Evolving New Chemistry: Biocatalysis for the Synthesis of Amine-Containing Pharmaceuticals
Abstract
1. Introduction
2. Transaminases
2.1. Overview
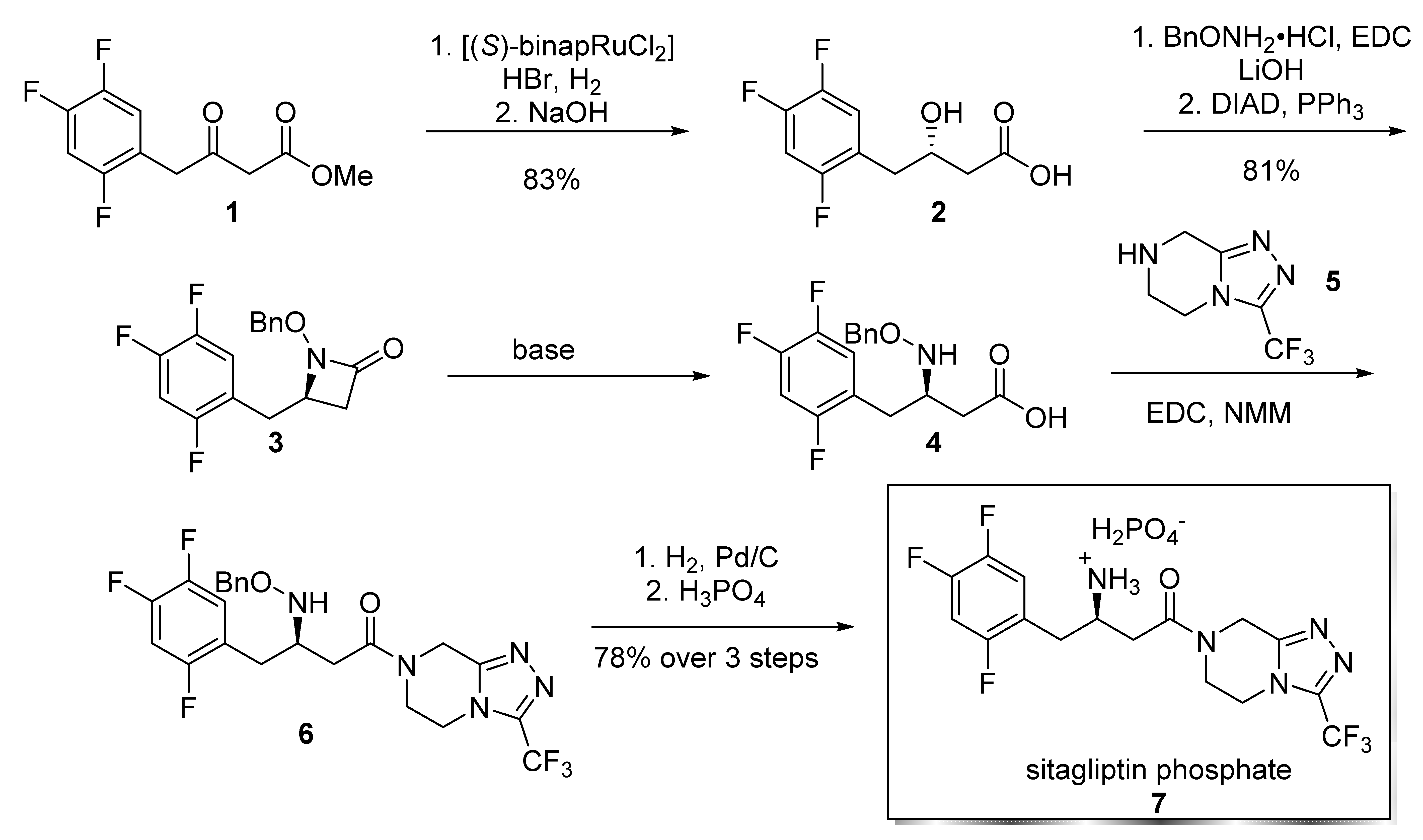
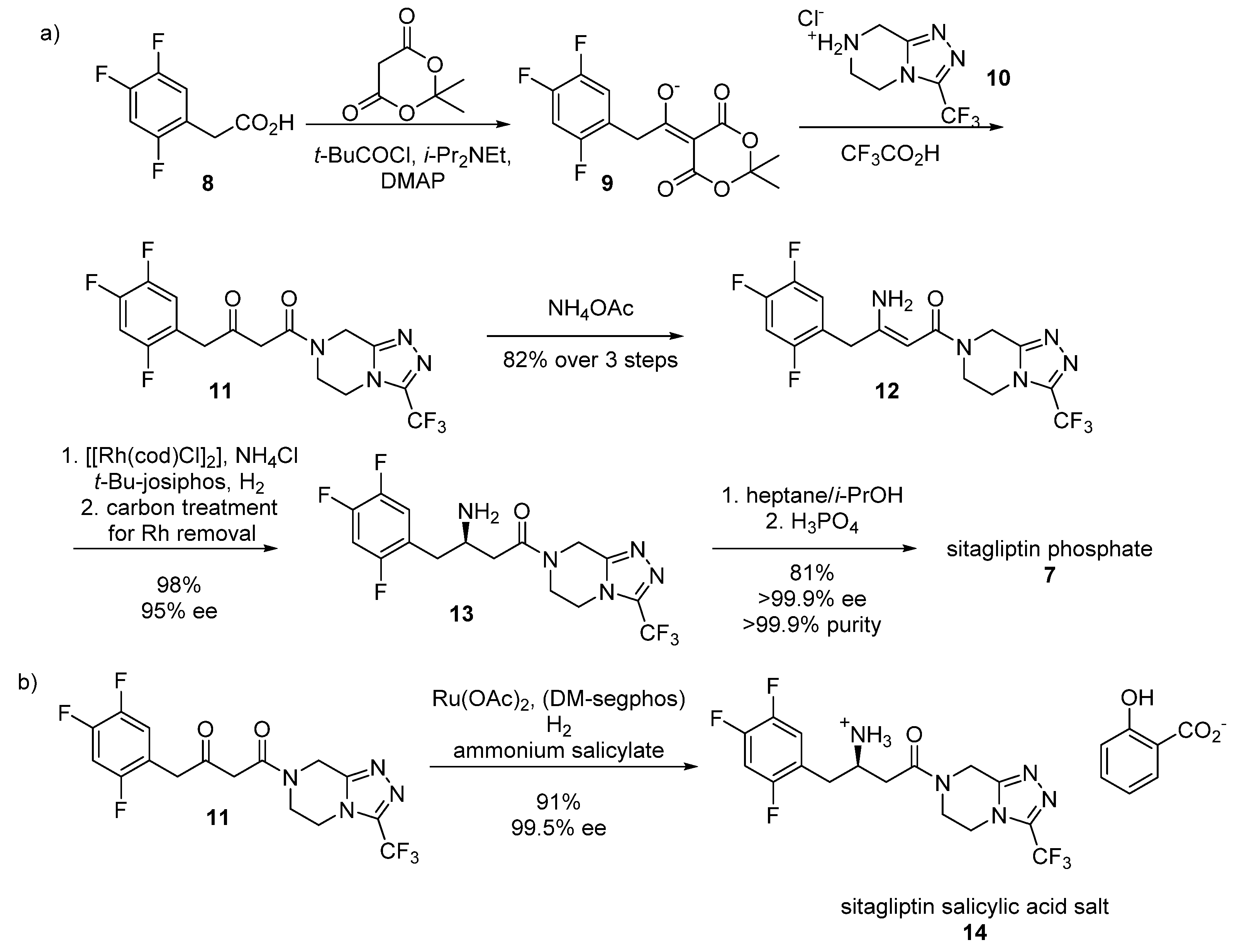
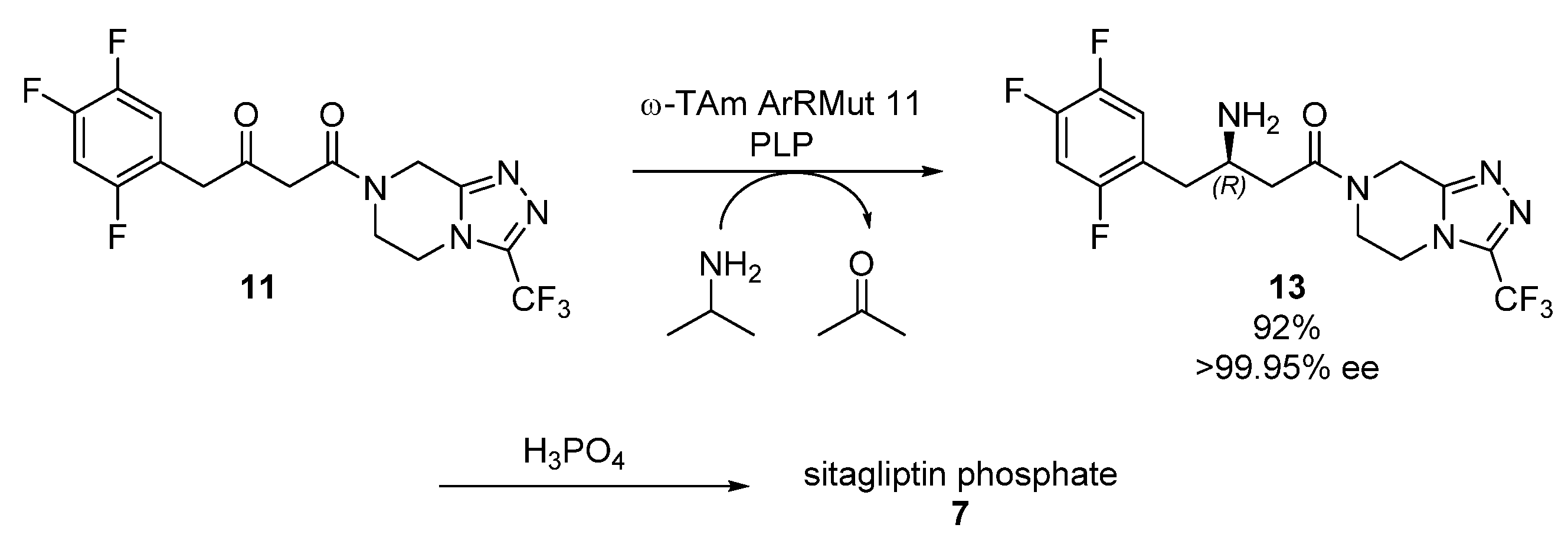
2.2. Sitagliptin
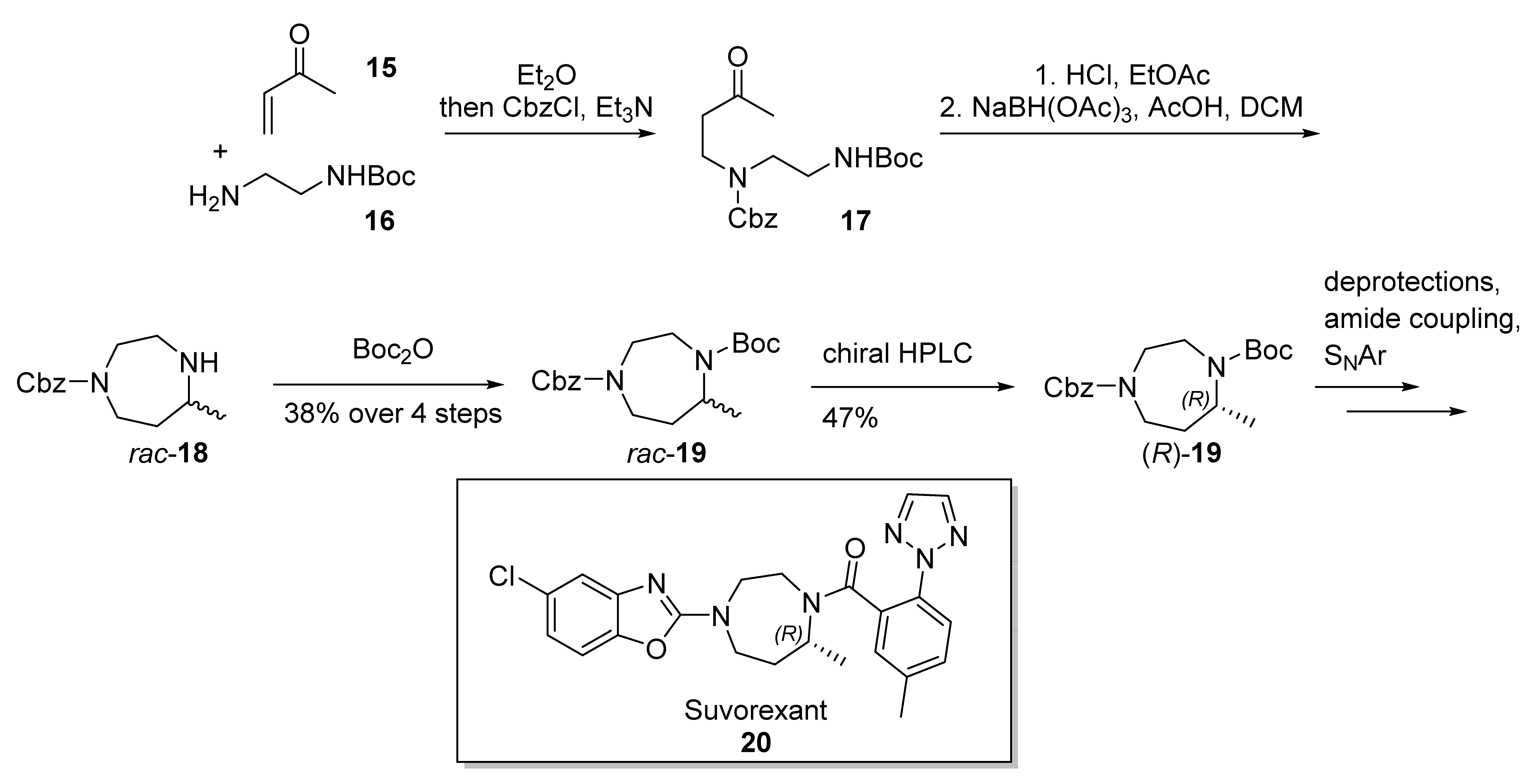
2.3. Suvorexant
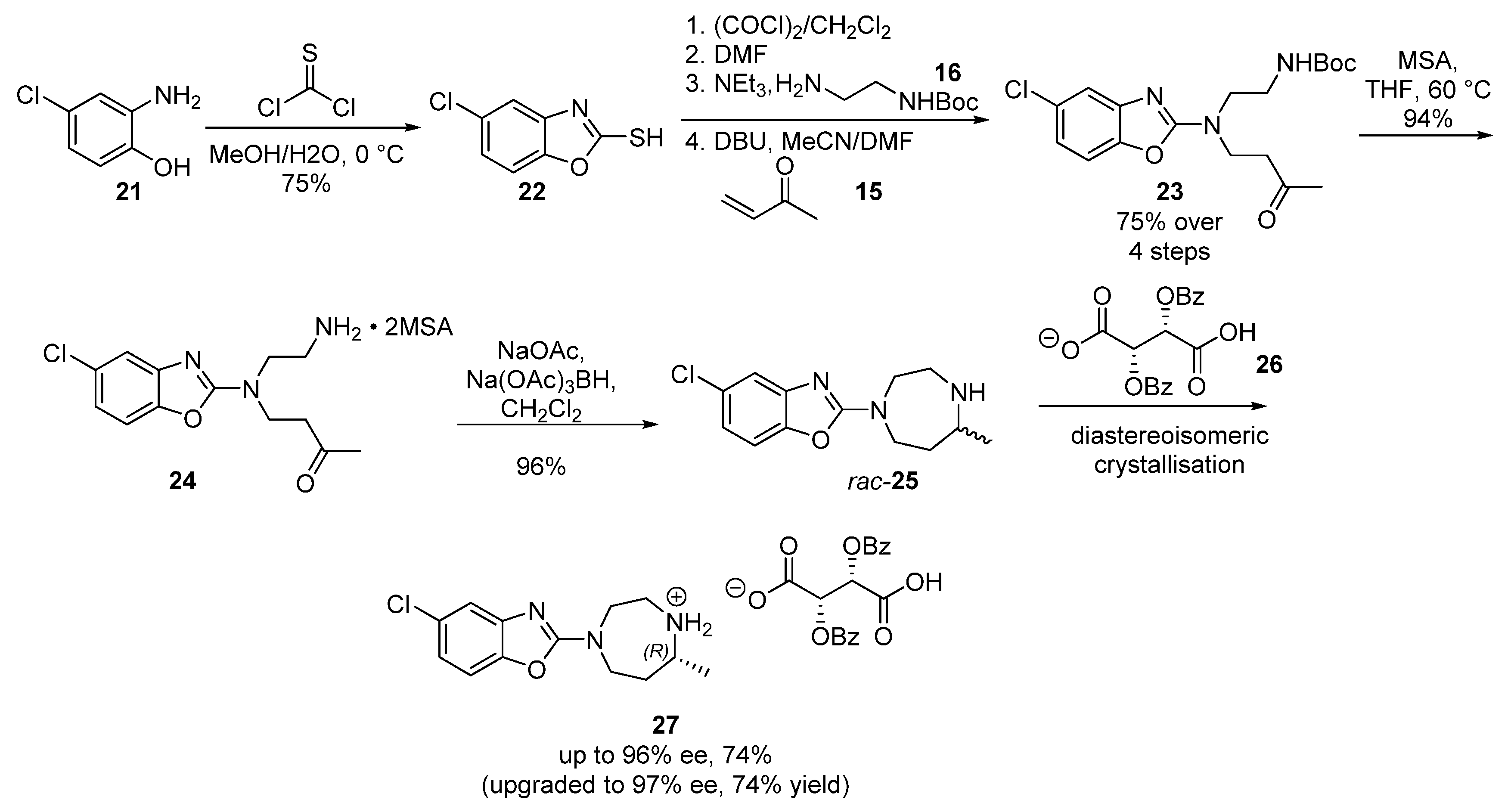
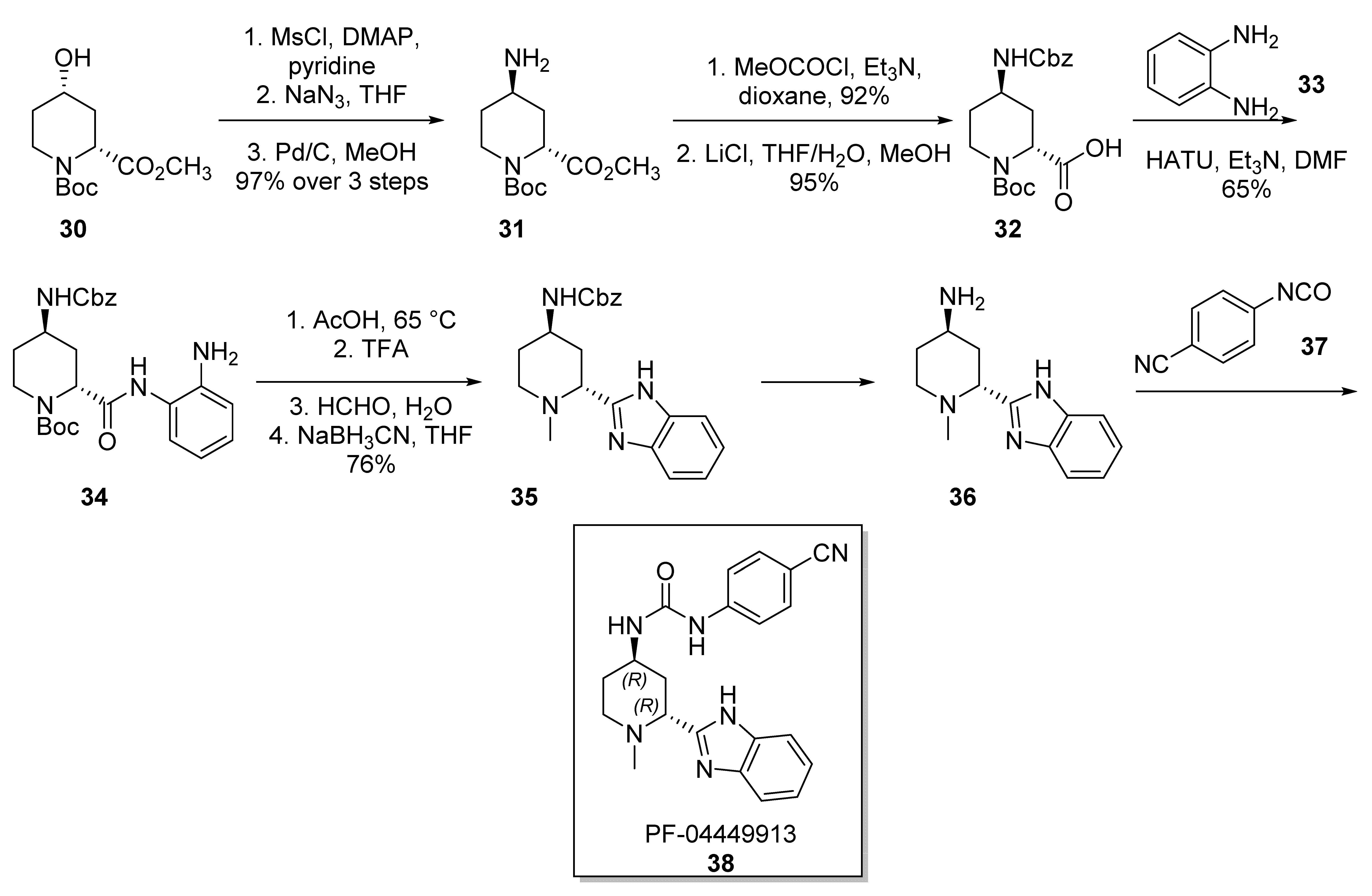
2.4. PF-0444913
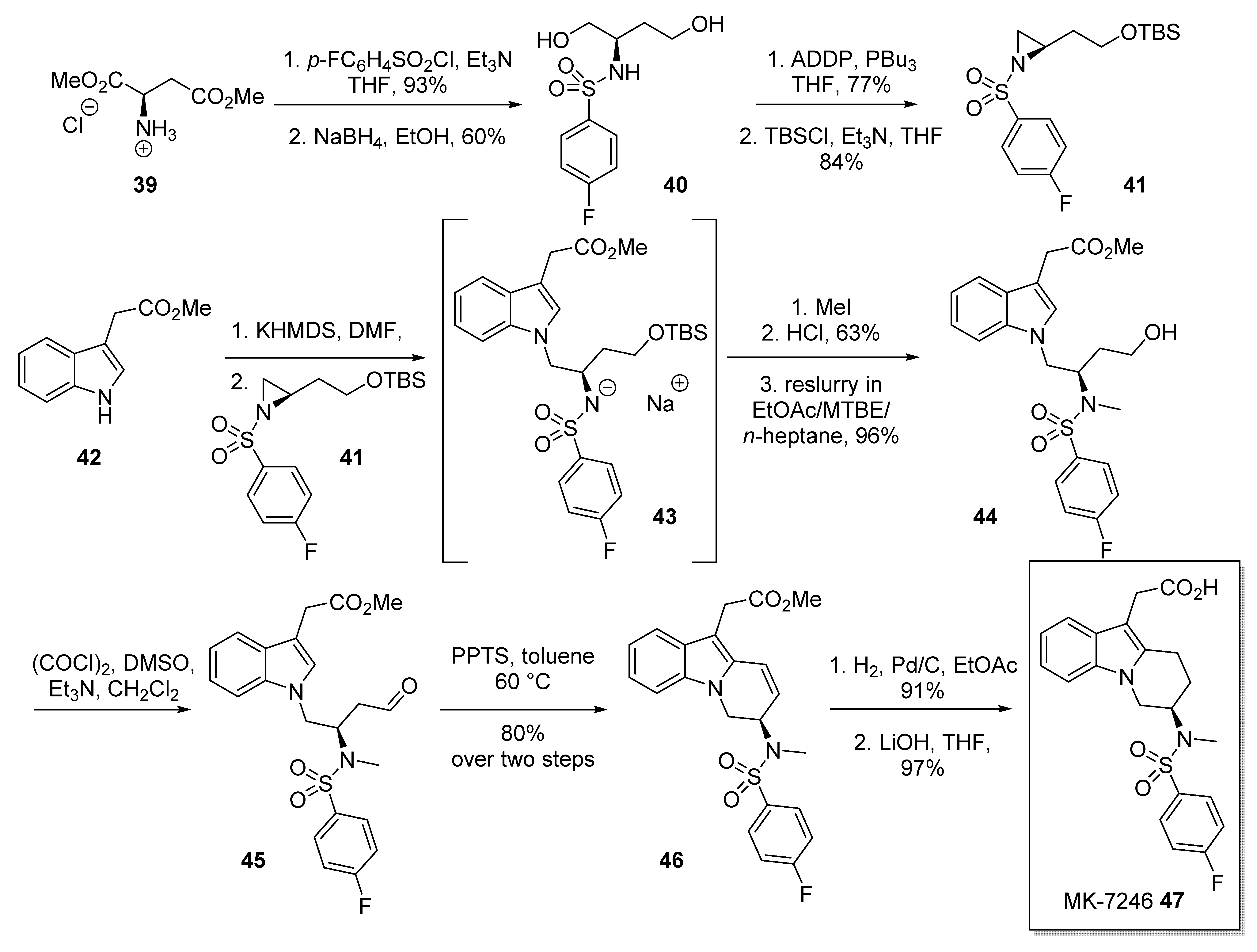
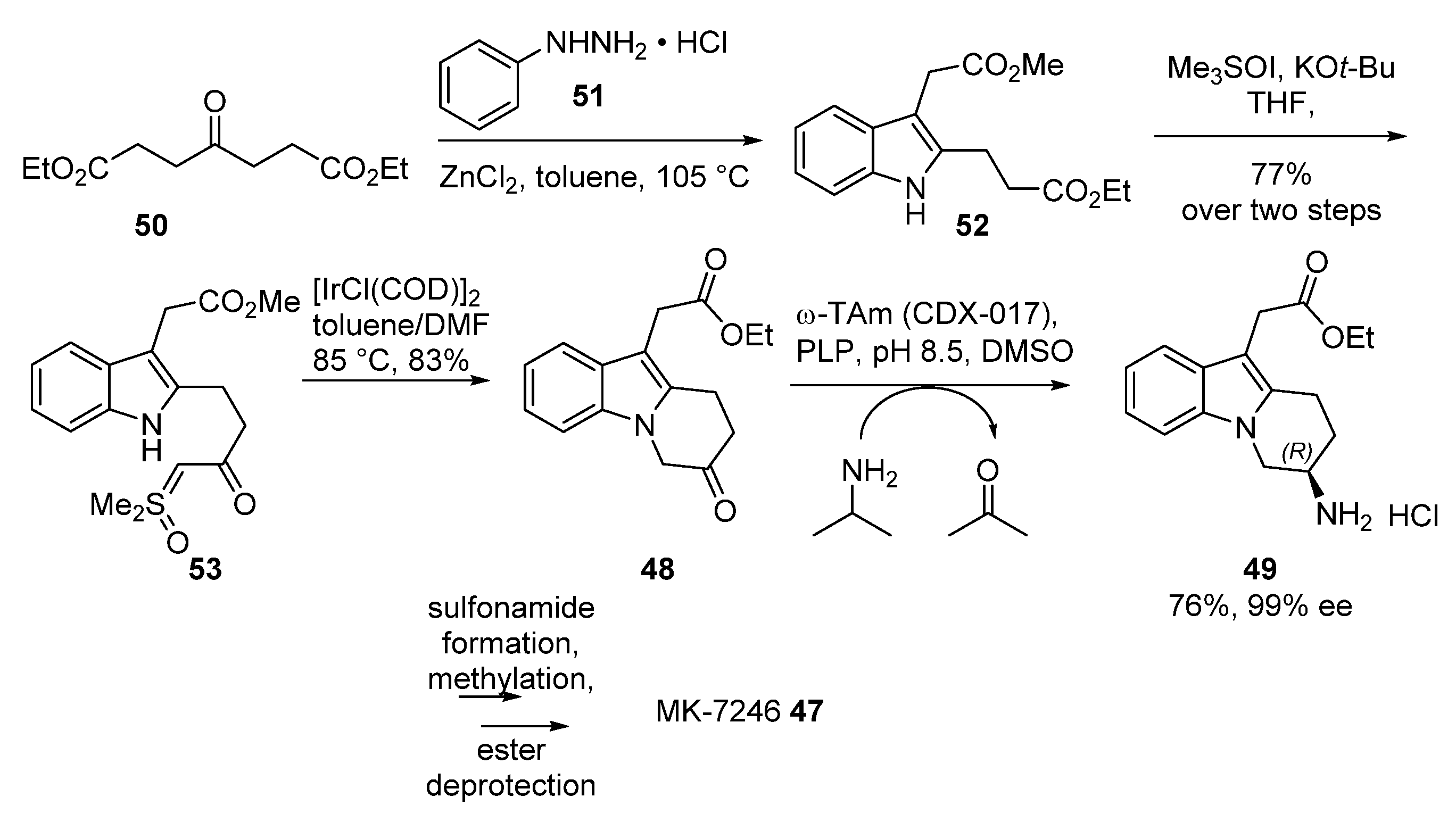
2.5. MK-7246
2.6. Vernakalant
3. Imine Reductases
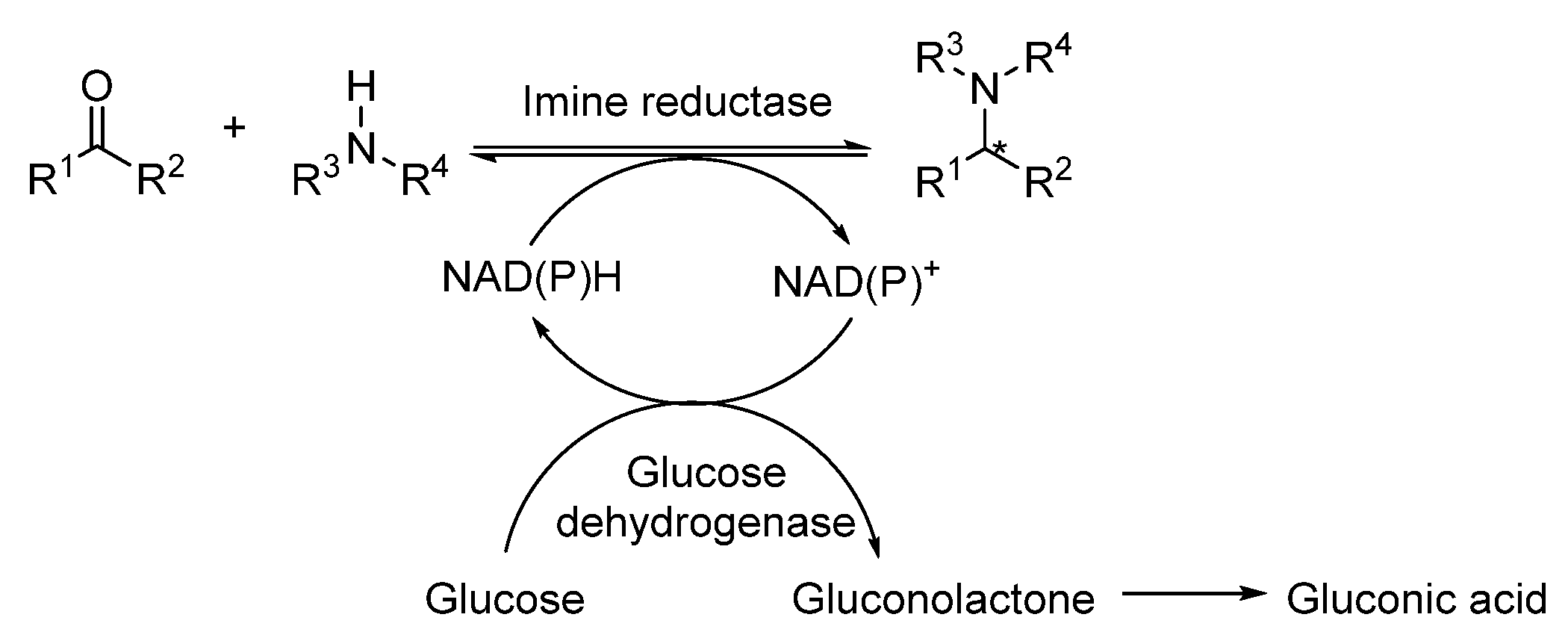
3.1. Overview
3.2. GSK2879552
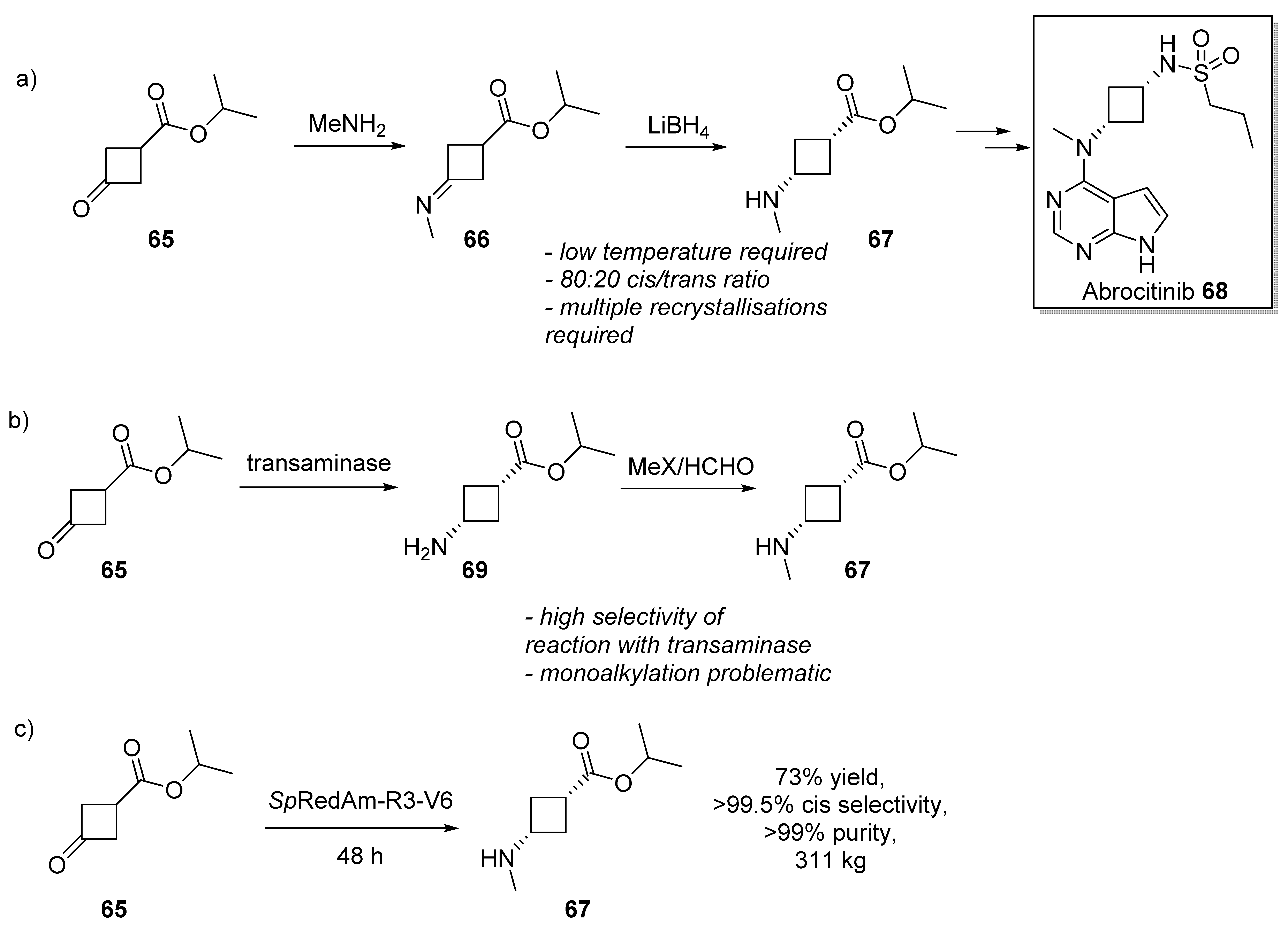
3.3. Abrocitinib
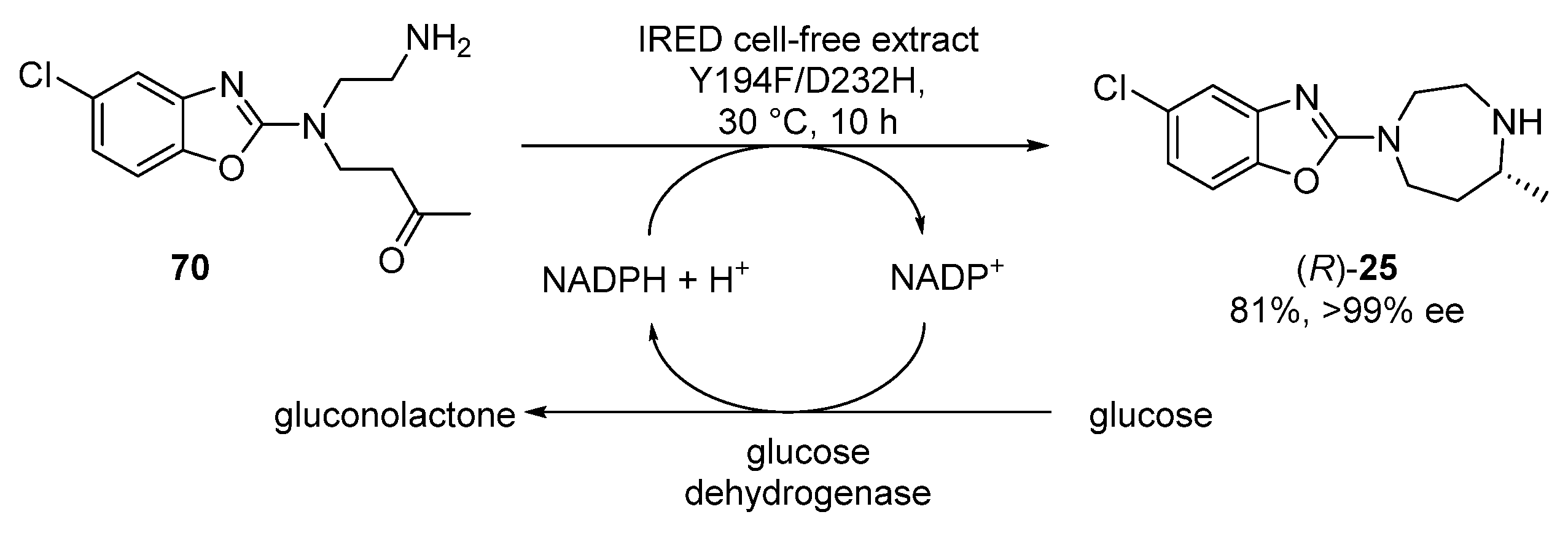
3.4. Suvorexant
4. Monoamine Oxidase
4.1. Overview
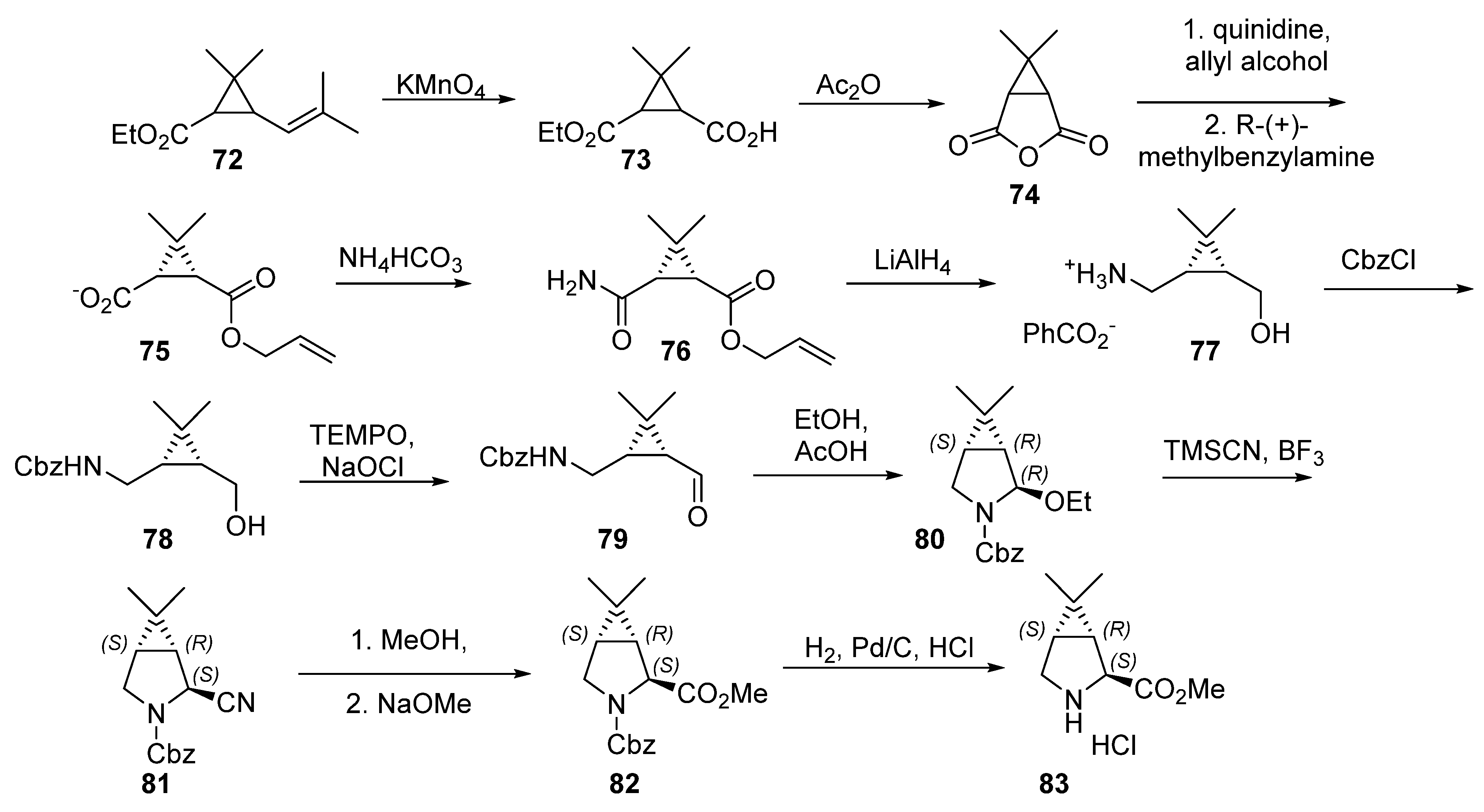
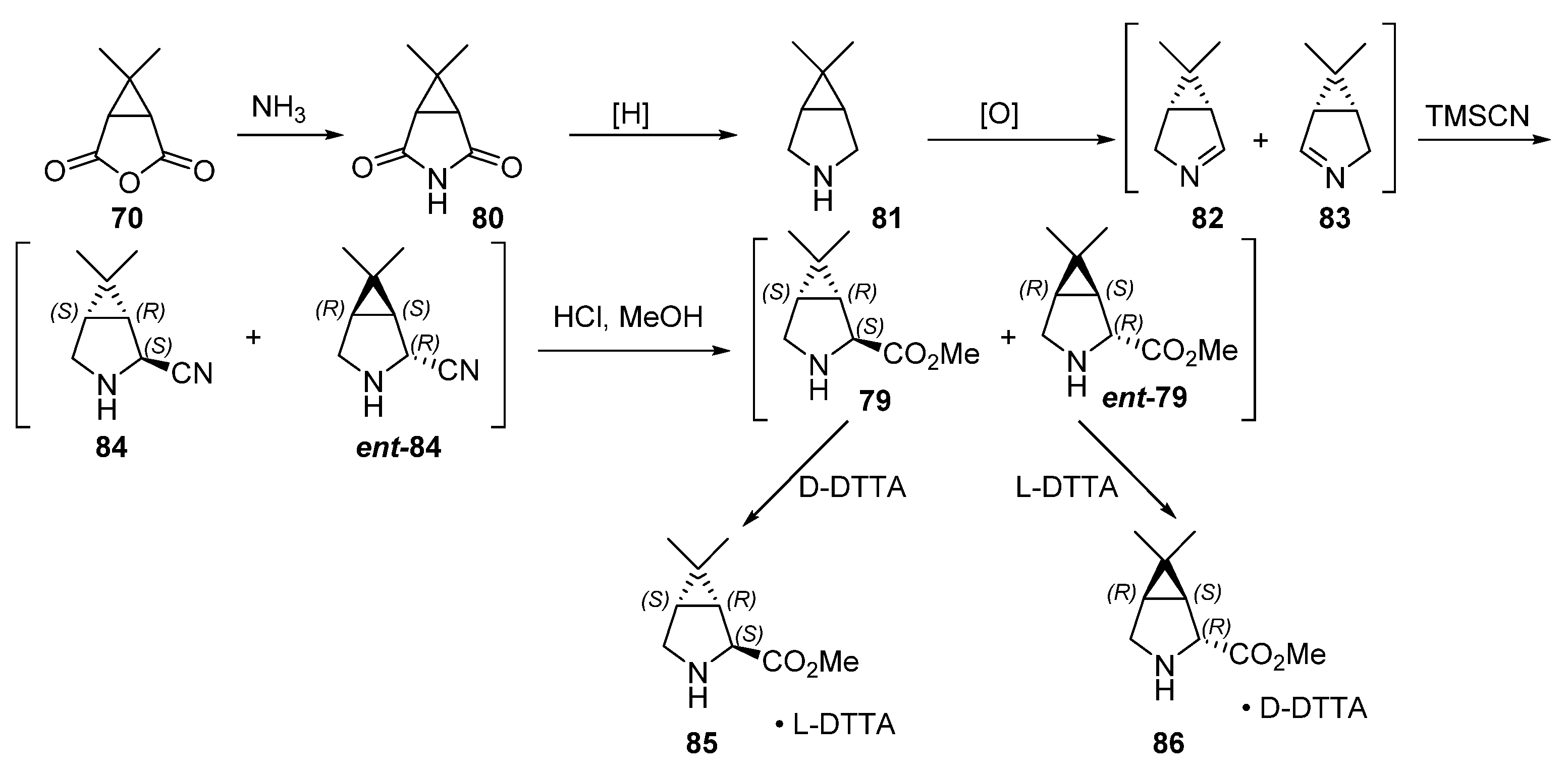
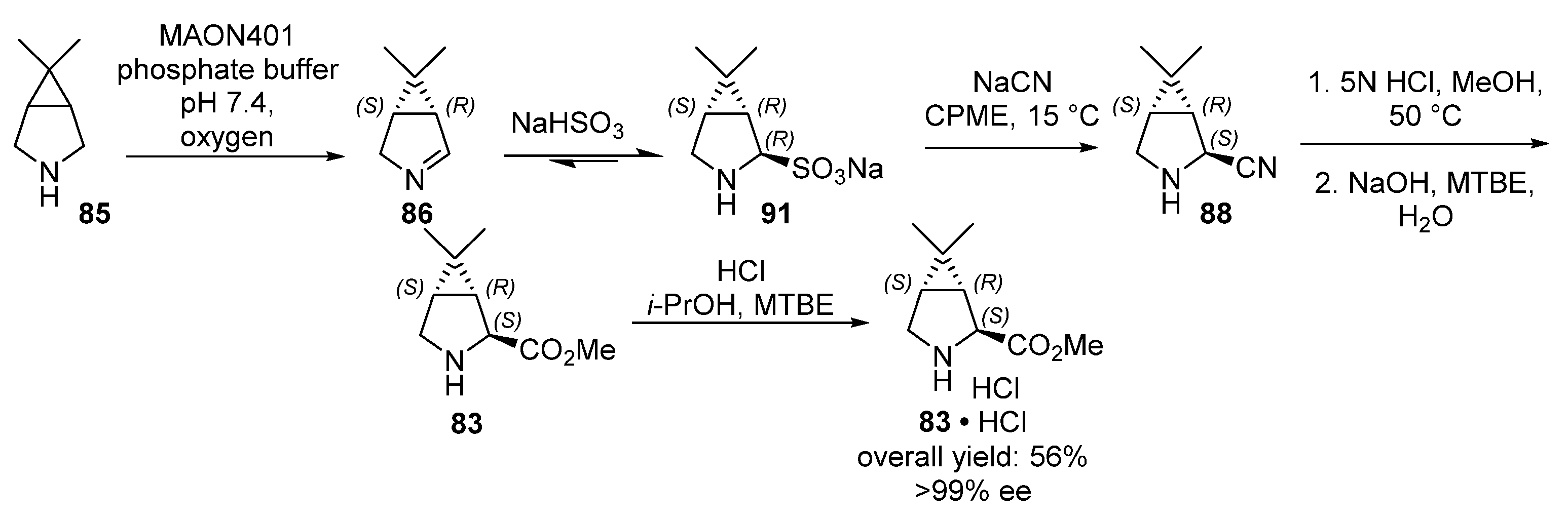
4.2. Boceprevir
5. Pictet-Spenglerases
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campos, K.R.; Coleman, P.J.; Alvarez, J.C.; Dreher, S.D.; Garbaccio, R.M.; Terrett, N.K.; Tillyer, R.D.; Truppo, M.D.; Parmee, E.R. The Importance of Synthetic Chemistry in the Pharmaceutical Industry. Science 2019, 363, eaat0805. [Google Scholar] [CrossRef] [PubMed]
- Lovato, K.; Fier, P.S.; Maloney, K.M. The Application of Modern Reactions in Large-Scale Synthesis. Nat. Rev. Chem. 2021, 5, 546–563. [Google Scholar] [CrossRef]
- Voight, E.A.; Greszler, S.N.; Kym, P.R. Fueling the Pipeline via Innovations in Organic Synthesis. ACS Med. Chem. Lett. 2021, 12, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Lasso, J.D.; Castillo-Pazos, D.J.; Li, C.J. Green Chemistry Meets Medicinal Chemistry: A Perspective on Modern Metal-Free Late-Stage Functionalization Reactions. Chem. Soc. Rev. 2021, 50, 10955–10982. [Google Scholar] [CrossRef]
- Blakemore, D.C.; Castro, L.; Churcher, I.; Rees, D.C.; Thomas, A.W.; Wilson, D.M.; Wood, A. Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem. 2018, 10, 383–394. [Google Scholar] [CrossRef]
- Boström, J.; Brown, D.G.; Young, R.J.; Keserü, G.M. Expanding the Medicinal Chemistry Synthetic Toolbox. Nat. Rev. Drug Discov. 2018, 17, 709–727. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, T.; Kang, D.; Zhang, J.; Song, Y.; Namasivayam, V.; Kongsted, J.; Pannecouque, C.; de Clercq, E.; Poongavanam, V.; et al. Overview of Recent Strategic Advances in Medicinal Chemistry. J. Med. Chem. 2019, 62, 9375–9414. [Google Scholar] [CrossRef]
