Protein Engineering of Pasteurella multocida α2,3-Sialyltransferase with Reduced α2,3-Sialidase Activity and Application in Synthesis of 3′-Sialyllactose
Abstract
1. Introduction
2. Results and Discussion
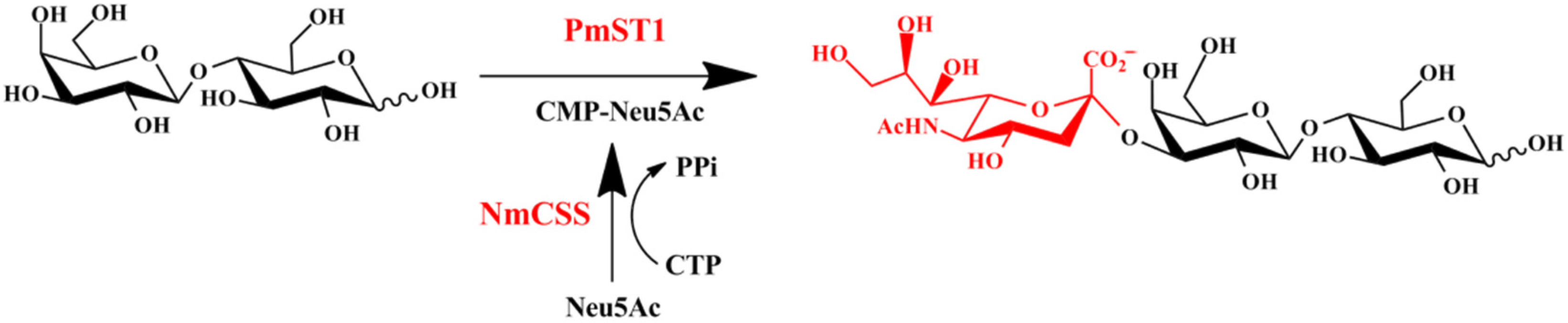
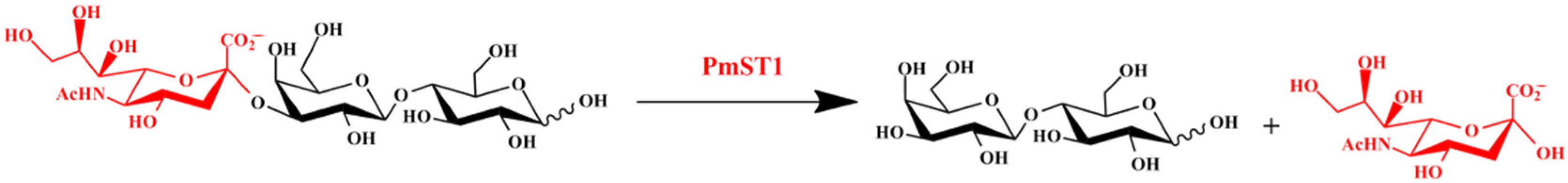
2.1. α2,3-Sialidase Activity of PmST1 M144D Led to Low Yield of 3′-SL
2.2. Design and Construction of M144D-Based First Generation of Mutants
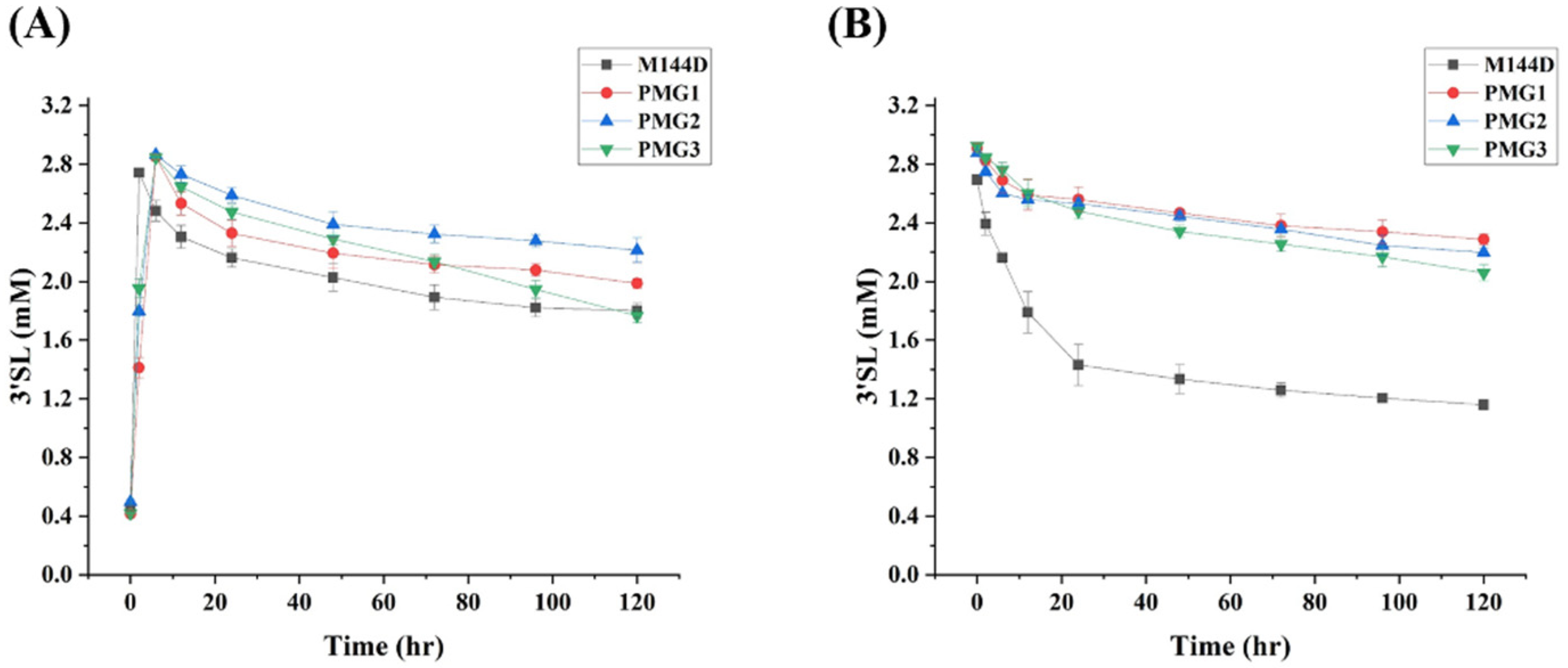
2.3. Characterization of M144D-Based First Generation of Mutants
2.4. Design, Construction and Characterization of Second Generation of Mutants
2.5. Preparative-Scale Synthesis of 3′-SL Using PMG2
3. Materials and Methods
3.1. General Information
3.2. Site-Directed Mutagenesis, Expression and Purification of PmST1 M144D Mutants
3.3. Analysis of α2,3-Sialyltransferase and α2,3-Sialidase Activity of PmST1 M144D Mutants by TLC
3.4. Kinetics of the α2,3-Sialyltransferase Activity of PmST1 M144D Mutants by LC-MS Analysis
3.5. Kinetics of the α2,3-Sialidase Activity of PmST1 M144D Mutants
3.6. Preparative-scale Synthesis of 3′-SL Using PMG2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 2007, 446, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Sialic Acid Is an Essential Nutrient for Brain Development and Cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef] [PubMed]
- Perdijk, O.; van Baarlen, P.; Fernandez-Gutierrez, M.M.; van den Brink, E.; Schuren, F.H.J.; Brugman, S.; Savelkoul, H.F.J.; Kleerebezem, M.; van Neerven, R.J.J. Sialyllactose and Galactooligosaccharides Promote Epithelial Barrier Functioning and Distinctly Modulate Microbiota Composition and Short Chain Fatty Acid Production In Vitro. Front. Immunol. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E.; Xu, L.Y.L.; Townsend, S.D. Prospecting Human Milk Oligosaccharides as a Defense Against Viral Infections. Acs Infect. Dis. 2021, 7, 254–263. [Google Scholar] [CrossRef]
- Kunz, C.; Rudloff, S.; Baier, W.; Klein, N.; Strobel, S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annu. Rev. Nutr. 2000, 20, 699–722. [Google Scholar] [CrossRef]
- Imai, M.; Kawaoka, Y. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr. Opin. Virol. 2012, 2, 160–167. [Google Scholar] [CrossRef]
