Advances in Enhancing the Stability of Cu-Based Catalysts for Methanol Reforming
Abstract
:1. Introduction
2. Deactivation Analysis of Copper-Based Catalysts
3. Method for Avoiding Deactivation of Cu-Based Catalysts
3.1. Improving Preparation Methods
3.2. Doping Promoters
3.3. Optimizing Supports
3.4. Emerging Materials and Technologies
3.4.1. Copper-Based Spinel Oxide
3.4.2. Plasmonic Copper-Based Catalysts
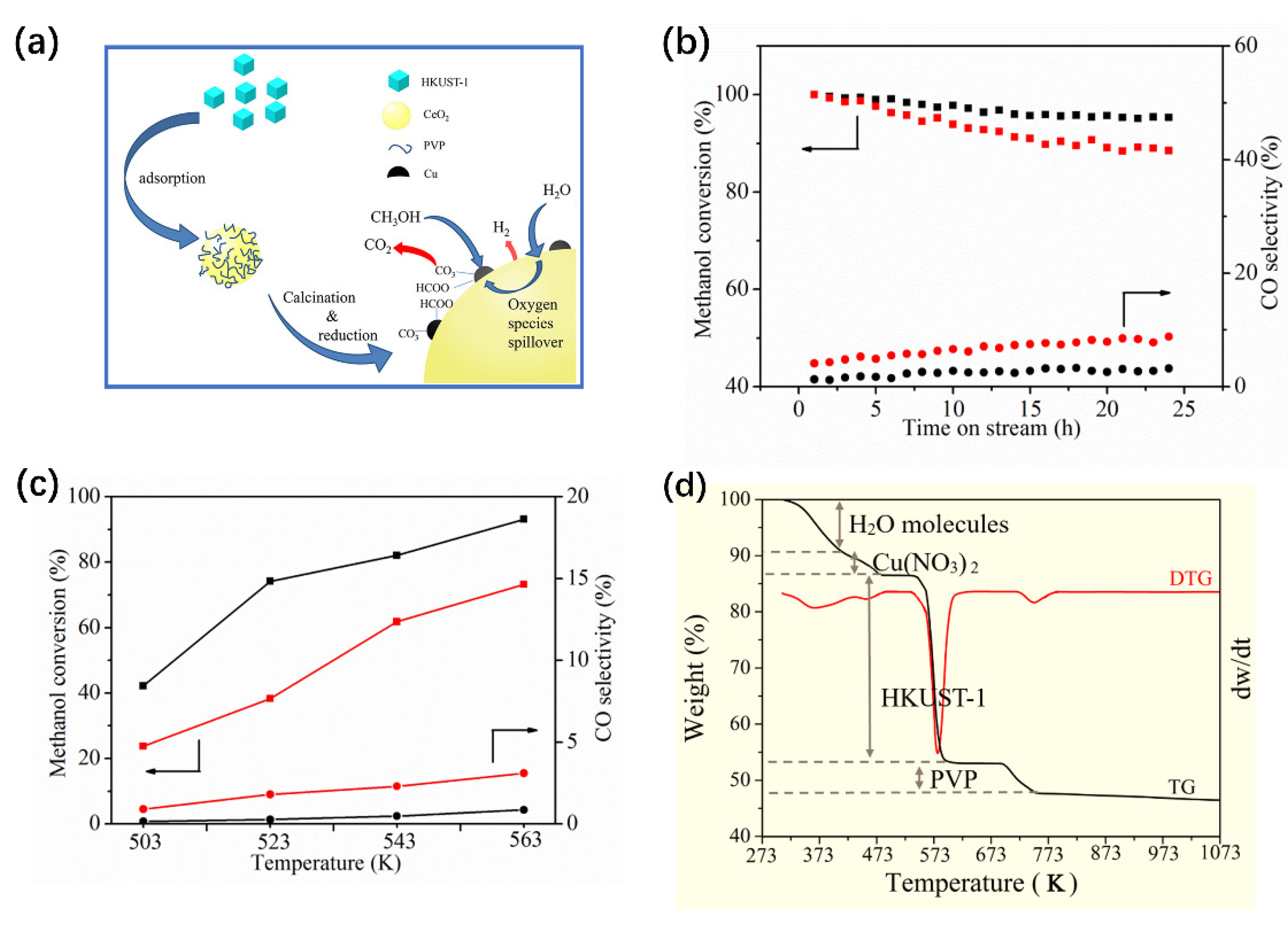
3.4.3. Metal-Organic Framework-Derived Cu-Based Catalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Lei, Z.; Ge, B.; Xiong, X.; Jin, Y.; Jiao, K.; Chen, F.; Peng, S. Development of catalytic combustion and CO2 capture and conversion technology. Int. J. Coal Sci. Technol. 2021, 8, 377–382. [Google Scholar] [CrossRef]
- Zhang, X.; Song, X.; Wang, J.; Su, W.; Zhou, B.; Bai, Y.; Yu, G. Physico-chemical structure evolution characteristics of coal char during gasification in the presence of iron-based waste catalyst. Int. J. Coal Sci. Technol. 2020, 7, 456–463. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q. Recovery of vanadium and tungsten from waste selective catalytic reduction catalysts by K2CO3 roasting and water leaching followed by CaCl2 precipitation. Int. J. Coal Sci. Technol. 2021, 8, 727–736. [Google Scholar] [CrossRef]
- Hwang, B.-Y.; Sakthinathan, S.; Chiu, T.-W. Production of hydrogen from steam reforming of methanol carried out by self-combusted CuCr1-xFexO2 (x = 0–1) nanopowders catalyst. Int. J. Hydrogen Energy 2019, 44, 2848–2856. [Google Scholar] [CrossRef]
- Xiang, Y.; He, J.; Sun, N.; Fan, Y.; Yang, L.; Fang, C.; Kuai, L. Hollow mesoporous CeO2 microspheres for efficient loading of Au single-atoms to catalyze the water-gas shift reaction. Micropor. Mesopor. Mater. 2020, 308, 110507. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, F.; Zhang, D.; Liao, Y.; Zhou, H. Plasma-based surface modification of g-C3N4 nanosheets for highly efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 495, 143520. [Google Scholar] [CrossRef]
- Guo, Y.; Mao, L.; Tang, Y.; Shang, Q.; Cai, X.; Zhang, J.; Hu, H.; Tan, X.; Liu, L.; Wang, H.; et al. Concentrating electron and activating H-OH bond of absorbed water on metallic NiCo2S4 boosting photocatalytic hydrogen evolution. Nano Energy 2022, 95, 107028. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, S.; Wang, J.; Zhang, R.; Wang, X. Cadmium sulfide 3D photonic crystal with hierarchically ordered macropores for highly efficient photocatalytic hydrogen generation. Inorg. Chem. 2022, 61, 2920–2928. [Google Scholar] [CrossRef]
- Yaakob, Z.; Kamarudin, S.K.; Daud, W.R.W.; Yosfiah, M.R.; Lim, K.L.; Kazemian, H. Hydrogen production by methanol-steam reforming using Ni-Mo-Cu/γ-alumina trimetallic catalysts. Asia-Pac. J. Chem. Eng. 2010, 5, 862–868. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Janssen, H.; Bringmann, J.C.; Emonts, B.; Schroeder, V. Safety-related studies on hydrogen production in high-pressure electrolysers. Int. J. Hydrogen Energy 2004, 29, 759–770. [Google Scholar] [CrossRef]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.-C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable hydrogen production. Int. J. Energ. Res. 2008, 32, 379–407. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Shi, R.; Li, Z.; Zhou, C.; Zhang, T. How to make use of methanol in green catalytic hydrogen production? Nano Select 2020, 1, 12–29. [Google Scholar] [CrossRef]
- Lin, L.; Yu, Q.; Peng, M.; Li, A.; Yao, S.; Tian, S.; Liu, X.; Li, A.; Jiang, Z.; Gao, R.; et al. Atomically dispersed Ni/α-MoC catalyst for hydrogen production from methanol/water. J. Am. Chem. Soc. 2021, 143, 309–317. [Google Scholar] [CrossRef]
- Huber, G.W.; Shabaker, J.W.; Dumesic, J.A. Raney Ni-Sn catalyst for H2 production from biomass-derived hydrocarbons. Science 2003, 300, 2075–2077. [Google Scholar] [CrossRef] [Green Version]
- Ribeirinha, P.; Mateos-Pedrero, C.; Boaventura, M.; Sousa, J.; Mendes, A. CuO/ZnO/Ga2O3 catalyst for low temperature MSR reaction: Synthesis, characterization and kinetic model. Appl. Catal. B Environ. 2018, 221, 371–379. [Google Scholar] [CrossRef]
- Wang, S.-S.; Su, H.-Y.; Gu, X.-K.; Li, W.-X. Differentiating intrinsic reactivity of copper, copper–zinc alloy, and copper/zinc oxide interface for methanol steam reforming by first-principles theory. J. Phys. Chem. C 2017, 121, 21553–21559. [Google Scholar] [CrossRef]
- Kim, W.; Mohaideen, K.K.; Seo, D.J.; Yoon, W.L. Methanol-steam reforming reaction over Cu-Al-based catalysts derived from layered double hydroxides. Int. J. Hydrogen Energy 2017, 42, 2081–2087. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Li, M.M.-J.; Tsang, S.C.E. Bimetallic catalysts for green methanol production via CO2 and renewable hydrogen: A mini-review and prospects. Catal. Sci. Technol. 2018, 8, 3450–3464. [Google Scholar] [CrossRef]
