Low-Temperature NH3-SCR on Cex-Mn-Tiy Mixed Oxide Catalysts: Improved Performance by the Mutual Effect between Ce and Ti
Abstract
:1. Introduction
2. Results and Discussion
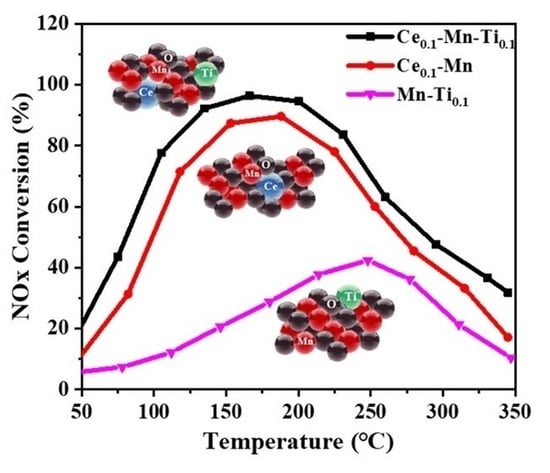
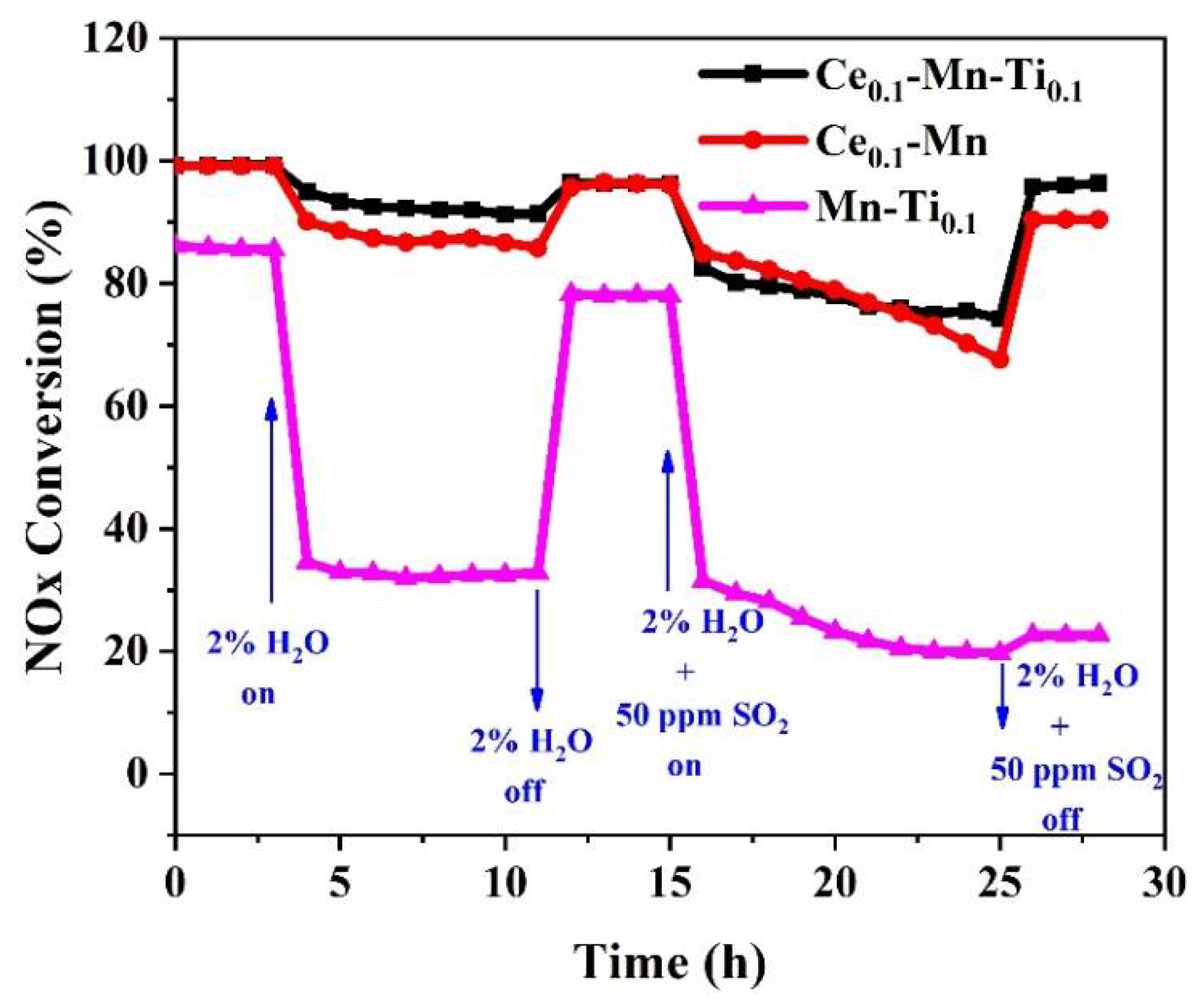
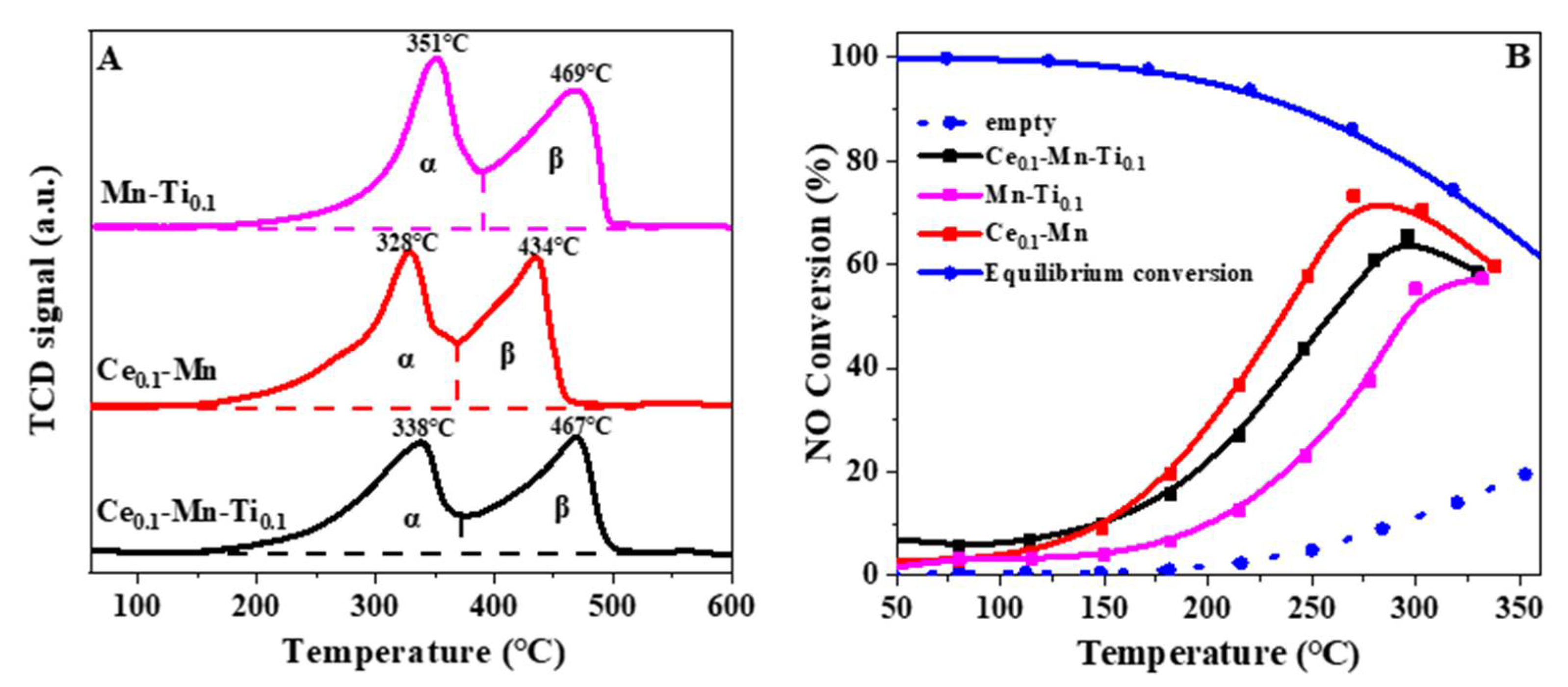
2.1. Effects of Ce and Ti on Catalytic Activity and SO2/H2O Resistance
2.2. Physicochemical Properties of Cex-Mn-Tiy Catalysts
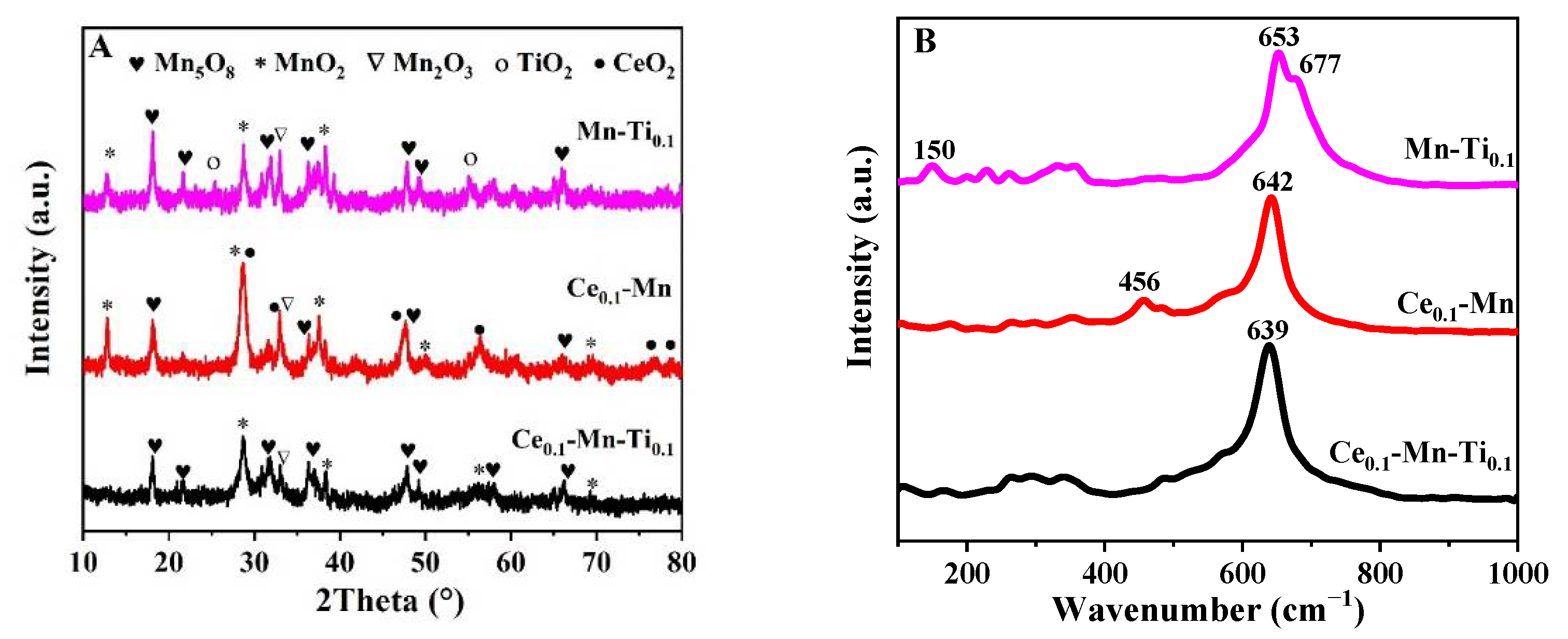
2.2.1. X-ray Diffraction
2.2.2. Raman Spectroscopy
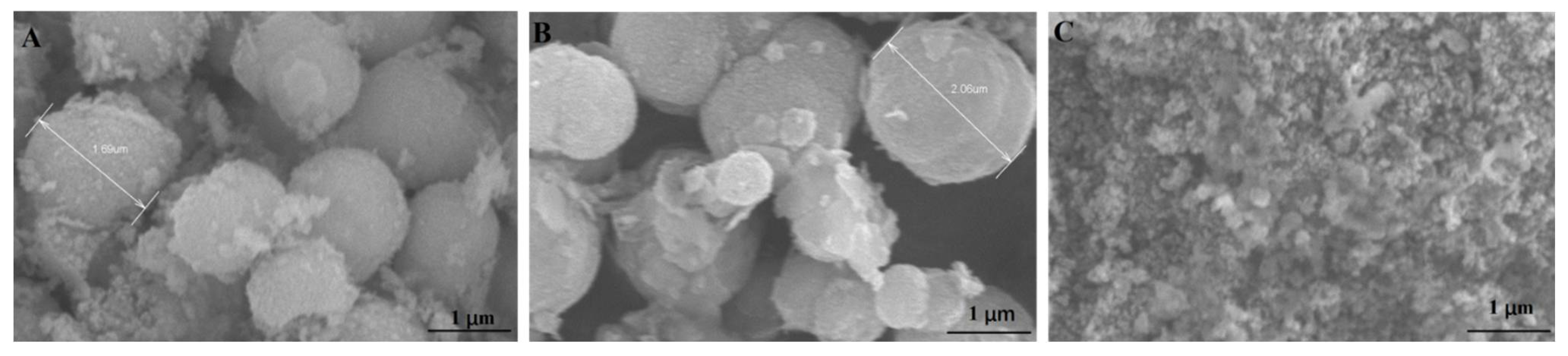
2.2.3. Scanning Electron Microscopy and N2 Adsorption–Desorption
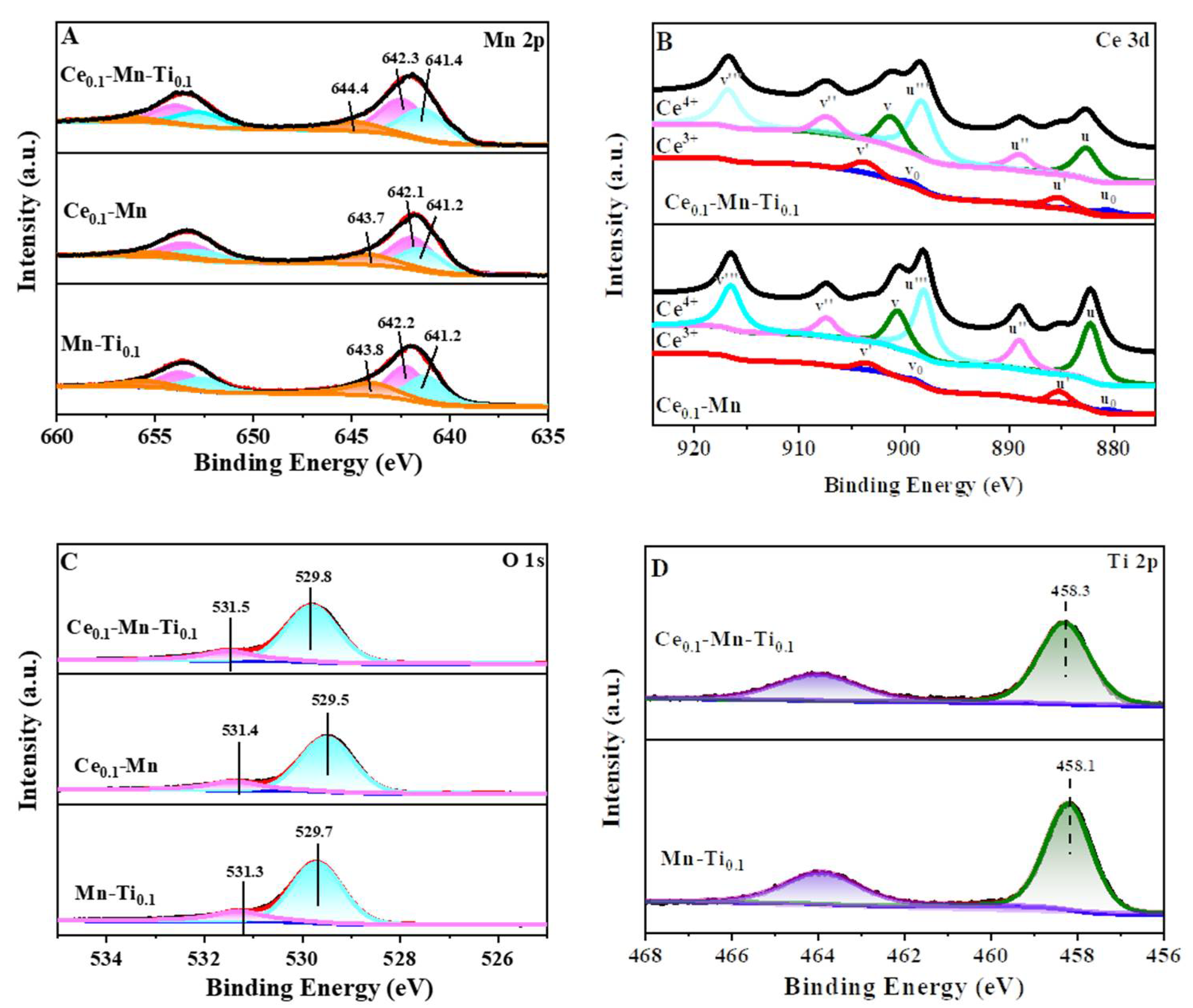
2.2.4. X-ray Photoelectron Spectroscopy
2.3. Redox Ability and Acidity of the Cex-Mn-Tiy Catalysts
2.3.1. H2 Temperature-Programmed Reduction and NO Oxidation Reaction
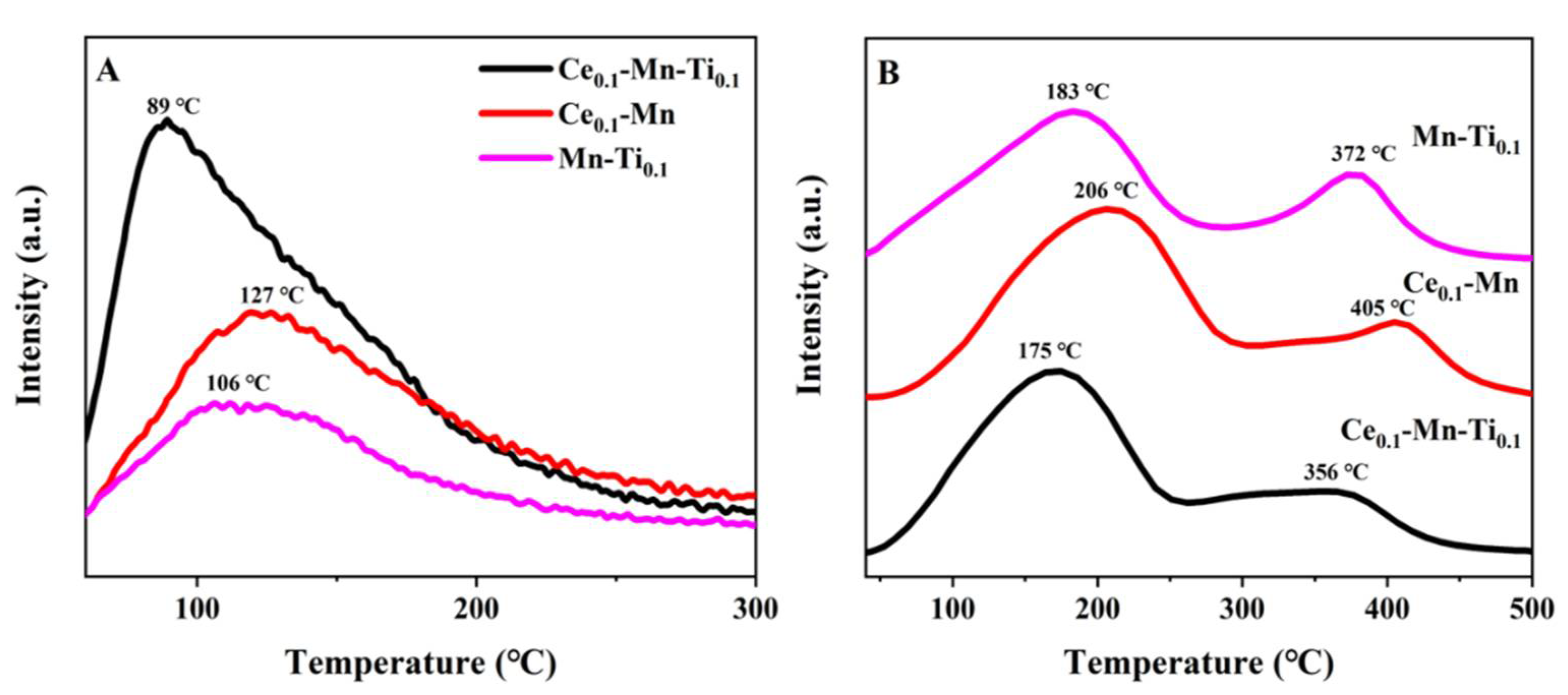
2.3.2. NH3/NO Temperature-Programmed Desorption
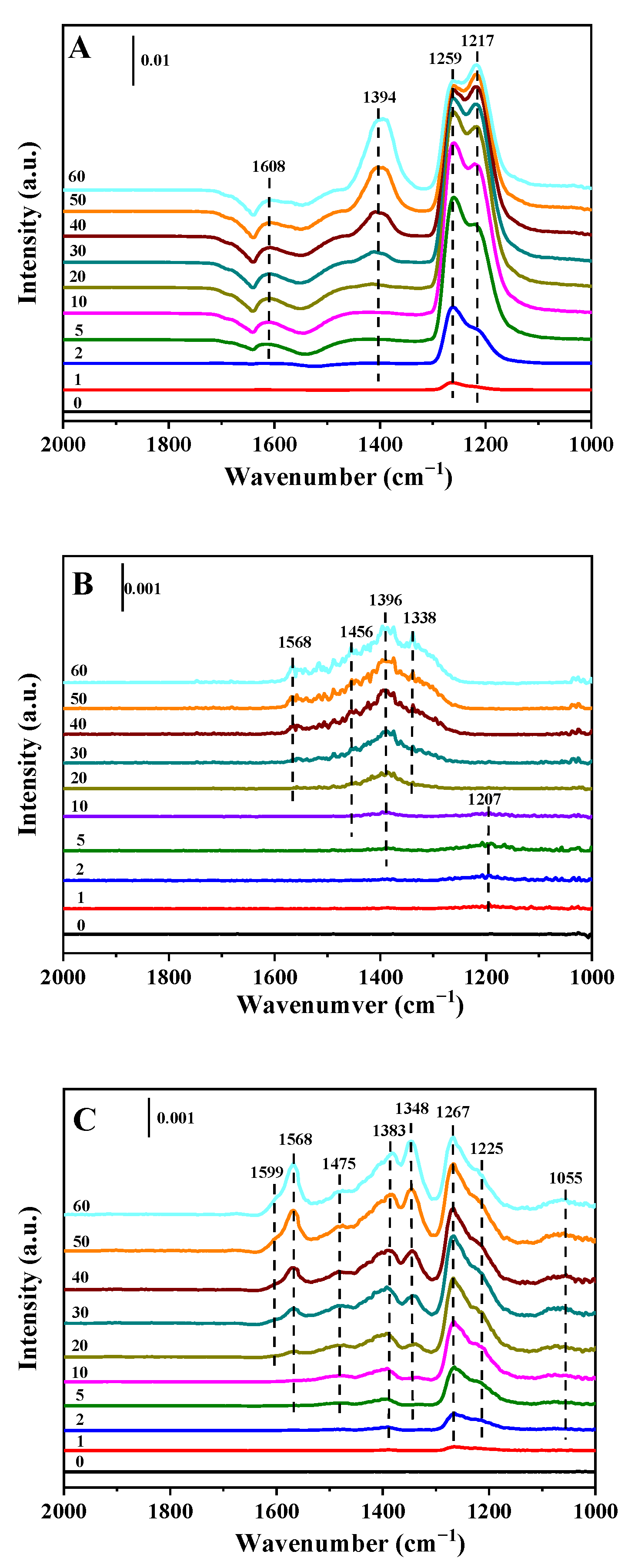
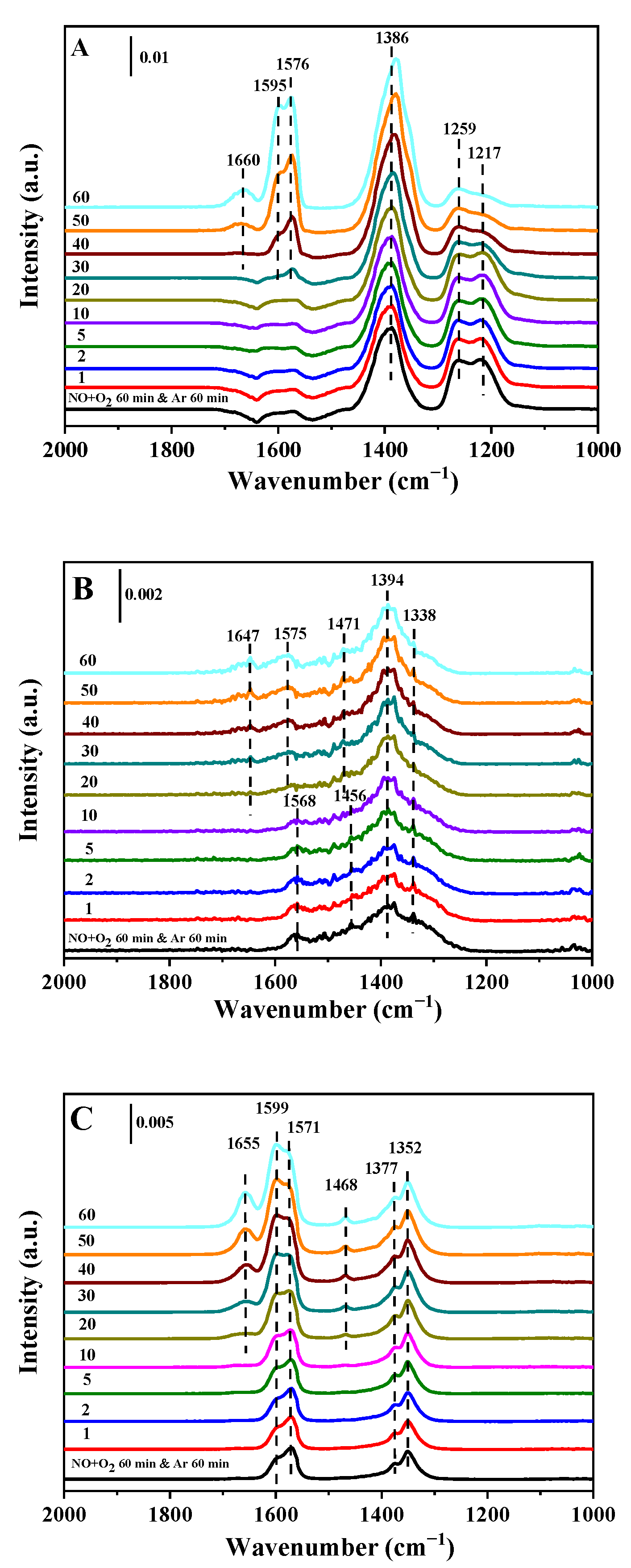
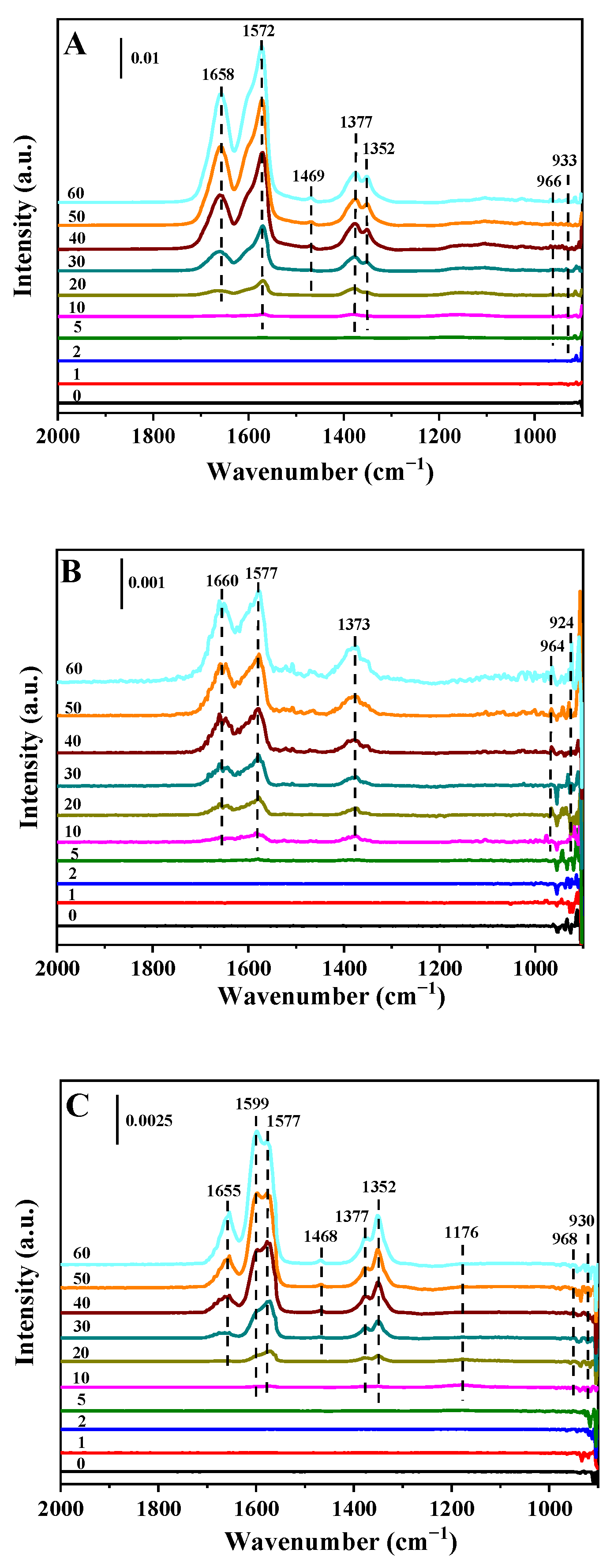
2.4. In Situ DRIFT Spectroscopy
2.4.1. Adsorption of NO + O2 Followed by NH3
2.4.2. Adsorption of NH3 Followed by NO + O2
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.L.; Ding, L.; Long, H.M.; Li, J.X.; Tan, W.; Ji, J.W.; Sun, J.F.; Tang, C.J.; Dong, L. Influence of CeO2 loading on structure and catalytic activity for NH3-SCR over TiO2-supported CeO2. J. Rare Earths 2020, 38, 883–890. [Google Scholar] [CrossRef]
- Han, M.J.; Jiao, Y.L.; Zhou, C.H.; Guo, Y.L.; Guo, Y.; Lu, G.Z.; Wang, L.; Zhan, W.C. Catalytic activity of Cu-SSZ-13 prepared with different methods for NH3-SCR reaction. Rare Met. 2018, 38, 210–220. [Google Scholar] [CrossRef]
- Zhang, N.Q.; Li, L.C.; Guo, Y.Z.; He, J.D.; Wu, R.; Song, L.Y.; Zhang, G.Z.; Zhao, J.S.; Wang, D.S.; He, H. A MnO2-based catalyst with H2O resistance for NH3-SCR: Study of catalytic activity and reactants-H2O competitive adsorption. Appl. Catal. B 2020, 270, 118860. [Google Scholar] [CrossRef]