- Izumi, M.; Shen, G.J.; Wacowich-Sgarbi, S.; Nakatani, T.; Plettenburg, O.; Wong, C.H. Microbial glycosyltransferases for carbohydrate synthesis: Alpha-2,3-sialyltransferase from Neisseria gonorrheae. J. Am. Chem. Soc. 2001, 123, 10909–10918. [Google Scholar] [CrossRef]
- Yu, H.; Chokhawala, H.; Karpel, R.; Yu, H.; Wu, B.Y.; Zhang, J.B.; Zhang, Y.X.; Jia, Q.; Chen, X. A multifunctional Pasteurella multocida sialyltransferase: A powerful tool for the synthesis of sialoside libraries. J. Am. Chem. Soc. 2005, 127, 17618–17619. [Google Scholar] [CrossRef]
- Chokhawala, H.A.; Huang, S.S.; Lau, K.; Yu, H.; Cheng, J.S.; Thon, V.; Hurtado-Ziola, N.; Guerrero, J.A.; Varki, A.; Chen, X. Combinatorial chemoenzymatic synthesis and high-through put screening of sialosides. Acs Chem. Biol. 2008, 3, 567–576. [Google Scholar] [CrossRef]
- Cao, H.Z.; Li, Y.H.; Lau, K.; Muthana, S.; Yu, H.; Cheng, J.S.; Chokhawala, H.A.; Sugiarto, G.; Zhang, L.; Chen, X. Sialidase substrate specificity studies using chemoenzymatically synthesized sialosides containing C5-modified sialic acids. Org. Biomol. Chem. 2009, 7, 5137–5145. [Google Scholar] [CrossRef] [PubMed]
- Drouillard, S.; Mine, T.; Kajiwara, H.; Yamamoto, T.; Samain, E. Efficient synthesis of 6′-sialyllactose, 6,6′-disialyllactose, and 6′-KDO-lactose by metabolically engineered E. coli expressing a multifunctional sialyltransferase from the Photobacterium sp JT-ISH-224. Carbohydr. Res. 2010, 345, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Schelch, S.; Zhong, C.; Petschacher, B.; Nidetzky, B. Bacterial sialyltransferases and their use in biocatalytic cascades for sialo-oligosaccharide production. Biotechnol. Adv. 2020, 44, 107613. [Google Scholar] [CrossRef]
- Fierfort, N.; Samain, E. Genetic engineering of Escherichia coli for the economical production of sialylated oligosaccharides. J. Biotechnol. 2008, 134, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, H.; Karpel, R.; Chen, X. Chemoenzymatic synthesis of CMP-sialic acid derivatives by a one-pot two-enzyme system: Comparison of substrate flexibility of three microbial CMP-sialic acid synthetases. Bioorg. Med. Chem. 2004, 12, 6427–6435. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.J.; Datta, A.K.; Izumi, M.; Koeller, K.M.; Wong, C.H. Expression of alpha 2,8/2,9-polysialyltransferase from Escherichia coli K92—Characterization of the enzyme and its reaction products. J. Biol. Chem. 1999, 274, 35139–35146. [Google Scholar] [CrossRef]
