The Effect of Potassium Inclusion in a Silver Catalyst for N2O-Mediated Oxidation of Soot in Oxidising Exhaust Gases
Abstract
:1. Introduction
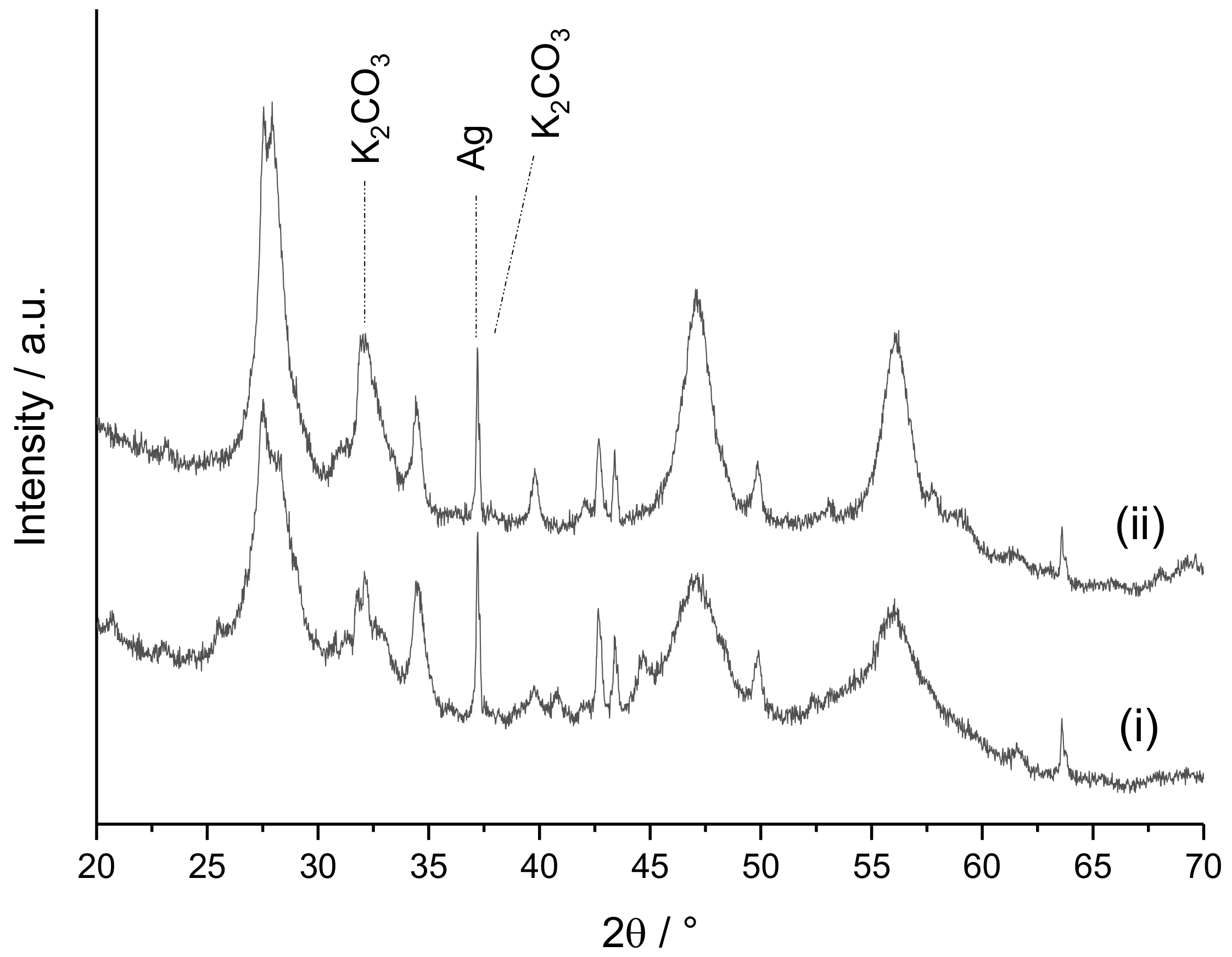
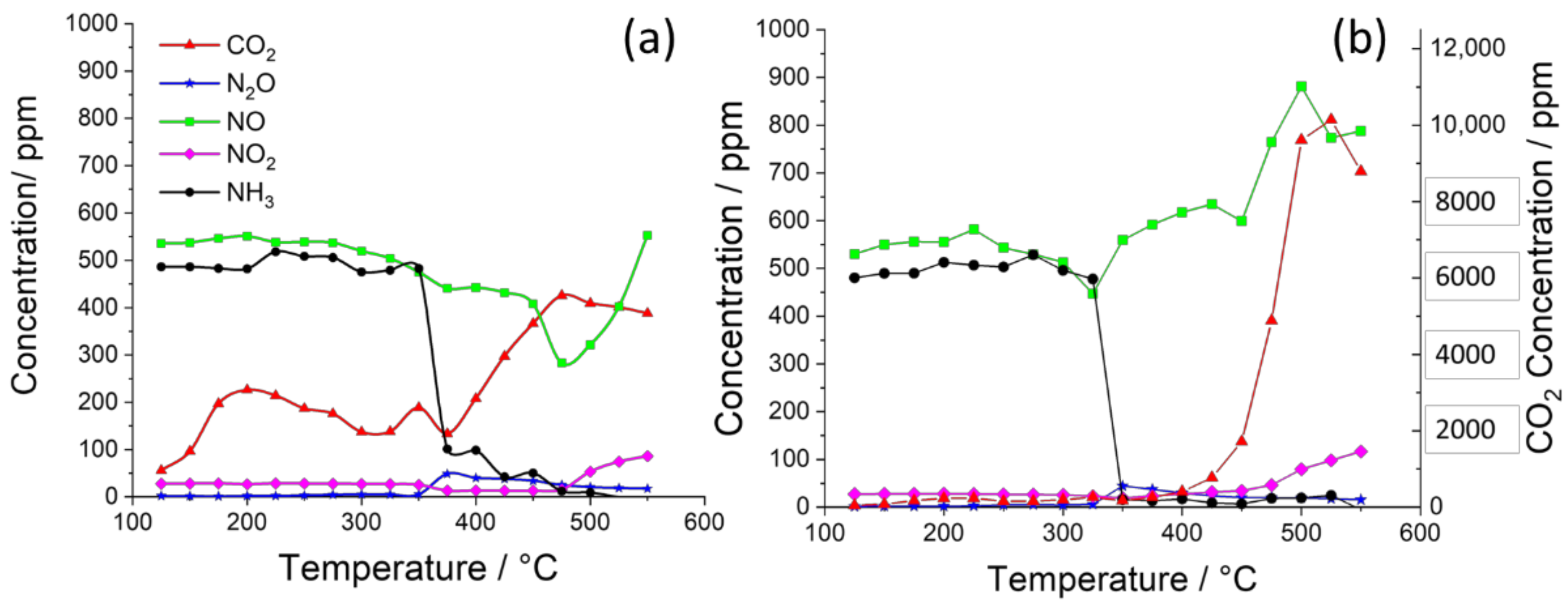
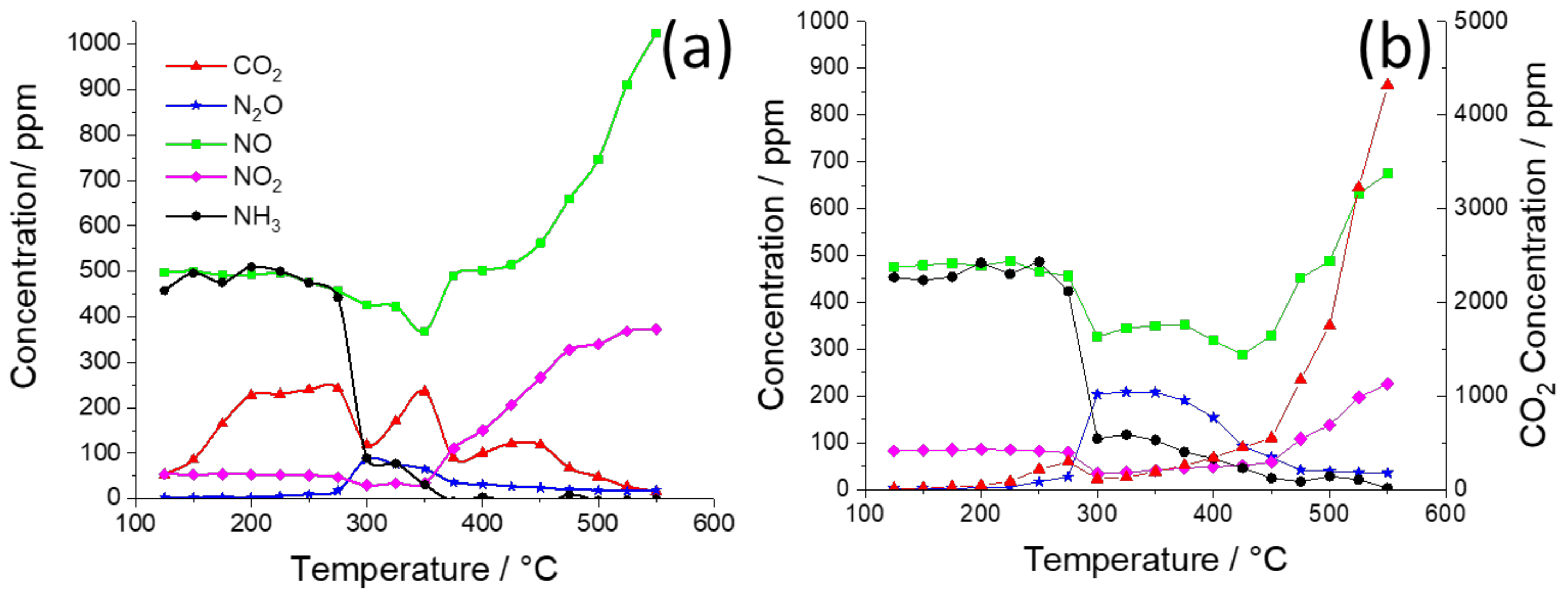
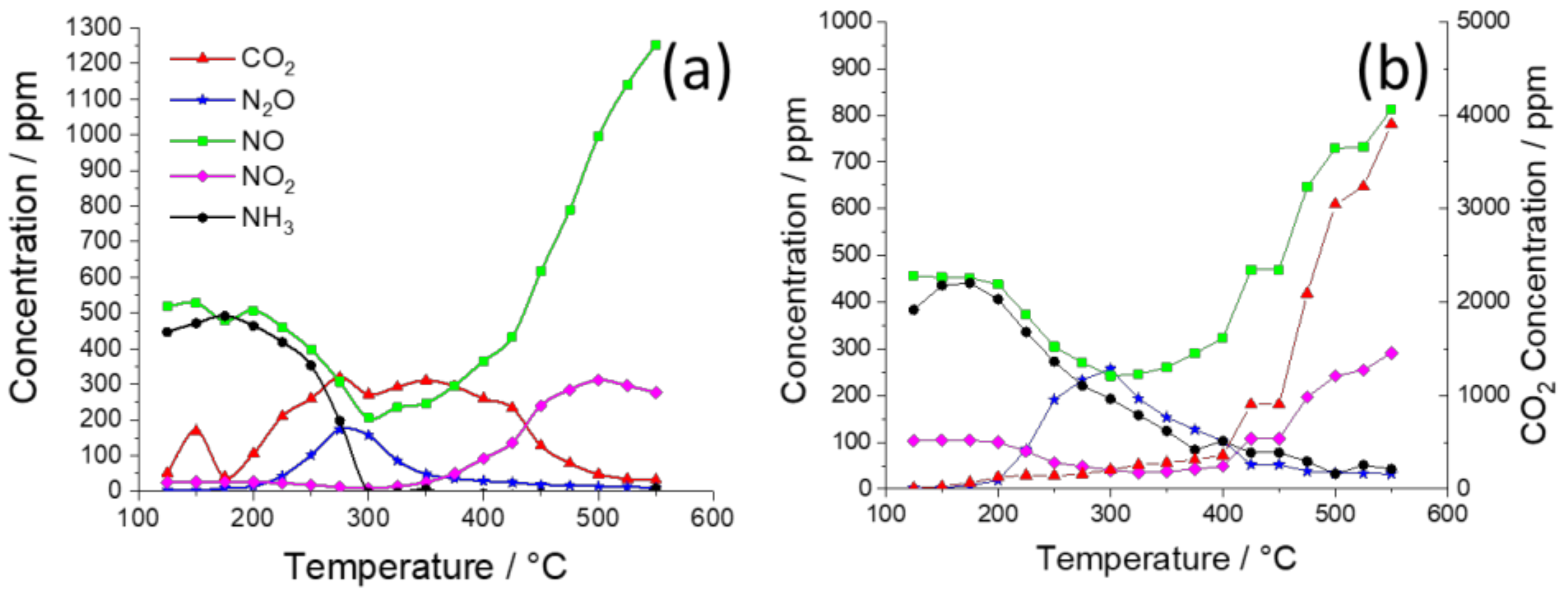
2. Results and Discussion
