Stimulation of Akt Phosphorylation and Glucose Transport by Metalloporphyrins with Peroxynitrite Decomposition Catalytic Activity
Abstract
1. Introduction
2. Results
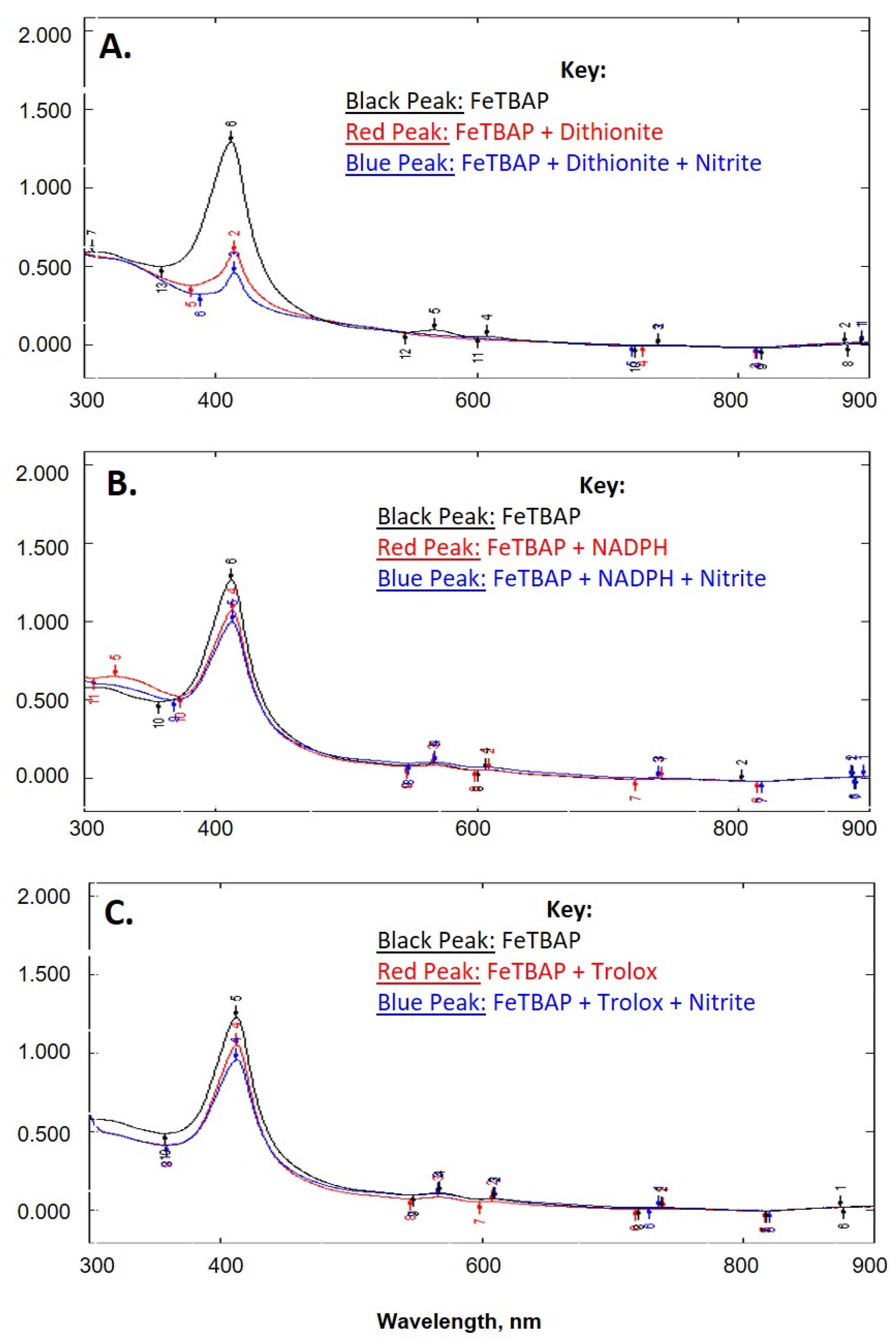
2.1. FeTBAP Does Not Act as a Nitrite Reductase
2.2. FeTBAP Acts as a Peroxynitrite Decomposition Catalyst
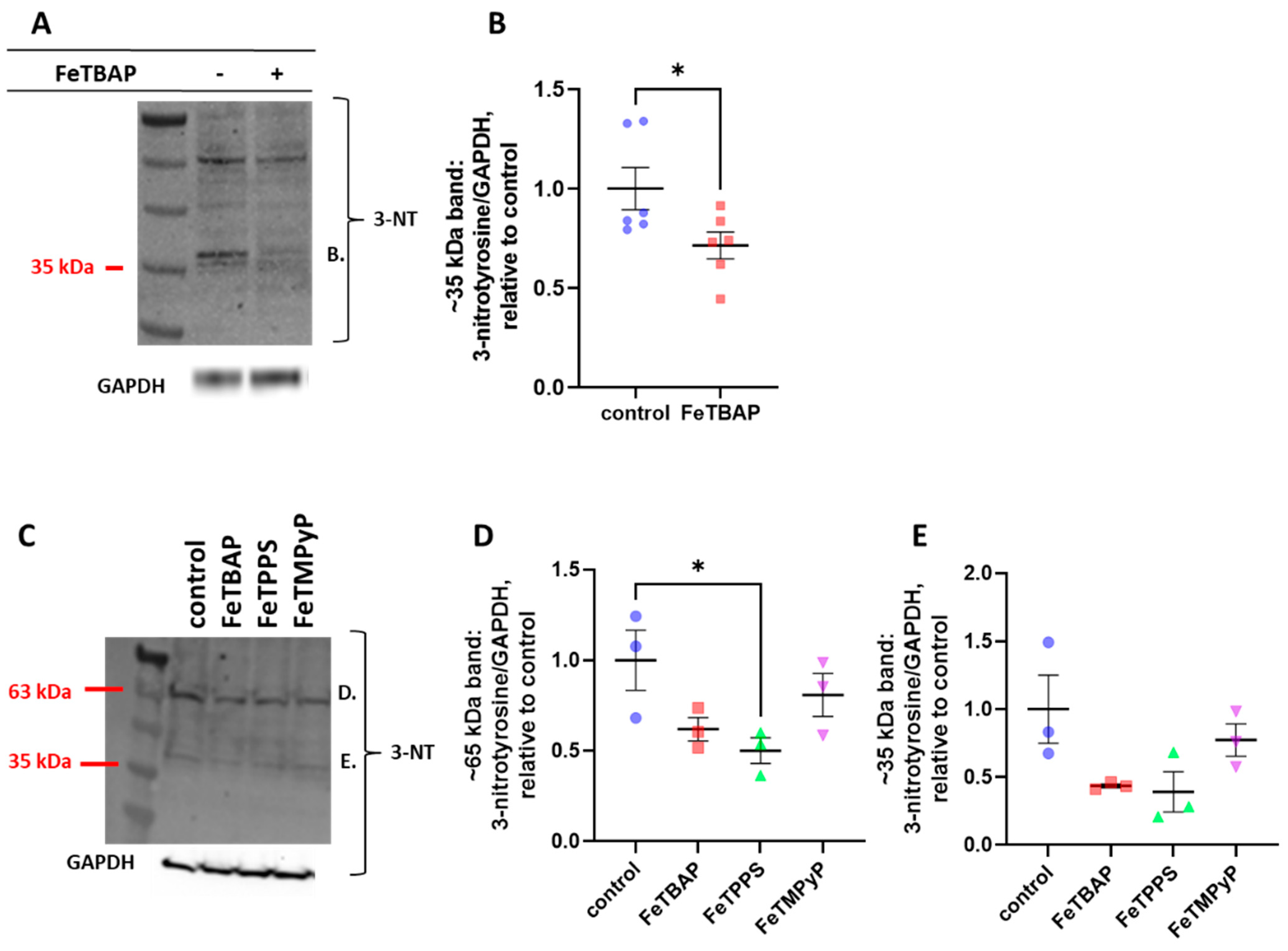
2.3. FeTBAP Decreases Nitration of Tyrosine Residues
2.4. FeTBAP and FeTPPS Increase Akt Phosphorylation and Glucose Transport
2.5. FeTPPS and FeTBAP Act as Peroxidases
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Potential Role of FeTBAP as a Nitrite Reductase
4.3. Cell Culture
4.4. Effect of FeTBAP on S-Nitrosylation
4.5. Western Blot Analysis
4.6. Peroxynitrite Decomposition Activity
4.7. Nitrotyrosine Levels in C2C12 Myotubes
4.8. Insulin Signaling
4.9. Glucose Transport
4.10. Peroxidase Activity
4.11. Statistics
4.12. Nitrated Mouse Proteins
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gladwin, M.T.; Kim-Shapiro, D.B. The functional nitrite reductase activity of the heme-globins. Blood 2008, 112, 2636–2647. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S.; Huang, Z.; Grubina, R.; Sun, J.; Ringwood, L.; MacArthur, P.H.; Xu, X.; Murphy, E.; Darley-Usmar, V.; Gladwin, M.T. Deoxymyoglobin Is a Nitrite Reductase That Generates Nitric Oxide and Regulates Mitochondrial Respiration. Circ. Res. 2007, 100, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Rassaf, T.; Flögel, U.; Drexhage, C.; Hendgen-Cotta, U.; Kelm, M.; Schrader, J. Nitrite Reductase Function of Deoxymyoglobin. Circ. Res. 2007, 100, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Filho, M.A.; Ueno, M.; Hirabara, S.M.; Seabra, A.B.; Carvalheira, J.B.; de Oliveira, M.G.; Velloso, L.A.; Curi, R.; Saad, M.J. