Abstract
Reaction coupling separation systems using calcium fumarate as a substrate can break the reaction equilibrium and promote the production of l-malate. However, the low reusability and stability of fumarase limit its further application. In this study, partially purified fumarase of Thermus thermophilus (87.0 U mg−1) was immobilized within konjac-κ-carrageenan beads. An amalgamation of konjac and carrageenan gum (2%) was used to form the beads, and polyethylene polyamine (0.2%) and glutaraldehyde (0.1%) were used as the cross-linking agents. The pH and temperature profiles of free and immobilized fumarases were remarkably similar. The diffusion limit of immobilized fumarase caused a decline in the maximal velocity (Vmax), whereas the kinetic constant (Km) value increased. Optimization of the parameters for biotransformation by immobilized fumarase showed that 88.3% conversion of 200 mM calcium fumarate could be achieved at 55 °C within 8 h. The beads were stored for 30 days at 4 °C with minimal loss in activity and were reusable for up to 20 cycles with 78.1% relative activity. By recycling the reaction supernatant, a total amount of 3.98 M calcium fumarate was obtained with a conversion of 99.5%, which is the highest value ever reported.
1. Introduction
l-Malic acid, a C4-dicarboxylic acid, is extensively applied in the chemical, pharmaceutical, and food industries [1]. The US Department of Energy has identified fumaric acid as one of the top 12 chemical building blocks that can that can be employed as a significant precursor for producing high-value products [2,3]. Nevertheless, l-malic acid is mainly manufactured via chemical synthesis, yielding a racemic mixture of d- and l-malic acid, and an efficient industrial biotechnological route for its production is yet to be developed.
In the context of industry, globally, seeking sustainable solutions for replacing compounds based on oil with renewable building blocks, the generation of l-malate by bio-derived microbial fermentation has aroused interest [4,5,6,7]. However, many issues still exist that need to be resolved prior to industrial production, for instance (i) it is often expensive and difficult to purify l-malate from the complex fermentation broth, (ii) strains are not stable enough, and (iii) the productivity and yield of l-malate remains low.
Compared with fermentation, biocatalysis is the preferred strategy for compound production because of its simplicity, convenience, and low cost of product recovery [8,9]. Fumarase (fumarate hydratase: EC 4.2.1.2) is found in eukaryotes and prokaryotes and catalyzes the reversible stereospecificity of fumarate to l-malate [10]. Fumarase with high activity from Dipodascus magnusii [11], Brevibacterium flavum [12], and Corynebacterium glutamicum [13], have been used as biocatalysts for the industrial production of l-malate. The temperature of reaction for l-malate production is usually set between 40 °C and 60 °C to minimize the generation of the unwanted byproduct succinic acid [14]. However, fumarase derived from mesophilic microorganisms rapidly deactivate at these temperatures [13]. Therefore, fumarase from thermophilic bacteria, which show high thermal stability, have drawn increasing attention for decades [15,16,17].
In an aqueous solution, the hydration of fumarate to l-malate is a reversible reaction with a conversion of approximately 80% [18]. The difficult separation of l-malate from the unreacted substrate greatly increases the cost of l-malate production. In this study, we tried to solve this problem by using calcium fumarate as a substrate, since the produced calcium l-malate continuously precipitates from the reaction mixture. In addition, in situ isolation of product from the reaction promoted the reaction to proceed in the forward direction. However, the inefficient recovery and reuse of fumarase makes the system uneconomic. Fumarase should be used in an immobilized form to match the low cost of hydrolysis. Purified enzymes entrapped in hydrogels [19] or immobilized within membrane reactors [20] have been applied to l-malate production. However, these immobilization methods do not apply to the reaction coupling separation systems. The system of konjac-κ-carrageenan gum-KCl is one of the most extensively utilized and inexpensive techniques for immobilizing enzymes and living cells [21,22]. The konjac-κ-carrageenan beads enhance enzyme stability and reusability, and protect enzymes from mechanical shear. Other techniques, such as cross-linking with glutaraldehyde and polyethylene polyamine, serve to further enhance the activity and stability of enzymes.
