Insight into Crystallization Features of MOR Zeolite Synthesized via Ice-Templating Method
Abstract
:1. Introduction
2. Results and Discussions
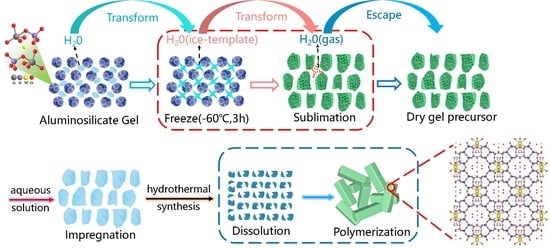
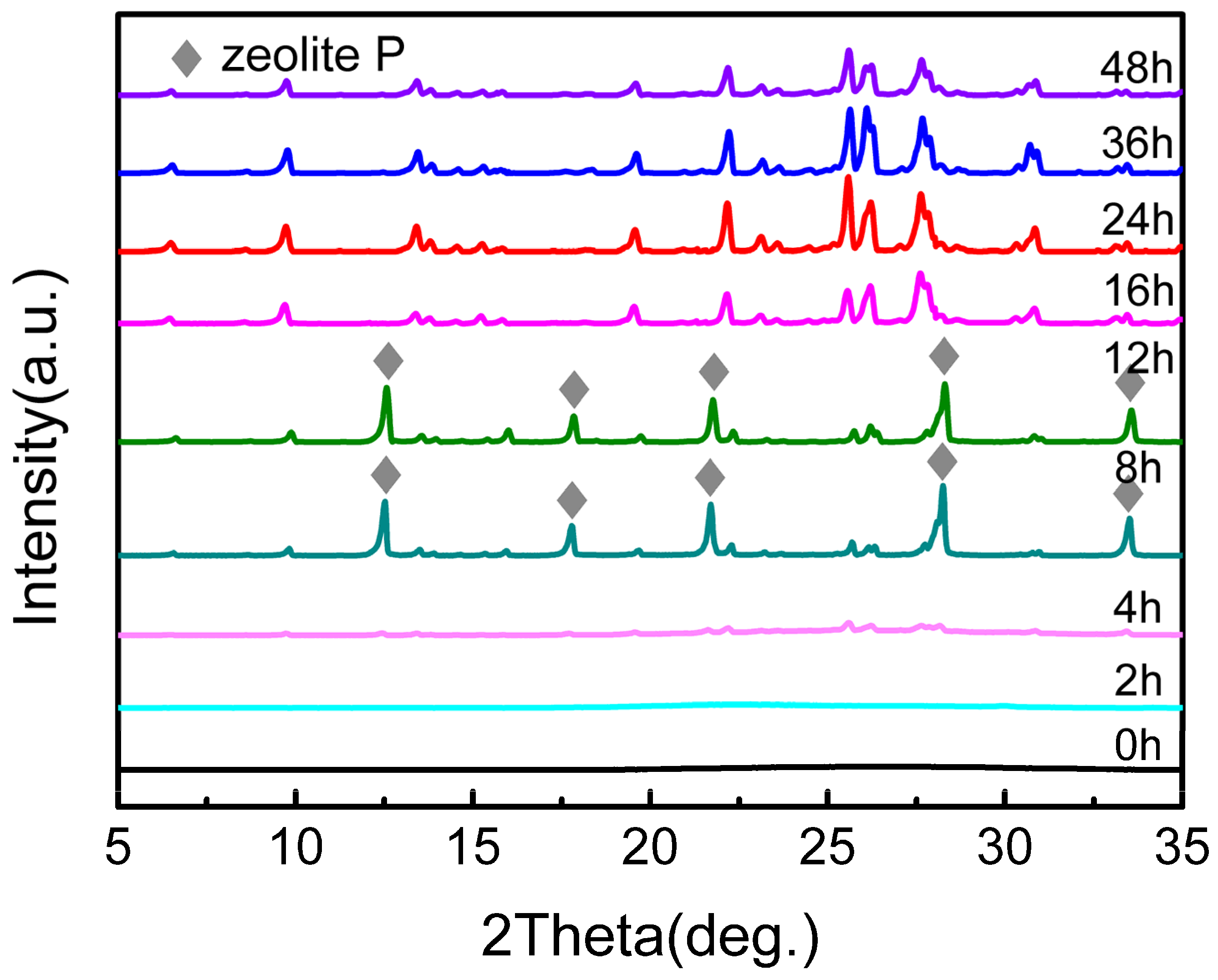
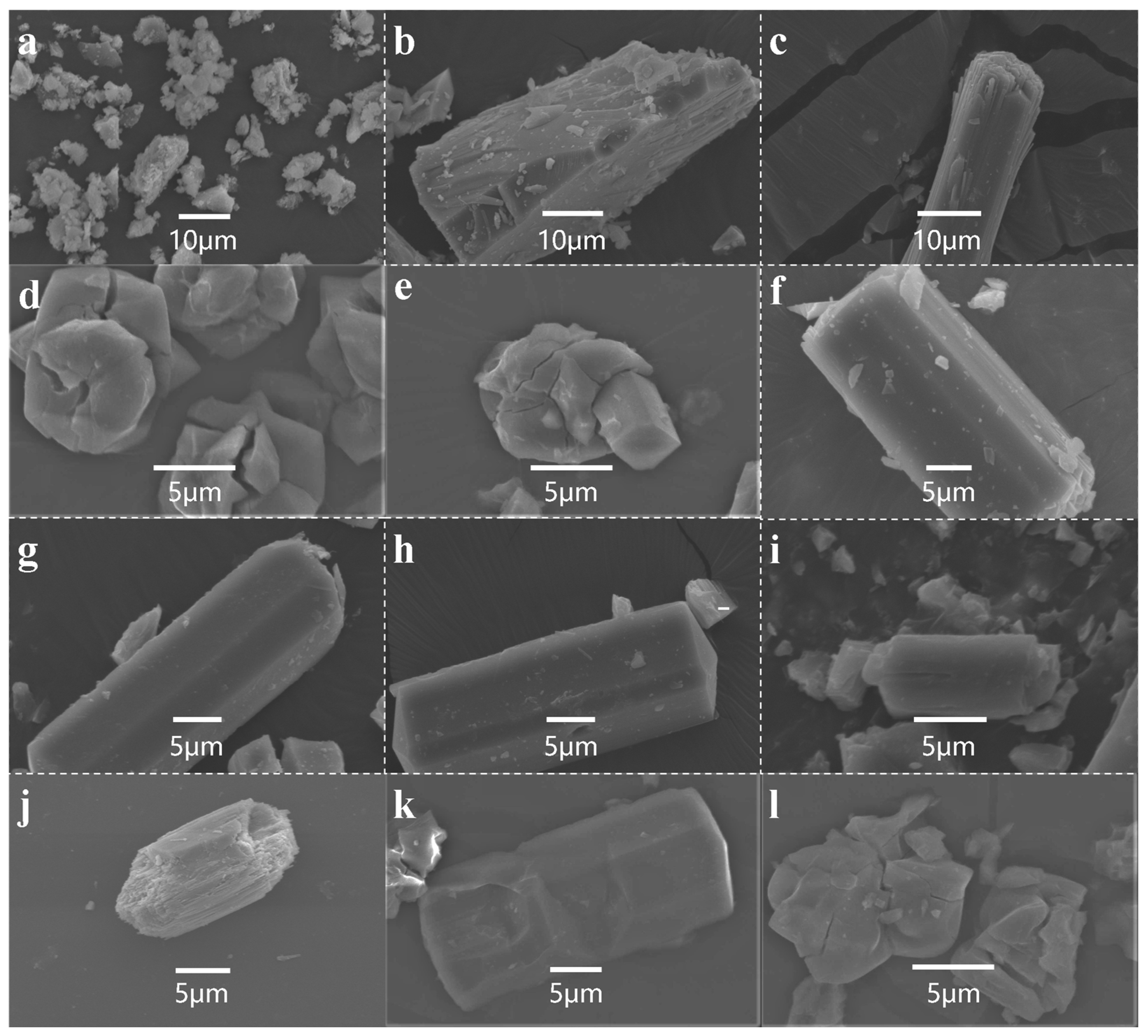
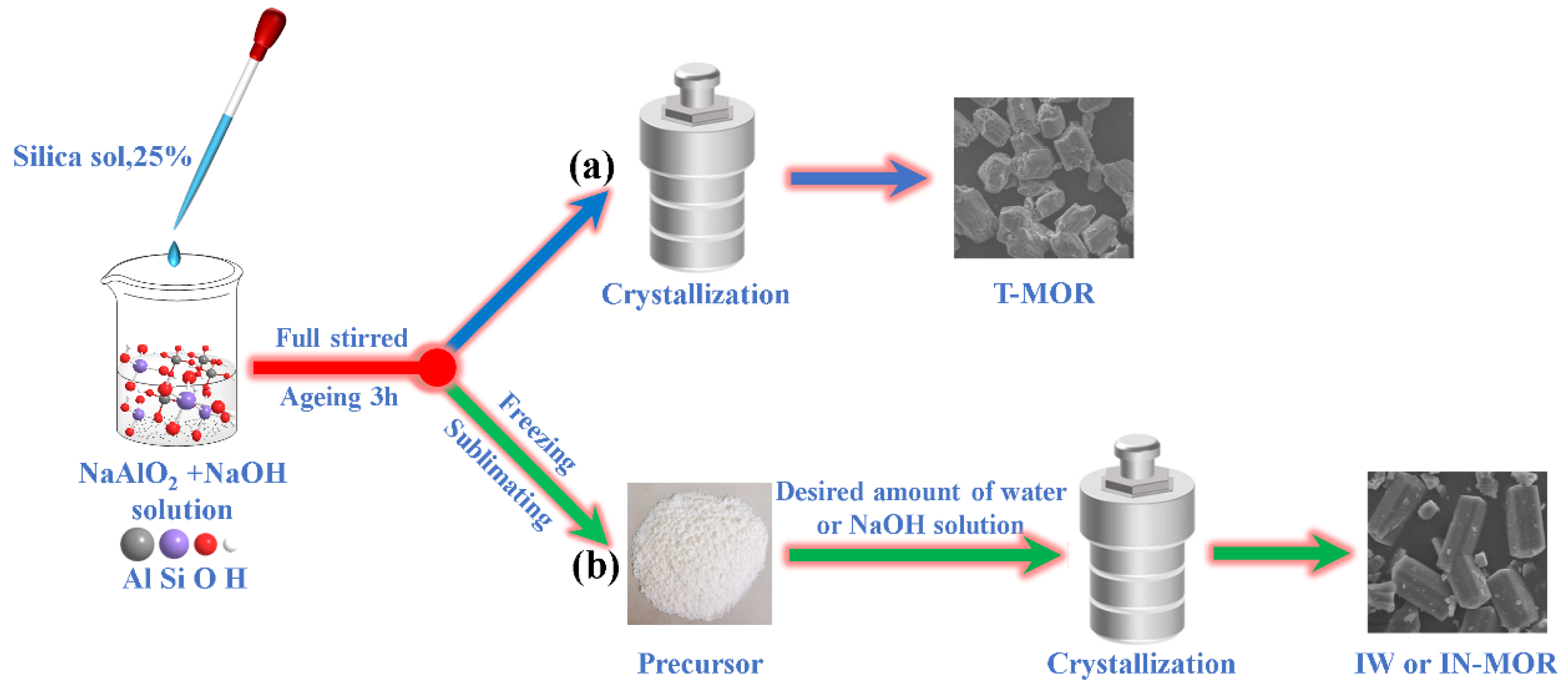
2.1. Formation of MOR Zeolite by Ice-Templating Method
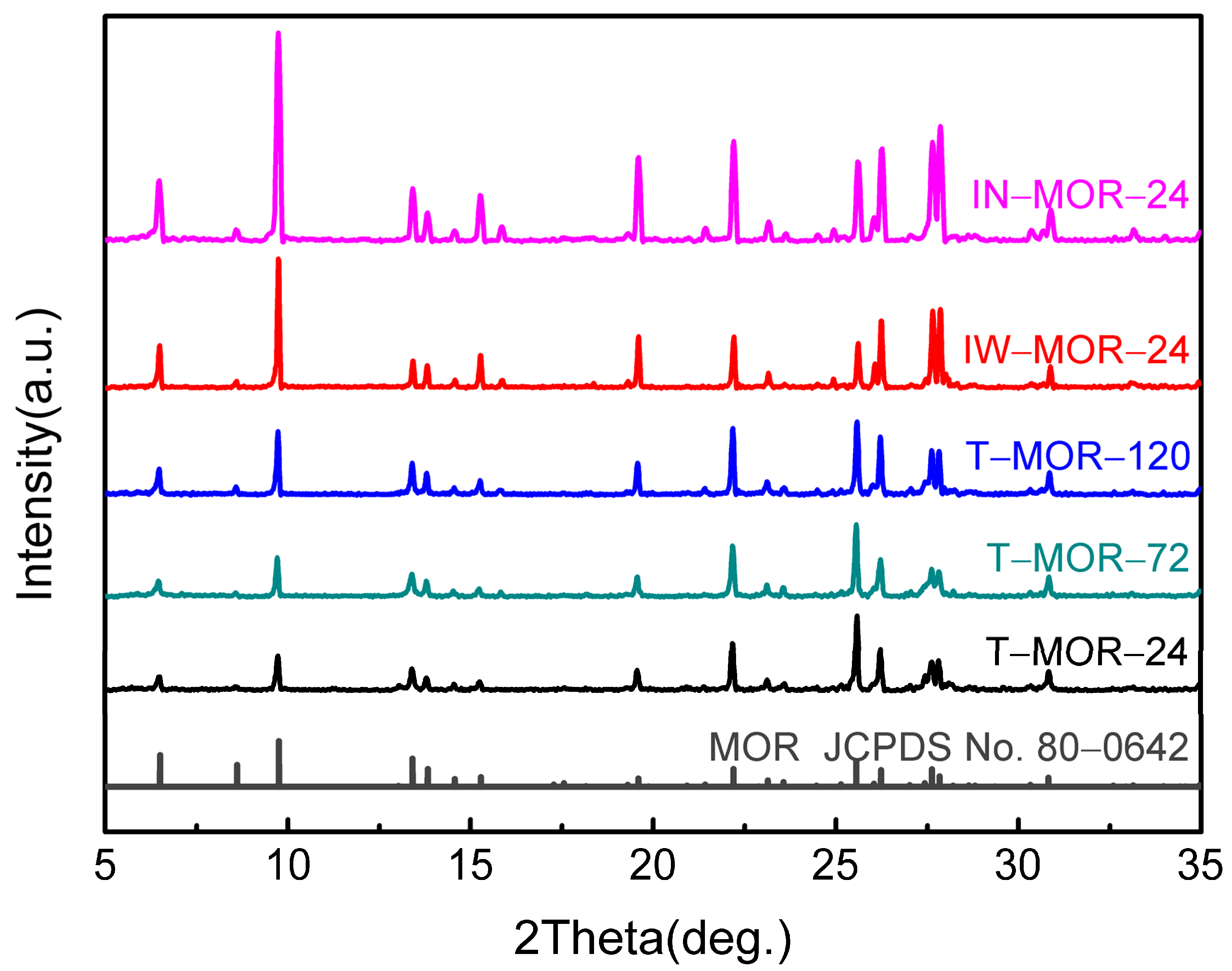
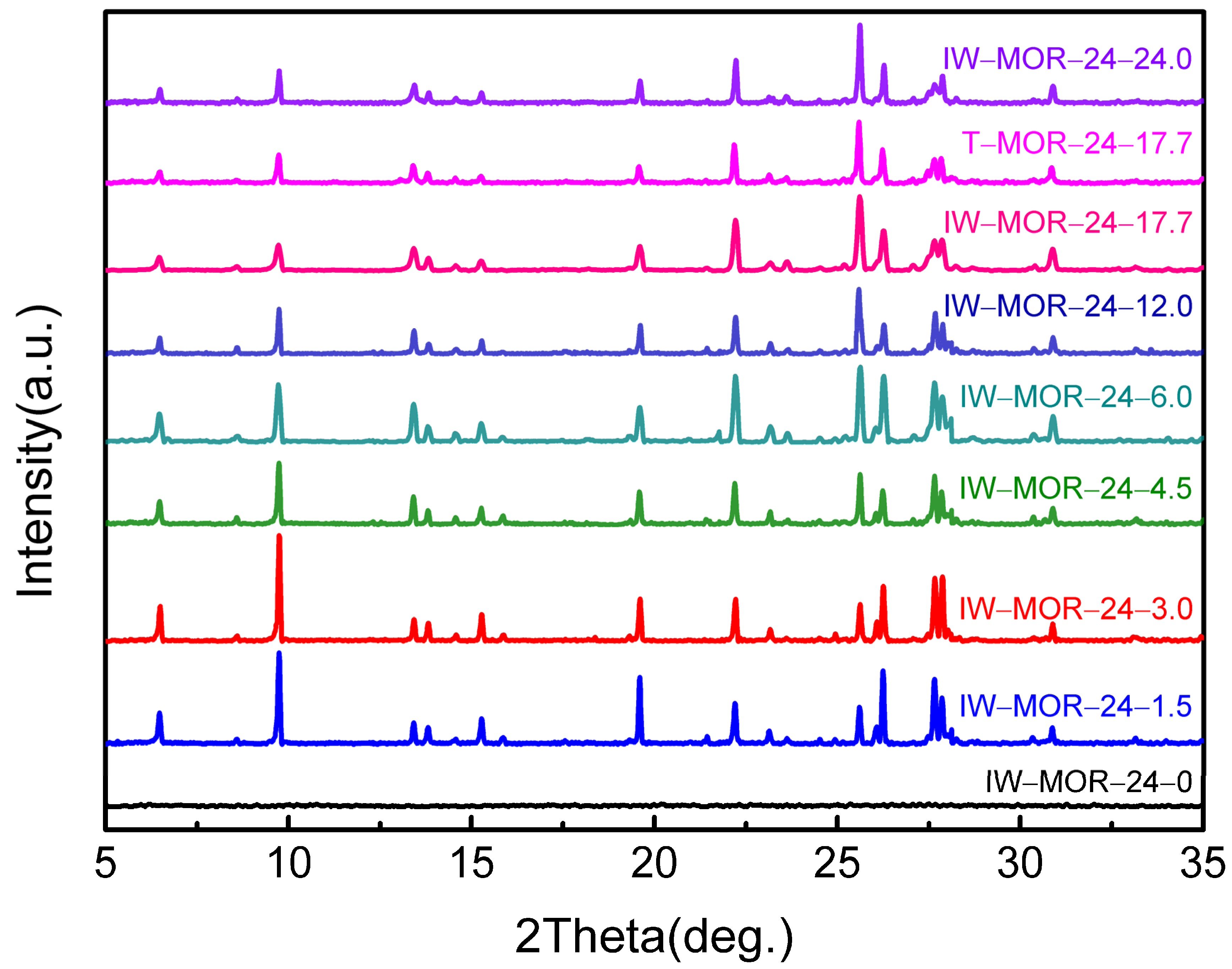
2.2. Influence of H2O/SiO2 Ratio during the Crystallization of Dry Gel
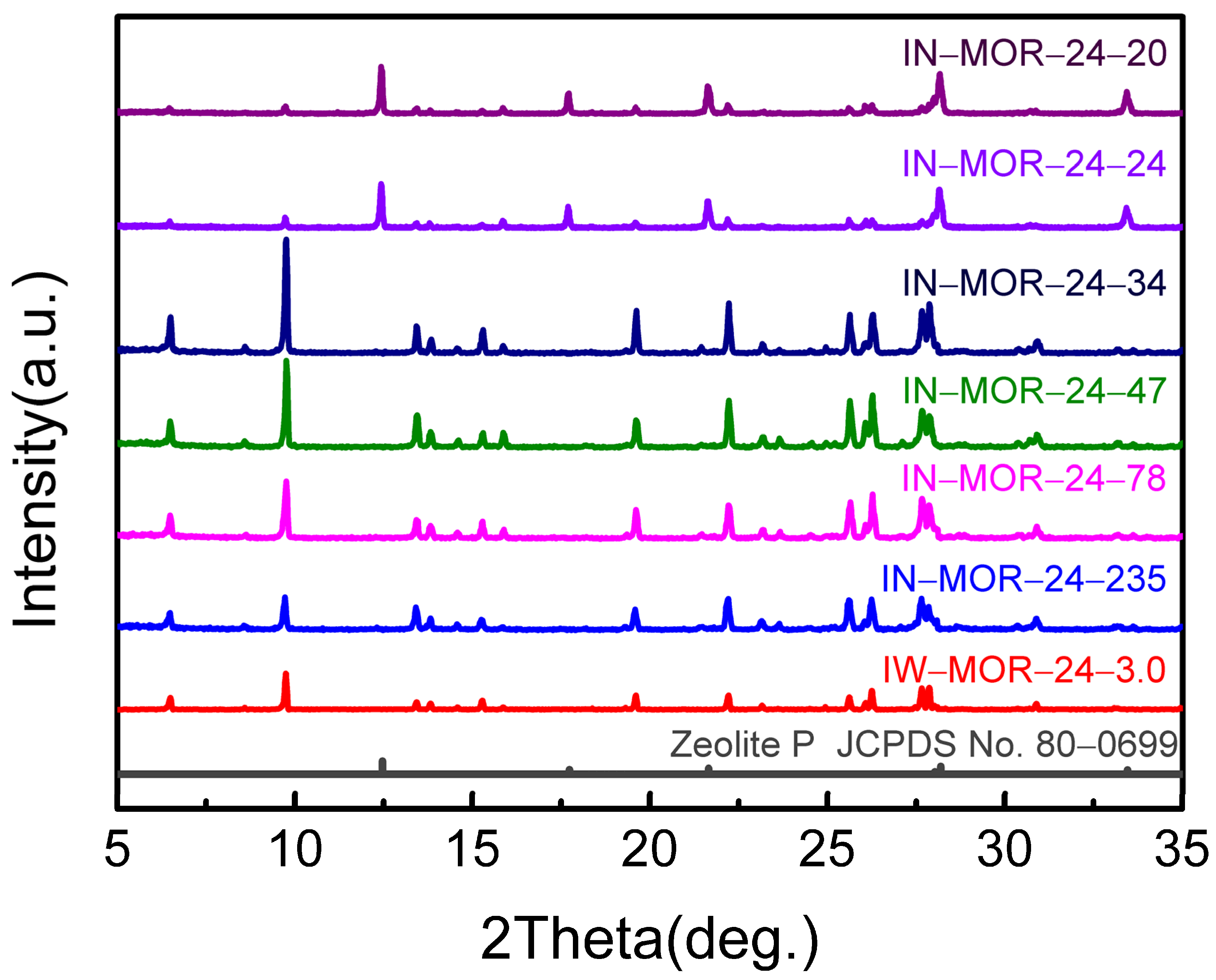
2.3. Influence of Alkaline Content during the Crystallization of Dry Gel
2.4. Elucidation of the Crystallization Behavior
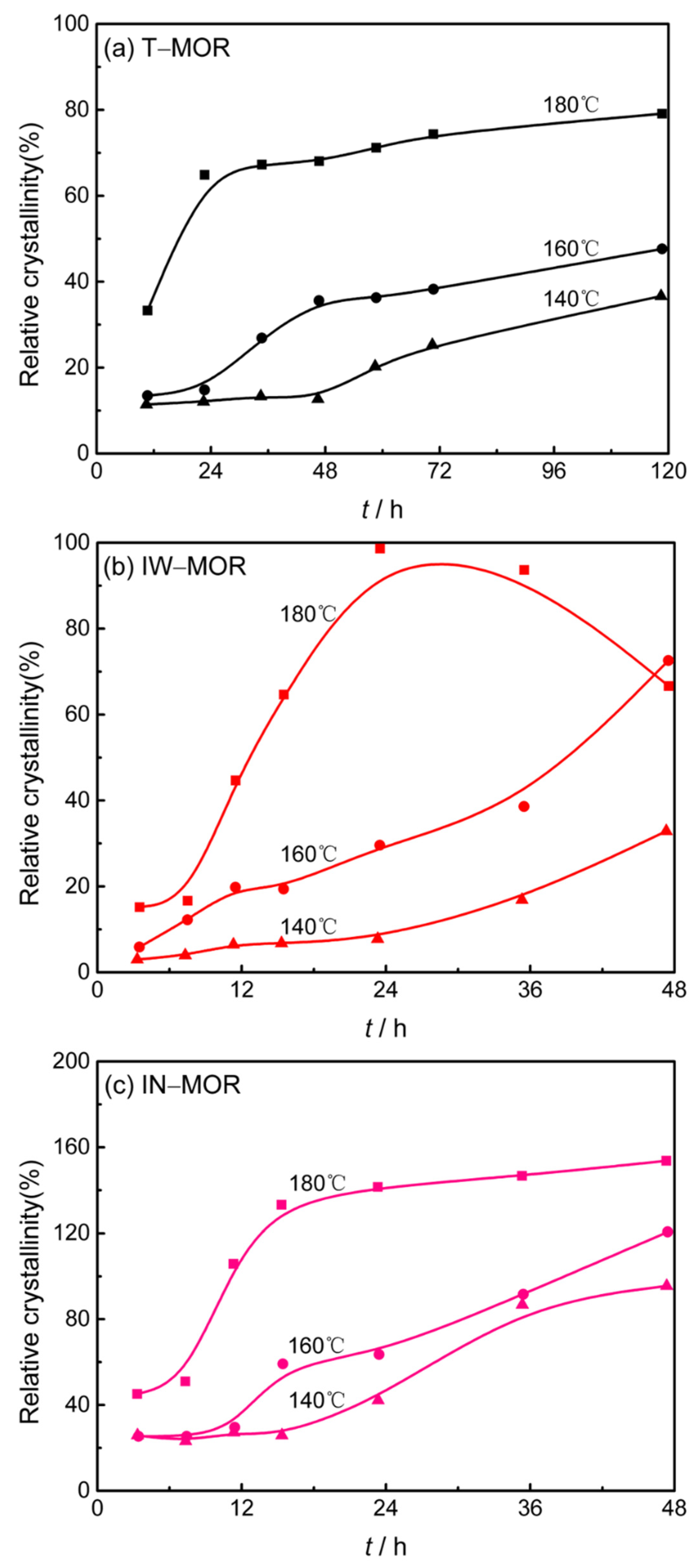
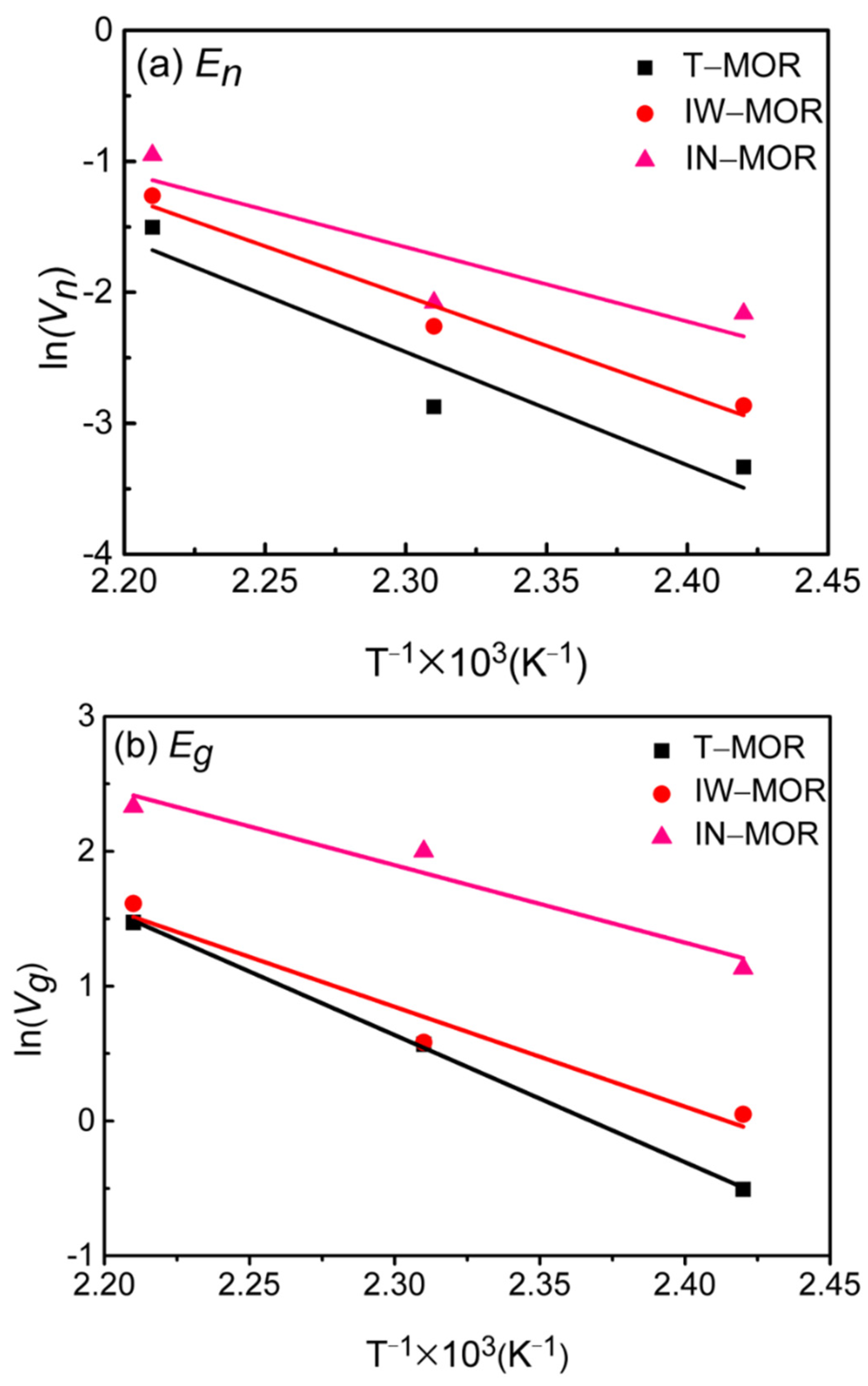
2.5. Crystallization Kinetics
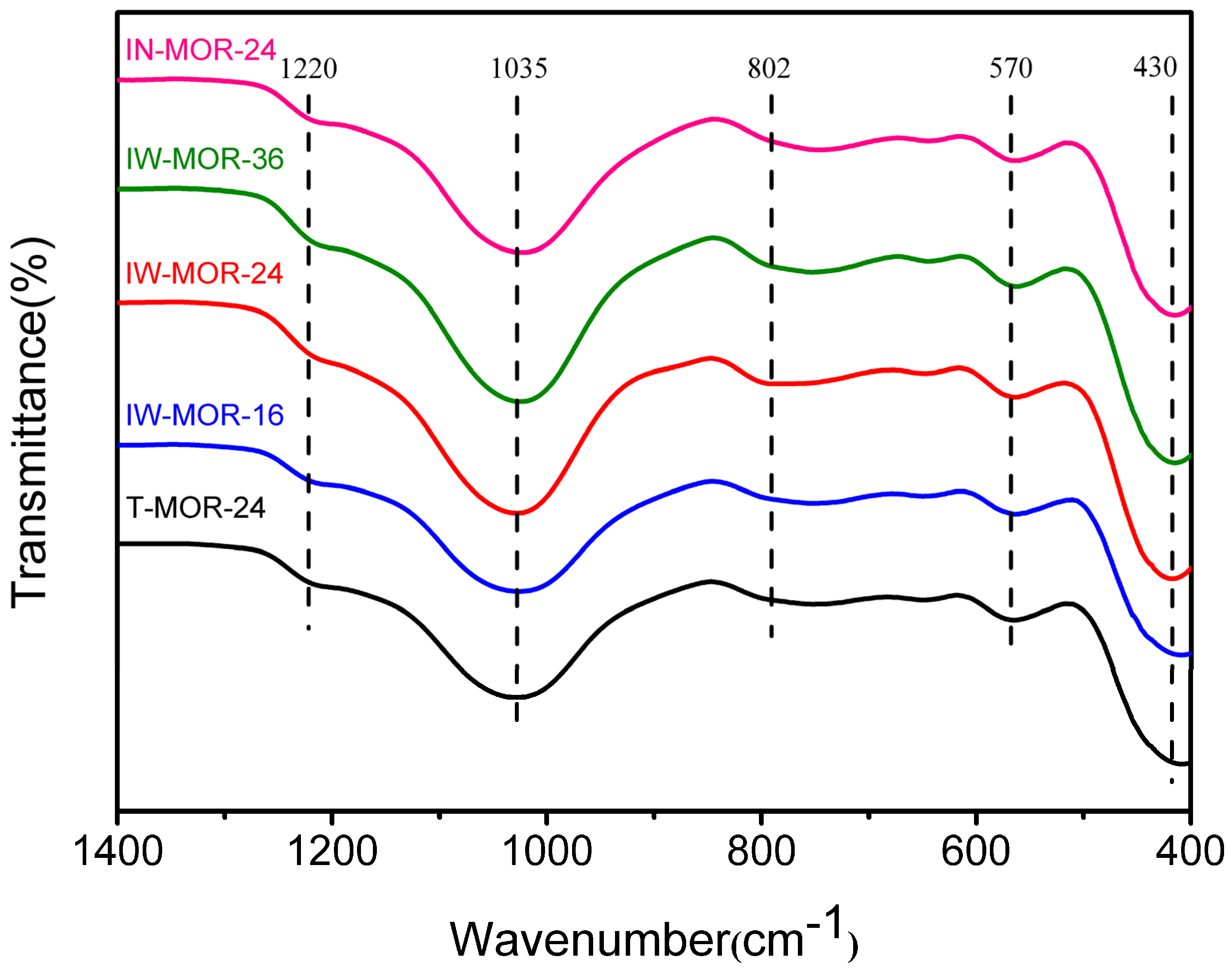
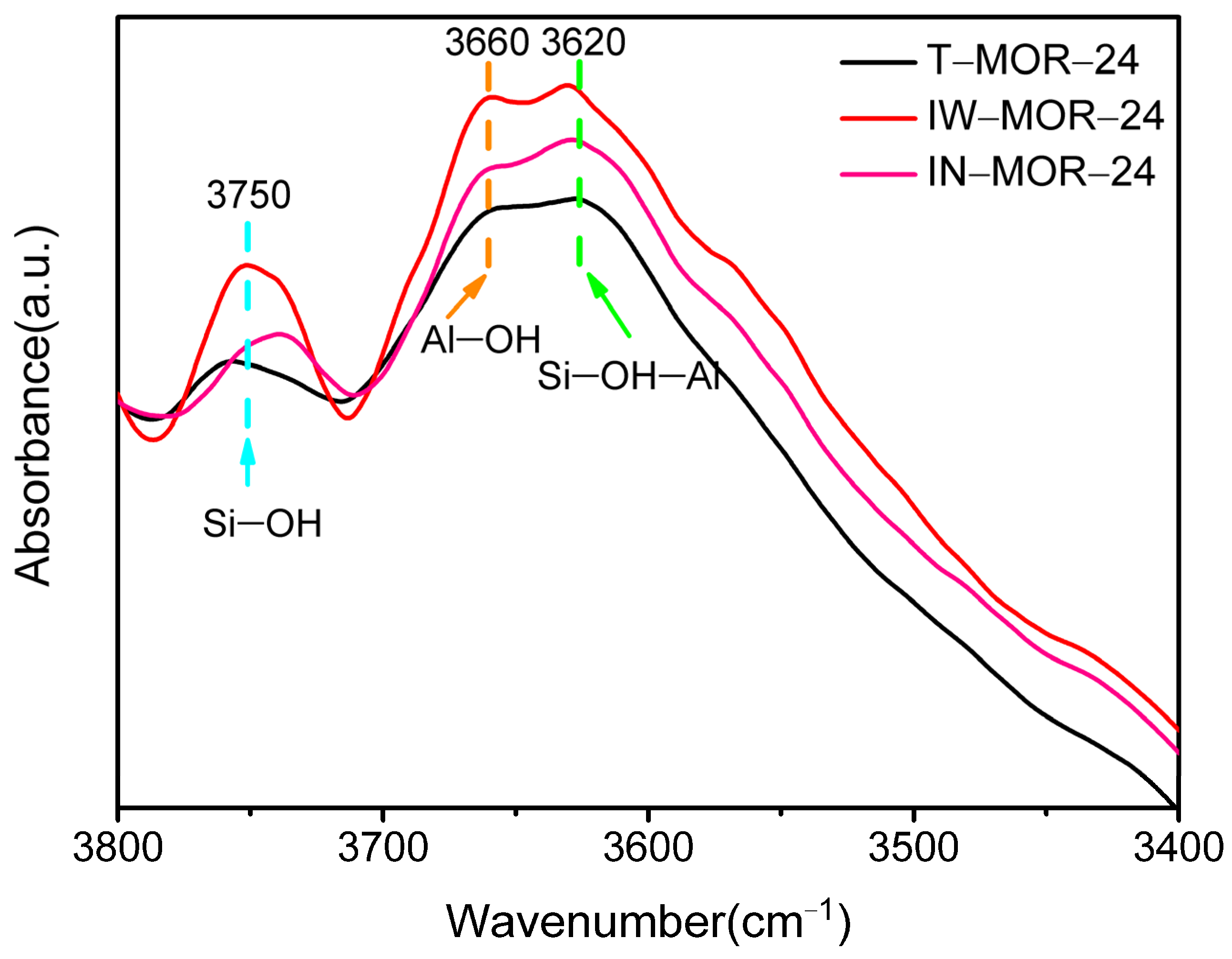
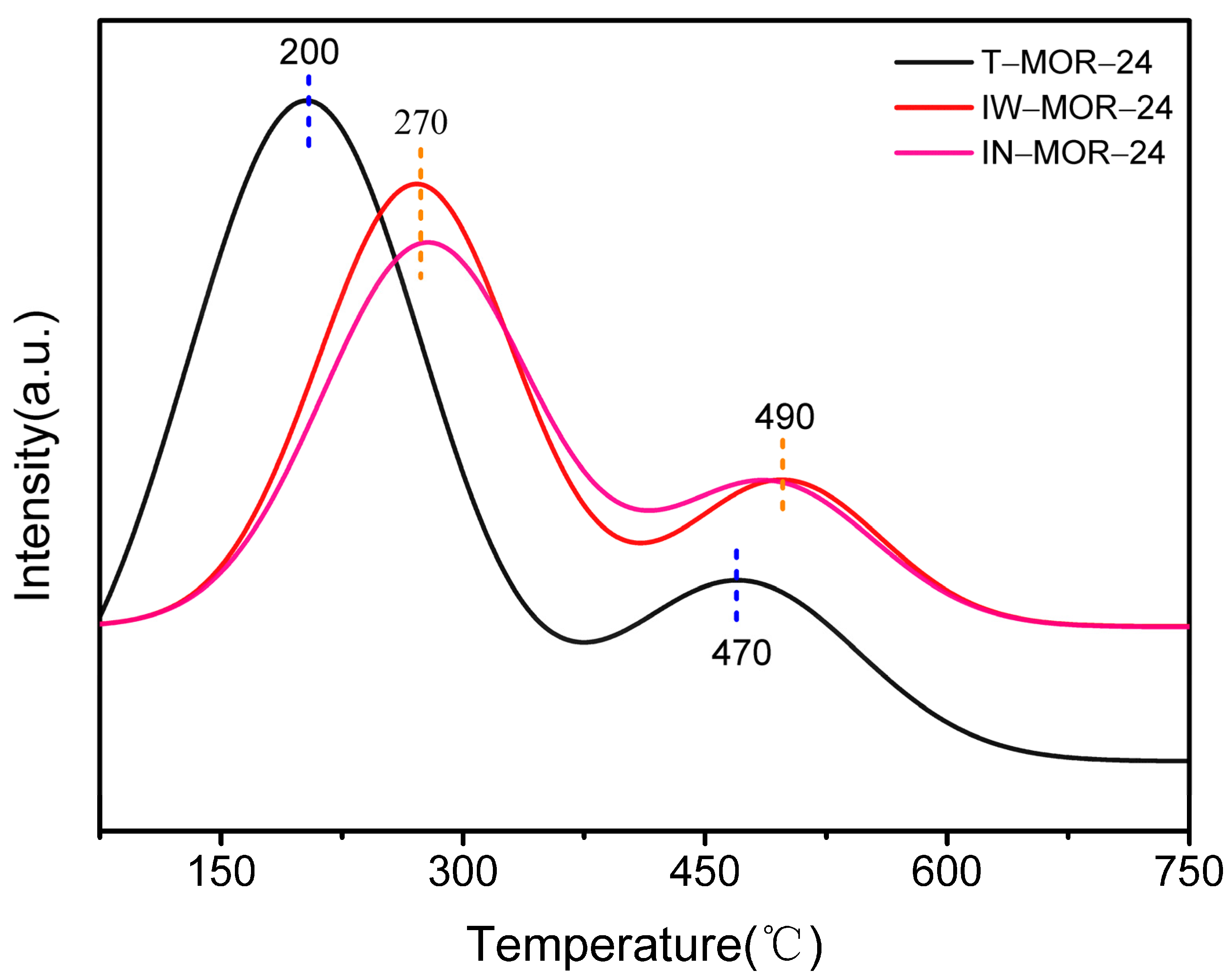
2.6. Acid Properties
2.7. Yield
3. Experimental Section
3.1. Materials
3.2. Synthesis of MOR Zeolite by Ice-Templating Method
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moliner, M.; Rey, F.; Corma, A. Towards the rational design of efficient organic structure–directing agents for zeolite synthesis. Angew. Chem. Int. Ed. 2013, 52, 13880–13889. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.M.; Liu, X.L.; Zhu, L.F.; Ding, L.H.; Gao, P.; Wang, X.; Pan, S.X.; Bian, C.Q.; Meng, X.J.; Xu, J.; et al. Solvent–free synthesis of zeolites from anhydrous starting raw solids. J. Am. Chem. Soc. 2015, 137, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Huang, J.; Ren, L.; Li, C.; Guan, Y.; Hu, B.; Xu, H.; Jiang, J.; Ma, Y.; Wu, P. High ethylene selectivity in methanol–to–olefin (MTO) reaction over MOR–zeolite nanosheets. Angew. Chem. Int. Ed. 2020, 59, 6258–6262. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Zhao, S.L.; Li, P.; Lv, Z.B.; Zhang, S.D. Generation of mesoporosity in MOR zeolites synthesized under perturbation conditions. Mater. Lett. 2011, 65, 2959–2962. [Google Scholar] [CrossRef]
