Modified MgH2 Hydrogen Storage Properties Based on Grapefruit Peel-Derived Biochar
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of BC and rGO
2.2. Preparation of MgH2 + BC and MgH2 + rGO Composites
2.3. Characterization
3. Result and Discussion
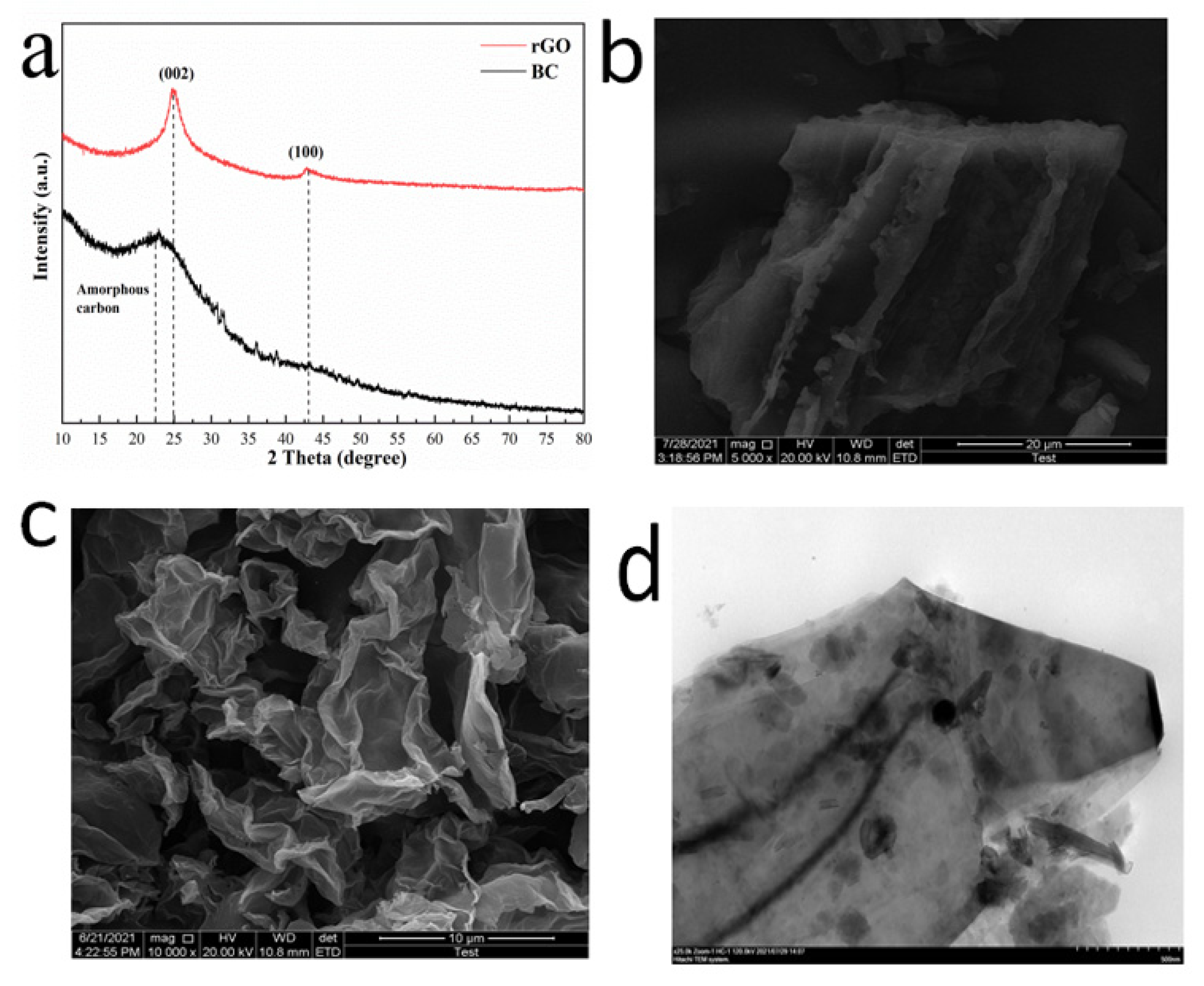
3.1. Characterization of the BC and rGO
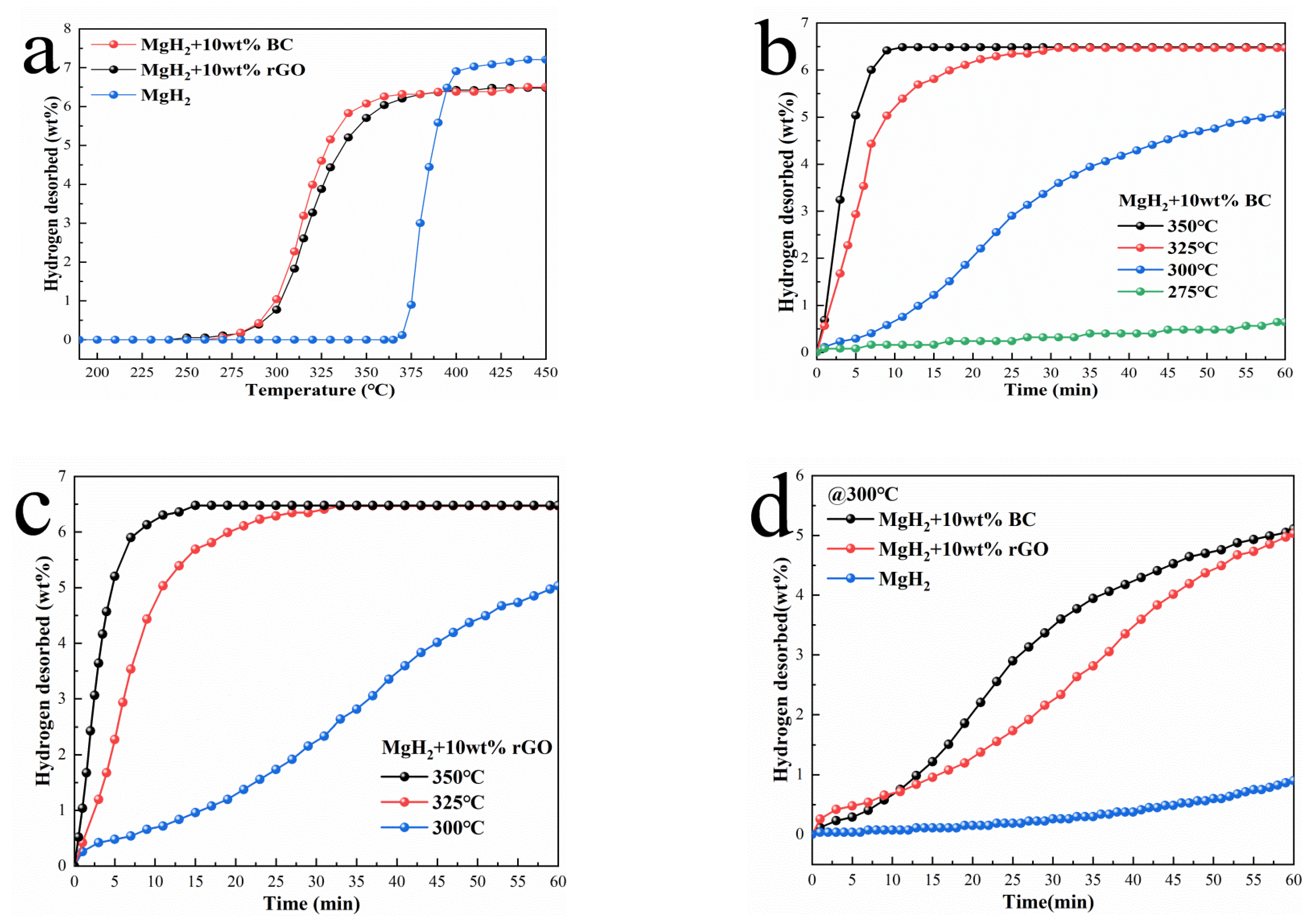
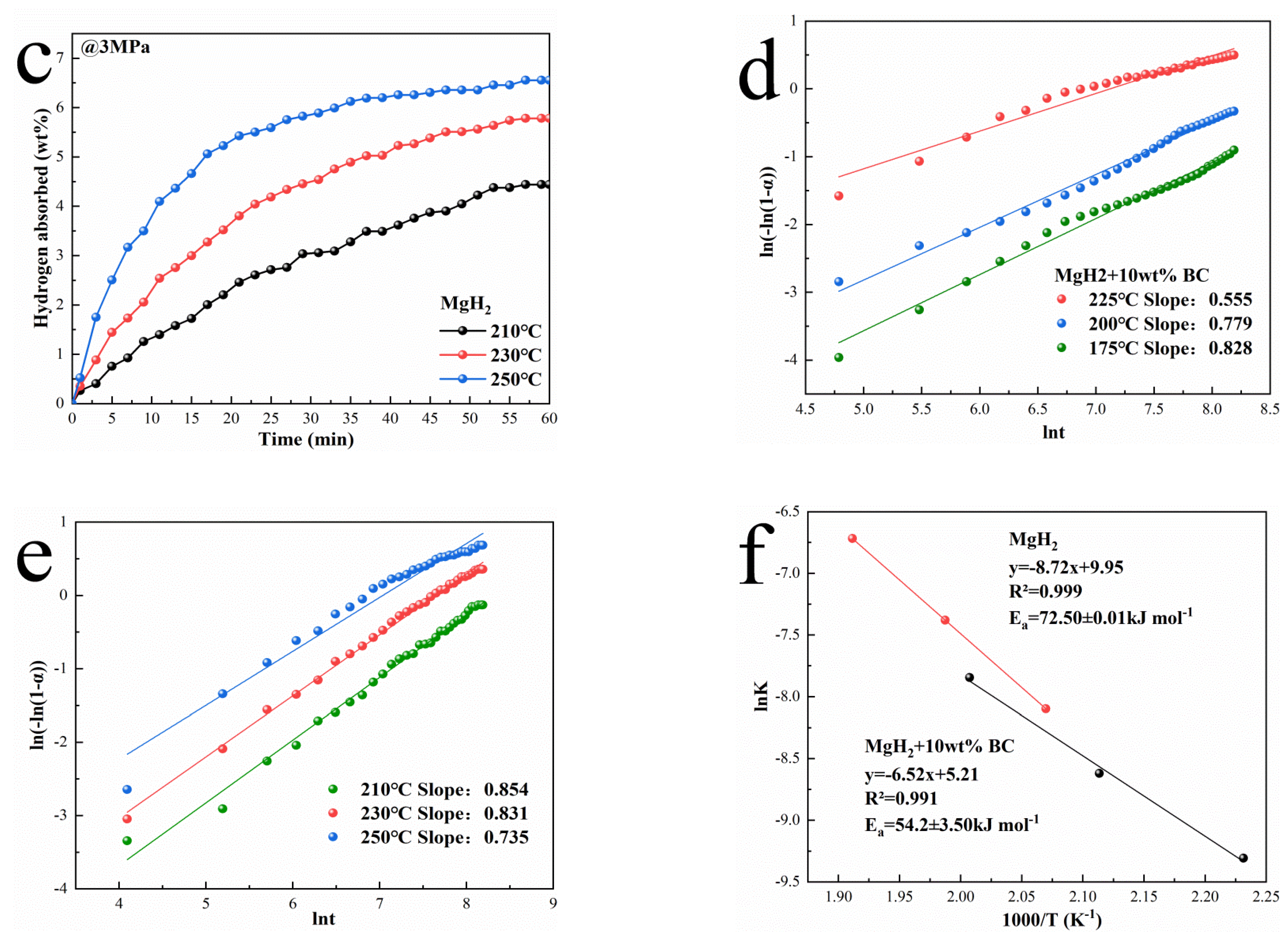
3.2. Hydrogen Desorption Performances of MgH2 + BC and MgH2 + rGO Composites
3.3. Hydrogen Absorption Performances of MgH2 + BC Composites
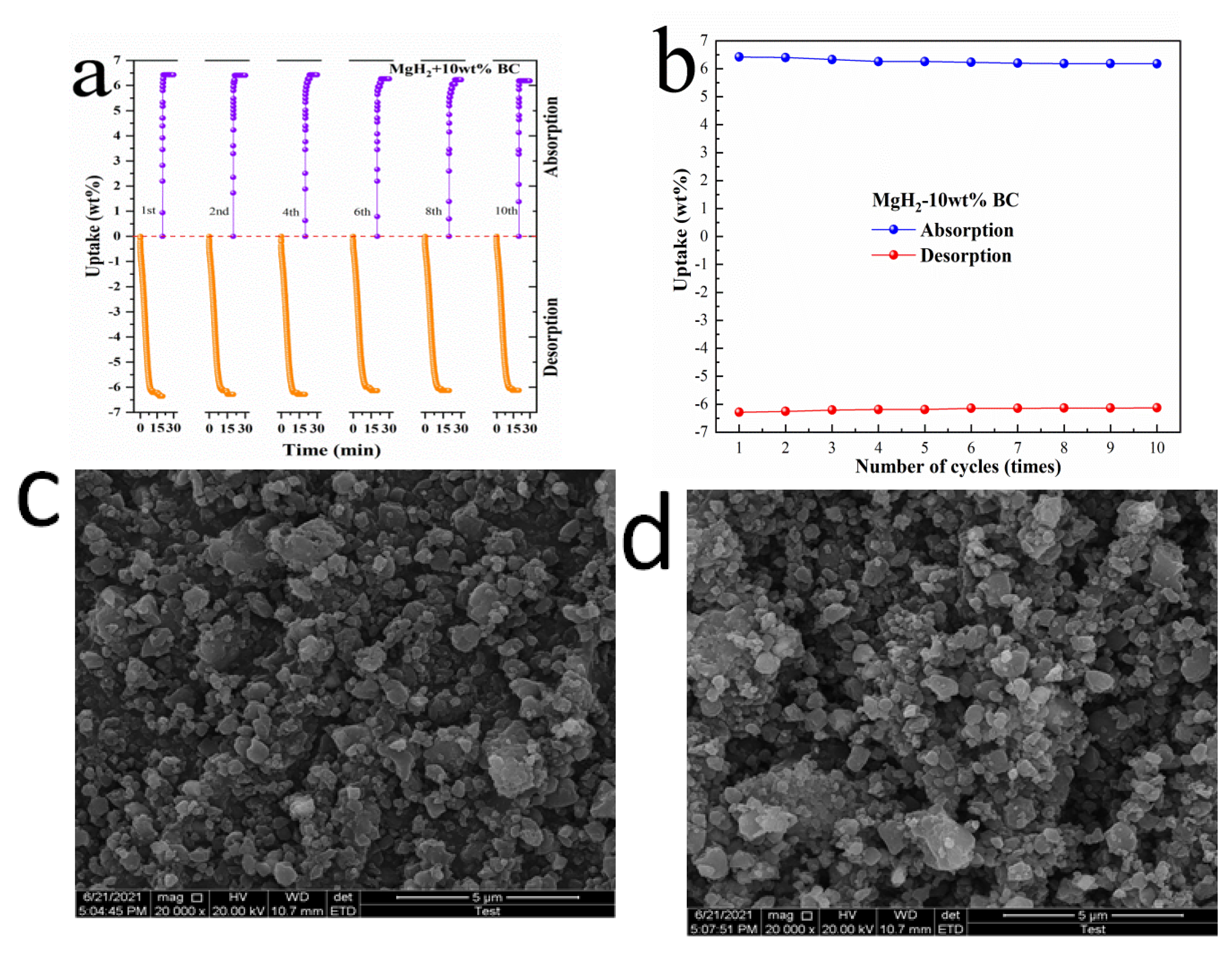
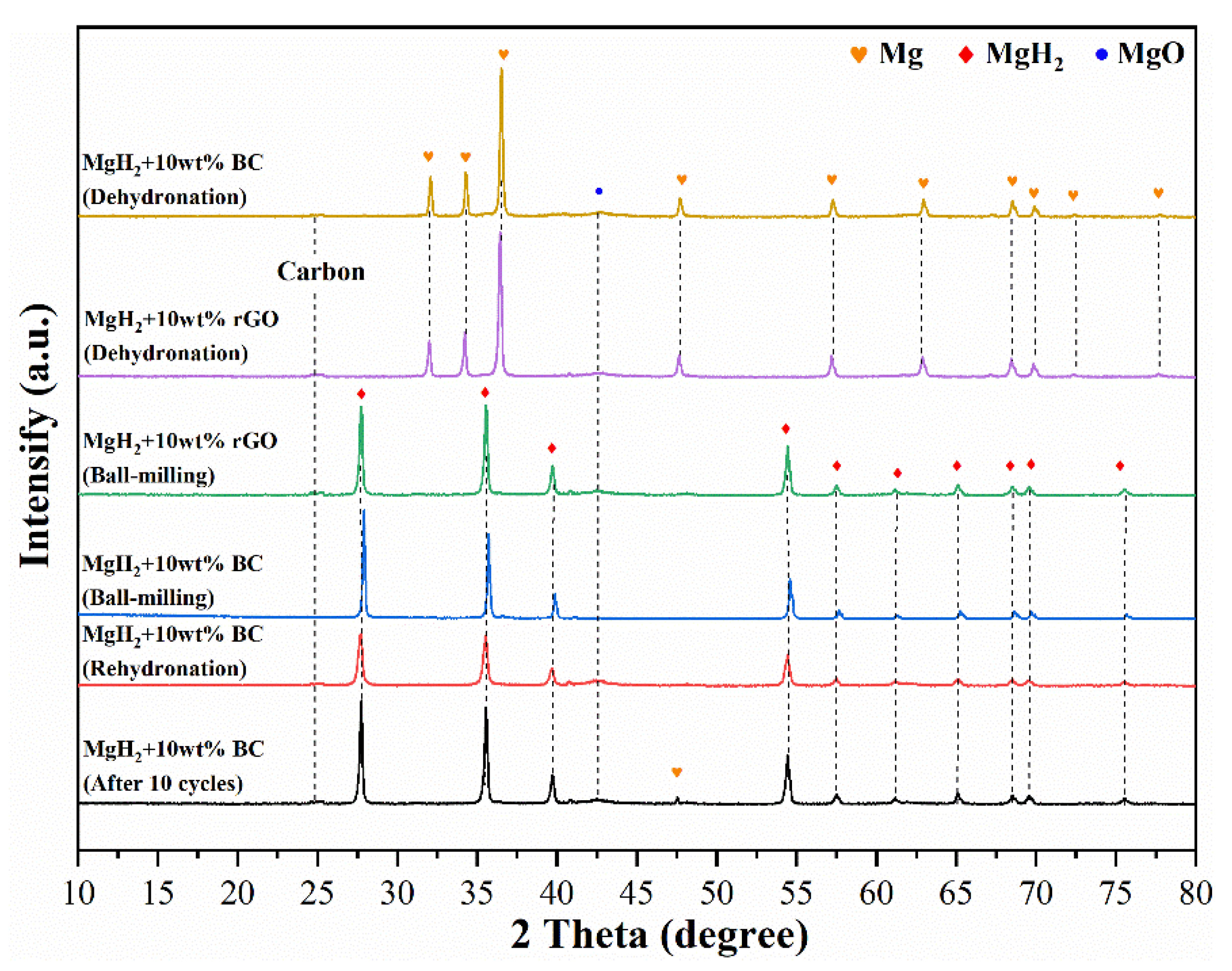
3.4. Cycling Performance of MgH2 + 10 wt%BC Composite System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atilhan, S.; Park, S.; El-Halwagi, M.M.; Atilhan, M.; Moore, M.; Nielsen, R.B. Green hydrogen as an alternative fuel for the shipping industry. Curr. Opin. Chem. Eng. 2021, 31, 100668. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Hou, Q.; Guo, X.; Xu, G. Improvement of Mg-Based Hydrogen Storage Materials by Metal Catalysts: Review and Summary. ChemistrySelect 2021, 6, 8809–8829. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Hu, J.; Gao, M.; Pan, H. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, Q.; Chang, J.; Zhang, D.; Peng, Y.; Yang, X. Improvement of hydrogen storage performance of MgH2 by MnMoO4 rod composite catalyst. Solid State Sci. 2021, 121, 106750. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. Mater. Int. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.; Guo, Z.; Aguey-Zinsou, K.; Cazorla-Amorós, D.; Linares-Solano, A. Effects of different carbon materials on MgH2 decomposition. Carbon 2008, 46, 126–137. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Ma, T.; Li, K.; Ye, F.; Wang, X.; Jiao, L.; Yuan, H.; Wang, Y. Facile synthesis of small MgH2 nanoparticles confined in different carbon materials for hydrogen storage. J. Alloy. Compd. 2020, 825, 153953. [Google Scholar] [CrossRef]
- Hou, Q.; Yang, X.; Zhang, J. Review on Hydrogen Storage Performance of MgH2: Development and Trends. ChemistrySelect 2021, 6, 1589–1606. [Google Scholar] [CrossRef]
- Wang, K.; Deng, Q. Constructing Core-Shell Co@N-Rich Carbon Additives Toward Enhanced Hydrogen Storage Performance of Magnesium Hydride. Front. Chem. 2020, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Lillo-Ródenas, M.A.; Aguey-Zinsou, K.F.; Cazorla-Amorós, D.; Linares-Solano, A.; Guo, Z.X. Effects of Carbon-Supported Nickel Catalysts on MgH2 Decomposition. J. Phys. Chem. C 2008, 112, 5984–5992. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Qiu, F.; Li, L.; Jiao, L.; Yuan, H. Synthesis of porous Ni@rGO nanocomposite and its synergetic effect on hydrogen sorption properties of MgH2. J. Mater. Chem. 2012, 22, 22542–22549. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, Z.; Ma, Z.; Ding, Y.; Zhu, Y.; Zhang, Y.; Zhang, J.; Li, L. Effect of rGO supported NiCu derived from layered double hydroxide on hydrogen sorption kinetics of MgH2. J. Alloy. Compd. 2019, 789, 768–776. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Liu, H.; He, T.; Wang, Y.; Li, S.; Yan, M. CNTs decorated with CoFeB as a dopant to remarkably improve the dehydrogenation/rehydrogenation performance and cyclic stability of MgH2. Int. J. Hydrogen Energy 2020, 45, 28964–28973. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Yu, H.; Lu, Z.; He, J.; Zheng, J.; Wu, F.; Chen, L. Achieving superior hydrogen storage properties of MgH2 by the effect of TiFe and carbon nanotubes. Chem. Eng. J. 2021, 422, 130101. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z.; Jiao, L.; Yuan, H.; Wang, Y. In situ preparation of nanocrystalline Ni@C and its effect on hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 18121–18129. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Z. The rational design of biomass-derived carbon materials towards next-generation energy storage: A review. Renew. Sustain. Energy Rev. 2020, 134, 110308. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef]
- De Barros Dias Moreira, J.; de Rezende, V.D.B.; Pasa, M.D. Deoxygenation of Macauba acid oil over Co-based catalyst supported on activated biochar from Macauba endocarp: A potential and sustainable route for green diesel and biokerosene production. Fuel 2020, 269, 117253. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Zhang, J.; Zhu, Z. Process Simulation of Preparing Biochar by Biomass Pyrolysis Via Aspen Plus and Its Economic Evaluation. Waste Biomass Valorization 2022, 13, 2609–2622. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef] [PubMed]
- Anto, S.; Sudhakar, M.; Ahamed, T.S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel 2021, 285, 119205. [Google Scholar] [CrossRef]
- Hou, Q.; Yang, X.; Zhang, J.; Yang, W.; Lv, E. Catalytic effect of NiO/C derived from Ni-UMOFNs on the hydrogen storage performance of magnesium hydride. J. Alloy. Compd. 2022, 899, 163314. [Google Scholar] [CrossRef]
- Yang, X.; Hou, Q.; Yu, L.; Zhang, J. Improvement of the hydrogen storage characteristics of MgH2 with a flake Ni nano-catalyst composite. Dalton Trans. 2021, 50, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhang, L.; Yang, X.; Zhu, X.; Chen, L. The remarkably improved hydrogen storage performance of MgH2 by the synergetic effect of an FeNi/rGO nanocomposite. Dalton Trans. 2020, 49, 4146–4154. [Google Scholar] [CrossRef]