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: A novel mechanism of insulin resistance. Diabetes 2005, 54, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Badal, S.; Brown, P.D.; Ragoobirsingh, D. Nitric oxide agents impair insulin-mediated signal transduction in rat skeletal muscle. BMC Biochem. 2006, 7, 17. [Google Scholar] [CrossRef][Green Version]
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef]
- Lane, P.; Gross, S.S. Cell signaling by nitric oxide. Semin. Nephrol. 1999, 19, 215–229. [Google Scholar] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef]
- Stern, M.K.; Jensen, M.P.; Kramer, K. Peroxynitrite Decomposition Catalysts. J. Am. Chem. Soc. 1996, 118, 8735–8736. [Google Scholar] [CrossRef]
- Salvemini, D.; Wang, Z.Q.; Stern, M.K.; Currie, M.G.; Misko, T.P. Peroxynitrite decomposition catalysts: Therapeutics for peroxynitrite-mediated pathology. Proc. Natl. Acad. Sci. USA 1998, 95, 2659–2663. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, K. Peroxynitrite mediates muscle insulin resistance in mice via nitration of IRbeta/IRS-1 and Akt. Toxicol. Appl. Pharmacol. 2009, 241, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Duplain, H.; Sartori, C.; Dessen, P.; Jayet, P.-Y.; Schwab, M.; Bloch, J.; Nicod, P.; Scherrer, U. Stimulation of peroxynitrite catalysis improves insulin sensitivity in high fat diet-fed mice. J. Physiol. 2008, 586, 4011–4016. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Singel, D.J.; Loscalzo, J. Biochemistry of nitric oxide and its redox-activated forms. Science 1992, 258, 1898–1902. [Google Scholar] [CrossRef]
- Stamler, J.S. Redox signaling: Nitrosylation and related target interactions of nitric oxide. Cell 1994, 78, 931–936. [Google Scholar] [CrossRef]
