Abstract
Various reaction mechanisms for the catalytic degradation of formaldehyde (HCHO) remain to be debated. Density functional theory (DFT) was applied to investigate whether the catalytic oxidation of HCHO on pristine Co3O4 (110) surface follows the Mars-van Krevelen (MvK) mechanism or the Langmuir–Hinshelwood (L-H) mechanism. Firstly, HCHO and O2 co-adsorb on the surface and two H atoms from HCHO are peculiarly prone to transfer to O2, forming CO and HOOH. For the MvK mechanism, CO2 is generated through CO grabbing a lattice oxygen. Meanwhile, the O–O bond of HOOH is broken into two OH groups. One OH fills the oxygen vacancy and its H atom moves to another OH group for H2O formation. For the L-H mechanism, CO directly obtains one OH group to generate COOH. Subsequently, the H atom of COOH transfers to another OH group along with CO2 and H2O generation. Both two mechanisms exhibit a similar maximum activation barrier. The lattice oxygen in the MvK mechanism and the surface-absorbed OH group in the L-H mechanism are the key reactive oxygen species. The small difference in energetic span further suggests that the catalytic cycle through the two mechanisms is feasible. This theoretical study provides new insight into the catalytic reaction path of HCHO oxidation on pristine Co3O4 surface.
1. Introduction
Formaldehyde (HCHO) is one of the most concerning indoor pollutants leading to hazardous effects on human health [1,2], and significant efforts have been made to settle indoor HCHO emissions. Room-temperature catalytic oxidation represents an attractive technology for complete conversion of HCHO to H2O and CO2 without the formation of harmful by-products or secondary pollutants, thus making it the most promising HCHO removal technique compared with conventional adsorption and photo-catalytic oxidation [3,4,5,6,7]. Supported noble metals (e.g., Pt [8], Pd [9], Au [10], and Ag [11]) are the most effective catalysts for HCHO catalytic oxidation at room temperature. Nevertheless, the high price and scarcity of noble metals may restrain their wide application in the long term. Transition metal oxides, such as MnOx [6], Co3O4 [12], CeO2 [13], TiO2 [14], and their composites [15,16,17], exhibit comparable catalytic performance and are cost-effective for low temperature HCHO oxidation. Among these catalysts, Co3O4-based catalysts have recently been attractive for low-temperature catalytic applications due to their strong oxidative capacity, high oxygen redox capability, and facile generation of reactive oxygen species (ROS) [18].
The catalytic path of HCHO oxidation can follow three mechanisms, including the Langmuir–Hinshelwood (L-H) mechanism, Eley–Rideal (E-R) mechanism, and Mars-van Krevelen (MvK) mechanism. Numerous experimental studies proved that the reaction path of HCHO oxidation on Co3O4-based catalysts mainly follows the L-H mechanism [19,20,21,22]. These studies utilized oxygen vacancy (OV) engineering or load noble metals to enhance the catalytic activity of Co3O4-based catalysts at room temperature. Surface-adsorbed ROS obtained by dissociating O2 from oxygen vacancies or noble metal sites are generally considered to be the most important ROS. For the L-H mechanism, the reaction starts from HCHO and O2 co-adsorption on surface to form carbonaceous intermediates and ROS, and then the adsorbed carbonaceous intermediates are oxidized by ROS into CO2 and H2O. Different from the L-H mechanism, the details of the E-R mechanism are that pre-adsorbed O2 first forms ROS, which then makes contact with HCHO and further oxidizes it into CO2 and H2O. However, the MvK mechanism that the catalytic oxidation of HCHO may follow is usually less frequently mentioned [11]. For the MvK mechanism, lattice oxygen (Olatt) oxidizes HCHO into CO2, accompanied by the formation of an oxygen vacancy. Then, adsorbed O2 dissociates on the oxygen vacancy into two active O atoms, one fills the O vacancy and the other combines with H atoms derived from HCHO for H2O generation. Thus, the biggest difference between the MvK mechanism and the L-H mechanism is whether surface adsorbed ROS or Olatt participates in HCHO oxidation, yet the possibility that these two routes are jointly involved in the catalytic oxidation of HCHO cannot be ignored.
In order to investigate the catalytic oxidation mechanism of HCHO on the Co3O4 surface, the selection of crystal facets with high reactivity is crucial. It is generally believed that increasing the exposure of the (110) surface facilitates the catalytic oxidation of various gaseous pollutants on Co3O4 [23,24,25], since the Co3O4 (110) surface possesses a large amount of Co3+ active sites, which can efficiently chemisorb reactants. Ma et al. indicated that mesoporous 2D-Co3O4 exhibited preferable HCHO oxidation activity compared to Co3O4 nanosheets, which can be explained by the dominance of the Co3+-rich (110) surface in 2D-Co3O4 [19]. Similarly, Bai et al. compared nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts and found that the high HCHO oxidation activity of 3D-Co3O4 was attributed to greater (110) surface exposure [12]. The theoretical study by Deng et al. suggested that the catalytic oxidation of HCHO followed the MvK mechanism on the perfect Co3O4 (110) surface, while on the Co3O4 (110) surface with oxygen vacancies it followed the L-H mechanism [26]. However, whether the HCHO oxidation on the pristine Co3O4 (110) surface follows the L-H mechanism remains to be further explored.
Herein, this work focuses on comparing the MvK mechanism and L-H mechanism of HCHO oxidation on the pristine Co3O4 (110) surface through density functional theory (DFT). The activation barriers, reaction energy, and maximum energetic span (δE) of elementary reactions for possible reaction paths have been examined. The results help to broaden the fundamental understanding of reaction mechanisms of HCHO oxidation over Co3O4-based catalysts.
2. Results and Discussion
2.1. Possible Adsorption Species
According to the experimental studies of the HCHO oxidation over Co3O4-based catalysts, a complete catalytic oxidation process is usually accompanied by the formation of intermediates such as CH2O2, HCOOH, and HCOO [17,19,27]. These intermediates are gradually oxidized by ROS (including O, OH,) and finally generate CO2 and H2O [20,28,29]. Therefore, there indeed exists different kinds of possible species in the reaction of HCHO oxidation to CO2 and H2O. In order to explore the reaction mechanism in detail, the (110)-B termination was chosen to model the Co3O4 surface (the reasons for choosing (110)-B termination are detailed in Section 3.1). The p() supercell slabs were utilized for the Co3O4 (110)-B surface, which are visualized in Figure 1.