- Sun, P.; Guo, R.T.; Liu, S.M.; Wang, S.X.; Pan, W.G.; Li, M.Y. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu. Appl. Catal. A 2017, 531, 129–138. [Google Scholar] [CrossRef]
- Shao, X.Z.; Wang, H.Y.; Yuan, M.L.; Yang, J.; Zhan, W.C.; Wang, L.; Guo, Y.; Lu, G.Z. Thermal stability of Si-doped V2O5/WO3-TiO2 for selective catalytic reduction of NOx by NH3. Rare Met. 2018, 38, 292–298. [Google Scholar] [CrossRef]
- Han, L.P.; Cai, S.X.; Gao, M.; Hasegawa, J.Y.; Wang, P.L.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Wang, H.; Chen, J.; Wen, J.; Xu, S.; Tian, X.; Gao, L.; Hou, Z.; Zhang, Q.; et al. Enhanced performance of iron-cerium NO reduction catalysts by sulfuric acid treatment: The synergistic effect of surface acidity and redox capacity. Appl. Catal. A 2021, 621, 118200. [Google Scholar] [CrossRef]
- Chen, Y.X.; Li, C.; Chen, J.X.; Tang, X.F. Self-Prevention of Well-Defined-Facet Fe2O3/MoO3 against Deposition of Ammonium Bisulfate in Low-Temperature NH3-SCR. Environ. Sci. Technol. 2018, 52, 11796–11802. [Google Scholar] [CrossRef]
- Huang, L.; Hu, X.N.; Yuan, S.; Li, H.R.; Yan, T.T.; Shi, L.Y.; Zhang, D.S. Photocatalytic preparation of nanostructured MnO2-(Co3O4)/TiO2 hybrids: The formation mechanism and catalytic application in SCR deNOx reaction. Appl. Catal. B 2017, 203, 778–788. [Google Scholar] [CrossRef]
- Meng, D.M.; Xu, Q.; Jiao, Y.L.; Guo, Y.; Guo, Y.L.; Wang, L.; Lu, G.Z.; Zhan, W.C. Spinel structured CoaMnbOx mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2018, 221, 652–663. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, C.; Pang, L.; Ming, S.J.; Liu, P.; Zhu, D.J.; Wang, J.Y.; Cai, X.; Chen, H.P.; Lai, Y.H.; et al. Direct synthesis of submicron Cu-SAPO-34 as highly efficient and robust catalyst for selective catalytic reduction of NO by NH3. Appl. Surf. Sci. 2018, 448, 671–680. [Google Scholar] [CrossRef]
- Huang, L.M.; Wang, X.M.; Yao, S.L.; Jiang, B.Q.; Chen, X.Y.; Wang, X. Cu-Mn bimetal ion-exchanged SAPO-34 as an active SCR catalyst for removal of NOx from diesel engine exhausts. Catal. Commun. 2016, 81, 54–57. [Google Scholar] [CrossRef]