- Eccardt, A.M.; Pelzel, R.J.; Mattathil, L.; Moon, Y.A.; Mannino, M.H.; Janowiak, B.E.; Fisher, J.S. A peroxidase mimetic protects skeletal muscle cells from peroxide challenge and stimulates insulin signaling. Am. J. Physiol. Cell Physiol. 2020, 318, C1214–C1225. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef]
- Hare, J.M.; Beigi, F.; Tziomalos, K. Chapter Twenty-One-Nitric Oxide and Cardiobiology-Methods for Intact Hearts and Isolated Myocytes. In Methods in Enzymology; Cadenas, E., Packer, L., Eds.; Academic Press: San Diego, CA, USA, 2008; Volume 441, pp. 369–392. [Google Scholar]
- Lauzier, B.; Sicard, P.; Bouchot, O.; Delemasure, S.; Moreau, D.; Vergely, C.; Rochette, L. A peroxynitrite decomposition catalyst: FeTPPS confers cardioprotection during reperfusion after cardioplegic arrest in a working isolated rat heart model. Fundam. Clin. Pharmacol. 2007, 21, 173–180. [Google Scholar] [CrossRef]
- Stadler, K. Peroxynitrite-driven mechanisms in diabetes and insulin resistance—The latest advances. Curr. Med. Chem. 2011, 18, 280–290. [Google Scholar] [CrossRef]
- Drel, V.R.; Pacher, P.; Vareniuk, I.; Pavlov, I.A.; Ilnytska, O.; Lyzogubov, V.V.; Bell, S.R.; Groves, J.T.; Obrosova, I.G. Evaluation of the peroxynitrite decomposition catalyst Fe(III) tetra-mesitylporphyrin octasulfonate on peripheral neuropathy in a mouse model of type 1 diabetes. Int. J. Mol. Med. 2007, 20, 783–792. [Google Scholar] [CrossRef][Green Version]