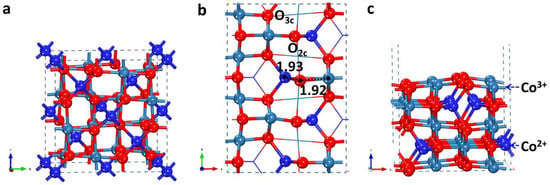
Figure 1.
(a) Bulk Co3O4, (b) top view and (c) side view of Co3O4 (110)-B surface. Co3+, Co2+, and O atoms are shown in light blue, dark blue, and red, respectively. In addition, in the top view of structures, the atoms at the top and sub layers are illustrated in ball-and-stick model, and the rest are in line model. These illustrations are utilized throughout this paper. The unit of bond lengths are Å.
The location of all possible intermediates on the Co3O4 (110) surface should be confirmed. The most stable adsorption configurations of HCHO and O2 have been given in Figure 2. In addition, Figure 3 presents the adsorption configurations of possible intermediates. The corresponding adsorption energies and key structural parameters are listed in Table 1.
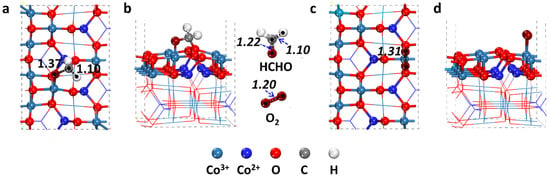
Figure 2.
The most stable adsorption configurations together with electron density difference of HCHO (a,b) and O2 (c,d) on the Co3O4 (110) surface. The unit of bond lengths are Å.
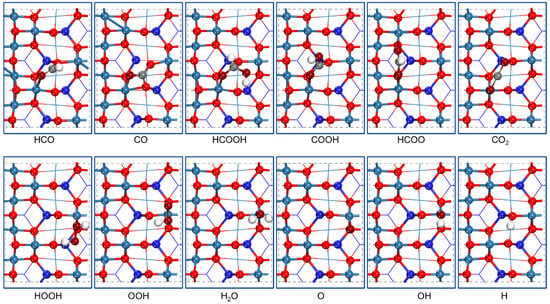
Figure 3.
The most stable adsorption configurations of possible intermediate species involved in HCHO oxidation to CO2 and H2O on Co3O4 (110) surface.

Table 1.
Adsorption energies and key structural parameters of the stable configurations for the possible adsorbed species involved in HCHO oxidation to CO2 and H2O on Co3O4 (110) surface.
HCHO, HCO, CO HCHO and HCO both adsorb on the surface by C atom bonding to the O2c site and O atom bonding to the Co3+ site, and their adsorption energies are 1.28 and 3.45 eV, respectively. CO adsorbs on the surface via the C atom located on the middle of two O2c atoms and the O atom bonded to a Co3+ site with the adsorption energy of 3.57 eV.
HCOOH, COOH, HCOO, CO2 HCOOH and COOH adsorb on the surface via C atom connecting to the O2c site and the O atom connecting to Co3+ site, and their adsorption energies are 0.76 and 3.71 eV, respectively. HCOO adsorbs on surface with two O atoms connecting to two Co3+ sites with the adsorption energy of 2.23 eV. CO2 weakly adsorbs on the surface by O atom bonding to the Co3+ site with the adsorption energy of 0.01 eV.
HOOH, OOH, O2 HOOH, OOH, and O2 are all located on the surface via O atom bonding to Co3+ site, and their adsorption energies are 1.13, 1.26, and 0.85 eV, respectively.
H2O, OH, O, H H2O, OH, and O adsorb on the surface via O atom connecting to Co3+ site, and their adsorption energies are 0.74, 2.35, and 2.87 eV, respectively. H atom connects to O2c with an adsorption energy of 3.74 eV.
To better understand the desorption behavior of the obtained product CO2 and H2O molecules at ambient conditions, the desorption time (τ) is implemented [30], which is expressed in Section 3.1. The calculated desorption time for CO2 and H2O molecules at ambient temperature are 1.14 × 10−3 and 1.76 s, respectively, indicating that CO2 and H2O molecules are physical adsorbed. Therefore, they can desorb easily from the Co3O4 surface, which facilitates the complete catalytic reaction.
2.2. Reactions Starting from HCHO and O2 Co-Adsorbing on Co3O4 (110) Surface
From the result of the absorption calculation, the adsorption energies of HCHO and O2 are 1.28 eV and 0.85 eV, respectively, suggesting that HCHO presents stronger adsorption capability than O2 on Co3O4 (110) surface. While for the co-adsorption configuration (Figure 4), HCHO and O2 locate on two different sites and maintain a distance of 2.35 Å. Therefore, HCHO and O2 can co-adsorb on Co3O4 (110) surface without rejection. The O–O bond length of the adsorbed O2 molecule is elongated to 1.29 Å, which is longer than that of free O2 (1.23 Å) and similar to O2 adsorption individually (1.31 Å). Based on the Mulliken analysis, the O atom as an intermediate has similar atomic charges to the individual adsorbed O2 molecule, which further confirms the strong chemical adsorption.
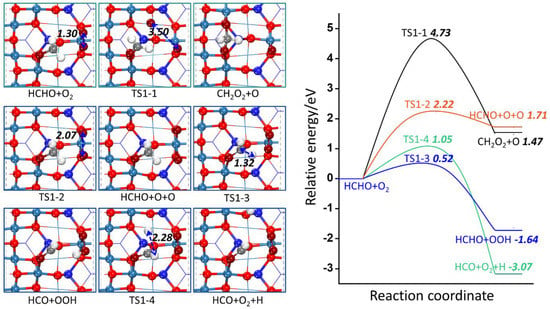
Figure 4.
The potential energy profile of the first step of HCHO oxidation together with the structures of TSs and FSs on Co3O4 (110) surface. The unit of bond lengths are Å.
We first considered the E-R mechanism on the Co3O4 (110) surface. The first element reaction is the contact of O2 with HCHO to form CH2O2 and an O atom. If gaseous O2 is contacted with HCHO pre-adsorbed on the surface, there will be no conversion into CH2O2 and O due to the lack of a transition state. Whereas, if HCHO in the gaseous phase is contacted with surface pre-adsorbed O2, it will convert into CH2O2 and an O atom. However, the activation energy can reach as high as 5.51 eV. Therefore, the E-R mechanism was excluded from consideration. Subsequently, the L-H mechanism and MvK mechanism were investigated in detail. From the co-adsorption of HCHO and O2 on the Co3O4 (110) surface to the CO2 and H2O production, HCHO would lose two H atoms and gain one O atom for the formation of CO2. Meanwhile, O2 would lose one O atom and gain two H atoms for the formation of H2O. Based on these two mechanisms, the corresponding activation barriers and reaction energy of all possible elementary reactions involved in HCHO oxidation have been listed in Table 2.

