The Impact of Amount of Cu on CO2 Reduction Performance of Cu/TiO2 with NH3 and H2O
Abstract
1. Introduction
- (1)
- To reveal the impact of loading weight of Cu on CO2 reduction characteristics using Cu/TiO2.
- (2)
- To reveal the effect of molar ratio of CO2 to reductants NH3 and H2O on CO2 reduction characteristics over Cu/TiO2.
2. Results and Discussion
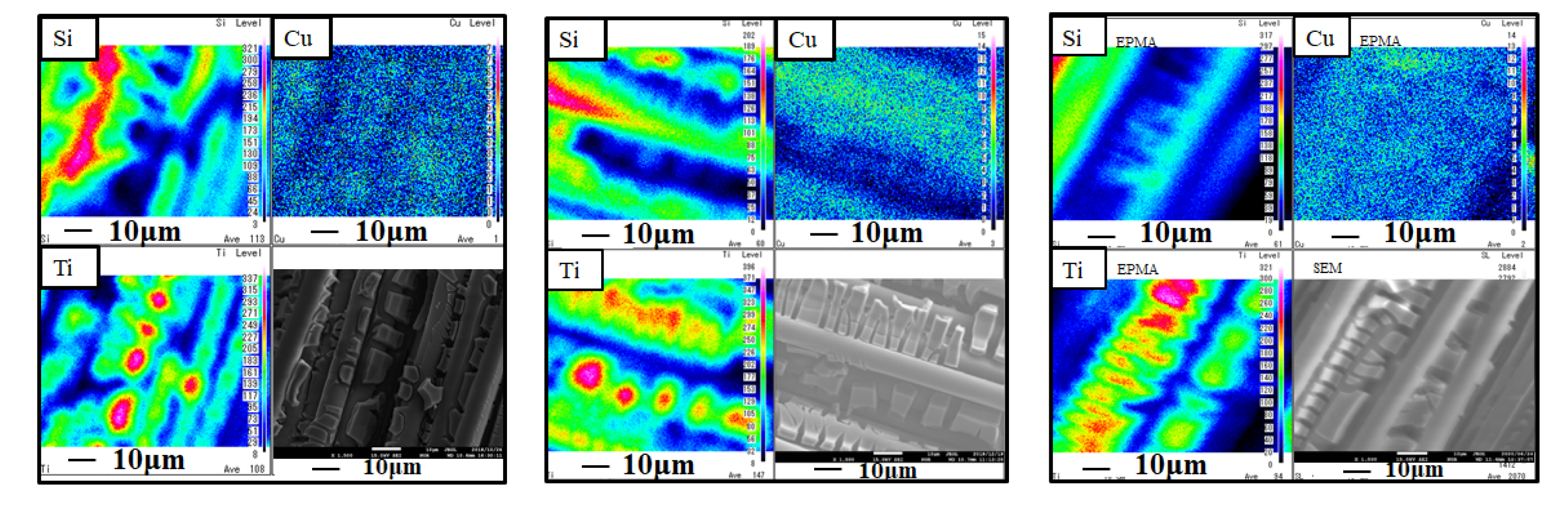
2.1. The Characterization Evaluation of Cu/TiO2 Film
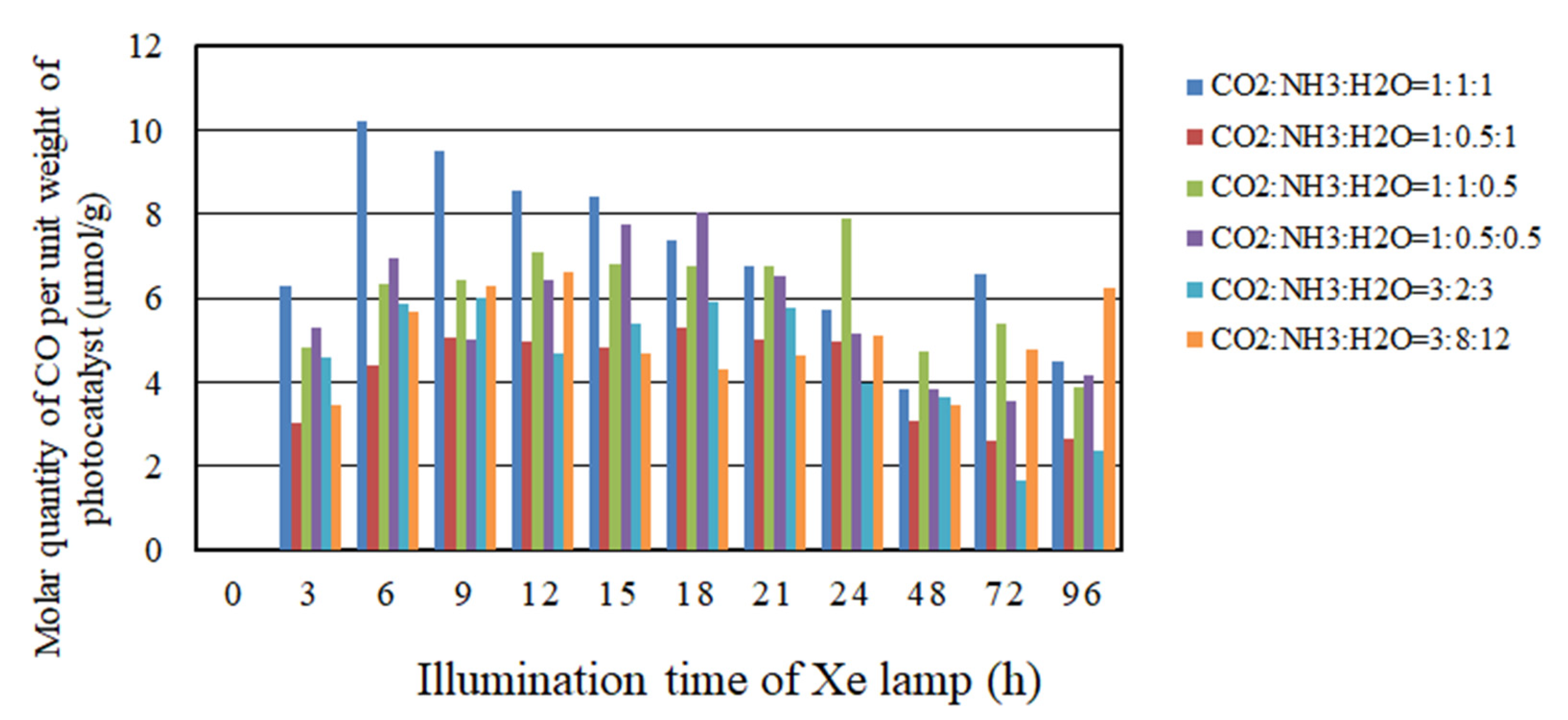
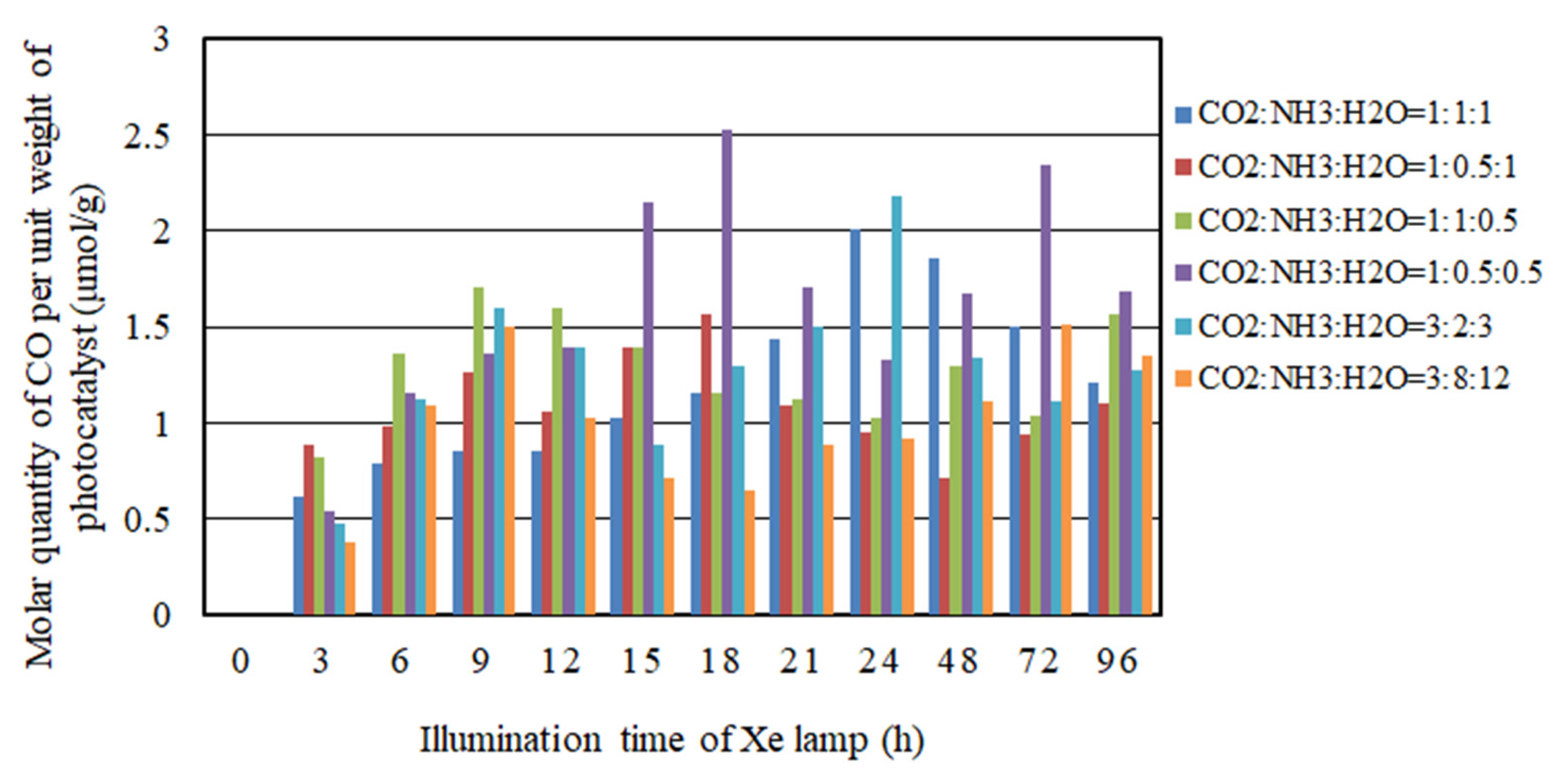