- Fryszkowska, A.; Devine, P.N. Biocatalysis in Drug Discovery and Development. In Current Opinion in Chemical Biology; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 151–160. [Google Scholar] [CrossRef]
- Truppo, M.D. Biocatalysis in the Pharmaceutical Industry: The Need for Speed. ACS Med. Chem. Lett. 2017, 8, 476–480. [Google Scholar] [CrossRef]
- Savile, C.K.; Janey, J.M.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J. Biocatalytic Asymmetric Synthesis of Sitagliptin Manufacture. Science 2010, 329, 305–310. [Google Scholar] [CrossRef]
- Ghislieri, D.; Turner, N.J. Biocatalytic Approaches to the Synthesis of Enantiomerically Pure Chiral Amines. Top. Catal. 2014, 57, 284–300. [Google Scholar] [CrossRef]
- Roughley, S.D.; Jordan, A.M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef] [PubMed]
- Shiri, P. Novel Hybrid Molecules Based on Triazole-β-Lactam as Potential Biological Agents. Mini-Rev. Med. Chem. 2021, 21, 536–553. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J. Directed Evolution Drives the next Generation of Biocatalysts. Nat. Chem. Biol. 2009, 5, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. In Angewandte Chemie—International Edition; Wiley-VCH: Weinheim, Germany, 2021; pp. 88–119. [Google Scholar] [CrossRef]
- Kobayashi, S.; Mori, Y.; Fossey, J.S.; Salter, M.M. Catalytic Enantioselective Formation of C—C Bonds by Addition to Imines and Hydrazones: A Ten-Year Update. Chem. Rev. 2011, 111, 2626–2704. [Google Scholar] [CrossRef] [PubMed]
- Robak, M.T.; Herbage, M.A.; Ellman, J.A. Synthesis and Applications of Tert-Butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740. [Google Scholar] [CrossRef] [PubMed]
- Cabré, A.; Verdaguer, X.; Riera, A. Recent Advances in the Enantioselective Synthesis of Chiral Amines via Transition Metal-Catalyzed Asymmetric Hydrogenation. Chem. Rev. 2022, 122, 269–339. [Google Scholar] [CrossRef]
- Yin, Q.; Shi, Y.; Wang, J.; Zhang, X. Direct Catalytic Asymmetric Synthesis of α-Chiral Primary Amines. Chem. Soc. Rev. 2020, 49, 6141–6153. [Google Scholar] [CrossRef]
- Hughes, D.L. Biocatalysis in Drug Development—Highlights of the Recent Patent Literature. Org. Process. Res. Dev. 2018, 22, 1063–1080. [Google Scholar] [CrossRef]
- Green, A.P.; Turner, N.J. Biocatalytic Retrosynthesis: Redesigning Synthetic Routes to High-Value Chemicals. Perspect. Sci. 2016, 9, 42–48. [Google Scholar] [CrossRef]