- Meng, D.M.; Zhan, W.C.; Guo, Y.; Guo, Y.L.; Wang, Y.S.; Wang, L.; Lu, G.Z. A highly effective catalyst of Sm-Mn mixed oxide for the selective catalytic reduction of NOx with ammonia: Effect of the calcination temperature. J. Mol. Catal. A 2016, 420, 272–281. [Google Scholar] [CrossRef]
- Tong, Y.M.; Li, Y.S.; Li, Z.B.; Wang, P.Q.; Zhang, Z.P.; Zhao, X.Y.; Yuan, F.L.; Zhu, Y.J. Influence of Sm on the low temperature NH3-SCR of NO activity and H2O/SO2 resistance over the SmaMnNi2Ti7Ox (a = 0.1, 0.2, 0.3, 0.4) catalysts. Appl. Catal. A 2020, 590, 117333. [Google Scholar] [CrossRef]
- Hu, H.; Cai, S.X.; Li, H.R.; Huang, L.; Shi, L.Y.; Zhang, D.S. Mechanistic Aspects of deNOx Processing over TiO2 Supported Co-Mn Oxide Catalysts: Structure-Activity Relationships and In Situ DRIFTs Analysis. ACS Catal. 2015, 5, 6069–6077. [Google Scholar] [CrossRef]
- Anand, A.; Manjuladevi, M.; Veena, R.; Veena, V.; Koshkid’ko, Y.; Sagar, S. The influence of Ti doping at the Mn site on structural, magnetic, and magnetocaloric properties of Sm0.6Sr0.4MnO3. J. Solid State Chem. 2022, 305, 122712. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Dai, Q.G.; Zhao, H.L.; Chai, G.T.; Guo, Y.L.; Guo, Y.; Wang, L.; Zhan, W.C. Catalytic oxidation of chlorinated volatile organic compounds over Mn-Ti composite oxides catalysts: Elucidating the influence of surface acidity. Appl. Catal. B 2021, 282, 119577. [Google Scholar] [CrossRef]
- Yan, Q.H.; Chen, S.N.; Zhang, C.; Wang, Q.; Louis, B. Synthesis and catalytic performance of Cu1Mn0.5Ti0.5Ox mixed oxide as low-temperature NH3-SCR catalyst with enhanced SO2 resistance. Appl. Catal. B 2018, 238, 236–247. [Google Scholar] [CrossRef]
- Li, L.; Li, P.; Tan, W.; Ma, K.; Zou, W.; Tang, C.; Dong, L. Enhanced low-temperature NH3-SCR performance of CeTiOx catalyst via surface Mo modification. Chin. J. Catal. 2020, 41, 364–373. [Google Scholar] [CrossRef]