- Gao, W.Z.; Amoo, C.C.; Zhang, G.H.; Javed, M.; Mazonde, B.; Lu, C.X.; Yang, R.Q.; Xing, C.; Tsubaki, N. Insight into solvent–free synthesis of MOR zeolite and its laboratory scale production. Microporous Mesoporous Mater. 2019, 280, 187–194. [Google Scholar] [CrossRef]
- Sano, T.; Wakabayashi, S.; Oumi, Y.; Uozumi, T. Synthesis of large mordenite crystals in the presence of aliphatic alcohol. Microporous Mesoporous Mater. 2001, 46, 67–74. [Google Scholar] [CrossRef]
- Kato, M.; Itabashi, K.; Matsumoto, A.; Tsutsumi, K. Characteristics of MOR–framework zeolites synthesized in fluoride–containing media and related ordered distribution of Al atoms in the framework. J. Phys. Chem. B 2003, 107, 1788–1797. [Google Scholar] [CrossRef]
- Santos, B.P.S.; Almeida, N.C.; Santos, I.S.; Marques, M.D.F.V.; Fernandes, L.D. Synthesis and characterization of mesoporous mordenite zeolite using soft templates. Catal. Lett. 2018, 148, 1870–1878. [Google Scholar] [CrossRef]
- Li, X.L.; Li, K.D.; Tao, S.; Ma, H.J.; Xu, R.S.; Wang, B.C.; Wang, P.; Tian, Z.J. Ionothermal synthesis of LTA–type aluminophosphate molecular sieve membranes with gas separation performance. Microporous Mesoporous Mater. 2016, 228, 45–53. [Google Scholar] [CrossRef]
- Musa, M.; Dawson, D.M.; Ashbrook, S.E.; Morris, R.E. Ionothermal synthesis and characterization of CoAPO–34 molecular sieve. Microporous Mesoporous Mater. 2017, 239, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Sajo, P.N.; Chiang, S.T.; Thompson, R.W. Synthesis of zeolitic mesoporous materials by dry gel conversion under controlled humidity. J. Phys. Chem. B 2003, 107, 7006–7014. [Google Scholar]