Table 2.
All possible elementary reactions with the corresponding activation barriers (Ea/eV) and reaction energies (ΔE/eV) involved in HCHO oxidation on Co3O4 (110) surface.
2.2.1. The Possible First Step of HCHO Oxidation
According to Table 2, all reactions on Co3O4 (110) surface are represented by Rn-m (n = 1~5; m = 1~10). The reactions from R1-1 to R1-4 represent four possible initial steps when HCHO and O2 co-adsorb on Co3O4 (110) surface. Figure 4 presents the potential energy profiles of possible pathways of the four possible initial steps together with transition states (TSs) and final states (FSs) on the Co3O4 (110) surface.
R1-1 goes through TS1-1 to form CH2O2 and O. Firstly, HCHO adsorbs at hollow site, and O2 adsorbs on the top Co3+ site with the O–O bond length of 1.31 Å. Then, one O atom tends to get close to the C atom of HCHO with the O–O distance extending to 3.50 Å. In the final state, the O=O distance is lengthened to 4.69 Å and becomes broken, forming CH2O2 with two O atoms connecting on two Co3+ sites, and the remaining O atom is adsorbed on another Co3+ site. The activation barrier and reaction energy of this elementary reaction are 4.73 eV and 1.47 eV, respectively. For R1-2, O2 can dissociate into two O atoms through TS1-2, where the O-O distance is elongated to 2.07 Å. In the final state, the O–O distance is lengthened to 2.76 Å and broken, forming two O atoms co-adsorbed on the Co3+ site. The activation barrier and reaction energy of this elementary reaction are 2.22 eV and 1.71 eV, respectively. R1-3 and R1-4 are reactions of HCHO dissociation into HCO and H atom, and the difference is whether the H atom adsorbs on the Co3O4 (110) surface or combines with O2 to generate OOH. In TS1-3, one H atom of HCHO tends to combine with O2 to form OOH with the C-H bond elongating to 1.32 Å and O-H bond shortening to 1.61 Å. In the final state, HCO adsorbs on the hollow site and OOH adsorbs on the Co3+ site. The activation barrier and reaction energy of this elementary reaction are 0.52 eV and −1.64 eV, respectively. In TS1-4, the C-H distance is elongated to 2.29 Å from 1.11 Å of the adsorbed HCHO. In the final state, HCO adsorbs on the hollow site with the H atom adsorbing on the Co3+ site. The activation barrier and reaction energy are 1.05 eV and −3.07 eV, respectively.
The case where HCHO obtains the Olatt for CH2O2 formation is also considered. However, the formed CH2O2 still adsorbs with the O atom filling the (OV), and the configuration is no different with HCHO adsorbing on the pristine surface similar to the final state of R1-1, thus this reaction is not considered. Through comparing the activation barrier and reaction energy, it is found that R1-3 of HCO and OOH formation is the most favorable reaction with the absolutely low activation barrier. Moreover, R1-3 is exothermic, indicating this step is not only thermodynamically but also kinetically favored. It is worth mentioning that although R1-4 is the most exothermic, its activation barrier is much higher than that of R1-3, which is kinetically hindered. Thus, the first step of HCHO reacting with O2 is in favor of forming HCO and OOH on the Co3O4 (110) surface, and the subsequent pathway of HCHO oxidation is considered starting from HCO and OOH.
In order to investigate why O2 comfortably accepts one H atom for OOH formation, the PDOS and charge density difference is displayed in Figure 5. The energy levels of Co minority α-spin d orbitals and O2 π* orbitals are well matched, leading to partial occupation of the formed d-π*orbitals. While the strong spin polarization provides large exchange stabilization energy for the majority β-spin orbitals, resulting in Co β-spin d-orbitals with energy levels about 1.0 eV lower than the π* orbitals of O2. Therefore, no conspicuous interaction of β orbitals is observed, indicating that only the α π* orbitals of O2 are partially occupied, which forces O2 to be of a radical nature, and active for hydrogenation. When O2 is hydrogenated to OOH, one electron transfers from the H atom to the β π* orbitals of O2, and both α and β DOS of Co 3d orbitals overlap with OOH’s π* orbitals, which further weakens the O–O bond. Therefore, the enhanced interaction and larger occupation of the π* orbitals in OOH leads to a lower O–O bond order, which is responsible for the lower dissociation barrier than that for O2.
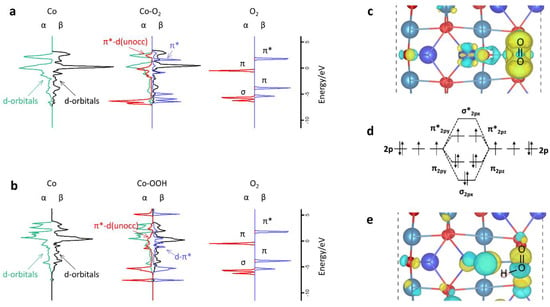
Figure 5.
Electronic structure analysis. (a) Projected electronic density of states (PDOS) and schematic illustrations of 3D orbitals of Co on Co3O4 (110) surface, 2p-orbitals of the O2 gas molecule, and their interaction within Co3O4-O2 configuration. (b) PDOS and schematic illustrations for Co3O4-OOH interaction configuration. As the O2 and OOH adsorb on a Co top site, the PDOS displays this Co atom and O2/OOH.(c,e) The charge density difference of O2 and OOH adsorb on Co3O4 (110) surface, respectively. (d) O2 molecular orbitals.
2.2.2. HCHO Catalytic Oxidation Mechanism on Co3O4 (110) Surface from HCO and OOH
- MvK Mechanism
The corresponding elementary reactions are revealed in Table 2, where the reactions related to MvK mechanism are marked R2-n and R3-n. The adsorbed HCO can react with Olatt to generate HCOO and OV, followed by the OOH dissociation into OH and an O atom, and O atom filling OV to restore the surface (R2-1). Subsequently, CO2 is formed by OH oxidation through C-H breaking away from HCOO (R2-2), and H atom migration to OH to produce H2O (R2-3). Another alternative reaction path is described below: HCO first dehydrogenates into CO (R3-1), which can pick up Olatt to form CO2 and OV (R3-2). Then, OOH is dissociated into OH and an O atom, which fills OV to restore Olatt (R3-3). Meanwhile, OH combines with H atom to form H2O (R3-4). In addition, Olatt can also adsorb H atom from HCO dehydrogenation to form COOH (R3-5), which is further oxidized to CO2 and H2O by OOH, accompanied by the recovery of OV to Olatt (R3-5 and R3-7). It is worth mentioning that the H atom of HCO can also transfer to OOH for CO and HOOH formation (R3-8). Then, CO can be oxidized by Olatt (R3-9), and the formed OV can be restored by an O atom produced from the HOOH decomposition, accompanied by the formation of H2O (R3-10). Therefore, four possible routes account for the MvK mechanism.
