Preparation and Electrocatalytic Application of Copper- and Cobalt-Carbon Composites Based on Pyrolyzed Polymer
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Metal Oxide-AFP Composites
3.3. Heat Treatment of the Metal Oxide-AFP Composites
3.4. Electrochemical Experiments
3.5. Physical-Chemical Investigations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodrigues-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–164. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef] [Green Version]
- Gerber, I.C.; Serp, P. A theory/experience description of support effects in carbon-supported catalysts. Chem. Rev. 2020, 120, 1250–1349. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Lyu, S.; Si, J.; Yang, B.; Li, Z.; Lei, L.; Wen, Z.; Wu, G.; Hou, Y. Nanostructures carbon based heterogeneous electrocatalysts for oxygen evolution reaction in alkaline media. ChemCatChem 2019, 11, 5855–5874. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Rozenberg, A.S.; Dzhardimalieva, G.I. Termolysis of metallopolymers and their precursors as a method for the preparation of nanocomposites. Russ. Chem. Rev. 2011, 80, 257–292. [Google Scholar] [CrossRef]
- Pavlenko, V.V.; Anurov, S.A.; Mansurov, Z.A.; Biisenbayev, M.A.; Azat, S.; Tanirbergenova, S.K. Thermo-oxidative modification of plant fiber. News Nat. Acad. Sci. Repub. Kazakhstan Ser. Chem. Technol. 2014, 5, 47–52. [Google Scholar]