- Zhou, D.; Lu, X.H.; Xu, J.; Yu, A.; Li, J.Y.; Deng, F.; Xia, Q.H. Dry gel conversion method for the synthesis of organic–inorganic hybrid MOR zeolites with modifiable catalytic activities. Chem. Mater. 2012, 24, 4160–4165. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Z.Y.; Hu, P.; Ding, L.P.; Xue, N.H.; Peng, L.M.; Guo, X.F.; Lin, M.; Ding, W.P. Platinum nanoparticles encapsulated in MFI zeolite crystals by a two–step dry gel conversion method as a highly selective hydrogenation catalyst. ACS Catal. 2015, 5, 6893–6901. [Google Scholar] [CrossRef]
- Wu, Q.M.; Meng, X.G.; Gao, X.G.; Xiao, F.S. Solvent–free synthesis of zeolites: Mechanism and utility. Acc. Chem. Res. 2018, 51, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Y.; Zhu, Y.D.; Pan, J.G.; Chen, X.H. Fast synthesis of hierarchical Al–free Ti–bea plate–like nanocrystals from low–templated dry gel via a steam–assisted conversion method. Green Chem. 2020, 22, 1681–1697. [Google Scholar] [CrossRef]
- Catalfamo, P.; Patrizia, F.C.; Primerano, P.; Pasquale, S.D. Study of the pre–crystallization stage of hydrothermally treated amorphous aluminosilicates through the composition of the aqueous phaset. J. Chem. Soc. Faraday Trans. 1993, 89, 171–175. [Google Scholar] [CrossRef]
- Ma, Z.P.; Xie, J.Y.; Zhang, J.L.; Zhang, W.; Zhou, Y.; Wang, J. Mordenite zeolite with ultrahigh SiO2/Al2O3 ratio directly synthesized from ionic liquid–assisted dry–gel–conversion. Microporous Mesoporous Mater. 2016, 224, 17–25. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, J.W.; Zhang, K.; Chen, G.L.; Yang, Y.F.; Liu, S.B.; Ding, C.M.; Meng, Y.Y.; Liu, P. Hierarchical ZSM–5 zeolite synthesized via dry gel conversion–steam assisted crystallization process and its application in aromatization of methanol. Powder Technol. 2018, 328, 415–429. [Google Scholar] [CrossRef]
- Luca, P.D.; Crea, F.; Fonseca, A.; Nagy, J.B. Direct formation of self–bonded pellets during the synthesis of mordenite and ZSM–11zeolites from low water content systems. Microporous Mesoporous Mater. 2001, 42, 37–48. [Google Scholar] [CrossRef]