The potential energy profile and possible reaction paths of TSs and FSs on Co3O4 (110) surface are shown in Figure 6 and Figure 7. The difference in line color indicates different paths. As shown in Figure 6, after HCO and OOH co-adsorption, HCO is more favorable for connecting to Olatt to form HCOO than dissociating into CO and H atom, because the dissociation barrier of 4.26 eV is much higher than that of HCOO formation with almost no activation barrier. Even so, the CO path is still considered, because concerning the catalytic cycles, one transition state does not determine the kinetics. The determining states are not necessarily the highest transition state and the lowest intermediate state. The condition that TOF-determining transition state (TDTS) and the TOF-determining intermediate (TDI) must maximize the energetic span, thus the determining states may be different from the extreme state of the energy profile. After HCOO formation via the oxidation of Olatt, the following oxygen vacancy filling, dehydrogenation and OH combining with H atom for H2O generation go through TS2-1, TS2-2 and TS2-3, and their activation barriers are 1.25 eV, 2.24 eV and 1.09 eV, respectively. For the paths of CO capturing Olatt or OH shown in Figure 7, the H atom of HCO first transfers to surface (R3-1) or OOH (R3-8) with the activation barrier of 4.26 eV and 1.62 eV, respectively, indicating that H atom preferentially bonds to OOH rather than surface.
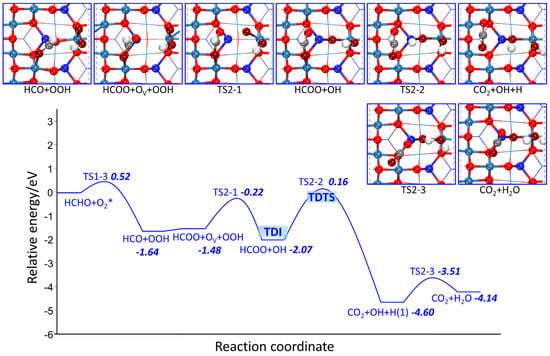
Figure 6.
The potential energy profile of HCHO oxidation based on MvK mechanism through HCO grabbing Olatt together with the structures of TSs and FSs on Co3O4 (110) surface.
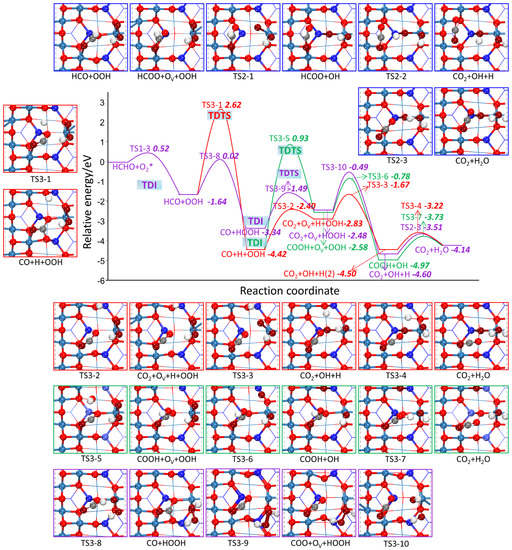
Figure 7.
The potential energy profile of HCHO oxidation based on MvK mechanism through CO grabbing Olatt together with the structures of TSs and FSs on Co3O4 (110) surface.
Starting from the reaction of CO + H + OOH (R3-2, R3-5), CO binds to Olatt for CO2 formation through TS3-2 and binds to lattice OH for COOH formation through TS3-5 with the activation barrier of 2.02 eV and 5.36 eV, respectively. The two reaction steps are endothermic with reaction energies of 1.59 and 1.84 eV, respectively, suggesting the combination of CO with lattice OH for COOH formation is blocked kinetically and thermodynamically. The following OV filling by OOH dissociation through TS3-3 and TS3-6 exhibits the activation barrier of 1.16 eV and 1.80 eV, respectively, which are different from that of R2-1, indicating that OOH dissociation is affected by co-adsorbents. As for the reaction of OH combining with H atom (TS3-4) and the reaction of H atom of COOH transferring to OH for H2O formation (TS3-7), their activation barriers are 1.40 eV and 1.24 eV, respectively. Starting from the reaction of CO+HOOH, CO reacts with Olatt to produce CO2, and HOOH dissociates into two OH groups. One OH occupies OV, the other OH reacts with H atom to produce H2O. The activation barrier for these three elementary reactions (R3-9, R3-10, R2-3) are 1.85, 1.00, and 1.09 eV, respectively. Through comparing the four reaction paths, the purple line in Figure 7 is the most preferable as the maximum activation barrier of 1.85 eV is much lower than other three paths with the maximum barrier of 4.26 eV.
As for the catalytic cycles, the TDTS for blue, red, green and purple line marked reaction paths are TS2-2, TS3-1, TS3-5, and TS3-9, respectively. Their corresponding TDI are HCOO + OH, HCOO + OV + OOH, CO + H + OOH and CO + HOOH, respectively. As the TDTS comes after the TDI, according to formula (5a), the δE for these four reaction routes are 2.24 eV, 4.26 eV, 5.36 eV, and 1.85 eV, respectively, which means that the efficiency of the catalytic cycles of the purple line marked reaction path are satisfactory. In short, irrespective of whether considering single route or catalytic cycles, the specific reaction paths representing the MvK mechanism are described below: firstly, two H atoms of HCHO continuously transfer to O2, forming CO and HOOH. Then, CO grabs Olatt to generate CO2, and HOOH dissociates into two OH groups with one of them occupying OV, and its H atom moves to another OH for H2O formation.