- Tackett, B.M.; Gomez, E.; Chen, J.G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2019, 2, 381–386. [Google Scholar] [CrossRef]
- Ali, S.; Sørensen, K.; Nielsen, M.P. Modeling a novel combined solid oxide electrolysis cell (SOEC)—Biomass gasification renewable methanol production system. Renew. Energy 2020, 154, 1025–1034. [Google Scholar] [CrossRef]
- Gautam, P.; Neha; Upadhyay, S.N.; Dubey, S.K. Bio-methanol as a renewable fuel from waste biomass: Current trends and future perspective. Fuel 2020, 273, 117783. [Google Scholar] [CrossRef]
- Lei, Y.; Luo, Y.; Li, X.; Lu, J.; Mei, Z.; Peng, W.; Chen, R.; Chen, K.; Chen, D.; He, D. The role of samarium on Cu/Al2O3 catalyst in the methanol steam reforming for hydrogen production. Catal. Today 2018, 307, 162–168. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Liu, Y. Effects of flow and operation parameters on methanol steam reforming in tube reactor heated by simulated waste heat. Int. J. Hydrogen Energy 2017, 42, 26270–26276. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Yaroslavtsev, A.B. The influence of the support composition and structure (MXZr1-XO2-δ) of bimetallic catalysts on the activity in methanol steam reforming. Int. J. Hydrogen Energy 2018, 43, 198–207. [Google Scholar] [CrossRef]
- Spassova, I.; Tsontcheva, T.; Velichkova, N.; Khristova, M.; Nihtianova, D. Catalytic reduction of NO with decomposed methanol on alumina-supported Mn–Ce catalysts. J. Colloid Interface Sci. 2012, 374, 267–277. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Gallo, A.; Scotti, N.; Dimitrov, M.; Delaigle, R.; Gaigneaux, E.M.; Kovacheva, D.; Dal Santo, V.; Ravasio, N. Optimization of the preparation procedure of cobalt modified silicas as catalysts in methanol decomposition. Appl. Catal. A Gen. 2012, 417–418, 209–219. [Google Scholar] [CrossRef]
- Mihaylov, M.; Tsoncheva, T.; Hadjiivanov, K. Structure sensitivity of methanol decomposition on Ni/SiO2 catalysts. J. Mater. Sci. 2011, 46, 7144–7151. [Google Scholar] [CrossRef]
- Li, C.-L.; Lin, Y.-C. Catalytic partial oxidation of methanol over copper–zinc based catalysts: A comparative study of alumina, zirconia, and magnesia as promoters. Catal. Lett. 2010, 140, 69–76. [Google Scholar] [CrossRef]
- Li, C.-L.; Jiang, B.-S.; Fanchiang, W.-L.; Lin, Y.-C. The effect of Pd content in LaMnO3 for methanol partial oxidation. Catal. Commun. 2011, 16, 165–169. [Google Scholar] [CrossRef]
- Kapran, A.Y.; Soloviev, S.O.; Orlyk, S.N. Decomposition and partial oxidation of methanol over metal oxide Cu–Zn–Ce-based monoliths. React. Kinet. Mech. Cat. 2010, 101, 343–353. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Erickson, P.; Yoon, H.C.; Liao, C.-H. Comparison of steam and autothermal reforming of methanol using a packed-bed low-cost copper catalyst. Int. J. Hydrogen Energy 2009, 34, 7656–7665. [Google Scholar] [CrossRef]
- Yong, S.T.; Hidajat, K.; Kawi, S. Reaction study of auto thermal steam reforming of methanol to hydrogen using a novel nano CuZnAl-catalyst. J. Power Sources 2004, 131, 91–95. [Google Scholar] [CrossRef]
- Yoon, H.C.; Erickson, P.A.; Kim, H.-M. Lowering the O2/CH3OH ratio in autothermal reforming of methanol by using a reduced copper-based catalyst. Int. J. Hydrogen Energy 2008, 33, 6619–6626. [Google Scholar] [CrossRef]
- Chen, W.-H.; Syu, Y.-J. Thermal behavior and hydrogen production of methanol steam reforming and autothermal reforming with spiral preheating. Int. J. Hydrogen Energy 2011, 36, 3397–3408. [Google Scholar] [CrossRef]
- Ploner, K.; Delir Kheyrollahi Nezhad, P.; Watschinger, M.; Schlicker, L.; Bekheet, M.F.; Gurlo, A.; Gili, A.; Doran, A.; Schwarz, S.; Stöger-Pollach, M.; et al. Steering the methanol steam reforming performance of Cu/ZrO2 catalysts by modification of the Cu-ZrO2 interface dimensions resulting from Cu loading variation. Appl. Catal. A Gen. 2021, 623, 118279. [Google Scholar] [CrossRef]
- Azenha, C.; Lagarteira, T.; Mateos-Pedrero, C.; Mendes, A. Production of hydrogen from methanol steam reforming using CuPd/ZrO2 catalysts–Influence of the catalytic surface on methanol conversion and CO selectivity. Int. J. Hydrogen Energy 2021, 46, 17490–17499. [Google Scholar] [CrossRef]
- Ruano, D.; Pabón, B.M.; Azenha, C.; Mateos-Pedrero, C.; Mendes, A.; Perez-Dieste, V.; Concepción, P. Influence of the ZrO2 crystalline phases on the nature of ctive sites in PdCu/ZrO2 catalysts for the methanol steam reforming reaction—An in situ spectroscopic study. Catalysts 2020, 10, 1005. [Google Scholar] [CrossRef]
- Li, G.; Gu, C.; Zhu, W.; Wang, X.; Yuan, X.; Cui, Z.; Wang, H.; Gao, Z. Hydrogen production from methanol decomposition using Cu-Al spinel catalysts. J. Clean. Prod. 2018, 183, 415–423. [Google Scholar] [CrossRef]