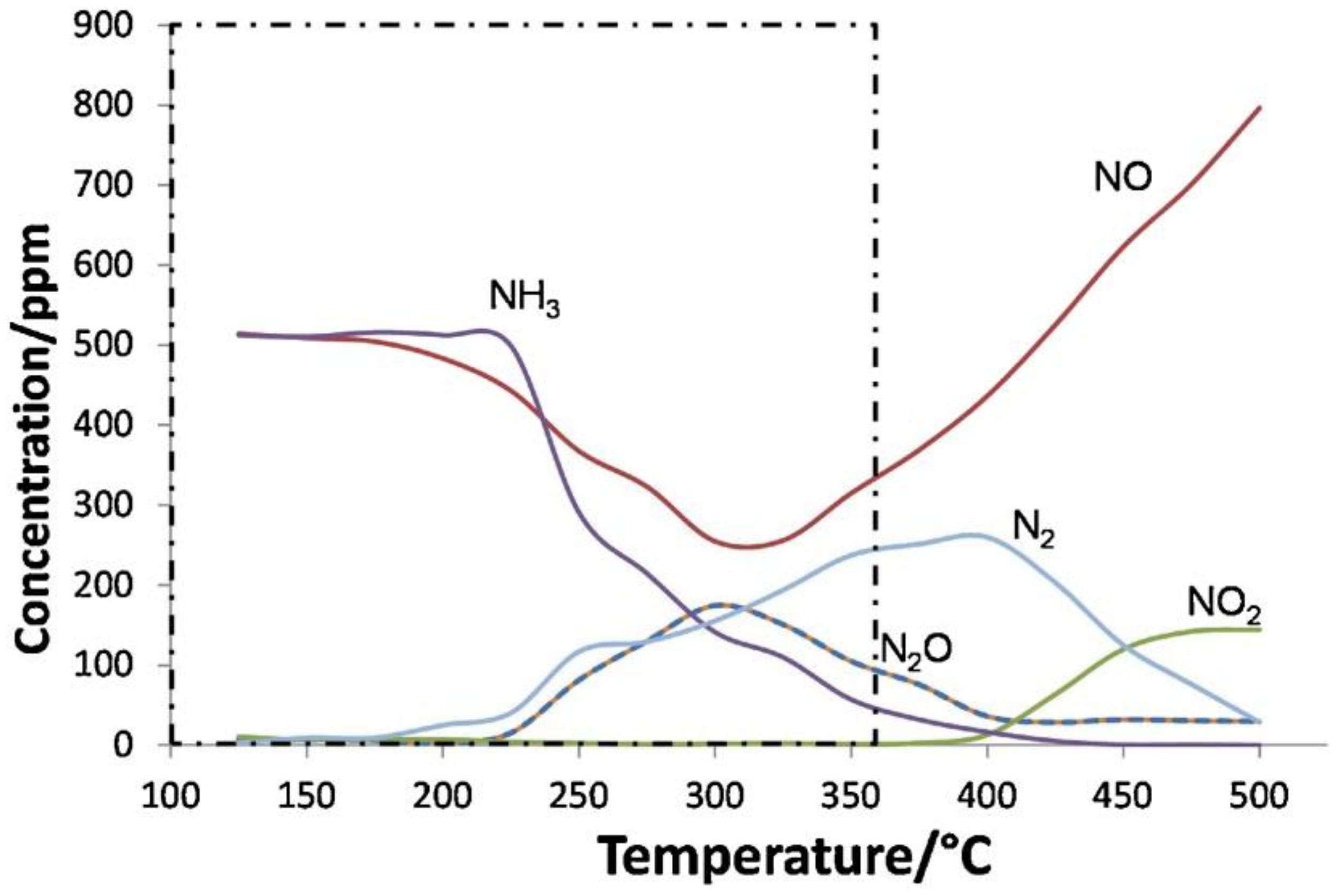
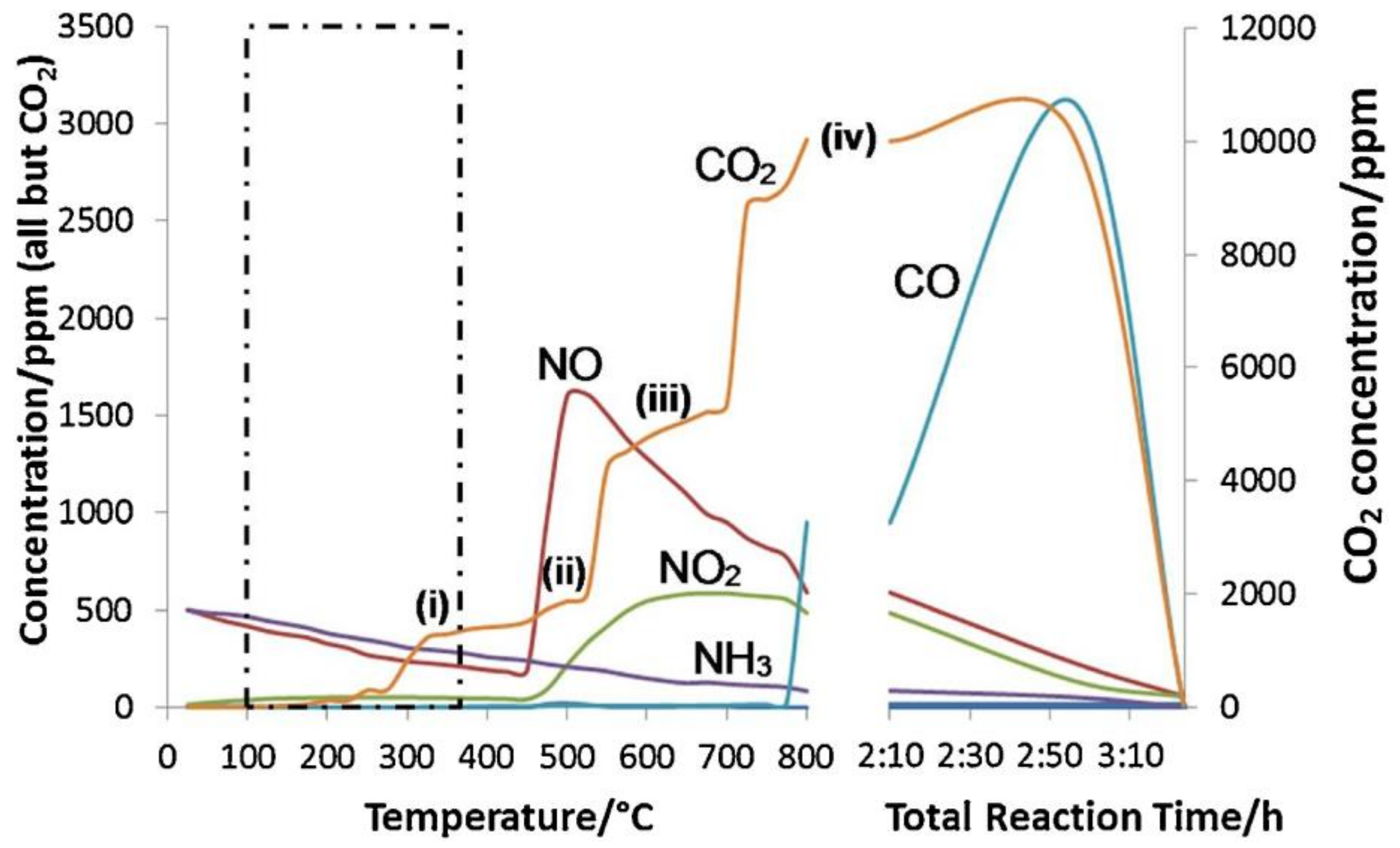
- (i)
- Catalysed oxidation by N2O to form CO2 and N2;
- (ii)
- Non-catalysed oxidation by NO2 to form CO2 and re-form NO;
- (iii)
- Catalysed oxidation by O2 to form CO2;
- (iv)
- Non-catalysed oxidation by O2 to form CO and CO2.
