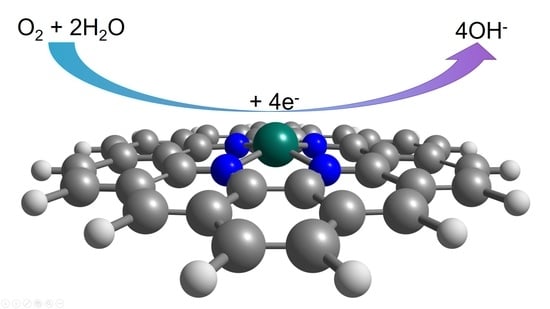
Quantum-Chemical Modeling of the Catalytic Activity of Graphene Doped with Metal Phthalocyanines in ORR
Abstract
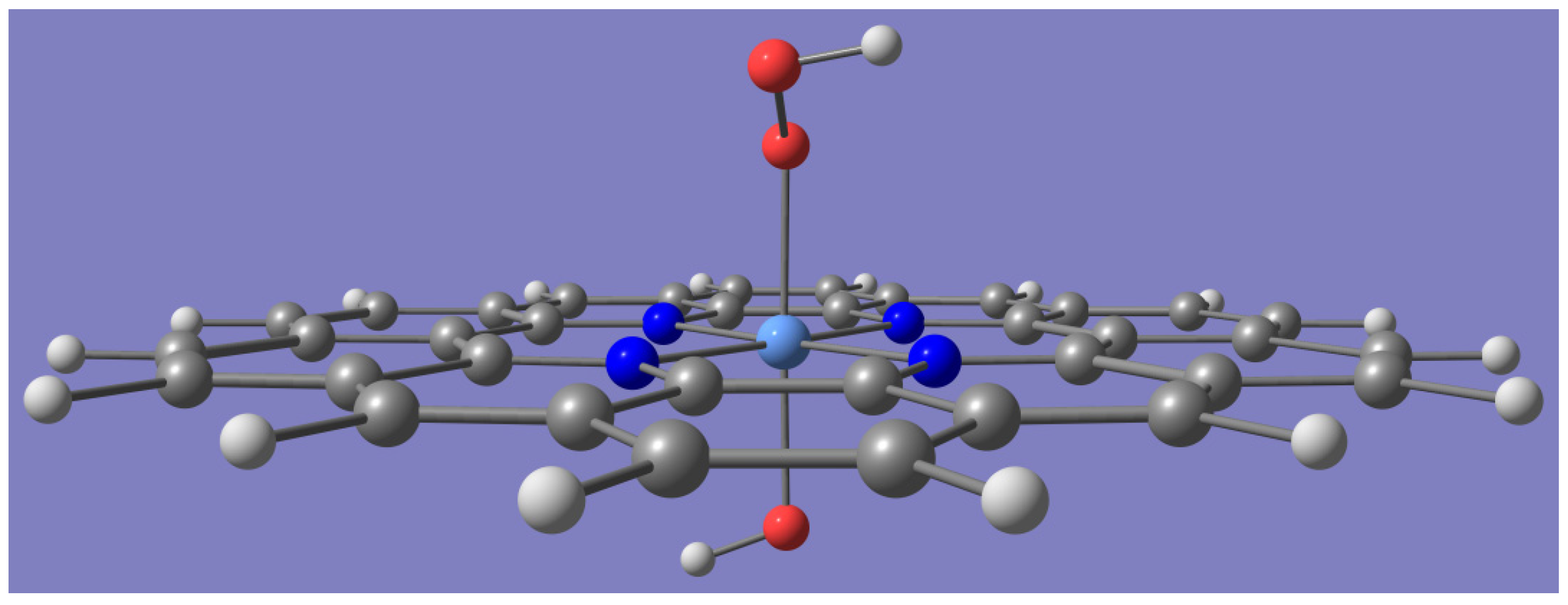
:1. Introduction
2. Results and Discussions
3. Model and Calculation Methods
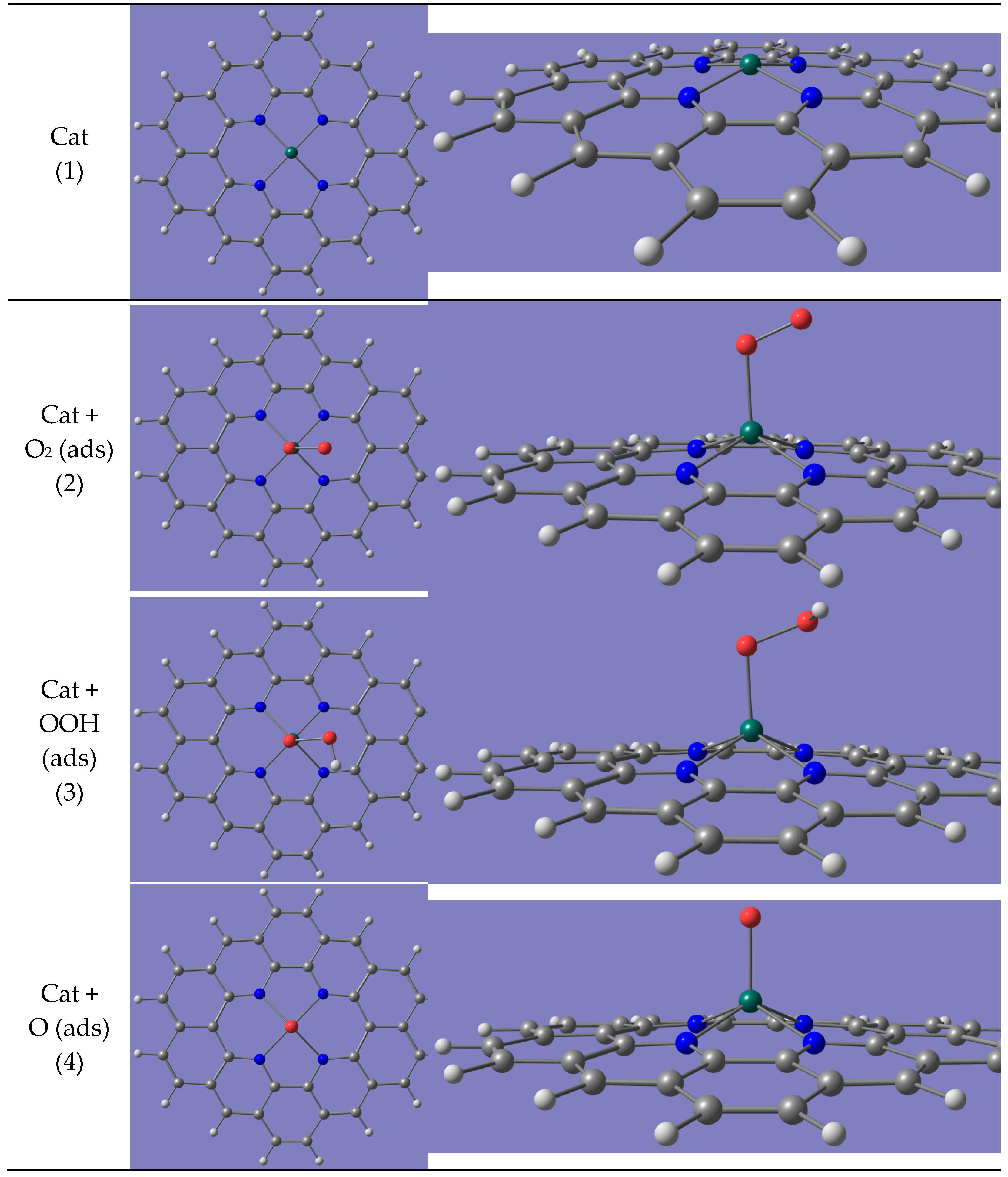
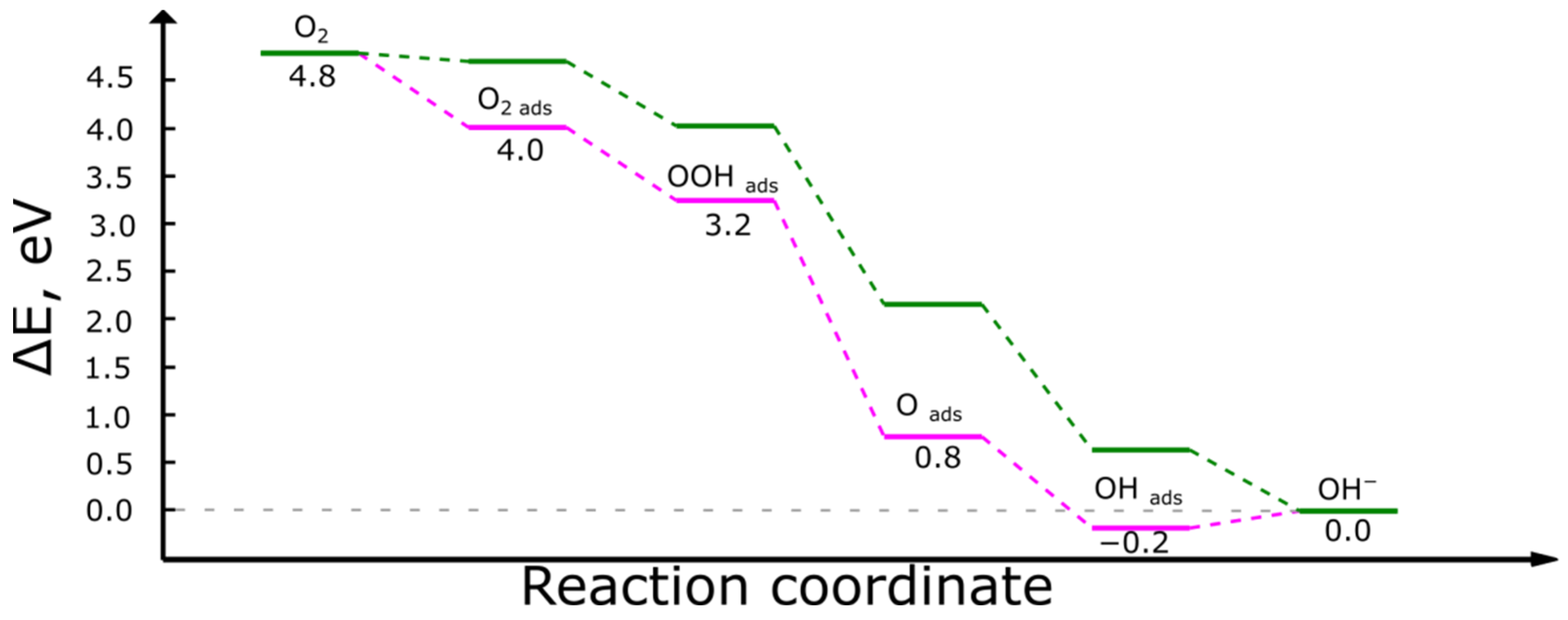
- O2 + 2 H2O
- O2(ads) + 2 H2O
- OOH(ads) + H2O + OH−
- O(ads) + H2O + 2 OH−
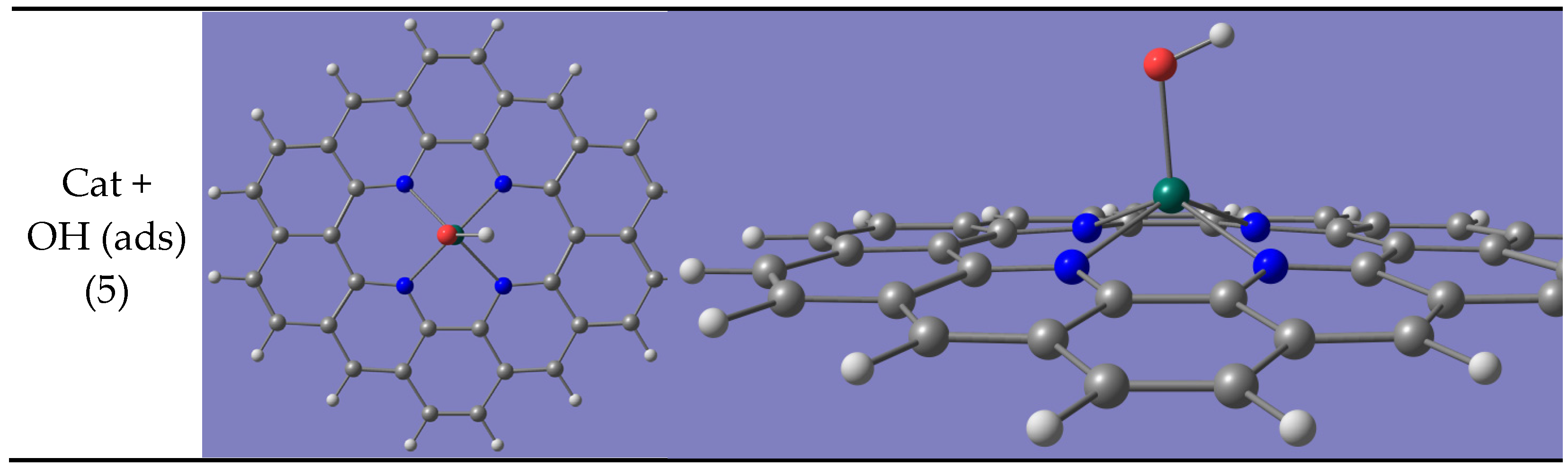
- OH(ads) + 3 OH−
- 4 OH−
- O2(ads) + H2O + e− → OOH(ads) + OH−
- OOH(ads) + e− → O(ads) + OH−
- O(ads) + H2O + e− → OH(ads) + OH−
- OH(ads) + e− → OH−
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Metal-free heteroatom-doped carbon-based catalysts for ORR: A critical assessment about the role of heteroatoms. Carbon 2020, 165, 434–454. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Vikkisk, M.; Joost, U.; Shulga, E.; Kink, I.; Tammeveski, K. Highly active nitrogen-doped few-layer graphene/carbon nanotube composite electrocatalyst for oxygen reduction reaction in alkaline media. Carbon 2014, 73, 361–370. [Google Scholar] [CrossRef]
- Bouleau, L.; Pérez-Rodríguez, S.; Quílez-Bermejo, J.; Izquierdo, M.T.; Xu, F.; Fierro, V.; Celzard, A. Best practices for ORR performance evaluation of metal-free porous carbon electrocatalysts. Carbon 2022, 189, 349–361. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, D.; Zhu, X.; Wang, S.; Zou, Y.; Lan, L.; Liao, Q. ZIF-67-derived Co nanoparticles embedded in N-doped porous carbon composite interconnected by MWCNTs as highly efficient ORR electrocatalysts for a flexible direct formate fuel cell. Chem. Eng. J. 2022, 432, 134192. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Liu, X.; Chen, Y.; Zhao, Y.; Gao, S. Fabricating N, S Co-Doped Hierarchical Macro-Meso-Micro Carbon Materials as pH-Universal ORR Electrocatalysts. Chem. Sel. 2022, 7, e202200044. [Google Scholar] [CrossRef]
- Ge, F.; Qiao, Q.; Chen, X.; Wu, Y. Probing the catalytic activity of M-N4—xOx embedded graphene for the oxygen reduction reaction by density functional theory. Front. Chem. Sci. Eng. 2021, 15, 1206–1216. [Google Scholar] [CrossRef]
- Kuzmin, A.; Shainyan, B. Exploring of catalytic oxygen reduction reaction activity of lattice carbons of vanadium and niobium doped nitrogen codoped carbon nanotubes by density functional theory. Authorea Prepr. 2022. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Su, Z.; Zhao, Z.; Cai, Q.; Zhao, J. Phthalocyanine-supported single-atom catalysts as a promising bifunctional electrocatalyst for ORR/OER: A computational study. Chem. Phys. Mater. 2022, 15, 237–245. [Google Scholar] [CrossRef]
- Yan, P.; Shu, S.; Zou, L.; Liu, Y.; Li, J.; Wei, F. Density functional theory study of active sites on nitrogen-doped graphene for oxygen reduction reaction. R. Soc. Open Sci. 2021, 8, 210272. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, Y.; Ding, S.; Su, Y. A DFT study on the activity origin of Fe-N-C sites for oxygen reduction reaction. Chem. Phys. Chem. 2022. [Google Scholar] [CrossRef]
- Lu, R.; Quan, C.; Zhang, C.; He, Q.; Liao, X.; Wang, Z.; Zhao, Y. Establishing a theoretical insight for penta-coordinated iron-nitrogen-carbon catalysts toward oxygen reaction. Nano Res. 2022, 15, 6067–6075. [Google Scholar] [CrossRef]