- Ren, L.M.; Wu, Q.M.; Yang, C.G.; Zhu, L.F.; Li, C.J.; Zhang, P.L.; Zhang, H.Y.; Meng, X.J.; Xiao, F.S. Solvent–free synthesis of zeolites from solid raw materials. J. Am. Chem. Soc. 2012, 134, 15173–15176. [Google Scholar] [CrossRef]
- Meng, X.J.; Xiao, F.S. Green routes for synthesis of zeolites. Chem. Rev. 2014, 114, 1521–1543. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, D.; Meggs, C.; Button, T.W. Porous Al2O3–ZrO2 composites fabricated by an ice template method. Scr. Mater. 2010, 62, 466–468. [Google Scholar] [CrossRef]
- Arabi, N.; Zamanian, A. Effect of cooling rate and gelatin concentration on the microstructural and mechanical properties of ice template gelatin scaffolds. Biotechnol. Appl. Biochem. 2013, 60, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.B.; Gao, H.L.; Yao, H.B.; Liu, L.; Cölfen, H.; Liu, G.; Chen, S.M.; Li, S.K.; Yan, Y.X.; Liu, Y.Y.; et al. Synthetic nacre by predesigned matrix–directed mineralization. Science 2016, 354, 107–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishihara, H.; Mukai, S.R.; Yamashita, D.; Tamon, H. Ordered macroporous silica by ice templating. Chem. Mater. 2005, 17, 683–689. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, D.Z.; Shi, W.H.; Liu, B.; Sim, G.J.; Ge, Q.; Yang, H.Y. Ice templated free–standing hierarchically WS2/CNT–Rgo aerogel for high–performance rechargeable lithium and sodium ion batteries. Adv. Energy Mater. 2016, 6, 1601057. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Q.Z.; Soomro, R.A.; He, S.Y.; Sun, N.; Qiao, N.; Xu, B. In situ ice template approach to fabricate 3D flexible Mxene film-based electrode for high performance supercapacitors. Adv. Funct. Mater. 2020, 30, 2000922. [Google Scholar] [CrossRef]
- Fajar, A.T.N.; Nurdin, F.A.; Mukti, R.R.; Subagjo; Rasrendra, C.B.; Kadja, G.T.M. Synergistic effect of dealumination and ceria impregnation to the catalytic properties of MOR zeolite. Mater. Today Chem. 2020, 17, 100313. [Google Scholar] [CrossRef]