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Performance Testing
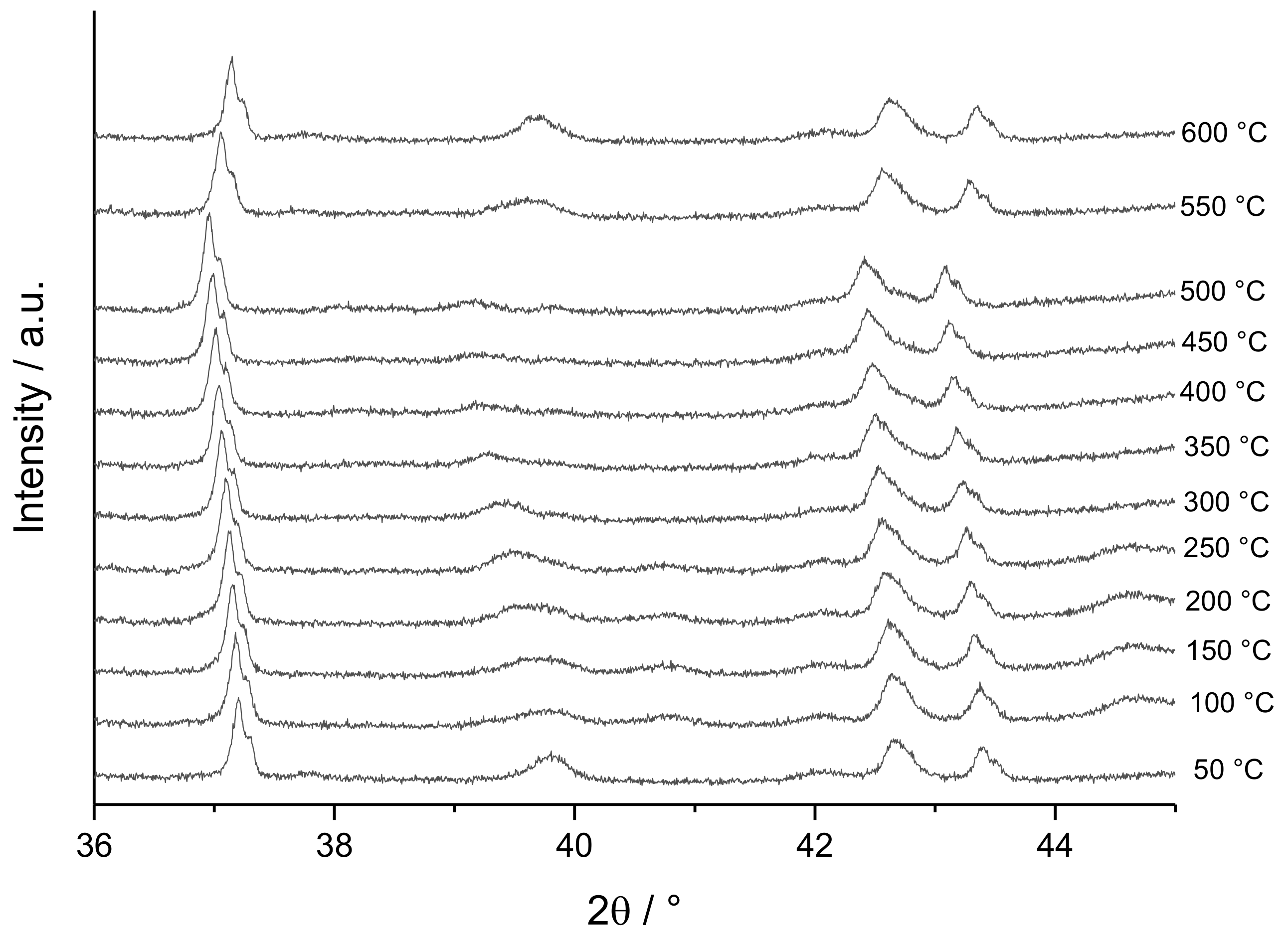
3.3. Temperature-Programmed XRD
3.4. XPS
4. Conclusions
- (i)
- Over the loading range of 5–15 wt% K, potassium blocks the majority of the NO-adsorption sites on the Ag and the low-temperature NH3 adsorption sites on the CZA, when it forms multiple layers over the surface during catalyst preparation. The NO-adsorption sites are partially restored on 2%Ag–5%K/CZA during testing in the presence of soot, when the potassium species become mobile and wet the soot particulate;
- (ii)
- At both the lowest (2 wt%) and highest (20 wt%) K-loading, most of the NO adsorption sites and low-temperature NH3 adsorption sites are available in the fresh catalysts, allowing NOx reduction to N2O to take place. This suggests that, during preparation of 2%Ag–20%K/CZA, the K2CO3 segregates to leave exposed regions on the Ag and CZA surfaces;
- (iii)
- Particularly at high K-loadings (15 and 20 wt%), the catalysts can function as NOx storage materials at temperatures > 300 °C, even when minimal NO adsorption is taking place. The likely pathway is by NH3 adsorption on CZA, followed by oxidation to nitrate species, which displace the carbonate from the potassium as CO2.