- Liu, D.; Men, Y.; Wang, J.; Kolb, G.; Liu, X.; Wang, Y.; Sun, Q. Highly active and durable Pt/In2O3/Al2O3 catalysts in methanol steam reforming. Int. J. Hydrogen Energy 2016, 41, 21990–21999. [Google Scholar] [CrossRef]
- Martinelli, M.; Jacobs, G.; Graham, U.M.; Davis, B.H. Methanol steam reforming: Na doping of Pt/YSZ provides fine tuning of selectivity. Catalysts 2017, 7, 148. [Google Scholar] [CrossRef] [Green Version]
- Köpfle, N.; Mayr, L.; Schmidmair, D.; Bernardi, J.; Knop-Gericke, A.; Hävecker, M.; Klötzer, B.; Penner, S. A comparative discussion of the catalytic activity and CO2-selectivity of Cu-Zr and Pd-Zr (Intermetallic) compounds in methanol steam reforming. Catalysts 2017, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Fornari, A.C.; Menechini Neto, R.; Lenzi, G.G.; dos Santos, O.A.A.; de Matos, L.M.J. Utilization of sol-gel CuO-ZnO-Al2O3 catalysts in the methanol steam reforming for hydrogen production. Can. J. Chem. Eng. 2017, 95, 2258–2271. [Google Scholar] [CrossRef]
- Mohtashami, Y.; Taghizadeh, M. Performance of the ZrO2 promoted CuZnO catalyst supported on acetic acid-treated MCM-41 in methanol steam reforming. Int. J. Hydrogen Energy 2019, 44, 5725–5738. [Google Scholar] [CrossRef]
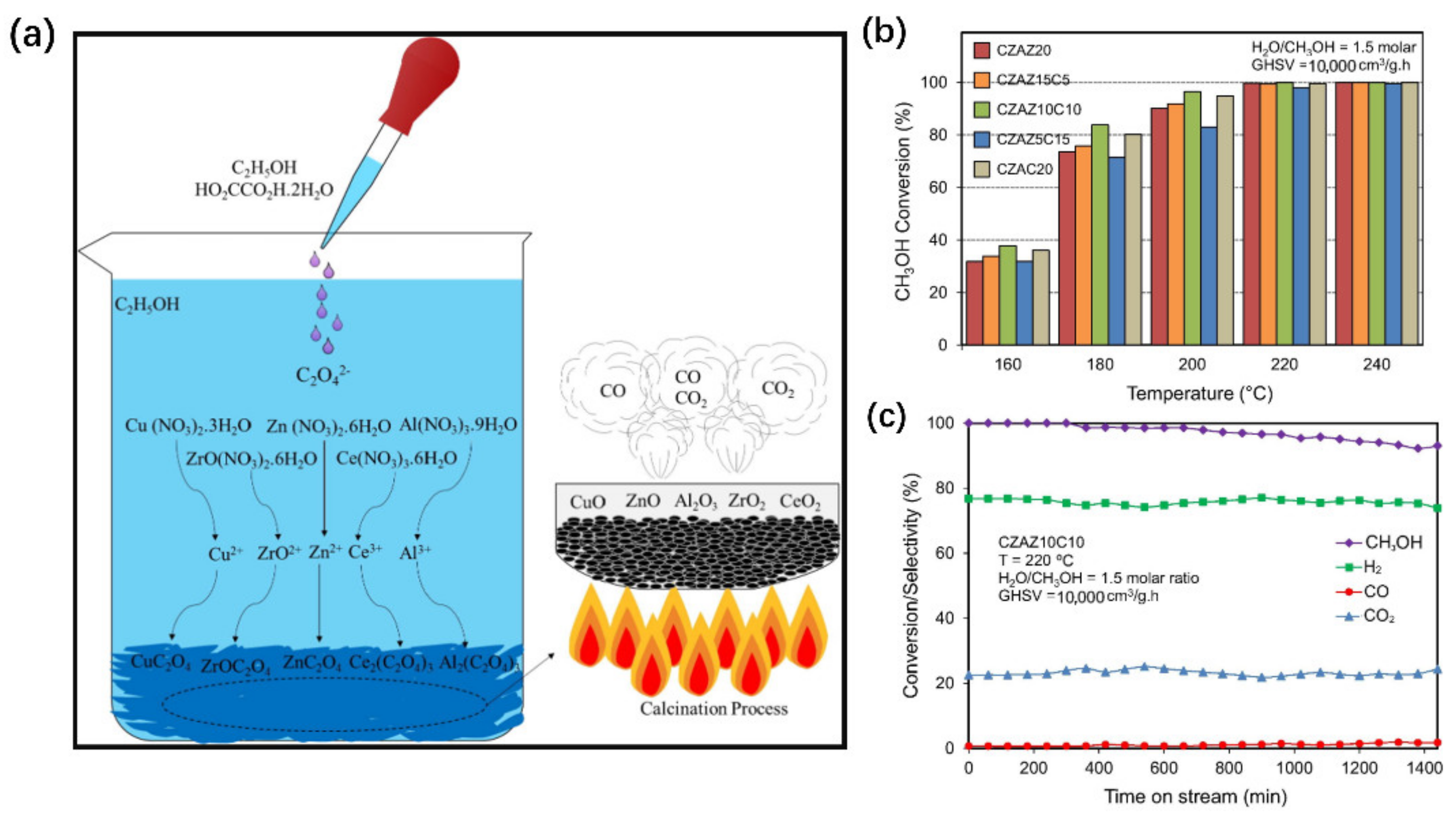
- Bagherzadeh, S.B.; Haghighi, M.; Rahemi, N. Novel oxalate gel coprecipitation synthesis of ZrO2-CeO2-promoted CuO-ZnO-Al2O3 nanocatalyst for fuel cell-grade hydrogen production from methanol: Influence of ceria-zirconia loading. Energy Convers Manag. 2017, 134, 88–102. [Google Scholar] [CrossRef]
- Dong, C.; Yu, Q.; Ye, R.P.; Su, P.; Liu, J.; Wang, G.H. Hollow carbon sphere nanoreactors loaded with PdCu nanoparticles: Void-confinement effects in liquid-phase hydrogenations. Angew. Chem. Int. Ed. Engl. 2020, 59, 18374–18379. [Google Scholar] [CrossRef]
- Yong, S.T.; Ooi, C.W.; Chai, S.P.; Wu, X.S. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes. Int. J. Hydrogen Energy 2013, 38, 9541–9552. [Google Scholar] [CrossRef]
- Ke, C.; Lin, Z. Density functional theory based micro- and macro-kinetic studies of Ni-catalyzed methanol steam reforming. Catalysts 2020, 10, 349. [Google Scholar] [CrossRef] [Green Version]
- Twigg, M.V.; Spencer, M.S. Deactivation of copper metal catalysts for methanol decomposition, methanol steam reforming and methanol synthesis. Top. Catal. 2003, 22, 191–203. [Google Scholar] [CrossRef]
- Khani, Y.; Bahadoran, F.; Soltanali, S.; Ahari, J.S. Hydrogen production by methanol steam reforming on a cordierite monolith reactor coated with Cu–Ni/LaZnAlO4 and Cu–Ni/γ-Al2O3 catalysts. Res. Chem. Intermed. 2018, 44, 925–942. [Google Scholar] [CrossRef]
- Kang, J.; Song, Y.; Kim, T.; Kim, S. Recent trends in the development of reactor systems for hydrogen production via methanol steam reforming. Int. J. Hydrogen Energy 2022, 47, 3587–3610. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Steam reforming of methanol for hydrogen production: A critical analysis of catalysis, processes, and scope. Ind. Eng. Chem. Res. 2021, 60, 89–113. [Google Scholar] [CrossRef]
- Kim, S.; Yun, S.-W.; Lee, B.; Heo, J.; Kim, K.; Kim, Y.-T.; Lim, H. Steam reforming of methanol for ultra-pure H2 production in a membrane reactor: Techno-economic analysis. Int. J. Hydrogen Energy 2019, 44, 2330–2339. [Google Scholar] [CrossRef]
- Zhuang, X.; Xu, X.; Li, L.; Deng, D. Numerical investigation of a multichannel reactor for syngas production by methanol steam reforming at various operating conditions. Int. J. Hydrogen Energy 2020, 45, 14790–14805. [Google Scholar] [CrossRef]
- Wang, G.; Wang, F.; Chen, B. Performance study on methanol steam reforming rib micro-reactor with waste heat recovery. Energies 2020, 13, 1564. [Google Scholar] [CrossRef] [Green Version]
- Mironova, E.Y.; Lytkina, A.A.; Ermilova, M.M.; Orekhova, N.V.; Zhilyaeva, N.A.; Roshan, N.R.; Ievlev, V.M.; Yaroslavtsev, A.B. Methanol steam reforming in a reactor with a palladium–copper membrane in the presence of a nickel–copper catalyst. Pet. Chem. 2020, 60, 1232–1238. [Google Scholar] [CrossRef]
- Li, X.; Rezaei, F.; Rownaghi, A.A. 3D-printed zeolite monoliths with hierarchical porosity for selective methanol to light olefin reaction. React. Chem. Eng. 2018, 3, 733–746. [Google Scholar] [CrossRef]
- Du, W.; Lee, M.-T.; Wang, Y.; Zhao, C.; Li, G.; Li, M. Design of a solar-driven methanol steam reforming receiver/reactor with a thermal storage medium and its performance analysis. Int. J. Hydrogen Energy 2020, 45, 33076–33087. [Google Scholar] [CrossRef]
- Liao, P.-H.; Yang, H.-M. Preparation of catalyst Ni–Cu/CNTs by chemical reduction with formaldehyde for steam reforming of methanol. Catal. Lett. 2008, 121, 274–282. [Google Scholar] [CrossRef]
