Biochemical Characterisation and Structure Determination of a Novel Cold-Active Proline Iminopeptidase from the Psychrophilic Yeast, Glaciozyma antarctica PI12
Abstract
1. Introduction
2. Results and Discussion
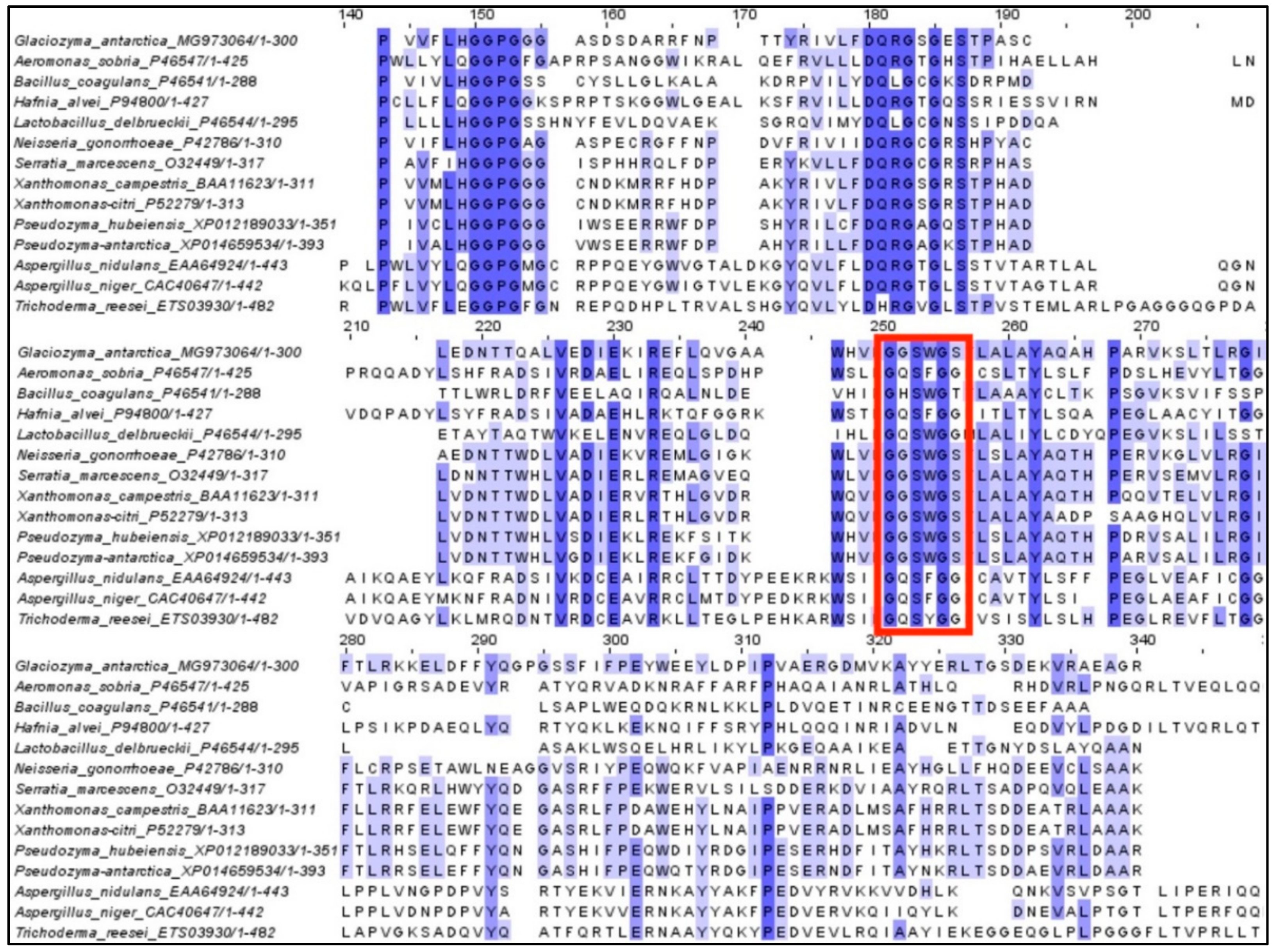
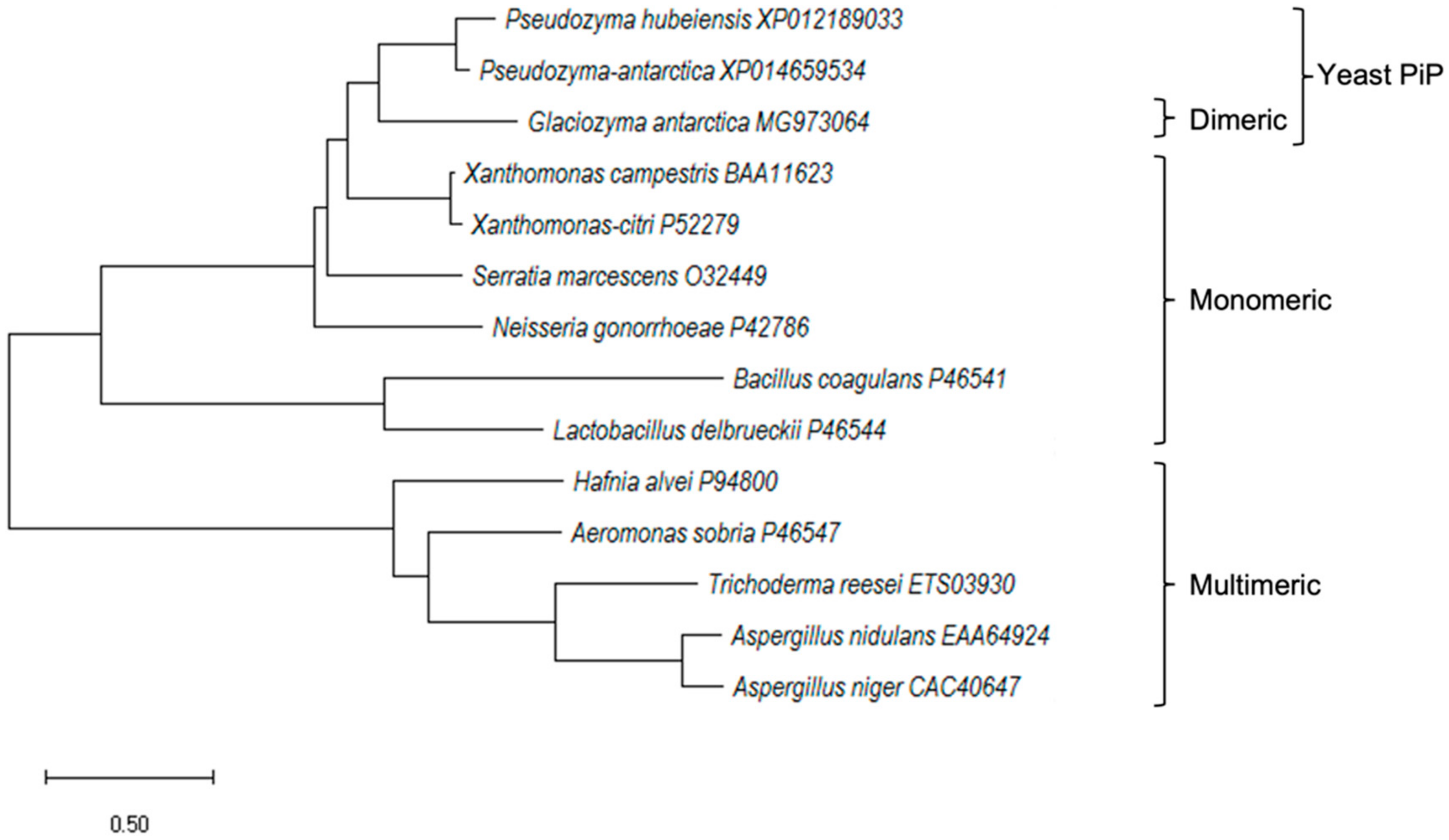
2.1. Cloning and In Silico Analysis of GaPIP G. antarctica
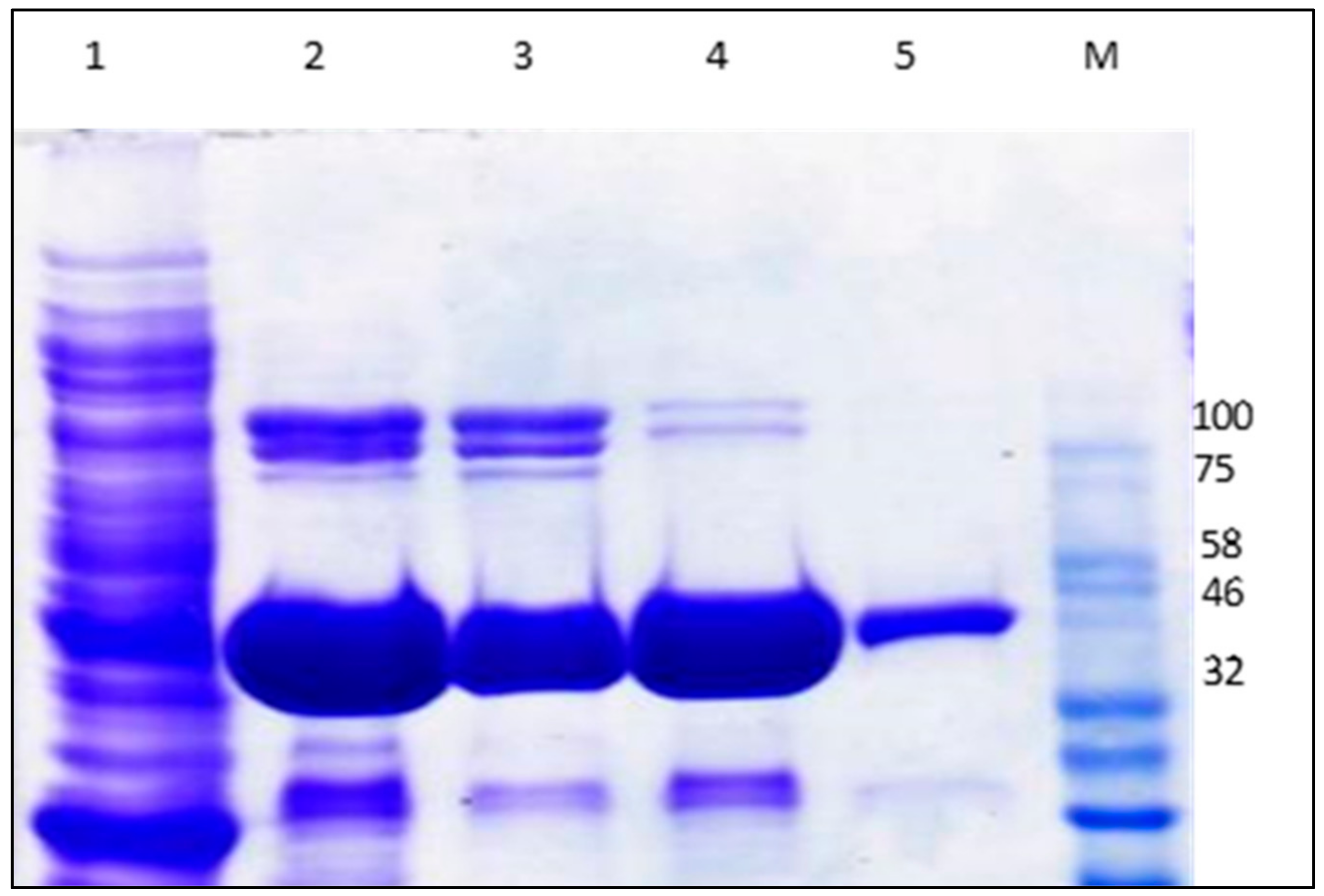
2.2. Expression of Recombinant GaPIP
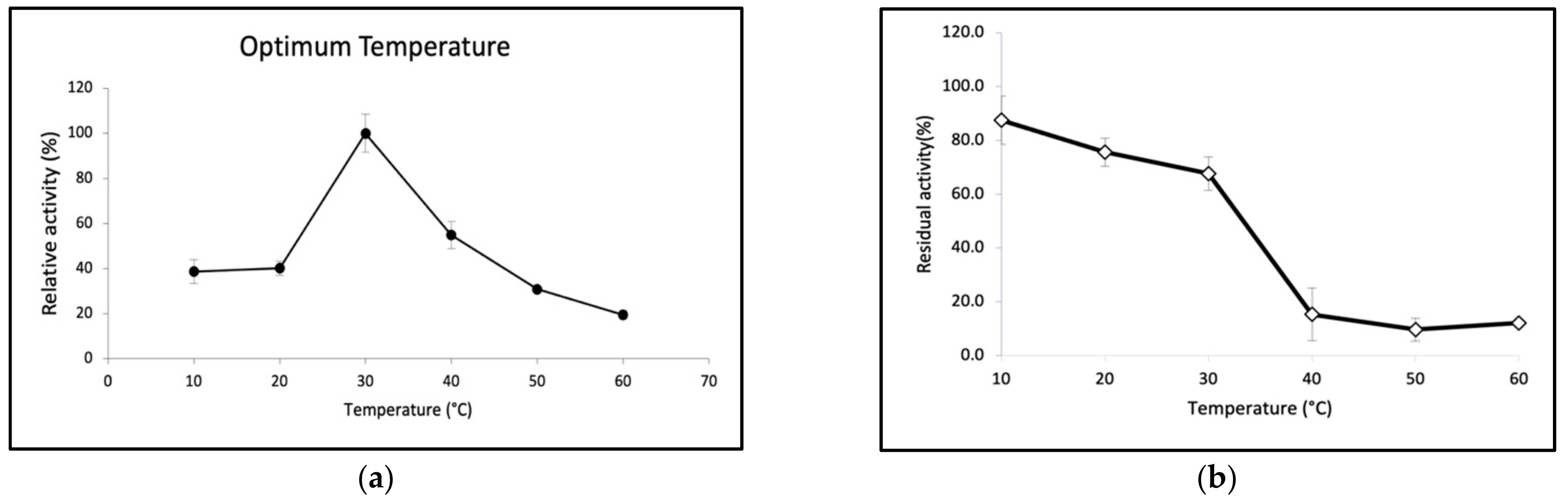
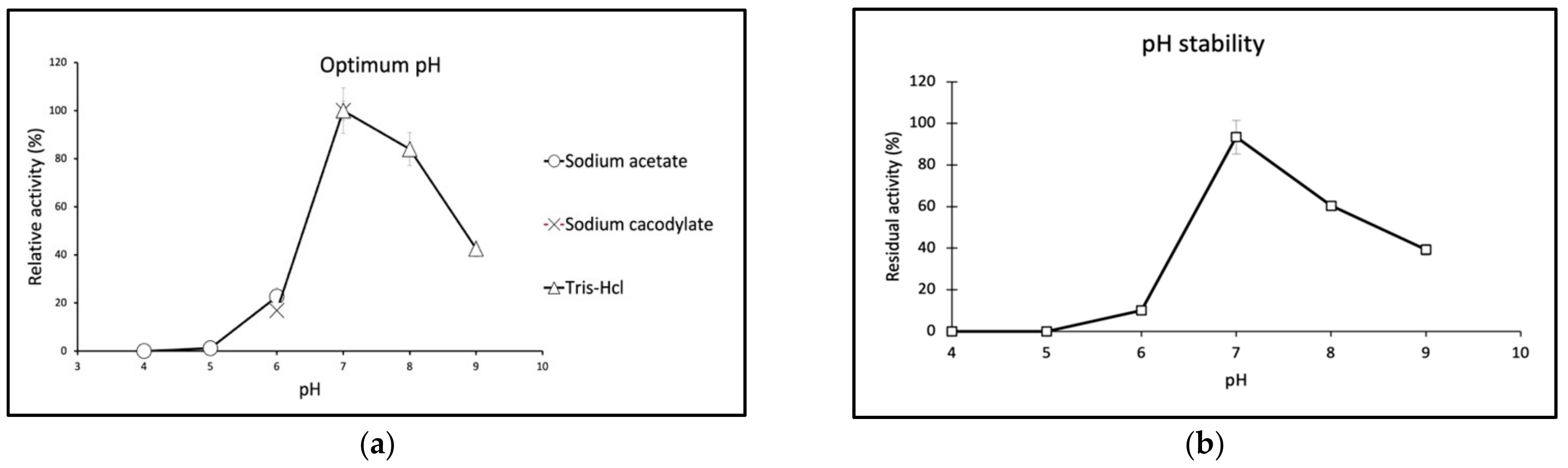
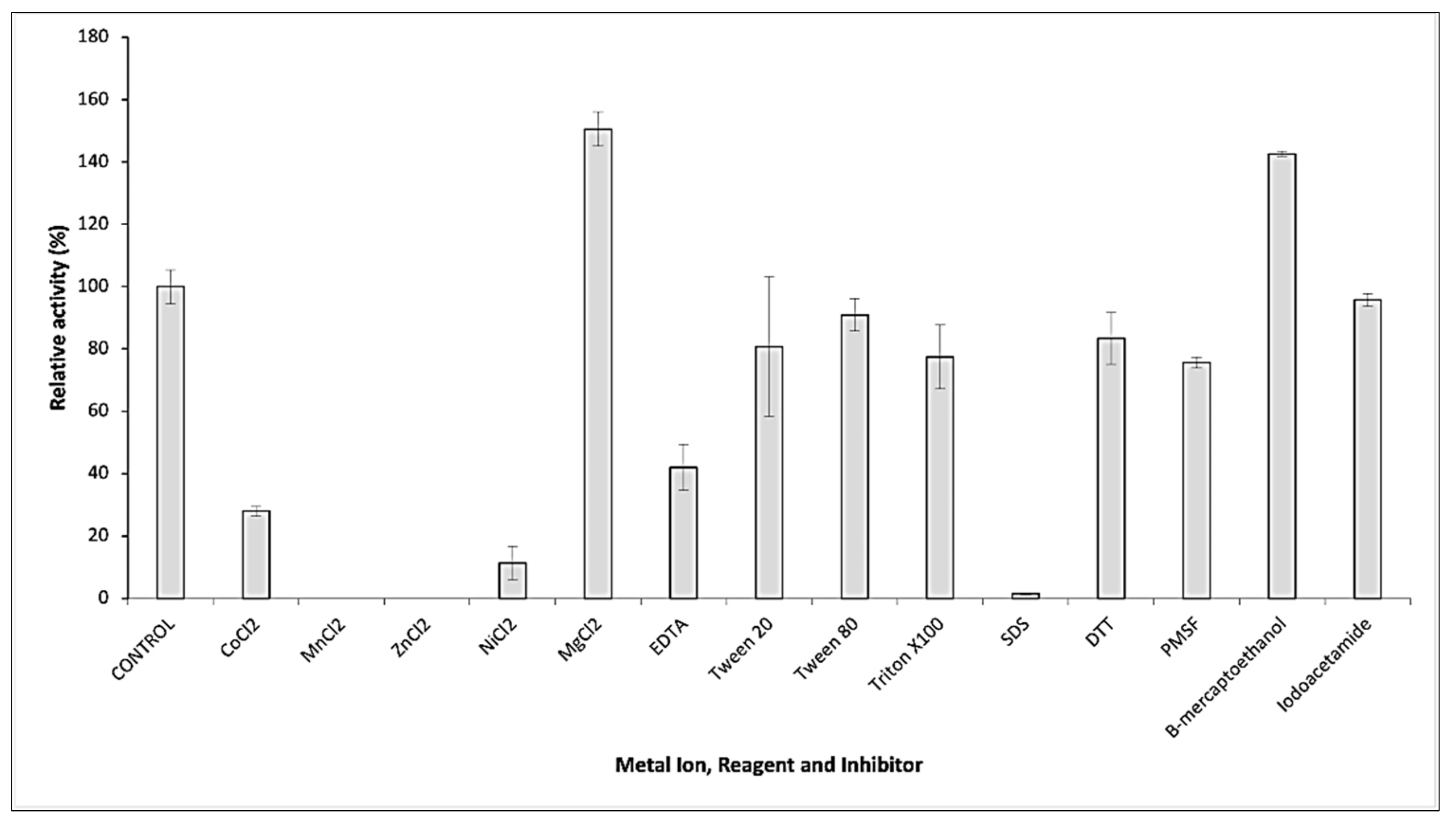
2.3. Biochemical Properties of GaPIP
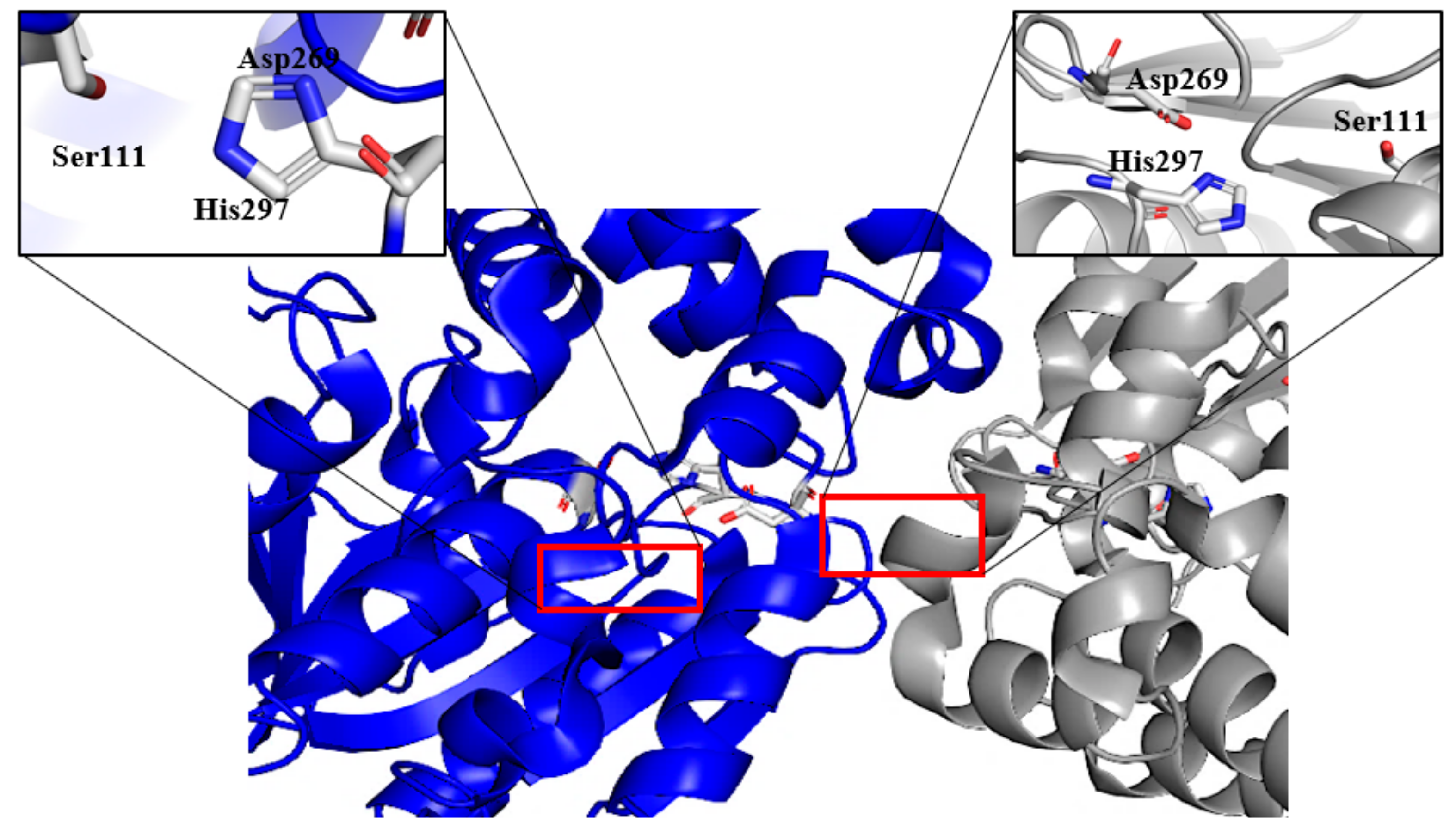
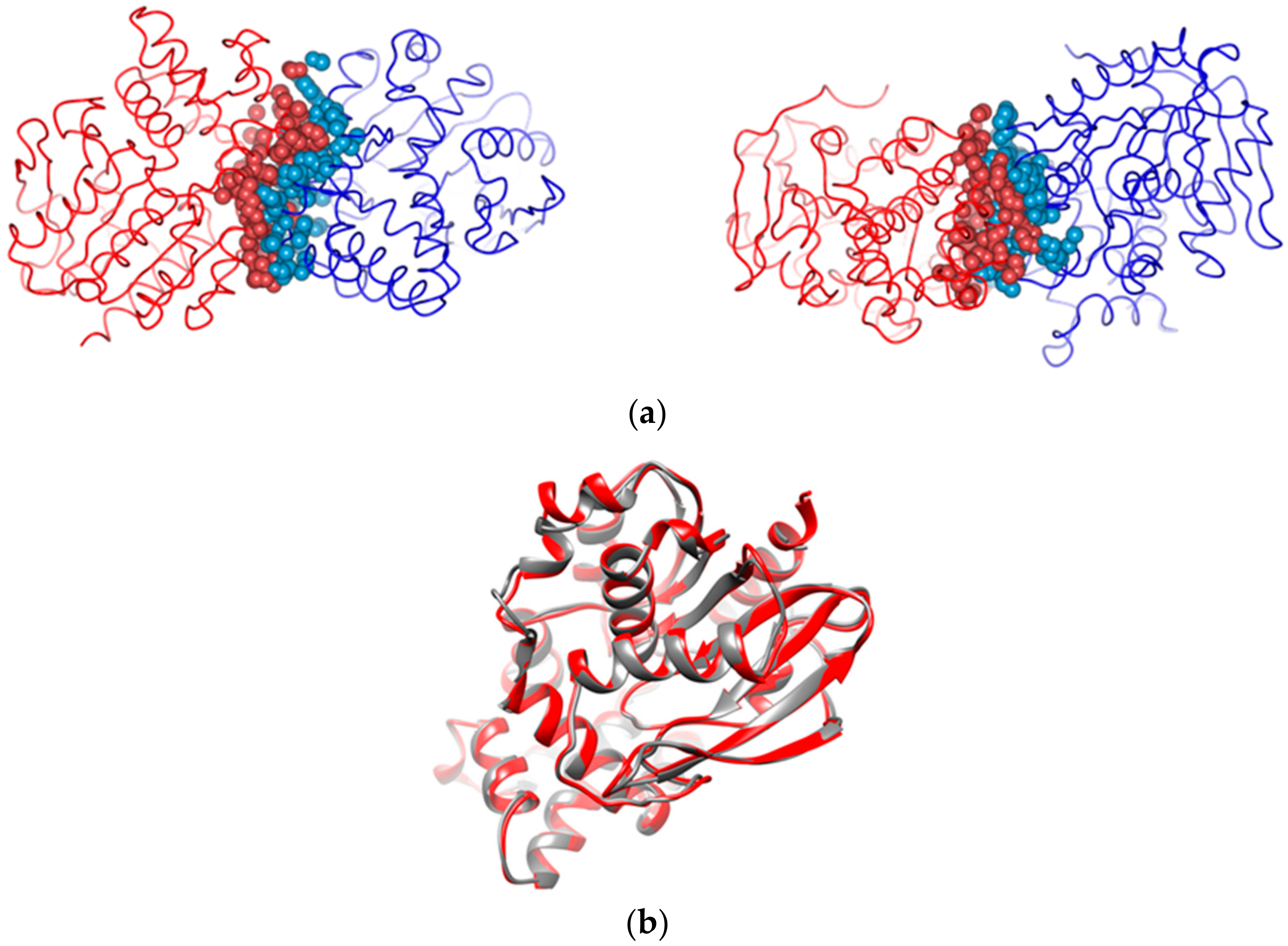
2.4. Structure Determination of GaPIP
3. Materials and Methods
3.1. Microorganism and Culture Conditions
3.2. Total RNA Isolation and cDNA Synthesis
3.3. Alignment of Protein Sequences and Phylogenetic Reconstruction
3.4. Construction of Expression Vectors
3.5. Expression of the GaPIP in E. coli BL21(DE3) Star
3.6. Purification of Recombinant GaPIP
3.7. Biochemical Characterisation of GaPIP
3.8. Substrate Specificity and Kinetic Parameters
3.9. Crystallisation, Data Collection, and Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and Hot Extremozymes: Industrial Relevance and Current Trends. Front. Bioeng. Biotechnol. 2015, 3, 148. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Kim, J.; Bae, E. Structural Analyses of Adenylate Kinases from Antarctic and Tropical Fishes for Understanding Cold Adaptation of Enzymes. Sci. Rep. 2017, 7, 16027. [Google Scholar] [CrossRef] [PubMed]
