Progress in Catalytic Conversion of Renewable Chitin Biomass to Furan-Derived Platform Compounds
Abstract
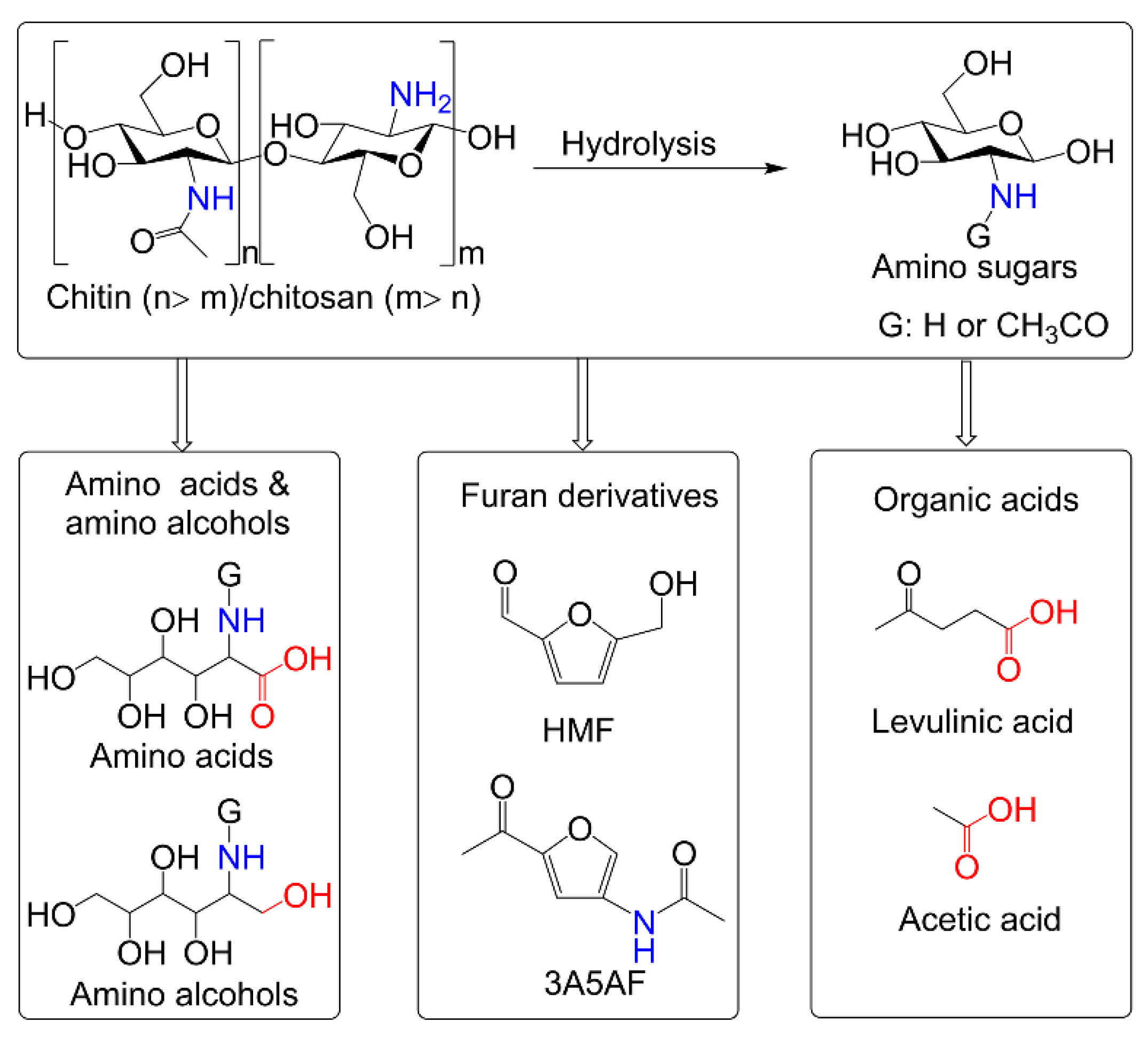
:1. Introduction
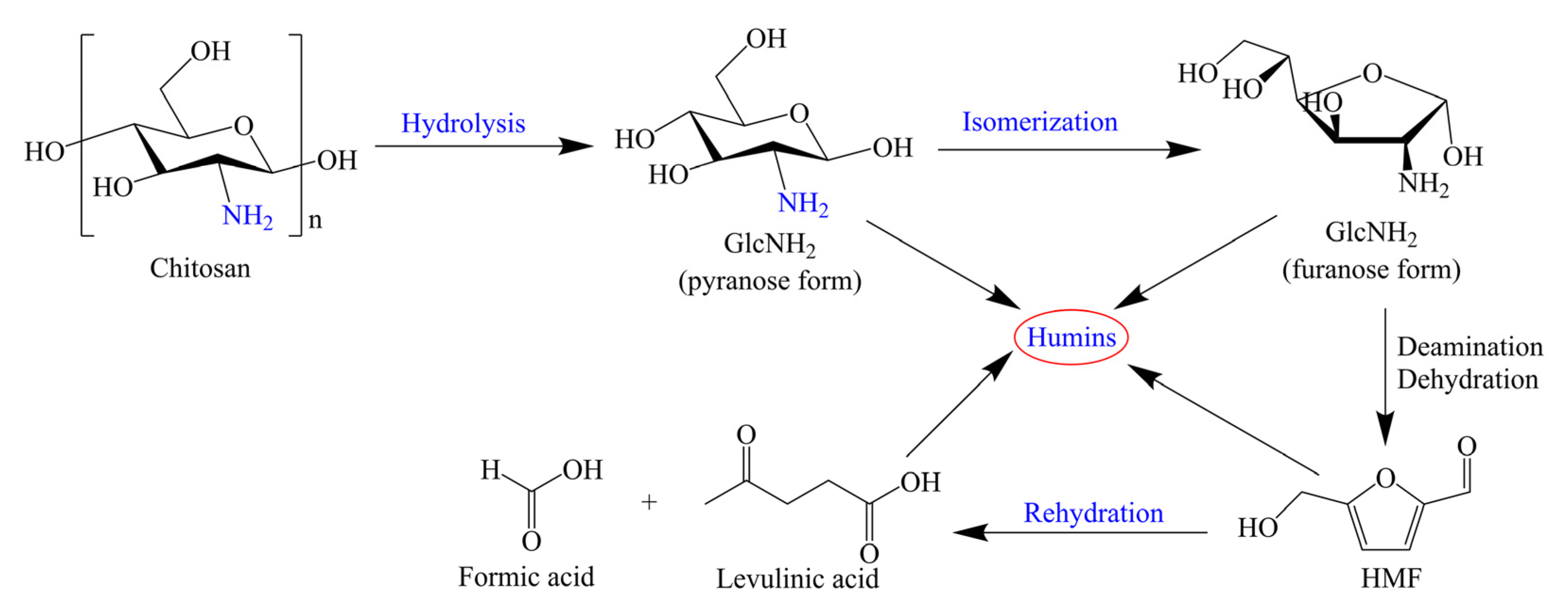
2. Conversion of Chitin Biomass to HMF
2.1. Bronsted Acid as Catalyst
2.2. Lewis Acid as Catalyst
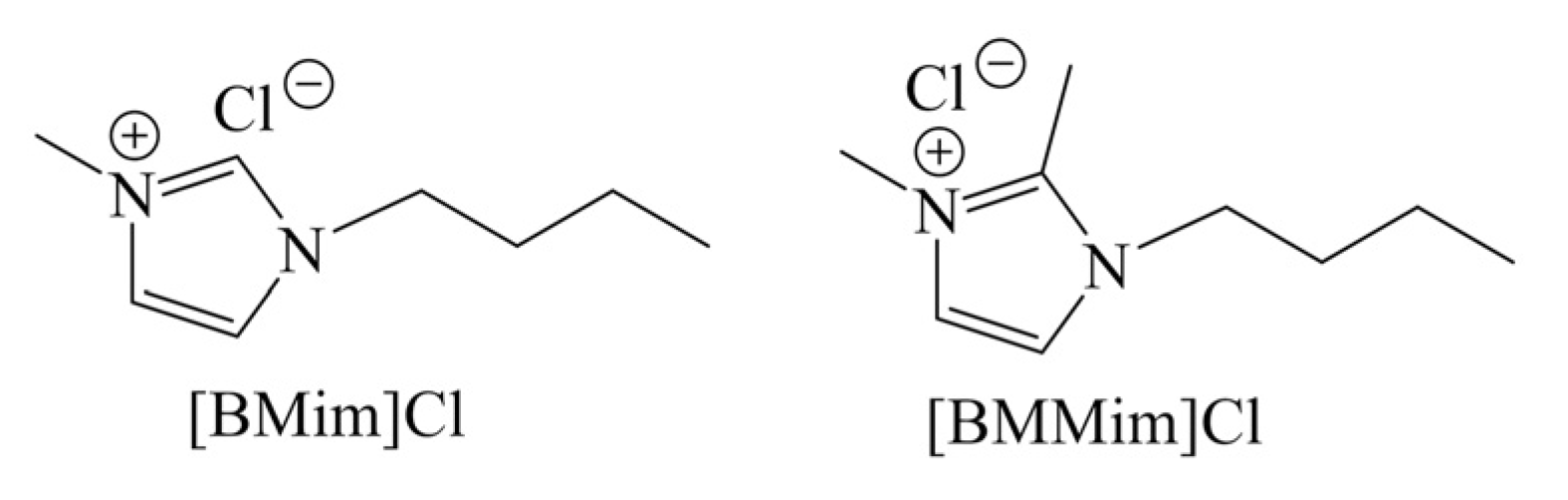
2.3. Ionic Liquid as Catalyst
2.4. Other Catalyst Types
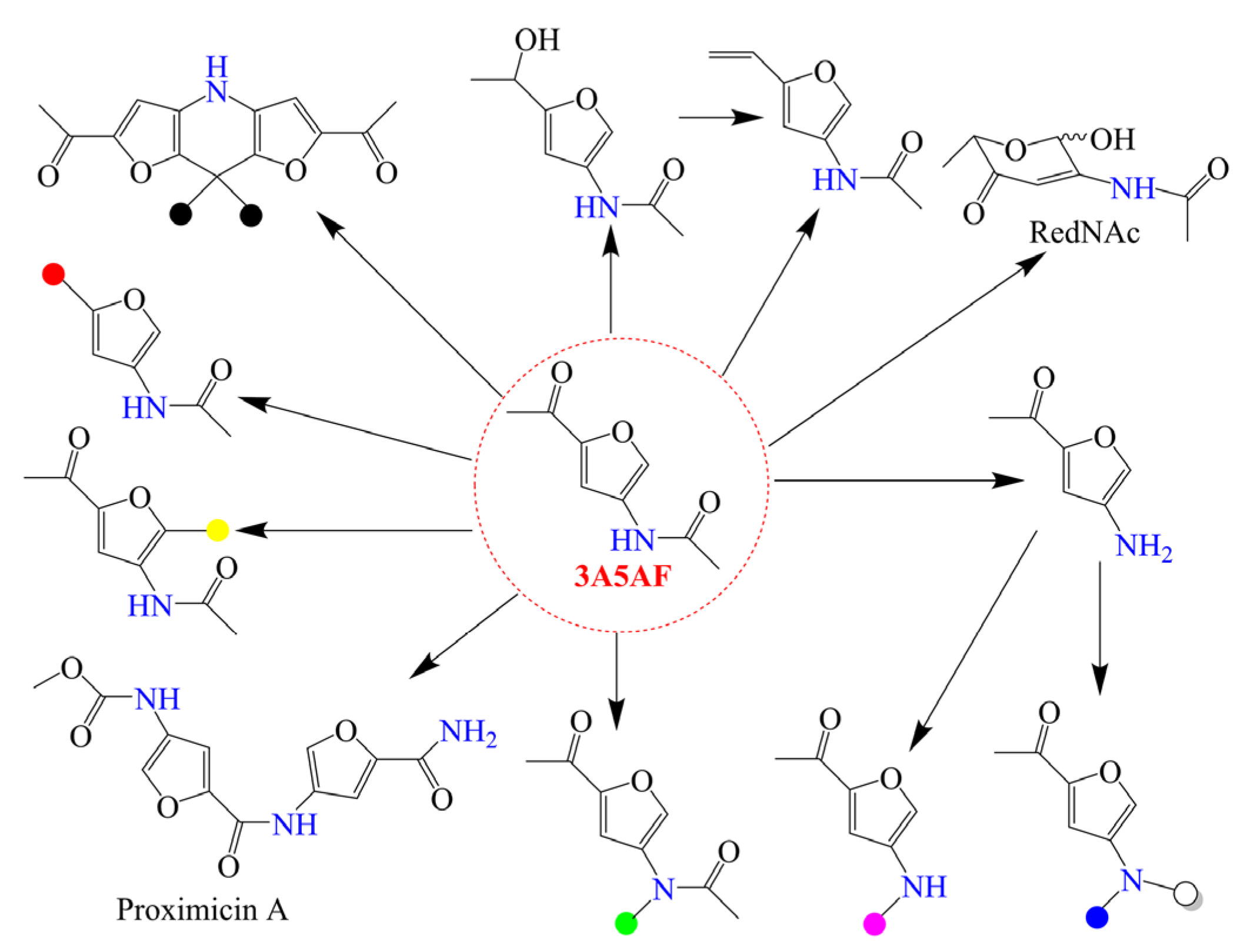
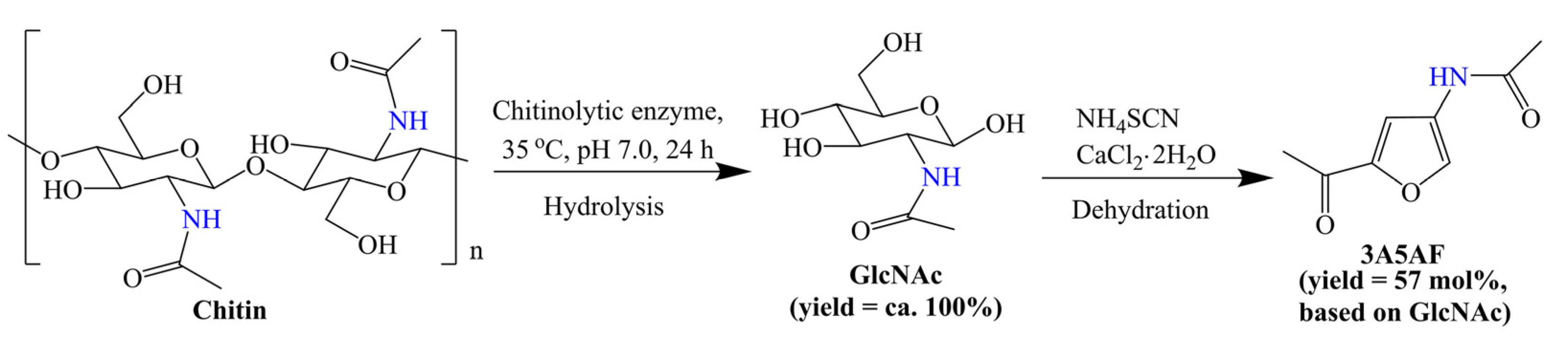
3. Conversion of Chitin Biomass to 3A5AF
3.1. GlcNAc as Reactant
3.2. Chitin as Reactant
4. Conclusions and Perspectives
4.1. Conclusions
4.2. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kang, Y.; Yang, Q.; Bartocci, P.; Wei, H.; Liu, S.S.; Wu, Z.; Zhou, H.; Yang, H.; Fantozzi, F.; Chen, H. Bioenergy in china: Evaluation of domestic biomass resources and the associated greenhouse gas mitigation potentials. Renew. Sustain. Energy Rev. 2020, 127, 109842. [Google Scholar] [CrossRef]
- Liao, Y.; Koelewijn, S.-F.; Van den Bossche, G.; Van Aelst, J.; Van den Bosch, S.; Renders, T.; Navare, K.; Nicolai, T.; Van Aelst, K.; Maesen, M.; et al. A sustainable wood biorefinery for low-carbon footprint chemicals production. Science 2020, 367, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, H.; Fang, Z.; Aida, T.M.; Smith, R.L., Jr. Cycloamination strategies for renewable N-heterocycles. Green Chem. 2020, 22, 582–611. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Li, C.-J. Perspectives on green synthesis and catalysis. Green Synth. Catal. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Han, B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem. Rev. 2017, 117, 6834–6880. [Google Scholar] [CrossRef]
- Duan, B.; Huang, Y.; Lu, A.; Zhang, L. Recent advances in chitin based materials constructed via physical methods. Prog. Polym. Sci. 2018, 82, 1–33. [Google Scholar] [CrossRef]
- Bastiaens, L.; Soetemans, L.; D’Hondt, E.; Elst, K. Sources of chitin and chitosan and their isolation. In Chitin and Chitosan; Wiley: Hoboken, NJ, USA, 2019; pp. 1–34. [Google Scholar]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; Barbosa de Lima, M.A.; de Oliveira Franco, L.; de Campos-Takaki, G.M. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Yan, N. Shell biorefinery: Dream or reality? Chem. Eur. J. 2016, 22, 13402–13421. [Google Scholar] [CrossRef]