- Khzouz, M.; Gkanas, E.I.; Du, S.; Wood, J. Catalytic performance of Ni-Cu/Al2O3 for effective syngas production by methanol steam reforming. Fuel 2018, 232, 672–683. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Cai, W.; Zhang, J.; Zhang, X. Hydrochlorination of acetylene using supported phosphorus-doped Cu-based catalysts. Catal. Sci. Technol. 2015, 5, 5174–5184. [Google Scholar] [CrossRef]
- Yao, C.-Z.; Wang, L.-C.; Liu, Y.-M.; Wu, G.-S.; Cao, Y.; Dai, W.-L.; He, H.-Y.; Fan, K.-N. Effect of preparation method on the hydrogen production from methanol steam reforming over binary Cu/ZrO2 catalysts. Appl. Catal. A Gen. 2006, 297, 151–158. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Wang, Y.; Hu, Y.; Nian, Y.; Li, W.; Zhang, J. Pyrrolidone ligand improved Cu-based catalysts with high performance for acetylene hydrochlorination. Appl. Organomet. Chem. 2021, 35, e6066. [Google Scholar] [CrossRef]
- Wu, G.-S.; Mao, D.-S.; Lu, G.-Z.; Cao, Y.; Fan, K.-N. The role of the promoters in Cu based catalysts for methanol steam reforming. Catal. Lett. 2009, 130, 177–184. [Google Scholar] [CrossRef]
- Cao, D.; Zeng, F.; Zhao, Z.; Cai, W.; Li, Y.; Yu, H.; Zhang, S.; Qu, F. Cu based catalysts for syngas production from ethanol dry reforming: Effect of oxide supports. Fuel 2018, 219, 406–416. [Google Scholar] [CrossRef]
- Valdés-Solís, T.; Marban, G.; Fuertes, A.B. Nanosized catalysts for the production of hydrogen by methanol steam reforming. Catal. Today 2006, 116, 354–360. [Google Scholar] [CrossRef]
- Prašnikar, A.; Likozar, B. Sulphur poisoning, water vapour and nitrogen dilution effects on copper-based catalyst dynamics, stability and deactivation during CO2 reduction reactions to methanol. React. Chem. Eng. 2022, 7, 1073–1082. [Google Scholar] [CrossRef]
- Peng, Y.; Si, W.; Li, X.; Chen, J.; Li, J.; Crittenden, J.; Hao, J. Investigation of the poisoning mechanism of lead on the CeO2—WO3 catalyst for the NH3–SCR reaction via in situ IR and raman spectroscopy measurement. Environ. Sci. Technol. 2016, 50, 9576–9582. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, H.; Chen, X.; Liu, Y.; Weng, X.; Wu, Z. Novel SCR catalyst with superior alkaline resistance performance: Enhanced self-protection originated from modifying protonated titanate nanotubes. J. Mater. Chem. A 2015, 3, 680–690. [Google Scholar] [CrossRef]
- Wang, P.; Yan, L.; Gu, Y.; Kuboon, S.; Li, H.; Yan, T.; Shi, L.; Zhang, D. Poisoning-resistant NOx reduction in the presence of alkaline and heavy metals over H-SAPO-34-supported Ce-promoted Cu-based catalysts. Environ. Sci. Technol. 2020, 54, 6396–6405. [Google Scholar] [CrossRef] [PubMed]
- Sanches, S.G.; Flores, J.H.; da Silva, M.I.P. Cu/ZnO and Cu/ZnO/ZrO2 catalysts used for methanol steam reforming. Mol. Catal. 2018, 454, 55–62. [Google Scholar] [CrossRef]
- Ajamein, H.; Haghighi, M. Influence of ambient gas on microwave-assisted combustion synthesis of CuO–ZnO–Al2O3 nanocatalyst used in fuel cell grade hydrogen production via methanol steam reforming. Ceram. Int. 2016, 42, 17978–17989. [Google Scholar] [CrossRef]
- Fasanya, O.O.; Atta, A.Y.; Myint, M.T.Z.; Dutta, J.; Jibril, B.Y. Effects of synthesis methods on performance of CuZn/MCM-41 catalysts in methanol steam reforming. Int. J. Hydrogen Energy 2021, 46, 3539–3553. [Google Scholar] [CrossRef]
- Liao, M.; Guo, C.; Guo, W.; Hu, T.; Xie, J.; Gao, P.; Xiao, H. One-step growth of CuO/ZnO/CeO2/ZrO2 nanoflowers catalyst by hydrothermal method on Al2O3 support for methanol steam reforming in a microreactor. Int. J. Hydrogen Energy 2021, 46, 9280–9291. [Google Scholar] [CrossRef]
- Ziaei-Azad, H.; Semagina, N. Bimetallic catalysts: Requirements for stabilizing PVP removal depend on the surface composition. Appl. Catal. A Gen. 2014, 482, 327–335. [Google Scholar] [CrossRef]
- Shahsavar, H.; Taghizadeh, M.; Kiadehi, A.D. Effects of catalyst preparation route and promoters (Ce and Zr) on catalytic activity of CuZn/CNTs catalysts for hydrogen production from methanol steam reforming. Int. J. Hydrogen Energy 2021, 46, 8906–8921. [Google Scholar] [CrossRef]
- Ye, R.-P.; Chen, Y.; Reina, T.R.; Cao, Z.; Xu, T.; Chen, X.; Jin, Y.; Zhang, X.L.; Liu, J. Fabrication method-engineered Cu–ZnO/SiO2 catalysts with highly dispersed metal nanoparticles toward efficient utilization of methanol as a hydrogen carrier. Renew. Sustain. Energy Rev. 2021, 2, 2100082. [Google Scholar]
- Matter, P.H.; Braden, D.J.; Ozkan, U.S. Steam reforming of methanol to H2 over nonreduced Zr-containing CuO/ZnO catalysts. J. Catal. 2004, 223, 340–351. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Wu, G.; Mao, D.; Lu, G. Influence of the component interaction over Cu/ZrO2 catalysts induced with fractionated precipitation method on the catalytic performance for methanol steam reforming. RSC Adv. 2016, 6, 30176–30183. [Google Scholar] [CrossRef]
