make this methodology cheaper, greener and sustainable. In 2021, Pilarski and co-workers were the first group to report a solvent free rhodium catalyzed C−H methylation under mechanochemical conditions [52]. Initially they used 2-phenylpyridine as a model substrate, five mol% of [Cp*RhCl2]2 as a catalyst, two equivalents of CH3B(OH)2 as methylating reagents, and 1.5 equivalents of Ag2CO3 as an oxidant. The reaction was performed in a stainless steel (SS) milling vessel equipped with a single SS ball using a mixer mill (MM), which operated at a frequency up to 36 Hz. This reaction condition was limited for the substrates capable of forming five-membered rhodacycle but using identical conditions for different types of substrates (phenoxypyridine), giving rise to the six-membered rhodacycle required re-optimization. CH3BF3K instead of CH3B(OH)2 with a sub-stoichiometric amount of AgSbF6 in Teflon milling vessels resulted in the best yield (Scheme 24). However, in the perspective of mechanochemical reactions, the influence of milling vessel material is still not fully understood. They have suggested that C(sp3)−H activation might have occurred by σ−bond metathesis. The usage of AgSbF6 improved the yield as it facilitates the transmetalation or reductive elimination step. The authors also performed some rhodacycle synthesis using different substrates and compared their protocol with conventional methods, i.e., with solvents like DMF or DCE. In the solvent reaction condition, rhodacycle was formed in a low yield, while under the mechanochemical conditions, rhodacycle was formed in a good yield, with an even shorter reaction time of one hour compared to 48 h. Mechanochemical methylation worked well with both e− rich and e− withdrawing groups like pyrazoles and oxazoles. Different bioactive molecules like etoxazole, oxaprozin, diflufenican, estrone, etc., were methylated in good yield.
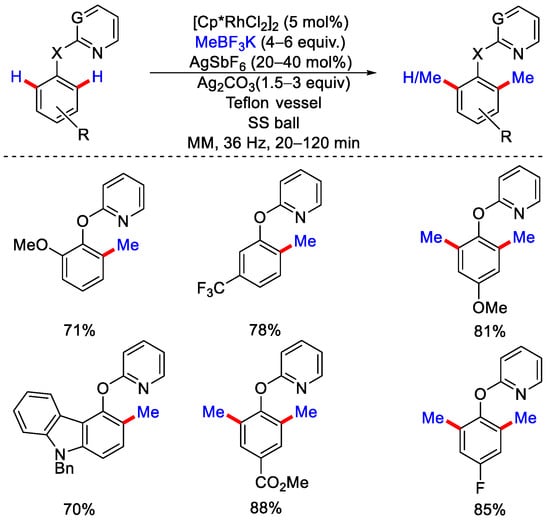
Scheme 24.
Rh catalyzed mechanochemical C−H methylation.
6. Metal-Free Approaches for C−H Methylation
C−H methylation of N-heterocycles can lead to the formation of a core structure for many biologically active compounds. In the realm of C−H functionalization of heteroarenes, numerous works have reported the utilization of metal complex as a catalyst or as an oxidant [53,54]. The purification of trace metal impurities from the reaction mixture always remains a challenging task. To avoid metal contamination, scientists tried to develop various approaches towards metal-free C−H functionalization. Achieving C−H methylation without the help of any metal catalyst or metal based oxidant can be a boon to the pharmaceutical industries since the removal of trace metal impurities will no longer be a problem [21,55]. Herein, we have summarized some of the metal-free approaches for C−H methylation.
In 2015, Li, Wu and co-workers reported metal-free methylation of pyridine N-oxide derivatives with dicumyl peroxide (DCP) as a methylating reagent under heat condition in inert atmosphere (Scheme 25) [56]. The authors reported that initially two equivalents of 2-phenyl propanoxy radical were generated by the thermal decomposition of DCP, which further yielded methyl radical upon elimination of 2-phenylpropan-2-ol. Methyl radical then attacked the N-oxide and formed another active radical species. This radical species interacts with DCP and generates the methylated product with 2-phenyl propanoxy radical and 2-phenyl propan-2-ol, which on losing an H2O molecule produces 2-phenyl-1-propene. As such, the 2-phenyl propanoxy radical generated above, takes part in the new reaction cycle (Scheme 26). The major concern regarding such a radical approach is the formation of inseparable mixtures of regioisomers. In 2016, Cho and co-workers negated the use of a radical approach and reported the C−2 methylation of pyridine-N-oxides by 1,1 diboryl-methane as methyl source, sodium methoxide as base, and toluene as solvent to obtain the methylated product in good yield (Scheme 27) [57]. This method was also applicable for the methylation of complex N-heterocycles substrates like 9-O-methylquinine N-oxide.
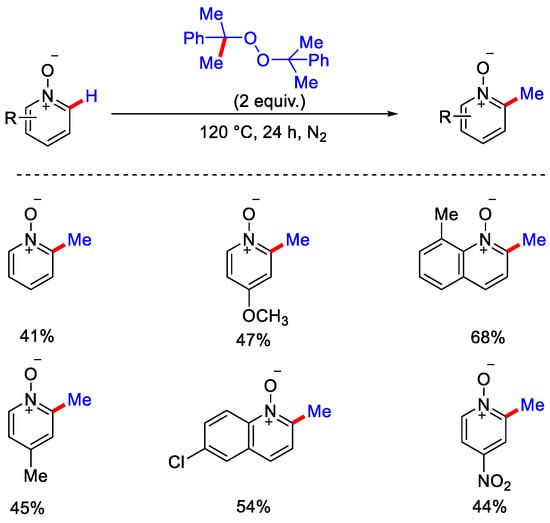
Scheme 25.
Metal-free radical based methylation of pyridine N-oxides.