- Li, H.; Ha, T.A.; Jiang, S.; Pozo-Gonzalo, C.; Wang, X.; Fang, J.; Wang, X.N. F and S doped carbon nanofibers generated from electrospun polymerized ionic liquids for metal-free bifunctional oxygen electrocatalysis. Electrochim. Acta 2021, 377, 138089. [Google Scholar] [CrossRef]
- She, Y.; Chen, J.; Zhang, C.; Lu, Z.; Ni, M.; Sit, P.H.L.; Leung, M.K. Nitrogen-doped graphene derived from ionic liquid as metal-free catalyst for oxygen reduction reaction and its mechanisms. Appl. Energy 2018, 225, 513–521. [Google Scholar] [CrossRef]
- Pham-Truong, T.N.; Ranjan, C.; Randriamahazaka, H.; Ghilane, J. Nitrogen doped carbon dots embedded in poly (ionic liquid) as high efficient metal-free electrocatalyst for oxygen reduction reaction. Catal. Today 2019, 335, 381–387. [Google Scholar] [CrossRef]
- Jiao, R.; Zhang, W.; Sun, H.; Zhu, Z.; Yang, Z.; Liang, W.; Li, A. N-and S-doped nanoporous carbon framework derived from conjugated microporous polymers incorporation with ionic liquids for efficient oxygen reduction reaction. Mater. Today Energy 2020, 16, 100382. [Google Scholar] [CrossRef]
- Martinaiou, I.; Wolker, T.; Shahraei, A.; Zhang, G.R.; Janßen, A.; Wagner, S.; Kramm, U.I. Improved electrochemical performance of Fe-NC catalysts through ionic liquid modification in alkaline media. J. Power Sources 2018, 375, 222–232. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Zhang, J.; Lim, J.; Gao, Y.; Zhang, S. Engineering the electronic structure of perovskite oxide surface with ionic liquid for enhanced oxygen reduction reaction. Appl. Catal. B Environ. 2021, 282, 119593. [Google Scholar] [CrossRef]