- Willis, L.M.; Gilbert, M.; Karwaski, M.F.; Blanchard, M.C.; Wakarchuk, W.W. Characterization of the alpha-2,8-polysialyltransferase from Neisseria meningitidis with synthetic acceptors, and the development of a self-priming polysialyltransferase fusion enzyme. Glycobiology 2008, 18, 177–186. [Google Scholar] [CrossRef][Green Version]
- Gilbert, M.; Brisson, J.R.; Karwaski, M.F.; Michniewicz, J.; Cunningham, A.M.; Wu, Y.Y.; Young, N.M.; Wakarchuk, W.W. Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384—Identification of the glycosyltransferase genes, enzymatic synthesis of model compounds, and characterization of nanomole amounts by 600-MHz H-1 and C-13 NMR analysis. J. Biol. Chem. 2000, 275, 3896–3906. [Google Scholar] [CrossRef]
- Fox, K.L.; Cox, A.D.; Gilbert, M.; Wakarchuk, W.W.; Li, J.J.; Makepeace, K.; Richards, J.C.; Moxon, E.R.; Hood, D.W. Identification of a bifunctional lipopolysaccharide sialyltransferase in Haemophilus influenzae—Incorporation of disialic acid. J. Biol. Chem. 2006, 281, 40024–40032. [Google Scholar] [CrossRef]
- Gilbert, M.; Watson, D.C.; Cunningham, A.M.; Jennings, M.P.; Young, N.M.; Wakarchuk, W.W. Cloning of the lipooligosaccharide alpha-2,3-sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J. Biol. Chem. 1996, 271, 28271–28276. [Google Scholar] [CrossRef]
- Kaniuk, N.A.; Monteiro, M.A.; Parker, C.T.; Whitfield, C. Molecular diversity of the genetic loci responsible for lipopolysaccharide core oligosaccharide assembly within the genus Salmonella. Mol. Microbiol. 2002, 46, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakashizuka, M.; Terada, I. Cloning and expression of a marine bacterial beta-galactoside alpha 2,6-sialyltransferase gene from Photobacterium damsela JT0160. J. Biochem. Tokyo 1998, 123, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Takakura, Y.; Tsukamoto, H.; Yamamoto, T. Molecular cloning, expression and properties of an alpha/beta-galactoside alpha 2,3-sialyltransferase from Vibrio sp JT-FAJ-16. J. Biochem. Tokyo 2007, 142, 403–412. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Takakura, Y.; Yamamoto, T. Purification, cloning, and expression of an alpha/beta-galactoside alpha-2,3-sialyltransferase from a luminous marine bacterium, Photobacterium phosphoreum. J. Biol. Chem. 2007, 282, 29794–29802. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, G.; Lau, K.; Qu, J.Y.; Li, Y.H.; Lim, S.; Mu, S.; Ames, J.B.; Fisher, A.J.; Chen, X. A Sialyltransferase Mutant with Decreased Donor Hydrolysis and Reduced Sialidase Activities for Directly Sialylating Lewis(X). Acs Chem. Biol. 2012, 7, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, G.; Lau, K.; Li, Y.H.; Khedri, Z.; Yu, H.; Le, D.T.; Chen, X. Decreasing the sialidase activity of multifunctional Pasteurella multocida alpha 2-3-sialyltransferase 1 (PmST1) by site-directed mutagenesis. Mol. Biosyst. 2011, 7, 3021–3027. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, J.H.; Park, J.H.; Lee, N.; Kim, D.H.; Jang, K.S.; Park, I.H.; Kim, B.G. Protein engineering of alpha 2,3/2,6-sialyltransferase to improve the yield and productivity of in vitro sialyllactose synthesis. Glycobiology 2014, 24, 159–169. [Google Scholar] [CrossRef]



| Variants | α2,3-Sialyltransferase | α2,3-Sialidase |
|---|---|---|
| M144D [25] | ↓a 94% | ↓99.98% |
| R313Y [27] | ↑b 25% | ↓83% |
| R313H [27] | ↑46% | ↓60% |
| R313N [27] | ↑131% | ↓33% |
| E271F [26] | ↑15% | ↓98% |
| T265S [27] | ↑16% | Not determined |
| R313N/T265S [27] | ↑116% | ↓93% |
| R313H/T265S [27] | ↑137% | ↓90% |
| Variants | Mutations |
|---|---|
| PGM1 | M144D/R313Y |
| PGM2 | M144D/R313H |
| PGM3 | M144D/R313N |
| PGM2-1 | M144D/R313H/T265S |
| PGM2-2 | M144D/R313H/E271F |
| PGM3-1 | M144D/R313N/T265S |
| PGM3-2 | M144D/R313N/E271F |
| Variants | Lac | CMP-Neu5Ac | |
|---|---|---|---|
| Km (mM) | M144D | 19.7 ± 1.8 | 1.93 ± 0.17 |
| PMG1 | 17.4 ± 1.5 | 1.60 ± 0.14 | |
| PMG2 | 19.6 ± 1.9 | 1.97 ± 0.19 | |
| PMG3 | 11.0 ± 0.9 | 0.55 ± 0.041 | |
| PMG2-1 | 9.7 ± 0.8 | 0.91 ± 0.079 | |
| PMG2-2 | 22.7 ± 2.1 | 0.80 ± 0.064 | |
| kcat (s−1) | M144D | 25.4 ± 2.2 | 4.64 ± 0.039 |
| PMG1 | 23.0 ± 1.7 | 3.54 ± 0.032 | |
| PMG2 | 35.1 ± 2.6 | 3.33 ± 0.028 | |
| PMG3 | 13.0 ± 1.1 | 1.56 ± 0.013 | |
| PMG2-1 | 8.97 ± 0.7 | 1.70 ± 0.013 | |
| PMG2-2 | 18.7 ± 1.5 | 1.75 ± 0.012 | |
| kcat/Km (s−1 mM−1) | M144D | 1.29 ± 0.089 | 2.44 ± 0.21 |
| PMG1 | 1.32 ± 0.083 | 2.20 ± 0.18 | |
| PMG2 | 1.79 ± 0.012 | 1.69 ± 0.15 | |
| PMG3 | 1.18 ± 0.011 | 2.84 ± 0.23 | |
| PMG2-1 | 0.93 ± 0.09 | 1.88 ± 0.17 | |
| PMG2-2 | 0.82 ± 0.076 | 2.20 ± 0.19 |
| Variants | 3′-SL | |
|---|---|---|
| Km (mM) | M144D | 16.4 ± 1.57 |
| PMG1 | 14.0 ± 1.1 | |