- Wu, Z.-Y.; Xu, S.-L.; Yan, Q.-Q.; Chen, Z.-Q.; Ding, Y.-W.; Li, C.; Liang, H.-W.; Yu, S.-H. Transition metal-assisted carbonization of small organic molecules toward functional carbon materials. Sci. Adv. 2018, 4, eaat0788. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Li, F.; Deng, G.; Guo, X.; Liu, H.; Jiang, W.; Wang, T. Polymerized complex method for preparation of supported bimetallic alloy and monometallic nanoparticles. Chem. Comm. 2016, 52, 2996–2999. [Google Scholar] [CrossRef]
- Rangraz, Y.; Heravi, V.V.; Elhampour, A. Recent advances in heteroatom-doped porous carbon/metal materials: Fascinating heterogeneous catalysts for organic transformations. Chem. Rec. 2021, 21, 1985–2073. [Google Scholar] [CrossRef]
- Mabena, L.F.; Ray, S.S.; Mhlanga, S.D.; Coville, N.J. Nitrogen-doped carbon nanotubes as a metal catalyst support. Appl. Nanosci. 2011, 1, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.Z.; Fan, W.B. Nitrogen-containing porous carbons: Synthesis and application. J. Mater. Chem. A 2013, 1, 999–1013. [Google Scholar] [CrossRef]
- Majeed, S.; Zhao, J.; Zhang, L.; Anjum, S.; Liu, Z.; Xu, G. Synthesis and electrochemical applications of nitrogen-doped carbon nanomaterials. Nanotechnol. Rev. 2013, 2, 615–635. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kurtyka, K.; Majumder, M.; Yang, X.; Ta, H.Q.; Bachmatiuk, A.; Liu, L.; Tizebicka, B.; Rummeli, M.H. Recent advances in boron- and nitrogen-doped carbon-based materials and their various applications. Adv. Mater. Interfaces 2022, 9, 2101964. [Google Scholar] [CrossRef]
- Pretschuh, C.; Schwarzinger, C.; Abdala, A.A.; Vukusic, S. Characterization of conductive nanographite melamine composites. Open J. Compos. Mater. 2014, 4, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, D.; Goel, C.; Bhunia, H.; Bajpai, P.K. Melamine-formaldehyde derived porous carbons for adsoption of CO2 capture. J. Environ. Manag. 