Photodegradation of Pharmaceutical Pollutants: New Photocatalytic Systems Based on 3D Printed Scaffold-Supported Ag/TiO2 Nanocomposite
Abstract
:1. Introduction
2. Results and Discussion
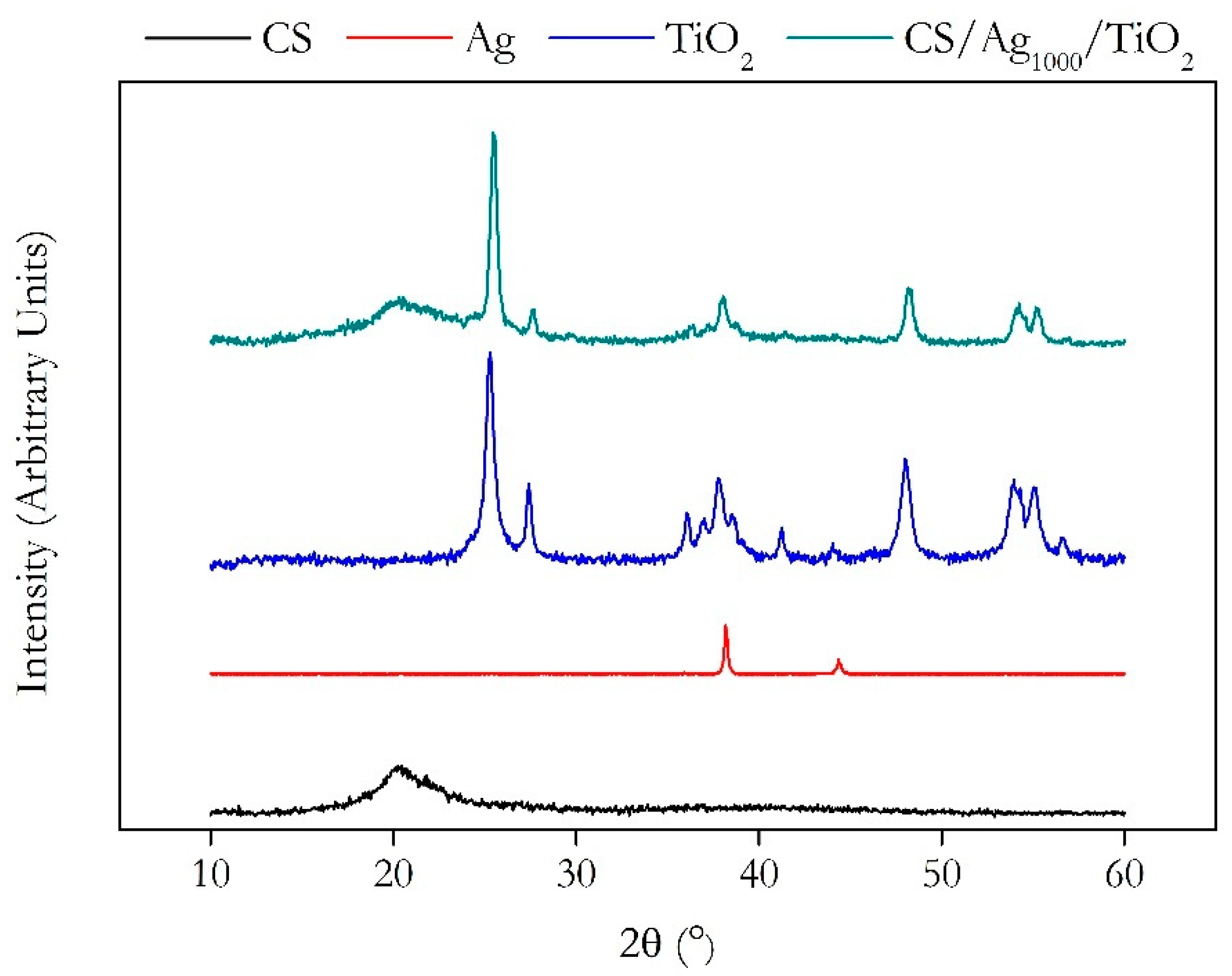
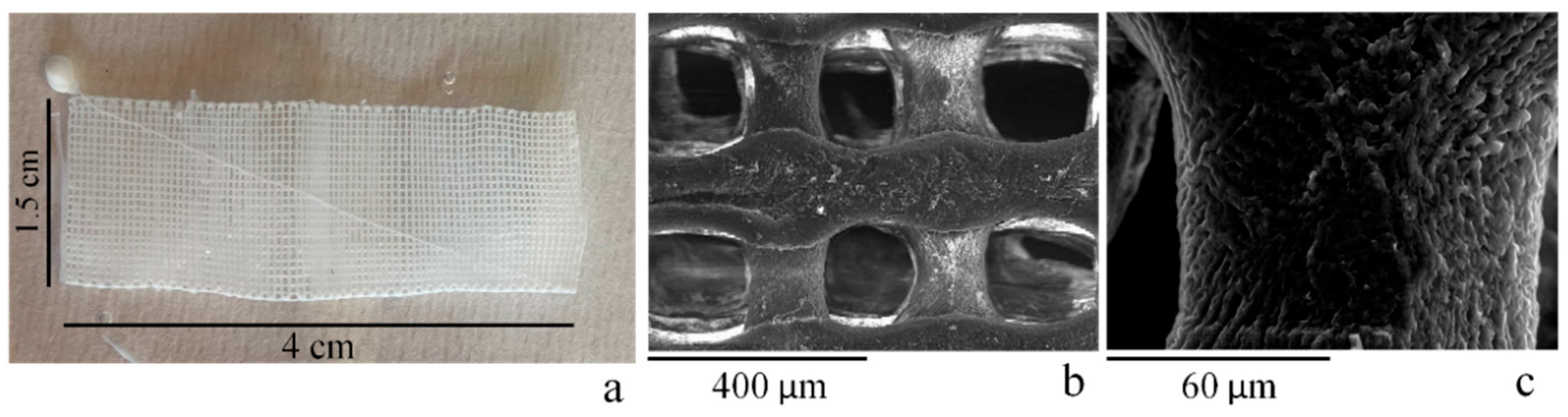
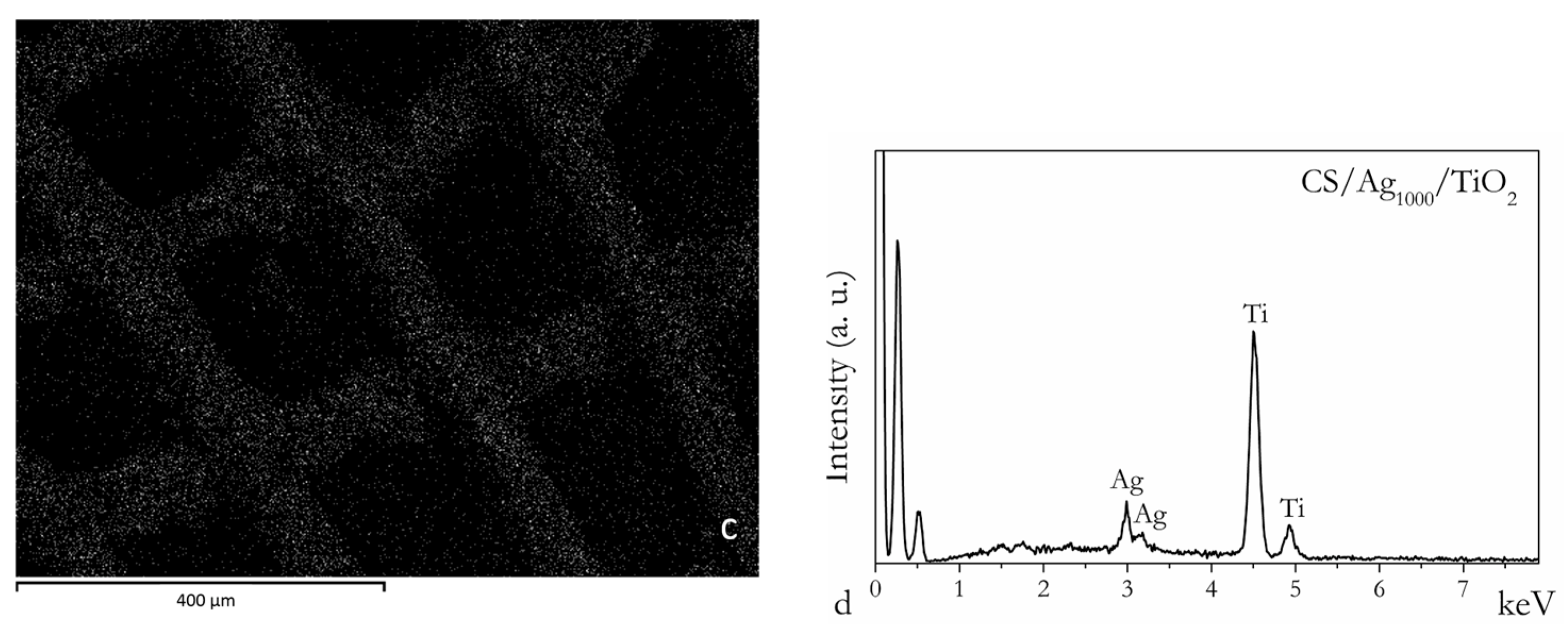
2.1. Chitosan Ag/TiO2 Scaffold Characterization
2.2. Adsorption and Photocatalytic Degradation Experiments
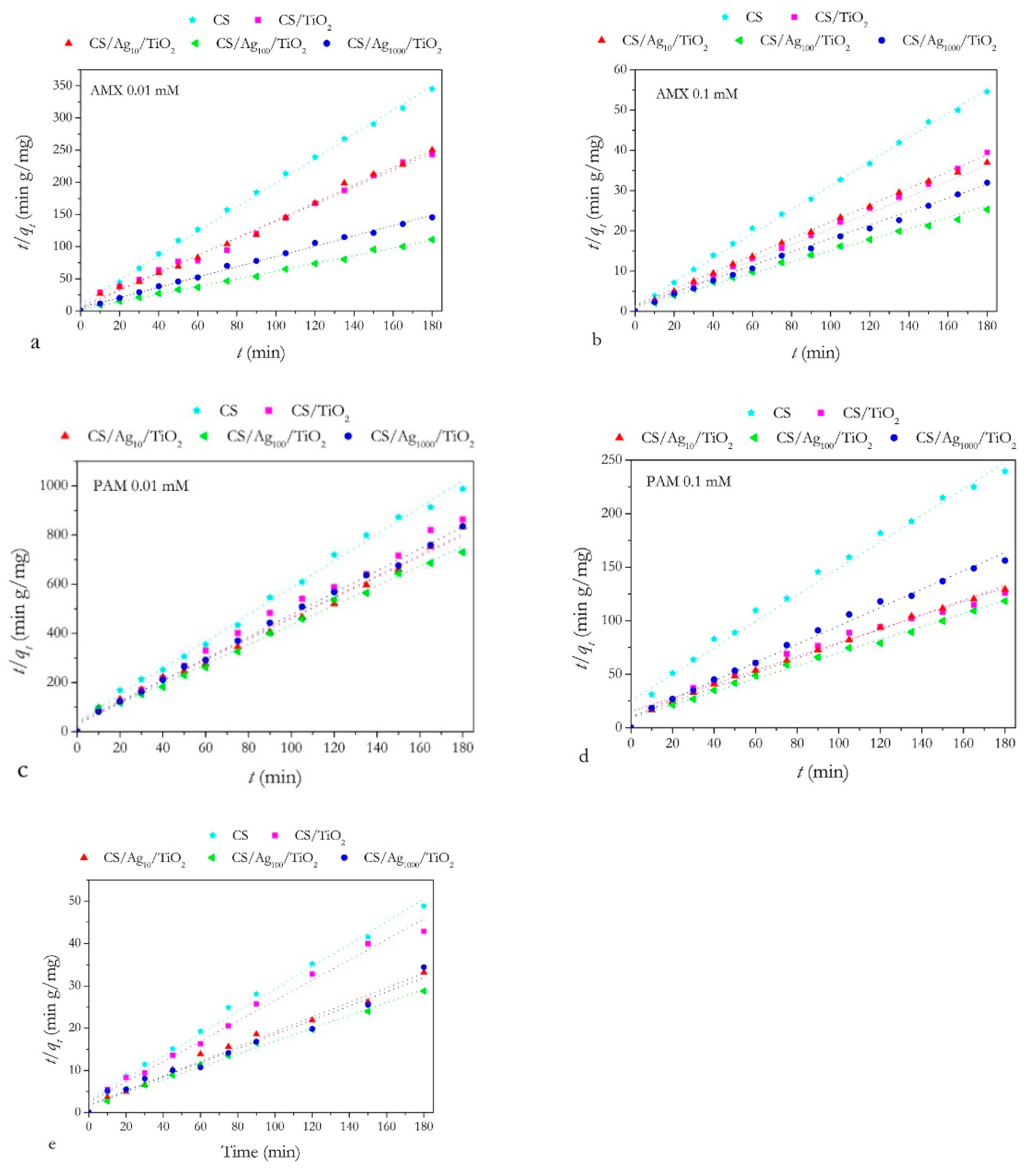
2.2.1. Equilibrium Adsorption Experiments
Adsorption Kinetics Studies
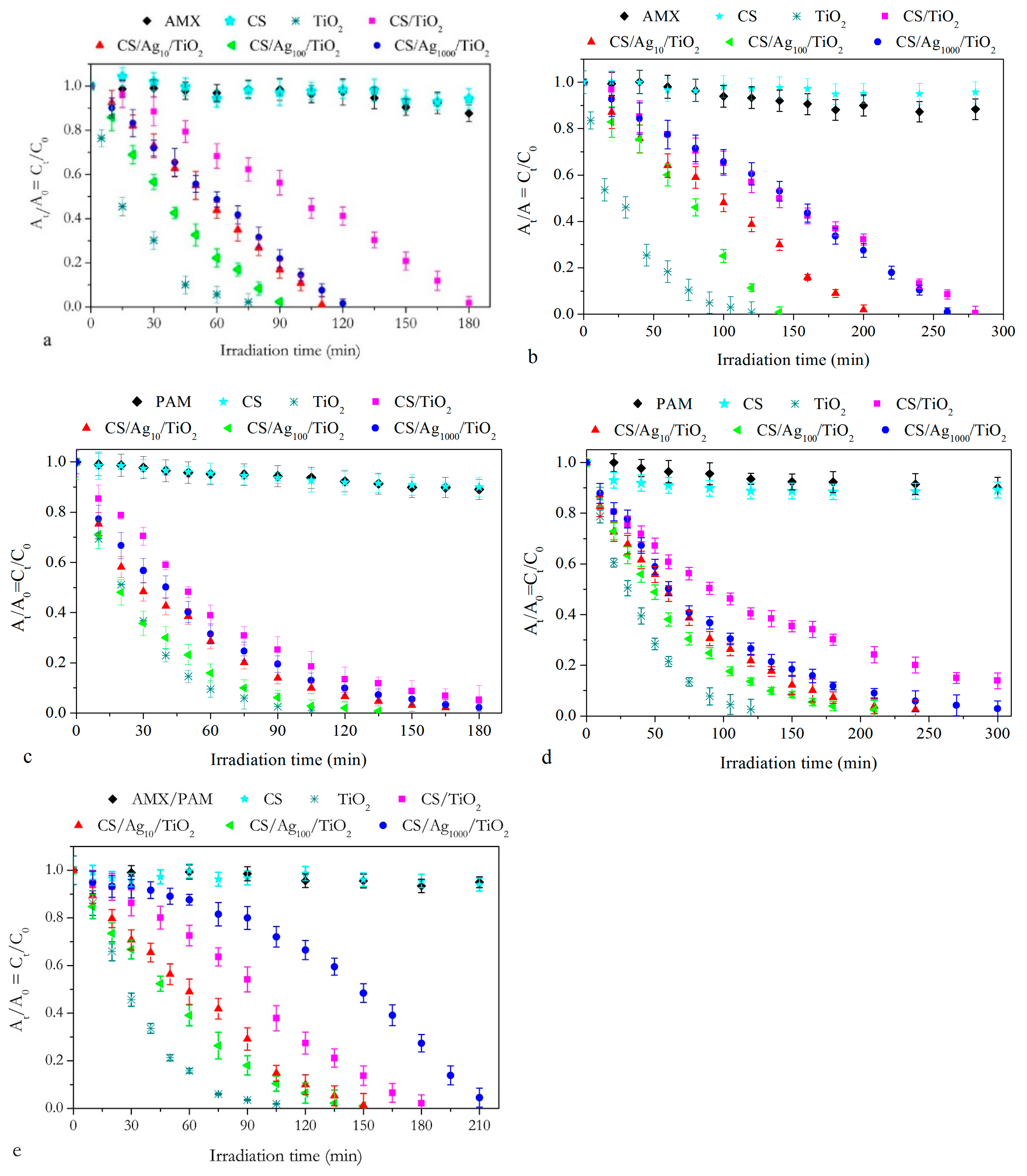
2.2.2. Photocatalytic Experiments
2.3. Liquid Chromatography–Mass Spectrometry Analysis of AMX and PAM Degradation Products
2.4. Residual Biocidal Activity of the PhCs Degradation Products
3. Materials and Methods
3.1. Chemicals
3.2. Chitosan/Ag/TiO2 Formulations
3.3. 3D Scaffold Design and Gelation Process
3.4. Characterization of Materials
3.5. Adsorption and Photocatalytic Degradation Experiments
3.5.1. Equilibrium Adsorption Experiments
3.5.2. Photocatalytic Degradation
3.6. Liquid Chromatography–Mass Spectrometry Analysis of AMX and PAM Degradation Products
3.7. Residual Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Lei, D.; Wu, D.; Xia, J.; Zhou, W.; Cui, C. Residual β-lactam antibiotics and ecotoxicity to Vibrio fischeri, Daphnia magna of pharmaceutical wastewater in the treatment process. J. Hazard. Mater. 2022, 425, 127840. [Google Scholar] [CrossRef] [PubMed]
- Giardina, S.; Castiglioni, S.; Corno, G.; Fanelli, R.; Maggi, C.; Migliore, L.; Sabbatucci, M.; Sesta, G.; Zaghi, C.; Zuccato, E. Approccio Ambientale All’antimicrobico-Resistenza; Rapporti ISTISAN 21/3; Istituto Superiore di Sanità: Rome, Italy, 2021; pp. 1123–3117. [Google Scholar]
- Tousova, Z.; Oswald, P.; Slobodnik, J.; Blaha, L.; Muz, M.; Hu, M.; Brack, W.; Krauss, M.; Di Paolo, C.; Tarcai, Z.; et al. European demonstration program on the effect-based and chemical identification and monitoring of organic pollutants in European surface waters. Sci. Total Environ. 2017, 601, 1849–1868. [Google Scholar] [CrossRef] [PubMed]
- Nippes, R.P.; Macruz, P.D.; da Silva, G.N.; Scaliante, M.H.N.O. A critical review on environmental presence of pharmaceutical drugs tested for the COVID-19 treatment. Process Saf. Environ. Prot. 2021, 152, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Igwegbe, C.A.; Aniagor, C.O.; Oba, S.N.; Yap, P.-S.; Iwuchukwu, F.U.; Liu, T.; de Souza, E.C.; Ighalo, J.O. Environmental protection by the adsorptive elimination of acetaminophen from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 104, 117–135. [Google Scholar] [CrossRef]