- Zhou, D.; Shen, D.; Lu, W.; Song, T.; Wang, M.; Feng, H.; Shentu, J.; Long, Y. Production of 5-hydroxymethylfurfural from chitin biomass: A review. Molecules 2020, 25, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascal, M.; Nikitin, E.B. Dramatic advancements in the saccharide to 5-(chloromethyl)furfural conversion reaction. ChemSusChem 2009, 2, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Liu, L. Production of chemicals from marine biomass catalysed by acidic ionic liquids. Green Chem. 2021, 23, 9800–9814. [Google Scholar] [CrossRef]
- Cai, X.; Wang, Z.; Ye, Y.; Wang, D.; Zhang, Z.; Zheng, Z.; Liu, Y.; Li, S. Conversion of chitin biomass into 5-hydroxymethylfurfural: A review. Renew. Sustain. Energy Rev. 2021, 150, 111452. [Google Scholar] [CrossRef]
- Hou, Q.; Qi, X.; Zhen, M.; Qian, H.; Nie, Y.; Bai, C.; Zhang, S.; Bai, X.; Ju, M. Biorefinery roadmap based on catalytic production and upgrading 5-hydroxymethylfurfural. Green Chem. 2021, 23, 119–231. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.; Fang, Z.; Kozinski, J.A.; Butler, I.S.; Xu, L.; Song, H.; Wei, X. Catalytic conversion of 5-hydroxymethylfurfural to some value-added derivatives. Green Chem. 2018, 20, 3657–3682. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Y.; Qin, H.; Qi, Z. Advances of ionic liquids and deep eutectic solvents in green processes of biomass-derived 5-hydroxymethylfurfura. ChemSusChem 2022, e202102635. [Google Scholar] [CrossRef]
- Dai, J.; Li, F.; Fu, X. Towards shell biorefinery: Advances in chemical-catalytic conversion of chitin biomass to organonitrogen chemicals. ChemSusChem 2020, 13, 6498–6508. [Google Scholar] [CrossRef]
- Jeong, G.-T. Production of levulinic acid from glucosamine by dilute-acid catalyzed hydrothermal process. Ind. Crops Prod. 2014, 62, 77–83. [Google Scholar] [CrossRef]