2017, 197, 415–427. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, H.; Liu, S.; Deng, C.; Wang, M. Nitrogen-enriched carbon from melamine resins with superior oxygen reduction reaction activity. ChemSusChem 2013, 6, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Y.; Chen, X.; Gao, Q.; Zhang, W. Functionalized metal-organic frameworks for effective removal of rocephin in aqueous solutions. J. Colloid Interface Sci. 2018, 514, 234–239. [Google Scholar]
- Choi, C.H.; Park, S.H.; Woo, S.I. N-doped carbon prepared by pyrolysis of dicyandiamide with various MeCl2·xH2O (Me = Co, Fe, and Ni) composites: Effect of type and amount of metal seed on oxygen reduction reactions. Appl. Catal. B Environ. 2012, 119–120, 123–131. [Google Scholar] [CrossRef]
- Daems, N.; Wouters, J.; Van Goethem, C.; Baert, K.; Poleunis, C.; Delcorte, A.; Hubin, A.; Vankelecom, I.F.J.; Pescarmona, P.P. Selective reduction of nitrobenzene to aniline over electrocatalysts based on nitrogen-doped carbons containing non-noble metals. Appl. Catal. B Environ. 2018, 226, 509–522. [Google Scholar] [CrossRef]
- Kozhitov, L.V.; Muratov, D.G.; Yakushko, E.V.; Kozhitov, S.L.; Savchenko, A.G.; Shchetinin, I.V.; Emelyanov, S.G.; Chervjakov, L.M. The synthesis of metalcarbon nanocomposite Ni/C on the basis of polyacrylonitrile. J. Nano- Electron. Phys. 2013, 5, 5855–5874. [Google Scholar]
- Zaporotskova, I.A.; Kozhitov, L.V.; Anikeev, N.A.; Davletova, O.A.; Popkova, A.V.; Muratov, D.G.; Yakushko, E.V. Metal-carbon nanocomposites based on pyrolysed polyacrylonitrile. Mod. Electron. Mater. 2015, 1, 43–49. [Google Scholar] [CrossRef]