- Roddan, R.; Ward, J.M.; Keep, N.H.; Hailes, H.C. Pictet–Spenglerases in Alkaloid Biosynthesis: Future Applications in Biocatalysis. In Current Opinion in Chemical Biology; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 69–76. [Google Scholar] [CrossRef]
- Grogan, G. Synthesis of Chiral Amines Using Redox Biocatalysis. In Current Opinion in Chemical Biology; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 15–22. [Google Scholar] [CrossRef]
- Kelly, S.A.; Pohle, S.; Wharry, S.; Mix, S.; Allen, C.C.R.; Moody, T.S.; Gilmore, B.F. Application of ω-Transaminases in the Pharmaceutical Industry. Chem. Rev. 2018, 118, 349–367. [Google Scholar] [CrossRef]
- Adams, J.P.; Brown, M.J.B.; Lba Diaz-Rodriguez, A.; Lloyd, R.C.; Roiban, G.-D. Biocatalysis: A Pharma Perspective. Adv. Synth. Catal. 2019, 361, 2421–2432. [Google Scholar] [CrossRef]
- Gomm, A.; Lewis, W.; Green, A.P.; O’Reilly, E. A New Generation of Smart Amine Donors for Transaminase-Mediated Biotransformations. Chem. Eur. J. 2016, 22, 12692–12695. [Google Scholar] [CrossRef] [PubMed]
- France, S.P.; Hussain, S.; Hill, A.M.; Hepworth, L.J.; Howard, R.M.; Mulholland, K.R.; Flitsch, S.L.; Turner, N.J. One-Pot Cascade Synthesis of Mono- and Disubstituted Piperidines and Pyrrolidines Using Carboxylic Acid Reductase (CAR), ω-Transaminase (ω-TA), and Imine Reductase (IRED) Biocatalysts. ACS Catal. 2016, 6, 3753–3759. [Google Scholar] [CrossRef]
- Simon, R.C.; Richter, N.; Busto, E.; Kroutil, W. Recent Developments of Cascade Reactions Involving ω-Transaminases. ACS Catal. 2014, 4, 129–143. [Google Scholar] [CrossRef]
- Cutlan, R.; de Rose, S.; Isupov, M.N.; Littlechild, J.A.; Harmer, N.J. Using Enzyme Cascades in Biocatalysis: Highlight on Transaminases and Carboxylic Acid Reductases. In Biochimica et Biophysica Acta—Proteins and Proteomics; Elsevier B.V.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- O’Reilly, E.O.; Iglesias, C.; Turner, N.J. Monoamine Oxidase—w-Transaminase Cascade for the Deracemisation and Dealkylation of Amines. ChemCatChem 2014, 6, 992–995. [Google Scholar] [CrossRef]
- O’Reilly, E.O.; Iglesias, C.; Ghislieri, D.; Hopwood, J.; Galman, J.L.; Lloyd, R.C.; Turner, N.J. A Regio- and Stereoselective w -Transaminase/Monoamine Oxidase Cascade for the Synthesis of Chiral 2, 5-Disubstituted Pyrrolidines ** Angewandte. Angew. Chem. Int. Ed. 2014, 53, 2447–2450. [Google Scholar] [CrossRef]