In the present study, partially purified fumarase from Thermus thermophilus was immobilized in a potassium konjac-κ-carrageenan matrix using an entrapment technique and cross-linked with polyethylene polyamine and glutaraldehyde. The characteristic catalytic properties of the immobilized enzymes were studied, including the optimum pH and temperature reaction conditions, thermostability, and storage stability. Finally, the reusability of immobilized fumarase in the reaction coupling separation system was investigated.
2. Results
2.1. Easy Obtainment of a Fraction with Fumarase Activity for Immobilization Purposes
Fumarase from Thermus thermophilus was successfully expressed as a soluble protein in Escherichia coli. A thick band of approximately 50 kDa in size was observed on the sodium dodecyl sulphate–polyacrylamide gel electrophoresis gel (SDS–PAGE) (Figure 1), similar to that described in previous studies [15]. According to previous reports, the use of purified fumarase is advantageous compared to the use of whole cells, as the former has 105-fold higher activity than the latter [18]. However, it is difficult to obtain a large amount of purified enzyme in an industrial setting. In this study, fumarase was purified from transformed E. coli using heat treatment due to its excellent enzyme stability. As shown in Figure 1, as the preheating temperature increased from 55 to 90 °C, most of the expressed fumarase remained in the soluble fraction. Activity analysis revealed that the highest specific activity (87.0 U mg−1) was obtained at 85 °C. A higher preheating temperature irreversibly inactivated fumarase, leading to a decrease in specific activity. Based on these results, the partially purified fumarase that had been heat-treated at 85 °C for 20 min was used for the following immobilization studies.
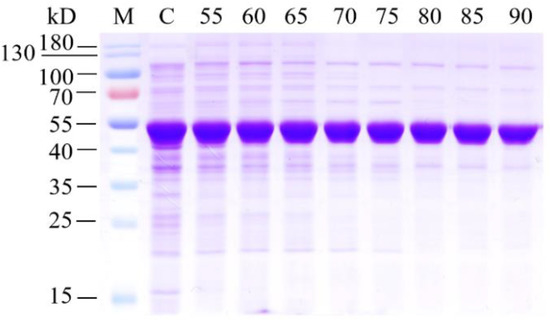
Figure 1.
Analysis of heatpurified fumarase using sodium dodecyl sulphate–polyacrylamide gel electrophoresis. Lane M, pre-stained protein molecular weight marker; lane C, crude cell extract without heat treatment; lanes 55–90, supernatant after heat treatment at 55–90 °C. The thick band at 50 kDa corresponds to the overexpressed fumarase.
Different parameters pertaining to the immobilization support were optimized. A konjac-κ-carrageenan concentration of 1% resulted in beads with higher initial fumarase activity that that achieved with higher percentages, probably because of a better mass transfer (Table 1). However, the bead strength was low and unsuitable for sustained biotransformation and recyclability. Therefore, 2% konjac-κ-carrageenan beads were used for the subsequent experiments. The optimum conditions for bead formation were determined using 3% KCl at room temperature. After encapsulation in konjac-κ-carrageenan, the beads were cross-linked with polyethylene polyamine and glutaraldehyde, as this treatment has been reported to enhance the stability of the immobilized enzyme by preventing enzyme leakage [23,24]. For the partially purified fumarase-immobilized system, a polyethylene polyamine concentration of 0.2% (w/v) and a glutaraldehyde concentration of 0.1% (w/v) resulted in an initial fumarase activity of 123.5 U/g beads with an activity recovery of 17.4% (Table 1). The highest concentrations of polyethylene polyamine and glutaraldehyde analyzed (0.24% and 0.25%, respectively) led to a slight decrease in enzyme activity compared to that at lower concentrations, but improved bead strength and maintained fumarase activity during prolonged storage.

