Hybrids of Reduced Graphene Oxide Aerogel and CNT for Electrochemical O2 Reduction
Abstract
:1. Introduction
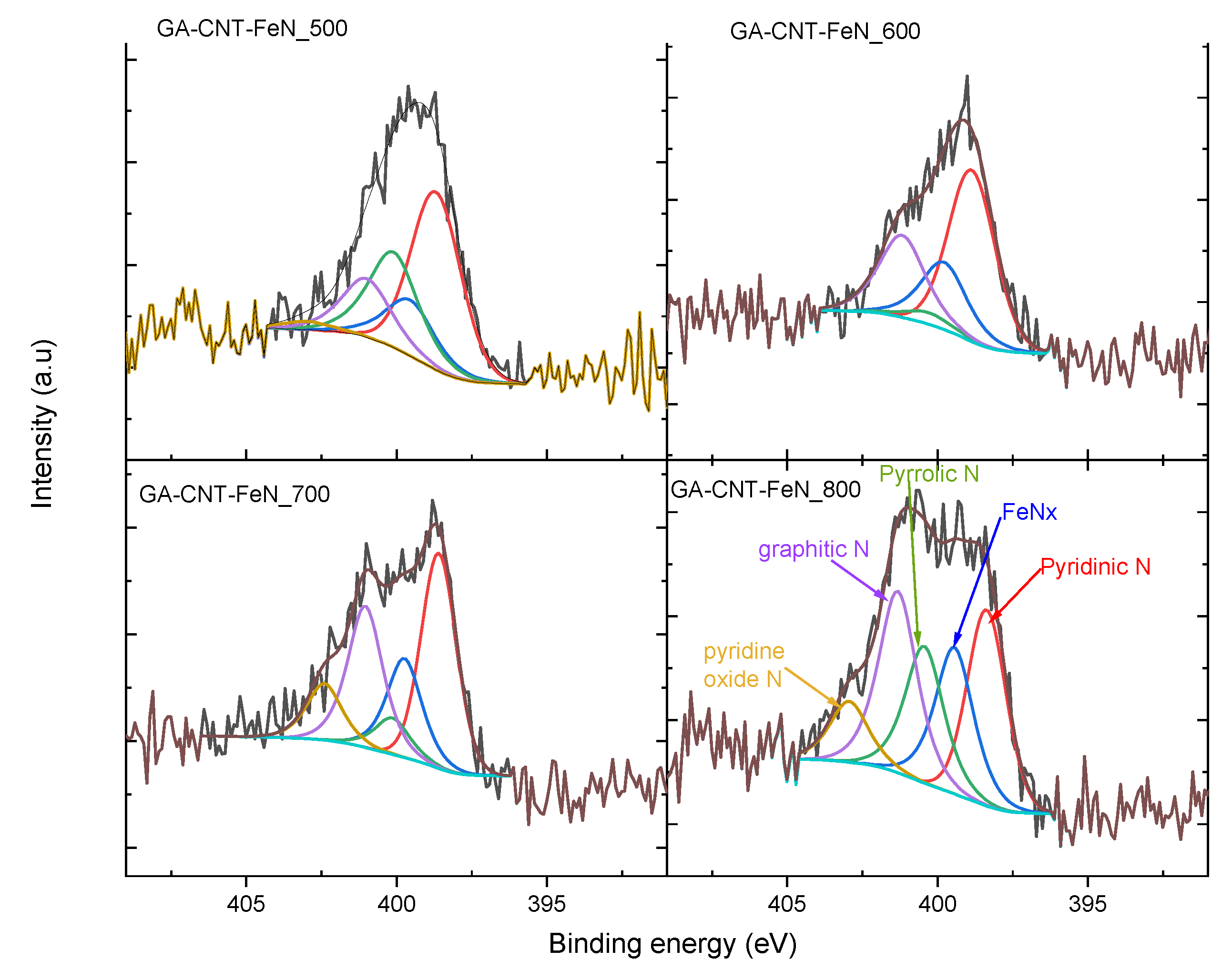
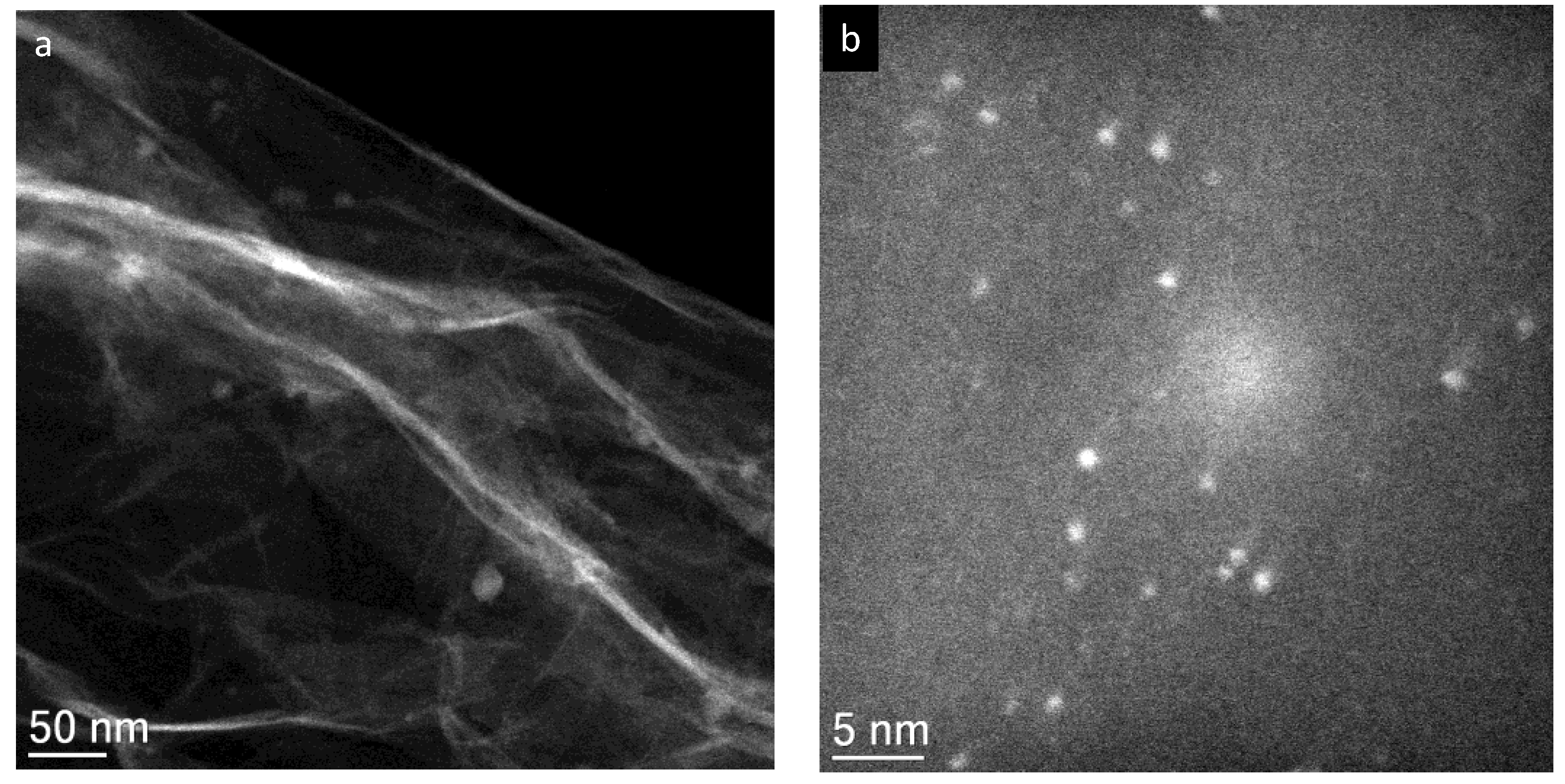
2. Results and Discussion
3. Materials and Methods
3.1. Aerogel Preparation and N-Doping
3.2. Electrochemical Characterisation
3.3. Electrocatalyst Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, e1804799. [Google Scholar] [CrossRef]
- Wang, D.-W.; Su, D. Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy Environ. Sci. 2013, 7, 576–591. [Google Scholar] [CrossRef]
- Inaba, M.; Yamada, H.; Tokunaga, J.; Matsuzawa, K.; Hatanaka, A.; Tasaka, A. Hydrogen Peroxide Formation as a Degradation Factor of Polymer Electrolyte Fuel Cells. ECS Trans. 2006, 1, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Siahrostami, S.; Villegas, S.J.; Mostaghimi, A.H.B.; Back, S.; Farimani, A.B.; Wang, H.; Persson, K.A.; Montoya, J. A Review on Challenges and Successes in Atomic-Scale Design of Catalysts for Electrochemical Synthesis of Hydrogen Peroxide. ACS Catal. 2020, 10, 7495–7511. [Google Scholar] [CrossRef]
- Jiang, Y.; Ni, P.; Chen, C.; Lu, Y.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective Electrochemical H2O2 Production through Two-Electron Oxygen Electrochemistry. Adv. Energy Mater. 2018, 8, 1801909. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirkovský, J.S.; Panas, I.; Ahlberg, E.; Halasa, M.; Romani, S.; Schiffrin, D.J. Single Atom Hot-Spots at Au–Pd Nanoalloys for Electrocatalytic H2O2 Production. J. Am. Chem. Soc. 2011, 133, 19432–19441. [Google Scholar] [CrossRef]
- Ba, H.; Liu, Y.; Truong-Phuoc, L.; Duong-Viet, C.; Nhut, J.-M.; Nguyen, D.L.; Ersen, O.; Tuci, G.; Giambastiani, G.; Pham-Huu, C. N-Doped Food-Grade-Derived 3D Mesoporous Foams as Metal-Free Systems for Catalysis. ACS Catal. 2016, 6, 1408–1419. [Google Scholar] [CrossRef]