- Costa, B.Z.; Galman, J.L.; Slabu, I.; France, S.P.; Marsaioli, A.J.; Turner, N.J. Synthesis of 2,5-Disubstituted Pyrrolidine Alkaloids via A One-Pot Cascade Using Transaminase and Reductive Aminase Biocatalysts. ChemCatChem 2018, 10, 4733–4738. [Google Scholar] [CrossRef]
- Kim, D.; Wang, L.; Beconi, M.; Eiermann, G.J.; Fisher, M.H.; He, H.; Hickey, G.J.; Kowalchick, J.E.; Leiting, B.; Lyons, K.; et al. (2R)-4-Oxo-4-[3-(Trifluoromethyl)-5,6-Dihydro[1,2,4]Triazolo[4,3-a] Pyrazin-7(8H)-Yl]-1-(2,4,5-Trifluorophenyl)Butan-2-Amine: A Potent, Orally Active Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2005, 48, 141–151. [Google Scholar] [CrossRef]
- Williams-Herman, D.; Round, E.; Swern, A.S.; Musser, B.; Davies, M.J.; Stein, P.P.; Kaufman, K.D.; Amatruda, J.M. Safety and Tolerability of Sitagliptin in Patients with Type 2 Diabetes: A Pooled Analysis. BMC Endocr. Disord. 2008, 8, 14. [Google Scholar] [CrossRef]
- Desai, A.A. Sitagliptin Manufacture: A Compelling Tale of Green Chemistry, Process Intensification, and Industrial Asymmetric Catalysis. Angew. Chem.—Int. Ed. 2011, 50, 1974–1976. [Google Scholar] [CrossRef]
- Hansen, K.B.; Balsells, J.; Dreher, S.; Hsiao, Y.; Kubryk, M.; Palucki, M.; Rivera, N.; Steinhuebel, D.; Armstrong, J.D.; Askin, D.; et al. First Generation Process for the Preparation of the DPP-IV Inhibitor Sitagliptin. Org. Process. Res. Dev. 2005, 9, 634–639. [Google Scholar] [CrossRef]
- Hansen, K.B.; Hsiao, Y.; Xu, F.; Rivera, N.; Clausen, A.; Kubryk, M.; Krska, S.; Rosner, T.; Simmons, B.; Balsells, J.; et al. Highly Efficient Asymmetric Synthesis of Sitagliptin. J. Am. Chem Soc. 2009, 131, 8798–8804. [Google Scholar] [CrossRef]
- Baxter, C.A.; Cleator, E.; Brands, K.M.J.; Edwards, J.S.; Reamer, R.A.; Sheen, F.J.; Stewart, G.W.; Strotman, N.A.; Wallace, D.J. The First Large-Scale Synthesis of MK-4305: A Dual Orexin Receptor Antagonist for the Treatment of Sleep Disorder. Org. Process. Res. Dev. 2011, 15, 367–375. [Google Scholar] [CrossRef]
- Cox, C.D.; Breslin, M.J.; Whitman, D.B.; Schreier, J.D.; McGaughey, G.B.; Bogusky, M.J.; Roecker, A.J.; Mercer, S.P.; Bednar, R.A.; Lemaire, W.; et al. Discovery of the Dual Orexin Receptor Antagonist [(7 R)-4-(5-Chloro-1,3-Benzoxazol-2-Yl)-7-Methyl-1,4-Diazepan-1-Yl][5-Methyl-2-(2 H-1,2,3-Triazol-2-Yl)Phenyl]Methanone (MK-4305) for the Treatment of Insomnia. J. Med. Chem. 2010, 53, 5320–5332. [Google Scholar] [CrossRef] [PubMed]