- Liu, Q.R.; Zhou, D.; Liu, H.; Li, H.; Lu, X.H.; Xia, Q.H. Synergistic roles of B/L acids and hierarchical micro–mesoporous structures for the unexpected isomerization of β–pinene over dual–modified MOR zeolite by inorganic/organic bases. Microporous Mesoporous Mater. 2021, 323, 111195. [Google Scholar] [CrossRef]
- Morris, R.E.; James, S.L. Solventless synthesis of zeolites. Angew. Chem. Int. Ed. 2013, 52, 2163–2165. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Feng, G.D.; Yan, W.F.; Boronat, B.; Li, X.; Su, J.H.; Wang, J.Y.; Li, Y.; Corma, A.; Xu, R.R.; Yu, J.H. Accelerated crystallization of zeolites via hydroxyl free radicals. Science 2016, 351, 1188–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time. Chem. Rev. 2003, 103, 663–701. [Google Scholar] [CrossRef]
- Mousavi, S.F.; Jafari, M.; Kazemimoghadam, M.; Mohammadi, T. Template free crystallization of zeolite Rho via hydrothermal synthesis: Effects of synthesis time, synthesis temperature, water content and alkalinity. Ceram. Int. 2013, 39, 7149–7158. [Google Scholar] [CrossRef]
- Huo, Z.P.; Xu, X.Y.; Lü, Z.; Song, J.Q.; He, M.Y.; Li, Z.F.; Wang, Q.; Yan, L.J. Synthesis of zeolite NaP with controllable morphologies. Microporous Mesoporous Mater. 2012, 158, 137–140. [Google Scholar] [CrossRef]
- Tang, Q.; Ge, Y.Y.; Wang, K.T.; He, Y.; Cui, X.M. Preparation of porous P–type zeolite spheres with suspension solidification method. Mater. Lett. 2015, 161, 558–560. [Google Scholar] [CrossRef]
- Mimura, H.; Akiba, K. Adsorption behavior of cesium and strontium on synthetic zeolite P. J. Nucl. Sci. Technol. 1993, 30, 436–443. [Google Scholar] [CrossRef]
- Du, Y.C.; Shi, S.L.; Dai, H.X. Water–bathing synthesis of high–surface–area zeolite P from diatomite. Particuology 2011, 9, 174–178. [Google Scholar] [CrossRef]