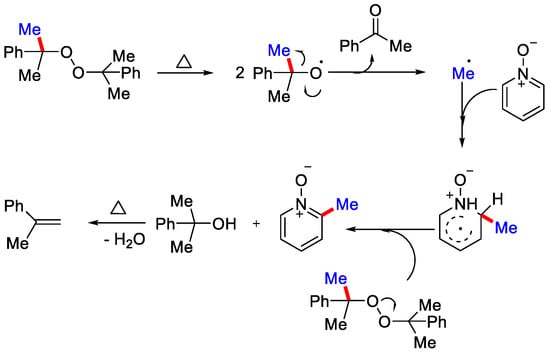
Scheme 26.
The mechanism involved for the metal-free methylation of pyridine N-oxides.
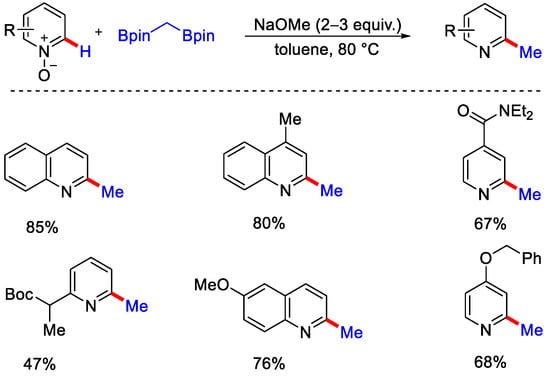
Scheme 27.
C−H methylation of pyridine N–oxides using 1,1 diboryl–methane.
The authors proposed that 1,1 diboryl-methane with NaOMe formed the α−boryl carbanion intermediate via a monoborate species. Then this carbanion intermediate attacked the C−2 position of pyridine N-oxide and formed another intermediate. Next, the migration of boron occurred to the N-oxide and formed another intermediate, which after the proton transfer to a benzylic position generated the desired methylated product by the re-aromatisation and elimination of NaOBpin (Scheme 28).
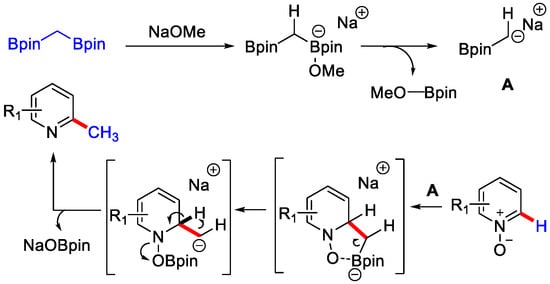
Scheme 28.
The mechanism involved for C−H methylation of pyridine N–oxides.
For the mechanistic studies, they performed deuterium labeling experiments, by which they observed 93% incorporation of deuterium at the benzylic position in the product, indicating that the C-2 H bond of heterocycle N-oxide acted as a proton source for the proto-deboronation, which occurred intramolecularly (shown by cross over experiments). This protocol proved to be a simple and scalable strategy for pyridine compounds.
Then in 2018, Han, Kim and co-workers reported the transition metal-free C−H alkylation of pyridine and quinolone N-oxides. Phosphonium salt as a methyl source, potassium tert-butoxide as a base and methyl tert-butyl ether as a solvent were used to get the methylated product in moderate to good yield (Scheme 29). The authors reported that phosphoniumylide was formed by the reaction of KOtBu with MePPh3I, then this ylide underwent intermolecular [3 + 2] annulation and formed an intermediate, which generated the methylated product after aromatization [58].
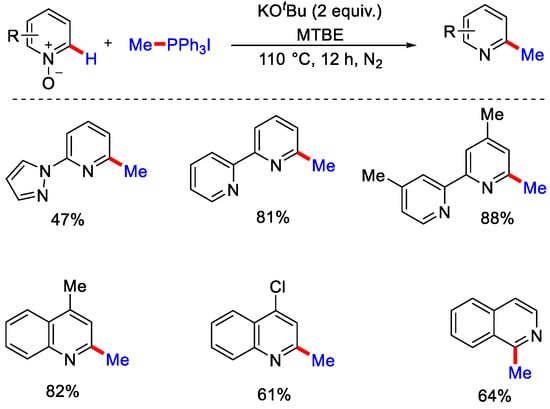
Scheme 29.
C−H methylation of quinolines and pyridine N-oxides with phosphonium ylides.
To confirm the formation of phosphoniumylides as the intermediate, the authors reported that when pyridine N-oxide was treated with bromo alkyl phosphonium salt under the optimal conditions, cycloalkylated product was formed, which confirms the formation of phosphoniumylide initially from bromo alkyl phosphonium salts, which further generated the corresponding ylide intermediate by reacting with a base. Furthermore, in 2019, Chung, Kim and co-workers extended this methodology to form diazine N-oxides using phosphonium ylides and got the desired methylated products in moderate to good yield (Scheme 30) [59].
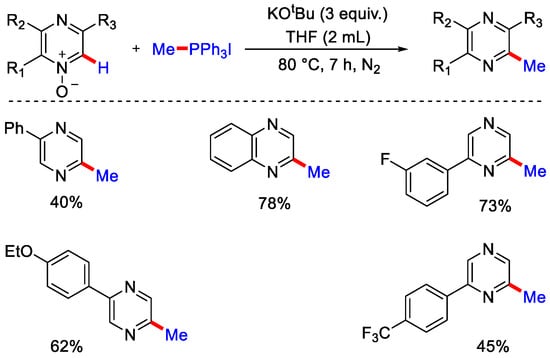
Scheme 30.
C−H methylation of N-pyrazines with phosphonium ylides.