- Strotman, N.A.; Baxter, C.A.; Brands, K.M.J.; Cleator, E.; Krska, S.W.; Reamer, R.A.; Wallace, D.J.; Wright, T.J. Reaction Development and Mechanistic Study of a Ruthenium Catalyzed Intramolecular Asymmetric Reductive Amination En Route to the Dual Orexin Inhibitor Suvorexant (MK-4305). J. Am. Chem Soc. 2011, 133, 8362–8371. [Google Scholar] [CrossRef] [PubMed]
- Mangion, I.K.; Sherry, B.D.; Yin, J.; Fleitz, F.J. Enantioselective Synthesis of a Dual Orexin Receptor Antagonist. Org. Lett. 2012, 14, 3458–3461. [Google Scholar] [CrossRef]
- Xu, Z.; Yao, P.; Sheng, X.; Li, J.; Li, J.; Yu, S.; Feng, J.; Wu, Q.; Zhu, D. Biocatalytic Access to 1,4-Diazepanes via Imine Reductase-Catalyzed Intramolecular Asymmetric Reductive Amination. ACS Catal. 2020, 10, 8780–8787. [Google Scholar] [CrossRef]
- Munchhof, M.J.; Li, Q.; Shavnya, A.; Borzillo, G.V.; Boyden, T.L.; Jones, C.S.; Lagreca, S.D.; Martinez-Alsina, L.; Patel, N.; Pelletier, K.; et al. Discovery of PF-04449913, a Potent and Orally Bioavailable Inhibitor of Smoothened. ACS Med. Chem. Lett. 2012, 3, 106–111. [Google Scholar] [CrossRef]
- Peng, Z.; Wong, J.W.; Hansen, E.C.; Puchlopek-Dermenci, A.L.A.; Clarke, H.J. Development of a Concise, Asymmetric Synthesis of a Smoothened Receptor (Smo) Inhibitor: Enzymatic Transamination of a 4-Piperidinone with Dynamic Kinetic Resolution. Org. Lett. 2014, 16, 860–863. [Google Scholar] [CrossRef]
- Gallant, M.; Beaulieu, C.; Berthelette, C.; Colucci, J.; Crackower, M.A.; Dalton, C.; Denis, D.; Ducharme, Y.; Friesen, R.W.; Guay, D.; et al. Discovery of MK-7246, a Selective CRTH2 Antagonist for the Treatment of Respiratory Diseases. Bioorganic Med. Chem. Lett. 2011, 21, 288–293. [Google Scholar] [CrossRef]
- Molinaro, C.; Bulger, P.G.; Lee, E.E.; Kosjek, B.; Lau, S.; Gauvreau, D.; Howard, M.E.; Wallace, D.J.; O’Shea, P.D. CRTH2 Antagonist MK-7246: A Synthetic Evolution from Discovery through Development. J. Org. Chem. 2012, 77, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Limanto, J.; Ashley, E.R.; Yin, J.; Beutner, G.L.; Grau, B.T.; Kassim, A.M.; Kim, M.M.; Klapars, A.; Liu, Z.; Strotman, H.R.; et al. A Highly Efficient Asymmetric Synthesis of Vernakalant. Org. Lett. 2014, 16, 2716–2719. [Google Scholar] [CrossRef] [PubMed]
- Plouvier, B.M.C.; Chou, D.T.H.; Jung, G.; Siu, L.; Choi, L.; Sheng, T.; Barrett, A.G.M.; Passafaro, M.S.; Kurz, M.; Moeckli, D.; et al. Synthetic Process for Aminocyclohexyl Ether Compounds. WO2006088525, 24 August 2006. [Google Scholar]