- The European Union. European commission implementation decision 2018/840. Establishing a watch list of substances for union-wide monitoring in the field of water policy pursuant to directive 2008/105/EC of the European Parliament and of the council and repealing commission implementing decision (EU) 2015/495. Off. J. Eur. Union 2018, 61, 9–12. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Anubha, M.; Jayashree, S. Degradation of toxic agrochemicals and pharmaceutical pollutants: Effective and alternative approaches toward photocatalysis. Environ. Pollut. 2022, 298, 118844. [Google Scholar] [CrossRef]
- Hiller, C.X.; Hübner, U.; Fajnorova, S.; Schwartz, T.; Drewes, J.E. Antibiotic microbial resistance (AMR) removal efficiencies by conventional and advanced wastewater treatment processes: A review. Sci. Total Environ. 2019, 685, 596–608. [Google Scholar] [CrossRef]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-002733-6. Available online: https://apps.who.int/iris/bitstream/handle/10665/341666/9789240027336-eng.pdf (accessed on 14 March 2022).
- Bassetti, S.; Tschudin-Sutter, S.; Egli, A.; Osthoff, M. Optimizing antibiotic therapies to reduce the risk of bacterial resistance. Eur. J. Intern. Med. 2022, 99, 7–12. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Teymoorian, T.; Teymourian, T.; Kowsari, E.; Ramakrishna, S. Direct and indirect effects of SARS-CoV-2 on wastewater treatment. J. Water Process Eng. 2021, 42, 102193. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ning, Q.; Dong, J.; Brooks, B.W.; You, J. Predicting mixture toxicity and antibiotic resistance of fluoroquinolones and their photodegradation products in Escherichia coli. Environ. Pollut. 2020, 262, 114275. [Google Scholar] [CrossRef] [PubMed]
- Nason, S.L.; Lin, E.; Eitzer, B.; Koelmel, J.; Peccia, J. Changes in sewage sludge chemical signatures during a COVID-19 community lockdown, part 1: Traffic, drugs, mental health, and disinfectants. Environ. Toxicol. Chem. 2021, 41, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, S. Detoxification and bioaugmentation potential for acetaminophen and its derivatives using Ensifer sp. isolated from activated sludge. Chemosphere 2020, 260, 127532. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamonti, L.; Gentili, S.; Acquotti, D.; Tegoni, M.; Lottici, P.P.; Graiff, C. Toxic metal sequential sequestration in water using new amido-aminoacid ligand as a model for the interaction with polyamidoamines. J. Hazard. Mater. 2021, 410, 124585. [Google Scholar] [CrossRef]
- Sescu, A.M.; Favier, L.; Lutic, D.; Soto-Donoso, N.; Ciobanu, G.; Harja, M. TiO2 doped with noble metals as an efficient solution for the photodegradation of hazardous organic water pollutants at ambient conditions. Water 2021, 13, 19. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Favier, L.; Simion, A.I.; Matei, E.; Grigoras, C.-G.; Kadmi, I.; Bouzaza, A. Photocatalytic oxidation of a hazardous phenolic compound over TiO2 in a batch system. Environ. Eng. Manag. J. 2016, 15, 1059–1067. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Comparison of different advanced oxidation processes for treatment of antibiotic aqueous solution. Desalination 2010, 256, 43–47. [Google Scholar] [CrossRef]