- Lee, S.-B.; Jeong, G.-T. Catalytic conversion of chitosan to 5-hydroxymethylfurfural under low temperature hydrothermal process. Appl. Biochem. Biotechnol. 2015, 176, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, M.-R.; Jeon, Y.J.; Kim, S.-K.; Hong, Y.-K.; Jeong, G.-T. Valorization of chitosan as food waste of aquatic organisms into 5-hydroxymethylfurfural by sulfamic acid-catalyzed conversion process. Energy Technol. 2018, 6, 1747–1754. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, M.-R.; Kim, S.-K.; Jeong, G.-T. Valorization of chitosan into levulinic acid by hydrothermal catalytic conversion with methanesulfonic acid. Korean J. Chem. Eng. 2018, 35, 1290–1296. [Google Scholar] [CrossRef]
- Park, M.-R.; Kim, H.S.; Kim, S.-K.; Jeong, G.-T. Thermo-chemical conversion for production of levulinic and formic acids from glucosamine. Fuel Process. Technol. 2018, 172, 115–124. [Google Scholar] [CrossRef]
- Omari, K.W.; Besaw, J.E.; Kerton, F.M. Hydrolysis of chitosan to yield levulinic acid and 5-hydroxymethylfurfural in water under microwave irradiation. Green Chem. 2012, 14, 1480–1487. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pedersen, C.M.; Deng, T.; Qiao, Y.; Hou, X. Direct conversion of chitin biomass to 5-hydroxymethylfurfural in concentrated ZnCl2 aqueous solution. Bioresour. Technol. 2013, 143, 384–390. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, X.; Hu, Y.; Hu, C. Controlling the reaction networks for efficient conversion of glucose into 5-hydroxymethylfurfural. ChemSusChem 2020, 13, 4812–4832. [Google Scholar] [CrossRef]
- Yu, S.; Zang, H.; Chen, S.; Jiang, Y.; Yan, B.; Cheng, B. Efficient conversion of chitin biomass into 5-hydroxymethylfurfural over metal salts catalysts in dimethyl sulfoxide -water mixture under hydrothermal conditions. Polym. Degrad. Stab. 2016, 134, 105–114. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Cellulose depolymerization over heterogeneous catalysts. Acc. Chem. Res. 2018, 51, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, J.; Liu, X.; Zhang, S. Towards a molecular understanding of cellulose dissolution in ionic liquids: Anion/cation effect, synergistic mechanism and physicochemical aspects. Chem. Sci. 2018, 9, 4027–4043. [Google Scholar] [CrossRef] [Green Version]