- Ploner, K.; Delir Kheyrollahi Nezhad, P.; Gili, A.; Kamutzki, F.; Gurlo, A.; Doran, A.; Cao, P.; Heggen, M.; Köwitsch, N.; Armbruster, M.; et al. The sol–gel autocombustion as a route towards highly CO2-selective, active and long-term stable Cu/ZrO2 methanol steam reforming catalysts. Mater. Chem. Front. 2021, 5, 5093–5105. [Google Scholar] [CrossRef]
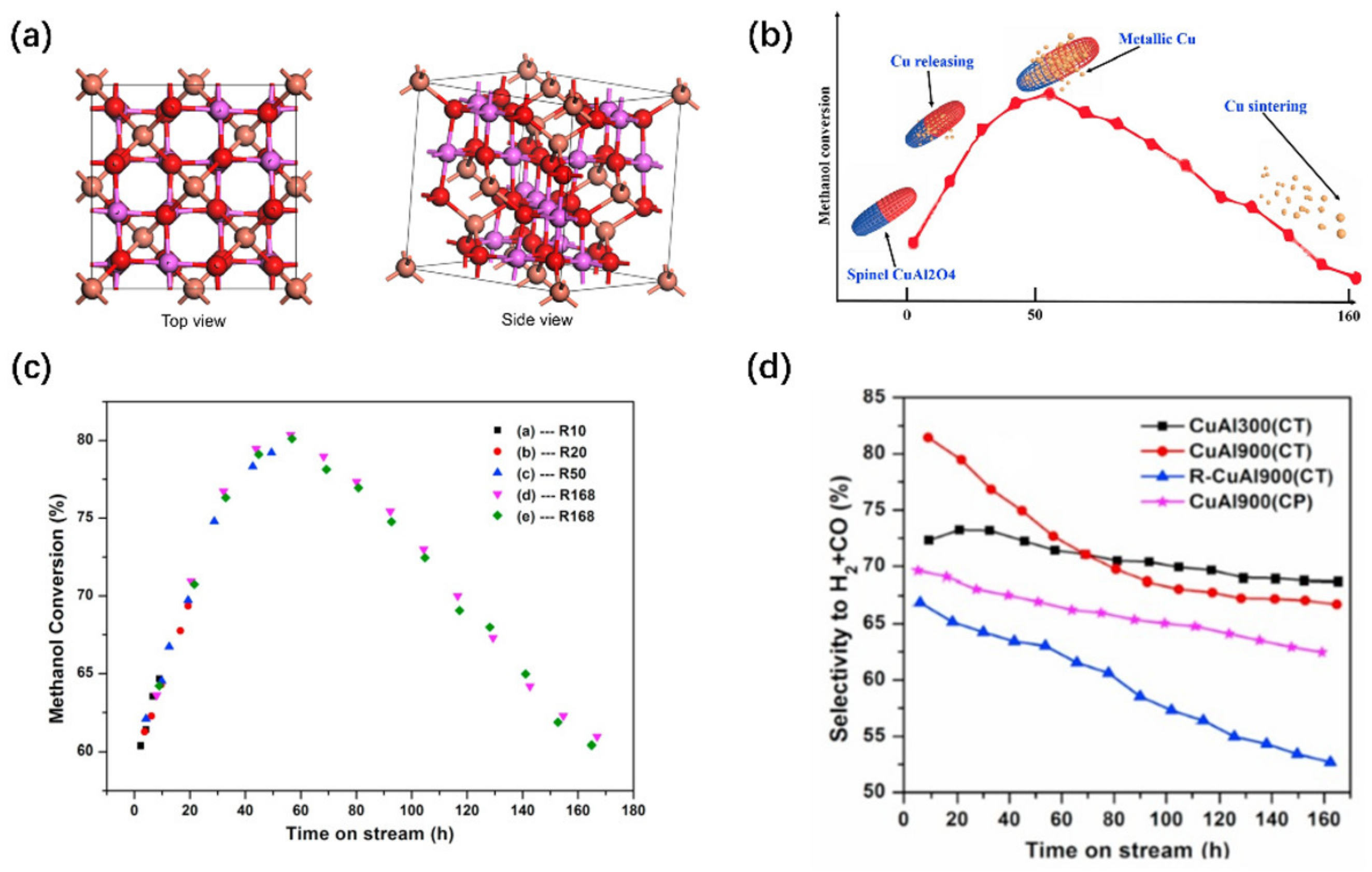
- Xi, H.; Hou, X.; Liu, Y.; Qing, S.; Gao, Z. Cu–Al spinel oxide as an efficient catalyst for methanol steam reforming. Angew. Chem. Int. Ed. Engl. 2014, 53, 11886–11889. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Combined steam reforming of methanol over Cu–Mn spinel oxide catalysts. J. Catal. 2007, 251, 7–20. [Google Scholar] [CrossRef]
- Tanaka, Y.; Utaka, T.; Kikuchi, R.; Takeguchi, T.; Sasaki, K.; Eguchi, K. Water gas shift reaction for the reformed fuels over Cu/MnO catalysts prepared via spinel-type oxide. J. Catal. 2003, 215, 271–278. [Google Scholar] [CrossRef]
- Dasireddy, V.D.B.C.; Likozar, B. Cu–Mn–O nano-particle/nano-sheet spinel-type materials as catalysts in methanol steam reforming (MSR) and preferential oxidation (PROX) reaction for purified hydrogen production. Renew. Energy 2022, 182, 713–724. [Google Scholar] [CrossRef]
- Parsaee, A.; Eliassi, A.; Ranjbar, M.; Kashi, E. Preparation of Cu-Zn-Ce-Al spinel catalyst for hydrogen production in micro-channel reactor and considering the geometrical effects of micro-channels on velocity distribution. Appl. Chem. 2017, 12, 71–82. [Google Scholar]
- Qin, F.-J.; Liu, Y.-J.; Qing, S.-J.; Hou, X.-N.; Gao, Z.-X. Cu-Al spinel as a sustained release catalyst for H2 production from methanol steam reforming: Effects of different copper sources. J. Fuel Chem. Technol. 2017, 45, 1481–1488. [Google Scholar] [CrossRef]
- Huang, H.-K.; Chih, Y.-K.; Chen, W.-H.; Hsu, C.-Y.; Lin, K.-J.; Lin, H.-P.; Hsu, C.-H. Synthesis and regeneration of mesoporous Ni–Cu/Al2O4 catalyst in sub-kilogram-scale for methanol steam reforming reaction. Int. J. Hydrogen Energy, 2021; in press. [Google Scholar] [CrossRef]
- Pu, Y.-C.; Li, S.-R.; Yan, S.; Huang, X.; Wang, D.; Ye, Y.-Y.; Liu, Y.-Q. An improved Cu/ZnO catalyst promoted by Sc2O3 for hydrogen production from methanol reforming. Fuel 2019, 241, 607–615. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Yang, T.; Zhang, Q.; Zhang, L. In-situ self-assembled Cu2O/ZnO core-shell catalysts synergistically enhance the durability of methanol steam reforming. Appl. Catal. A Gen. 2021, 616, 118072. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, W.; Lan, G.; Sun, X.; Wang, X.; Jiang, C.; Li, Y. High-performance Cu/ZnO/Al2O3 catalysts for methanol steam reforming with enhanced Cu-ZnO synergy effect via magnesium assisted strategy. J. Energy. Chem. 2021, 63, 550–557. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Chen, H.-I.; Huang, Y.-J. The promotional effects of ZrO2 and Au on the CuZnO catalyst regarding the durability and activity of the partial oxidation of methanol. Catalysts 2018, 8, 345. [Google Scholar] [CrossRef] [Green Version]
- Lorenzut, B.; Montini, T.; De Rogatis, L.; Canton, P.; Benedetti, A.; Fornasiero, P. Hydrogen production through alcohol steam reforming on Cu/ZnO-based catalysts. Appl. Catal. B Environ. 2011, 101, 397–408. [Google Scholar] [CrossRef]
- Bossola, F.; Roongcharoen, T.; Coduri, M.; Evangelisti, C.; Somodi, F.; Sementa, L.; Fortunelli, A.; Dal Santo, V. Discovering indium as hydrogen production booster for a Cu/SiO2 catalyst in steam reforming of methanol. Appl. Catal. B Environ. 2021, 297, 120398. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Li, H.; Liu, T.; Sang, S.; Chen, S.; Duan, L.; Zeng, L.; Xiang, W.; Gong, J. Chemical looping oxidative steam reforming of methanol: A new pathway for auto-thermal conversion. Appl. Catal. B Environ. 2020, 269, 118758. [Google Scholar] [CrossRef]
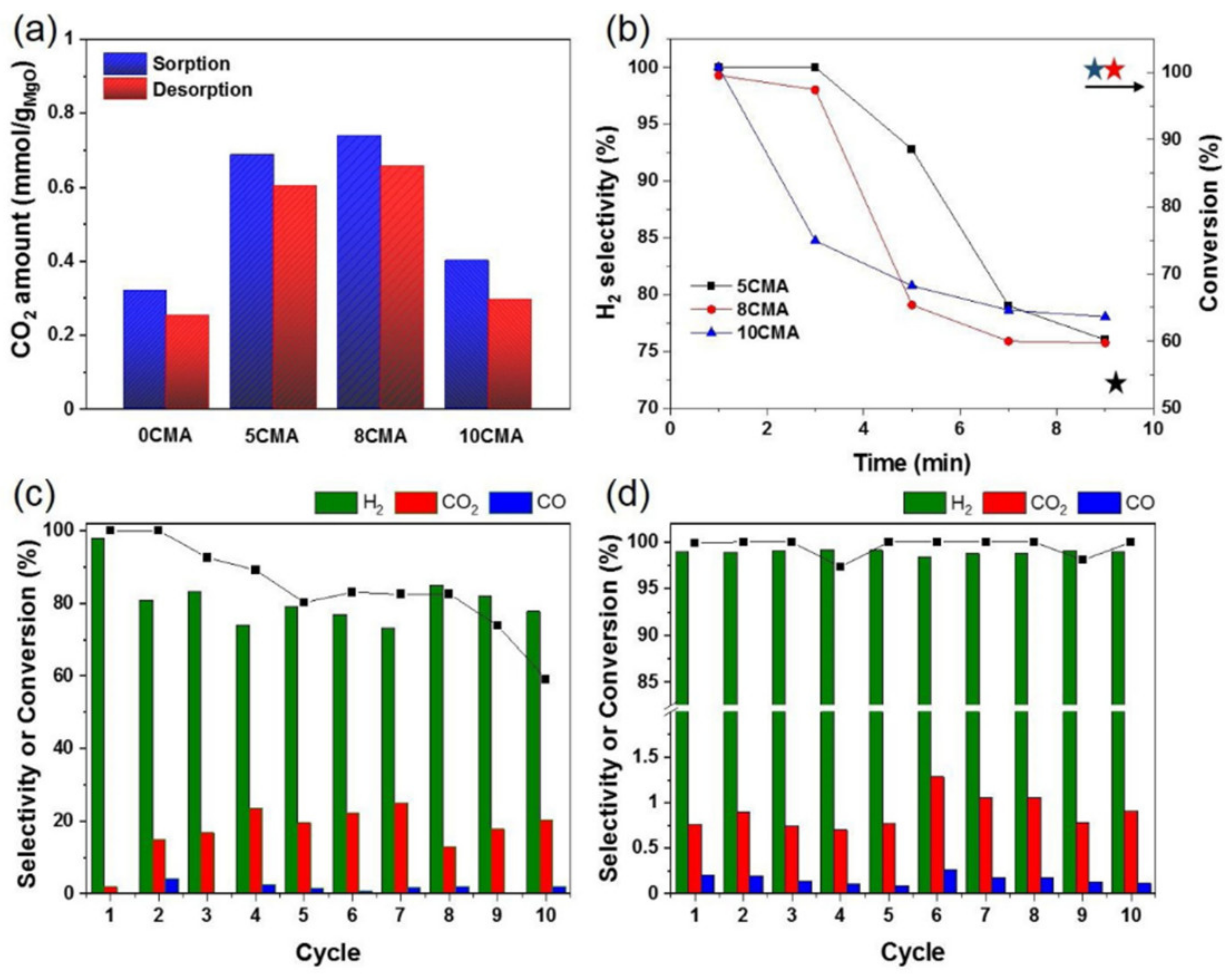
- Li, H.; Tian, H.; Chen, S.; Sun, Z.; Liu, T.; Liu, R.; Assabumrungrat, S.; Saupsor, J.; Mu, R.; Pei, C.; et al. Sorption enhanced steam reforming of methanol for high-purity hydrogen production over Cu-MgO/Al2O3 bifunctional catalysts. Appl. Catal. B Environ. 2020, 276, 119052. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, J.; Zhang, R.; Wu, Y.; Li, H.; Toan, S.; Sun, Z. Fabricating Ga doped and MgO embedded nanomaterials for sorption-enhanced steam reforming of methanol. J. Mater. Chem. A 2022, 10, 7300–7313. [Google Scholar] [CrossRef]
- Kang, L.; Han, L.; He, J.; Li, H.; Yan, T.; Chen, G.; Zhang, J.; Shi, L.; Zhang, D. Improved NOx reduction in the presence of SO2 by using Fe2O3-promoted halloysite-supported CeO2–WO3 catalysts. Environ. Sci. Technol. 2019, 53, 938–945. [Google Scholar] [CrossRef]