This method was applicable to a wide range of substrates and had a high functional group tolerance with high selectivity. The utility of this method was described by sequential C−H alkylation. Furthermore, the late stage functionalization of complex molecules highlighted the significance of this with respect to drug discovery. Again in 2020, the same group reported the C−2 methylation of heterocycle N-oxide with sulfonium ylides as methyl source. Heterocyclic N-oxide reacted with sulfonium ylide to form betaine intermediate instead of aza-oxathiolane due to the weak S−O bond. Then, this intermediate underwent E−2 elimination, followed by aromatization to generate a C−2 methylated product (Scheme 31) [60].
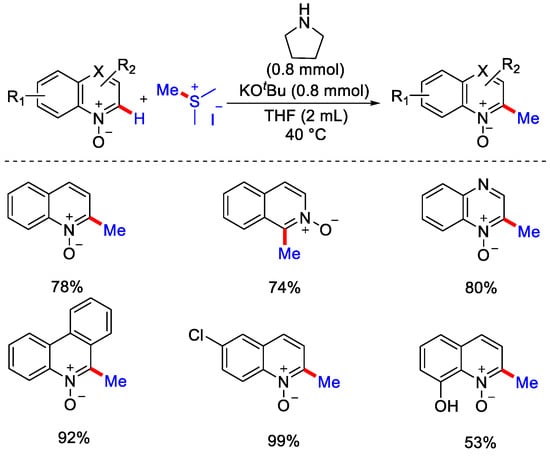
Scheme 31.
C−H methylation of N-heterocycles with sulphonium ylides.
Under a deuterium labeled experiment with quinoline N-oxide, no deuterium incorporation was observed at a benzylic position, indicating there was no intramolecular migration of deuterium at the benzylic position. Recently they have extended the use of sulfur ylides, like trimethylsulphonium ylides, for the methylation of iminoamidodiazines and triazines heterocycles by negating the use of organic solvents. Use of green solvent (H2O) and economical base (KOH) made this protocol important for pharmaceutical industries. Also, a wide range of N-alkylated and N-benzylated pyrazinones were methylated with good yield (Scheme 32) [61].
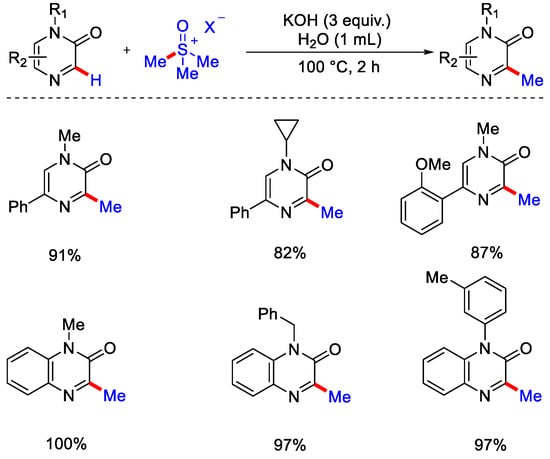
Scheme 32.
C−H methylation of N−heterocycles via sulphonium ylides in H2O as solvent.
Some bioactive molecules like pyrazinone, quinoxalinone and azauracils were also well versed under this methodology. The Zhang group reported the metal-free, radical mediated methylation of pyrimidinones and pyridinones utilizing dicumyl peroxide (DCP). Pyrimidinone derivatives and DCP in HOAc as solvent were stirred at 120 °C (Scheme 33) [62]. Due to the involvement of a large amount of peroxide in the reaction vessel precautions should be taken while heating. The waste obtained during reaction process was minimal as the solvent (HOAc) and the DCP derived acetophenone as byproduct were easily recovered by distillation and the product was purified by crystallization. On heating DCP produces cumyloxy radical, then the methyl radical gets generated by β−scission of alkoxy radical. Then, this methyl radical selectively attacks the fifth position of the pyridinone scaffold to yield another radical species, which gets aromatized to form the corresponding methylated product (Scheme 34).
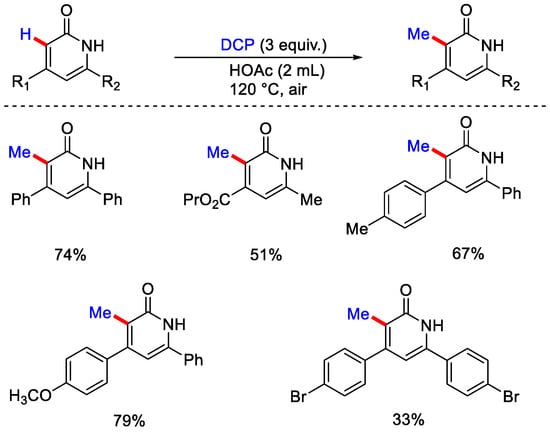
Scheme 33.
C−H methylation of pyrimidinones and pyridinones using DCP.
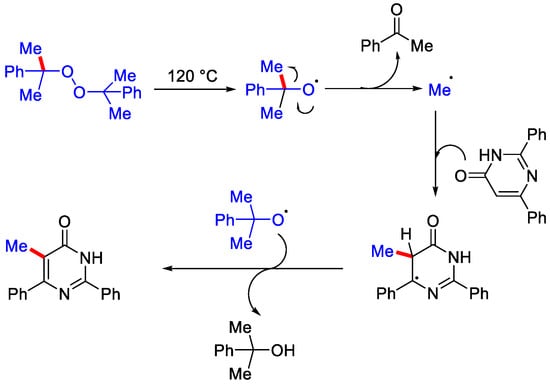
Scheme 34.
Mechanism involved for the methylation of pyrimidinones.