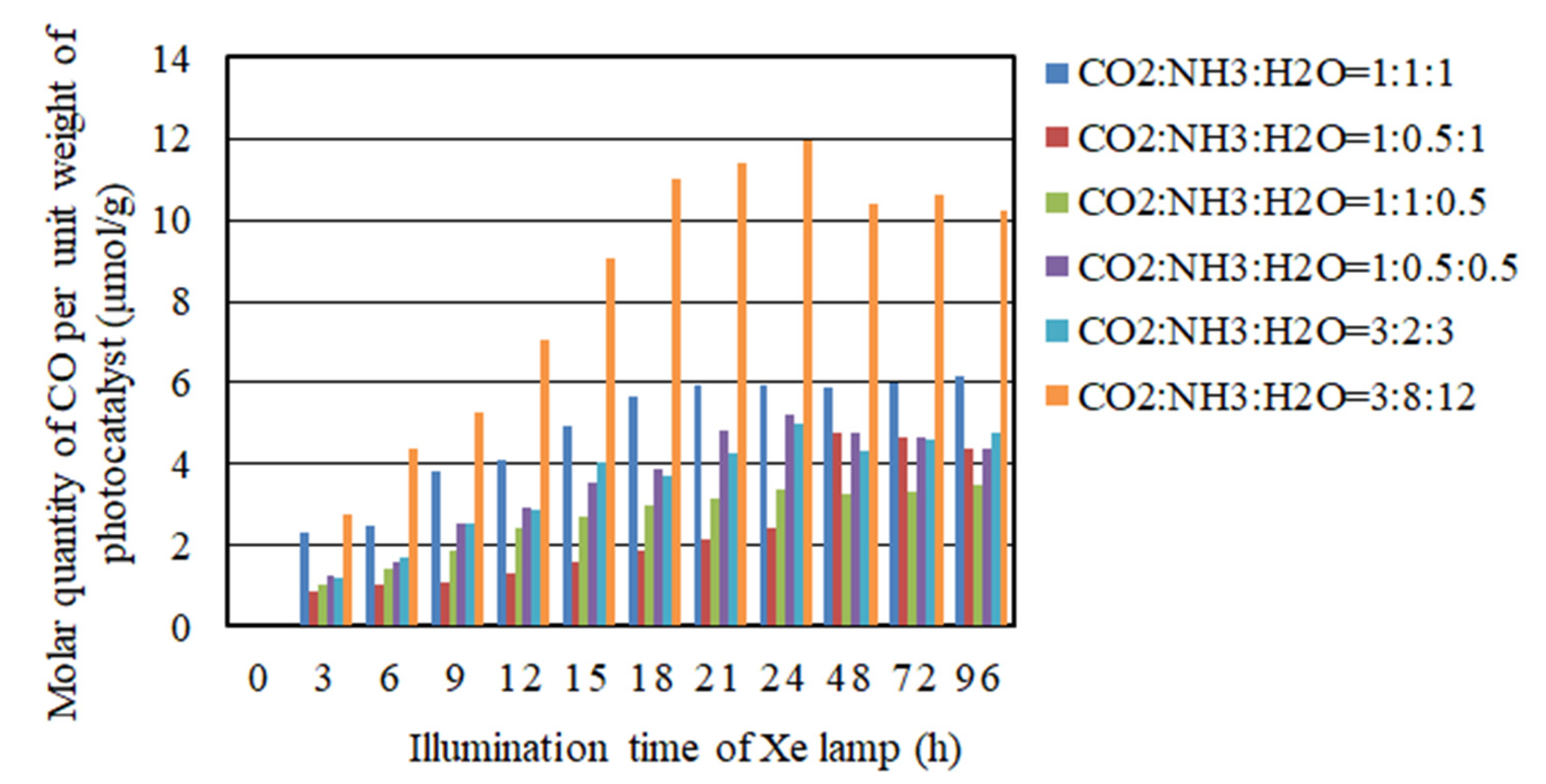
2.2. The CO2 Reduction Characteristics over Cu/TiO2 for a Pulse Number of 100
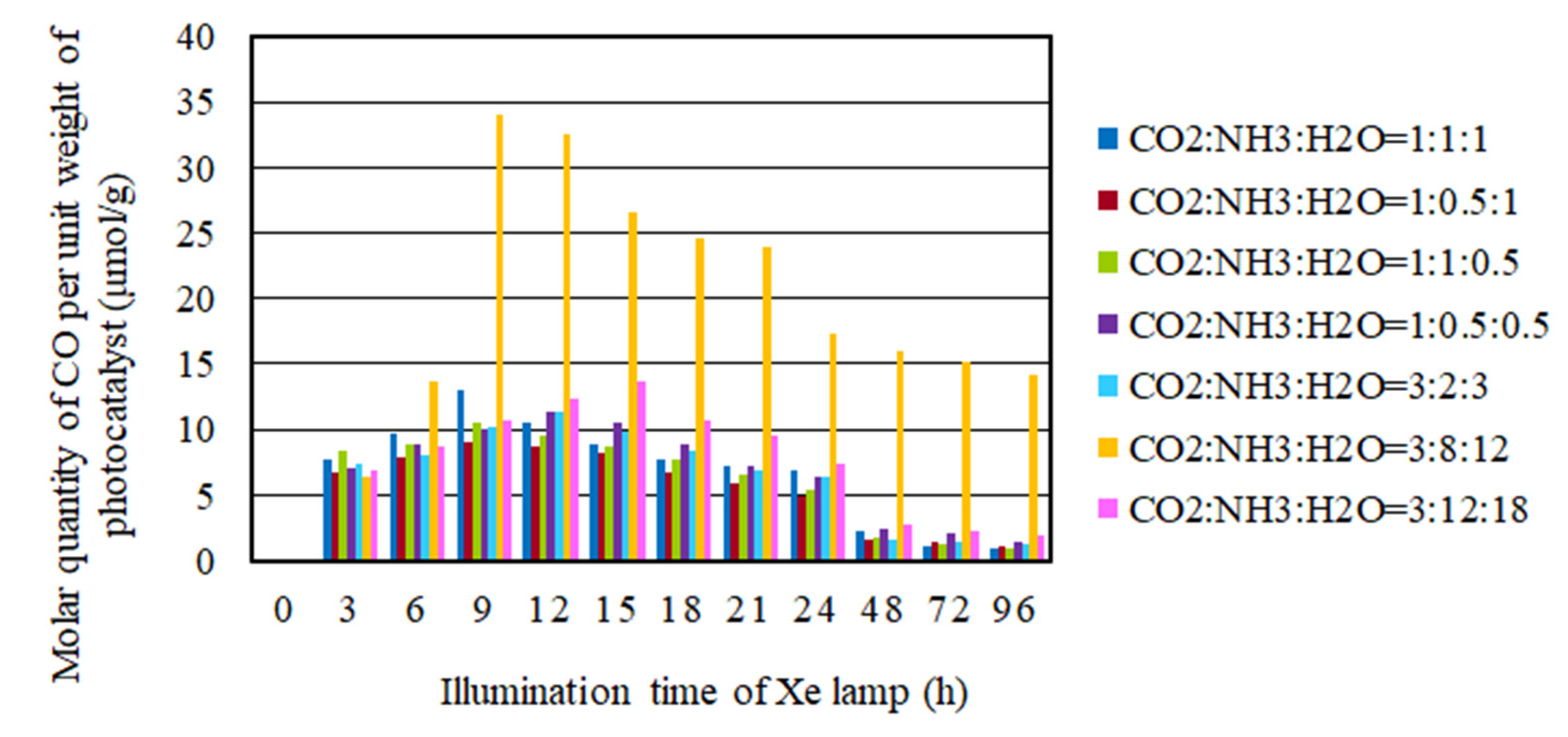
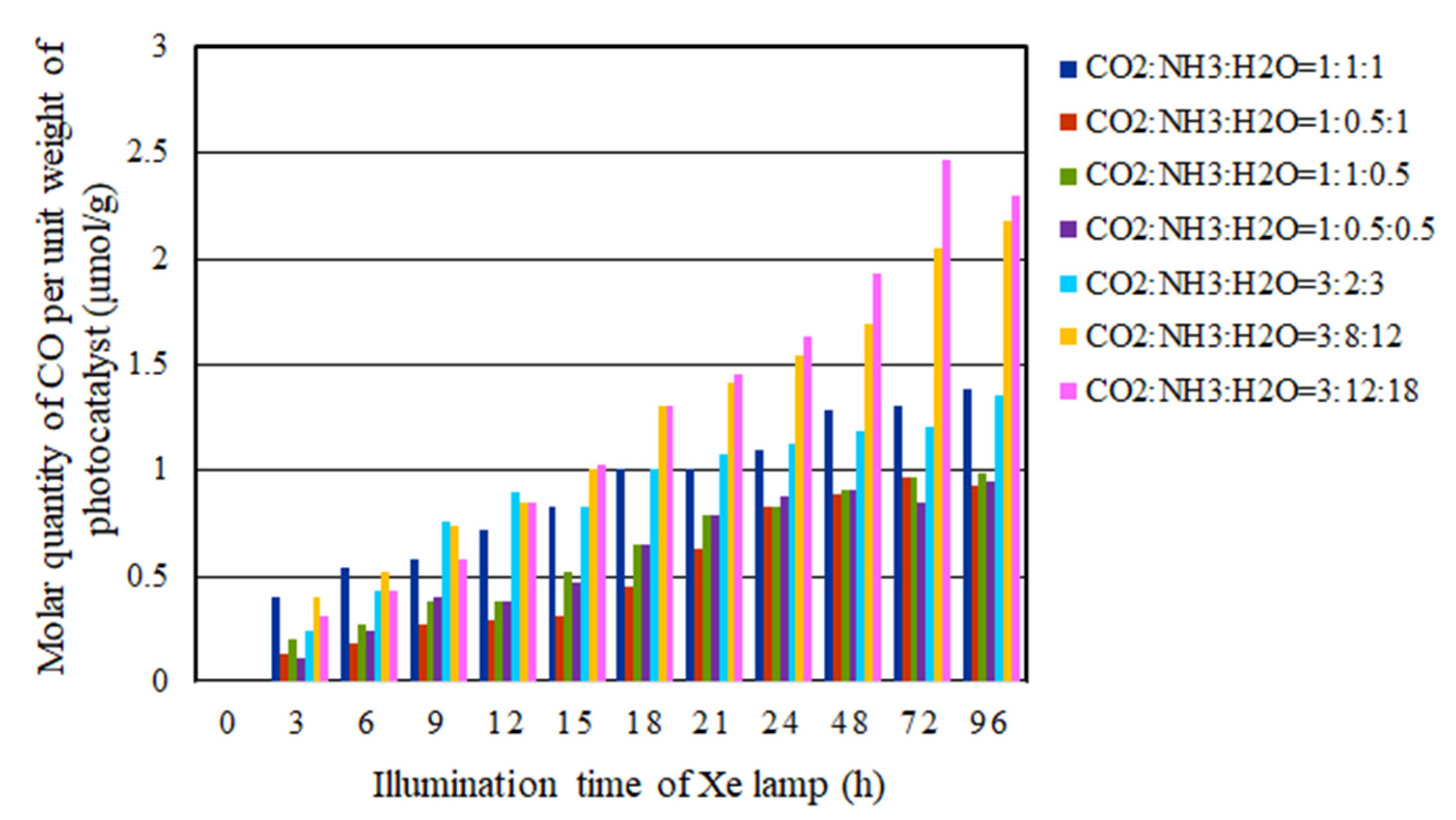
2.3. The CO2 Reduction Characteristics over Cu/TiO2 for a Pulse Number of 200