- Raddadi, N.; Cherif, A.; Daffonchio, D.; Neifar, M.; Fava, F. Biotechnological Applications of Extremophiles, Extremozymes and Extremolytes. Appl. Microbiol. Biotechnol. 2015, 19, 7907–7913. [Google Scholar] [CrossRef] [PubMed]
- Firdaus-Raih, M.; Hashim, N.H.F.; Bharudin, I.; Abu Bakar, M.F.; Huang, K.K.; Alias, H.; Lee, B.K.B.; Mat Isa, M.N.; Mat-Sharani, S.; Sulaiman, S. The Glaciozyma antarctica Genome Reveals an Array of Systems That Provide Sustained Responses towards Temperature Variations in a Persistently Cold Habitat. PLoS ONE 2018, 13, e0189947. [Google Scholar] [CrossRef] [PubMed]
- Struvay, C.; Feller, G. Optimization to Low Temperature Activity in Psychrophilic Enzymes. Int. J. Mol. Sci. 2012, 13, 11643–11665. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.A.; Hashim, N.H.F.; Bharudin, I. Cold Adaptation Strategies and the Potential of Psychrophilic Enzymes from the Antarctic Yeast, Glaciozyma antarctica Pi12. J. Fungi 2021, 7, 528. [Google Scholar] [CrossRef]
- Åqvist, J.; Isaksen, G.V.; Brandsdal, B.O. Computation of Enzyme Cold Adaptation. Nat. Rev. Chem. 2017, 1, 51. [Google Scholar] [CrossRef]
- Siddiqui, K.S. Some like It Hot, Some like It Cold: Temperature Dependent Biotechnological Applications and Improvements in Extremophilic Enzymes. Biotechnol. Adv. 2015, 33, 1912–1922. [Google Scholar] [CrossRef]
- Marx, J.C.; Blaise, V.; Collins, T.; D’Amico, S.; Delille, D.; Gratia, E.; Hoyoux, A.; Huston, A.L.; Sonan, G.; Feller, G. A Perspective on Cold Enzymes: Current Knowledge and Frequently Asked Questions. Cell. Mol. Biol. (Noisy-Grand) 2004, 50, 643–655. [Google Scholar]