- Yao, X.; Cao, J.; Chen, L.; Kang, K.; Chen, Y.; Tian, M.; Yang, F. Doping effect of cations (Zr4+, Al3+, and Si4+) on MnOx/CeO2 nano-rod catalyst for NH3-SCR reaction at low temperature. Chin. Catal. 2019, 40, 733–743. [Google Scholar] [CrossRef]
- Xu, Q.; Fang, Z.L.; Chen, Y.Y.; Guo, Y.L.; Guo, Y.; Wang, L.; Wang, Y.S.; Zhang, J.S.; Zhan, W.C. Titania-Samarium-Manganese composite oxide for the low-temperature selective catalytic reduction of NO with NH3. Environ. Sci. Technol. 2020, 54, 2530–2538. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, J.; Li, J.; Ma, L.; Woo, S.I. Novel Mn-Ce-Ti mixed-oxide catalyst for the selective catalytic reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2014, 6, 14500–14508. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yao, X.J.; Cao, J.; Yang, F.M.; Tang, C.J.; Dong, L. Effect of Ti4+ and Sn4+ co-incorporation on the catalytic performance of CeO2-MnOx catalyst for low temperature NH3-SCR. Appl. Surf. Sci. 2019, 476, 283–292. [Google Scholar] [CrossRef]
- Jiang, H.X.; Wang, J.; Zhou, J.L.; Chen, Y.F.; Zhang, M.H. Effect of Promoters on the Catalytic Performance and SO2/H2O Resistance of α-MnO2 Catalysts for Low Temperature NH3-SCR. Ind. Eng. Chem. Res. 2019, 58, 1760–1768. [Google Scholar] [CrossRef]
- Shi, Y.R.; Yi, H.H.; Gao, F.Y.; Zhao, S.Z.; Xie, Z.L.; Tang, X.L. Facile synthesis of hollow nanotube MnCoOx catalyst with superior resistance to SO2 and alkali metal poisons for NH3-SCR removal of NOx. Sep. Purif. Technol. 2021, 265, 118517. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Liu, X.Q.; Qi, K.; Xie, J.L.; Li, F.X.; Yu, C.Q. Low temperature NH3-SCR of NO over an unexpected Mn-based catalyst: Promotional effect of Mg doping. Appl. Surf. Sci. 2018, 427, 45–55. [Google Scholar] [CrossRef]
- Ding, J.B.; Yang, X.W.; Wang, A.Y.; Yang, C.; Guo, Y.L.; Guo, Y.; Wang, L.; Zhan, W.C. Sm-MnOx catalysts for low-temperature selective catalytic reduction of NO with NH3: Effect of precipitation agent. J. Rare Earths 2021. [Google Scholar] [CrossRef]
