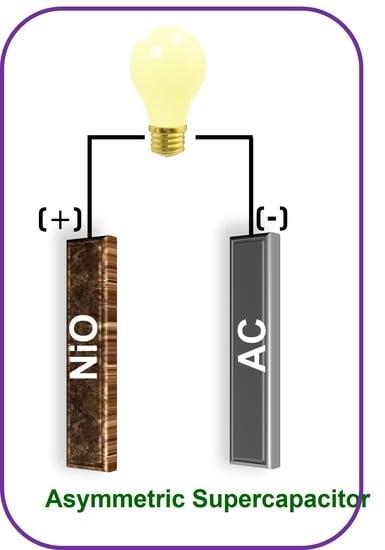
Fabrication of High-Performance Asymmetric Supercapacitor Consists of Nickel Oxide and Activated Carbon (NiO//AC)
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
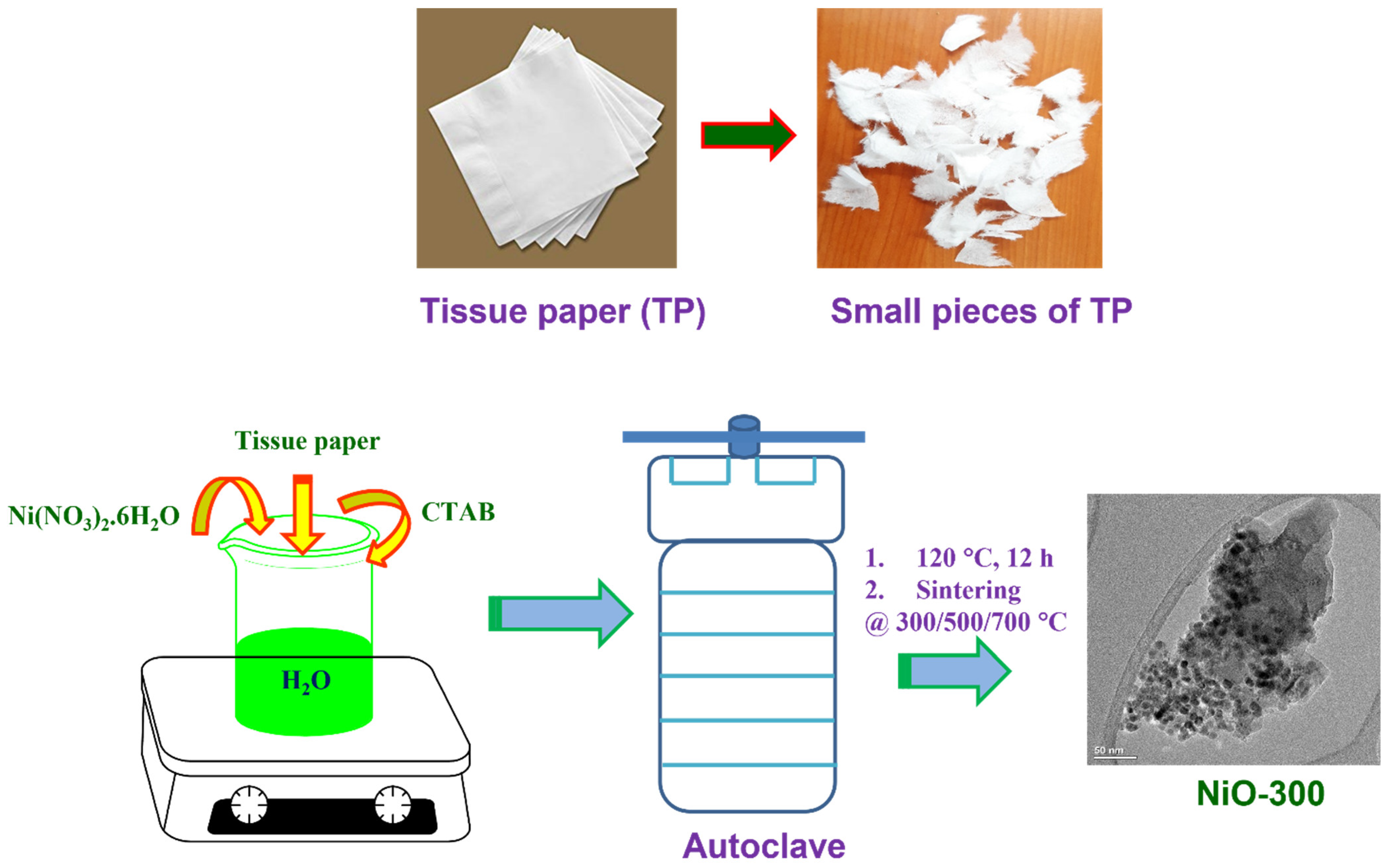
2.2. Synthesis of NiO Nanoparticles
2.3. Characterization Techniques
2.4. Electrochemical Measurements
2.5. Fabrication of ASC Device
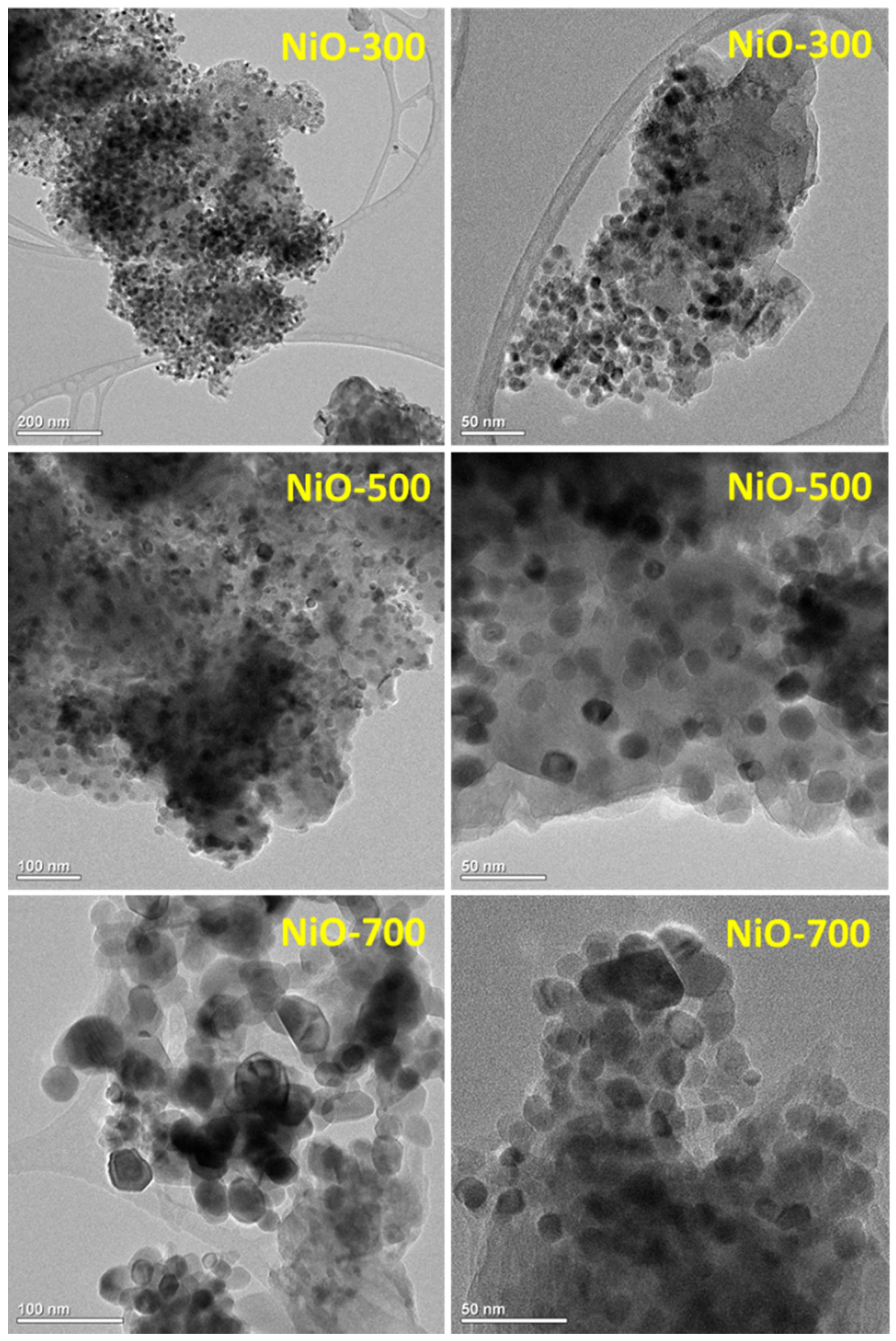
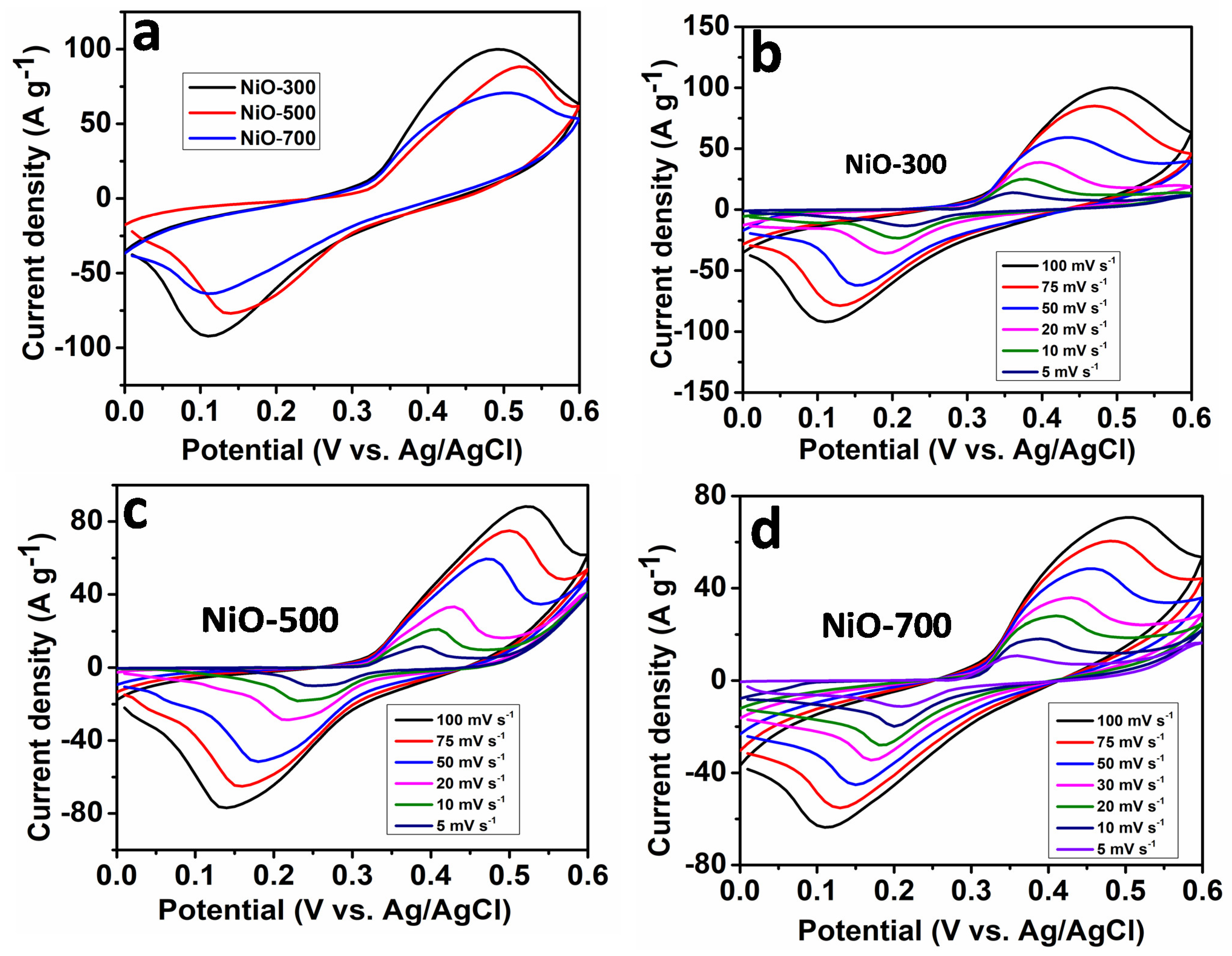
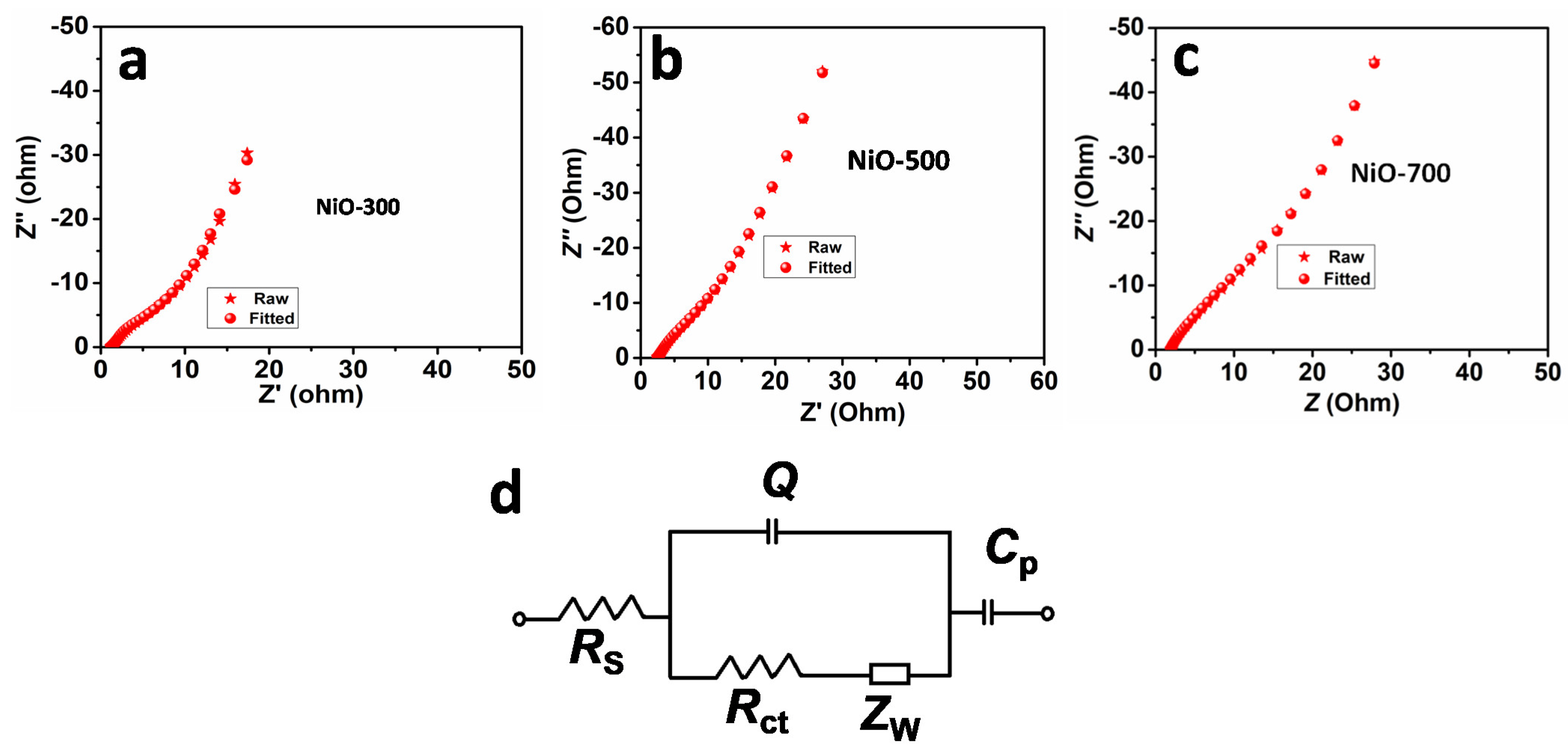
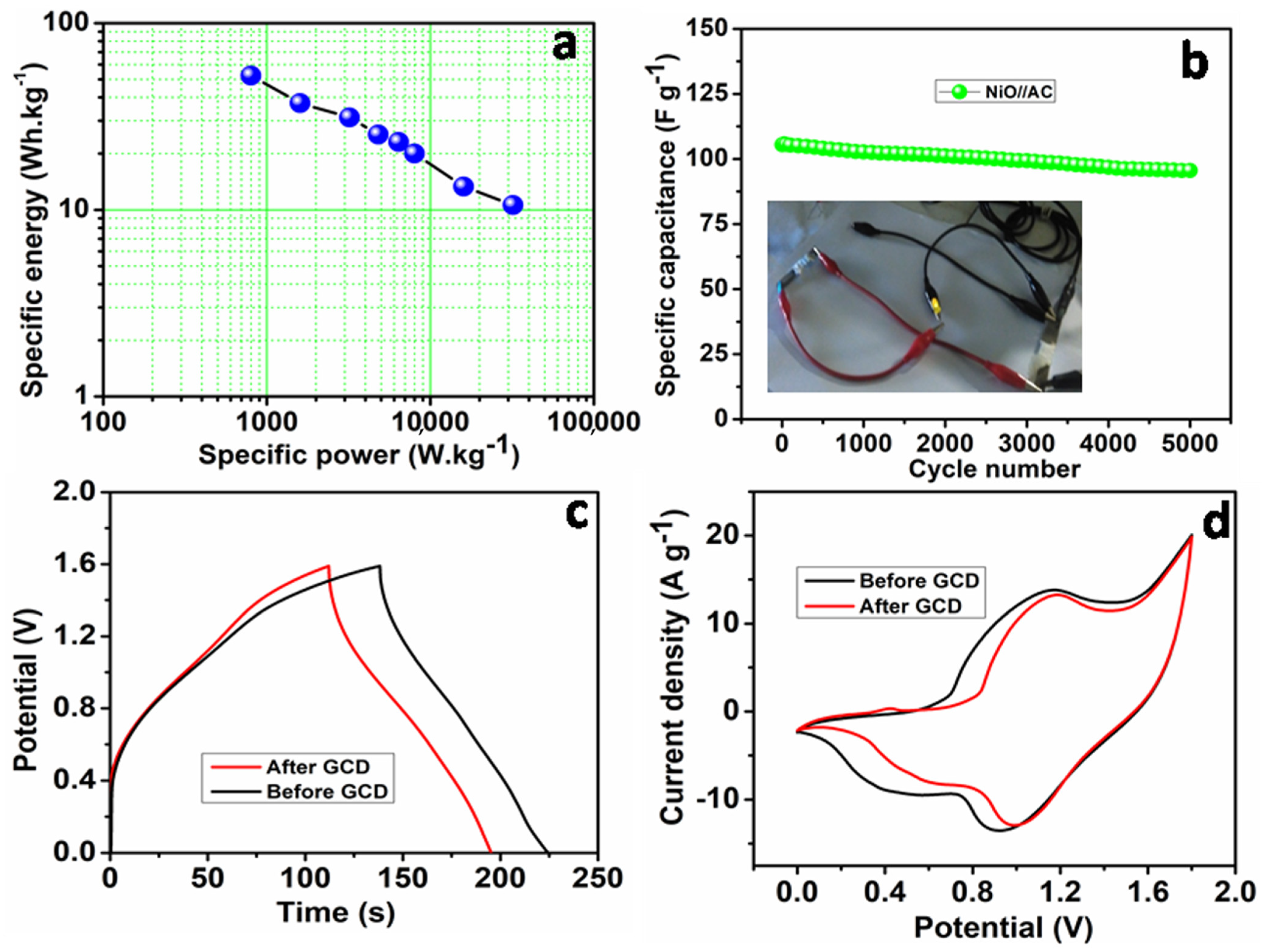
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Hu, L.; Vosgueritchian, M.; Wang, H.; Xie, X.; McDonough, J.R.; Cui, X.; Cui, Y.; Bao, Z. Solution-Processed Graphene/MnO2 Nanostructured Textiles for High-Performance Electrochemical Capacitors. Nano Lett. 2011, 11, 2905–2911. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinodh, R.; Sasikumar, Y.; Kim, H.-J.; Atchudan, R.; Yi, M. Chitin and chitosan based biopolymer derived electrode materials for supercapacitor applications: A critical review. J. Ind. Eng. Chem. 2021, 104, 155–171. [Google Scholar] [CrossRef]
- Kim, I.; Vinodh, R.; Gopi, C.V.V.M.; Kim, H.-J.; Babu, R.S.; Deviprasath, C.; Devendiran, M.; Kim, S. Novel porous carbon electrode derived from hypercross-linked polymer of poly(divinylbenzene-co-vinyl benzyl chloride) for supercapacitor applications. J. Energy Storage 2021, 43, 103287. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kim, H.-J. Recent progress of advanced energy storage materials for flexible and wearable supercapacitor: From design and development to applications. J. Energy Storage 2020, 27, 101035. [Google Scholar]
- Vinodh, R.; Babu, R.S.; Gopi, C.V.V.M.; Deviprasath, C.; Atchudan, R.; Samyn, L.M.; De Barros, A.L.F.; Kim, H.-J.; Yi, M. Influence of annealing temperature in nitrogen doped porous carbon balls derived from hypercross-linked polymer of anthracene for supercapacitor applications. J. Energy Storage 2020, 28, 101196. [Google Scholar] [CrossRef]