- Trogadas, P.; Fuller, T.F.; Strasser, P. Carbon as catalyst and support for electrochemical energy conversion. Carbon 2014, 75, 5–42. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M.; Wong, C.-P. Facile Synthesis of Nitrogen-Doped Graphene via Pyrolysis of Graphene Oxide and Urea, and its Electrocatalytic Activity toward the Oxygen-Reduction Reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, S.; Zhang, Y.; Cao, M. A simple and green pathway toward nitrogen and sulfur dual doped hierarchically porous carbons from ionic liquids for oxygen reduction. J. Power Sources 2014, 259, 138–144. [Google Scholar] [CrossRef]
- She, Y.; Lu, Z.-G.; Ni, M.; Li, L.; Leung, M.K. Facile Synthesis of Nitrogen and Sulfur Codoped Carbon from Ionic Liquid as Metal-Free Catalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 7214–7221. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sinev, I.; Ju, W.; Bergmann, A.; Dresp, S.; Kühl, S.; Spoeri, C.; Schmies, H.; Wang, H.; Bernsmeier, D.; et al. Efficient Electrochemical Hydrogen Peroxide Production from Molecular Oxygen on Nitrogen-Doped Mesoporous Carbon Catalysts. ACS Catal. 2018, 8, 2844–2856. [Google Scholar] [CrossRef]
- Fellinger, T.-P.; Hasché, F.; Strasser, P.; Antonietti, M. Mesoporous Nitrogen-Doped Carbon for the Electrocatalytic Synthesis of Hydrogen Peroxide. J. Am. Chem. Soc. 2012, 134, 4072–4075. [Google Scholar] [CrossRef]
- Iglesias, D.; Giuliani, A.; Melchionna, M.; Marchesan, S.; Criado, A.; Nasi, L.; Bevilacqua, M.; Tavagnacco, C.; Vizza, F.; Prato, M.; et al. N-Doped Graphitized Carbon Nanohorns as a Forefront Electrocatalyst in Highly Selective O2 Reduction to H2O2. Chem 2018, 4, 106–123. [Google Scholar] [CrossRef]