- Machiya, K.; Ike, K.; Watanabe, M.; Yoshino, T.; Okamoto, T.; Morinaga, Y.; Mizobata, S.; Astellas Pharma Inc. Production Method of Optically Active Cyclohexane Ether Compounds. WO2006075778, 20 July 2007. [Google Scholar]
- Jung, G.; Yee, J.G.K.; Chou, D.T.H.; Plouvier, B.M.C.; Cardiome Pharma Corp. Synthetic Processes for the Preparation of Aminocyclohexyl Ether Compounds. WO2006138673, 28 February 2006. [Google Scholar]
- Ye, H.; Yu, C.; Zhong, W. New Procedure for the Preparation of (1 R,2 R)-2-[(R)-3-(Benzyloxy)Pyr- Rolidin-1-Yl]Cyclohexanol. Synthesis 2012, 44, 51–56. [Google Scholar] [CrossRef][Green Version]
- Zawodny, W.; Montgomery, S.L.; Marshall, J.R.; Finnigan, J.D.; Turner, N.J.; Clayden, J. Chemoenzymatic Synthesis of Substituted Azepanes by Sequential Biocatalytic Reduction and Organolithium-Mediated Rearrangement. J. Am. Chem Soc. 2018, 140, 17872–17877. [Google Scholar] [CrossRef]
- Mitsukura, K.; Suzuki, M.; Tada, K.; Yoshida, T.; Nagasawa, T. Asymmetric Synthesis of Chiral Cyclic Amine from Cyclic Imine by Bacterial Whole-Cell Catalyst of Enantioselective Imine Reductase. Org. Biomol. Chem. 2010, 8, 4533–4535. [Google Scholar] [CrossRef]
- Mitsukura, K.; Suzuki, M.; Shinoda, S.; Kuramoto, T.; Yoshida, T.; Nagasawa, T. Purification and Characterization of a Novel (R)-Imine Reductase from Streptomyces Sp. GF3587. Biosci. Biotechnol. Biochem. 2011, 75, 1778–1782. [Google Scholar] [CrossRef]
- Schober, M.; MacDermaid, C.; Ollis, A.A.; Chang, S.; Khan, D.; Hosford, J.; Latham, J.; Ihnken, L.A.F.; Brown, M.J.B.; Fuerst, D.; et al. Chiral Synthesis of LSD1 Inhibitor GSK2879552 Enabled by Directed Evolution of an Imine Reductase. Nat. Catal. 2019, 2, 909–915. [Google Scholar] [CrossRef]
- Kumar, R.; Karmilowicz, M.J.; Burke, D.; Burns, M.P.; Clark, L.A.; Connor, C.G.; Cordi, E.; Do, N.M.; Doyle, K.M.; Hoagland, S.; et al. Biocatalytic Reductive Amination from Discovery to Commercial Manufacturing Applied to Abrocitinib JAK1 Inhibitor. Nat. Catal. 2021, 4, 775–782. [Google Scholar] [CrossRef]
- Johnson, N.W.; Kasparec, J.; GlaxoSmithKline. Cyclopropylamines as LSD1 Inhibitors. U.S. Patent 8853408, 4 October 2012. [Google Scholar]
- Köhler, V.; Bailey, K.R.; Znabet, A.; Raftery, J.; Helliwell, M.; Turner, N.J. Enantioselective Biocatalytic Oxidative Desymmetrization of Substituted Pyrrolidines. Angew. Chem. Int. Ed. 2010, 49, 2182–2184. [Google Scholar] [CrossRef]