Non-phenyl substituted pyrimidinones did not give any methylated product due to their radical intermediates having less stability. Ghosh and Sen developed another metal-free approach for the methylation of quinolone N-oxides with tert-butanol as a methylating reagent, hypervalent iodine reagent PhI(OAc)2 as an oxidant and K2CO3 as a base (Scheme 35) [63].
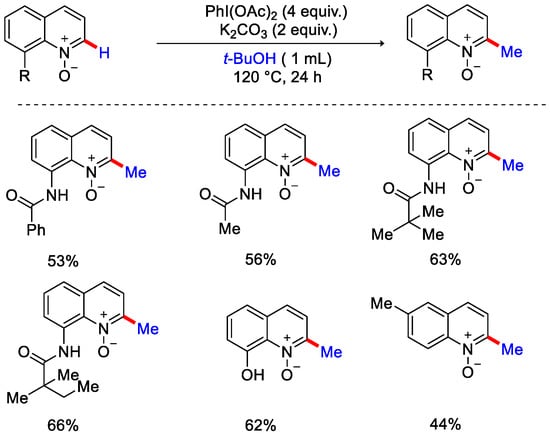
Scheme 35.
C−H methylation of N−heterocycles using tert butanol as methyl source.
Simple 8-hydroxy quinoline (N-oxide free) gave no reaction under optimal reaction conditions signifying that N-oxide was crucial for this transformation. Furthermore, when they added BHT and TEMPO, the reaction was inhibited, which shows that the reaction underwent a SET pathway. In the proposed mechanism, PhI(OAc)2 reacts with tert-butanol to form an intermediate, which reacts with the substrate. It generates another intermediate followed by the homolytic cleavage of C−C bond of alcohol via a SET pathway. Finally, after methylation and aromatization to yield the corresponding 2-methylated product. Zard and co-workers showcased radical methylation of heteroarenes using carboxylic xanthate along with dilauroyl peroxide (DLP) to form α−carboxymethyl radical. This α−carboxymethyl radical adds to heteroarene to give radical adduct, which oxidize by e− transfer to the peroxide followed by aromatization to give the addition product. This finally underwent decarboxylation to produce methylated heteroarene (Scheme 36) [64].
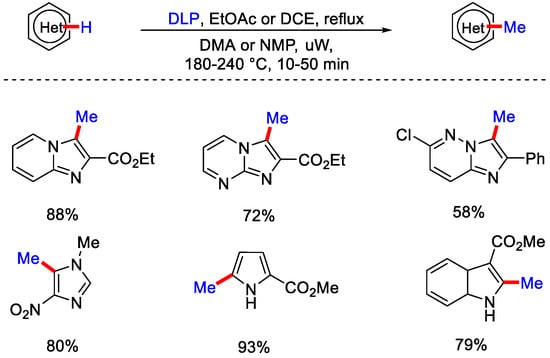
Scheme 36.
C−H methylation of N-heterocycles using DLP.
In 2020, Yan and co-workers reported the methylation of quinoxalin-2-(1H)-one and imidazo[1,2-a] pyridine derivatives with DCP as a methyl source in acetic acid under inert atmosphere and metal-free conditions at 120 °C for 24 h [65]. Both e− withdrawing and e− donating groups containing substrates produced the desired methylated product in moderate to good yield (Scheme 37). However, substrate with a methyl group at C−3 position of imidazo[1,2-a] pyridine and tert-butyl group at C−2 position did not give methylated product under the optimal reaction condition. Moreover, 2-thiophene substituted imidazo[1,2-a] pyridines gave a product with poor yield. The authors tested the radical pathway by using radical scavengers such as TEMPO, BHT and ethane-1-1-diyldibenzene. The addition of these reagents suppressed the formation of methylated product, confirming radical mechanism.
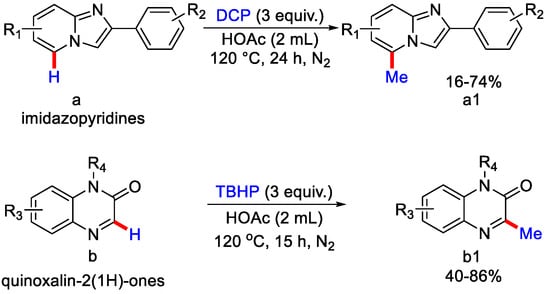
Scheme 37.
Methylation of quinoxalin–2–(1H)–one and imidazo[1,2–a] pyridine derivatives.
As per the proposed mechanism, upon heating, peroxides form an alkoxy radical, followed by the generation of methyl radical via β-scission. This methyl radical attacks the C−5 position of 2-phenyl imidazo[1,2-a] pyridines: (a) to form C−C intermediate, then this intermediate with 2-phenyl propanoxy radical generates the corresponding methylated product while with quinoxalin-2-(1H)-ones; (b), methyl radical reacts and forms a radical intermediate, then after SET and deprotonation generates the methylated product (Scheme 38). Metal-free C−3 methylation of quinoxalin-2-(1H)ones using tertbutylhydroperoxide (TBHP) as methylating reagent with iodine molecule and Na2SO3 as additive in acetonitrile as solvent was reported by Gu, Xia and co-workers [66]. Under the optimized reaction conditions, the authors reported the C−3 methylation of many N-substituted quinoxaline moderate yields (Scheme 39) except for N-propynyl substrate, which gave the product a lower yield due to the high reactivity of the terminal alkyne moiety.