- Meloni, E.G.; Ocone, L.R.; Block, B.P. Phathalocyaninato (2--) chromium (III) phosphinates. Inorg. Chem. 1967, 6, 424–425. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Dursun, S.; Akay, R.G.; Yazici, M.S. CVD graphene supported cobalt (II) phthalocyanine as cathode electrocatalyst for PEM fuel cells. Int. J. Hydrogen Energy 2020, 45, 34837–34844. [Google Scholar] [CrossRef]
- Kramm, U.I.; Herrmann-Geppert, I.; Behrends, J.; Lips, K.; Fiechter, S.; Bogdanoff, P. On an easy way to prepare metal–nitrogen doped carbon with exclusive presence of MeN4-type sites active for the ORR. J. Am. Chem. Soc. 2016, 138, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhang, X.; Qin, J.; Liu, R. TMN4 complex embedded graphene as bifunctional electrocatalysts for high efficiency OER/ORR. J. Energ. Chem. 2021, 55, 437–443. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Ghildina, A.R.; Zavershinskiy, I.P.; Mebel, A.M.; Vinogradov, K.Y.; Bulanova, A.V.; Zhu, H. Theoretical Study of the Mechanism and Kinetics of the Oxidation of Cyclopenta[a]Naphthalenyl Radical C13H9 with Molecular Oxygen. J. Phys. Chem. A 2021, 125, 6796–6804. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [Green Version]
- Vinogradov, K.Y.; Bulanova, A.V.; Shafigulin, R.V.; Tokranova, E.O.; Mebel, A.M.; Zhu, H. Density Functional Theory Study of the Oxygen Reduction Reaction Mechanism on Graphene Doped with Nitrogen and a Transition Metal. ACS Omega 2022, 7, 7066–7073. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Melle-Franco, M.; San-Fabián, E.; Morallón, E.; Cazorla-Amorós, D. Towards understanding the active sites for the ORR in N-doped carbon materials through fine-tuning of nitrogen functionalities: An experimental and computational approach. J. Mater. Chem. A 2019, 7, 24239–24250. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Pan, X.; Cao, X.; Hu, P.; Bao, X. Oxygen reduction reaction mechanism on nitrogen-doped graphene: A density functional theory study. J. Cat. 2011, 282, 183–190. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, J.; Luo, B.; Tian, E.; Waterhouse, G.I.N. Central metal and ligand effects on oxygen electrocatalysis over 3d transition metal single-atom catalysts: A theoretical investigation. Chem. Eng. J. 2022, 427, 132038. [Google Scholar] [CrossRef]
- Yang, Y.; Qi, W.; Niu, J.; Chen, F.; Li, W. Understanding active sites and mechanism of oxygen reduction reaction on FeN4–doped graphene from DFT study. Int. J. Hydrogen Energy 2020, 45, 15465–15475. [Google Scholar] [CrossRef]
- Wei, P.; Li, X.; He, Z.; Sun, X.; Liang, Q.; Wang, Z.; Fang, C.; Li, Q.; Yang, H.; Han, J.; et al. Porous N, B co-doped carbon nanotubes as efficient metal-free electrocatalysts for ORR and Zn-air batteries. Chem. Eng. J. 2021, 422, 130134. [Google Scholar] [CrossRef]
- Vashchenko, A.V.; Kuzmin, A.V.; Shainyan, B.A. Si-Doped Single-Walled Carbon Nanotubes as Potential Catalysts for Oxygen Reduction Reactions. Russ. J. Gen. Chem. 2020, 90, 454–459. [Google Scholar] [CrossRef]





| Catalyst | CuN4 | NiN4 | MnN4 | CoN4 | ZnN4 | Cr(OH)N4 |
|---|---|---|---|---|---|---|
| Gads, eV | 0.15 | 0.23 | −0.21 | 0.12 | −0.43 | −0.08 |
| dMe-O, Å | 2.17 | 1.94 | 1.87 | 2.07 | 1.99 | 2.01 |
| dO-O, Å | 1.31 | 1.32 | 1.32 | 1.33 | 1.34 | 1.32 |
| Catalyst | CuN4 | NiN4 | MnN4 | CoN4 | ZnN4 | Cr(OH)N4 |