- Uto, T.; Idenoue, S.; Yamamoto, K.; Kadokawa, J.-i. Understanding dissolution process of chitin crystal in ionic liquids: Theoretical study. Phys. Chem. Chem. Phys. 2018, 20, 20669–20677. [Google Scholar] [CrossRef]
- Li, M.; Zang, H.; Feng, J.; Yan, Q.; Yu, N.; Shi, X.; Cheng, B. Efficient conversion of chitosan into 5-hydroxymethylfurfural via hydrothermal synthesis in ionic liquids aqueous solution. Polym. Degrad. Stab. 2015, 121, 331–339. [Google Scholar] [CrossRef]
- Zhang, M.; Zang, H.; Ma, B.; Zhang, X.; Xie, R.; Cheng, B. Green synthesis of 5-hydroxymethylfurfural from chitosan biomass catalyzed by benzimidazole-based ionic liquids. ChemistrySelect 2017, 2, 10323–10328. [Google Scholar] [CrossRef]
- Zang, H.; Yu, S.; Yu, P.; Ding, H.; Du, Y.; Yang, Y.; Zhang, Y. Hydrothermal conversion of N-acetyl-D-glucosamine to 5-hydroxymethylfurfural using ionic liquid as a recycled catalyst in a water-dimethyl sulfoxide mixture. Carbohydr. Res. 2017, 442, 1–8. [Google Scholar] [CrossRef]
- Islam, M.S.; Nakamura, M.; Rabin, N.N.; Rahman, M.A.; Fukuda, M.; Sekine, Y.; Beltramini, J.N.; Kim, Y.; Hayami, S. Microwave-assisted catalytic conversion of chitin to 5-hydroxymethylfurfural using polyoxometalate as catalyst. RSC Adv. 2021, 12, 406–412. [Google Scholar] [CrossRef]
- Kobayashi, H.; Techikawara, K.; Fukuoka, A. Hydrolytic hydrogenation of chitin to amino sugar alcohol. Green Chem. 2017, 19, 3350–3356. [Google Scholar] [CrossRef] [Green Version]
- Kalane, N.D.; Krishnan, R.A.; Yadav, V.D.; Jain, R.; Dandekar, P. Synergistic effect of hetero- and homo-catalysts on the “green” synthesis of 5-hydroxymethylfurfural from chitosan biomass. Cellulose 2019, 26, 2805–2819. [Google Scholar] [CrossRef]
- Qi, M.; Chen, X.; Zhong, H.; Wu, J.; Jin, F. Base-free, vanadium-catalyzed conversion of chitin into acetic acid under low oxygen pressure. ACS Sustain. Chem. Eng. 2020, 8, 18661–18670. [Google Scholar] [CrossRef]
- Su, H.; Wang, J.; Yan, L. Homogeneously synchronous degradation of chitin into carbon dots and organic acids in aqueous solution. ACS Sustain. Chem. Eng. 2019, 7, 18476–18482. [Google Scholar] [CrossRef]