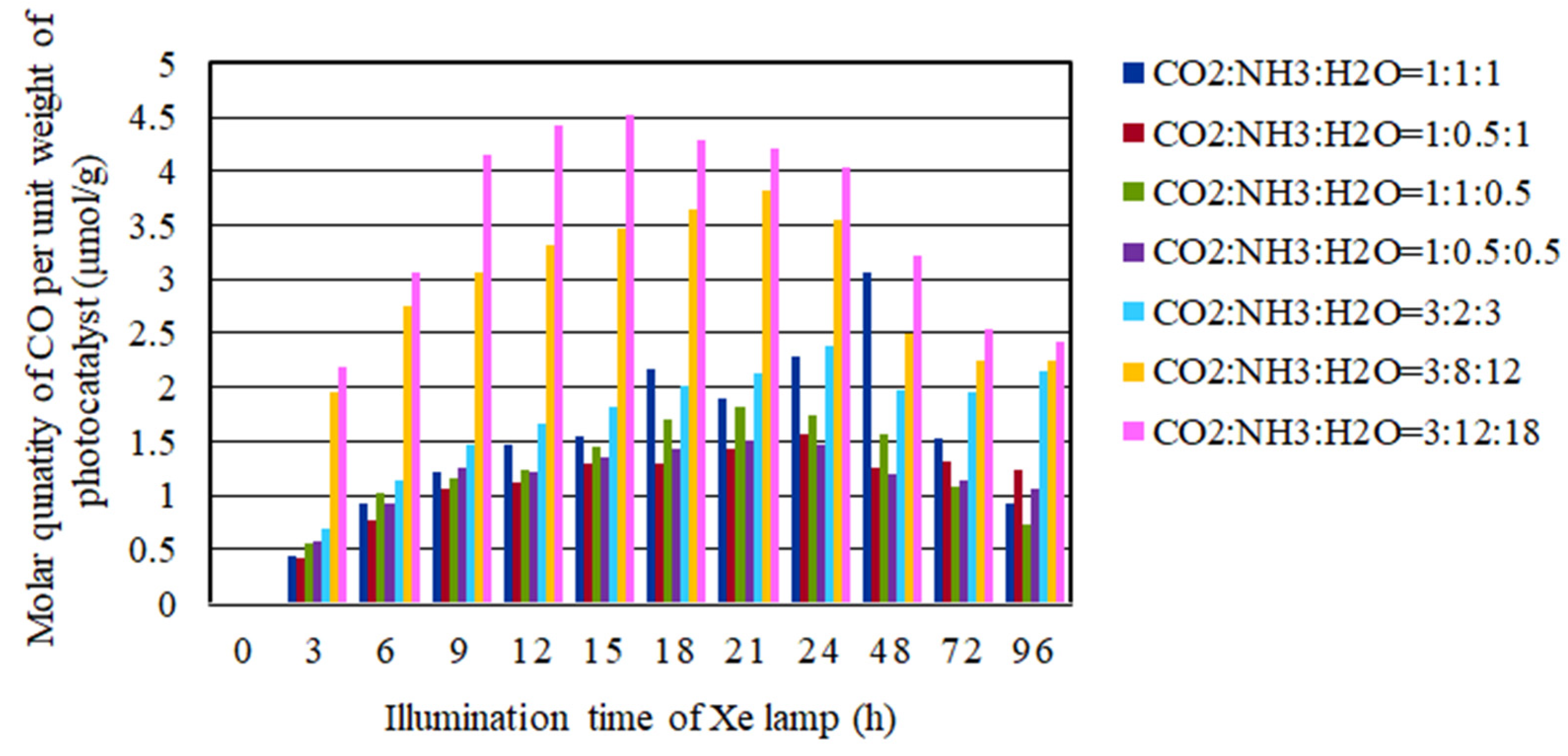
2.4. The CO2 Reduction Characteristics over Cu/TiO2 for a Pulse Number of 500
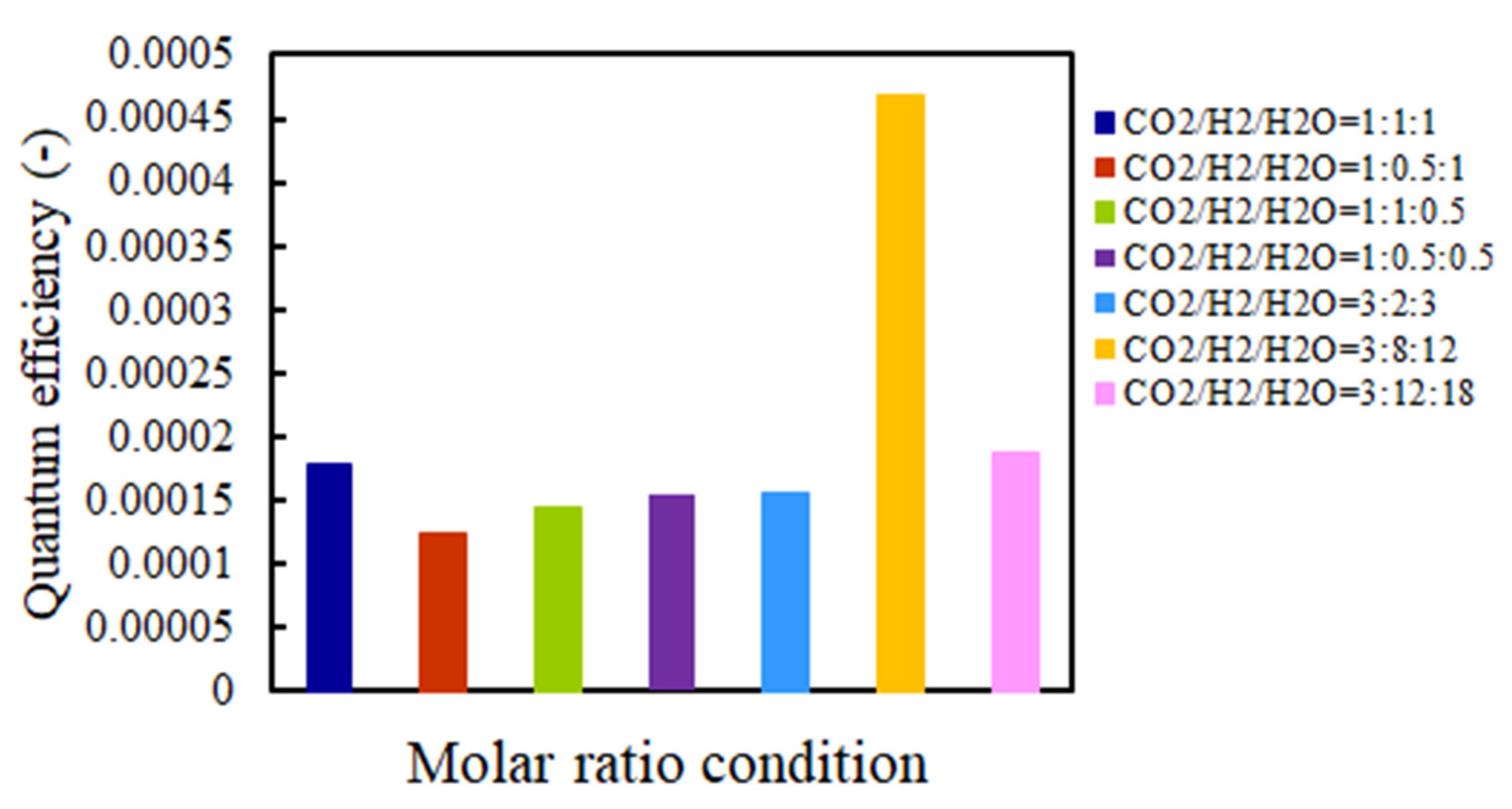
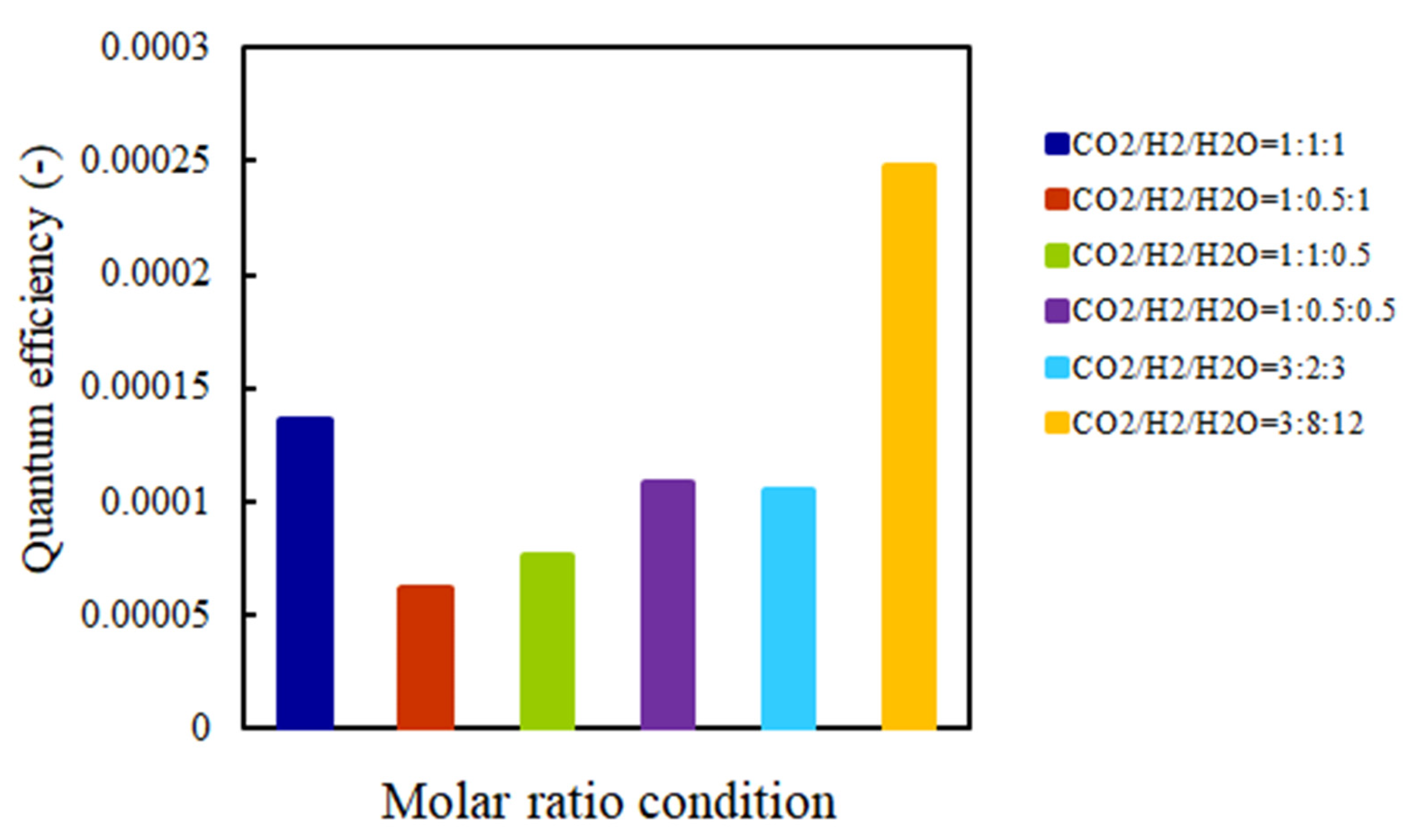
2.5. The Quantum Efficiency Evaluation
3. Experiments