- Hussain, I.; Jalil, A.A.; Hassan, N.S.; Hambali, H.U.; Jusoh, N.W.C. Fabrication and characterization of highly active fibrous silica–mordenite (FS@SiO2–MOR) cockscomb shaped catalyst for enhanced CO2 methanation. Chem. Eng. Sci. 2020, 228, 115978. [Google Scholar] [CrossRef]
- Culfaz, A.L.I.; Sand, L.B. Mechanism of nucleation and crystallization of zeolites from gels. Mol. Sieves 1973, 121, 140–151. [Google Scholar]
- Grizzetti, R.; Artioli, G. Kinetics of nucleation and growth of zeolite LTA from clear solution by in situ and exsitu XRPD. Microporous Mesoporous Mater. 2002, 54, 105–112. [Google Scholar] [CrossRef]
- Sun, C.T.; Xue, D.F. Polymorph growth of inorganic functional materials. Funct. Mater. Lett. 2017, 10, 1741001. [Google Scholar] [CrossRef]
- Wu, P.; Yashima, T. IR and MAS NMR studies on the incorporation of aluminum atoms into defect sites of dealuminated mordenites. J. Phys. Chem. 1995, 99, 10923–10931. [Google Scholar] [CrossRef]
- Pastvova, J.; Pilar, R.; Moravkova, J.; Kaucky, D.; Rathousky, J.; Sklenak, S.; Sazama, P. Tailoring the structure and acid site accessibility of mordenite zeolite for hydroisomerisation of n–hexane. Appl. Catal. A 2018, 562, 159–172. [Google Scholar] [CrossRef]
- Cao, K.P.; Fan, D.; Li, L.Y.; Fan, B.H.; Wang, L.Y.; Zhu, D.L.; Wang, Q.Y.; Tian, P.; Liu, Z.M. Insights into the pyridine–modified MOR zeolite catalysts for DME carbonylation. ACS Catal. 2020, 10, 3372–3380. [Google Scholar] [CrossRef]
- Cao, K.P.; Fan, D.; Zeng, S.; Fan, B.H.; Chen, N.; Gao, M.B.; Zhu, D.L.; Wang, L.Y.; Tian, P.; Liu, Z.M. Organic–free synthesis of MOR nanoassemblies with excellent DME carbonylation performance. Chin. J. Catal. 2021, 42, 1468–1477. [Google Scholar] [CrossRef]
- Zholobenko, V.L.; Makarova, M.A.; Dwyer, J. Inhomogeneity of brónsted acid sites in H–mordenite. J. Phys. Chem. 1993, 97, 5962–5964. [Google Scholar] [CrossRef]