Table 1.
Optimization of different parameters for the immobilization of partially purified fumarase in konjac-κ-carrageenan beads.
2.2. Characterization of Free and Immobilized Fumarase
Temperature and pH play vital roles in the proper functioning of immobilized enzymes. In the current study, both free and immobilized fumarases showed maximum initial activity at an optimum pH of 7.5 and temperature of 85 °C (Figure 2). Furthermore, immobilized fumarase showed higher activity than free fumarase over wider pH and temperature ranges (Figure 3). Both free and immobilized fumarase beads were thermostable in the range of 40–70 °C (Figure 3). After 2 h of incubation, free fumarase retained 82.6% and 65.0% residual activity at 80 °C and 90 °C, respectively. Immobilized fumarase showed higher stability at both temperature conditions, with 96.0% and 87.1% residual activity at 80 °C and 90 °C, respectively.
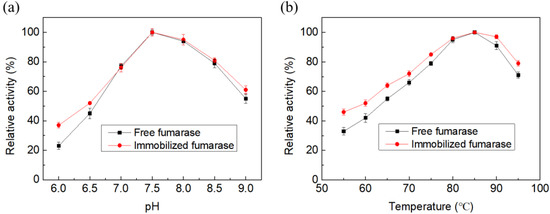
Figure 2.
The relative initial activity of immobilized and free fumarase at different pH (a) and temperature (b) conditions.
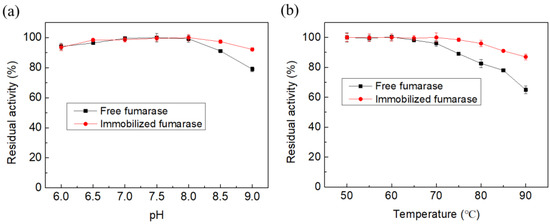
Figure 3.
The residual activity of immobilized and free fumarase incubated at different pH (a) and temperature (b) conditions for 2 h.
The kinetic parameters, kinetic constant value (Km) and maximal velocity (Vmax), of free and immobilized fumarase were determined using a Lineweaver–Burk plot. The Km values of free fumarase and fumarase entrapped in konjac-κ-carrageenan beads were 5.6 and 16.5 mM, respectively (Figure 4). The values of Vmax of free fumarase and fumarase entrapped in konjac-κ-carrageenan beads were 188.7 mM min−1 and 99.0 mM min−1, respectively. The increase in Km value indicated a reduced affinity of the immobilized enzyme for its substrate compared to that of free fumarase. The observed decrease in the Vmax value, which indicated a lower reaction velocity, might be due to changes in the conformation of the enzyme following immobilization or due to the diffusional limitation of the substrate to the active site of the enzyme.
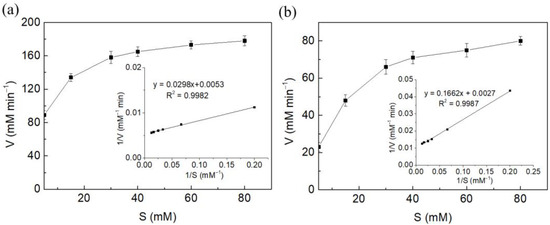
Figure 4.
Lineweaver–Burk and Michaelis–Menten plots of free (a) and immobilized (b) fumarase.
2.3. Establishment of a Reaction–Separation System
Considering that the solubility of calcium l-malate is lower than that of calcium fumarate (Figure S1), the produced calcium l-malate should continuously precipitate from the system, promoting the reaction to proceed in the forward direction. As shown in Figure 5a, 100 mM calcium fumarate was successfully converted to l-malate at 55 °C with a conversion of 83.6%, which was 4.2% higher than that using sodium fumarate as the substrate. In addition, the conversion increased as the calcium fumarate concentration increased (Figure 5b). Therefore, the system required higher substrate concentrations to achieve an increased conversion. However, stirring became difficult after the addition of over 200 mM substrate. Fixing the substrate concentration at 200 mM resulted in a conversion of 88.3% (Figure 5b) [18]. The reaction temperature was also investigated. Higher reaction temperatures improved the efficiency of the reaction but did not further increase the conversion (Figure 5c). In addition, we found that a higher temperature was unfavorable for crystal shape fixing (Figure 6). To ensure rapid isolation of calcium l-malate crystals, 55 °C was selected for subsequent biotransformation.