- Vinayagam, M.; Babu, R.S.; Sivasamy, A.; De Barros, A.L.F. Biomass-derived porous activated carbon from Syzygium cumini fruit shells and Chrysopogon zizanioides roots for high-energy density symmetric supercapacitors. Biomass Bioenergy 2020, 143, 105838. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Babu, R.S.; Sundramoorthy, A.K.; Renita, A.A.; Lee, Y.R. Facile synthesis of nitrogen-doped porous carbon materials using waste biomass for energy storage applications. Chemosphere 2022, 289, 133225. [Google Scholar] [CrossRef]
- Babu, R.S.; Vinodh, R.; De Barros, A.L.F.; Samyn, L.M.; Prasanna, K.; Maier, M.A.; Alves, C.H.F.; Kim, H.-J. Asymmetric supercapacitor based on carbon nanofibers as the anode and two-dimensional copper cobalt oxide nanosheets as the cathode. Chem. Eng. J. 2019, 366, 390–403. [Google Scholar] [CrossRef]
- Vinayagam, M.; Babu, R.S.; Sivasamy, A.; De Barros, A.L.F. Biomass-derived porous activated carbon nanofibers from Sapindus trifoliatus nut shells for high-performance symmetric supercapacitor applications. Carbon Lett. 2021, 31, 1133–1143. [Google Scholar] [CrossRef]
- Lu, X.; Dou, H.; Yang, S.; Hao, L.; Zhang, L.; Shen, L.; Zhang, F.; Zhang, X. Fabrication and electrochemical capacitance of hierarchical graphene/polyaniline/carbon nanotube ternary composite film. Electrochim. Acta 2011, 56, 9224–9232. [Google Scholar] [CrossRef]
- Feng, X.M.; Li, R.M.; Ma, Y.W.; Chen, R.F.; Shi, N.E.; Fan, Q.L.; Huang, W. One-Step Electrochemical Synthesis of Graphene/Polyaniline Composite Film and Its Applications. Adv. Funct. Mater. 2011, 21, 2989–2996. [Google Scholar] [CrossRef]
- Song, C.-S.; Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Kalla, R.M.N.; Obaidat, I.M.; Kim, H.-J. Morphology-dependent binder-free CuNiO2 electrode material with excellent electrochemical performances for supercapacitors. J. Energy Storage 2019, 26, 101037. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Sambasivam, S.; Vinodh, R.; Kim, H.-J.; Obaidat, I.M. Nanostructured Ni-doped CuS thin film as an efficient counter electrode material for high-performance quantum dot-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2020, 31, 975–982. [Google Scholar] [CrossRef]