- Korfinyi, T.I.; Föttinger, K.; Vinek, H.; Nagy, J.B. Characterization of aluminium siting in MOR and BEA zeolites by 27A1, 29Si NMR and FTIR spectroscopy. Stud. Surf. Sci. Catal. 2005, 158, 765–772. [Google Scholar]
- Allian, B.A.D.; Sunley, G.J.; Law, D.J.; Iglesia, E. Specificity of sites within eight–membered ring zeolite channels for carbonylation of methyls to acetyls. J. Am. Chem. Soc. 2007, 129, 4919–4924. [Google Scholar]
- Iglesia, B.E. A link between reactivity and local structure in acid catalysis on zeolites. Acc. Chem. Res. 2008, 41, 559–567. [Google Scholar]
- Gutiérrez, A.; Larrubia, M.A.; Ramirez, J.; Busca, G. FT–IR evidence of the interaction of benzothiophene with the hydroxyl groups of H–MFI and H–MOR zeolites. Vib. Spectrosc. 2006, 41, 42–47. [Google Scholar] [CrossRef]
- Cai, K.; Huang, S.Y.; Li, Y.; Cheng, Z.Z.; Lv, J.; Ma, X.B. Influence of acid strength on the reactivity of dimethyl ether carbonylation over H–MOR. ACS Sustain. Chem. Eng. 2018, 7, 2027–2034. [Google Scholar] [CrossRef]
- Chen, Z.W.; Li, Z.; Zhang, Y.; Chevella, D.; Li, G.M.; Chen, Y.P.; Guo, X.H.; Liu, J.; Yu, J.Q. A green route for the synthesis of nano–sized hierarchical ZSM–5 zeolite with excellent DTO catalytic performance. Chem. Eng. J. 2020, 388, 124322. [Google Scholar] [CrossRef]
- He, S.B.; Muizebelt, I.; Heeres, A.; Schenk, N.J.; Blees, R.; Heeres, H.J. Catalytic pyrolysis of crude glycerol over shaped ZSM–5/bentonite catalysts for bio–BTX synthesis. Appl. Catal. B 2018, 235, 45–55. [Google Scholar] [CrossRef]
- Jin, Y.Y.; Sun, Q.; Qi, G.D.; Yang, C.G.; Xu, J.; Chen, F.; Meng, X.J.; Deng, F.; Xiao, F.S. Solvent–free synthesis of silicoaluminophosphate zeolites. Angew. Chem. Int. Ed. 2013, 52, 9172–9175. [Google Scholar] [CrossRef]
| Samples | ||||
|---|---|---|---|---|
| T–MOR–24 | T–MOR–72 | T–MOR–120 | IW–MOR–24 | IN–MOR–24 |
| 39% | 42% | 53% | 64% | 100% |
| Temperature (℃) | Samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T–MOR | IW–MOR | IN–MOR | ||||||||||