| PMG2 | 8.6 ± 0.73 | |
| PMG3 | 5.1 ± 0.46 | |
| PMG2-1 | 26.0 ± 2.2 | |
| PMG2-2 | 32.8 ± 2.8 | |
| kcat (s−1) | M144D | 2.0 ± 0.19 |
| PMG1 | 0.25 ± 0.021 | |
| PMG2 | 0.26 ± 0.023 | |
| PMG3 | 0.18 ± 0.016 | |
| PMG2-1 | 2.25 ± 0.17 | |
| PMG2-2 | 2.05 ± 0.19 | |
| kcat/Km (s−1 mM−1) | M144D | 0.120 ± 0.011 |
| PMG1 | 0.018 ± -0.0014 | |
| PMG2 | 0.030 ± 0.0024 | |
| PMG3 | 0.035 ± 0.0029 | |
| PMG2-1 | 0.086 ± 0.0083 | |
| PMG2-2 | 0.063 ± 0.0057 |
| Gene | Primer | Sequence (5′→3′) |
|---|---|---|
| PmST1 M144D | R313YF | CAAGGGTCATCCGTACGGTGGCGAAATTAATG |
| R313YR | CATTAATTTCGCCACCGTACGGATGACCCTTG | |
| R313HF | CAAGGGTCATCCGCACGGTGGCGAAATTAATG | |
| R313HR | CATTAATTTCGCCACCGTGCGGATGACCCTTG | |
| R313NF | CAAGGGTCATCCGAACGGTGGCGAAATTAATG | |
| R313NR | CATTAATTTCGCCACCGTTCGGATGACCCTTG | |
| PMG2 (M144D/R313H)/PMG3 (M144D/R313N) | T265S F | GCAGGCAAAATTCATTTTTAGCGGCACCACCACC |
| T265S R | GGTGGTGGTGCCGCTAAAAATGAATTTTGCCTGC | |
| E271FF | ACCGGCACCACCACCTGGTTTGGCAATACCGATG | |
| E271FR | TTCGCGCACATCGGTATTGCCAAACCAGGTGGTGGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Gong, M.; Jiao, S.; Han, J.; Feng, C.; Pei, M.; Zhou, Z.; Du, Y.; Li, J. Protein Engineering of Pasteurella multocida α2,3-Sialyltransferase with Reduced α2,3-Sialidase Activity and Application in Synthesis of 3′-Sialyllactose. Catalysts 2022, 12, 579. https://doi.org/10.3390/catal12060579
Yang R, Gong M, Jiao S, Han J, Feng C, Pei M, Zhou Z, Du Y, Li J. Protein Engineering of Pasteurella multocida α2,3-Sialyltransferase with Reduced α2,3-Sialidase Activity and Application in Synthesis of 3′-Sialyllactose. Catalysts. 2022; 12(6):579. https://doi.org/10.3390/catal12060579
Chicago/Turabian StyleYang, Rui, Mengge Gong, Siming Jiao, Juntian Han, Cui Feng, Meishan Pei, Zhongkai Zhou, Yuguang Du, and Jianjun Li. 2022. "Protein Engineering of Pasteurella multocida α2,3-Sialyltransferase with Reduced α2,3-Sialidase Activity and Application in Synthesis of 3′-Sialyllactose" Catalysts 12, no. 6: 579. https://doi.org/10.3390/catal12060579
APA StyleYang, R., Gong, M., Jiao, S., Han, J., Feng, C., Pei, M., Zhou, Z., Du, Y., & Li, J. (2022). Protein Engineering of Pasteurella multocida α2,3-Sialyltransferase with Reduced α2,3-Sialidase Activity and Application in Synthesis of 3′-Sialyllactose. Catalysts, 12(6), 579. https://doi.org/10.3390/catal12060579