- Sadiq, A.D.; Chen, X.; Yan, N.; Sperry, J. Towards the shell biorefinery: Sustainable synthesis of the anticancer alkaloid proximicin A from chitin. ChemSusChem 2018, 11, 532–535. [Google Scholar] [CrossRef]
- Wolter, F.E.; Schneider, K.; Davies, B.P.; Socher, E.R.; Nicholson, G.; Seitz, O.; Süssmuth, R.D. Total synthesis of proximicin A−C and synthesis of new furan-based DNA binding agents. Org. Lett. 2009, 11, 2804–2807. [Google Scholar] [CrossRef]
- Brucoli, F.; Natoli, A.; Marimuthu, P.; Borrello, M.T.; Stapleton, P.; Gibbons, S.; Schätzlein, A. Efficient synthesis and biological evaluation of proximicins A, B and C. Bioorg. Med. Chem. 2012, 20, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Thuy Trang, P.; Lindsay, A.C.; Kim, S.-W.; Persello, L.; Chen, X.; Yan, N.; Sperry, J. Two-step preparation of diverse 3-amidofurans from chitin. ChemistrySelect 2019, 4, 10097–10099. [Google Scholar]
- Pham, T.T.; Lindsay, A.C.; Chen, X.; Gözaydin, G.; Yan, N.; Sperry, J. Transferring the biorenewable nitrogen present in chitin to several N-functional groups. Sustain. Chem. Pharm. 2019, 13, 100143. [Google Scholar] [CrossRef]
- Shao, Y.; Ding, Y.; Dai, J.; Long, Y.; Hu, Z.-T. Synthesis of 5-hydroxymethylfurfural from dehydration of biomass-derived glucose and fructose using supported metal catalysts. Green Synth. Catal. 2021, 2, 187–197. [Google Scholar] [CrossRef]
- Franich, R.A.; Goodin, S.J.; Wilkins, A.L. Acetamidofurans, acetamidopyrones, and acetamidoacetaldehyde from pyrolysis of chitin and N-acetylglucosamine. J. Anal. Appl. Pyrolysis 1984, 7, 91–100. [Google Scholar] [CrossRef]
- Omari, K.W.; Dodot, L.; Kerton, F.M. A simple one-pot dehydration process to convert N-acetyl-D-glucosamine into a nitrogen-containing compound, 3-acetamido-5-acetylfuran. ChemSusChem 2012, 5, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- Padovan, D.; Kobayashi, H.; Fukuoka, A. Facile preparation of 3-acetamido-5-acetylfuran from N-acetyl-D-glucosamine by using commercially available aluminum salts. ChemSusChem 2020, 13, 3594–3598. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Zhang, A.; Chen, K.; Cao, F.; Ouyang, P. Conversion of N-acetyl-D-glucosamine into 3-acetamido-5-acetylfuran using cheap ammonium chloride as catalyst. ChemistrySelect 2022, 7, e202104574. [Google Scholar] [CrossRef]
