Development of Monodisperse Mesoporous Microballs Composed of Decahedral Anatase Nanocrystals
Abstract
:1. Introduction
2. Experimental Part
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Lee, C.; Javed, H.; Yu, P.; Kim, J.H.; Alvarez, P.J. Easily recoverable, micrometer-sized TiO2 hierarchical spheres decorated with cyclodextrin for enhanced photocatalytic degradation of organic micropollutants. Environ. Sci. Technol. 2018, 52, 12402–12411. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.H.; Lim, J.; Lee, J.H.; Choi, W.; Jang, H.M. Enhanced photocatalytic activity of {101}-oriented bipyramidal TiO2 agglomerates through interparticle charge transfer. Appl. Catal. B 2018, 176, 76–82. [Google Scholar] [CrossRef]
- Trang, N.T.H.; Ali, Z.; Kang, D.J. Mesoporous TiO2 spheres interconnected by multiwalled carbon nanotubes as an anode for high-performance lithium ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 3676–3683. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Chen, J.; Nie, X.; Li, G.; Zhang, H.; Liu, X.; Zhao, H. Synthesis of carbon nanotube–anatase TiO2 sub-micrometer-sized sphere composite photocatalyst for synergistic degradation of gaseous styrene. ACS Appl. Mater. Interfaces 2021, 4, 5988–5996. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Liang, Y.; Yuan, C.; Yu, T.; Li, Q.; Li, Q. Enhanced photocatalytic performance of Ag decorated hierarchical micro/nanostructured TiO2 microspheres. J. Alloys Compd. 2015, 652, 386–392. [Google Scholar] [CrossRef]
- Eiden-Assmann, S.; Widoniak, J.; Maret, G. Synthesis and Characterization of Porous and Nonporous Monodisperse Colloidal TiO2 Particles. Chem. Mater. 2004, 16, 6–11. [Google Scholar] [CrossRef]
- Pan, J.H.; Han, G.; Zhou, R.; Zhao, X.S. Hierarchical N-doped TiO2 hollow microspheres consisting of nanothorns with exposed anatase {101} facets. Chem. Commun. 2021, 47, 6942–6944. [Google Scholar] [CrossRef]