- Park, T.Y.; Gopi, C.V.V.M.; Vinodh, R.; Kim, H.-J. Facile synthesis of highly efficient V2O5@NiCo2O4 as battery-type electrode material for high-performance electrochemical supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 13519–13524. [Google Scholar] [CrossRef]
- Maier, M.A.; Babu, R.S.; Sampaio, D.M.; De Barros, A.L.F. Binder-free polyaniline interconnected metal hexacyanoferrates nanocomposites (Metal = Ni, Co) on carbon fibers for flexible supercapacitors. J. Mater. Sci. Mater. Electron. 2017, 28, 17405–17413. [Google Scholar] [CrossRef]
- Babu, R.S.; De Barros, A.L.F.; Maier, M.A.; Sampaio, D.M.; Balamurugan, J.; Lee, J.H. Novel polyaniline/manganese hexacyanoferrate nanoparticles on carbon fiber as binder-free electrode for flexible supercapacitors. Compos. Part B Eng. 2018, 143, 141–147. [Google Scholar] [CrossRef]
- Samyn, L.M.; Babu, R.S.; Devendiran, M.; De Barros, A.L.F. Electropolymerization of p-phenylenediamine films on carbon fiber fabrics electrode for flexible supercapacitors: Surface and electrochemical characterizations. Ionics 2020, 26, 3041–3050. [Google Scholar] [CrossRef]
- Samyn, L.M.; Babu, R.S.; Devendiran, M.; De Barros, A.L.F. One-step electropolymerization of methylene blue films on highly flexible carbon fiber electrode as supercapacitors. Micro Nano Syst. Lett. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Santos, R.S.; Babu, R.S.; Devendiran, M.; Haddad, D.B.; De Barros, A.L.F. Facile synthesis of transition metal (M = Cu, Co) oxide grafted graphitic carbon nitride nanosheets for high performance asymmetric supercapacitors. Mater. Lett. 2022, 308, 131156. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.M.; Liu, P.; Zhong, Y.L.; Chong, K.F. W18O49 nanowires-graphene nanocomposite for asymmetric supercapacitors employing AlCl3 aqueous electrolyte. Chem. Eng. J. 2021, 409, 128216. [Google Scholar] [CrossRef]