- Wang, J.; Zang, H.; Jiao, S.; Wang, K.; Shang, Z.; Li, H.; Lou, J. Efficient conversion of N-acetyl-D-glucosamine into nitrogen-containing compound 3-acetamido-5-acetylfuran using amino acid ionic liquid as the recyclable catalyst. Sci. Total Environ. 2020, 710, 136293. [Google Scholar] [CrossRef]
- Du, Y.; Zang, H.; Feng, Y.; Wang, K.; Lv, Y.; Liu, Z. Efficient catalytic system for converting N-acetyl-D-glucosamine into valuable chemical 3-acetylamino-5-acetylfuran. J. Mol. Liq. 2022, 347, 117970. [Google Scholar] [CrossRef]
- Zang, H.; Li, H.; Jiao, S.; Lou, J.; Du, Y.; Huang, N. Green conversion of N-acetylglucosamine into valuable platform compound 3-acetamido-5-acetylfuran using ethanolamine ionic liquids as recyclable catalyst. ChemistrySelect 2021, 6, 3848–3857. [Google Scholar] [CrossRef]
- Zang, H.; Lou, J.; Jiao, S.; Li, H.; Du, Y.; Wang, J. Valorization of chitin derived N-acetyl-D-glucosamine into high valuable N-containing 3-acetamido-5-acetylfuran using pyridinium-based ionic liquids. J. Mol. Liq. 2021, 330, 115667. [Google Scholar] [CrossRef]
- Drover, M.W.; Omari, K.W.; Murphy, J.N.; Kerton, F.M. Formation of a renewable amide, 3-acetamido-5-acetylfuran, via direct conversion of N-acetyl-D-glucosamine. RSC Adv. 2012, 2, 4642–4644. [Google Scholar] [CrossRef] [Green Version]
- Gozaydin, G.; Song, S.; Yan, N. Chitin hydrolysis in acidified molten salt hydrates. Green Chem. 2020, 22, 5096–5104. [Google Scholar] [CrossRef]
- Chen, X.; Chew, S.L.; Kerton, F.M.; Yan, N. Direct conversion of chitin into a N-containing furan derivative. Green Chem. 2014, 16, 2204–2212. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, Y.; Kerton, F.M.; Yan, N. Conversion of chitin and N-acetyl-D-glucosamine into a N-containing furan derivative in ionic liquids. RSC Adv. 2015, 5, 20073–20080. [Google Scholar] [CrossRef]
- Chen, X.; Gao, Y.; Wang, L.; Chen, H.; Yan, N. Effect of treatment methods on chitin structure and its transformation into nitrogen-containing chemicals. ChemPlusChem 2015, 80, 1565–1572. [Google Scholar] [CrossRef]