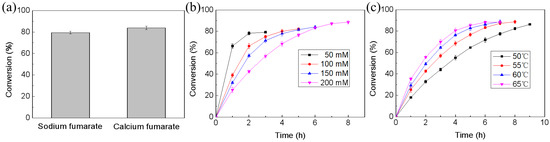
Figure 5.
Transformation of fumarate to l-malate by immobilized fumarase. (a) The effects of substrate types on fumarate conversion; (b) The effects of substrate concentration on calcium fumarate conversion; (c) The effects of temperature on calcium fumarate conversion.
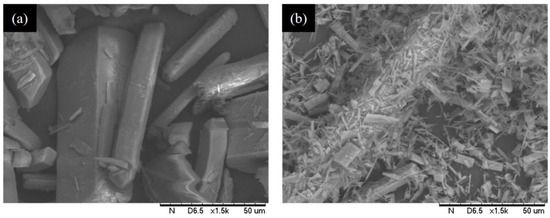
Figure 6.
Scanning electron micrographs of calcium l-malate crystals. (a) Calcium l-malate precipitated at 55 °C reaction. (b) Calcium l-malate precipitated at 65 °C reaction.
2.4. Storage and Recyclability of Immobilized Fumarase
To determine the viability and usability of the enzyme after storage, the free and immobilized fumarase preparations were stored in assay buffer at 4 °C for 30 days, and the activity was recorded. Immobilized fumarases showed better retention of activity during the whole storage (Figure 7a). Free fumarase retained 68.3% of its original activity after 30 days, whereas fumarase entrapped in the konjac-κ-carrageenan membrane retained 91.3% of its initial activity during the same storage period. These results revealed that the immobilized enzyme remained stable when stored at 4 °C.
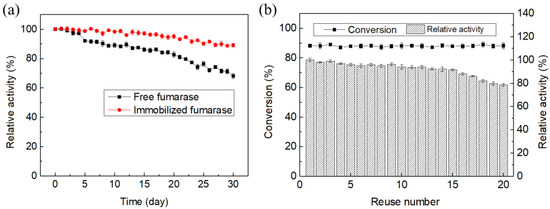
Figure 7.
Storage (a) and recyclability (b) of immobilized and free fumarase.
The inefficient recovery and reuse of immobilized fumarase significantly increases the cost of industrial l-malic acid production. Therefore, we assessed the reusability of immobilized fumarase. Reactions were conducted at 55 °C and pH 7.5 for 10 h using 200 mM fumarate as the substrate. The immobilized fumarase was recovered after each reaction and reused for the subsequent batch reaction (Figure 8). After 15 repeated cycles, the immobilized fumarase retained at least 90.1% of its original activity with no surface damage. In the 20th cycle, the immobilized fumarase retained 78.1% of its initial activity (Figure 7b). The decrease in activity with successive cycles was likely due to the loss of bead strength, which resulted in leakage of the enzyme. The calcium fumarate produced after each reaction was isolated by filtration. The filtered fluid, which contained approximately 23 mM calcium fumarate and a minimal amount of calcium l-malate, was recycled for the subsequent batch reaction. After 20 repeated cycles, a total amount of 4.0 M calcium fumarate was successfully converted to calcium l-malate with a conversion of 99.5%, which was much higher than previously reported (~80%) [18,25].