- Santiago, M.; Ramírez-Sarmiento, C.A.; Zamora, R.A.; Parra, L.P. Discovery, Molecular Mechanisms, and Industrial Applications of Cold-Active Enzymes. Front. Microbiol. 2016, 7, 1408. [Google Scholar] [CrossRef]
- Tattersall, G.J.; Sinclair, B.J.; Withers, P.C.; Fields, P.A.; Seebacher, F.; Cooper, C.E.; Maloney, S.K. Coping with Thermal Challenges: Physiological Adaptations to Environmental Temperatures. Compr. Physiol. 2012, 2, 2151–2202. [Google Scholar] [PubMed]
- Nandanwar, S.K.; Borkar, S.B.; Lee, J.H.; Kim, H.J. Taking Advantage of Promiscuity of Cold-Active Enzymes. Appl. Sci. 2020, 10, 8128. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Salvesen, G. Handbook of Proteolytic Enzymes; Elsevier: Amsterdam, The Netherlands, 2013; Volume 3, pp. 2491–2523. [Google Scholar]
- Omar, M.N.; Rahman, R.N.Z.R.A.; Noor, N.D.M.; Latip, W.; Knight, V.F.; Ali, M.S.M. Structure-Function and Industrial Relevance of Bacterial Aminopeptidase p. Catalysts 2021, 11, 1157. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; O’Cuinn, G. Enzymatic Debittering of Food Protein Hydrolysates. Biotechnol. Adv. 2006, 24, 234–237. [Google Scholar] [CrossRef]
- Cheung, L.K.Y.; Aluko, R.E.; Cliff, M.A.; Li-Chan, E.C.Y. Effects of Exopeptidase Treatment on Antihypertensive Activity and Taste Attributes of Enzymatic Whey Protein Hydrolysates. J. Funct. Foods 2015, 13, 262–275. [Google Scholar] [CrossRef]