- Chen, K.; Wu, C.; Wang, C.; Zhang, A.; Cao, F.; Ouyang, P. Chemo-enzymatic protocol converts chitin into a nitrogen-containing furan derivative, 3-acetamido-5-acetylfuran. Mol. Catal. 2021, 516, 112001. [Google Scholar] [CrossRef]

| Entry | Substrates | Reaction Conditions | HMF Yield (mol%) | Ref |
|---|---|---|---|---|
| 1 | GlcNH2·HCl | 90 g/L substrate, 2 wt% H2SO4, 175 °C, 5 min | 3.0 | [20] |
| 2 | Chitosan | 2.2 wt% H2SO4, 174 °C, 36.9 min | 15 | [21] |
| 3 | Chitosan | 3 wt% chitosan, 0.7 M sulfamic acid, 200 °C, 2 min | 27 | [22] |
| 4 | Chitosan | 2 wt% substrate, 0.1 M methanesulfonic acid, 200 °C, 15 min | 19 | [23] |
| 5 | Chitosan | 2 wt% substrate, 0.1 M methanesulfonic acid, 200 °C, 30 min | 8.2 | [23] |
| 6 | GlcNH2 | 100 g/L substrate, 0.1 M methanesulfonic acid, 160 °C, 40 min | 2.3 | [24] |
| Entry | Substrates | Reaction Conditions | HMF Yield (mol%) | Ref |
|---|---|---|---|---|
| 1 | Chitosan with different molecular weight | 100 mg chitosan, 42 mg SnCl4·5H2O, 5 mL water, in sealed vessels, under microwave irradiation, 200 °C, 30 min | Ca. 13 | [25] |
| 2 | GlcNH2 | 1 g substrate, 20 g ZnCl2, 10 g water, 200 °C, 90 min | 22 | [26] |
| 3 | GlcNAc | 2.8 | [26] | |
| 4 | Chitosan-1K | 10 | [26] | |
| 5 | GlcNAc | 100 mg substrate, 628 mg FeCl2·4H2O, 20 g solvent (4 g DMSO and 16 g water), 180 °C, 5 h | 38 | [28] |
| 6 | GlcNH2·HCl | 100 mg substrate, 646 mg FeCl2·4H2O, 20 g solvent (4 g DMSO and 16 g water), 180 °C, 5 h | 24 | [28] |
| 7 | Chitosan-350K | 100 mg substrate, 1111 mg FeCl2·4H2O, 20 g solvent (8 g DMSO and 12 g water), 190 °C, 6 h | 27 | [28] |
| 8 | Chitosan-1050K | 21 | [28] | |
| 9 | Chitin-400K | 100 mg substrate, 881 mg FeCl2·4H2O, 20 g solvent (8 g DMSO and 12 g water), 190 °C, 6 h | 19 | [28] |
| Entry | Substrates | Reaction Conditions | HMF Yield (mol%) | Ref |
|---|---|---|---|---|
| 1 | Low-molecular-weight chitosan | 100 mg substrate, 20 mL water, 4 wt% concentration of [MIM]HSO4 aqueous solution, 180 °C, 5 h | 30 | [32] |
| 2 | Chitin | 19 | [32] | |
| 3 | Chitosan-350K | 100 mg substrate, 2.5 wt% [Hbim]Cl, 20 g water, 180 °C, 3 h 100 mg substrate, 2.5 wt% [Hbim]Cl, 18 g water, 2 g DMSO,180 °C, 3 h | 31 | [33] |