- (iv)
- The main soot oxidation reaction over all these catalysts is C+O2, which has an onset temperature of around 375 °C. The only other apparent soot oxidation route is by reaction with the nitrates formed during NOx storage; this reaction re-forms NO at the same time as forming CO2;
- (v)
- Potassium prevents N2O-mediated oxidation of soot, which is the main mechanism by which soot destruction occurs over K-free Ag/CZA at the low temperatures typical of the exhaust emitted by light-duty diesel engines.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mali, B.; Shrestha, A.; Chapagain, A.; Bishwokarma, R.; Kumar, P.; Gonzalez-Longatt, F. Challenges in the penetration of electric vehicles in developing countries with a focus on Nepal. Renew. Energy Focus 2022, 40, 1–12. [Google Scholar] [CrossRef]
- Chaturvedi, B.K.; Nautiyal, A.; Kandpal, T.C.; Yaqoot, M. Projected transition to electric vehicles in India and its impact on stakeholders. Energy Sustain. Dev. 2022, 66, 189–200. [Google Scholar] [CrossRef]
- Garcia, A.; Monsalve-Serrano, J.; Villalta, D.; Tripathi, S. Electric Vehicles vs e-Fuelled ICE Vehicles: Comparison of Potentials for Life Cycle CO2 Emission Reduction; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2022. [Google Scholar]
- Joshi, A. Review of Vehicle Engine Efficiency and Emissions; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2022. [Google Scholar]
- Twigg, M.V. Catalytic control of emissions from cars. Catal. Today 2011, 163, 33–41. [Google Scholar] [CrossRef]
- Lambert, C.K. Current state of the art and future needs for automotive catalysis. Nat. Catal. 2019, 21, 554–557. [Google Scholar] [CrossRef]
- Granger, P. Challenges and breakthroughs in post-combustion catalysis: How to match future stringent regulations. Catal. Sci. Technol. 2017, 7, 5195–5211. [Google Scholar] [CrossRef]
- Martinovic, F.; Castoldi, L.; Deorsola, F.A. Aftertreatment technologies for diesel engines: An overview of the combined systems. Catalysts 2021, 11, 653. [Google Scholar] [CrossRef]
- Purfürst, M.; Naumov, S.; Langeheinecke, K.-J.; Gläser, R. Influence of soot on ammonia adsorption and catalytic DeNOx-properties of diesel particulate filters coated with SCR-catalysts. Chem. Eng. Sci. 2017, 168, 423–436. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Fino, D.; Russo, N. Catalysis in diesel engine NOx aftertreatment: A review. Catal. Struct. React. 2015, 1, 155–173. [Google Scholar]
- Urán, L.; Gallego, J.; Ruiz, W.; Bailón-García, E.; Bueno-López, A.; Santamaría, A. Monitoring intermediate species formation by DRIFT during the simultaneous removal of soot and NOx over LaAgMnO3 catalyst. Appl. Catal. A Gen. 2019, 588, 117280. [Google Scholar] [CrossRef]
- Wang, R.; Zhong, C.; Li, D.; Yu, X.; Zhao, Z.; Sojka, Z.; Kotarba, A.; Wei, Y.; Liu, J. Preparation of 3DOM ZrTiO4 support, WxCeMnOδ/3DOM ZrTiO4 catalysts, and their catalytic performance for the simultaneous removal of soot and NOx. Front. Chem. 2022, 10, 880884. [Google Scholar] [CrossRef]