- Ghislieri, D.; Green, A.P.; Pontini, M.; Willies, S.C.; Rowles, I.; Frank, A.; Grogan, G.; Turner, N.J. Engineering an Enantioselective Amine Oxidase for the Synthesis of Pharmaceutical Building Blocks and Alkaloid Natural Products. J. Am. Chem. Soc. 2013, 135, 10863–10869. [Google Scholar] [CrossRef]
- Alexeeva, M.; Enright, A.; Dawson, M.J.; Mahmoudian, M.; Turner, N.J. Deracemization of a-Methylbenzylamine Using an Enzyme Obtained by In Vitro Evolution. Angew. Chem. Int. Ed. 2002, 41, 3177–3180. [Google Scholar] [CrossRef]
- Znabet, A.; Zonneveld, J.; Janssen, E.; de Kanter, F.J.J.; Helliwell, M.; Turner, N.J.; Ruijter, E.; Orru, R.V.A. Asymmetric Synthesis of Synthetic Alkaloids by a Tandem Biocatalysis/Ugi/Pictet-Spengler-Type Cyclization Sequence. Chem. Commun. 2010, 46, 7706–7708. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liang, J.; Ambrogelly, A.; Brennan, T.; Gloor, G.; Huisman, G.; Lalonde, J.; Lekhal, A.; Mijts, B.; Muley, S.; et al. Efficient, Chemoenzymatic Process for Manufacture of the Boceprevir Bicyclic [3.1.0] Proline Intermediate Based on Amine Oxidase-Catalyzed Desymmetrization. J. Am. Chem Soc. 2012, 134, 6467–6472. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Sudhakar, A.; Wong, G.S.; Chen, M.; Weber, J.; Yang, X.; Kwok, D.-I.; Jeon, I.; Raghavan, R.R.; Tamarez, T.; et al. Process and Intermediates for the Preparation of (1R,2S,5S)-6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylates or Salts Thereof. WO2004113295, 29 December 2004. [Google Scholar]
- Wu, G.; Chen, F.X.; Rashatasakhon, P.; Eckert, J.M.; Wong, G.S.; Lee, H.-C.; Erickson, N.C.; Vance, J.A.; Nirchio, P.C.; Weber, J.; et al. Process for the Preparation of 6,6-dimethyl-3-azabicyclo-[3.1.0]-hexane Compounds and Enantiomeric Salts Thereof. WO2007075790, 5 July 2007. [Google Scholar]
- Kwok, D.-L.; Lee, H.-C.; Zavialov, I.A.; Schering Corporation. Dehydrohalogenation Process for the Preparation of Intermediates Useful in Providing 6,6-dimethyl-3-azabicyclo-[3.1.0]-hexane Compounds. WO2009073380, 11 June 2009. [Google Scholar]
- Mijts, B.; Muley, S.; Liang, J.; Neman, L.M.; Zhang, X.; Lalonde, J.; Clay, M.; Zhu, J.; Gruber, J.M.; Colbeck, J.; et al. Biocatalytic Processes for the Preparation of Substantially Stereomerically Pure Fused Bicyclic Proline Compounds. WO2010008828, 21 January 2010. [Google Scholar]