- Faleiros, A.C.; Rabelo, T.N.; Thim, G.P.; Oliveira, M. Kinetics of phase change. Mater. Res. 2000, 3, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Ji, L.; Yan, N.; Sun, Z.; Lu, X.; Zhang, L.; Zhu, X.; Chen, L. Superior catalytic effects of FeCo nanosheets on MgH2 for hydrogen storage. Dalton Trans. 2019, 48, 12699–12706. [Google Scholar] [CrossRef]
- Singh, M.K.; Bhatnagar, A.; Pandey, S.K.; Mishra, P.; Srivastava, O. Experimental and first principle studies on hydrogen desorption behavior of graphene nanofibre catalyzed MgH2. Int. J. Hydrogen Energy 2017, 42, 960–968. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, Q.; Guo, X.; Yang, X. Achieve high-efficiency hydrogen storage of MgH2 catalyzed by nanosheets CoMoO4 and rGO. J. Alloy. Compd. 2022, 911, 165153. [Google Scholar] [CrossRef]
- Hou, Q.; Zhang, J.; Guo, X.; Yang, X. Improved MgH2 kinetics and cyclic stability by fibrous spherical NiMoO4 and rGO. J. Taiwan Inst. Chem. Eng. 2022, 134, 104311. [Google Scholar] [CrossRef]