- Hou, X.; Qing, S.; Liu, Y.; Li, L.; Gao, Z.; Qin, Y. Enhancing effect of MgO modification of Cu–Al spinel oxide catalyst for methanol steam reforming. Int. J. Hydrogen Energy 2020, 45, 477–489. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Wang, Q.; Yang, T.; Zhang, Q.; Zhang, L. Fast start-up structured CuFeMg/Al2O3 catalyst applied in microreactor for efficient hydrogen production in methanol steam reforming. Chem. Eng. J. 2021, 426, 130644. [Google Scholar] [CrossRef]
- Li, D.; Xu, F.; Tang, X.; Dai, S.; Pu, T.; Liu, X.; Tian, P.; Xuan, F.; Xu, Z.; Wachs, I.E.; et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol. Nat. Catal. 2022, 5, 99–108. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; Azenha, C.; Pacheco Tanaka, D.A.; Sousa, J.M.; Mendes, A. The influence of the support composition on the physicochemical and catalytic properties of Cu catalysts supported on Zirconia-Alumina for methanol steam reforming. Appl. Catal. B Environ. 2020, 277, 119243. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Petriev, I.S.; Baryshev, M.G.; Yaroslavtsev, A.B. Methanol steam reforming over ZrO2-supported catalysts in conventional and membrane reactors. Pet. Chem. 2017, 57, 1219–1227. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Ni, C.; Sun, T.; Zhao, S.; Wang, S.; Wang, A.; Hu, Y. CeO2–ZrO2-promoted CuO/ZnO catalyst for methanol steam reforming. Int. J. Hydrogen Energy 2013, 38, 4397–4406. [Google Scholar] [CrossRef]
- Wang, W.; Qu, Z.; Song, L.; Fu, Q. CO2 hydrogenation to methanol over Cu/CeO2 and Cu/ZrO2 catalysts: Tuning methanol selectivity via metal-support interaction. J. Energy. Chem. 2020, 40, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Ploner, K.; Watschinger, M.; Kheyrollahi Nezhad, P.D.; Götsch, T.; Schlicker, L.; Köck, E.-M.; Gurlo, A.; Gili, A.; Doran, A.; Zhang, L.; et al. Mechanistic insights into the catalytic methanol steam reforming performance of Cu/ZrO2 catalysts by in situ and operando studies. J. Catal. 2020, 391, 497–512. [Google Scholar] [CrossRef]
- Liang, F.; Yu, Y.; Zhou, W.; Xu, X.; Zhu, Z. Highly defective CeO2 as a promoter for efficient and stable water oxidation. J. Mater. Chem. A 2015, 3, 634–640. [Google Scholar] [CrossRef]
- Tonelli, F.; Gorriz, O.; Tarditi, A.; Cornaglia, L.; Arrua, L.; Abello, M.C. Activity and stability of a CuO/CeO2 catalyst for methanol steam reforming. Int. J. Hydrogen Energy 2015, 40, 13379–13387. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.; Wang, X.; Ma, H.; Assabumrungrat, S.; Gong, J. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B Environ. 2015, 176–177, 532–541. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Liu, Y.; Zhang, L.; Chen, Y.; Wang, H.; Tian, Y.; Zhang, C.; Liu, D. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming. Int. J. Hydrogen Energy 2019, 44, 7252–7261. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.; Zhang, L.; Li, Y.; Pan, L. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on γ-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming. Int. J. Hydrogen Energy 2017, 42, 9930–9937. [Google Scholar] [CrossRef]
- Khani, Y.; Bahadoran, F.; Safari, N.; Soltanali, S.; Taheri, S.A. Hydrogen production from steam reforming of methanol over Cu-based catalysts: The behavior of ZnxLaxAl1-xO4 and ZnO/La2O3/Al2O3 lined on cordierite monolith reactors. Int. J. Hydrogen Energy 2019, 44, 11824–11837. [Google Scholar] [CrossRef]
- Aslam, S.; Subhan, F.; Yan, Z.; Peng, P.; Qiao, K.; Xing, W.; Bai, P.; Ullah, R.; Etim, U.J.; Zeng, J.; et al. Facile fabrication of Ni-based KIT-6 for adsorptive desulfurization. Chem. Eng. J. 2016, 302, 239–248. [Google Scholar] [CrossRef]
- Tao, M.; Meng, X.; Lv, Y.; Bian, Z.; Xin, Z. Effect of impregnation solvent on Ni dispersion and catalytic properties of Ni/SBA-15 for CO methanation reaction. Fuel 2016, 165, 289–297. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Akhoundzadeh, H.; Rezayan, A.; Sadeghian, M. Excellent catalytic performance of 3D-mesoporous KIT-6 supported Cu and Ce nanoparticles in methanol steam reforming. Int. J. Hydrogen Energy 2018, 43, 10926–10937. [Google Scholar] [CrossRef]
- Chen, M.; Sun, G.; Wang, Y.; Liang, D.; Li, C.; Wang, J.; Liu, Q. Steam reforming of methanol for hydrogen production over attapulgite-based zeolite-supported Cu-Zr catalyst. Fuel 2022, 314, 122733. [Google Scholar] [CrossRef]
- Hou, X.; Qing, S.; Liu, Y.; Zhang, L.; Zhang, C.; Feng, G.; Wang, X.; Gao, Z.; Qin, Y. Cu1−xMgxAl3 spinel solid solution as a sustained release catalyst: One-pot green synthesis and catalytic performance in methanol steam reforming. Fuel 2021, 284, 119041. [Google Scholar] [CrossRef]