- Chanalia, P.; Gandhi, D.; Attri, P.; Dhanda, S. Extraction, Purification and Characterization of Low Molecular Weight Proline Iminopeptidase from Probiotic L. plantarum for Meat Tenderization. Int. J. Biol. Macromol. 2018, 109, 651–663. [Google Scholar] [CrossRef]
- Dunaevsky, Y.E.; Tereshchenkova, V.F.; Belozersky, M.A.; Filippova, I.Y.; Oppert, B.; Elpidina, E.N. Review Effective Degradation of Gluten and Its Fragments by Gluten-Specific Peptidases: A Review on Application for the Treatment of Patients with Gluten Sensitivity. Pharmaceutics 2021, 13, 1603. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, D.; Huang, Q.; Gu, L.; Zhou, N.; Tian, Y. Mutagenesis for Improvement of Activity and Stability of Prolyl Aminopeptidase from Aspergillus oryzae. Appl. Biochem. Biotechnol. 2020, 191, 1483–1498. [Google Scholar] [CrossRef]
- Li, N.; Wu, J.; Zhang, L.; Zhang, Y.; Feng, H. Biochimie Characterization of a Unique Proline Iminopeptidase from White-Rot Basidiomycetes Phanerochaete chrysosporium. Biochimie 2010, 92, 779–788. [Google Scholar] [CrossRef]
- Alonso, J.; Garća, J.L. Proline Iminopeptidase Gene from Xanthomonas campestris pv. citri. Microbiology 1996, 142, 2951–2957. [Google Scholar] [CrossRef][Green Version]
- Basten, D.E.J.W.; Moers, A.P.H.A.; van Ooyen, A.J.J.; Schaap, P.J. Characterisation of Aspergillus niger Prolyl Aminopeptidase. Mol. Genet. Genom. 2005, 272, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Kitazono, A.; Kitano, A.; Tsuru, D.; Yoshimoto, T. Isolation and Characterization of the Prolyl Aminopeptidase Gene (pap) from Aeromonas sobria: Comparison with the Bacillus coagulans Enzyme. J. Biochem. 1994, 116, 818–825. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Feller, G. Psychrophilic Enzymes: From Folding to Function and Biotechnology. Scientifica 2013, 2013, 512840. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R.; Charlton, T.; Ertan, H.; Omar, S.M.; Siddiqui, K.S.; Williams, T.J. Biotechnological Uses of Enzymes from Psychrophiles. Microb. Biotechnol. 2011, 4, 449–460. [Google Scholar] [CrossRef]
- Ding, G.W.; Zhou, N.D.; Tian, Y.P. Over-Expression of a Proline Specific Aminopeptidase from Aspergillus oryzae JN-412 and Its Application in Collagen Degradation. Appl. Biochem. Biotechnol. 2014, 173, 1765–1777. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Inoue, T.; Kabashima, T.; Kanada, N.; Huang, H.S.; Ma, X.; Azmi, N.; Azab, E.; Yoshimoto, T. Substrate Recognition Mechanism of Prolyl Aminopeptidase from Serratia marcescens. J. Biochem. 2000, 128, 673–678. [Google Scholar] [CrossRef]
- Medrano, F.J.; Alonso, J.; Garcia, J.L.; Bode, W.; Gomis-Ruth, F.X. Crystallization and Preliminary X-Ray Diffraction Analysis of Proline Iminopeptidase from Xanthomonas campestris pv. citri. FEBS Lett. 1997, 400, 91–93. [Google Scholar] [CrossRef][Green Version]
- Paredes, D.; Watters, K.; Pitman, J.D.; Bystroff, C.; Dordick, S.J. Comparative void-volume analysis of psychrophilic and mesophilic enzymes: Structural bioinformatics of psychrophilic enzymes reveals sources of core flexibility. BMC Struct. Biol. 2011, 11, 42. [Google Scholar] [CrossRef]
- Gou, R.; Cang, Z.; Yao, J.; Kim, M.; Deans, E.; Wei, G.; Kang, S. Structural cavities are critical to balancing stability and activity of a membrane-integral enzyme. Proc. Natl. Acad. Sci. USA 2020, 117, 22146–22156. [Google Scholar]