- Davies, C.; Thompson, K.; Cooper, A.; Golunski, S.; Taylor, S.H.; Bogarra Macias, M.; Doustdar, O.; Tsolakis, A. Simultaneous removal of NOx and soot particulate from diesel exhaust by in-situ catalytic generation and utilisation of N2O. Appl. Catal. B Environ. 2018, 239, 10–15. [Google Scholar] [CrossRef]
- Cooper, A.; Davies, T.E.; Morgan, D.J.; Golunski, S.; Taylor, S.H. Influence of the preparation method of Ag-K/CeO2-ZrO2-Al2O3 catalysts on their structure and activity for the simultaneous removal of soot and NOx. Catalysts 2020, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, J.; Ge, M. The poisoning effect of alkali metals doping over nano V2O5–WO3/TiO2 catalysts on selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2011, 170, 531–537. [Google Scholar] [CrossRef]
- Rinkenburger, A.; Toriyama, T.; Yasuda, K.; Niessner, R. Catalytic Effect of Potassium Compounds in Soot Oxidation. ChemCatChem 2017, 9, 3513–3525. [Google Scholar] [CrossRef]
- Ramdas, R.; Nowicka, E.; Jenkins, R.; Sellick, D.; Davies, C.; Golunski, S. Using real particulate matter to evaluate combustion catalysts for direct regeneration of diesel soot filters. Appl. Catal. B Environ. 2015, 176–177, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Gao, X.; Zhang, Y.; Wu, W.; Luo, Z.; Cen, K. Effect of KCl on the Selective Catalytic Reduction of NO with NH3 over Vanadia-Based Catalysts for Biomass Combustion. Environ. Prog. Sustain. Energy 2014, 33, 390–395. [Google Scholar] [CrossRef]
- Twigg, M.V. An essay book review of ‘Urea-SCR technology for deNOx after treatment of diesel exhausts’. Johns. Matthey Technol. Rev. 2015, 59, 221–232. [Google Scholar] [CrossRef]
- Janoš, P.; Hladík, T.; Kormunda, M.; Ederer, J.; Šťastný, M. Thermal treatment of cerium oxide and its properties: Adsorption ability versus degradation efficiency. Adv. Mater. Sci. Eng. 2014, 2014, 706041. [Google Scholar] [CrossRef] [Green Version]
- Davies, C.; Mayer, A.; Gabb, J.; Walls, J.M.; Degirmenci, V.; Thompson, P.B.J.; Cibin, G.; Golunski, S.; Kondrat, S.A. Operando potassium K-edge X-ray absorption spectroscopy: Investigating potassium catalysts during soot oxidation. Phys. Chem. Chem. Phys. 2020, 22, 18976–18988. [Google Scholar] [CrossRef]
- Gill, L.J.; Blakeman, P.G.; Twigg, M.V.; Walker, A.P. The use of NOx adsorber catalysts on diesel engines. Top. Catal. 2004, 28, 157–164. [Google Scholar] [CrossRef]
- Jayakumar, G.; Irudayaraj, A.; Raj, A.D.; Irudayaraj, A.A. Particle size effect on the properties of cerium oxide (CeO2) nanoparticles synthesized by hydrothermal method. Mech. Mater. Sci. Eng. 2017, 9, 127–131. [Google Scholar]
- Ghamsari, M.S.; Mahzar, Z.A.S.; Radiman, S.; Hamid, A.M.A.; Khalilabad, S.R. Facile route for preparation of highly crystalline γ-Al2O3 nanopowder. Mater. Lett. 2012, 72, 32–35. [Google Scholar] [CrossRef]
- Halvarsson, M.; Langer, V.; Vuorinen, S. Determination of the thermal expansion of K-Al2O3 by high temperature XRD. Surf. Coat. Technol. 1995, 76–77, 358–362. [Google Scholar] [CrossRef]