- Wei, L.S.; Li, J.H.; Tang, X.F. NOx Storage at Low Temperature over MnOx-SnO2 Binary Metal Oxide Prepared Through Different Hydrothermal Process. Catal. Lett. 2008, 127, 107–112. [Google Scholar] [CrossRef]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.H.; Guang, J.Y.; Guo, D.W.; Wang, L.F.; Li, X.H. Ceria modified FeMnOx-Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B Environ. 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Skala, T.; Sutara, F.; Skoda, M.; Prince, K.C.; Matolin, V. Palladium interaction with CeO2, Sn-Ce-O and Ga-Ce-O layers. J. Phys. Condens Matter. 2009, 21, 055005. [Google Scholar] [CrossRef]
- Sun, P.; Huang, S.X.; Guo, R.T.; Li, M.Y.; Liu, S.M.; Pan, W.G.; Fu, Z.G.; Liu, S.W.; Sun, X.; Liu, J. The enhanced SCR performance and SO2 resistance of Mn/TiO2 catalyst by the modification with Nb: A mechanistic study. Appl. Surf. Sci. 2018, 447, 479–488. [Google Scholar] [CrossRef]
- Li, G.; Mao, D.S.; Chao, M.X.; Li, G.H.; Yu, J.; Guo, X.M. Low-temperature NH3-SCR of NO over MnCeOx/TiO2 catalyst: Enhanced activity and SO2 tolerance by modifying TiO2 with Al2O3. J. Rare Earths 2021, 39, 805–816. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, C.; Zhao, J.C.; Yang, L.Z.; Sun, K.A.; Zeng, L.Y.; Pan, Y.; Liu, Y.Q.; Liu, C.G. Study on the NO2 production pathways and the role of NO2 in fast selective catalytic reduction DeNOx at low-temperature over MnOx/TiO2 catalyst. Chem. Eng. J. 2020, 379, 122288. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Gao, F.Y.; Yang, C.; Tang, X.L.; Yi, H.H.; Wang, C.Z. Co- or Ni-modified Sn-MnOx low-dimensional multi-oxides for high-efficient NH3-SCR De-NOx: Performance optimization and reaction mechanism. J. Environ. Sci. 2022, 113, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.J.; Liang, Y.; Liu, W.Z.; Wu, H.L.; Aldahri, T.; Carrero, D.S.; Liu, Q.C. Synergistic effect and mechanism of FeOx and CeOx co-doping on the superior catalytic performance and SO2 tolerance of Mn-Fe-Ce/ACN catalyst in low-temperature NH3-SCR of NOx. J. Environ. Chem. Eng. 2021, 9, 106360. [Google Scholar] [CrossRef]