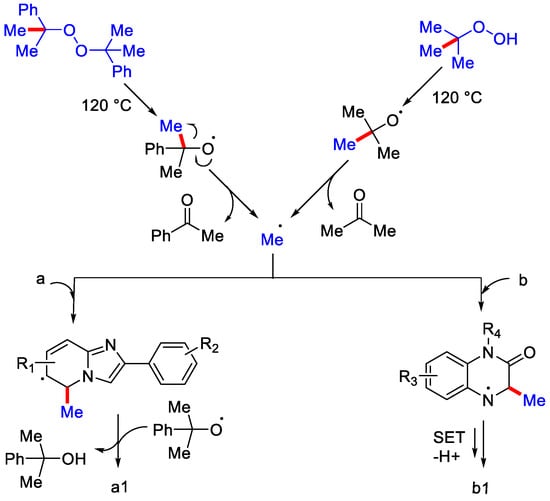
Scheme 38.
The mechanism involved in the methylation of quinoxalin-2-(1H)-ones and imidazo[1,2-a] pyridines.
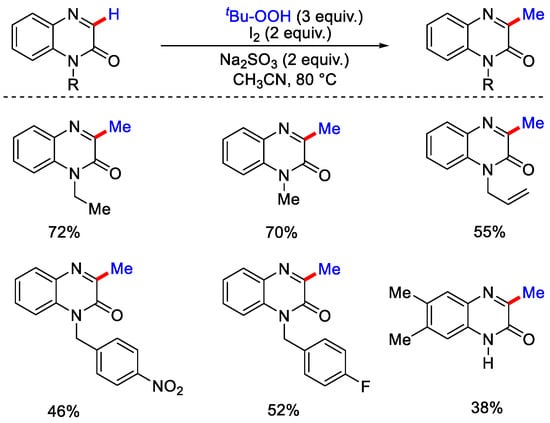
Scheme 39.
Methylation of quinoxalin-2-(1H)ones using TBHP.
Furthermore, quinoxalin-2-(1H)ones containing a halide group and strong e− withdrawing group, such as NO2, were also well tolerated under this reaction. After performing this reaction under dark and inert conditions, yields remained unaffected, confirming that visible light and oxygen are not essential for this protocol. The addition of TEMPO did not lead to the desired product, which indicates that this transformation occurs via a radical pathway. As per the plausible mechanism, the first TBHP undergoes thermal decomposition to generate the tert-butoxy radical and hydroxyl radical. Then, the methyl radical is generated from the tert-butoxy radical by β−scission. I2 and hydroxyl radical oxidizes Na2SO3 to Na2SO4, simultaneously generate the iodine radical. Methyl radical assisted by iodine radical selectively attacks on C−3 position of quinoxalin-2-(1H)ones and forms an intermediate, which by eliminating HI molecule produces the desired methylated product. For biologically active molecules, quinolones and pyridines are important structural motifs. Therefore, the development of new methodologies for the synthetic transformation of these molecules has received considerable efforts.
7. Conclusions
The interest in C−H methylation has grown rapidly due to its biological importance in medicinal chemistry, which is popularly called the “Magic Methyl Effect”. The installation of a methyl group on heterocycles at a strategic position modulates its potency. A radical based approach for the alkylation of heterocycles was first proposed by Francesco Minisci. Later on, various groups came up with modified Minisci-type reactions via different strategies viz. photo-redox, electrochemical, mechanochemical, transition metal catalyzed as well as metal-free. So far various methyl sources have been discovered, e.g., CH3OH, CH3COOH, DMSO, CH3B(OH)2, CH4, DCP, TBHP, etc. Photo-redox and electrochemical approaches usually negate the use of the harsh oxidants. Mechanochemical protocol helps in minimizing the solvent waste. Metal-free approaches are providing a new direction; to methylate the carbon centers by avoiding the use of metal catalyst. This helps in reducing the metal contamination. These sustainable approaches for C−H methylation of both sp2 and sp3 carbon centers fueled the remarkable development in its applications. However, these methodologies still have lots of shortcomings and there is still plenty of potential in the area of C−H methylation. Photo-redox C−H methylation is mainly limited to a metal based photo catalyst, mostly Ir and Ru salts, and those are quite expensive. There is much scope to replace the Ir and Ru based photocatalysts by organophotocatalysts, which are relatively cheaper and greener. Electrochemical and mechanochemical methodologies are limited to proximal C−H bond methylation. Distal aliphatic and aromatic C−H methylation can still be explored. Artificial metalloenzyme catalyzed asymmetric C−H methylation can lead to synthesis of drugs precursors. Carbon dioxide as methylating reagent can help to achieve net-zero. C−H methylation of arenes and heteroarenes at distal sites via directed or non-directed strategy is still elusive. Development of 3D metal catalyst for methylation is an emerging area on which to focus. So to address these inadequacies, scientists need to come up with new design of ligands, catalysts, and methylating reagents to achieve C−H methylation in a more productive and greener fashion.
Author Contributions
Original draft preparation, I.A. and G.P.; Writing—review and editing, I.A., G.P., S.A.A.-T., M.M. and D.M.; Supervision, M.M., D.M. and S.A.A.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by SERB India (TTR/2021/000108).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are thankful to SERB India (TTR/2021/000108) for financial support. Financial support from CSIR India (Fellowship to G.P.) is gratefully acknowledged.
Conflicts of Interest
There are no conflict to declare.