- Amor, C.; Marchão, L.; Lucas, M.S.; Peres, J.A. Application of advanced oxidation processes for the treatment of recalcitrant agro-industrial wastewater: A review. Water 2019, 11, 205. [Google Scholar] [CrossRef] [Green Version]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.N.; Bhopate, D.P.; Kondalkar, V.V.; Majhi, S.M.; Bathula, C.D.; Tran, A.V.; Lee, S.W. Facile synthesis of Ag-ZnO core–shell nanostructures with enhanced photocatalytic activity. J. Ind. Eng. Chem. 2018, 61, 78–86. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Vo, T.-T.T.; Do, H.H.; Le, V.T.; Nguyen, T.D.; Vo, T.K.; Nguyen, B.-S.; Nguyen, T.T.; Phung, T.K.; Tran, V.A. Ag@ ZnO porous nanoparticle wrapped by rGO for the effective CO2 electrochemical reduction. Chem. Eng. Sci. 2021, 232, 116381. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Tran, K.N.T.; Van Tan, L.; Tran, V.A.; Doan, V.D.; Lee, T.; Nguyen, T.D. Microwave-assisted solvothermal synthesis of bimetallic metal-organic framework for efficient photodegradation of organic dyes. Mater. Chem. Phys. 2021, 272, 125040. [Google Scholar] [CrossRef]
- Ounnar, A.; Favier, L.; Bouzaza, A.; Bentahar, F.; Trari, M. Kinetic study of spiramycin removal from aqueous solution using heterogeneous photocatalysis. Kinet. Catal. 2016, 57, 200–206. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Calvete, M.J.; Piccirillo, G.; Vinagreiro, C.S.; Pereira, M.M. Hybrid materials for heterogeneous photocatalytic degradation of antibiotics. Coord. Chem. Rev. 2019, 395, 63–85. [Google Scholar] [CrossRef]
- Romão, J.; Barata, D.; Ribeiro, N.; Habibovic, P.; Fernandes, H.; Mul, G. High throughput screening of photocatalytic conversion of pharmaceutical contaminants in water. Environ. Pollut. 2017, 220, 1199–1207. [Google Scholar] [CrossRef]
- Bergamonti, L.; Alfieri, I.; Lorenzi, A.; Montenero, A.; Predieri, G.; Di Maggio, R.; Girardi, F.; Lazzarini, L.; Lottici, P.P. Characterization and photocatalytic activity of TiO2 by sol-gel in acid and basic environments. J. Sol-Gel Sci. Technol. 2015, 73, 91–102. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269. [Google Scholar] [CrossRef]
- Herrmann, J.M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–pyrolytic method for TiO2 polymorphs production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Sun, X.; Yan, X.; Su, H.; Sun, L.; Zhao, L.; Shi, J.; Wang, Z.; Niu, J.; Qian, H.; Duan, E. Non-stacked γ-Fe2O3/C@TiO2 double-layer hollow nanoparticles for enhanced photocatalytic applications under visible light. Nanomaterials 2022, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Kokorin, A.I.; Sviridova, T.V.; Konstantinova, E.A.; Sviridov, D.V.; Bahnemann, D.W. Dynamics of photogenerated charge carriers in TiO2/MoO3, TiO2/WO3 and TiO2/V2O5 photocatalysts with mosaic structure. Catalysts 2020, 10, 1022. [Google Scholar] [CrossRef]