- Oh, S.H.; Nazar, L.F. Direct synthesis of electroactive mesoporous hydrous crystalline RuO2 templated by a cationic surfactant. J. Mater. Chem. 2010, 20, 3834–3839. [Google Scholar] [CrossRef]
- Ye, J.-S.; Cui, H.F.; Liu, X.; Lim, T.M.; Zhang, W.-D.; Sheu, F.-S. Preparation and characterization of aligned carbon nanotubes-ruthenium oxide nanocomposites for supercapacitors. Small 2005, 1, 560–565. [Google Scholar] [CrossRef]
- Li, Y.; Huang, K.; Liu, S.; Yao, Z.; Zhuang, S. Meso-macroporous Co3O4 electrode prepared by polystyrene spheres and carbowax templates for supercapacitors. J. Solid State Electrochem. 2011, 15, 587–592. [Google Scholar] [CrossRef]
- Xu, M.-W.; Zhao, D.-D.; Bao, S.-J.; Li, H.-L. Mesoporous amorphous MnO2 as electrode material for supercapacitor. J. Solid State Electrochem. 2007, 11, 1101–1107. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Hu, Z.A.; Zhang, Z.Y.; Zhang, F.H.; Zhang, Y.J.; Liang, P.J.; Zhang, H.Y.; Wu, H.Y. Reduced graphene oxide–nickel oxide composites with high electrochemical capacitive performance. Mater. Chem. Phys. 2012, 133, 363–368. [Google Scholar] [CrossRef]
- Zhao, B.; Song, J.; Liu, P.; Xu, W.; Fang, T.; Jiao, Z.; Zhang, H.; Jiang, Y. Monolayer graphene/NiO nanosheets with two-dimension structure for supercapacitors. J. Mater. Chem. 2011, 21, 18792–18798. [Google Scholar] [CrossRef]
- Ge, C.; Hou, Z.; He, B.; Zeng, F.; Cao, J.; Liu, Y.; Kuang, Y. Three-dimensional flowerlike nickel oxide supported on graphene sheets as electrode material for supercapacitors. J. Sol-Gel Sci. Technol. 2012, 63, 146–152. [Google Scholar] [CrossRef]
- Fan, L.; Tang, L.; Gong, H.; Yao, Z.; Guo, R. Carbon–nanoparticles encapsulated in hollow nickel oxides for supercapacitor application. J. Mater. Chem. 2012, 22, 16376–16381. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Zhang, W.; Zheng, H.; Su, G. Charge-discharge process of MnO2 supercapacitor. Trans. Nonferr. Met. Soc. China 2007, 17, 649–653. [Google Scholar] [CrossRef]