3.1. The Preparation Proceure of Cu/TiO2 Film
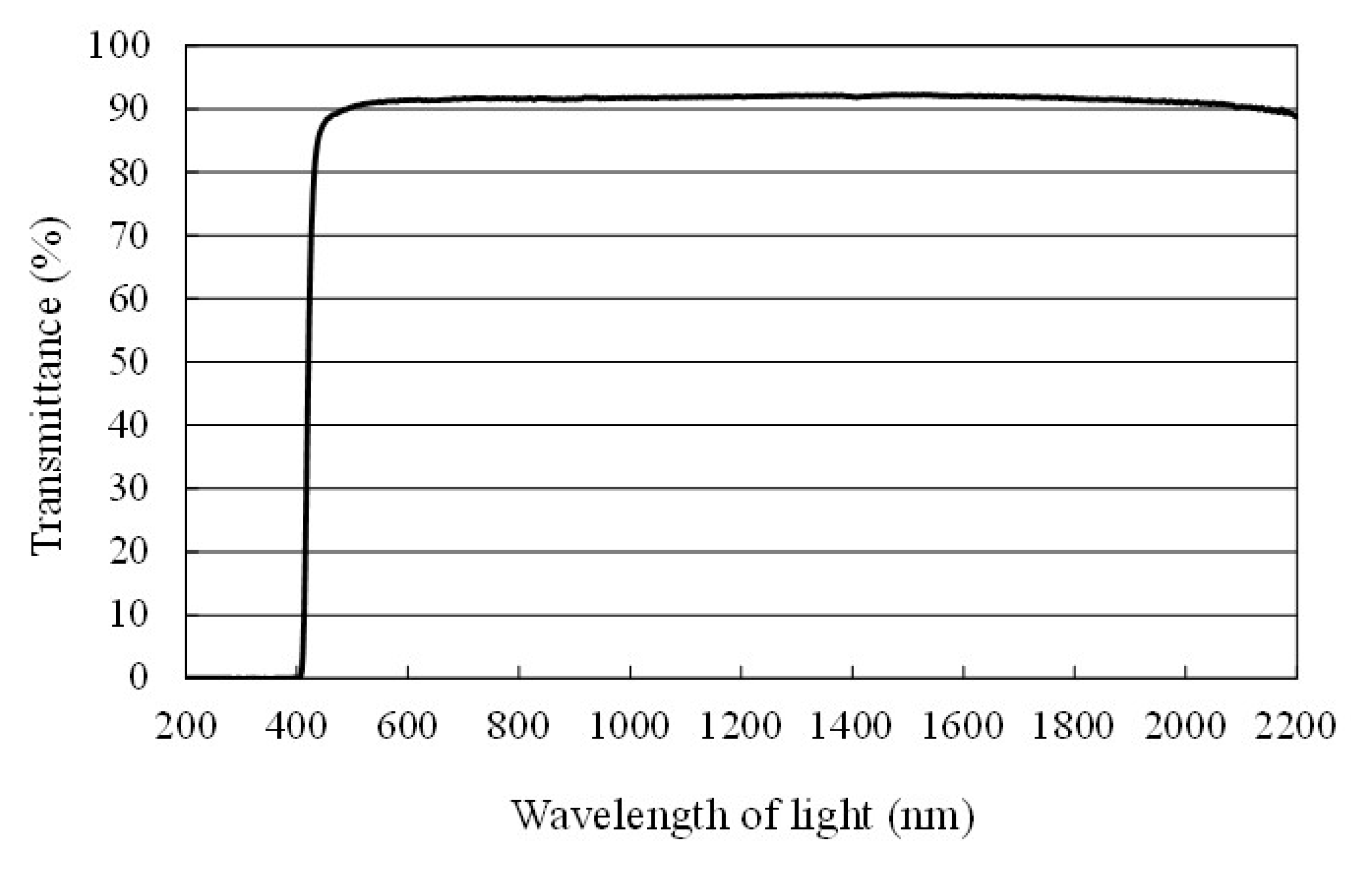
3.2. The Characterization Procedure of Cu/TiO2 Film
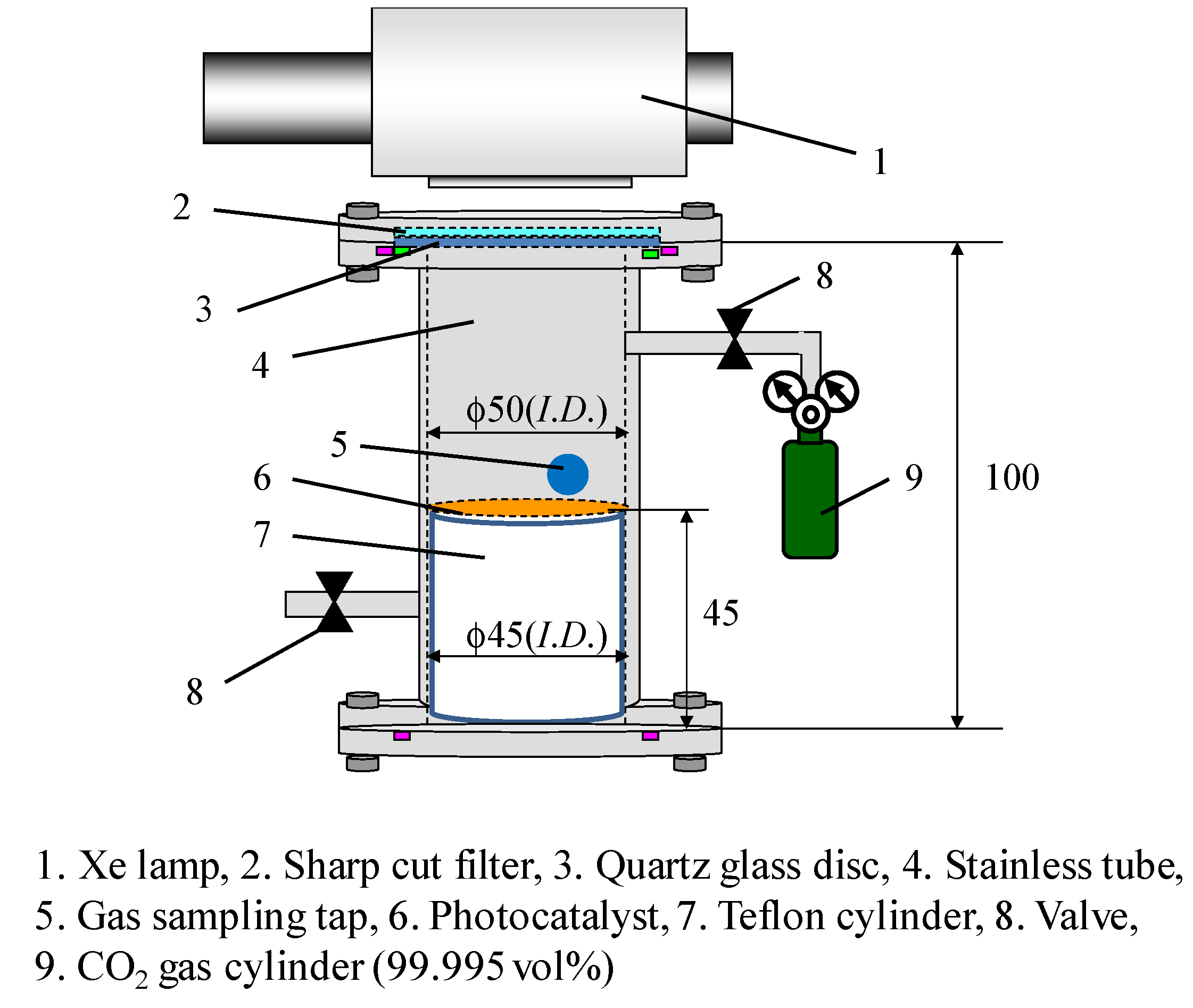
3.3. The Exprimental Procedure of CO2 Reduciton
4. Conclusions
- (i)
- Cu particles whose size is nano-scale could be loaded on TiO2 uniformly by the pulse arc plasma gun process. The weight percentages of element Cu within the Cu/TiO2 film for pulse numbers of 100, 200, and 500 increase with the increase in the pulse number, which are 1.62 wt%, 4.57 wt%, and 7.95 wt%, respectively.