- Ye, J.; Liu, W.; Cai, J.; Chen, S.; Zhao, X.; Zhou, H.; Qi, L. Nanoporous anatase TiO2 mesocrystals: Additive-free synthesis, remarkable crystalline-phase stability, and improved lithium insertion behavior. J. Am. Chem. Soc. 2011, 133, 933–940. [Google Scholar] [CrossRef]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous synthesis of TiO2 nanocrystals using TiF4 to engineer morphology, oxygen vacancy concentration, and photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef]
- Buonsanti, R.; Carlino, E.; Giannini, C.; Altamura, D.; De Marco, L.; Giannuzzi, R.; Manca, M.; Gigli, G.; Cozzoli, P.D. Hyperbranched anatase TiO2 nanocrystals: Nonaqueous synthesis, growth mechanism, and exploitation in dye-sensitized solar cells. J. Am. Chem. Soc. 2011, 133, 19216–19239. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, B.; Wang, J.; Shao, Z. Non-aqueous hybrid supercapacitors fabricated with mesoporous TiO2 microspheres and activated carbon electrodes with superior performance. J. Power Sources 2014, 253, 80–89. [Google Scholar] [CrossRef]
- Lü, X.; Ding, S.; Xie, Y.; Huang, F. Non-Aqueous Preparation of High-Crystallinity Hierarchical TiO2 Hollow Spheres with Excellent Photocatalytic Efficiency. Eur. J. Inorg. Chem. 2011, 2011, 2879–2883. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mino, L.; Pellegrino, F.; Rades, S.; Radnik, J.; Hodoroaba, V.D.; Spoto, G.; Maurino, V.; Martra, G. Beyond shape engineering of TiO2 nanoparticles: Post-synthesis treatment dependence of surface hydration, hydroxylation, Lewis acidity and photocatalytic activity of TiO2 anatase nanoparticles with dominant {001} or {101} facets. ACS Appl. Nano Mater. 2018, 1, 5355–5365. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.-E.; Niu, X.; Bai, Y.; Qi, H.; Guo, Y.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Synthesis of anatase TiO2 nanocrystals with defined morphologies from exfoliated nanoribbons: Photocatalytic performance and application in dye-sensitized solar cell. ChemistrySelect 2019, 4, 4443–4457. [Google Scholar] [CrossRef]