References
- Moir, M.; Danon, J.J.; Reekie, T.A.; Kassiou, M. An overview of late-stage functionalization in today’s drug discovery. Expert Opin. Drug Discov. 2019, 14, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Jana, R.; Begam, H.M.; Dinda, E. The emergence of the C–H functionalization strategy in medicinal chemistry and drug discovery. Chem. Commun. 2021, 57, 10842–10866. [Google Scholar] [CrossRef]
- Dutta, U.; Maiti, S.; Bhattacharya, T.; Maiti, D. Arene Diversification through Distal C(sp2)−H Functionalization. Science 2021, 372, eabd5992. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.M.L.; Bois, J.D.; Yu, J.-Q. C–H Functionalization in Organic Synthesis. Chem. Soc. Rev. 2011, 40, 1855–1856. [Google Scholar] [CrossRef]
- Ali, W.; Prakash, G.; Maiti, D. Recent Development in Transition Metal-Catalysed C–H Olefination. Chem. Sci. 2021, 12, 2735–2759. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.; Paul, N.; Oliver, G.A.; Werz, D.B.; Maiti, D. C–H Deuteration of Organic Compounds and Potential Drug Candidates. Chem. Soc. Rev. 2022, 51, 3123–3163. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.J.; Kemmerle, A.E.; Fraga, C.A.M. The Methylation Effect in Medicinal Chemistry. Chem. Rev. 2011, 111, 5215–5246. [Google Scholar] [CrossRef] [PubMed]
- Schçnherr, H.; Cernak, T. Profound Methyl Effects in Drug Discovery and a Call for New C–H Methylation Reactions. Angew. Chem. Int. Ed. 2013, 52, 12256–12267. [Google Scholar] [CrossRef]
- Ritchie, T.J.; Macdonald, S.J.F.; Pickett, S.D. Insights into the impact of N-and O-methylation on aqueous solubility and lipophilicity using matched molecular pair analysis. Med. Chem. Comm. 2015, 6, 1787–1797. [Google Scholar] [CrossRef]
- Kuntz, K.W.; Campbell, J.E.; Keilhack, H.; Pollock, R.M.; Knutson, S.K.; Porter-Scott, M.; Richon, V.M.; Sneeringer, C.J.; Wigle, T.J.; Allain, C.J.; et al. The importance of being me: Magic methyls, methyl transferase inhibitors, and the discovery of tazemetostat. J. Med. Chem. 2016, 59, 1556–1564. [Google Scholar] [CrossRef]
- Vitaku, E.; Ilardi, E.A.; Njarðarson, J.T. Top 200 Pharmaceutical Products by US Retail Sales in 2018; University of Arizona: Tucson, AZ, USA. Available online: https://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/2018Top200PharmaceuticalRetailSalesPosterLowResFinalV2.pdf (accessed on 2 May 2018).
- McGrath, N.A.; Brichacek, M.; Njarðarson, J.T. A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Educ. 2010, 87, 1348. [Google Scholar] [CrossRef]
- Sinha, S.K.; Guin, S.; Maiti, S.; Biswas, J.P.; Porey, S.; Maiti, D. Toolbox for Distal C–H bond functionalizations in organic molecules. Chem. Rev. 2022, 6, 5682–5841. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.; Prakash, G.; Goswami, N.; Maiti, D. Traditional and Sustainable Approaches for the Construction of C–C Bonds by Harnessing C–H Arylation. Nat. Commun. 2022, 13, 1085. [Google Scholar] [CrossRef]
- Uygur, M.; Mancheno, O.G. Visible light-mediated organophotocatalysed C–H bond functionalization reactions. Org. Biomol. Chem. 2019, 17, 5475–5489. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Marzo, L.; Das, A.; Shaikh, R.; König, B. Visible light-mediated Photo-redox Catalytic Arylation Reactions. Acc. Chem. Res. 2016, 49, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Fang, P.; Mei, T.-S. Recent Advances in C–H Functionalization Using Electrochemical Transition Metal Catalysis. ACS Catal. 2018, 8, 7179–7189. [Google Scholar] [CrossRef]
- Hernández, J.G. Mechanochemical Palladium-Catalysed Carbonylative Reactions Using Mo(CO)6. Chem. Eur. J. 2017, 23, 17157–17165. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Liu, C.; Zhao, Y. Recent advances in mechanochemical C–H functionalization reactions. Tetrahedron Lett. 2018, 59, 317–324. [Google Scholar] [CrossRef]
- Ranu, B.C.; Ghosh, T.; Jalal, S. Recent developments in CH functionalization via CH bond activation using ball milling and transition-metal catalysts. Arkivoc 2019, 2019, 79–92. [Google Scholar] [CrossRef]
- Shamsabadi, A.; Chudasama, V. Recent advances in metal-free aerobic C–H activation. Org. Biomol. Chem. 2019, 17, 2865–2872. [Google Scholar] [CrossRef] [Green Version]
- Samanta, R.; Matcha, K.; Antonchick, A.P. Metal-Free Oxidative Carbon-Heteroatom Bond Formation Through C–H Bond Functionalization. Eur. J. Org. Chem. 2013, 2013, 5769–5804. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Dixon, J.A.; O’Hara, F.; Funder, E.D.; Dixon, D.D.; Rodriguez, R.A.; Baxter, R.D.; Herle, B.; Sach, N.; Collins, M.R.; et al. C–H Methylation of Heteroarenes Inspired by Radical SAM Methyl Transferase. Nature 2012, 492, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Punta, C.; Minisci, F. Minisci Reaction: A Friedel-Crafts Type Process with Opposite Reactivity and Selectivity. Selective Homolytic Alkylation, Acylation, Carboxylation and Carbamoylation of Heterocyclic Aromatic Bases. Trends Heterocycl. Chem. 2008, 13, 1–68. [Google Scholar] [CrossRef]