- Yang, S.; Qi, F.; Xiong, S.; Dang, H.; Liao, Y.; Wong, P.K.; Li, J. MnOx supported on Fe–Ti spinel: A novel Mn based low temperature SCR catalyst with a high N2 selectivity. Appl. Catal. B Environ. 2016, 181, 570–580. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H.; Chen, W.; Chen, D.; Yu, S.; Liu, A.; Dong, L.; Feng, S. Insights into the Sm/Zr co-doping effects on N2 selectivity and SO2 resistance of a MnOx-TiO2 catalyst for the NH3-SCR reaction. Chem. Eng. J. 2018, 347, 27–40. [Google Scholar] [CrossRef]
- Sounak, R.; Viswanath, B.; Hegde, M.S.; Girigdar, M. Low-Temperature Selective Catalytic Reduction of NO with NH3 over Ti0.9M0.1O2−δ (M= Cr, Mn, Fe, Co, Cu). J. Phys. Chem. C 2008, 112, 6002–6012. [Google Scholar]
- Wang, X.B.; Duan, R.B.; Liu, W.; Wang, D.W.; Wang, B.R.; Xu, Y.R.; Niu, C.H.; Shi, J.W. The insight into the role of CeO2 in improving low-temperature catalytic performance and SO2 tolerance of MnCoCeOx microflowers for the NH3-SCR of NOx. Appl. Surf. Sci. 2020, 510, 145517. [Google Scholar] [CrossRef]
- Li, Y.L.; Han, X.J.; Hou, Y.Q.; Guo, Y.P.; Liu, Y.J.; Cui, Y.; Huang, Z.G. Role of CTAB in the improved H2O resistance for selective catalytic reduction of NO with NH3 over iron titanium catalyst. Chem. Eng. J. 2018, 347, 313–321. [Google Scholar] [CrossRef]
- Xiong, S.C.; Peng, Y.; Wang, D.; Huang, N.; Zhang, Q.F.; Yang, S.J.; Chen, J.J.; Li, J.H. The role of the Cu dopant on a Mn3O4 spinel SCR catalyst: Improvement of low-temperature activity and sulfur resistance. Chem. Eng. J. 2020, 387, 124090. [Google Scholar] [CrossRef]
- Guo, R.T.; Li, M.Y.; Sun, P.; Pan, W.G.; Liu, S.M.; Liu, J.; Sun, X.; Liu, S.W. Mechanistic Investigation of the Promotion Effect of Bi Modification on the NH3-SCR Performance of Ce/TiO2 Catalyst. J. Phys. Chem. C 2017, 121, 27535–27545. [Google Scholar] [CrossRef]