- Raymond-Bouchard, I.; Goordial, J.; Zolotarov, Y.; Ronholm, J.; Stromvik, M.; Bakermans, C.; Whyte, G.L. Conserved genomic and amino acid traits of cold adaptation in subzero-growing Arctic permafrost bacteria. FEMS Microbiol. Ecol. 2018, 94, fiy023. [Google Scholar] [CrossRef] [PubMed]
- Bharudin, I.; Zaki, N.Z.; Abu Bakar, F.D.; Mahadi, N.M.; Najimudin, N.; Illias, R.M.; Murad, A.M.A. Comparison of RNA extraction methods for transcript analysis from the psychrophilic yeast, Glaciozyma antarctica. Malays. Appl. Biol. 2014, 3, 71–80. [Google Scholar]
- Edgar, R.C. MUSCLE: A Multiple Sequence Alignment Method with Reduced Time and Space Complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Jaafar, N.R.; Littler, D.; Beddoe, T.; Rossjohn, J.; Illias, R.M.; Mahadi, N.M.; Mackeen, M.M.; Murad, A.M.A.; Abu Bakar, F.D. Crystal Structure of Fuculose Aldolase from the Antarctic Psychrophilic Yeast Glaciozyma antarctica PI12. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016, 72, 831–839. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Echols, N.; Headd, J.J.; Hung, L.-W.; Jain, S.; Kapral, G.J.; Grosse Kunstleve, R.W.; et al. The Phenix Software for Automated Determination of Macromolecular Structures. Methods 2011, 55, 94–106. [Google Scholar] [CrossRef]
| Substrate | Km (mM) | Vmax (nmole min−1) | Kcat (s−1) | Kcat/Km (mM−1 s−1) | Specific Activity (Umg−1) |
|---|---|---|---|---|---|
| L-Proline-p-nitroanilide-trifluoroacetate | 1.012 | 3.561 | 2.08 | 2.05 | 3561 |