- Perazzolo, V.; Durante, C.; Pilot, R.; Paduano, A.; Zheng, J.; Rizzi, G.A.; Martucci, A.; Granozzi, G.; Gennaro, A. Nitrogen and sulfur doped mesoporous carbon as metal-free electrocatalysts for the in situ production of hydrogen peroxide. Carbon 2015, 95, 949–963. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Li, F.; Chang, K.-H.; Hu, C.-C.; Ohsaka, T. Novel synthesis of N-doped porous carbons from collagen for electrocatalytic production of H2O2. Appl. Catal. B Environ. 2012, 126, 208–214. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, T.; Yin, K.; Liu, C.; Wei, Y. Selective H2O2 production on N-doped porous carbon from direct carbonization of metal organic frameworks for electro-Fenton mineralization of antibiotics. Chem. Eng. J. 2019, 383, 123184. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Odedairo, T.; Zhao, X.; Jin, Z.; Zhu, Z.; Yao, X. Activated carbon becomes active for oxygen reduction and hydrogen evolution reactions. Chem. Commun. 2016, 52, 8156–8159. [Google Scholar] [CrossRef]
- Oh, J.; Park, S.; Jang, D.; Shin, Y.; Lim, D.; Park, S. Metal-free N-doped carbon blacks as excellent electrocatalysts for oxygen reduction reactions. Carbon 2019, 145, 481–487. [Google Scholar] [CrossRef]
- Kim, H.W.; Ross, M.B.; Kornienko, N.; Zhang, L.; Guo, J.; Yang, P.; McCloskey, B.D. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal. 2018, 1, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Yin, G.; Zhang, J.; Gao, Y. Comparative investigation of the resistance to electrochemical oxidation of carbon black and carbon nanotubes in aqueous sulfuric acid solution. Electrochim. Acta 2006, 51, 5853–5857. [Google Scholar] [CrossRef]
- You, P.; Kamarudin, S. Recent progress of carbonaceous materials in fuel cell applications: An overview. Chem. Eng. J. 2017, 309, 489–502. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Preuss, K.; Fuertes, A.B.; Sevilla, M.; Titirici, M.-M. The influence of pore size distribution on the oxygen reduction reaction performance in nitrogen doped carbon microspheres. J. Mater. Chem. A 2016, 4, 2581–2589. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2020, 116, 100717. [Google Scholar] [CrossRef]