- Brousse, T.; Toupin, M.; Dugas, R.; Athouel, L.; Crosnier, O.; Belanger, D. Crystalline MnO2 as possible alternatives to amorphous compounds in electrochemical supercapacitors. J. Electrochem. Soc. 2006, 153, A2171–A2180. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y. Electrochemical capacitance characterization of NiO with ordered mesoporous structure synthesized by template SBA-15. Electrochim. Acta 2006, 51, 3223–3227. [Google Scholar] [CrossRef]
- Lang, J.-W.; Kong, L.-B.; Wu, W.-J.; Luo, Y.-C.; Kang, L. Facile approach to prepare loose-packed NiO nano-flakes materials for supercapacitors. Chem. Commun. 2008, 35, 4213–4215. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, G.; Liu, J.; Qiao, S.; Ahn, H. Highly ordered mesoporous NiO anode material for lithium ion batteries with an excellent electrochemical performance. J. Mater. Chem. 2011, 21, 3046–3052. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Zhang, Y.; Wang, S.; Zhang, Z.; Fei, L.; Qian, Y. High-yield synthesis of NiO nanoplatelets and their excellent electrochemical performance. Cryst. Growth Des. 2006, 6, 2163–2165. [Google Scholar] [CrossRef]
- Justin, P.; Meher, S.K.; Rao, G.R. Tuning of capacitance behavior of NiO using anionic, cationic, and nonionic surfactants by hydrothermal synthesis. J. Phys. Chem. C 2010, 114, 5203–5210. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Singh, S.; Kim, H.-J. Co9S8-Ni3S2/CuMn2O4-NiMn2O4 and MnFe2O4-ZnFe2O4/graphene as binder-free cathode and anode materials for high energy density supercapacitors. Chem. Eng. J. 2020, 381, 122640. [Google Scholar] [CrossRef]
- Vinodh, R.; Gopi, C.V.V.M.; Kummara, V.G.R.; Atchudan, R.; Ahamad, T.; Sambasivam, S.; Yi, M.; Obaidat, I.M.; Kim, H.-J. A review on porous carbon electrode material derived from hypercross-linked polymers for supercapacitor applications. J. Energy Storage 2020, 32, 101831. [Google Scholar] [CrossRef]
- Wardani, M.; Yulizar, Y.; Abdullah, I.; Apriandanu, D.O.B. Synthesis of NiO nanoparticles via green route using Ageratum conyzoides L. leaf extract and their catalytic activity. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012077. [Google Scholar] [CrossRef]