- Szikra, D.; Dublinszky, K.; Cserhati, C.; Nagy, I.P. Elaboration of composite electrodes based on Raney Nickel and Pd/C for electrosynthesis application. J. New Mater. Electrochem. Syst. 2010, 13, 133–140. [Google Scholar]
- Bondue, C.J.; Koper, M.T. Electrochemical reduction of the carbonyl functional group: The importance of adsorption geometry, molecular structure, and electrode surface structure. J. Am. Chem. Soc. 2019, 141, 12071–12078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilar, M.; Oliveiro, J.L.; Navarro, M. Investigation of the hydrogenation reactivity of some organic substrates using an electrocatalytic method. Appl. Catal. A Gen. 2010, 372, 1–7. [Google Scholar] [CrossRef]
- Villalba, M.; del Pozo, M.; Calvo, E.J. Electrocatalytic hydrogenation of acetophenone and benzophenone using palladium electrodes. Electrochem. Acta. 2015, 164, 125–131. [Google Scholar] [CrossRef]
- Yadav, G.D.; Mewada, R.K. Selective hydrogenation of acetophenone to 1-phenyl ethanol over nanofibrous Ag-OMS-2 catalysts. Catal. Today 2012, 198, 330–337. [Google Scholar] [CrossRef]
- Lin, S.; Liu, J.; Ma, L. Ni@C catalyzed hydrogenation of acetophenone to phenyl ethanol under industrial mild conditions in a flow reactor. React. Chem. Eng. 2022, 7, 1126–1135. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Chen, G.; He, Z. Highly loaded and dispersed Ni2P/Al2O3 catalyst with high selectivity for hydrogenation of acetophenone. Catalysts 2018, 8, 309. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.B.; Lu, W.N.; Wang, J.L.; Liao, B.H.; Qin, Y.H.; Zhang, Y.J.; Zhang, B.; Xin, S.A. Selective catalytic hydrogenation of acetophenone to 1-phenylethanol over Co/mordenite in water. Catal. Commun. 2019, 119, 124–128. [Google Scholar] [CrossRef]
- Pei, F.; Wang, Z.; Lv, J.; Li, F.; Xue, W. Efficient catalytic transfer hydrogenation of acetophenone to 1-phenylethanol over Cu-Zn-Al catalysts. Ind. Eng. Chem. Res. 2022, 61, 5419–5428. [Google Scholar] [CrossRef]
- Bertero, N.M.; Apesteguia, C.R.; Marchi, A.J. Catalytic and kinetic study of the liquid-phase hydrogenation of acetophenone over Cu/SiO2 catalyst. Appl. Catal. A Gen. 2008, 349, 100–109. [Google Scholar] [CrossRef]
- Saini, L.; Patra, M.K.; Dhaka, M.K.; Jani, R.K.; Gupta, G.K.; Dixit, A.; Vadera, S.R. Ni/Graphitic core-shell nanostructured based light weight elastomeric composites for Ku-band microwave absorbing applications. Cryst. Eng. Comm. 2018, 20, 4630–4640. [Google Scholar] [CrossRef]
- Chaudhary, J.; Tailor, G.; Verma, D.; Verma, R. Synthesis and characterization of cobalt nanocomposite using aniline-formaldehyde resin. Compos. Comm. 2020, 18, 13–18. [Google Scholar] [CrossRef]
- Chaudhary, J.; Tailor, G.; Tomer, N. Synthesis, characterization and studies on antimicrobial properties of copper nanocomposite. Mat. Sci. Ind. J. 2018, 16, 130. [Google Scholar]
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J. Phys. Chem. C 2010, 114, 111–119. [Google Scholar] [CrossRef]
- Ivanova, N.M.; Visurkhanova, Y.A.; Soboleva, E.A.; Pavlenko, N.A.; Muldakhmetov, Z.M. Structure and electrocatalytic activity of aniline-formaldehyde polymer doped with copper(II) chloride. ChemistrySelect 2016, 1, 5304–5309. [Google Scholar] [CrossRef]