- Anthony, S.M.; Tona, V.; Zou, Y.; Morrill, L.A.; Billingsley, J.M.; Lim, M.; Tang, Y.; Houk, K.N.; Garg, N.K. Total Synthesis of (-)-Strictosidine and Interception of Aryne Natural Product Derivatives “Strictosidyne” and “Strictosamidyne”. J. Am. Chem Soc. 2021, 143, 7471–7479. [Google Scholar] [CrossRef]
- Brown, S.; Clastre, M.; Courdavault, V.; O’Connor, S.E. De Novo Production of the Plant-Derived Alkaloid Strictosidine in Yeast. Proc. Natl. Acad. Sci. USA 2015, 112, 3205–3210. [Google Scholar] [CrossRef]
- Qu, G.; Li, A.; Acevedo-Rocha, C.G.; Sun, Z.; Reetz, M.T. The Crucial Role of Methodology Development in Directed Evolution of Selective Enzymes. In Angewandte Chemie—International Edition; Wiley-VCH: Weinheim, Germany, 2020; pp. 13204–13231. [Google Scholar] [CrossRef]












| Compound | Enzyme Class | Enzyme Used |
|---|---|---|
 sitagliptin | Transaminase | ω-TAm ArRMut11 |
 suvorexant | Transaminase | ω-TAm (CDX-017) |
| Imine reductase | IR1 Leishmania major from Y194F/D232H | |
 PF-0444913 | Transaminase | ATA-036 |
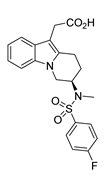 MK-7246 | Transaminase | ω-TAm (CDX-017) |
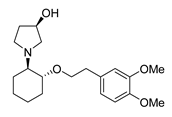 vernakalant | Transaminase | ATA-303 |
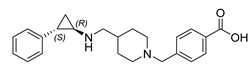 GSK2879552 | Imine reductase | IRED-M3 |
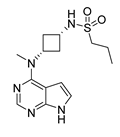 abrocitinib | Imine reductase | SpRedAm-V6 |
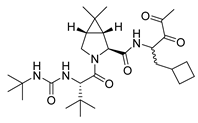 boceprevir | Monoamine oxidase | MAON401 |
 (−)-strictosidine | Picter-Spenglerase | Strictosidine synthase cell-free lysate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawodny, W.; Montgomery, S.L. Evolving New Chemistry: Biocatalysis for the Synthesis of Amine-Containing Pharmaceuticals. Catalysts 2022, 12, 595. https://doi.org/10.3390/catal12060595
Zawodny W, Montgomery SL. Evolving New Chemistry: Biocatalysis for the Synthesis of Amine-Containing Pharmaceuticals. Catalysts. 2022; 12(6):595. https://doi.org/10.3390/catal12060595
Chicago/Turabian StyleZawodny, Wojciech, and Sarah Louise Montgomery. 2022. "Evolving New Chemistry: Biocatalysis for the Synthesis of Amine-Containing Pharmaceuticals" Catalysts 12, no. 6: 595. https://doi.org/10.3390/catal12060595
APA StyleZawodny, W., & Montgomery, S. L. (2022). Evolving New Chemistry: Biocatalysis for the Synthesis of Amine-Containing Pharmaceuticals. Catalysts, 12(6), 595. https://doi.org/10.3390/catal12060595