- Hou, Q.; Zhang, J.; Guo, X.; Xu, G.; Yang, X. Synthesis of low-cost biomass charcoal-based Ni nanocatalyst and evaluation of their kinetic enhancement of MgH2. Int. J. Hydrogen Energy 2022, 47, 15209–15223. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Hou, Q.; Guo, X.; Yang, X. Modified MgH2 Hydrogen Storage Properties Based on Grapefruit Peel-Derived Biochar. Catalysts 2022, 12, 517. https://doi.org/10.3390/catal12050517
Zhang J, Hou Q, Guo X, Yang X. Modified MgH2 Hydrogen Storage Properties Based on Grapefruit Peel-Derived Biochar. Catalysts. 2022; 12(5):517. https://doi.org/10.3390/catal12050517
Chicago/Turabian StyleZhang, Jiaqi, Quanhui Hou, Xintao Guo, and Xinglin Yang. 2022. "Modified MgH2 Hydrogen Storage Properties Based on Grapefruit Peel-Derived Biochar" Catalysts 12, no. 5: 517. https://doi.org/10.3390/catal12050517
APA StyleZhang, J., Hou, Q., Guo, X., & Yang, X. (2022). Modified MgH2 Hydrogen Storage Properties Based on Grapefruit Peel-Derived Biochar. Catalysts, 12(5), 517. https://doi.org/10.3390/catal12050517