- Sajan, C.P.; Wageh, S.; Al-Ghamdi, A.; Yu, J.; Cao, S. TiO2 nanosheets with exposed {001} facets for photocatalytic applications. Nano Res. 2016, 9, 3–27. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Wei, Z.; Kowalska, E.; Ohtani, B. Enhanced photocatalytic activity by particle morphology: Preparation and photocatalytic activities of octahedral anatase titania particles. Chem. Lett. 2014, 143, 346–348. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Kowalska, E.; Verrett, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral-shaped anatase titania particles prepared by ultrasonication-hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef] [Green Version]
- Amano, F.; Prieto-Mahaney, O.O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral single-crystalline particles of anatase titanium (IV) oxide with high photocatalytic activity. Chem. Mater. 2009, 21, 2601–2603. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for crystal-face-dependent TiO2 photocatalysis from single-molecule imaging and kinetic analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.; Balcytis, A.; Nitta, A.; Medrano, M.G.M.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B 2018, 237, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.; Kim, B.J.; Park, Y.K.; Kim, S.C.; Jung, S.C. Photocatalytic properties of amorphous N-doped TiO2 photocatalyst under visible light irradiation. Catalysts 2021, 11, 1010. [Google Scholar] [CrossRef]
- Wei, Z.; Kowalska, E.; Ohtani, B. Influence of post-treatment operations on structural properties and photocatalytic activity of octahedral anatase particles prepared by an ulrasonication hydrothermal method. Molecules 2014, 19, 19573–19587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Liu, M.; Feng, S.; Li, X.; Wang, J.; Shen, D.; Li, Y.; Sun, Z.; Elzatahry, A.A.; Lu, H.; et al. Template-free synthesis of uniform magnetic mesoporous TiO2 nanospindles for highly selective enrichment of phosphopeptides. Mater. Horiz. 2014, 1, 439–445. [Google Scholar] [CrossRef]
- Chen, D.; Cao, L.; Huang, F.; Imperia, P.; Cheng, Y.-B.; Caruso, R.A. Synthesis of monodisperse mesoporous titania beads with controllable diameter, high surface areas, and variable pore diameters (14–23 nm). J. Am. Chem. Soc. 2010, 132, 4438–4444. [Google Scholar] [CrossRef]
- Chang, Y.; He, P.; Wei, Z.; Chen, Y.; Wang, H.; Wu, C.; Zhou, Z.; Huang, H.; Kowalska, E.; Dong, S. Three-dimensional monodispersed TiO2 microsphere network formed by a sub-zero sol-gel method. Mater. Lett. 2020, 268, 127592. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Bian, Z.; Xie, S.; Cai, C.; Wang, J.; Yang, H.; Li, H. Solvothermally controllable synthesis of anatase TiO2 nanocrystals with dominant {001} facets and enhanced photocatalytic activity. Crystengcomm 2010, 12, 2219–2224. [Google Scholar] [CrossRef]
- Janczarek, M.; Wei, Z.; Mogan, T.R.; Wang, L.; Wang, K.; Nitta, A.; Ohtani, B.; Kowalska, E. Does Symmetry Control Photocatalytic Activity of Titania-Based Photocatalysts? Symmetry 2021, 13, 1682. [Google Scholar] [CrossRef]
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of titania nanosheets with a high percentage of Exposed (001) facets and related photocatalytic properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Zhang, L.; Yu, J. Direct sonochemical preparation and characterization of highly active mesoporous TiO2. Chem. Mater. 2002, 14, 4647–4653. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, M.; Cheng, B.; Yu, H.; Zhao, X. Ultrasonic preparation of mesoporous titanium dioxide nanocrystalline photocatalysts and evaluation of photocatalytic activity. J. Mol. Catal. A Chem. 2005, 227, 75–80. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Z.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photoch. Photobio. A 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with dominant {001} facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, J.; Jaroniec, M. Tunable photocatalytic selectivity of hollow TiO2 microspheres composed of anatase polyhedra with exposed {001} facets. J. Am. Chem. Soc. 2010, 132, 11914–11916. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Z.; Zhao, H.; Zheng, M.; Du, P.; Zhao, J.; Fan, H. Phase control of hierarchically structured mesoporous anatase TiO2 microspheres covered with {001} facets. J. Mater. Chem. 2012, 22, 21965–21971. [Google Scholar] [CrossRef]
- Roy, N.; Sohn, Y.; Pradhan, D. Synergy of low-energy {101} and high-energy {001} TiO2 crystal facets for enhanced photocatalysis. ACS Nano 2013, 7, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Sung, Y.M. Synthesis of anatase nanosheets with exposed (001) facets via chemical vapor deposition. Cryst. Growth Des. 2012, 12, 5792–5795. [Google Scholar] [CrossRef]
- Janczarek, M.; Wei, Z.; Endo, M.; Ohtani, B.; Kowalska, E. Silver- and copper-modifed decahedral anatase titania particles as visible light-reponsive plasmonic photocatalytst. J. Photon. Energy 2017, 7, 012008. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Janczarek, M.; Wang, K.; Zheng, S.; Kowalska, E. Morphology-governed performance of plasmonic photocatalysts. Catalysts 2020, 10, 1070. [Google Scholar] [CrossRef]
- Wei, Z.; Endo, M.; Wang, K.; Charbit, E.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Noble metal-modified octahedral anatase titania particles with enhanced activity for decomposition of chemical and microbiological pollutants. Chem. Eng. J. 2017, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Endo-Kimura, M.; Wang, K.; Colbeau-Justin, C.; Kowalska, E. Influence