- L-H Mechanism
The difference between the L-H mechanism and the MvK mechanism is that the ROS binding to carbon intermediates are derived from surface adsorbed oxygen species, rather than Olatt. The specific reactions related to the L-H mechanism are marked R4-n and R5-n in Table 2. Starting from HCO and OOH absorbed on Co3O4 (110) surface, the O–O bond of OOH dissociates into OH and O atom (R4-1), which can bind to HCO for HCOO (R4-2) or HCOOH formation (R4-5). HCOO can be oxidized by OH to produce CO2 and H2O (R4-3 and R4-4); whereas HCOOH combines with O atom to form HCOO and OH (R4-6 and R4-7), which in turn react to produce CO2 and H2O (R4-8). In addition, HCO can be dehydrogenated to form CO (R5-1). The O–O bond of OOH decomposes into O atom and an OH group (R5-2), the former combines with CO to form CO2 (R5-3) and the latter combines with H atom to form H2O (R5-4). Moreover, OH can also combine with CO to form COOH, which is subsequently converted into CO2 (R5-5 and R5-6). The H atom from HCO dehydrogenation can bind to OOH for HOOH formation (R5-7). It reacts with CO to form COOH and OH (R5-8), which are finally converted to CO2 and H2O (R5-9). Therefore, five possible routes account for the L-H mechanism.
The potential energy profile and possible reaction pathways of TSs and FSs on the Co3O4 (110) surface are shown in Figure 8 and Figure 9. As shown in Figure 8, HCO follows the L-H mechanism to grab surface adsorbed oxygen species via two reaction paths. After HCO and OOH co-adsorption, O–O bond of OOH is broken to form an O atom and OH adsorbed on Co3+ site through TS4-1. The dissociation barrier is 0.68 eV and the reaction energy is 0.21 eV. In TS4-2, an O atom approaches HCO, and the C-O bond length is shortened from 3.36 Å to 1.98 Å; In TS4-5, OH rotates and moves to HCO, and the C-O distance is shortened from 3.13 Å to 2.11 Å. The activation barrier of the two transition states are 4.05 eV and 1.11 eV, respectively, and the reaction energies are −0.65 eV and 0.04 eV, respectively, indicating that the combination of HCO to OH is kinetically feasible. This is against that of the MvK mechanism, in which HCO preferentially combines with Olatt, suggesting that the lattice oxygen species and adsorbed oxygen species possess different electronic properties that affect their reaction capability. In addition, for transition states (TS4-6, TS4-7, and TS4-8) through which HCOOH is converted to CO2 and H2O, their energy barriers are lower than those through HCOO paths (TS4-3 and TS4-4), indicating that the route starting from HCO combining with OH is preferable.
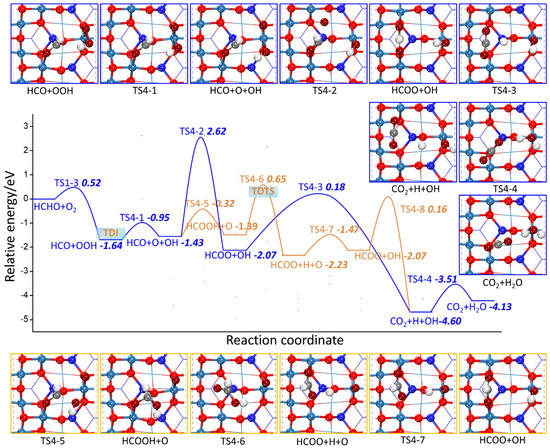
Figure 8.
The potential energy profile of HCHO oxidation based on the L-H mechanism through HCO grabbing the surface adsorbed oxygen species together with the structures of TSs and FSs on Co3O4 (110) surface.
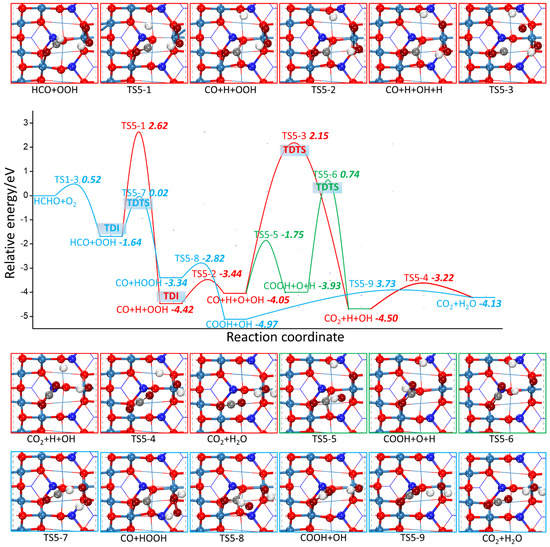
Figure 9.
The potential energy profile of HCHO oxidation based on the L-H mechanism through CO grabbing the surface adsorbed oxygen species together with the structures of TSs and FSs on Co3O4 (110) surface.
As revealed in Figure 9, another three possible reaction paths start with the dehydrogenation of adsorbed HCO to form CO, which further follows the L-H mechanism to grab surface adsorbed oxygen species. The H atom generated by the dehydrogenation of HCO can be combined with lattice oxygen through TS5-1,whose activation barrier and reaction energy are 4.26 eV and 2.78 eV, respectively. The H atom can also be combined with OOH through TS5-7 to form HOOH, whose activation barrier and reaction energy are 1.62 eV and −1.70 eV, respectively. In this reaction, the C-H distance is elongated to 1.49 Å along with the H-O distance shortened to 1.28 Å. Therefore, it can be inferred that H atom preferentially bonds with OOH rather than other locations on the surface after cleaving away from HCO. Starting from the reaction of CO + H + OOH, the O–O bond of OOH ruptures again through TS5-2, whose activation barrier and reaction energy are 0.98 eV and 0.37 eV, respectively. CO can react with O atom to form CO2 via TS5-3 or OH to form COOH via TS5-5. Their activation barriers are 6.20 eV and 2.30 eV, respectively, and their reaction energies are −0.55 eV and 0.12 eV, respectively. This suggests that the CO reaction with OH is more preferable than O atom, which is consistent with the HCO reaction with OH or O, further indicating that surface adsorbed OH is an important ROS. The following COOH dehydrogenation by O atom via TS5-6 may be prevented by the high activation barrier of 4.68 eV. Starting from the reaction of CO+HOOH, CO can directly capture OH from HOOH for COOH formation via TS5-8 with the activation barrier as low as 0.52 eV. Meanwhile, the O–O distance is elongated 2.28 Å and C-O distance is shortened to 2.24 Å. This reaction step is also kinetically favored with reaction energy of −1.63 eV. The H atom of COOH can transfer to OH through TS5-9 with the activation barrier and reaction energy of 1.24 and 0.84 eV, respectively. Comparing to R5-6 with dehydrogenation of COOH with the H atom adsorbed on the O atom, H atom prefers to be close to OH, which is similar to the fact that the H atom of HCO preferentially reacts directly with the OOH rather than bond with the surface absorbed O atom. In conclusion, among five reaction paths, the light blue line marked route in Figure 9 is the most favorable, because the maximum energy barrier is much lower than that of other four paths.