- Zarzuela, R.; Moreno-Garrido, I.; Gil, M.A.; Mosquera, M.J. Effects of surface functionalization with alkylalkoxysilanes on the structure, visible light photoactivity and biocidal performance of Ag-TiO2 nanoparticles. Powder Technol. 2021, 383, 381–395. [Google Scholar] [CrossRef]
- Fornasini, L.; Bergamonti, L.; Bondioli, F.; Bersani, D.; Lazzarini, L.; Paz, Y.; Lottici, P.P. Photocatalytic N-doped TiO2 for self-cleaning of limestones. Eur. Phys. J. Plus 2019, 134, 539. [Google Scholar] [CrossRef]
- Luna, M.; Mosquera, M.J.; Vidal, H.; Gatica, J.M. Au-TiO2/SiO2 photocatalysts for building materials: Self-cleaning and de-polluting performance. Build. Environ. 2019, 164, 106347. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Torkian, N.; Bahrami, A.; Hosseini-Abari, A.; Momeni, M.M.; Abdolkarimi-Mahabadi, M.; Bayat, A.; Hajipour, P.; Rourani, H.A.; Abbasi, M.S.; Torkian, S.; et al. Synthesis and characterization of Ag-ion-exchanged zeolite/TiO2 nanocomposites for antibacterial applications and photocatalytic degradation of antibiotics. Environ. Res. 2021, 207, 112157. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Kockler, J.; Motti, C.A.; Glass, B.D.; Oelgemöller, M. Titanium dioxide/zeolite integrated photocatalytic adsorbents for the degradation of amoxicillin. Appl. Catal. B Environ. 2015, 166, 45–55. [Google Scholar] [CrossRef]
- Lee, J.; Lee, M. Improved light harvest in diffraction grating-embedded TiO2 nanoparticle film. Appl. Phys. A 2017, 123, 737. [Google Scholar] [CrossRef]
- Bergamonti, L.; Bergonzi, C.; Graiff, C.; Lottici, P.P.; Bettini, R.; Elviri, L. 3D printed chitosan scaffolds: A new TiO2 support for the photocatalytic degradation of amoxicillin in water. Water Res. 2019, 163, 114841. [Google Scholar] [CrossRef] [PubMed]
- Elviri, L.; Bianchera, A.; Bergonzi, C.; Bettini, R. Controlled local drug delivery strategies from chitosan hydrogels for wound healing. Expert Opin. Drug Deliv. 2017, 14, 897–908. [Google Scholar] [CrossRef]
- Galli, C.; Parisi, L.; Elviri, L.; Bianchera, A.; Smerieri, A.; Lagonegro, P.; Lumetti, S.; Manfredi, E.; Bettini, R.; Macaluso, G.M. Chitosan scaffold modified with D-(+) raffinose and enriched with thiol-modified gelatin for improved osteoblast adhesion. Biomed. Mater. 2016, 11, 015004. [Google Scholar] [CrossRef] [Green Version]
- Elviri, L.; Asadzadeh, M.; Cucinelli, R.; Bianchera, A.; Bettini, R. Macroporous chitosan hydrogels: Effects of sulfur on the loading and release behaviour of amino acid-based compounds. Carbohydr. Polym. 2015, 132, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, C.; Remaggi, G.; Graiff, C.; Bergamonti, L.; Potenza, M.; Ossiprandi, M.C.; Zanotti, I.; Bernini, F.; Bettini, R.; Elviri, L. Three-dimensional (3D) printed silver nanoparticles/alginate/nanocrystalline cellulose hydrogels: Study of the antimicrobial and cytotoxicity efficacy. Nanomaterials 2020, 10, 844. [Google Scholar] [CrossRef]