- (ii)
- As to the pulse number of 100, the CO2 reduction characteristic under the condition of the molar ratio of CO2/NH3/H2O = 1:1:1 is the highest where the molar quantity of CO produced for per unit weight of photocatalyst is 10.2 mol/g with UV light. However, the CO2 reduction characteristic under the condition of the molar ratio of CO2/NH3/H2O = 1:0.5:0.5 is the highest where the molar quantity of CO produced for per unit weight of photocatalyst is 2.52 mol/g without UV light.
- (iii)
- As to the pulse number of 200, the CO2 reduction characteristics under the condition of the molar ratio of CO2/NH3/H2O = 3:8:12 is the highest under the illumination conditions both with and without UV light, where the molar quantity of CO produced per unit weight of photocatalyst are 34.1 mol/g and 12.0 mol/g, respectively.
- (iv)
- In the case of pulse number of 500, the CO2 reduction characteristic under the condition of the molar ratio of CO2/NH3/H2O = 3:12:18 under the illumination conditions both with and without UV light is the highest where the molar quantity of CO produced per unit weight of photocatalyst are 4.4 mol/g and 2.5 mol/g, respectively. It is revealed that the optimum loading weight of Cu is 4.57 wt% in order to promote CO2 reduction characteristic with NH3 and H2O.
- (v)
- This study clarifies that the highest quantum efficiencies with and without UV light illumination are 4.69 × 10–4 and 2.47 × 10–4, respectively.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Monitoring Laboratory. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/global.html (accessed on 9 December 2020).
- Matavos-Aramyan, S.; Soukhakian, S.; Jazebizadeh, H.M.; Moussavi, M.; Hojjati, M.R. On Engineering Strategies for Photoselective CO2 Reduction—A through Review. Appl. Mater. Today 2020, 18, 1–40. [Google Scholar]
- Remiro-Buenamanana, S.; Garcia, H. Photoassisted CO2 Conversion to Fuels. Chem. Cat Chem. Minirev. 2019, 11, 342–356. [Google Scholar]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 Photocatalyst for CO2 Photocatalytic Reduction: An Overview. J. CO2 Util. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Sohn, Y.; Huang, W.; Tagipour, F. Recent progress and perspectives in the photocatalytic CO2 reduction of Ti-oxide-based nanomaterials. Appl. Surf. Sci. 2017, 396, 1696–1711. [Google Scholar] [CrossRef]
- Nahar, S.; Zain, M.F.M.; Kadhum, A.A.H.; Abu, H.H. Advances in Photocatalytic CO2 Reduction with Water: A review. Materials 2017, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Amin, N.S. Indium-doped TiO2 Nanoparticles for Photocatalytic CO2 Reduction with H2O Vapors to CH4. Appl. Catal. B Envion. 2015, 162, 98–109. [Google Scholar] [CrossRef]
- Su, K.Y.; Chen, C.Y.; Wu, R.J. Preparation of Pd/TiO2 Nanowires for the Photoreduction of CO2 into Renewable Hydrocarbon Fuels. J. Taiwan Inst. Chem. Eng. 2019, 96, 409–418. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Y.; Wu, X.; Zheng, H.; Zhao, Z.; Liu, J.; Li, J. Graphene-wrapped Pt/TiO2 Photocatalysts with Enhanced Photogenerated Charges Separation and Reactant Adsorption for High Selective Photoreduction of CO2 to CH4. Appl. Catal. B Environ. 2018, 226, 360–372. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, X.; Zhao, Y.; Wang, L.; Zhao, Z.; Huang, X.; Liu, J.; Li, J. Efficient Photocatalysts of TiO2 Nanocrystals-supported PtRu Alloy Nanoparticles for CO2 Reduction with H2O: Synergetic Effect of Pt-Ru. Appl. Catal. B Environ. 2018, 236, 445–457. [Google Scholar] [CrossRef]
- Zeng, S.; Vahidzadeh, E.; VanEssen, C.G.; Kar, P.; Kisslinger, R.; Goswami, A.; Zhang, Y.; Mahdi, N.; Riddell, S.; Kobryn, A.E.; et al. Optical Control of Selectivity of High Rate CO2 Photoreduction via Interband- or Hot Electron Z-scheme Reaction Pathways in Au-TiO2 Plasmonic Photonic Crystal Photocatalyst. Appl. Catal. B Environ. 2020, 267, 1–11. [Google Scholar] [CrossRef]
- Hashermizadeh, I.; Golovko, V.B.; Choi, J.; Tsang, D.C.W.; Yip, A.C.K. Photocatalytic Reduction of CO2 to Hydrocarbons Using Bio-templated Porous TiO2 Architectures under UV and Visible Light. Chem. Eng. J. 2018, 347, 64–73. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Borkar, R.; Bansiwal, A.; Labhsetwar, N.; Jain, S.L. Metal-organic Hybrid: Photoreduction of CO2 Using Graphic Carbon Nitride Supported Heteroleptic Iridium Complex under Visible Light Irradiation. Carbon 2017, 123, 371–379. [Google Scholar] [CrossRef]
- Camarillo, R.; Toston, S.; Martinez, F.; Jimenez, C.; Rincon, J. Preparation of TiO2-based Catalyst with Supercritical Fluid Technology: Characterization and Photocatalytic Activity in CO2 Reduction. J. Chem. Tech. Biotech. 2017, 92, 1710–1720. [Google Scholar] [CrossRef]
- Lin, L.Y.; Nie, Y.; Kavadiya, S.; Soundappan, T.; Biswas, P. N-doped Reduced Graphene Oxide Promoted Nano TiO2 as a Bifunctional Adsorbent/Photocatalyst for CO2 Photoreduction: Effect of N Species. Chem. Eng. J. 2017, 316, 449–460. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 Heterostructure for CO2 Reduction through a Direct Z-scheme: Protecting Cu2O from Photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Kavil, Y.N.; Shaban, Y.A.; Farawati, R.K.A.; Orif, M.I.; Zobidi, M.; Khan, S.U.M. Photocatalytic Conversion of CO2 into Methanol over Cu-C/TiO2 Nanoparticles under UV Light and Natural Sunlight. J. Photochem. Photobiol. A Chem. 2017, 347, 244–253. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, X.; Li, Z.; Ye, X.; Liu, Y.; Chen, Y.; Yang, L.; Chen, M.; Zhang, D.; Li, G.; et al. Cooperation between Inside and Outside of TiO2: Lattice Cu+ Accelerates Carrier Migration to the Surface of Metal Copper for Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2020, 264, 1–10. [Google Scholar] [CrossRef]