- Bi, H.; Yin, K.; Xie, X.; Zhou, Y.; Wan, N.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Low Temperature Casting of Graphene with High Compressive Strength. Adv. Mater. 2012, 24, 5124–5129. [Google Scholar] [CrossRef]
- Garcia-Bordejé, E.; Benito, A.; Maser, W. Graphene aerogels via hydrothermal gelation of graphene oxide colloids: Fine-tuning of its porous and chemical properties and catalytic applications. Adv. Colloid Interface Sci. 2021, 292, 102420. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Huang, Y.; Zhang, F.; Yang, X.; Ma, Y.; Chen, Y. Preventing Graphene Sheets from Restacking for High-Capacitance Performance. J. Phys. Chem. C 2011, 115, 23192–23197. [Google Scholar] [CrossRef]
- Liao, Y.; Mustonen, K.; Tulić, S.; Skákalová, V.; Khan, S.A.; Laiho, P.; Zhang, Q.; Li, C.; Monazam, M.R.A.; Kotakoski, J.; et al. Enhanced Tunneling in a Hybrid of Single-Walled Carbon Nanotubes and Graphene. ACS Nano 2019, 13, 11522–11529. [Google Scholar] [CrossRef]
- Dong, X.; Li, B.; Wei, A.; Cao, X.; Chan-Park, M.; Zhang, H.; Li, L.-J.; Huang, W.; Chen, P. One-step growth of graphene–carbon nanotube hybrid materials by chemical vapor deposition. Carbon 2011, 49, 2944–2949. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Víctor-Román, S.; Sanahuja-Parejo, O.; Benito, A.M.; Maser, W.K. Control of the microstructure and surface chemistry of graphene aerogels via pH and time manipulation by a hydrothermal method. Nanoscale 2018, 10, 3526–3539. [Google Scholar] [CrossRef]
- Rodríguez-Mata, V.; Hernández-Ferrer, J.; Carrera, C.; Benito, A.M.; Maser, W.K.; García-Bordejé, E. Towards high-efficient microsupercapacitors based on reduced graphene oxide with optimized reduction degree. Energy Storage Mater. 2020, 25, 740–749. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, T.-Y.; Qian, Y.-H.; Li, S.-S.; Yu, S.-H. A Nitrogen-Doped Graphene/Carbon Nanotube Nanocomposite with Synergistically Enhanced Electrochemical Activity. Adv. Mater. 