- Molander, G.A.; Colombel, V.; Braz, V.A. Direct alkylation of heteroaryls using potassium alkyl-and alkoxymethyltrifluoroborates. Org. Lett. 2011, 13, 1852–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiRocco, D.A.; Dykstra, K.; Krska, S.; Vachal, P.; Conway, D.V.; Tudge, M. Late-Stage Functionalization of Biologically Active Heterocycles Through Photo-redox Catalysis. Angew. Chem. Int. Ed. 2014, 53, 4802–4806. [Google Scholar] [CrossRef]
- Jin, J.; MacMillan, D.W.C. Alcohols as alkylating agents in heteroarene C–H functionalization. Nature 2015, 525, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Huff, C.A.; Cohen, R.D.; Dykstra, K.D.; Fuss, E.S.; DiRocco, D.A.; Krska, S.W.J. Photo-redox-Catalysed Hydroxymethylation of Heteroaromatic Bases. Org. Chem. 2016, 16, 6980–6987. [Google Scholar] [CrossRef]
- Liu, W.; Yang, X.; Zhou, Z.-Z.; Li, C.-J. Simple and Clean Photo-induced Methylation of Heteroarenes with MeOH. Chem 2017, 2, 688–702. [Google Scholar] [CrossRef] [Green Version]
- McCallum, T.; Pitre, S.P.; Morin, M.; Scaiano, J.C.; Barriault, L. The Photochemical Alkylation and Reduction of Heteroarenes. Chem. Sci. 2017, 8, 7412–7418. [Google Scholar] [CrossRef] [Green Version]
- Zidan, M.; Morris, A.O.; McCallum, T.; Barriault, L. The Alkylation and Reduction of Heteroarenes with Alcohols Using Photo-redox Catalysed Hydrogen Atom Transfer via Chlorine Atom Generation. Eur. J. Org. Chem. 2020, 2020, 1453–1458. [Google Scholar] [CrossRef]
- Cheng, W.-M.; Shang, R.; Fu, M.-C.; Fu, Y. Photo-redox-Catalysed Decarboxylative Alkylation of N-Heteroarenes with N-(Acyloxy)phthalimides. Chem. Eur. J. 2017, 23, 2537–2541. [Google Scholar] [CrossRef] [PubMed]
- Genovino, J.; Lian, Y.; Zhang, Y.; Hope, T.O.; Juneau, A.; Gagné, Y.; Ingle, G.; Frenette, M. Metal-Free-Visible Light C−H Alkylation of Heteroaromatics via Hypervalent Iodine-Promoted Decarboxylation. Org. Lett. 2018, 20, 3229–3232. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, T.C.; Li, N.; Yazdani, A.N.; Dhar, T.G.M.J. Organocatalysed, Visible-Light Photo-redox-Mediated, One-Pot Minisci Reaction Using Carboxylic Acids via N-(Acyloxy)phthalimides. Org. Chem. 2018, 83, 3000–3012. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Su, Y.; Wang, K.-H.; Zhang, R.; Feng, Y.; Cao, L.; Huang, D.; Hu, Y. Visible-light induced decarboxylative alkylation of quinoxalin-2(1H)-ones at the C3-position. Org. Biomol. Chem. 2019, 17, 6654–6661. [Google Scholar] [CrossRef]
- Lai, X.; Shu, X.; Song, J.; Xu, H. Electrophotocatalytic Decarboxylative C–H Functionalization of Heteroarenes. Angew. Chem. Int. Ed. 2020, 59, 10626–10632. [Google Scholar] [CrossRef]
- Garza-Sanchez, R.A.; Patra, T.; Tlahuext-Aca, A.; Strieth-Kalthoff, F.; Glorius, F. DMSO as a Switchable Alkylating Agent in Heteroarene C–H Functionalization. Chem. A Eur. J. 2018, 24, 10064–10068. [Google Scholar] [CrossRef]
- Jiang, S.; Yang, Z.; Guo, Z.; Li, Y.; Chen, L.; Zhu, Z.; Chen, X. Transition Metal-Free α−Methylation of 1,8-Naphthyridine Derivatives Using DMSO as Methylation Reagent. Org. Biomol. Chem. 2019, 17, 7416–7424. [Google Scholar] [CrossRef]
- Li, G.-X.; Morales-Rivera, C.A.; Wang, Y.; Gao, F.; He, G.; Liu, P.; Chen, G. Photo-redox-Mediated Minisci C−H Alkylation of NHeteroarenes Using Boronic Acids and Hypervalent Iodine. Chem. Sci. 2016, 7, 6407–6412. [Google Scholar] [CrossRef] [Green Version]
- Hu, A.; Guo, J.-J.; Pan, H.; Zuo, Z. Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis. Science 2018, 361, 668–672. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, B.-J.; Shi, Z.-J. Challenge and progress: Palladium-catalyzed sp3 C–H activation. Catal. Sci. Technol. 2011, 1, 191–206. [Google Scholar] [CrossRef]
- Le, C.; Liang, Y.; Evans, R.W.; Li, X.; MacMillan, D.W.C. Selective sp3 C–H alkylation via polarity-matchbased cross-coupling. Nature 2017, 547, 79–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Qiu, Z.; Tan, L.; Rashid, R.T.; Chu, S.; Cen, Y.; Luo, Z.; Khaliullin, R.Z.; Mi, Z.; Li, C.-J. Photocatalytic Methylation of Non activated sp3 and sp2 C−H Bonds Using Methanol on GaN. ACS Catal. 2020, 10, 6248–6253. [Google Scholar] [CrossRef]