- Ivanova, N.M.; Soboleva, E.A.; Visurkhanova, Y.A.; Lazareva, E.S. Structure-phase changes in polymer composites doped with silver nitrate and their electrocatalytic activity. Russ. J. Electrochem. 2018, 54, 1010–1017. [Google Scholar] [CrossRef]
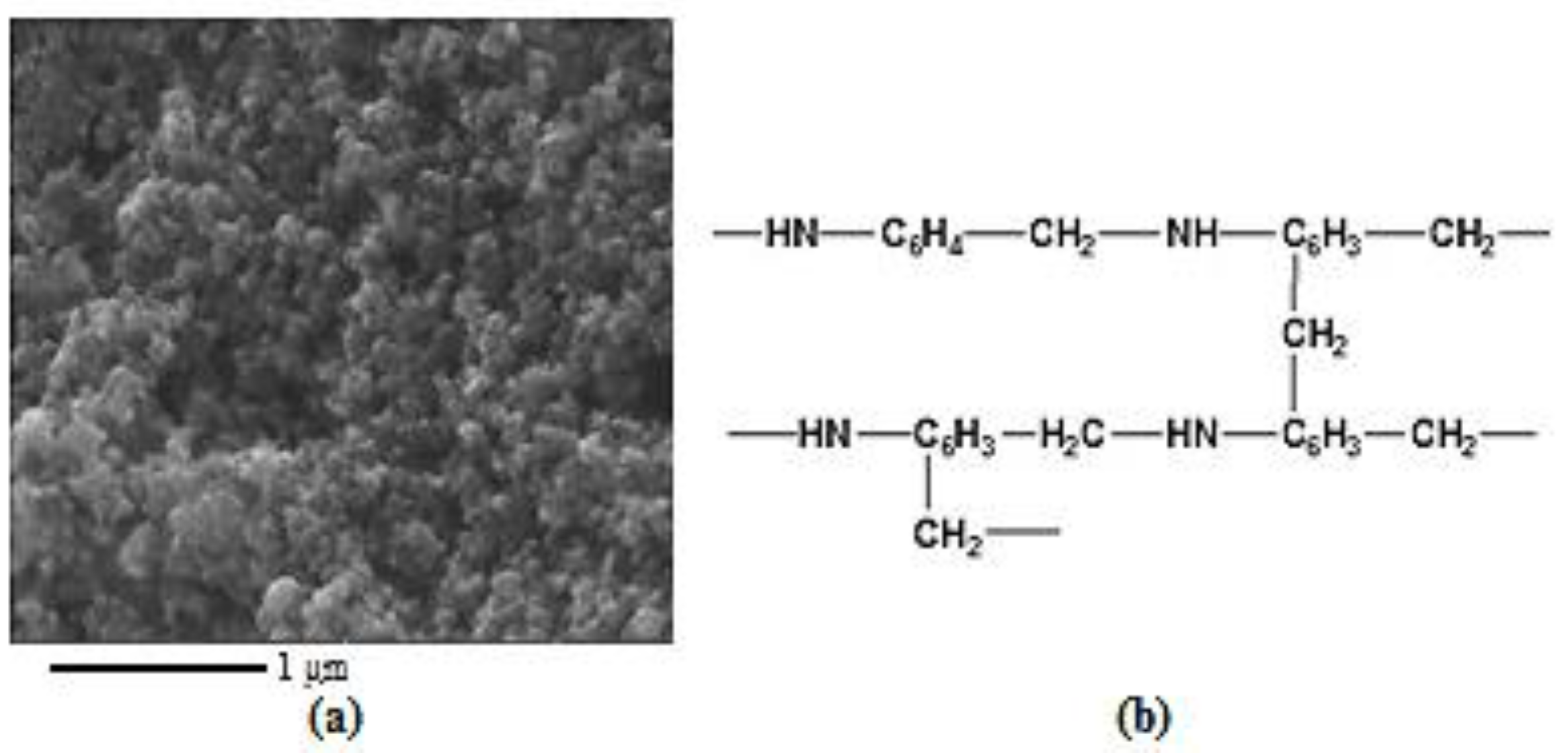

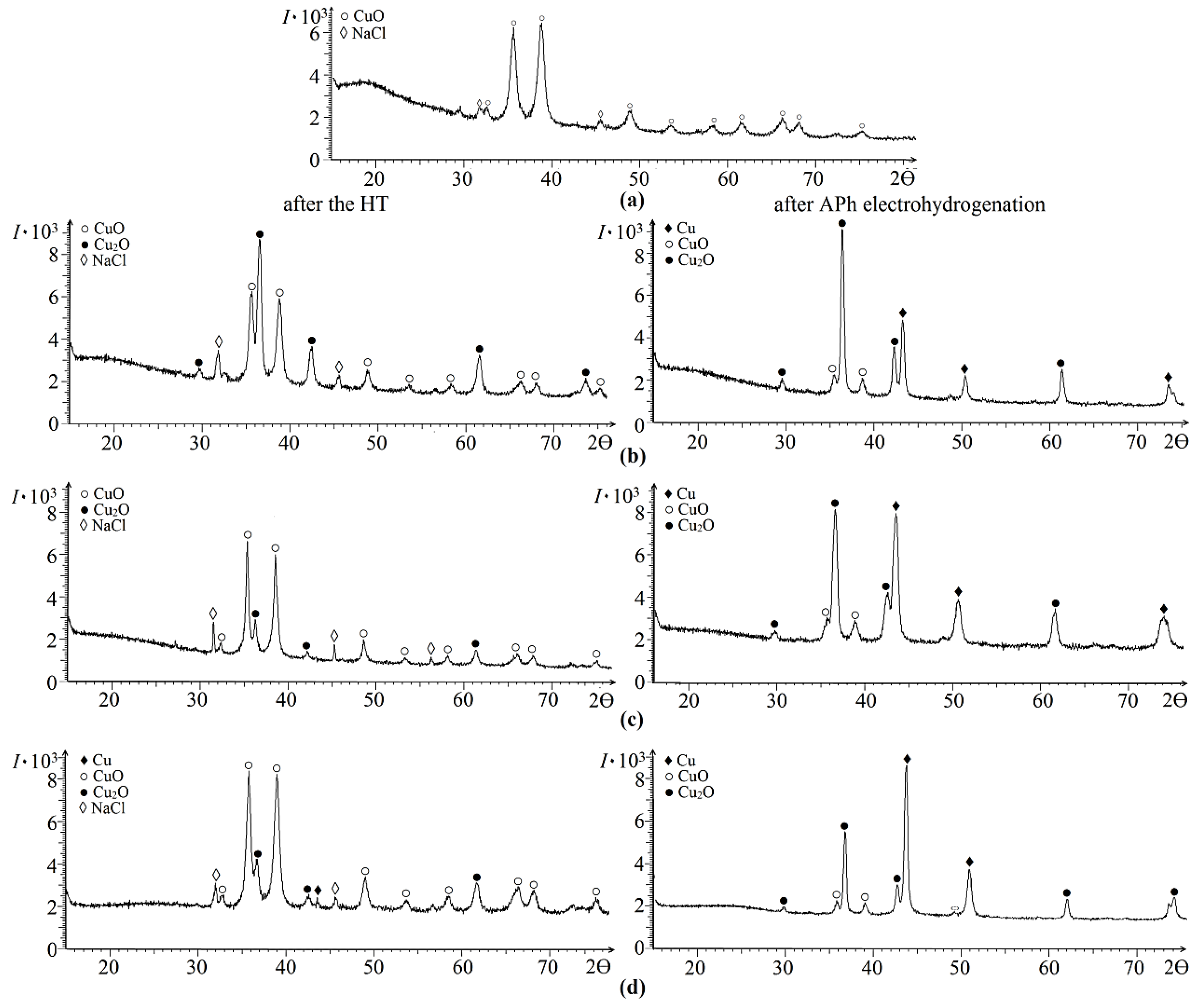
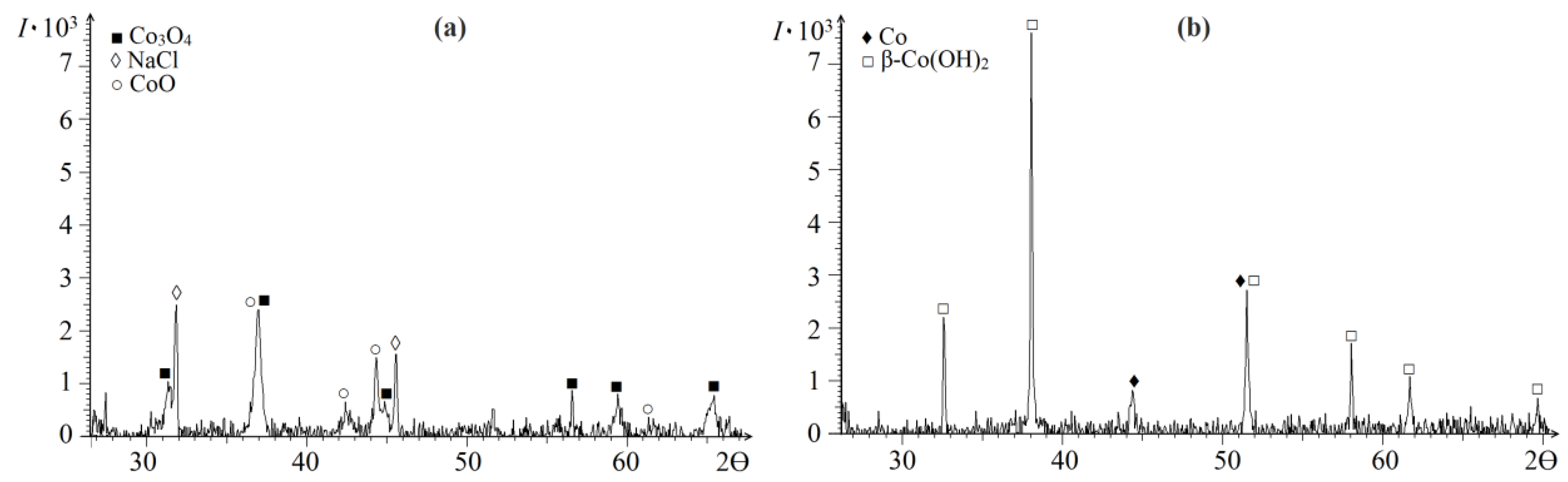
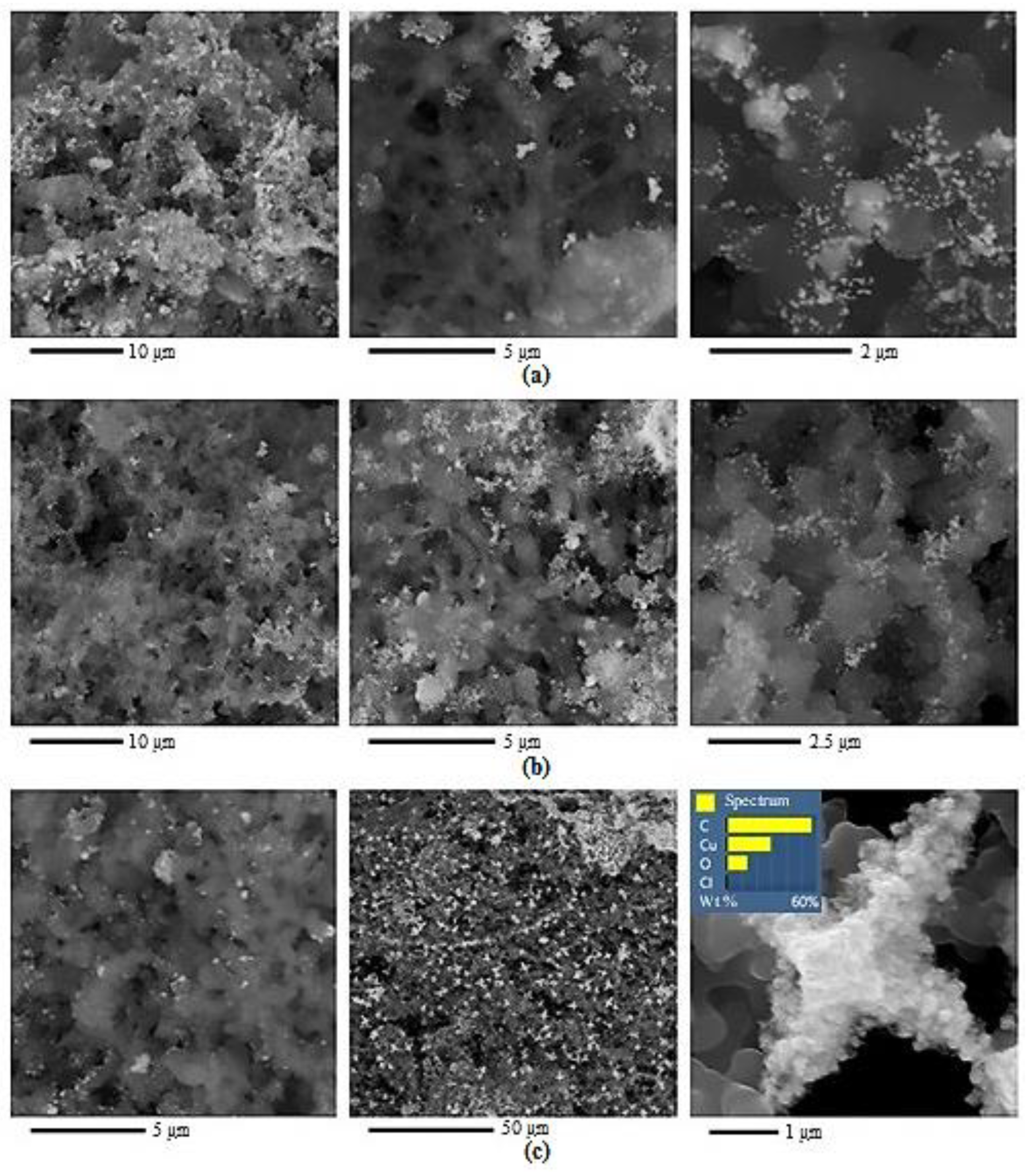

| Polymer after HT | Ssp., m2/g | VƩ Pores, cm3/g | Pore Size, nm | Pore Size Distribution, % |
|---|---|---|---|---|
| AFP (400 °C) | 44.8 ± 23.7 | 0.439 | 3.5 29.4 56.1 79.6 | 22.7 35.4 20.6 21.3 |
| AFP (500 °C) | 23.0 ± 2.7 | 0.161 | 56.1 79.6 | 56.2 43.8 |
| AFP (700 °C) | 13.9 ± 0.6 | 0.348 | 5.9 56.1 79.6 | 30.5 59.6 9.9 |
| Composites | Metal Content in 1 g of Composites, g | VH2,1 mL | Electrocatalytic Hydrogenation of APh | Product Compositions, % | |||
|---|---|---|---|---|---|---|---|
| W,2 mL H2/min | α,3 % | 1-PhE | APh | PCs | |||
| Cu cathode (cell 1) | - | 0.0 | 2.0 | 28.1 | 4.4 | 49.9 | 45.7 |
| AFP + Cu(NO3)2 (400 °C) AFP + Cu(NO3)2 (500 °C) AFP + Cu(NO3)2 (700 °C) AFP + Cu(NO3)2 (800 °C) | 0.355 0.408 0.509 0.600 | 13.0 43.3 60.7 106.8 | 4.4 4.8 5.2 6.3 | 89.0 94.3 95.1 89.7 | 95.1 96.5 97.6 96.9 | 4.0 2.8 2.4 3.1 | 0.9 0.7 - - |
| Cu cathode (cell 2) | - | 0.0 | 1.7 | 15.0 | 12.9 | 83.6 | 3.5 |
| AFP + Cu(NO3)2 AFP + Cu(NO3)2 (500 °C) AFP + Cu(NO3)2 (500 °C) AFP + Cu(NO3)2 (500 °C) | 0.234 0.408 <0.408 <0.408 | 67.3 46.0 0.0 0.0 | 7.7 8.8 8.4 7.4 | 99.5 99.6 99.8 99.6 | 98.5 99.0 99.6 99.2 | 1.2 1.0 0.4 0.5 | 0.3 - - 0.3 |