- Wang, B.; Wang, M.X.; Han, L.N.; Hou, Y.Q.; Bao, W.R.; Zhang, C.M.; Feng, G.; Chang, L.P.; Huang, Z.G.; Wang, J.C. Improved Activity and SO2 Resistance by Sm-Modulated Redox of MnCeSmTiOx Mesoporous Amorphous Oxides for Low-Temperature NH3-SCR of NO. ACS Catal. 2020, 10, 9034–9045. [Google Scholar] [CrossRef]
- Wang, F.; Xie, Z.B.; Liang, J.S.; Fang, B.Z.; Piao, Y.A.; Hao, M.; Wang, Z.S. Tourmaline-Modified FeMnTiOx Catalysts for Improved Low-Temperature NH3-SCR Performance. Environ. Sci. Technol. 2019, 53, 6989–6996. [Google Scholar] [CrossRef]
- Sun, X.; Guo, R.T.; Liu, J.; Fu, Z.G.; Liu, S.W.; Pan, W.G.; Shi, X.; Qin, H.; Wang, Z.Y.; Liu, X.Y. The enhanced SCR performance of Mn/TiO2 catalyst by Mo modification: Identification of the promotion mechanism. Int. J. Hydrogen Energy 2018, 43, 16038–16048. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.W.; Fan, Z.Y.; Wang, B.R.; Wang, Y.; He, C.; Wang, X.B.; Li, J.; Niu, C.M. “Fast SCR” reaction over Sm-modified MnOx-TiO2 for promoting reduction of NOx with NH3. Appl. Catal. A Gen. 2018, 564, 102–112. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Ren, S.; Chen, Z.C.; Zhou, Y.H.; Liu, W.Z. Effects of Sm modification on biochar supported Mn oxide catalysts for low-temperature NH3-SCR of NO. J. Energy Inst. 2021, 98, 234–243. [Google Scholar] [CrossRef]
- Qiu, M.Y.; Zhan, S.H.; Yu, H.B.; Zhu, D.D.; Wang, S.Q. Facile preparation of ordered mesoporous MnCo2O4 for low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2015, 7, 2568–2577. [Google Scholar] [CrossRef]
- Guo, M.Y.; Zhao, P.P.; Liu, Q.L.; Liu, C.X.; Han, J.F.; Ji, N.; Song, C.F.; Ma, D.G.; Lu, X.B.; Liang, X.Y.; et al. Improved Low-Temperature Activity and H2O Resistance of Fe-Doped Mn-Eu Catalysts for NO Removal by NH3-SCR. ChemCatChem 2019, 11, 4954–4965. [Google Scholar] [CrossRef]


| Sample | SBET (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | Mole Ratio a | |
|---|---|---|---|---|---|
| Ce/Mn | Ti/Mn | ||||
| Ce0.1-Mn-Ti0.1 | 52.46 | 17.7 | 0.23 | 0.12 | 0.12 |