- Liu, Y.; Qing, S.; Hou, X.; Qin, F.; Wang, X.; Gao, Z.; Xiang, H. Cu−Ni−Al spinel oxide as an efficient durable catalyst for methanol steam reforming. ChemCatChem. 2018, 10, 5698–5706. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Kang, H.-F.; Hou, X.-N.; Zhang, L.; Qing, S.-J.; Gao, Z.-X.; Xiang, H.-W. Cu-Ni-Al spinel catalyzed methanol steam reforming for hydrogen production: Effect of Al content. J. Fuel. Chem. Technol. 2020, 48, 1112–1121. [Google Scholar] [CrossRef]
- Qing, S.; Hou, X.; Liu, Y.; Li, L.; Wang, X.; Gao, Z.; Fan, W. Strategic use of CuAlO2 as a sustained release catalyst for production of hydrogen from methanol steam reforming. Chem. Commun. 2018, 54, 12242–12245. [Google Scholar] [CrossRef]
- Liu, Y.; Qing, S.; Hou, X.; Qin, F.; Wang, X.; Gao, Z.; Xiang, H. Temperature dependence of Cu–Al spinel formation and its catalytic performance in methanol steam reforming. Catal. Sci. Technol. 2017, 7, 5069–5078. [Google Scholar] [CrossRef]
- Li, L.; Shi, L.; Yu, X.; Qing, S.; Gao, Z.; Luo, Q.; Feng, G.; Zhang, R. Adsorption of Nin (n = 1–4) clusters on perfect and O-defective CuAl2O4 surfaces: A DFT study. Chin. Chem. Lett. 2019, 30, 1147–1152. [Google Scholar] [CrossRef]
- Feng, L.S.G. High coverage H2O adsorption on CuAl2O4 surface: A DFT study. Appl. Surf. Sci. 2019, 507, 145162. [Google Scholar]
- Li, L.; Gan, Y.-M.; Lu, Z.-H.; XiaohuYu; Qing, S.; Gao, Z.; Zhang, R.; Feng, G. The effects of Fe, Co and Ni doping in CuAl2O4 spinel surface and bulk: A DFT study. Appl. Surf. Sci. 2020, 521, 146478. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Wang, S.-F.; Tsai, A.-P.; Kameoka, S. Reduction behaviors and catalytic properties for methanol steam reforming of Cu-based spinel compounds CuX2O4 (X = Fe, Mn, Al, La). Ceram. Int. 2014, 40, 4541–4551. [Google Scholar] [CrossRef]
- Tan, R.; Hwang, S.W.; Sivanantham, A.; Cho, I.S. Solution synthesis and activation of spinel CuAl2O4 film for solar water-splitting. J. Catal. 2021, 400, 218–227. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting—The untamed dream: A review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-T.; Chan, A.; Al-Azri, Z.H.N.; Dosado, A.G.; Nadeem, M.A.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I.N. Effect of TiO2 polymorph and alcohol sacrificial agent on the activity of Au/TiO2 photocatalysts for H2 production in alcohol–water mixtures. J. Catal. 2015, 329, 499–513. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Swearer, F.D.; Zhao, H.; Zhou, L.; Zhang, C.; Robatjazi, H.; Martirez, P.J.M.; Krauter, M.C.; Yazdi, S.; McClain, J.M.; Ringe, E.; et al. Heterometallic antenna−reactor complexes for photocatalysis. Proc. Nat. Acad. Sci. USA 2016, 113, 8916–8920. [Google Scholar] [CrossRef] [Green Version]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Zhou, L.; Swearer, F.D.; Zhang, C.; Robatjazi, H.; Zhao, H.; Henderson, L.; Dong, L.; Christopher, P.; Carter, A.E.; Nordlander, P.; et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 2018, 362, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Aslam, U.; Rao, V.G.; Chavez, S.; Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat. Catal. 2018, 1, 656–665. [Google Scholar] [CrossRef]
- Zhou, L.; Martirez, J.M.P.; Finzel, J.; Zhang, C.; Swearer, D.F.; Tian, S.; Robatjazi, H.; Lou, M.; Dong, L.; Henderson, L.; et al. Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nat. Energy 2020, 5, 61–70. [Google Scholar] [CrossRef]
- Wang, Z.-j.; Song, H.; Pang, H.; Ning, Y.; Dao, T.D.; Wang, Z.; Chen, H.; Weng, Y.; Fu, Q.; Nagao, T.; et al. Photo-assisted methanol synthesis via CO2 reduction under ambient pressure over plasmonic Cu/ZnO catalysts. Appl. Catal. B Environ. 2019, 250, 10–16. [Google Scholar] [CrossRef]
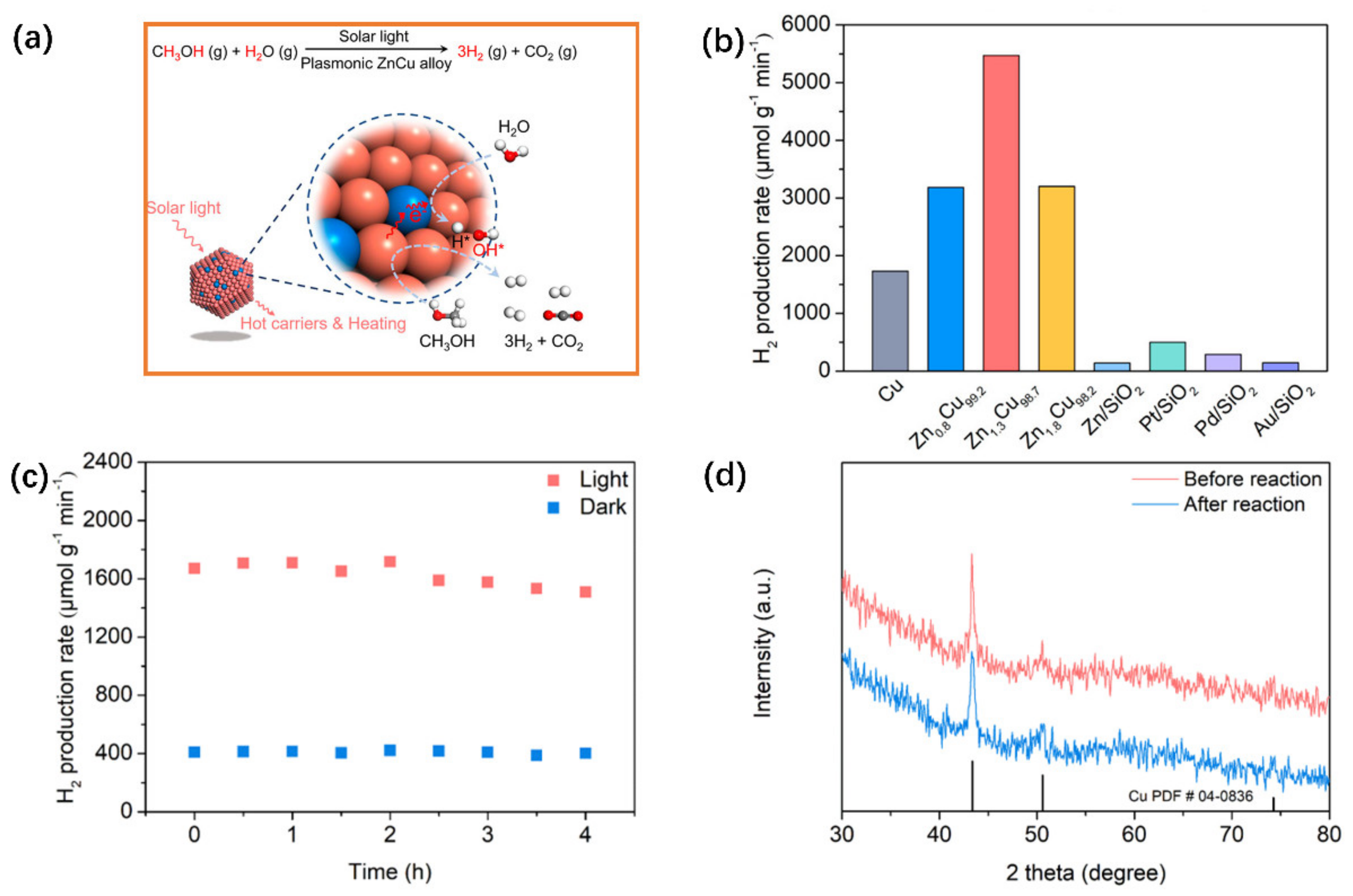
- Luo, S.; Lin, H.; Wang, Q.; Ren, X.; Hernández-Pinilla, D.; Nagao, T.; Xie, Y.; Yang, G.; Li, S.; Song, H.; et al. Triggering water and methanol activation for solar-driven H2 production: Interplay of dual active sites over plasmonic ZnCu alloy. J. Am. Chem. Soc. 2021, 143, 12145–12153. [Google Scholar] [CrossRef]
- Luo, S.; Song, H.; Philo, D.; Oshikiri, M.; Kako, T.; Ye, J. Solar-driven production of hydrogen and acetaldehyde from ethanol on Ni-Cu bimetallic catalysts with solar-to-fuels conversion efficiency up to 3.8%. Appl. Catal. B Environ. 2020, 272, 118965. [Google Scholar] [CrossRef]