|---|---|---|---|---|---|---|
| Gads OOH, eV | −0.37 | 0.01 | −1.04 | −0.60 | −1.24 | −0.79 |
| Gads O, eV | −1.52 | −1.89 | −3.58 | −3.12 | −2.39 | −2.74 |
| Gads OH, eV | −1.53 | −1.09 | −2.30 | −1.81 | −2.52 | −2.02 |
| Catalyst | CuN4 | NiN4 | MnN4 | CoN4 | ZnN4 | Cr(OH)N4 |
|---|---|---|---|---|---|---|
| O2→OOH | 0.48 | 0.18 | 0.80 | 0.68 | 0.77 | 0.67 |
| OOH→O | 1.08 | 1.83 | 2.47 | 2.45 | 1.08 | 1.88 |
| O→OH | 2.26 | 1.44 | 0.96 | 0.93 | 2.37 | 1.52 |
| OH→OH− | 1.12 | 1.57 | 0.36 | 0.85 | 0.14 | 0.63 |
| Overpotential, V | 0.72 | 1.02 | 0.84 | 0.52 | 1.06 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinogradov, K.Y.; Bulanova, A.V.; Shafigulin, R.V.; Tokranova, E.O.; Zhu, H. Quantum-Chemical Modeling of the Catalytic Activity of Graphene Doped with Metal Phthalocyanines in ORR. Catalysts 2022, 12, 786. https://doi.org/10.3390/catal12070786
Vinogradov KY, Bulanova AV, Shafigulin RV, Tokranova EO, Zhu H. Quantum-Chemical Modeling of the Catalytic Activity of Graphene Doped with Metal Phthalocyanines in ORR. Catalysts. 2022; 12(7):786. https://doi.org/10.3390/catal12070786
Chicago/Turabian StyleVinogradov, Kirill Y., Anzhela V. Bulanova, Roman V. Shafigulin, Elena O. Tokranova, and Hong Zhu. 2022. "Quantum-Chemical Modeling of the Catalytic Activity of Graphene Doped with Metal Phthalocyanines in ORR" Catalysts 12, no. 7: 786. https://doi.org/10.3390/catal12070786
APA StyleVinogradov, K. Y., Bulanova, A. V., Shafigulin, R. V., Tokranova, E. O., & Zhu, H. (2022). Quantum-Chemical Modeling of the Catalytic Activity of Graphene Doped with Metal Phthalocyanines in ORR. Catalysts, 12(7), 786. https://doi.org/10.3390/catal12070786