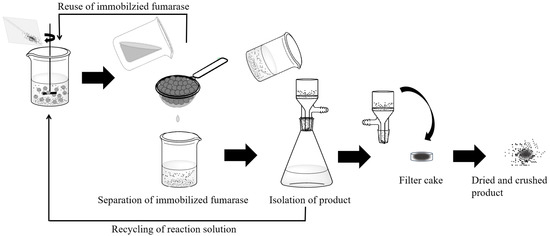
Figure 8.
Scheme of recycling of immobilized fumarase and isolation of calcium l-malate crystals.
In general, enzymatic hydration is one of the most promising methods for producing l-malate. The reaction coupling separation system using calcium fumarate as a substrate can not only ease the isolation of the product but also break the reaction equilibrium and promote the production of l-malate. However, inefficient recovery and reuse of fumarase in the reaction coupling separation system increases its costs, making them non-competitive options. Due to the high mechanical shear, there are no studies that have reported recycling fumarase from the reaction coupling separation system. In this study, we successfully entrapped fumarase in konjac-κ-carrageenan beads with high strength. The high stability and reusability of the beads make the system more suitable for the industrial production of l-malate.
3. Materials and Methods
3.1. Bacterial Strains, Chemicals
Competent cells of E. coli BL21(DE3) were purchased from Beijing Biomed Co., Ltd. (Beijing, China). Gene of T. thermophilus fumarase was codon-optimized and cloned into pET-28a(+) by Sangon Biotech (Shanghai, China). Konjac gum and κ-carrageenan gum were procured from Sangon Biotech (Shanghai, China). All other chemicals were provided by Aladdin (Shanghai, China), Sangon Biotech (Shanghai, China), Sigma Aldrich (Shanghai, China), TCI Chemicals (Shanghai, China) or Macklin (Shanghai, China). All chemicals were of analytical or HPLC grade.
3.2. Expression and Preparation of Thermus thermophilus Fumarase
The pET-28a(+) containing fumarase gene was transformed into E. coli BL21(DE3) by heat shock. The recombinant E. coli strain was transferred into liquid LB medium (50 mL) involving 50 μg mL–1 of kanamycin and then grown at a temperature of 37 °C, at 200 rpm for twelve hours. The preculture (2 mL) was utilized for inoculating culture medium (200 mL) involving 50 μg mL–1 of kanamycin and next grown under a temperature of 37 °C, at 200 rpm. When OD600 was between 0.6 and 0.8, 0.2 mM Isopropyl β-d-1-thiogalactopyranoside (IPTG) was added. The cells were subsequently grown under a temperature of 18 °C overnight for 20 h and gathered through centrifugation at 8000 rpm under a temperature of 4 °C for fifteen minutes. Cell disruption was achieved by ultra-sonication (50% amplitude, 3 s pulse, and 7 s pause for 15 min). At a temperature of 85 °C, the supernatant was heated for twenty minutes and the debris was removed by centrifugation (4 °C, 10,000 rpm, and 20 min). The fumarase, partially purified by heat treatment, was used for immobilization.
3.3. Immobilization of Fumarase
The partially purified fumarase was immobilized by the entrapment method using konjac-κ-carrageenan beads. Cell disruption was achieved by ultrasonication from 5 mL water that contained 1.0 g wet cells. The supernatant was heated at 85 °C for twenty minutes and the debris was removed through centrifugation. The partially purified enzyme was mixed in equal volume (1:1) with konjac-κ-carrageenan solution prepared in water (with a konjac gum: κ-carrageenan gum ratio of 3:7). The fumarase-konjac-κ-carrageenan mixture was extruded drop by drop with a fine needle into 3% of KCl solution generated in distilled water. The immobilized beads were hardened in an identical solution for 60 min and maintained in a fresh solution of KCl at 4 ° C for twelve hours. The beads were subsequently cleaned with water in order to remove any loosely bound or unbound enzymes. Cross-linking of the fumarase was implemented by immersing the beads in polyethylene polyamine (pH 7.5) and glutaraldehyde solution, respectively, prepared in water and shaken for 60 min. The beads were isolated via filtration and next cleaned repeatedly by water in order to remove the unbound glutaraldehyde and polyethylene polyamine. Immobilization was optimized utilizing different concentrations of konjac-κ-carrageenan (1–3%, w/v), polyethylene polyamine (0–0.3%, w/v), and KCl (0–0.3%, w/v).