| 4 | Chitosan-350K | 35 | [33] | |
| 5 | GlcNAc | 100 mg substrate, the molar ratio of [Hmim][HSO4] with substrate as 20:1, 12 g water, 8 g DMSO, 180 °C, 6 h | 65 | [34] |
| 6 | GlcNH2·HCl | 55 | [34] | |
| 7 | Chitosan | 35 | [34] | |
| 8 | Chitin | 26 | [34] |
| Entry | Catalyst and Additive | Solvent | Reaction Conditions | 3A5AF Yield (mol%) | Ref |
|---|---|---|---|---|---|
| 1 | 100 mol% B(OH)3, 200 mol% NaCl | DMA | MW 1, 220 °C, 15 min | 77 | [47] |
| 2 | 100 mol%AlCl3·6H2O | DMF | CH 2, 120 °C, 30 min | 30 | [48] |
| 3 | 200 mol% NH4C, 400 mol% LiCl | DMF | CH 2, 160 °C, 5 min | 43 | [49] |
| 4 | glycine chloride (100 wt%) | DMA | CH 2, 200 °C, 10 min | 43 | [50] |
| 5 | glycine chloride (100 wt%), CaCl2 (100 wt%) | DMA | CH 2, 200 °C, 10 min | 53 | [50] |
| 6 | 200 mol% pyrazine hydrochloride, 100 mol% B(OH)3, 100 mol% CaCl2 | DMA | CH 2, 190 °C, 60 min | 70 | [51] |
| 7 | None | [BMim]Cl | MW 1, 180 °C, 3 min | 34 | [54] |
| 8 | Non | [BMMim]Cl | MW 1, 180 °C, 3 min | 33 | [54] |
| 9 | 200 mol% B(OH)3 | [Bmim]Cl | CH 2, 180 °C, 60 min | 79 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Du, Z.; Dai, J.; Yang, R.; Yang, D.; Gu, X.; Li, N.; Li, F. Progress in Catalytic Conversion of Renewable Chitin Biomass to Furan-Derived Platform Compounds. Catalysts 2022, 12, 653. https://doi.org/10.3390/catal12060653
Xu B, Du Z, Dai J, Yang R, Yang D, Gu X, Li N, Li F. Progress in Catalytic Conversion of Renewable Chitin Biomass to Furan-Derived Platform Compounds. Catalysts. 2022; 12(6):653. https://doi.org/10.3390/catal12060653
Chicago/Turabian StyleXu, Benjing, Ziting Du, Jinhang Dai, Ronghe Yang, Delong Yang, Xingxing Gu, Ning Li, and Fukun Li. 2022. "Progress in Catalytic Conversion of Renewable Chitin Biomass to Furan-Derived Platform Compounds" Catalysts 12, no. 6: 653. https://doi.org/10.3390/catal12060653
APA StyleXu, B., Du, Z., Dai, J., Yang, R., Yang, D., Gu, X., Li, N., & Li, F. (2022). Progress in Catalytic Conversion of Renewable Chitin Biomass to Furan-Derived Platform Compounds. Catalysts, 12(6), 653. https://doi.org/10.3390/catal12060653