| Ce0.1-Mn | 46.61 | 16.9 | 0.20 | 0.12 | - |
| Mn-Ti0.1 | 29.45 | 25.1 | 0.18 | - | 0.12 |
| Sample | Surface Atom Concentration b | ||||
|---|---|---|---|---|---|
| Ce/Mn | Ti/Mn | Mn4+/(Mn4+ + Mn3+) | OS/(OS + OL) | Ce3+/(Ce4+ + Ce3+) | |
| Ce0.1-Mn-Ti0.1 | 0.29 | 0.14 | 60.1% | 16.6% | 14.7% |
| Ce0.1-Mn | 0.32 | - | 58.9% | 12.6% | 6.9% |
| Mn-Ti0.1 | - | 0.16 | 55.7% | 13.2% | - |
| Sample | α (T/°C) | Curve Area β (T/°C) | α/β | H2 Consumption (mmol/g) |
|---|---|---|---|---|
| Ce0.1-Mn-Ti0.1 | 2.79 (338) | 2.56 (467) | 1.08 | 5.35 |
| Ce0.1-Mn | 3.65 (328) | 2.91 (434) | 1.25 | 6.56 |
| Mn-Ti0.1 | 3.46 (351) | 3.12 (469) | 1.11 | 6.58 |
| Sample | Curve Area of NH3-TPD × 103 | Area/SBET(NH3) | Curve Area of NO-TPD × 107 | Area/SBET(NO) × 105 |
|---|---|---|---|---|
| Ce0.1-Mn-Ti0.1 | 9.6 | 183 | 1.8 | 3.5 |
| Ce0.1-Mn | 6.0 | 129 | 2.0 | 4.3 |
| Mn-Ti0.1 | 3.5 | 118 | 1.6 | 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Wang, A.; Zhang, J.; Guo, Y.; Guo, Y.; Wang, L.; Zhan, W. Low-Temperature NH3-SCR on Cex-Mn-Tiy Mixed Oxide Catalysts: Improved Performance by the Mutual Effect between Ce and Ti. Catalysts 2022, 12, 471. https://doi.org/10.3390/catal12050471
Zhu Q, Wang A, Zhang J, Guo Y, Guo Y, Wang L, Zhan W. Low-Temperature NH3-SCR on Cex-Mn-Tiy Mixed Oxide Catalysts: Improved Performance by the Mutual Effect between Ce and Ti. Catalysts. 2022; 12(5):471. https://doi.org/10.3390/catal12050471
Chicago/Turabian StyleZhu, Qianwen, Aiyong Wang, Jinshui Zhang, Yanglong Guo, Yun Guo, Li Wang, and Wangcheng Zhan. 2022. "Low-Temperature NH3-SCR on Cex-Mn-Tiy Mixed Oxide Catalysts: Improved Performance by the Mutual Effect between Ce and Ti" Catalysts 12, no. 5: 471. https://doi.org/10.3390/catal12050471
APA StyleZhu, Q., Wang, A., Zhang, J., Guo, Y., Guo, Y., Wang, L., & Zhan, W. (2022). Low-Temperature NH3-SCR on Cex-Mn-Tiy Mixed Oxide Catalysts: Improved Performance by the Mutual Effect between Ce and Ti. Catalysts, 12(5), 471. https://doi.org/10.3390/catal12050471