3.4. Determination of Enzyme Activity
The activity of fumarase was evaluated at pH 7.5 and 55 °C by determining the generation of l-malate by absorbance variations of the fumarate bond at 255 nm. The standard test mixture was composed of diluted enzyme preparation, sodium fumarate (50 mM) as well as potassium phosphate buffer (50 mM, with a pH of 7.5). The enzyme activity producing sodium l-malate (1 μmol) per minute was the unit of fumarate enzyme activity. At 250 nm, the molar coefficient of sodium fumarate is 1.479 × 103 M−1 cm−1 [26]. Utilizing BSA as the standard [27], Lowry’s approach was applied to estimate the protein. Three replicates were employed and the data were expressed as mean ± SD.
3.5. Characterization of Free and Immobilized Fumarase
To determine the values of Km and Vmax for the free and immobilized fumarase preparations, a Lineweaver–Burk plot was made using various substrate concentrations (5–80 mM) in phosphate buffer (50 mM; pH 7.5). The pH activity was determined in 50 mM citrate phosphate buffer (pH 4.0–6.0), phosphate buffer (pH 6.0–8.0), or Tris-HCl buffer (pH 8.0–9.0). The temperature activity was assayed at 30–90 °C for 10 min at pH 7.5. The relative activity was calculated with the activity at optimal pH or temperature as 100%. Temperature stability was determined by pre-incubating the enzyme at 30–80 °C in phosphate buffer (pH 7.5) without substrate for 2 h, and then activity was measured at 55 °C. The residual activity was calculated with the activity before incubation as 100%.
3.6. Storage and Reusability Studies
In order to assess the stability of immobilized and free enzymes in the process of storage, beads with retained fumarase and purified fumarase were stored in 50 mm phosphate buffer (pH 7.5) at 4 °C and extracted equally every other day. The relationship between the percentage of residual activity and the storage time (days) was plotted. With the hydrolysis of fumarate (200 mM, pH 7.5 in water) at 55 ° C, the reusability of the immobilized system could be assessed. After each hydrolysis cycle (10 h), the immobilized fumarase and reaction supernatant were taken out and put into the next cycle with fresh substrate. The remaining activity of fumarase was counted as 100% of enzyme activity in the first cycle. The recycling process needed to be conducted several times.
3.7. HPLC Analysis
The samples were diluted to ultrapure water and filtered with a membrane (0.25 μm). At 30 °C, HPLC with an Aminex HPX-87H column (BioRad, Hercules, CA, USA) and an Agilent 1260 VWD UV detector (Palo Alto, CA, USA) were utilized to determine l-malate and fumarate at 210 nm wavelength. According to the above methods, the mobile phase was 5 mM H2SO4 and the constant flow rate was 0.6 mL min−1.
4. Conclusions
In this study, T. thermophilus fumarase was successfully immobilized in konjac-κ-carrageenan beads and cross-linked with polyethylene polyamine and glutaraldehyde. The stability of immobilized fumarase increased over a wide range of temperatures (50–90 °C) and pH values (4.0–9.0). A kinetic study demonstrated that the immobilized enzyme presented a lower Vmax and higher Km than the free form. The biocatalyst beads could be reused in the reaction–separation system for 20 batch cycles, retaining 78.1% of the initial activity. The beads could be stored for 30 days at 4 °C with minimal loss in activity. By recycling the reaction supernatant, a total amount of 3.98 M calcium fumarate was obtained with a conversion of 99.5%, which is the highest value ever reported. The high stability and reusability of fumarase in the konjac-κ-carrageenan beads highlight it as a promising method for preparing l-malate in reaction coupling separation systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12060587/s1, Figure S1: Solubility of calcium fumarate and calcium l-malate at different temperatures.