In Figure 8 and Figure 9, the TDTS and TDI marked in different colors represent different paths of TDTS and TDI. As the TDTS comes after the TDI for the five routes, according to formula (5a), the δE for these five routes are 4.26 eV, 2.29 eV, 6.57 eV, 5.16 eV, and 1.65 eV, respectively, which means that the efficiency of catalytic cycles of the light blue line marked path is satisfactory. Therefore, the optimal reaction path of the HCHO oxidation process is as follows: two H atoms of HCHO are successively transferred directly to O2 to form HOOH. Subsequently, the O–O bond of HOOH is broken to generate two OH. One OH binds to CO to form COOH, whose H atom further combines with another OH to finally generate CO2 and H2O.
2.2.3. Comparison of HCHO Catalytic Oxidation through MvK and L-H Mechanism
Starting from HCHO and O2 co-adsorption, the most favorable reaction paths that belong to MvK and L-H mechanism are shown in Figure 10 and Scheme 1. Two H atoms of HCHO transfer to O2 continuously for CO and HOOH formation are the first two favorable steps. For MvK mechanism, CO grabs Olatt to generate CO2 firstly. The maximum activation barrier is 1.85 eV in this reaction path. The subsequent steps are the O–O bond of HOOH breaking and O vacancy filling by O atom of OH. Finally, H atom move to another OH for H2O formation. For L-H mechanism, COOH is formed through CO drawing OH from HOOH, and then H atom of COOH is taken away by another OH for CO2 and H2O formation. The maximum activation barrier of this reaction path is 1.65 eV, corresponding to the second H atom of HCHO dehydrogenation, which is slightly lower than that of MvK mechanism. Therefore, the highest energy barriers to overcome for the MvK and L-H mechanisms are similar, making both mechanisms feasible. The δE of the two paths correspond exactly to the activation barrier, suggesting that the catalytic cycle through the two mechanisms are equivalent to some extent. It is worth noting that due to the relatively strong exotherm of the reaction, HCHO cannot be oxidized smoothly at room temperature if the energy released in the previous cycles cannot be fully utilized for subsequent cycles. In addition, the maximum δE cannot be affected by the reaction energy according to formula (5b), resulting in TDTS appearing after TDI in all possible paths through MvK and L-H mechanism.
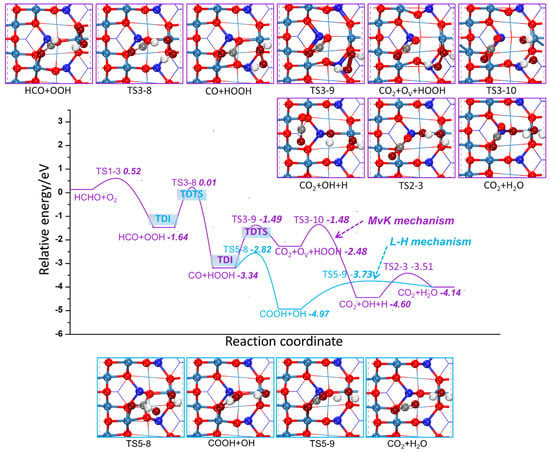
Figure 10.
Potential energy diagram for the most favorable pathways of HCHO catalytic oxidation through the MvK and L-H mechanism.
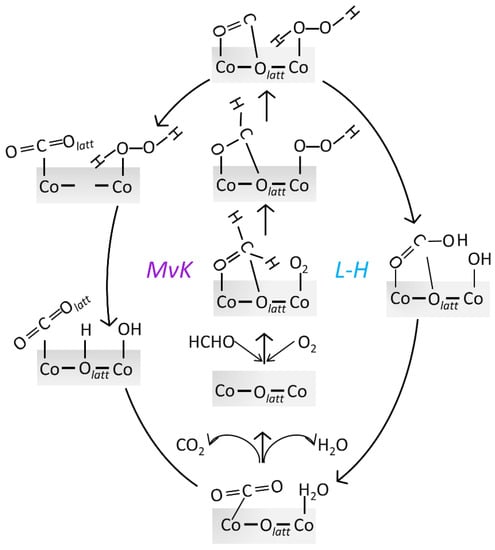
Scheme 1.
Elementary reaction steps of HCHO catalytic oxidation based on MvK and L-H mechanism on Co3O4 (110) surface.
3. Computational Details
3.1. Surface Models
Co3O4 has a spinel structure (Fd3m) which contains half-filled octahedral sites with Co3+ cations and tetrahedral sites with Co2+ cations. For the Co3O4 (110) surface, it bears two different terminations, usually denoted as the A and B terminations: the (110)-A termination exposes two types of cations (Co2+ and Co3+) and one type of anion (three-fold coordinated O3c), whereas the (110)-B termination has only one type of cation (Co3+) and two types of anions (two-fold coordinated O2c and three-fold coordinated O3c). Chen et al. [31] have reported the curves of Gibbs surface energy of these two terminations against oxygen chemical potential and found that the terminal B surface possessed a lower Gibbs surface energy in oxygen-rich conditions. Considering the oxygen chemical potential under the common work conditions of HCHO oxidation (PO2/P0 = 0.2; T = 300~330 K), the (110)-B termination was energetically more stable than the (110)-A termination. Therefore, the (110)-B termination was chosen to model the catalyst surface. We simulated symmetric slabs with an odd number of five layers, for which the total dipole moment was zero. This slab was stoichiometric and symmetric along the surface normal plane. The p() supercell slabs were utilized for Co3O4 (110)-B surface, which can be seen in Figure 1. The bottom two Co-O layers are fixed. Whereas the topmost three Co-O layers and the adsorbates are fully relaxed in all calculations.