- Shen, L.; Che, Q.; Li, H.; Zhang, X. Mesoporous NiCo2O4 nanowire arrays grown on carbon textiles as binder-free flexible electrodes for energy storage. Adv. Funct. Mater. 2014, 24, 2630–2637. [Google Scholar] [CrossRef]
- Santhoshkumar, P.; Prasanna, K.; Jo, Y.N.; Sivagami, I.N.; Kang, S.H.; Lee, C.W. Hierarchically structured mesoporous bimetallic oxides as a potential anode material for rechargeable lithium batteries. J. Alloys Compd. 2019, 771, 555–564. [Google Scholar] [CrossRef]
- Jahromi, S.P.; Pandikumar, A.; Goh, B.T.; Lim, Y.S.; Basirun, W.J.; Lim, H.N.; Huang, N.M. Influence of particle size on performance of a nickel oxide nanoparticle-based supercapacitor. RSC Adv. 2015, 5, 14010–14019. [Google Scholar] [CrossRef]
- Babu, R.S.; Prabhu, P.; Narayanan, S.S. Green synthesized nickel nanoparticles modified electrode in ionic liquid medium and its application towards determination of biomolecules. Talanta 2013, 110, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, A.I.; Kim, Y.; Pawar, S.M.; Kim, J.H.; Im, H.; Kim, H. Chemically grown, porous, nickel oxide thin-film for electrochemical supercapacitors. J. Power Sources 2011, 196, 2393–2397. [Google Scholar] [CrossRef]
- Han, D.; Xu, P.; Jing, X.; Wang, J.; Song, D.; Liu, J.; Zhang, M. Facile approach to prepare hollow core-shell NiO microspherers for supercapacitor electrodes. J. Solid State Chem. 2013, 203, 60–67. [Google Scholar] [CrossRef]
- Yan, X.; Tong, X.; Wang, J.; Gong, C.; Zhang, M.; Liang, L. Rational synthesis of hierarchically porous NiO hollow spheres and their supercapacitor application. Mater. Lett. 2013, 95, 1–4. [Google Scholar] [CrossRef]
- Xu, J.; Gao, L.; Cao, J.; Wang, W.; Chen, Z. Electrochemical capacitance of nickel oxide nanotubes synthesized in anodic aluminum oxide templates. J. Solid State Electrochem. 2010, 15, 2005–2011. [Google Scholar] [CrossRef]