- She, H.; Zhao, Z.; Bai, W.; Huang, J.; Wang, L.; Wang, Q. Enhanced Performance of Photocatalytic CO2 Reduction with Synergistic Effect between Chitosan and Cu: TiO2. Mater. Res. Bull. 2020, 124, 1–7. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kumar, M.; Dash, T.; Saran, S.; Bhandari, S.; Kumar, U. Self-organized Copper Impregnation and Doping in TiO2 with Enhanced Photocatalytic Conversion of H2O and CO2 to Fuel. Int. J. Hydrog. Energy 2018, 43, 19468–19480. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Advances in Visible Light Responsive Titanium Oxide Based Photocatalysts for CO2 Conversion to Hydrocarbon Fuels. Energy Convers. Manag. 2013, 76, 194–214. [Google Scholar] [CrossRef]
- Goren, Z.; Willner, I.; Nelson, A.J. Selective Photoreduction of CO2/HCO3− to formate by aqueous suspensions and colloids of Pd-TiO2. J. Physic. Chem. 1990, 94, 3784–3790. [Google Scholar] [CrossRef]
- Tseng, I.H.; Chang, W.C.; Wu, J.C.S. Photoreduction of CO2 Using Sol-gel Derived Titania and Titania-supported Copper Catalysts. Appl. Catal. B 2002, 37, 37–38. [Google Scholar] [CrossRef]
- Izumi, Y. Recent Advances in the Photocatalytic Conversion of Carbon Dioxide to Fuels with Water and/or Hydrogen Using Solar Energy and Beyond. Coord. Chem. Rev. 2013, 257, 171–186. [Google Scholar] [CrossRef]
- Lo, C.C.; Hung, C.H.; Yuan, C.S.; Wu, J.F. Photoreduction of Carbon Dioxide with H2 and H2O over TiO2 and ZrO2 in a Circulated Photocatalytic Reactor. Sol. Energy Mater. Sci. 2007, 91, 1765–1774. [Google Scholar] [CrossRef]
- Nishimura, A.; Ishida, N.; Tatematsu, D.; Hirota, M.; Koshio, A.; Kokai, F.; Hu, E. Effect of Fe Loading Condition and Reductants on CO2 Reduction Performance with Fe/TiO2 Photocatalyst. Int. J. Photoenergy 2017, 2017, 1625274. [Google Scholar] [CrossRef]
- Nishimura, A.; Sakakibara, Y.; Inoue, T.; Hirota, M.; Koshio, A.; Kokai, F.; Hu, E. Impact of Molar Ratio of NH3 and H2O on CO2 Reduction Performance over Cu/TiO2 Photocatalyst. Phys. Astron. Int. J. 2019, 3, 176–182. [Google Scholar]
- Ambrozova, N.; Reli, M.; Sihor, M.; Kurstrowski, P.; Wu, J.C.S.; Koci, K. Copper and Platinum Doped Titania for Photocatalytic Reduction of Carbon Dioxide. Appl. Surf. Sci. 2018, 430, 475–487. [Google Scholar] [CrossRef]
- Nemoto, J.; Goken, N.; Ueno, K. Photodecomposition of Ammonia to Dinitrogen and Dihydrogen on Platinized TiO2 Nanoparticles in an Aqueous Solution. J. Photochem. Photobiol. A Chem. 2007, 185, 295–300. [Google Scholar] [CrossRef]
- Japan Society of Mechanical Engineering. Heat Transfer Hand Book, 1st ed.; Maruzen: Tokyo, Japan, 1993; pp. 367–369. [Google Scholar]
- Paulino, P.N.; Salim, V.M.M.; Resende, N.S. Zu-Cu Promoted TiO2 Photocatalyst for CO2 Reduction with H2O under UV Light. Appl. Catal. B Environ. 2016, 185, 362–370. [Google Scholar]
- Tahir, M.; Amin, N.A.S. Photo-induced CO2 Reduction by Hydrogen for Selective CO Evolution in a Dynamic Monolith Photoreactor Loaded with Ag-Modified TiO2 Nanocatalyst. Int. J. Hydrog. Energy 2017, 42, 15507–15522. [Google Scholar] [CrossRef]
- Zhao, H.; Rao, G.; Wang, L.; Xu, J.; Liu, L.; Li, Y. Synthesis of Novel MgAl Layered Double Oxide Grafted TiO2 Cuboids and their Photocatalytic Activity on CO2 Reduction with Water Vapor. Catal. Sci. Technol. 2015, 5, 3288–3295. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, Z.; Li, C.; Zuo, Y.; Zhou, Y. Monolithic g-C3N4/Reduced Graphene Oxide Aerogel with in Situ Embedding of Pd Nanoparticles for Hydrogeneration of CO2 to CH4. Appl. Suf. Sci. 2019, 475, 953–960. [Google Scholar] [CrossRef]
- Camarillo, R.; Toston, S.; Martinez, F.; Jimenez, C.; Rincon, J. Enhancing the Photocatalytic Reduction of CO2 through Engineering of Catalysts with High Pressure Technology: Pd/TiO2 Photocatalsyts. J. Supercrit. Fluids 2017, 123, 18–27. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Yuan, Z.; Chen, J.; Huang, B.; Dionysiou, D.D.; Yang, G. Enhanced Photocatalytic CO2 Reduction via the Synergistic Effect between Ag and Activated Carbon in TiO2/AC-Ag Ternary Composite. Chem. Eng. J. 2018, 348, 592–598. [Google Scholar] [CrossRef]
- Hoque, M.A.; Guzman, M.J. Photocatalytic Activity: Experimental Features to Report in Heterogeneous Photocatalysis. Materials 2018, 11, 1990. [Google Scholar] [CrossRef]
- Nishimura, A.; Inoue, T.; Sakakibara, Y.; Hirota, M.; Koshio, A.; Kokai, F.; Hu, E. Optimum Molar Ratio of H2 and H2O to Reduce CO2 Using Pd/TiO2. Aims Mater. Sci. 2019, 6, 464–483. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.N.; Zhan, Z.; Woo, M.H.; Wu, C.Y.; Biswas, P. Photocatalytic Reduction of CO2 with H2O on Mesoporous Silica Supported Cu/TiO2 Catalysts. Appl. Catal. B Environ. 2010, 100, 386–392. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, S.; Asif, M. Layered Double Hydroxide (LDH) Based Photocatalysts: An Outstanding Strategy for Efficient Photocatalytic CO2 Conversion. Catalysts 2020, 10, 1185. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, A.; Sakakibara, Y.; Koshio, A.; Hu, E. The Impact of Amount of Cu on CO2 Reduction Performance of Cu/TiO2 with NH3 and H2O. Catalysts 2021, 11, 610. https://doi.org/10.3390/catal11050610
Nishimura A, Sakakibara Y, Koshio A, Hu E. The Impact of Amount of Cu on CO2 Reduction Performance of Cu/TiO2 with NH3 and H2O. Catalysts. 2021; 11(5):610. https://doi.org/10.3390/catal11050610
Chicago/Turabian StyleNishimura, Akira, Yoshito Sakakibara, Akira Koshio, and Eric Hu. 2021. "The Impact of Amount of Cu on CO2 Reduction Performance of Cu/TiO2 with NH3 and H2O" Catalysts 11, no. 5: 610. https://doi.org/10.3390/catal11050610
APA StyleNishimura, A., Sakakibara, Y., Koshio, A., & Hu, E. (2021). The Impact of Amount of Cu on CO2 Reduction Performance of Cu/TiO2 with NH3 and H2O. Catalysts, 11(5), 610. https://doi.org/10.3390/catal11050610