of semiconductor morphology on photocatalytic activity of plasmonic photocatalysts: Titanate nanowires and octahedral anatase nanoparticles. Nanomaterials 2019, 9, 1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, A.L.; Dragoe, D.; Wang, K.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Uribe, D.B.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Photocatalytic hydrogen evolution using Ni-Pd/TiO2: Correlation of light absorption, charge-carrier dynamics, and quantum efficiency. J. Phys. Chem. C 2017, 121, 14302–14311. [Google Scholar] [CrossRef]

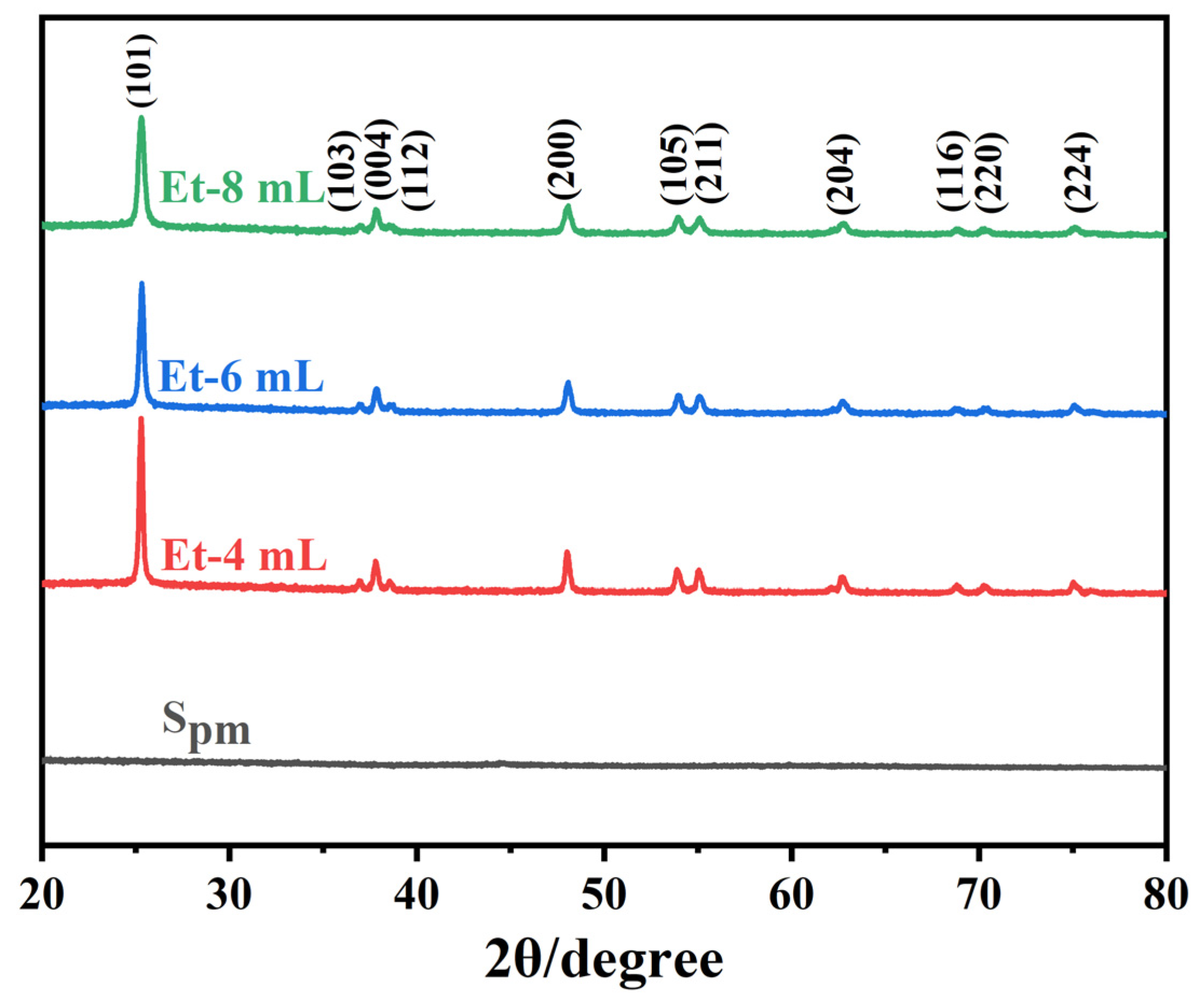
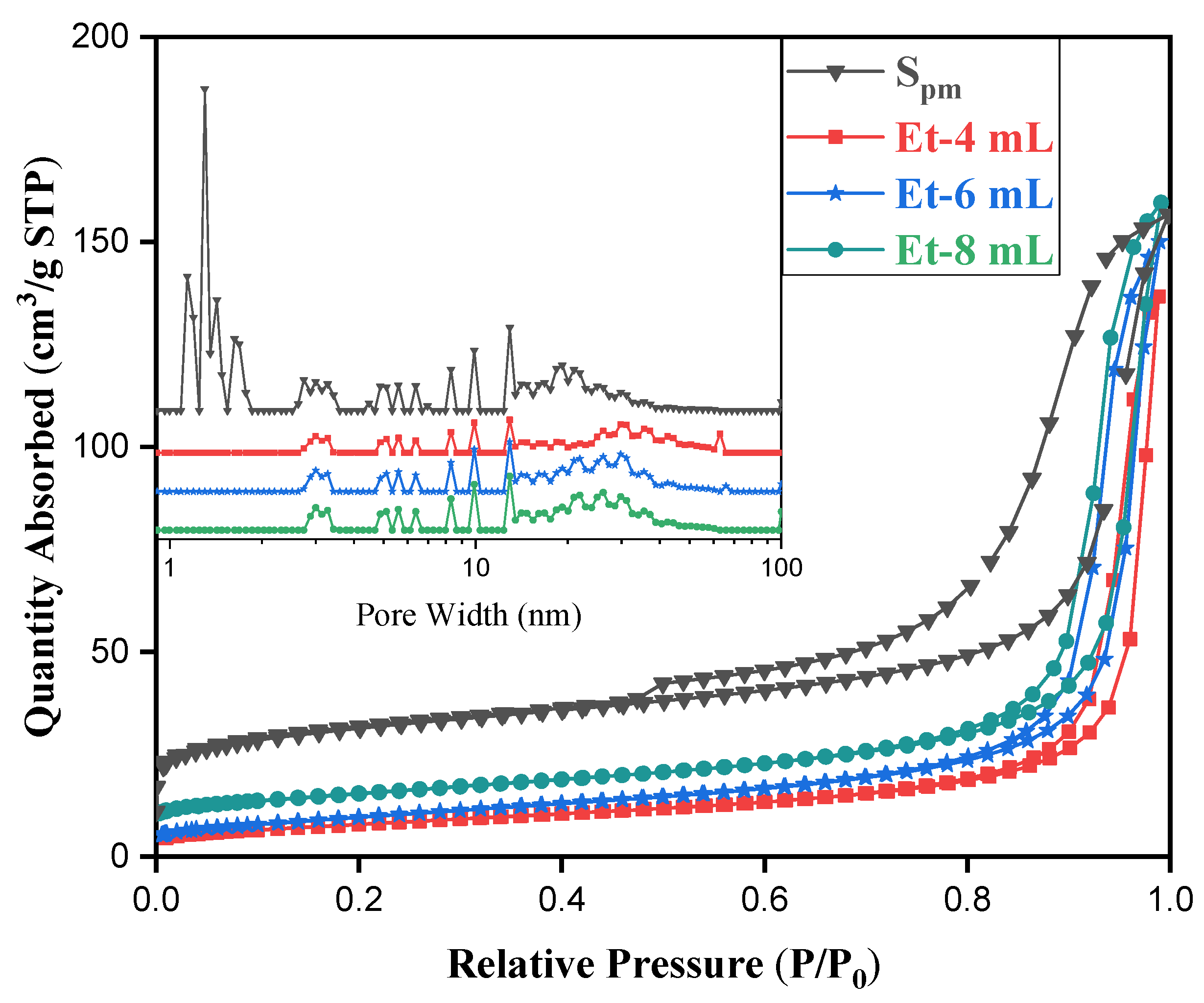
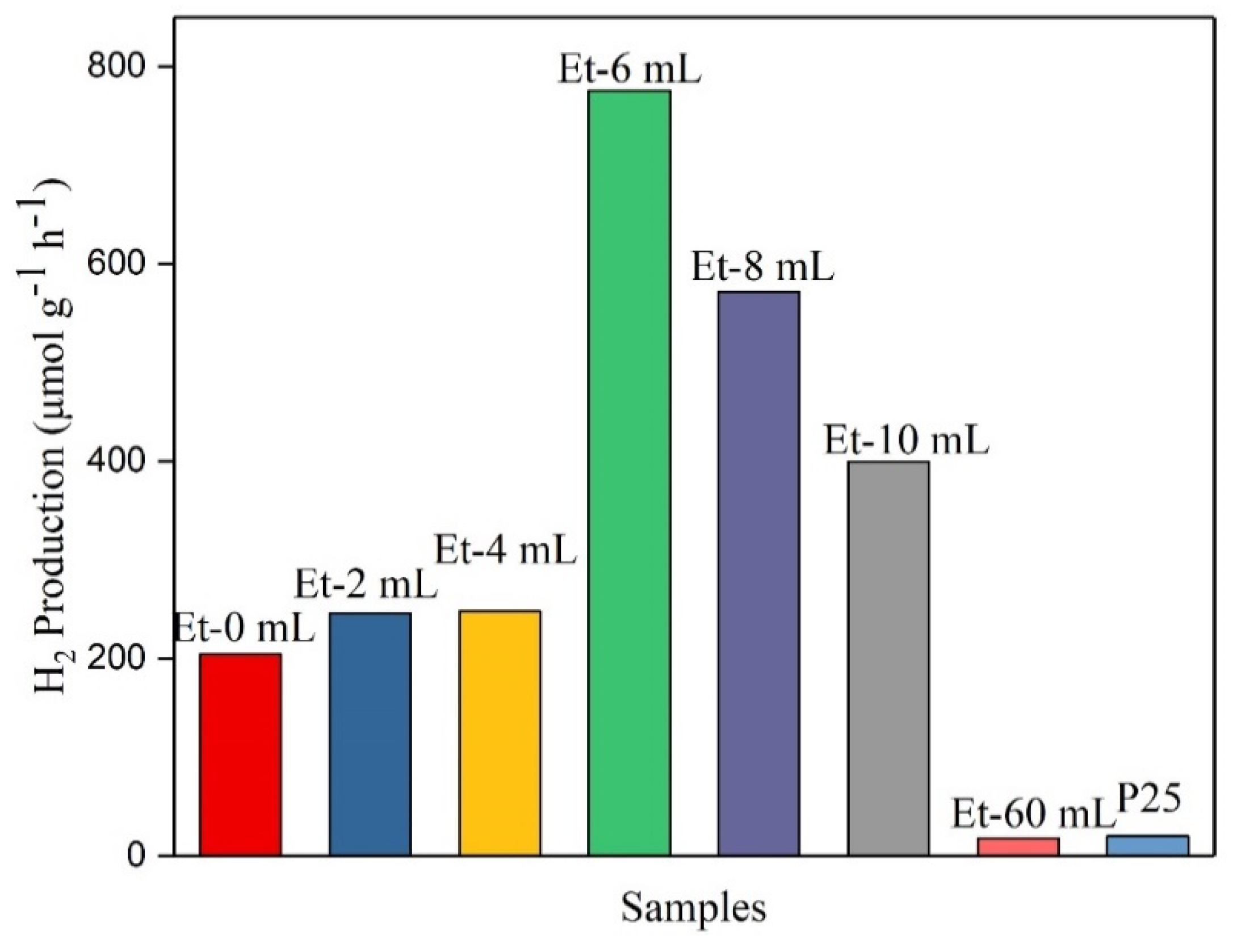
| Sample | EtOH/H2O | (001)/nm | (100)/nm | PAR | SSA/m2 g−1 |
|---|---|---|---|---|---|
| Spm | - | - | - | - | 89.1 |
| Et-4 mL | 56:4 | 48.6 | 131.1 | 1.07 | 29.2 |
| Et-6 mL | 54:6 | 43.2 | 57.4 | 0.51 | 35.9 |
| Et-8 mL | 52:8 | 38.9 | 32.8 | 0.46 | 38.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Jiang, J.; Wei, Z.; Kowalska, E. Development of Monodisperse Mesoporous Microballs Composed of Decahedral Anatase Nanocrystals. Catalysts 2022, 12, 408. https://doi.org/10.3390/catal12040408
Chang Y, Jiang J, Wei Z, Kowalska E. Development of Monodisperse Mesoporous Microballs Composed of Decahedral Anatase Nanocrystals. Catalysts. 2022; 12(4):408. https://doi.org/10.3390/catal12040408
Chicago/Turabian StyleChang, Ying, Jiayi Jiang, Zhishun Wei, and Ewa Kowalska. 2022. "Development of Monodisperse Mesoporous Microballs Composed of Decahedral Anatase Nanocrystals" Catalysts 12, no. 4: 408. https://doi.org/10.3390/catal12040408
APA StyleChang, Y., Jiang, J., Wei, Z., & Kowalska, E. (2022). Development of Monodisperse Mesoporous Microballs Composed of Decahedral Anatase Nanocrystals. Catalysts, 12(4), 408. https://doi.org/10.3390/catal12040408