- Ren, B.; Fan, M.; Liu, Q.; Wang, J.; Song, D.; Bai, X. Hollow NiO nanofibers modified by citric acid and the performances as supercapacitor electrode. Electrochim. Acta 2013, 92, 197–204. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, S.; Zhang, E.; Wei, J.; Suo, H.; Zhao, C.; Zhao, X. One-pot synthesis of nickel oxide–carbon composite microspheres on nickel foam for supercapacitors. J. Mater. Sci. 2011, 47, 2182–2187. [Google Scholar] [CrossRef]
- Chen, W.; Gui, D.; Liu, J. Nickel oxide/graphene aerogel nanocomposite as a supercapacitor electrode material with extremely wide working potential window. Electrochim. Acta 2014, 222, 1424–1429. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, H.; Li, Z.; Sun, P.; Liu, F.; Dong, C.; Wang, J.; Li, Z.; Wu, M.; Zhang, C.; et al. Synthesis of delaminated layered double hydroxides and their assembly with graphene oxide for supercapacitor application. J. Alloys Compd. 2017, 711, 31–41. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.; Yin, Z.; Wang, X.; Tu, J.; Li, J. Periodic stacking of 2D charged sheets: Self-assembled superlattice of Ni-Al layered double hydroxide (LDH) and reduced graphene oxide. Nano Energy 2016, 20, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Shi, N.; Liu, Q.; Wang, J.; Yang, B.; Wang, B.; Yan, H.; Sun, Y.; Jing, X. Facile synthesis of exfoliated Co-Al LDH-carbon nanotube composites with high performance as supercapacitor electrodes. Phys. Chem. Chem. Phys. 2014, 16, 17936–17942. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, Z.; Xu, Y.; Sun, J.; Hong, W.; Liu, X.; Wang, J.; Yang, S. Simple synthesis of amorphous NiWO4 nanostructure and its application as a novel cathode material for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 8044–8052. [Google Scholar] [CrossRef] [PubMed]




| Electrode | Electrolyte | Specific Capacitance | Energy Density (Wh kg−1) | Power Density (W kg−1) | Stability (Cycles/Capacitance Retention) | Ref. |
|---|---|---|---|---|---|---|
| Porous NiO film | 1 M NaOH | 104.2 F g−1 @ 1 mA cm−1 | --- | --- | 5000 cycles/--- | [45] |
| NiO core/shell | 5 M KOH | 448 F g−1 @ 0.5 A g−1 | --- | --- | 500 cycles/--- | [46] |
| NiO hollow spheres | 2 M KOH | 346 F g−1 @ 1 A g−1 | --- | --- | 5000 cycles/--- | [47] |