3.2. Computational Details
All the calculations were performed in the framework of the density functional theory (DFT) by using the Cambridge Sequential Total Energy Package (CASTEP) in Material Studio 8.0 of Accelrys. The generalized gradient approximation (GGA) was chosen to represent the exchange-correlation potential in the formulation of the Perdew–Burke–Ernzerhof (PBE) [32]. Owing to the magnetic properties of Co, all calculations were spin-polarized with an energy cutoff of 380 eV. The Brillouin zone was sampled by a 2 × 2 × 1 k-points grid generated via the Monkhorst-Pack procedure [33]. The geometry optimization was converged when the energy differences between two electronic optimization steps were smaller than 10−5 eV, and the forces for ions were less than 0.05 eV/Å. For the transition state (TS) search calculations of all elementary reactions in HCHO oxidation process, the complete LST/QST was adopted for search protocol, and the convergence tolerance of RMS convergence resorted to 0.25 eV/Å. In order to further confirm that the transition from reactant to product went through a transition state structure, the obtained transition state from TS search will be further checked via TS confirmation. The output of a TS confirmation calculation is another trajectory document, which follows the Intrinsic Reaction Path (IRP) as discussed in reaction paths.
The adsorption energy of all reactants, intermediates, and product species can be calculated through the following formula:
where Especies/slab represents the total energy of Co3O4 (110) surface adsorbing certain species, Especies represents the energy of sole species, Eslab represents the slab energy of Co3O4 (110) surface.
Eads = Especies/slab − Especies − Eslab
Based on the calculation results, all the adsorption energies are negative. Therefore, we take absolute values to compare the adsorption energies of different surface species.
The desorption time (τ) was calculated through the following formula:
where A is bond vibration frequency in the range of 1012 Hz, kB is the Boltzmann constant (8.63 × 10−5 eV/K) and T is the room temperature of 298.15 K. Edes is the desorption energy.
For a reaction such as R (reactant) →·P (product) on catalyst surface, the activation barrier (Ea) and reaction energy (ΔE) were calculated according to the following formulas:
Ea = ETS − ER
ΔE = EP − ER
Where ER and EP are the total energies of the adsorbed reactant and product, respectively, and ETS represent the total energies of the transition state.
The energetic span (δE) model was also employed to elucidate the efficiency of the catalytic cycles. According to the literature, there were only two states to determine δE, namely determining transition state (DTS) and determining intermediate state (DIS), the smaller the energy span, the faster the reaction. δE is shown as:
4. Conclusions
DFT calculations have been performed to investigate possible HCHO catalytic oxidation paths on pristine Co3O4 (110) surface for comparisons of different reaction mechanisms. In addition, the maximum δE is calculated to state the catalytic cycle. The following results were obtained. HCHO and O2 prefer to co-adsorb on the Co3O4 (110) surface. The transfer of the H atom of HCHO to O2 for HCO and OOH formation represents the first preferable step rather than O2 dissociation. The electron property of PDOS analysis further suggests that only the α π* orbitals of O2 are partially occupied, forcing O2 to be of a radical nature, which is active for hydrogenation. HCO is further prone to transfer its H atom to OOH to generate CO and HOOH. Regarding the MvK mechanism, through CO grabbing Olatt, CO2 is produced and the O vacancy is filled by one OH group originating from HOOH bond breakage. Meanwhile, the H atom approaches in close proximity to OH to form H2O. For the L-H mechanism, CO obtains one OH from HOOH, then COOH gives an H atom to another OH for the formation of CO2 and H2O. Comparing the maximum activation barrier and δE, both the MvK and L-H mechanism are found to be feasible. Lattice oxygen in the MvK mechanism and surface OH in the L-H mechanism are both key ROS for oxidation of CO intermediates, and the catalytic cycle is almost equivalent.
Author Contributions
Conceptualization, Y.H., R.L. and T.H.; methodology, R.L. and T.H.; software, R.L. and T.H.; validation, R.L. and T.H.; formal analysis, R.L. and T.H.; data curation, R.L.; writing—original draft preparation, R.L. and T.H.; writing—R.L.; visualization, T.H.; supervision, Y.H., M.C. and W.H.; project administration, S.-c.L. and J.C.; funding acquisition, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by National Science Foundation of China, China [grant Nos. 51878644 and 41573138], the Strategic Priority Research Program of the Chinese Academy of Sciences, China [grant Nos. XDA23010300 and XDA23010000], the National Key Research and Development Program of China, China [grant No. 2016YFA0203000], and the Plan for “National Youth Talents” of the Organization Department of the Central Committee.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the Indoor Environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Ho, K.F.; Lee, S.C.; Yu, J.Z.; Louie, P.K.K. Characteristics and health impacts of VOCs and carbonyls associated with residential cooking activities in Hong Kong. J. Hazard. Mater. 2011, 186, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Yu, J.; Jaroniec, M.; Tao, F.F. Room-temperature catalytic oxidation of formaldehyde on catalysts. Catal. Sci. Technol. 2016, 6, 3649–3669. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Jiang, C.; Zhou, P.; Zhang, P.; Yu, J. The effect of manganese vacancy in birnessite-type MnO2 on room-temperature oxidation of formaldehyde in air. Appl. Catal. B Environ. 2017, 204, 147–155. [Google Scholar] [CrossRef]