| L-Methionine-p-nitroanilide | 1.321 | 1.783 | 1.04 | 0.79 | 1783 |
| L-Alanine-p-nitroanilide | 4.088 | 4.891 | 14.3 | 3.49 | 48,900 |
| L-Leucine-p-nitroanilide | N.D. | N.D. | N.D. | N.D. | N.D. |
| N-succinyl-Ala-Ala-Pro-Leu-p-nitroanilide | N.D. | N.D. | N.D. | N.D. | N.D. |
| GaPIP | 1AZW | |
|---|---|---|
| Total number of amino acids Glycine (%) Proline (%) Arginine (%) | 8.2 6.3 5.6 | 7.7 5.1 7.3 |
| Hydrophobic interactions | 526 | 528 |
| Hydrogen bonds (MC-MC) | 807 | 769 |
| Hydrogen bonds (MC-SC) | 271 | 285 |
| Hydrogen bonds (SC-SC) | 202 | 290 |
| Ionic interactions | 89 | 86 |
| Aromatic-aromatic interactions | 50 | 40 |
| Aromatic-sulphur interactions | 4 | 5 |
| Cation-pi interactions | 10 | 6 |
| Intraprotein disulphide bridges | 0 | 0 |
| Pocket (cavity) numbers | 5 | 4 |
| Exposed surface area (Å2) Total Polar Non-polar | 23,374.4 3636.0 14,489.7 | 23,500.9 3747.7 13,361.8 |
| Group | Nomenclature Species | Accession No |
|---|---|---|
| Bacteria | Aeromonas sobria Bacillus coagulans Hafnia alvei Lactobacillus delbrueckii Neisseria gonorrhoeae Serratia marcescens | P46547 P46541 P94800 P46544 P42786 O32449 |
| Xanthomonas campestris Xanthomonas citri | BAA11623 P52279 | |
| Yeasts | Pseudozyma hubeiensis Pseudozyma antarctica | XP 012189033 XP 014659534 |
| Fungi | Aspergillus nidulans Aspergillus niger | EAA64924 CAC40647 |