2013, 25, 3192–3196. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Jiang, S.P. Are metal-free pristine carbon nanotubes electrocatalytically active? Chem. Commun. 2015, 51, 13764–13767. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect Chemistry of Nonprecious-Metal Electrocatalysts for Oxygen Reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Z.; Wang, H.; Wang, Y.; Meng, L.; Song, S. Tuning nondoped carbon nanotubes to an efficient metal-free electrocatalyst for oxygen reduction reaction by localizing the orbital of the nanotubes with topological defects. Nanoscale 2014, 6, 14262–14269. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, L.; Du, A.; Gao, G.; Chen, J.; Yan, X.; Brown, C.L.; Yao, X. Defect Graphene as a Trifunctional Catalyst for Electrochemical Reactions. Adv. Mater. 2016, 28, 9532–9538. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.M.; Soares, O.S.G.P.; Figueiredo, J.L.; Freire, C.; Pereira, M.F.R. Bifunctionality of the pyrone functional group in oxidized carbon nanotubes towards oxygen reduction reaction. Catal. Sci. Technol. 2017, 7, 1868–1879. [Google Scholar] [CrossRef]
- Seredych, M.; Szczurek, A.; Fierro, V.; Celzard, A.; Bandosz, T.J. Electrochemical Reduction of Oxygen on Hydrophobic Ultramicroporous PolyHIPE Carbon. ACS Catal. 2016, 6, 5618–5628. [Google Scholar] [CrossRef]
- Kaneko, K. Determination of pore size and pore size distribution: 1. Adsorbents and catalysts. J. Membr. Sci. 1994, 96, 59–89. [Google Scholar] [CrossRef]
- Balbuena, P.B.; Gubbins, K.E. Classification of adsorption behavior: Simple fluids in pores of slit-shaped geometry. Fluid Phase Equilibria 1992, 76, 21–35. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems. In Handbook of Heterogenous Catalysis; Wiley: Hoboken, NJ, USA, 2008; pp. 1217–1230. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Zhu, D. Chemical doping of graphene. J. Mater. Chem. 2010, 21, 3335–3345. [Google Scholar] [CrossRef]