| Vn (h−1) | Vg (h−1) | En (kJ/mol) | Eg (kJ/mol) | Vn (h−1) | Vg (h−1) | En (kJ/mol) | Eg (kJ/mol) | Vn (h−1) | Vg (h−1) | En (kJ/mol) | Eg (kJ/mol) | |
| 140 | 0.04 | 0.60 | 71.8 | 78.4 | 0.05 | 1.05 | 63.2 | 61.5 | 0.12 | 3.10 | 47.2 | 47.8 |
| 160 | 0.06 | 1.77 | 0.14 | 1.79 | 0.13 | 7.39 | ||||||
| 180 | 0.22 | 4.35 | 0.28 | 5.01 | 0.39 | 10.28 | ||||||
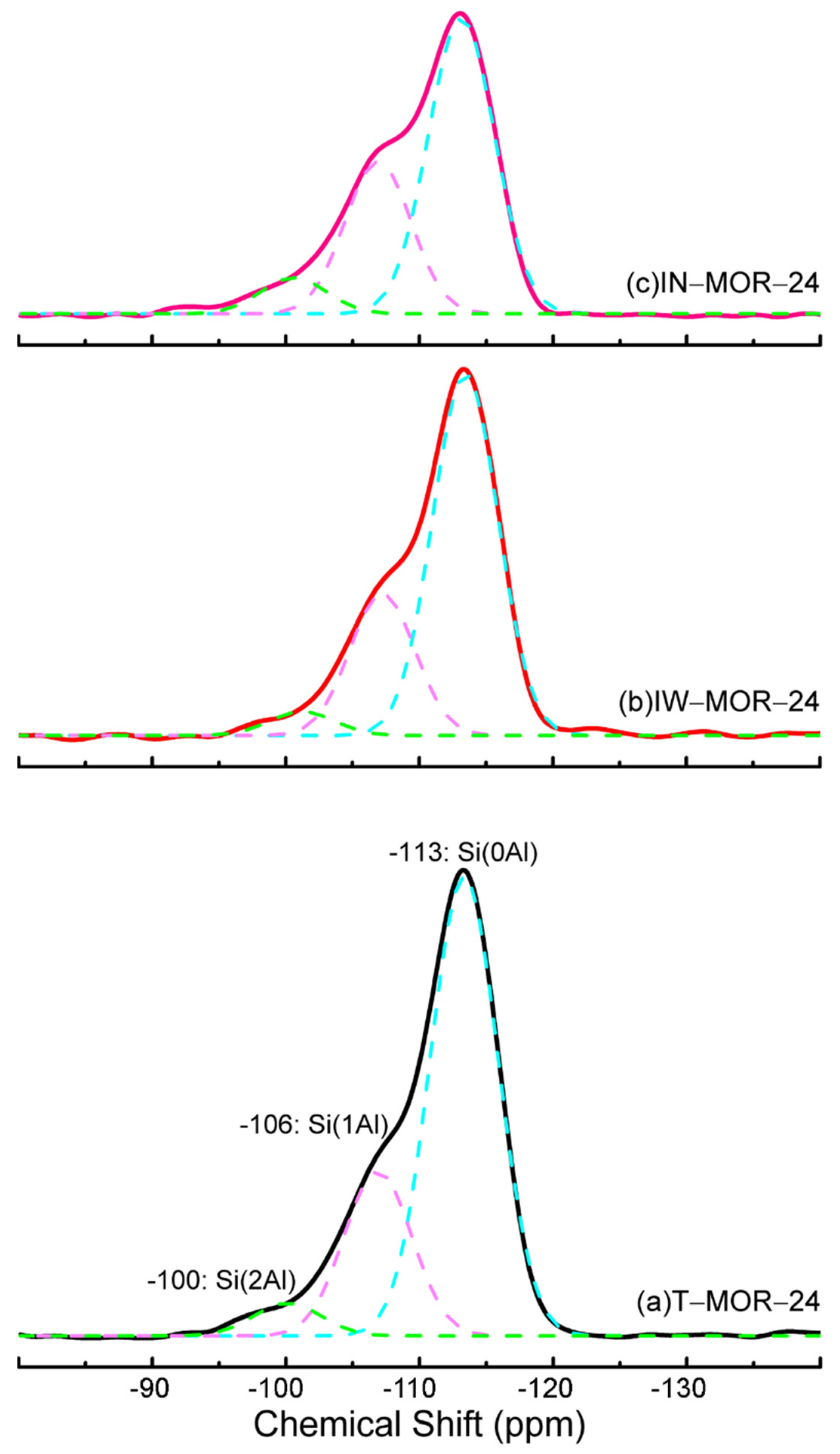
| Samples | Chemical Shift (ppm) | Peak Area |
|---|---|---|
| T–MOR–24 | −113(Q4) | 23,230 |
| −106(Q3) | 8315 | |
| −100(Q2) | 1658 | |
| IW–MOR–24 | −113(Q4) | 17,600 |
| −107(Q3) | 6871 | |
| −101(Q2) | 1159 | |
| IN–MOR–24 | −113(Q4) | 14,610 |
| −106(Q3) | 7508 | |
| −100(Q2) | 1781 |
| Samples | Raw Materials | Yield/g | |||
|---|---|---|---|---|---|
| Silica sol/g | NaAlO2/g | NaOH/g | H2O/g | ||
| T–MOR–24–17.7 | 52.29 | 2.312 | 3.4 | 30 | 9.56 |
| IW–MOR–24–3.0 | 52.29 | 2.312 | 3.4 | 12 | 19.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Li, H.; Zhang, W.; Wang, B.; Yang, X.; Peng, Y.; Zhang, Y.; Li, Z. Insight into Crystallization Features of MOR Zeolite Synthesized via Ice-Templating Method. Catalysts 2022, 12, 301. https://doi.org/10.3390/catal12030301
Zhao S, Li H, Zhang W, Wang B, Yang X, Peng Y, Zhang Y, Li Z. Insight into Crystallization Features of MOR Zeolite Synthesized via Ice-Templating Method. Catalysts. 2022; 12(3):301. https://doi.org/10.3390/catal12030301
Chicago/Turabian StyleZhao, Shangqing, Haiwei Li, Weilong Zhang, Bo Wang, Xiaolong Yang, Yanhua Peng, Yan Zhang, and Zhuo Li. 2022. "Insight into Crystallization Features of MOR Zeolite Synthesized via Ice-Templating Method" Catalysts 12, no. 3: 301. https://doi.org/10.3390/catal12030301
APA StyleZhao, S., Li, H., Zhang, W., Wang, B., Yang, X., Peng, Y., Zhang, Y., & Li, Z. (2022). Insight into Crystallization Features of MOR Zeolite Synthesized via Ice-Templating Method. Catalysts, 12(3), 301. https://doi.org/10.3390/catal12030301