- Sayed, M.; El-Maghraby, H.F.; Bondioli, F.; Naga, S.M. 3D carboxymethyl cellulose/hydroxyapatite (CMC/HA) scaffold composites based on recycled eggshell. J. Appl. Pharm. Sci. 2018, 8, 23–30. [Google Scholar] [CrossRef]
- Elviri, L.; Foresti, R.; Bergonzi, C.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R. Highly defined 3D printed chitosan scaffolds featuring improved cell growth. Biomed. Mater. 2017, 12, 045009. [Google Scholar] [CrossRef]
- Sciancalepore, C.; Moroni, F.; Messori, M.; Bondioli, F. Acrylate-based silver nanocomposite by simultaneous polymerization-reduction approach via 3D stereolithography. Compos. Commun. 2017, 6, 11–16. [Google Scholar] [CrossRef]
- Amin, K.A.M. Reinforced materials based on chitosan, TiO2 and Ag composites. Polymers 2012, 4, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Sobana, N.; Muruganadham, M.; Swaminathan, M. Nano-Ag particles doped TiO2 for efficient photodegradation of direct azo dyes. J. Mol. Catal. A Chem. 2006, 258, 124–132. [Google Scholar] [CrossRef]
- Taspika, M.; Desiati, R.D.; Mahardika, M.; Sugiarti, E.; Abral, H. Influence of TiO2/Ag particles on the properties of chitosan film. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 015017. [Google Scholar] [CrossRef]
- Jbeli, A.; Hamden, Z.; Bouattour, S.; Ferraria, A.; Conceição, D.; Ferreira, L.V.; Chehimi, M.; Rego, A.B.D.; Vilar, M.R.; Boufi, S. Chitosan-Ag-TiO2 films: An effective photocatalyst under visible light. Carbohydr. Polym. 2018, 199, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Mankad, V.; Gupta, S.K.; Jha, P.K. Size distribution of silver nanoparticles: UV-visible spectroscopic assessment. Nanosci. Nanotechnol. Lett. 2012, 4, 30–34. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- Amirjani, A.; Firouzi, F.; Haghshenas, D.F. Predicting the size of silver nanoparticles from their optical properties. Plasmonics 2020, 15, 1077–1082. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Hernández-Iturriaga, M.; Mota-Morales, J.D.; Vázquez-Lepe, M.; Kovalenko, Y.; Sanchez, I.C.; Luna-Bárcenas, G. Chitosan/silver nanocomposites: Synergistic antibacterial action of silver nanoparticles and silver ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Semwal, A.; Singh, R.; Dutta, P.K. Chitosan: A promising substrate for pharmaceuticals. J. Chitin Chitosan Sci. 2013, 1, 87–102. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Adriano, W.S.; Veredas, V.; Santana, C.C.; Gonçalves, L.B. Adsorption of amoxicillin on chitosan beads: Kinetics, equilibrium and validation of finite bath models. Biochem. Eng. J. 2005, 27, 132–137. [Google Scholar] [CrossRef]