- Guerrero-Medina, J.; Mass-Gonzalez, G.; Pacheco-Londoño, L.; Hernandez-Rivera, S.P.; Fu, R.; Hernandez-Maldonado, A.J. Long and local range structural changes in M [(bdc)(ted)0.5] (M = Zn, Ni or Cu) metal organic frameworks upon spontaneous thermal dispersion of LiCl and adsorption of carbon dioxide. Micropor. Mesopor. Mater. 2015, 212, 8–17. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic methods for the functionalization of metal–organic frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, G.; Geng, J.; Jing, D.; Hong, X.; Guo, L. A high-performance PdZn alloy catalyst obtained from metal-organic framework for methanol steam reforming hydrogen production. Int. J. Hydrogen Energy 2019, 44, 24387–24397. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Pérez, N.C.; Miró, E.E.; Casado, C.; Seoane, B.; Tellez, C.; Coronas, J. HKUST-1 MOF: A matrix to synthesize CuO and CuO–CeO2 nanoparticle catalysts for CO oxidation. Chem. Eng. J. 2012, 195–196, 180–187. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Aerobic oxidation of benzylic alcohols catalyzed by metal−organic frameworks assisted by TEMPO. ACS Catal. 2011, 1, 48–53. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Liu, B.; Akita, T.; Haruta, M.; Sakurai, H.; Xu, Q. Au@ZIF-8: CO oxidation over gold nanoparticles deposited to metal−organic framework. J. Am. Chem. Soc. 2009, 131, 11302–11303. [Google Scholar] [CrossRef]
- Zou, R.-Q.; Sakurai, H.; Han, S.; Zhong, R.-Q.; Xu, Q. Probing the lewis acid sites and CO catalytic oxidation activity of the porous metal−organic polymer [Cu(5-methylisophthalate)]. J. Am. Chem. Soc. 2007, 129, 8402–8403. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Lv, S.; Huang, Y. A novel synthetic route for MOF-derived CuO-CeO2 catalyst with remarkable methanol steam reforming performance. Catal. Commun. 2021, 149, 106215. [Google Scholar] [CrossRef]
- Ye, R.-P.; Lin, L.; Chen, C.-C.; Yang, J.-X.; Li, F.; Zhang, X.; Li, D.-J.; Qin, Y.-Y.; Zhou, Z.; Yao, Y.-G. Synthesis of robust MOF-derived Cu/SiO2 catalyst with low copper loading via sol–gel method for the dimethyl oxalate hydrogenation reaction. ACS Catal. 2018, 8, 3382–3394. [Google Scholar] [CrossRef]
- Varmazyari, M.; Khani, Y.; Bahadoran, F.; Shariatinia, Z.; Soltanali, S. Hydrogen production employing Cu(BDC) metal–organic framework support in methanol steam reforming process within monolithic micro-reactors. Int. J. Hydrogen Energy 2021, 46, 565–580. [Google Scholar] [CrossRef]





| Entry | Catalyst | Preparation | Conv.CH3OH/% | Select.H2/% | T/°C | Coke Formation/% | Lifetime/h | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Cu-ZnO-ZrO2/MCM-41 | Modified impregnation method | 97.8 | 99.0 | 300 | 3.11 | 60 | [48] |
| 2 | Cu-ZnO-ZrO2/MCM-41 | Sol-gel | 96.3 | 98.8 | 300 | 3.89 | 40 | [48] |
| 3 | Cu-ZnO-ZrO2/MCM-41 | Conventional impregnation | 94.0 | 98.5 | 300 | 4.69 | 20 | [48] |
| 4 | CuO/ZnO/CeO2/ZrO2 | One step hydrothermal process | 99.8 | - | 330 | 2.2 | 30 | [78] |
| 5 | Ni-Cu/Al2O4 | Green template-free method | >99.0 | 42.17 | 300 | <1.0 | 30 | [91] |
| Entry | Catalyst | Dopant Species | Conv.CH3OH/% | Select.H2/% | T/°C | Dispersion/% | Lifetime/h | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Cu/Sc2O3-ZnO | Sc2O3 | 95.0 | - | 220–600 | 6.9 | 16 | [92] |
| 2 | Cu-Al spinel oxide | MgO | 96.5 | 96.5 | 255 | - | 450 | [102] |
| 3 | CuZn/CNTs | Ce.Zr | 94.2 | 98.2 | 300 | 18 | 48 | [80] |
| 4 | Cu-MgO/Al2O3 | MgO | ~100 | 99.3 | 220 | 62 | 30 | [99] |
| 5 | Cu/SiO2 | In | 57.1 | ~100 | 260 | 56.4 | 18 | [97] |
| 6 | CuFeMg/Al2O3 | FeOx | 85 | 97.8 | 250 | 24.1 | 100 | [103] |
| Entry | Catalyst | Support | Conv.CH3OH/% | Select.H2/% | T/°C | Dispersion/% | Lifetime/h | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Cu-Zr/AZ | Zeolite-supported | - | 90.6 | 400 | - | 50 | [119] |
| 2 | Ce-Cu/KIT-6 | KIT-6 | ~92 | 99.0 | 300 | 9.8 | 24 | [118] |
| 3 | CuO/CeO2 | CeO2 | 100 | - | 280 | 15.33 | 40 | [111] |
| 4 | CuZrAlX | ZrO2-Al2O3 | 96.0 | - | 270 | 23.1 | 30 | [105] |
| 5 | Cu/Zn1.11La1.26Al0.5O4.27 | Ceramic support | 97 | 91 | 300 | 11.5 | 12 | [115] |
| 6 | 10% Cu/g-Al@MMO | ZnAl-LDHs/g-Al2O3 | 99.98 | 97.8 | 300 | 14.16 | 20 | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, R.; Xiao, S.; Lai, Q.; Wang, D.; Huang, Y.; Feng, G.; Zhang, R.; Wang, T. Advances in Enhancing the Stability of Cu-Based Catalysts for Methanol Reforming. Catalysts 2022, 12, 747. https://doi.org/10.3390/catal12070747
Ye R, Xiao S, Lai Q, Wang D, Huang Y, Feng G, Zhang R, Wang T. Advances in Enhancing the Stability of Cu-Based Catalysts for Methanol Reforming. Catalysts. 2022; 12(7):747. https://doi.org/10.3390/catal12070747
Chicago/Turabian StyleYe, Runping, Shuwei Xiao, Qinghua Lai, Dashan Wang, Yuanyuan Huang, Gang Feng, Rongbin Zhang, and Tao Wang. 2022. "Advances in Enhancing the Stability of Cu-Based Catalysts for Methanol Reforming" Catalysts 12, no. 7: 747. https://doi.org/10.3390/catal12070747
APA StyleYe, R., Xiao, S., Lai, Q., Wang, D., Huang, Y., Feng, G., Zhang, R., & Wang, T. (2022). Advances in Enhancing the Stability of Cu-Based Catalysts for Methanol Reforming. Catalysts, 12(7), 747. https://doi.org/10.3390/catal12070747