| Cu cathode (cell 1) | - | 0.0 | 2.0 | 28.1 | 4.4 | 49.9 | 45.7 |
| AFP + Co(NO3)2 (400 °C) AFP + Co(NO3)2 (500 °C) AFP + Co(NO3)2 (700 °C) | 0.238 0.285 0.357 | 0.0 0.0 29.4 | 3.5 3.8 5.1 | 80.8 69.8 85.4 | 90.4 88.8 93.9 | 9.3 11.2 6.1 | 0.2 - - |
| Cu cathode (cell 2) | - | 0.0 | 1.7 | 15.0 | 12.9 | 83.6 | 3.5 |
| AFP + Co(NO3)2 AFP + Co(NO3)2 (500 °C) | 0.171 0.285 | 41.8 0.0 | 4.2 7.3 | 99.7 99.8 | 97.5 97.9 | 2.5 2.1 | - - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muldakhmetov, Z.M.; Ivanova, N.M.; Vissurkhanova, Y.A.; Soboleva, Y.A. Preparation and Electrocatalytic Application of Copper- and Cobalt-Carbon Composites Based on Pyrolyzed Polymer. Catalysts 2022, 12, 862. https://doi.org/10.3390/catal12080862
Muldakhmetov ZM, Ivanova NM, Vissurkhanova YA, Soboleva YA. Preparation and Electrocatalytic Application of Copper- and Cobalt-Carbon Composites Based on Pyrolyzed Polymer. Catalysts. 2022; 12(8):862. https://doi.org/10.3390/catal12080862
Chicago/Turabian StyleMuldakhmetov, Zainulla M., Nina M. Ivanova, Yakha A. Vissurkhanova, and Yelena A. Soboleva. 2022. "Preparation and Electrocatalytic Application of Copper- and Cobalt-Carbon Composites Based on Pyrolyzed Polymer" Catalysts 12, no. 8: 862. https://doi.org/10.3390/catal12080862
APA StyleMuldakhmetov, Z. M., Ivanova, N. M., Vissurkhanova, Y. A., & Soboleva, Y. A. (2022). Preparation and Electrocatalytic Application of Copper- and Cobalt-Carbon Composites Based on Pyrolyzed Polymer. Catalysts, 12(8), 862. https://doi.org/10.3390/catal12080862