- Guha, P.; Juluri, R.R.; Bhukta, A.; Ghosh, A.; Maiti, S.; Bhattacharyya, A.; Srihari, V.; Satyam, P.V. In situ synchrotron X-ray diffraction study of coherently embedded silver nanostructure growth in silicon. CrystEngComm 2017, 19, 6811–6820. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, C.-B.; Wachs, I.E. Reaction induced spreading of metal oxides: In Situ Raman spectroscopic studies during oxidation reactions. Stud. Surf. Sci. Catal. 1997, 110, 255–264. [Google Scholar]
- Fino, D.; Bensaid, S.; Piumetti, M.; Russo, N. A review on the catalytic combustion of soot in diesel particulate filters for automotive applications: From powder catalysts to structured reactors. Appl. Catal. A Gen. 2016, 509, 75–96. [Google Scholar] [CrossRef]
- Foo, R.; Vazhnova, T.; Lukyanov, D.B.; Millington, P.; Collier, J.; Rajaram, R.; Golunski, S. Formation of reactive Lewis acid sites on Fe/WO3-ZrO2 catalysts for higher temperature SCR applications. Appl. Catal. B Environ. 2015, 162, 174–179. [Google Scholar] [CrossRef]
- Jabłońska, M.; Palkovits, R. It is no laughing matter: Nitrous oxide formation in diesel engines and advances in its abatement over rhodium-based catalysts. Catal. Sci. Technol. 2016, 6, 7671–7687. [Google Scholar] [CrossRef]
- Matsukata, M.; Fujikawa, T.; Kikuchi, E.; Morita, Y. Interaction between potassium carbonate and carbon substrate at subgasification temperatures. Migration of potassium into the carbon matrix. Energy Fuels 1988, 2, 750–756. [Google Scholar] [CrossRef]


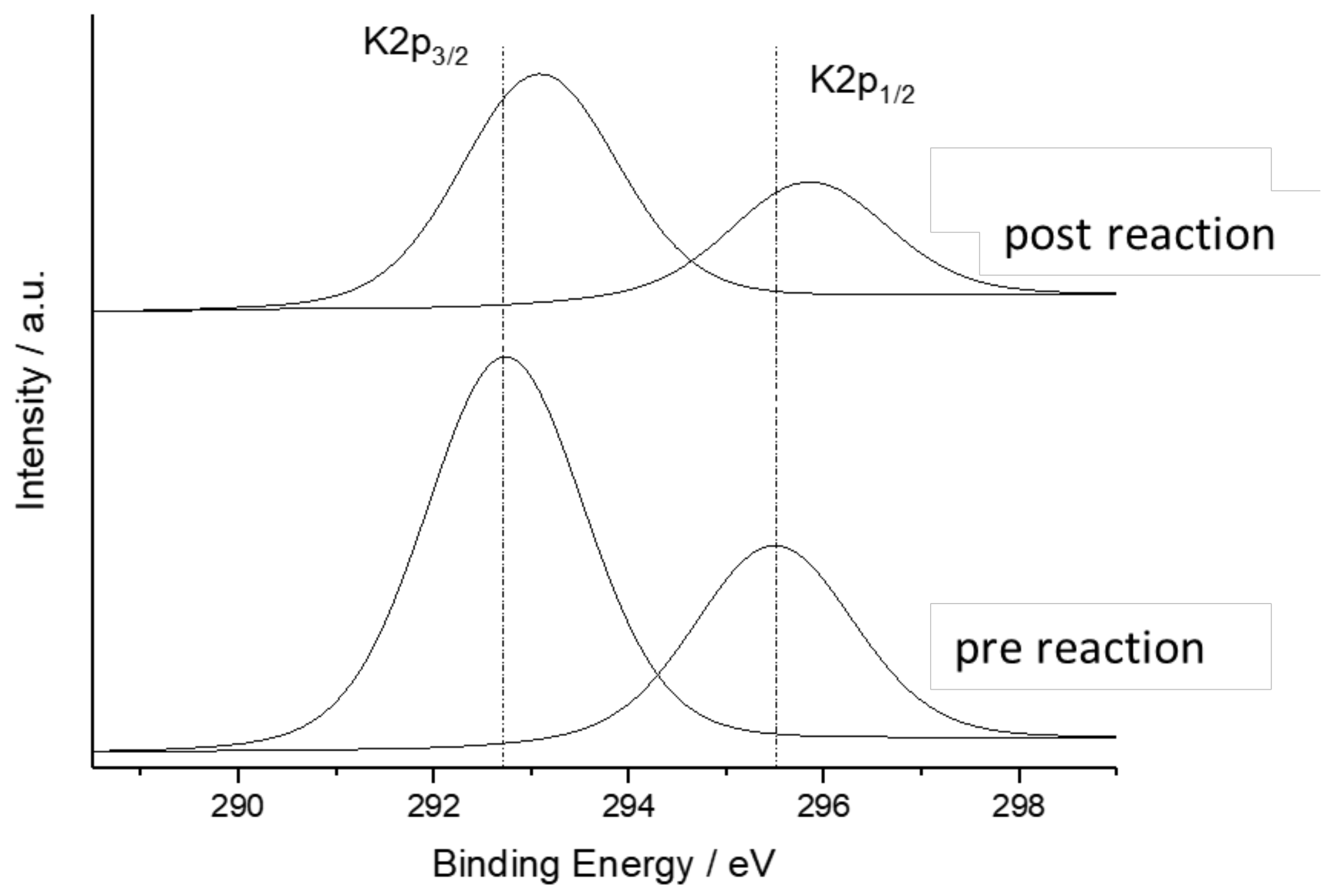
| Ag | C (Carbonate) | K | Ce | Al | O | Zr | |
|---|---|---|---|---|---|---|---|
| Before testing | 1.0 | 5.6 | 18.6 | 12.9 | 16.5 | 37.6 | 3.3 |
| After testing | 0.6 | 3.7 | 12.3 | 24.1 | 13.0 | 32.1 | 5.6 |
| K Loading in 2%Ag–K/CZA | Maximum NOx Conversion (to N2O and N2) | Onset Temperatures of Steps in Soot Oxidation |
|---|---|---|
| 0% | 50% at 320 °C | 220 °C: C+N2O 450 °C: C+NO2 525 °C: C+O2 |
| 2% | 45% at 300 °C | 375 °C: C+O2 |
| 5% | 42% at 425 °C | 375 °C: C+O2 |
| 10% | 19% at 350 °C | 375 °C: C+O2 |
| 15% | 12% at 325 °C | 375 °C: C+O2 |
| 20% | 20% at 375 °C | 300 °C: C+nitrate 375 °C: C+O2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, A.; Golunski, S.; Taylor, S.H. The Effect of Potassium Inclusion in a Silver Catalyst for N2O-Mediated Oxidation of Soot in Oxidising Exhaust Gases. Catalysts 2022, 12, 753. https://doi.org/10.3390/catal12070753
Cooper A, Golunski S, Taylor SH. The Effect of Potassium Inclusion in a Silver Catalyst for N2O-Mediated Oxidation of Soot in Oxidising Exhaust Gases. Catalysts. 2022; 12(7):753. https://doi.org/10.3390/catal12070753
Chicago/Turabian StyleCooper, Anna, Stan Golunski, and Stuart H. Taylor. 2022. "The Effect of Potassium Inclusion in a Silver Catalyst for N2O-Mediated Oxidation of Soot in Oxidising Exhaust Gases" Catalysts 12, no. 7: 753. https://doi.org/10.3390/catal12070753
APA StyleCooper, A., Golunski, S., & Taylor, S. H. (2022). The Effect of Potassium Inclusion in a Silver Catalyst for N2O-Mediated Oxidation of Soot in Oxidising Exhaust Gases. Catalysts, 12(7), 753. https://doi.org/10.3390/catal12070753