- Ju, T.J.; Kwon, W.Y.; Kim, Y.W.; Kim, J.Y.; Kim, Y.D.; Lee, I.K.; Park, S.Y. Hemin improves insulin sensitivity in skeletal muscle in high fat-fed mice. J. Pharm. Sci. 2014, 126, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Dłużewicz, K.; Sadoch, J.; Szkudelska, K. Effects of the activation of heme oxygenase-1 on hormonal and metabolic changes in rats fed a high-fat diet. Biomed. Pharm. 2017, 87, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Zhang, F.; Cheng, Y.; Liu, J.; Huang, R.; Yan, M.; Wang, Y.; He, Z.; Lai, H.; Wang, H.; et al. Hemin Improves Insulin Sensitivity and Lipid Metabolism in Cultured Hepatocytes and Mice Fed a High-Fat Diet. Nutrients 2017, 9, 805. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.J.; Buehler, P.W.; Alayash, A.I.; Belcher, J.D.; Vercellotti, G.M. Hemolysis and free hemoglobin revisited: Exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 2013, 121, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Batthyány, C.; Bartesaghi, S.; Mastrogiovanni, M.; Lima, A.; Demicheli, V.; Radi, R. Tyrosine-Nitrated Proteins: Proteomic and Bioanalytical Aspects. Antioxid. Redox Signal 2017, 26, 313–328. [Google Scholar] [CrossRef]
- DeepNitro. Available online: http://deepnitro.renlab.org/ (accessed on 15 July 2022).
- Xie, Y.; Luo, X.; Li, Y.; Chen, L.; Ma, W.; Huang, J.; Cui, J.; Zhao, Y.; Xue, Y.; Zuo, Z.; et al. DeepNitro: Prediction of Protein Nitration and Nitrosylation Sites by Deep Learning. Genom. Proteom. Bioinform. 2018, 16, 294–306. [Google Scholar] [CrossRef]
- Abello, N.; Kerstjens, H.A.; Postma, D.S.; Bischoff, R. Protein tyrosine nitration: Selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. J. Proteome Res. 2009, 8, 3222–3238. [Google Scholar] [CrossRef]
- National Library of Medicine (US). National Center for Biotechnology Information, PubChem Compound Summary for CID 6610341, Mntbap. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6610341#section=2D-Structure (accessed on 15 July 2022).