- Okamoto, Y. First-principles molecular dynamics simulation of O2 reduction on nitrogen-doped carbon. Appl. Surf. Sci. 2009, 256, 335–341. [Google Scholar] [CrossRef]
- Roldán, L.; Truong-Phuoc, L.; Ansón-Casaos, A.; Pham-Huu, C.; García-Bordejé, E. Mesoporous carbon doped with N,S heteroatoms prepared by one-pot auto-assembly of molecular precursor for electrocatalytic hydrogen peroxide synthesis. Catal. Today 2018, 301, 2–10. [Google Scholar] [CrossRef]
- Wang, J.; Kim, J.; Choi, S.; Wang, H.; Lim, J. A Review of Carbon-Supported Nonprecious Metals as Energy-Related Electrocatalysts. Small Methods 2020, 4, 2000621. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Surkus, A.-E.; Junge, H.; Pohl, M.-M.; Radnik, J.; Rabeah, J.; Huan, H.; Schünemann, V.; Brückner, A.; Beller, M. Nanoscale Fe2O3-Based Catalysts for Selective Hydrogenation of Nitroarenes to Anilines. Science 2013, 342, 1073–1076. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Junge, H.; Beller, M. Green synthesis of nitriles using non-noble metal oxides-based nanocatalysts. Nat. Commun. 2014, 5, 4123. [Google Scholar] [CrossRef] [Green Version]
- Jagadeesh, R.V.; Natte, K.; Junge, H.; Beller, M. Nitrogen-Doped Graphene-Activated Iron-Oxide-Based Nanocatalysts for Selective Transfer Hydrogenation of Nitroarenes. ACS Catal. 2015, 5, 1526–1529. [Google Scholar] [CrossRef]
- Geniès, L.; Faure, R.; Durand, R. Electrochemical reduction of oxygen on platinum nanoparticles in alkaline media. Electrochim. Acta 1998, 44, 1317–1327. [Google Scholar] [CrossRef]
- Wu, J.; Xia, F.; Pan, M.; Zhou, X.-D. Oxygen Reduction Reaction on Active and Stable Nanoscale TiSi2Supported Electrocatalysts. J. Electrochem. Soc. 2012, 159, B654–B660. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Stamenkovic, V.; Arenz, M.; Markovic, N.; Ross, P. Oxygen electrocatalysis in alkaline electrolyte: Pt(hkl), Au(hkl) and the effect of Pd-modification. Electrochim. Acta 2002, 47, 3765–3776. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, H.; Liu, Z.; Han, B.; Zhang, X. A Highly Efficient Chemical Sensor Material for H2S: α-Fe2O3 Nanotubes Fabricated Using Carbon Nanotube Templates. Adv. Mater. 2005, 17, 2993–2997. [Google Scholar] [CrossRef]
- Matanovic, I.; Artyushkova, K.; Atanassov, P. Understanding PGM-free catalysts by linking density functional theory calculations and structural analysis: Perspectives and challenges. Curr. Opin. Electrochem. 2018, 9, 137–144. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, X.; Dubois, M.; Herraiz, M.; Chenitz, R.; Lefèvre, M.; Cherif, M.; Vidal, F.; Glibin, V.P.; Sun, S.; et al. Non-PGM electrocatalysts for PEM fuel cells: Effect of fluorination on the activity and stability of a highly active NC_Ar + NH3 catalyst. Energy Environ. Sci. 2019, 12, 3015–3037. [Google Scholar] [CrossRef]
- Yu, X.; Lai, S.; Xin, S.; Chen, S.; Zhang, X.; She, X.; Zhan, T.; Zhao, X.; Yang, D. Coupling of iron phthalocyanine at carbon defect site via π-π stacking for enhanced oxygen reduction reaction. Appl. Catal. B Environ. 2020, 280, 119437. [Google Scholar] [CrossRef]
- Mata, V.R.; González-Domínguez, J.M.; Benito, A.M.; Maser, W.K.; García-Bordejé, E. Reduced Graphene Oxide Aerogels with Controlled Continuous Microchannels for Environmental Remediation. ACS Appl. Nano Mater. 2019, 2, 1210–1222. [Google Scholar] [CrossRef]
- Paulus, U.; Wokaun, A.; Scherer, G.; Schmidt, T.J.; Stamenkovic, V.; Markovic, N.; Ross, P. Oxygen reduction on high surface area Pt-based alloy catalysts in comparison to well defined smooth bulk alloy electrodes. Electrochim. Acta 2002, 47, 3787–3798. [Google Scholar] [CrossRef]