| NiO nanotubes | 6 M KOH | 266 F g−1 @ 0.1 A g−1 | --- | --- | 2000 cycles/93% | [48] |
| NiO nanofibers | 6 M KOH | 336 F g−1 @ 5 mA cm−1 | --- | --- | 1000 cycles/87% | [49] |
| NiO-carbon | 6 M KOH | 265 F g−1 @ 0.25 A g−1 | --- | --- | 1000 cycles/70% | [50] |
| 3D-NiO/Graphene | 6 M KOH | 587.3 F g−1 @ 1 A g−1 | --- | --- | 1000 cycles/98.5% | [51] |
| NiAl-LDHs//AC | 6 M KOH | 959 F g−1 @ 1 A g−1 | 21.0 | 700 | 6000 cycles/63% | [52] |
| NiAl-LDHs-rGO//AC | 6 M KOH | 129 A h kg−1 @ 1 A g−1 | 15.4 | 342 | 10,000 cycles/72.7% | [53] |
| CoAl LDHCNTs//AC | 2 M KOH | 884 F g−1 @ 0.86 A g−1 | 28.0 | 444.1 | 1000 cycles/68% | [54] |
| NiWO4//AC | 2 M KOH | 586.2 F g−1 @ 0.5 A g−1 | 25.3 | 200 | 5000 cycles/91.4% | [55] |
| NiO nanoflakes//AC | 2 M KOH | 568.7F g−1@ 0.5 A g−1 | 52.4 | 32,000 | 5000 cycles/90.6% | [This work] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinodh, R.; Babu, R.S.; Atchudan, R.; Kim, H.-J.; Yi, M.; Samyn, L.M.; de Barros, A.L.F. Fabrication of High-Performance Asymmetric Supercapacitor Consists of Nickel Oxide and Activated Carbon (NiO//AC). Catalysts 2022, 12, 375. https://doi.org/10.3390/catal12040375
Vinodh R, Babu RS, Atchudan R, Kim H-J, Yi M, Samyn LM, de Barros ALF. Fabrication of High-Performance Asymmetric Supercapacitor Consists of Nickel Oxide and Activated Carbon (NiO//AC). Catalysts. 2022; 12(4):375. https://doi.org/10.3390/catal12040375
Chicago/Turabian StyleVinodh, Rajangam, Rajendran Suresh Babu, Raji Atchudan, Hee-Je Kim, Moonsuk Yi, Leandro Marques Samyn, and Ana Lucia Ferreira de Barros. 2022. "Fabrication of High-Performance Asymmetric Supercapacitor Consists of Nickel Oxide and Activated Carbon (NiO//AC)" Catalysts 12, no. 4: 375. https://doi.org/10.3390/catal12040375
APA StyleVinodh, R., Babu, R. S., Atchudan, R., Kim, H.-J., Yi, M., Samyn, L. M., & de Barros, A. L. F. (2022). Fabrication of High-Performance Asymmetric Supercapacitor Consists of Nickel Oxide and Activated Carbon (NiO//AC). Catalysts, 12(4), 375. https://doi.org/10.3390/catal12040375