| Trichoderma reesei | ETS03930 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaruddin, S.; Ahmad Redzuan, R.; Minor, N.; Seman, W.M.K.W.; Md Tab, M.; Jaafar, N.R.; Ahmad Rodzli, N.; Jonet, M.A.; Bharudin, I.; Yusof, N.A.; et al. Biochemical Characterisation and Structure Determination of a Novel Cold-Active Proline Iminopeptidase from the Psychrophilic Yeast, Glaciozyma antarctica PI12. Catalysts 2022, 12, 722. https://doi.org/10.3390/catal12070722
Kamaruddin S, Ahmad Redzuan R, Minor N, Seman WMKW, Md Tab M, Jaafar NR, Ahmad Rodzli N, Jonet MA, Bharudin I, Yusof NA, et al. Biochemical Characterisation and Structure Determination of a Novel Cold-Active Proline Iminopeptidase from the Psychrophilic Yeast, Glaciozyma antarctica PI12. Catalysts. 2022; 12(7):722. https://doi.org/10.3390/catal12070722
Chicago/Turabian StyleKamaruddin, Shazilah, Rohaiza Ahmad Redzuan, Nurulermila Minor, Wan Mohd Khairulikhsan Wan Seman, Mahzan Md Tab, Nardiah Rizwana Jaafar, Nazahiyah Ahmad Rodzli, Mohd Anuar Jonet, Izwan Bharudin, Nur Athirah Yusof, and et al. 2022. "Biochemical Characterisation and Structure Determination of a Novel Cold-Active Proline Iminopeptidase from the Psychrophilic Yeast, Glaciozyma antarctica PI12" Catalysts 12, no. 7: 722. https://doi.org/10.3390/catal12070722
APA StyleKamaruddin, S., Ahmad Redzuan, R., Minor, N., Seman, W. M. K. W., Md Tab, M., Jaafar, N. R., Ahmad Rodzli, N., Jonet, M. A., Bharudin, I., Yusof, N. A., Xia, D. Q. H., Mahadi, N. M., Abdul Murad, A. M., & Abu Bakar, F. D. (2022). Biochemical Characterisation and Structure Determination of a Novel Cold-Active Proline Iminopeptidase from the Psychrophilic Yeast, Glaciozyma antarctica PI12. Catalysts, 12(7), 722. https://doi.org/10.3390/catal12070722