- Li, H.; Huang, T.; Lu, Y.; Cui, L.; Wang, Z.; Zhang, C.; Lee, S.; Huang, Y.; Cao, J.; Ho, W. Unraveling the mechanisms of room-temperature catalytic degradation of indoor formaldehyde and its biocompatibility on colloidal TiO2-supported MnOx–CeO2. Environ. Sci. Nano 2018, 5, 1130–1139. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Wang, W.; Chen, M.; Li, H.; Lee, S.-C.; Ho, W.; Huang, T.; Cao, J. Oxygen vacancy–engineered δ-MnOx/activated carbon for room-temperature catalytic oxidation of formaldehyde. Appl. Catal. B Environ. 2020, 278, 119294. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, M.; Huang, Y.; Li, R.; Huang, T.; Cao, J.-j.; Shen, Z.; Lee, S.C. FeCo alloy encased in nitrogen-doped carbon for efficient formaldehyde removal: Preparation, electronic structure, and d-band center tailoring. J. Hazard. Mate. 2022, 424, 127593. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Tanaka, K.-I. Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B Environ. 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Jabłońska, M.; Król, A.; Kukulska-Zając, E.; Tarach, K.; Girman, V.; Chmielarz, L.; Góra-Marek, K. Zeolites Y modified with palladium as effective catalysts for low-temperature methanol incineration. Appl. Catal. B Environ. 2015, 166–167, 353–365. [Google Scholar] [CrossRef]
- Chen, B.-B.; Zhu, X.-B.; Crocker, M.; Wang, Y.; Shi, C. FeOx-supported gold catalysts for catalytic removal of formaldehyde at room temperature. Appl. Catal. B Environ. 2014, 154–155, 73–81. [Google Scholar] [CrossRef]
- Bai, B.; Li, J. Positive Effects of K+ Ions on Three-Dimensional Mesoporous Ag/Co3O4 Catalyst for HCHO Oxidation. ACS Catal. 2014, 4, 2753–2762. [Google Scholar] [CrossRef]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl. Catal. B Environ. 2013, 142–143, 677–683. [Google Scholar] [CrossRef]
- Huang, Y.; Long, B.; Tang, M.; Rui, Z.; Balogun, M.-S.; Tong, Y.; Ji, H. Bifunctional catalytic material: An ultrastable and high-performance surface defect CeO2 nanosheets for formaldehyde thermal oxidation and photocatalytic oxidation. Appl. Catal. B Environ. 2016, 181, 779–787. [Google Scholar] [CrossRef]
- Zeng, L.; Song, W.; Li, M.; Zeng, D.; Xie, C. Catalytic oxidation of formaldehyde on surface of HTiO2/HCTiO2 without light illumination at room temperature. Appl. Catal. B Environ. 2014, 147, 490–498. [Google Scholar] [CrossRef]
- Li, H.; Ho, W.; Cao, J.; Park, D.; Lee, S.-C.; Huang, Y. Active Complexes on Engineered Crystal Facets of MnOx–CeO2 and Scale-Up Demonstration on an Air Cleaner for Indoor Formaldehyde Removal. Environ. Sci. Technol. 2019, 53, 10906–10916. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cui, L.; Lu, Y.; Huang, Y.; Cao, J.; Park, D.; Lee, S.-C.; Ho, W. In Situ Intermediates Determination and Cytotoxicological Assessment in Catalytic Oxidation of Formaldehyde: Implications for Catalyst Design and Selectivity Enhancement under Ambient Conditions. Environ. Sci. Technol. 2019, 53, 5230–5240. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Huang, Y.; Zhu, D.; Ho, W.; Cao, J.; Lee, S. Improved Oxygen Activation over a Carbon/Co3O4 Nanocomposite for Efficient Catalytic Oxidation of Formaldehyde at Room Temperature. Environ. Sci. Technol. 2021, 55, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Huang, Y.; Zhu, D.; Ho, W.; Lee, S.; Cao, J. A Review of Co3O4-based Catalysts for Formaldehyde Oxidation at Low Temperature: Effect Parameters and Reaction Mechanism. Aerosol Sci. Eng. 2020, 4, 147–168. [Google Scholar] [CrossRef]
- Ma, C.; Wang, D.; Xue, W.; Dou, B.; Wang, H.; Hao, Z. Investigation of Formaldehyde Oxidation over Co3O4−CeO2 and Au/Co3O4−CeO2 Catalysts at Room Temperature: Effective Removal and Determination of Reaction Mechanism. Environ. Sci. Technol. 2011, 45, 3628–3634. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, L.; Jiang, D. Surface oxygen vacancies on Co3O4 mediated catalytic formaldehyde oxidation at room temperature. Catal. Sci. Technol. 2016, 6, 3845–3853. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Cheng, B.; Jiang, C. Co3O4 nanorod-supported Pt with enhanced performance for catalytic HCHO oxidation at room temperature. Appl. Surf. Sci. 2017, 404, 426–434. [Google Scholar] [CrossRef]
- Zha, K.; Sun, W.; Huang, Z.; Xu, H.; Shen, W. Insights into High-Performance Monolith Catalysts of Co3O4 Nanowires Grown on Nickel Foam with Abundant Oxygen Vacancies for Formaldehyde Oxidation. ACS Catal. 2020, 10, 12127–12138. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, J.; Guo, Y.; Liu, J. Ultrathin, Polycrystalline, Two-Dimensional Co3O4 for Low-Temperature CO Oxidation. ACS Catal. 2019, 9, 2558–2567. [Google Scholar] [CrossRef]
- Bae, J.; Shin, D.; Jeong, H.; Choe, C.; Choi, Y.; Han, J.W.; Lee, H. Facet-Dependent Mn Doping on Shaped Co3O4 Crystals for Catalytic Oxidation. ACS Catal. 2021, 11, 11066–11074. [Google Scholar] [CrossRef]
- Xiao, M.; Yu, X.; Guo, Y.; Ge, M. Boosting Toluene Combustion by Tuning Electronic Metal–Support Interactions in In Situ Grown Pt@Co3O4 Catalysts. Environ. Sci. Technol. 2022, 56, 1376–1385. [Google Scholar] [CrossRef]
- Deng, J.; Song, W.; Chen, L.; Wang, L.; Jing, M.; Ren, Y.; Zhao, Z.; Liu, J. The effect of oxygen vacancies and water on HCHO catalytic oxidation over Co3O4 catalyst: A combination of density functional theory and microkinetic study. Chem. Eng. J. 2019, 355, 540–550. [Google Scholar] [CrossRef]
- Wang, X.; Ying, J.; Mai, Y.; Zhang, J.; Chen, J.; Wen, M.; Yu, L. MOF-derived metal oxide composite Mn2Co1Ox/CN for efficient formaldehyde oxidation at low temperature. Catal. Sci. Technol. 2019, 9, 5845–5854. [Google Scholar] [CrossRef]
- Chen, J.; Huang, M.; Chen, W.; Tang, H.; Jiao, Y.; Zhang, J.; Wang, G.; Wang, R. Defect Engineering and Synergistic Effect in Co3O4 Catalysts for Efficient Removal of Formaldehyde at Room Temperature. Ind. Eng. Chem. Res. 2020, 59, 18781–18789. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Z.; Luo, T.; Yan, Z.; Jin, M.; Shi, L. Pt Anchored on Mn(Co)CO3/MnCo2O4 Heterostructure for Complete Oxidation of Formaldehyde at Room Temperature. ChemistrySelect 2020, 5, 10537–10548. [Google Scholar] [CrossRef]
- Jiang, Q.G.; Ao, Z.M.; Li, S.; Wen, Z. Density functional theory calculations on the CO catalytic oxidation on Al-embedded graphene. RSC Adv. 2014, 4, 20290–20296. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Selloni, A. Electronic states and magnetic structure at the Co3O4 (110) surface: A first-principles study. Phys. Rev. B 2012, 85, 085306. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef] [PubMed]
- Pack, J.D.; Monkhorst, H.J. “Special points for Brillouin-zone integrations”—A reply. Phys. Rev. B 1977, 16, 1748–1749. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).