Author Contributions
Experimental procedures and writing—original draft preparation G.L.; Conceptualization, B.L. and N.C.; methodology, F.G., Q.X. and M.M.; investigation, L.Z.; resources, F.Z. and X.Y.; funding acquisition, F.Z. and X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the High-Level Talent project of West Anhui University (WGKQ2021025), the Natural Science Foundation of Anhui Province (2008085QB96), the China Postdoctoral Science Foundation (2020M680134), and the Guangdong Basic and Applied Basic Research Foundation (2019A1515111051).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Acknowledgments
We gratefully thank Wei Wang for the HPLC analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chi, Z.; Wang, Z.-P.; Wang, G.-Y.; Khan, I.; Chi, Z.-M. Microbial biosynthesis and secretion of l-malic acid and its applications. Crit. Rev. Biotechnol. 2016, 36, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhou, Y.; Luo, H.; Cheng, X.; Zhang, R.; Teng, W. A comparative evaluation of different types of microbial electrolysis desalination cells for malic acid production. Bioresour. Technol. 2015, 198, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. Microbial production of organic acids: Expanding the markets. Trends Biotechnol. 2008, 26, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Dai, Z.; Peng, W.; Zhang, S.; Zhou, J.; Ma, J.; Dong, W.; Xin, F.; Zhang, W.; Jiang, M. Metabolic engineering of Pichia pastoris for malic acid production from methanol. Biotechnol. Bioeng. 2021, 118, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, Y.; Cao, W.; Liu, H. Improved Production of Malic Acid in Aspergillus niger by Abolishing Citric Acid Accumulation and Enhancing Glycolytic Flux. ACS Synth. Biol. 2020, 9, 1418–1425. [Google Scholar] [CrossRef]
- Koevilein, A.; Kubisch, C.; Cai, L.; Ochsenreither, K. Malic acid production from renewables: A review. J. Chem. Technol. Biotechnol. 2020, 95, 513–526. [Google Scholar] [CrossRef] [Green Version]
- Jantama, K.; Haupt, M.J.; Svoronos, S.A.; Zhang, X.; Moore, J.C.; Shanmugam, K.T.; Ingram, L.O. Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate. Biotechnol. Bioeng. 2008, 99, 1140–1153. [Google Scholar] [CrossRef]
- Li, G.; Lian, J.; Xue, H.; Jiang, Y.; Wu, M.; Lin, J.; Yang, L. Enzymatic preparation of pyruvate by a whole-cell biocatalyst coexpressing L-lactate oxidase and catalase. Process Biochem. 2020, 96, 113–121. [Google Scholar] [CrossRef]
- Li, G.; Lian, J.; Xue, H.; Jiang, Y.; Ju, S.; Wu, M.; Lin, J.; Yang, L. Biocascade Synthesis of L-Tyrosine Derivatives by Coupling a Thermophilic Tyrosine Phenol-Lyase and L-Lactate Oxidase. Eur. J. Org. Chem. 2020, 2020, 1050–1054. [Google Scholar] [CrossRef]
- Cernia, E.; Libori, R.; Marconi, W.; Soro, S. Study of fumarase activity in non-conventional media. Part I. J. Mol. Catal. B-Enzym. 1996, 1, 81–88. [Google Scholar] [CrossRef]
- Rosenberg, M.; Mikova, H.; Kristofikova, L. Formation of L-malic acid by yeasts of the genus Dipodascus. Lett. Appl. Microbiol. 1999, 29, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Takata, I.; Tosa, T.; Chibata, I. Stability of fumarase activity of Brevibacterium flavum immobilized with k-carrageenan and Chinese gallotannin. Appl. Microbiol. Biotechnol. 1984, 19, 85–90. [Google Scholar] [CrossRef]
