Metal Oxides as Catalyst/Supporter for CO2 Capture and Conversion, Review
Abstract
1. Introduction
2. Background
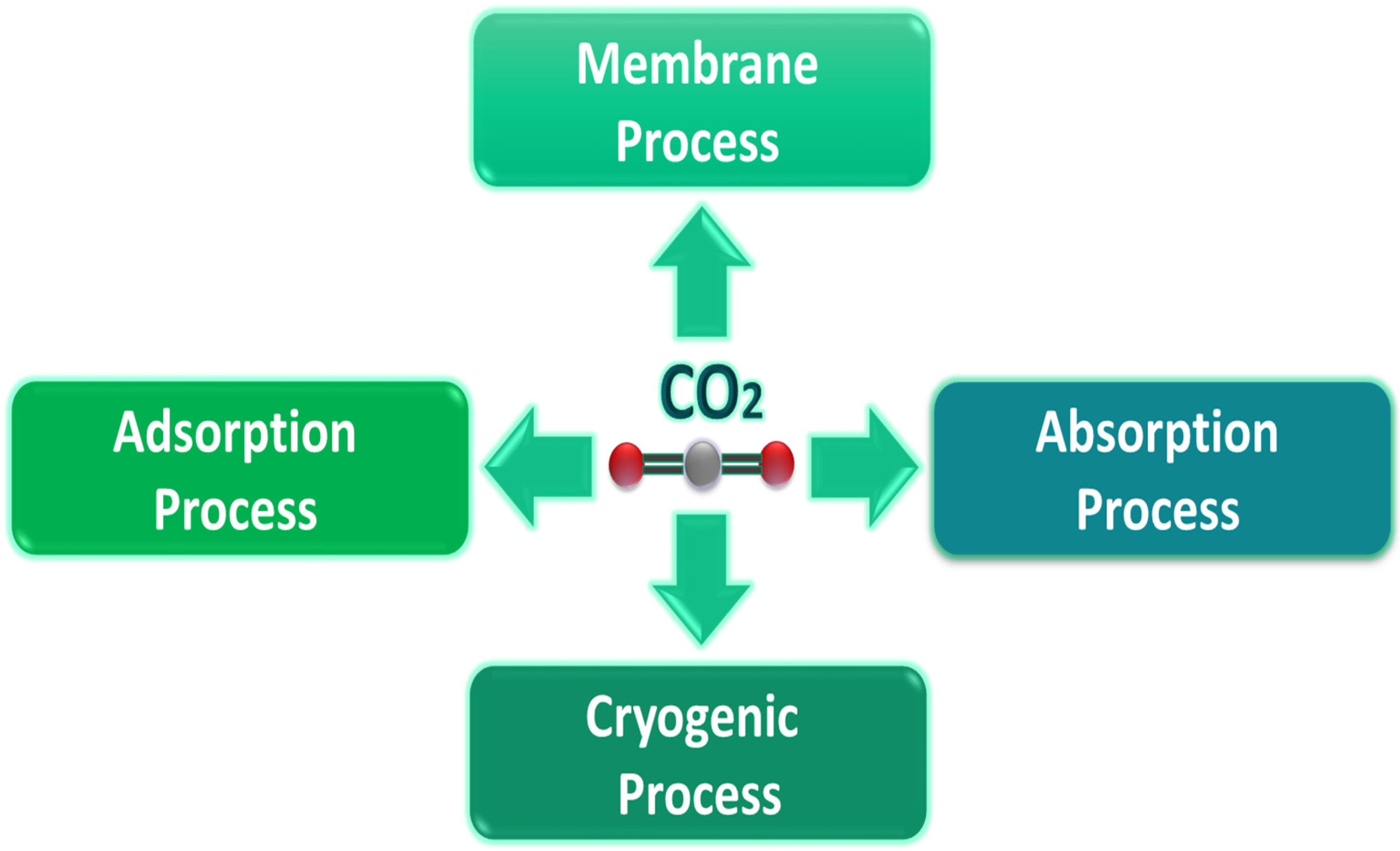
3. Carbon Dioxide Capturing Processes
4. Carbon Dioxide Absorption
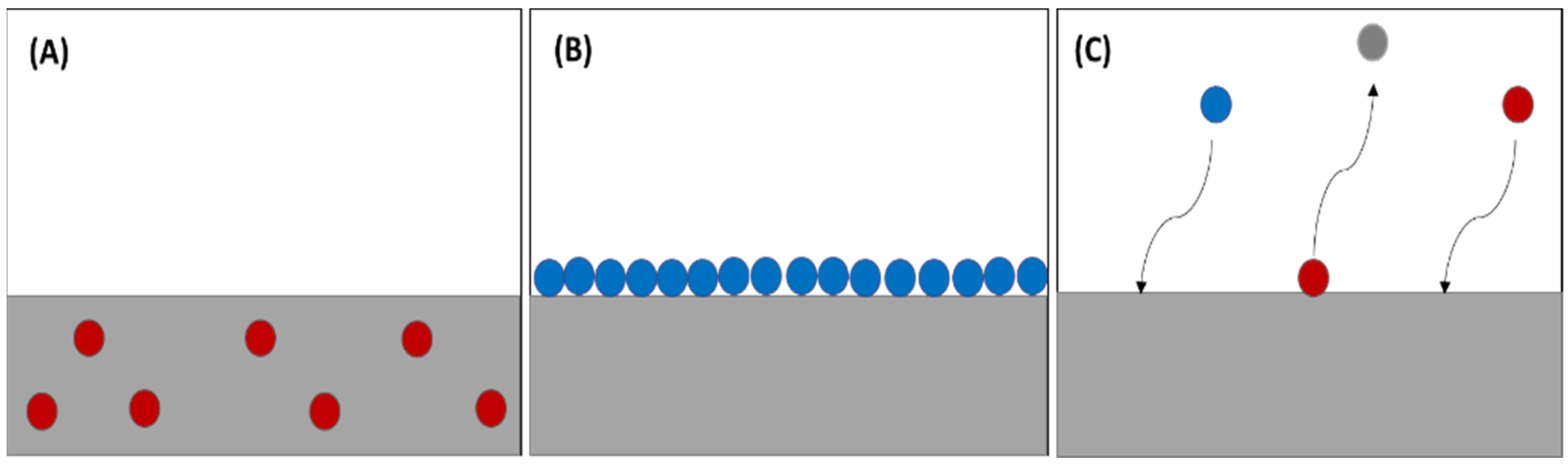
5. Metal Oxides for CO2 Uptake
6. CO2 Conversion
6.1. CO2 Conversion Using Catalyst-Supporting Materials
6.2. CO2 Conversion Using Single Metal Oxide
6.3. Binary Metal Oxides for CO2 Conversion
6.4. Metal Oxide Nanoparticles for CO2 Conversion
7. Various Techniques for the Conversion of Carbon Dioxide
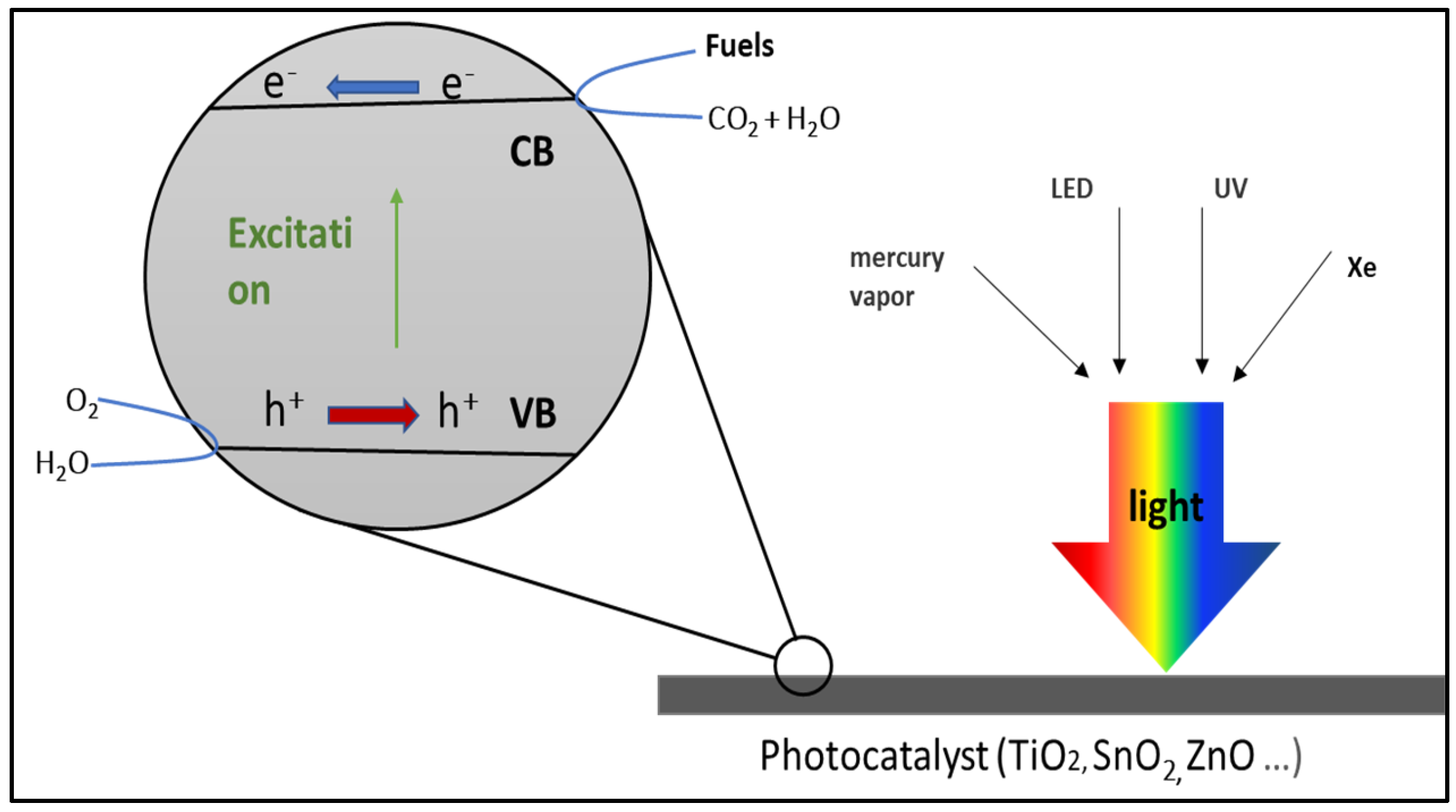
7.1. Photocatalytic Applications of Carbon Dioxide Conversion
7.2. Carbon Dioxide Conversion by Plasma Catalysis
7.3. Electrocatalyst Applications in Converting Carbon Dioxide