- Lotfollahzadeh, R.; Yari, M.; Sedaghat, S.; Delbari, A.S. Biosynthesis and characterization of silver nanoparticles for the removal of amoxicillin from aqueous solutions using Oenothera biennis water extract. J. Nanostruct. Chem. 2021, 11, 693–706. [Google Scholar] [CrossRef]
- Wang, A.; Zhu, Q.; Xing, Z. Multifunctional quaternized chitosan@ surface plasmon resonance Ag/N-TiO2 core-shell microsphere for synergistic adsorption-photothermal catalysis degradation of low-temperature wastewater and bacteriostasis under visible light. Chem. Eng. J. 2020, 393, 124781. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Tran, H.N.; Juang, R.-S.; Dat, N.D.; Tomul, F.; Ivanets, A.; Woo, S.H.; Hosseini-Bandegharaei, A.; Nguyen, V.P.; Chao, H.-P. Adsorption process and mechanism of acetaminophen onto commercial activated carbon. J. Environ. Chem. Eng. 2020, 8, 104408. [Google Scholar] [CrossRef]
- Rahmanifar, B.; Dehaghi, S.M. Removal of organochlorine pesticides by chitosan loaded with silver oxide nanoparticles from water. Clean Technol. Environ. 2014, 16, 1781–1786. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/desorption of Cd (II), Cu (II) and Pb (II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.S.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef] [Green Version]
- Tran, V.A.; Vo, T.T.T.; Nguyen, P.A.; Don, T.N.; Vasseghian, Y.; Phan, H.; Lee, S.W. Experimental and computational investigation of a green Knoevenagel condensation catalyzed by zeolitic imidazolate framework-8. Environ. Res. 2022, 204, 112364. [Google Scholar] [CrossRef] [PubMed]
- Bergamonti, L.; Potenza, M.; Poshtiri, A.H.; Lorenzi, A.; Sanangelantoni, A.M.; Lazzarini, L.; Lottici, P.P.; Graiff, C. Ag-functionalized nanocrystalline cellulose for paper preservation and strengthening. Carbohydr. Polym. 2020, 231, 115773. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Herrmann, J.M. Influence of metallic silver and of platinum-silver bimetallic deposits on the photocatalytic activity of titania (anatase and rutile) in organic and aqueous media. J. Photochem. Photobiol. A 1998, 113, 181–188. [Google Scholar] [CrossRef]
- Guerra, M.H.; Alberola, I.O.; Rodriguez, S.M.; López, A.A.; Merino, A.A.; Alonso, J.Q. Oxidation mechanisms of amoxicillin and paracetamol in the photo-Fenton solar process. Water Res. 2019, 156, 232–240. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, D.; Wu, J. Photodegradation of Norfloxacin in aqueous solution containing algae. J. Environ. Sci. 2012, 24, 743–749. [Google Scholar] [CrossRef]
- Ilisz, I.; László, Z.; Dombi, A. Investigation of the photodecomposition of phenol in near-UV-irradiated aqueous TiO2 suspensions. I: Effect of charge-trapping species on the degradation kinetics. Appl. Catal. A Gen. 1999, 180, 25–33. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Q.L.; Zhou, X.J.; Cao, H.O.; Du, J.S.; Yin, R.L.; Ren, N.Q. Enhanced amoxicillin treatment using the electro-peroxone process: Key factors and degradation mechanism. RSC Adv. 2015, 5, 52695–52702. [Google Scholar] [CrossRef]