- Vasilopoulos, A.; Krska, S.W.; Stahl, S.S. C(sp3)–H methylation enabled by peroxide photosensitization and Ni-mediated radical coupling. Science 2021, 372, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel, S.R.; Lips, S.; Selt, M.; Riehl, B.; Kampf, C.J. Electrochemical arylation reaction. Chem. Rev. 2018, 118, 6706–6765. [Google Scholar] [CrossRef] [PubMed]
- Röckl, J.L.; Pollok, D.; Franke, R.; Waldvogel, S.R. A decade of electrochemical dehydrogenative C, C-coupling of aryls. Acc. Chem. Res. 2020, 53, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhao, C.-Q.; Li, Y.-Q.; Zhang, L.-P.; Xu, X.-T.; Zhang, K.; Mei, T.-S. Palladium-catalysed C–H activation/C–C cross-coupling reactions via electrochemistry. Chem. Commun. 2017, 53, 12189–12192. [Google Scholar] [CrossRef]
- Yang, Q.-L.; Li, C.-Z.; Zhang, L.-W.; Li, Y.-Y.; Tong, X.; Wu, X.-Y.; Mei, T.-S. Palladium-Catalysed Electrochemical C−H Alkylation of Arenes. Organometallics 2019, 38, 1208–1212. [Google Scholar] [CrossRef]
- Novaes, L.F.T.; Ho, J.S.K.; Mao, K.; Liu, K.; Tanwar, M.; Neurock, M.; Villemure, E.; Terrett, J.A.; Lin, S. Exploring Electrochemical C(sp3)−H Oxidation for the Late-Stage Methylation of Complex Molecules. J. Am. Chem. Soc. 2022, 3, 1187–1197. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A force of synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [Green Version]
- James, S.L.; Friscic, T. Mechanochemistry. Chem. Soc. Rev. 2013, 42, 7494–7496. [Google Scholar] [CrossRef]
- Ni, S.; Hribersek, M.; Baddigam, S.K.; Ingner, F.J.L.; Orthaber, A.; Gates, P.J.; Pilarski, L.T. Mechanochemical Solvent-Free Catalytic C—H Methylation. Angew. Chem. Int. Ed. 2021, 60, 6660–6666. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Gevorgyan, V. Direct Transition Metal-Catalyzed Functionalization of Heteroaromatic Compounds. Chem. Soc. Rev. 2007, 36, 1173. [Google Scholar] [CrossRef] [PubMed]
- Urbina, K.; Tresp, D.; Sipps, K.; Szostak, M. Recent Advances in Metal-Catalyzed Functionalization of Indoles. Adv. Synth. Catal. 2021, 363, 2723–2739. [Google Scholar] [CrossRef]
- Dalton, T.; Faber, T.; Glorius, F. C–H Activation: Toward Sustainability and Applications. ACS Cent. Sci. 2021, 7, 245–261. [Google Scholar] [CrossRef]
- Li, G.; Yang, S.; Lv, B.; Han, Q.; Ma, X.; Sun, K.; Wang, Z.; Zhao, F.; Lv, Y.; Wu, H. Metal-free Methylation of Pyridine N-Oxides C–H Bond by Using Peroxides. Org. Biomol. Chem. 2015, 13, 11184–11188. [Google Scholar] [CrossRef]
- Jo, W.; Kim, J.; Choi, S.; Cho, S.H. Transition-Metal-Free Regioselective Alkylation of Pyridine N-Oxides Using 1,1-Diborylalkanes as Alkylating Reagents. Angew. Chem. Int. Ed. 2016, 55, 9690–9694. [Google Scholar] [CrossRef]
- Han, S.; Chakrasali, P.; Park, J.; Oh, H.; Kim, S.; Kim, K.; Pandey, A.K.; Han, S.H.; Han, S.B.; Kim, I.S. Reductive C2-Alkylation of Pyridine and Quinoline N-Oxides Using Wittig Reagents. Angew. Chem. Int. Ed. 2018, 57, 12737–12740. [Google Scholar] [CrossRef]
- Ghosh, P.; Kwon, N.Y.; Han, S.; Kim, S.; Han, S.H.; Mishra, N.K.; Jung, Y.H.; Chung, S.J.; Kim, I.S. Site-Selective C−H Alkylation of Diazine N-Oxides Enabled by Phosphonium Ylides. Org. Lett. 2019, 21, 6488–6493. [Google Scholar] [CrossRef]
- An, W.; Choi, S.B.; Kim, N.; Kwon, N.Y.; Ghosh, P.; Han, S.H.; Mishra, N.K.; Han, S.; Hong, S.; Kim, I.S. C2-Selective C−H Methylation of Heterocyclic N-Oxides with Sulfonium Ylides. Org. Lett. 2020, 22, 9004–9009. [Google Scholar] [CrossRef]
- Ghosh, P.; Kwon, N.Y.; Kim, S.; Han, S.; Lee, S.H.; An, W.; Mishra, N.K.; Han, S.B.; Kim, I.S. C–H Methylation of Iminoamido Heterocycles with Sulfur Ylides. Angew. Chem. Int. Ed. 2021, 60, 191–196. [Google Scholar] [CrossRef]
- Zhang, P.-Z.; Li, J.-A.; Zhang, L.; Shoberu, A.; Zou, J.-P.; Zhang, W. Metal-free radical C–H methylation of pyrimidinones and pyridinones with dicumyl peroxide. Green Chem. 2017, 19, 919–923. [Google Scholar] [CrossRef]
- Sen, C.; Ghosh, S.C. Transition-Metal-Free Regioselective Alkylation of Quinoline N-Oxides via Oxidative Alkyl Migration and C-C Bond Cleavage of tert-/sec-Alcohols. Adv. Synth. Catal. 2018, 360, 905–910. [Google Scholar] [CrossRef]
- Huang, Q.; Zard, S.Z. Inexpensive Radical Methylation and Related Alkylations of Heteroarenes. Org. Lett. 2018, 20, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yao, H.; Lin, S.; You, X.; Yang, Y.; Yan, Z. Peroxide-mediated site-specific C–H methylation of imidazo [1, 2-a]pyridines and quinoxalin-2(1H)-ones under metal-free conditions. Org. Biomol. Chem. 2020, 18, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Jin, L.; Gu, Y.; Liang, G.; Xia, Q. Transition-Metal-free Radical C–H Methylation of Quinoxalinones with TBHP. Asian J. Org. Chem. 2020, 9, 185–188. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).