- National Library of Medicine (US). National Center for Biotechnology Information, PubChem Substance Record for SID 26758697. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/26758697#section=2D-Structure (accessed on 15 July 2022).
- National Library of Medicine (US). National Center for Biotechnology Information, PubChem Compound Summary for CID 16760420, FeTMPyP. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/16760420#section=2D-Structure (accessed on 15 July 2022).
- Andrisse, S.; Patel, G.D.; Chen, J.E.; Webber, A.M.; Spears, L.D.; Koehler, R.M.; Robinson-Hill, R.M.; Ching, J.K.; Jeong, I.; Fisher, J.S. ATM and GLUT1-S490 Phosphorylation Regulate GLUT1 Mediated Transport in Skeletal Muscle. PLoS ONE 2013, 8, e66027. [Google Scholar] [CrossRef]
- Eccardt, A.M.; Bell, T.P.; Mattathil, L.; Prasad, R.; Kelly, S.C.; Fisher, J.S. Trans-Plasma Membrane Electron Transport and Ascorbate Efflux by Skeletal Muscle. Antioxidants 2017, 6, 89. [Google Scholar] [CrossRef]
- Mirazizi, F.; Bahrami, A.; Haghbeen, K.; Shahbani Zahiri, H.; Bakavoli, M.; Legge, R.L. Rapid and direct spectrophotometric method for kinetics studies and routine assay of peroxidase based on aniline diazo substrates. J. Enzym. Inhib. Med. Chem. 2016, 31, 1162–1169. [Google Scholar] [CrossRef]
- UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res 2021, 49, D480–D489. [CrossRef] [PubMed]
- UniProt. Available online: https://www.uniprot.org/ (accessed on 15 July 2022).




| Fe-Porphyrin | Author(s) | Conditions | Results |
|---|---|---|---|
| FeTPPS | Zhou et al. [12] | Insulin resistant high fat diet-fed (HFD) mice | Administration of FeTPPS improved muscle insulin signaling and whole body insulin sensitivity |
| FeTPPS | Duplain et al. [13] | Insulin resistant high fat diet-fed mice | FeTPPS treatment restored insulin signaling and glucose uptake. Diminished HFD-induced insulin resistance in mice |
| FeTMPS | Drel et al. [21] | Streptozotocin induced type 1 diabetic mice | Alleviated various symptoms associated with diabetic neuropathy including manifest motor and sensory nerve conduction velocity deficits |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eccardt, A.M.; Pelzel, R.J.; Bell, T.P.; Fisher, J.S. Stimulation of Akt Phosphorylation and Glucose Transport by Metalloporphyrins with Peroxynitrite Decomposition Catalytic Activity. Catalysts 2022, 12, 849. https://doi.org/10.3390/catal12080849
Eccardt AM, Pelzel RJ, Bell TP, Fisher JS. Stimulation of Akt Phosphorylation and Glucose Transport by Metalloporphyrins with Peroxynitrite Decomposition Catalytic Activity. Catalysts. 2022; 12(8):849. https://doi.org/10.3390/catal12080849
Chicago/Turabian StyleEccardt, Amanda M., Ross J. Pelzel, Thomas P. Bell, and Jonathan S. Fisher. 2022. "Stimulation of Akt Phosphorylation and Glucose Transport by Metalloporphyrins with Peroxynitrite Decomposition Catalytic Activity" Catalysts 12, no. 8: 849. https://doi.org/10.3390/catal12080849
APA StyleEccardt, A. M., Pelzel, R. J., Bell, T. P., & Fisher, J. S. (2022). Stimulation of Akt Phosphorylation and Glucose Transport by Metalloporphyrins with Peroxynitrite Decomposition Catalytic Activity. Catalysts, 12(8), 849. https://doi.org/10.3390/catal12080849