- Moctezuma, E.; Leyva, E.; Aguilar, C.A.; Luna, R.A.; Montalvo, C. Photocatalytic degradation of paracetamol: Intermediates and total reaction mechanism. J. Hazard. Mater. 2012, 243, 130–138. [Google Scholar] [CrossRef]
- NCCLS. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard, 8th ed.; NCCLS Document M2-A8; NCCLS: Wayne, PA, USA, 2003; ISBN 1-56238-485-6. [Google Scholar]



| AMX | ||||||
| [C] mM | CS | CS/TiO2 | CS/Ag10/TiO2 | CS/Ag100/TiO2 | CS/Ag1000/TiO2 | |
| 0.01 0.1 | Ads (%) | 10.8 (9) | 12.5 (8) | 14.4 (5) | 18.4 (3) (9) | 16.4 (8) |
| 0.1 | 7.6 (4) | 8.9 (6) | 11.0 (3) | 14.3 (7) | 10.4 (6) | |
| 0.01 0.1 | qe (mg/g) | 0.52 (3) | 0.66 (1) | 0.73 (1) | 1.62 (2) | 1.24 (2) |
| 0.1 | 3.3 (6) | 4.6 (4) | 4.9 (4) | 7.2 (5) | 5.7 (3) | |
| 0.01 | k2 (g/(mg min)) | 0.35 (2) | 0.31 (3) | 0.25 (6) | 0.17 (2) | 0.13 (1) |
| 0.1 | 0.069 (7) | 0.13 (5) | 0.073 (3) | 0.015 (4) | 0.031 (4) | |
| PAM | ||||||
| [C] mM | CS | CS/TiO2 | CS/Ag10/TiO2 | CS/Ag100/TiO2 | CS/Ag1000/TiO2 | |
| 0.01 0.1 | Ads (%) | 7.3 (5) | 8.3 (4) | 8.3 (4) | 9.8 (1) | 8.5 (3) |
| 0.1 | 3.6 (4) | 6.1 (7) | 5.8 (4) | 6.4 (1) | 5.7 (4) | |
| 0.01 0.1 | qe (mg/g) | 0.18 (4) | 0.22 (1) | 0.23 (2) | 0.25 (4) | 0.21 (5) |
| 0.1 | 0.78 (2) | 1.42 (9) | 1.39 (4) | 1.52 (8) | 1.15 (9) | |
| 0.01 | k2 (g/(mg min)) | 0.75 (8) | 0.68 (5) | 0.66 (7) | 0.41(6) | 0.64 (6) |
| 0.1 | 0.077 (3) | 0.038 (5) | 0.043 (2) | 0.035 (5) | 0.065 (3) | |
| AMX/PAM | ||||||
| [C] mM | CS | CS/TiO2 | CS/Ag10/TiO2 | CS/Ag100/TiO2 | CS/Ag1000/TiO2 | |
| 0.1 | Ads (%) | 5.6 (2) | 6.4 (3) | 8.2 (1) | 9.1 (2) | 8.7 (5) |
| 0.1 | qe (mg/g) | 3.7 (4) | 4.2 (3) | 5.4 (1) | 6.3 (3) | 5.2 (6) |
| 0.1 | k2 (g/(mg min)) | 0.026 (2) | 0.023 (2) | 0.019 (2) | 0.015 (3) | 0.021 (2) |
| Amoxicillin | |||||
| AMX:TiO2 | kapp (min−1) | k0 (min−1) | t1/2 (min) | r2 | |
| PFOpht | PZOpht | ||||
| TiO2 | 1/100 1/10 | 0.049 (1) 0.036 (2) | - - | 14.2 (0.3) 19 (1) | 0.99 0.98 |
| CS/TiO2 | 1/100 1/10 | - - | 0.0052 (3) 0.0034 (1) | 96 (6) 147 (5) | 0.98 0.99 |
| CS/Ag10/TiO2 | 1/100 1/10 | - - | 0.0091 (5) 0.0051 (2) | 55 (3) 98 (4) | 0.99 0.99 |
| CS/Ag100/TiO2 | 1/100 1/10 | - - | 0.0132 (6) 0.0056 (2) | 38 (2) 89 (3) | 0.97 0.96 |
| CS/Ag1000/TiO2 | 1/100 1/10 | - - | 0.0085 (4) 0.0028 (1) | 59 (3) 178 (6) | 0.99 0.96 |
| Paracetamol | |||||
| PAM:TiO2 | kapp (min−1) | k0 (min−1) | t1/2 (min) | r2 | |
| PFOpht | PZOpht | ||||
| TiO2 | 1/100 1/10 | 0.039 (6) 0.028 (4) | - - | 18 (3) 25 (3) | 0.99 0.99 |
| CS/TiO2 | 1/100 1/10 | 0.016 (2) 0.007 (1) | - - | 43 (5) 99 (10) | 0.99 0.99 |
| CS/Ag10/TiO2 | 1/100 1/10 | 0.023 (1) 0.014 (2) | - - | 30 (1) 50 (7) | 0.98 0.98 |
| CS/Ag100/TiO2 | 1/100 1/10 | 0.033 (4) 0.017 (2) | - - | 21 (3) 41 (5) | 0.98 0.99 |