- Tuci, G.; Zafferoni, C.; Rossin, A.; Milella, A.; Luconi, L.; Innocenti, M.; Phuoc, L.T.; Duong-Viet, C.; Pham-Huu, C.; Giambastiani, G. Chemically Functionalized Carbon Nanotubes with Pyridine Groups as Easily Tunable N-Decorated Nanomaterials for the Oxygen Reduction Reaction in Alkaline Medium. Chem. Mater. 2014, 26, 3460–3470. [Google Scholar] [CrossRef]

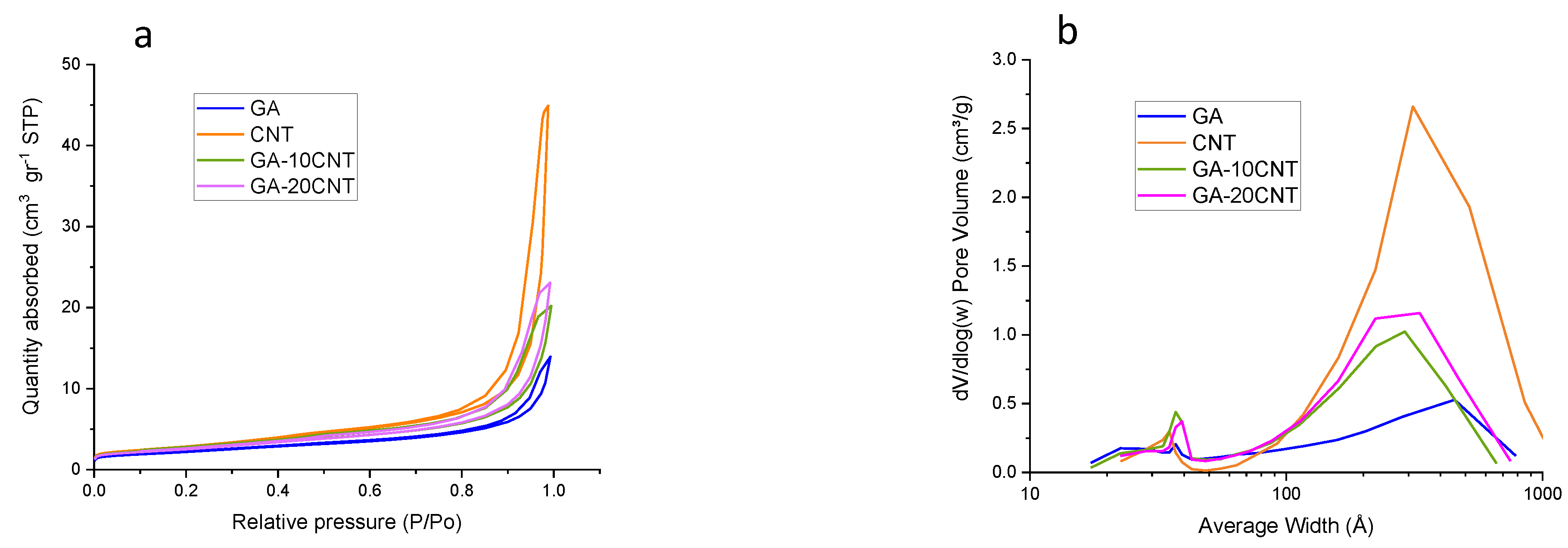
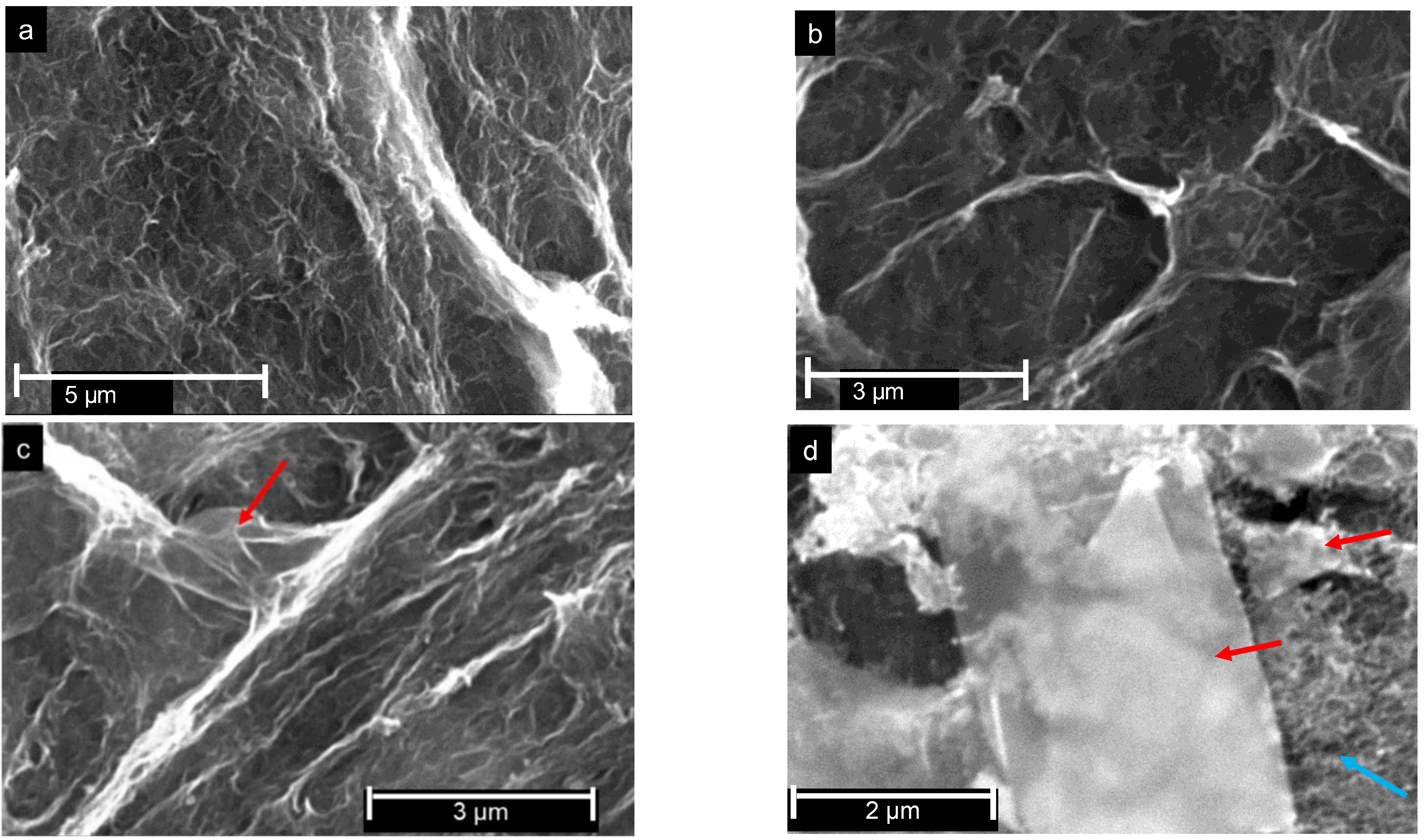
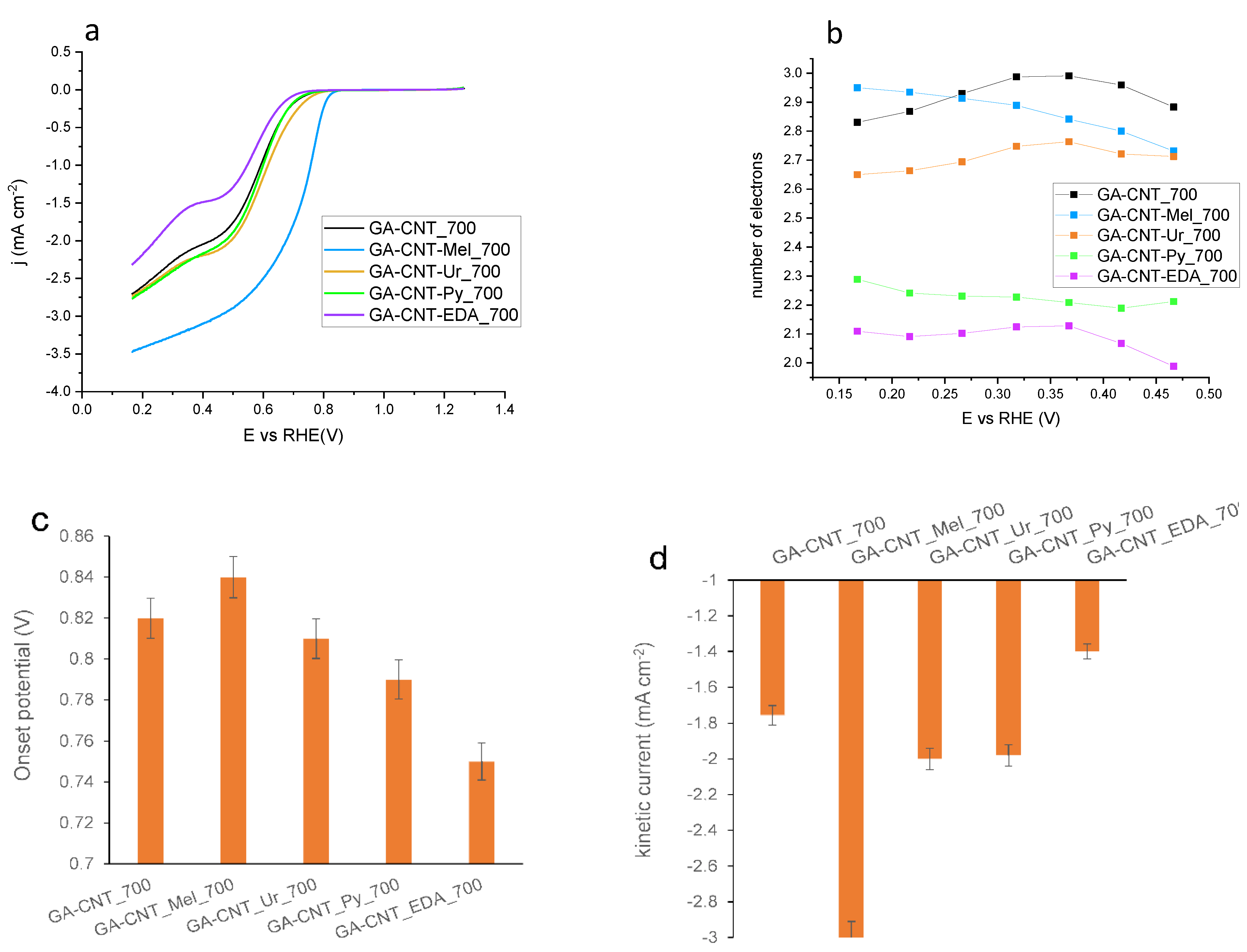


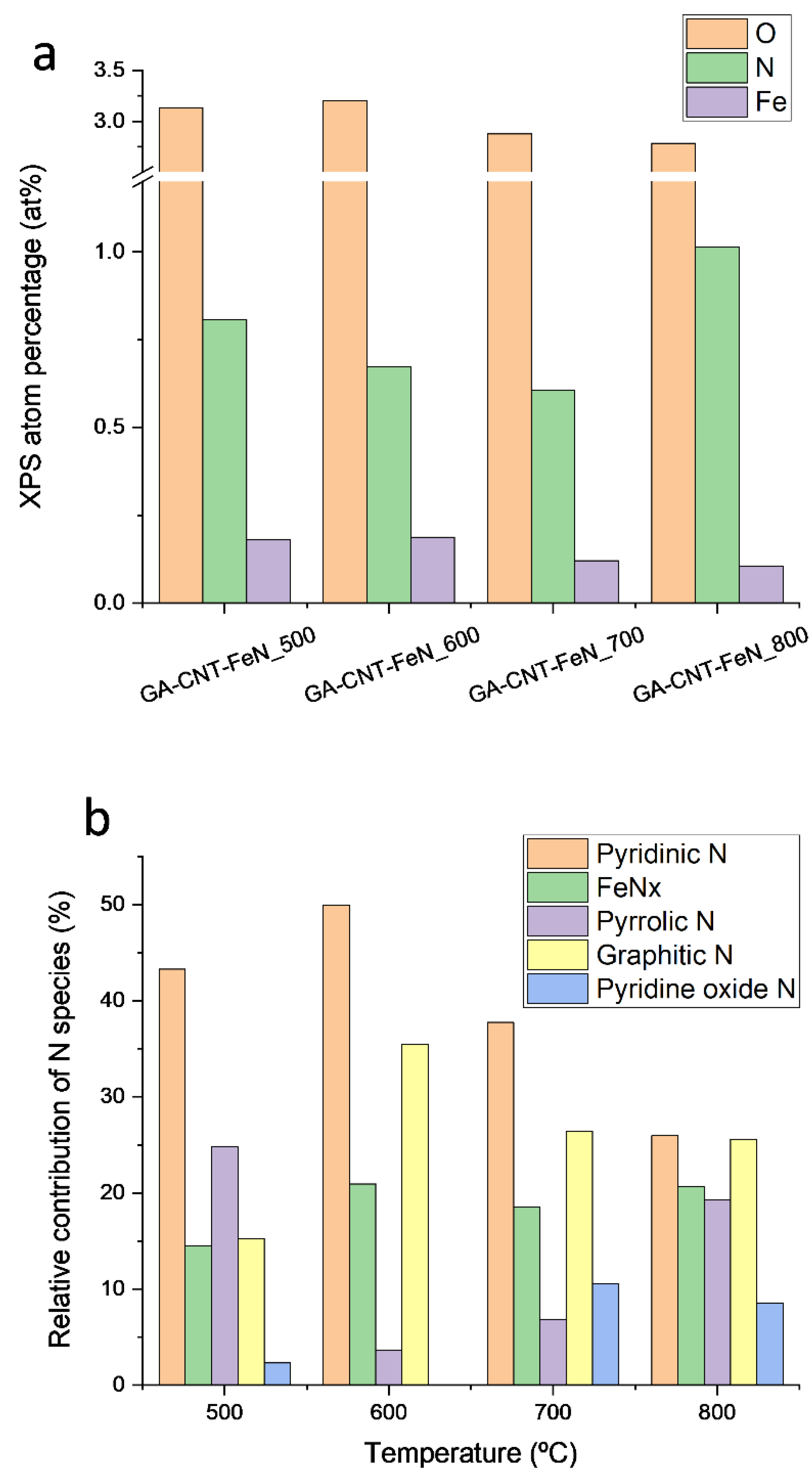
| Catalyst | C | O | N | H |
|---|---|---|---|---|
| wt% | wt% | wt% | wt% | |
| CNT | 93.7 | 5.1 | 0.6 | 0.6 |
| GA | 70 | 21.1 | 8.3 | 0.9 |
| GA-CNT | 81.8 | 13 | 4.4 | 0.8 |
| CNT_700 | 97.6 | 1.6 | 0.4 | 0.4 |
| GA_700 | 83 | 7.9 | 8 | 0.8 |
| GA-CNT_700 | 90.4 | 4.8 | 4.2 | 0.6 |
| Sample | Surface Area | Pore Volume | Micropore Surface Area * (Smic) | External Surface Area (Smes) |
|---|---|---|---|---|
| m2/g | cm3/g | m2/g | m2/g | |
| CNT | 228 | 1.55 | 63 | 165 |
| GA | 173 | 0.48 | 43 | 130 |
| GA-CNT (10 mg) | 208 | 0.7 | 42 | 165 |
| GA-CNT (20 mg) | 200 | 0.8 | 21 | 178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ferrer, J.; Benito, A.M.; Maser, W.K.; García-Bordejé, E. Hybrids of Reduced Graphene Oxide Aerogel and CNT for Electrochemical O2 Reduction. Catalysts 2021, 11, 1404. https://doi.org/10.3390/catal11111404
Hernández-Ferrer J, Benito AM, Maser WK, García-Bordejé E. Hybrids of Reduced Graphene Oxide Aerogel and CNT for Electrochemical O2 Reduction. Catalysts. 2021; 11(11):1404. https://doi.org/10.3390/catal11111404
Chicago/Turabian StyleHernández-Ferrer, Javier, Ana M. Benito, Wolfgang K. Maser, and Enrique García-Bordejé. 2021. "Hybrids of Reduced Graphene Oxide Aerogel and CNT for Electrochemical O2 Reduction" Catalysts 11, no. 11: 1404. https://doi.org/10.3390/catal11111404
APA StyleHernández-Ferrer, J., Benito, A. M., Maser, W. K., & García-Bordejé, E. (2021). Hybrids of Reduced Graphene Oxide Aerogel and CNT for Electrochemical O2 Reduction. Catalysts, 11(11), 1404. https://doi.org/10.3390/catal11111404