- Genda, T.; Watabe, S.; Ozaki, H. Purification and characterization of fumarase from Corynebacterium glutamicum. Biosci. Biotechnol. Biochem. 2006, 70, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Su, R.-R.; Wang, A.; Hou, S.-T.; Gao, P.; Zhu, G.-P.; Wang, W. Identification of a novel fumarase C from Streptomyces lividans TK54 as a good candidate for malate production. Mol. Biol. Rep. 2014, 41, 497–504. [Google Scholar] [CrossRef]
- Mizobata, T.; Fujioka, T.; Yamasaki, F.; Hidaka, M.; Nagai, J.; Kawata, Y. Purification and characterization of a thermostable class II fumarase from Thermus thermophilus. Arch. Biochem. Biophys. 1998, 355, 49–55. [Google Scholar] [CrossRef]
- Kosuge, T.; Umehara, K.; Hoshino, T. Cloning and sequence analysis of fumarase and superoxide dismutase genes from an extreme thermophile Thermus thermophilus HB27. J. Ferment. Bioeng. 1998, 86, 125–129. [Google Scholar] [CrossRef]
- Liu, Y.; Song, J.; Tan, T.; Liu, L. Production of fumaric acid from L-malic acid by solvent engineering using a recombinant thermostable fumarase from Thermus thermophilus HB8. Appl. Biochem. Biotechnol. 2015, 175, 2823–2831. [Google Scholar] [CrossRef]
- Presecki, A.V.; Zelic, B.; Vasic-Racki, D. Comparison of the L-malic acid production by isolated fumarase and fumarase in permeabilized baker’s yeast cells. Enzym. Microb. Technol. 2007, 41, 605–612. [Google Scholar] [CrossRef]
- Marconi, W.; Faiola, F.; Piozzi, A. Catalytic activity of immobilized fumarase. J. Mol. Catal. B-Enzym. 2001, 15, 93–99. [Google Scholar] [CrossRef]
- Presecki, A.V.; Zelic, B.; Vasic-Racki, D. Modelling of continuous L-malic acid production by porcine heart fumarase and fumarase in yeast cells. Chem. Biochem. Eng. Q. 2009, 23, 519–525. [Google Scholar]
- Wang, X.; Zhou, D.; Guo, Q.; Liu, C. Textural and structural properties of a kappa-carrageenan-konjac gum mixed gel: Effects of kappa-carrageenan concentration, mixing ratio, sucrose and Ca2+ concentrations and its application in milk pudding. J. Sci. Food Agric. 2021, 101, 3021–3029. [Google Scholar] [CrossRef] [PubMed]
- van de Velde, F.; Lourenco, N.D.; Pinheiro, H.M.; Bakker, M. Carrageenan: A food-grade and biocompatible support for immobilisation techniques. Adv. Synth. Catal. 2002, 344, 815–835. [Google Scholar] [CrossRef]
- Aharwar, A.; Parihar, D.K. Talaromyces verruculosus tannase immobilization, characterization, and application in tea infusion treatment. Biomass Convers. Biorefin. 2021, 11, 112–125. [Google Scholar] [CrossRef]
- Shi, X.; Tao, M.; Lin, H.; Zhang, W. Application of the polyacrylonitrile fiber as a support for the green heterogeneous base catalyst and supported phase-transfer catalyst. RSC Adv. 2014, 4, 64347–64353. [Google Scholar] [CrossRef]
- Stojkovic, G.; Plazl, I.; Znidarsic-Plazl, P. L-Malic acid production within a microreactor with surface immobilised fumarase. Microfluid. Nanofluid. 2011, 10, 627–635. [Google Scholar] [CrossRef]
- Ohare, M.C.; Doonan, S. Purification and structural comparisons of the cytosolic and mitochondrial isoenzyme of fumarase from pig-liver. Biochim. Biophys. Acta 1985, 827, 127–134. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).