| CS/Ag1000/TiO2 | 1/100 1/10 | 0.019 (1) 0.012 (2) | - - | 36 (2) 58 (9) | 0.99 0.99 |
| Amoxicillin/Paracetamol | |||||
| AMX/PAM:TiO2 | kapp (min−1) | k0 (min−1) | t1/2 (min) | r2 | |
| PFOpht | PZOpht | ||||
| TiO2 | 1/10 | 0.035 (1) | - | 20 (1) | 0.98 |
| CS/TiO2 | 1/10 | - | 0.0056 (1) | 89 (1) | 0.98 |
| CS/Ag10/TiO2 | 1/10 | - | 0.0082 (1) 0.0030 (1) | 61 (1) 167 (3) | 0.99 0.99 |
| CS/Ag100/TiO2 | 1/10 | - | 0.0103 (3) 0.0034 (4) | 49 (1) 147 (8) | 0.99 0.92 |
| CS/Ag1000/TiO2 | 1/10 | - | 0.0025 (1) 0.0071 (2) | 200 (4) 70 (1) | 0.96 0.99 |
| Compound | Formula | Molecular Mass | m/z |
|---|---|---|---|
| amoxicillin (AMX) | C16H19N3O5S | 365 | 366 |
| A1 (dihydroxylated AMX) | C16H19N3O6S | 381 | 382 |
| A2 (derivative of hydroxylated AMX) | C15H18N2O7S | 382 | 383 |
| A3 (penicilloic acid) | C16H21N3O6S | 383 | 384 |
| A4 (derivative of penicilloic acid) | C15H19N3O5S | 353 | 354 |
| A5 (derivative of penicilloic acid) | C15H21N3O4S | 339 | 340 |
| A6 (derivative of hydroxylated AMX) | C16H19N3O8S | 411 | 412 |
| A7 (tetrahydroxylated AMX) | C16H17N3O9S | 427 | 428 |
| A8 (derivative from destruction of lactamic bond) | C8H10N2O4S | 230 | 231 |
| A9 (derivative of penicilloic acid) | C6H9NO2S | 159 | 160 |
| paracetamol (PAR) | C8H9NO2 | 151 | 152 |
| P1 (hydroquinone) | C6H6O2 | 110 | 111 |
| P2 (1,2,4-trihydroxybenzene) | C6H6O3 | 126 | 127 |
| P3 (benzoquinone) | C6H4O2 | 108 | 109 |
| P4 (p-nitrophenol) | C6H5NO3 | 139 | 140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergamonti, L.; Graiff, C.; Bergonzi, C.; Potenza, M.; Reverberi, C.; Ossiprandi, M.C.; Lottici, P.P.; Bettini, R.; Elviri, L. Photodegradation of Pharmaceutical Pollutants: New Photocatalytic Systems Based on 3D Printed Scaffold-Supported Ag/TiO2 Nanocomposite. Catalysts 2022, 12, 580. https://doi.org/10.3390/catal12060580
Bergamonti L, Graiff C, Bergonzi C, Potenza M, Reverberi C, Ossiprandi MC, Lottici PP, Bettini R, Elviri L. Photodegradation of Pharmaceutical Pollutants: New Photocatalytic Systems Based on 3D Printed Scaffold-Supported Ag/TiO2 Nanocomposite. Catalysts. 2022; 12(6):580. https://doi.org/10.3390/catal12060580
Chicago/Turabian StyleBergamonti, Laura, Claudia Graiff, Carlo Bergonzi, Marianna Potenza, Cinzia Reverberi, Maria Cristina Ossiprandi, Pier Paolo Lottici, Ruggero Bettini, and Lisa Elviri. 2022. "Photodegradation of Pharmaceutical Pollutants: New Photocatalytic Systems Based on 3D Printed Scaffold-Supported Ag/TiO2 Nanocomposite" Catalysts 12, no. 6: 580. https://doi.org/10.3390/catal12060580
APA StyleBergamonti, L., Graiff, C., Bergonzi, C., Potenza, M., Reverberi, C., Ossiprandi, M. C., Lottici, P. P., Bettini, R., & Elviri, L. (2022). Photodegradation of Pharmaceutical Pollutants: New Photocatalytic Systems Based on 3D Printed Scaffold-Supported Ag/TiO2 Nanocomposite. Catalysts, 12(6), 580. https://doi.org/10.3390/catal12060580