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alsarhan, L.M.; Alayyar, A.S.; Alqahtani, N.B.; Khdary, N.H. Circular Carbon Economy (CCE): A Way to Invest CO2 and Protect the Environment, a Review. Sustainability 2021, 13, 11625. [Google Scholar] [CrossRef]
- Zhong, W.; Haigh, J.D. The greenhouse effect and carbon dioxide. Weather 2013, 68, 100–105. [Google Scholar] [CrossRef]
- Streck, C.; Keenlyside, P.; Von Unger, M. The Paris agreement: A new beginning. J. Eur. Environ. Plan. Law 2016, 13, 3–29. [Google Scholar] [CrossRef]
- Hasan, M.M.F.; First, E.L.; Boukouvala, F.; Floudas, C.A. A multi-scale framework for CO2 capture, utilization, and sequestration: CCUS and CCU. Comput. Chem. Eng. 2015, 81, 2–21. [Google Scholar] [CrossRef]
- Ali, K.A.; Ahmad, M.I.; Yusup, Y. Issues, impacts, and mitigations of carbon dioxide emissions in the building sector. Sustainability 2020, 12, 7427. [Google Scholar]
- Takht Ravanchi, M.; Sahebdelfar, S. Catalytic conversions of CO2 to help mitigate climate change: Recent process developments. Process Saf. Environ. Prot. 2021, 145, 172–194. [Google Scholar] [CrossRef]
- Ozorio, L.P.; Mota, C.J.A. Direct Carbonation of Glycerol with CO2 Catalyzed by Metal Oxides. ChemPhysChem 2017, 18, 3260–3265. [Google Scholar] [CrossRef]
- Song, C. CO2 Conversion and Utilization: An Overview. ACS Symp. Ser. 2002, 809, 2–30. [Google Scholar]
- Babhare, S.; Raskar, R.; Bobade, K.; Gaikwad, A. The applications of mixed metal oxides to capture the CO2 and convert to syn-gas. Bull. Chem. React. Eng. Catal. 2015, 10, 125–142. [Google Scholar] [CrossRef]
- Alami, A.H.; Hawili, A.A.; Tawalbeh, M.; Hasan, R.; Mahmoud, L.A.; Chibib, S.; Mahmood, A.; Aokal, K.; Rattanapanya, P. Materials and logistics for carbon dioxide capture, storage and utilization. Sci. Total Environ. 2020, 717, 137221. [Google Scholar] [CrossRef]
- Alazmi, A.; El Tall, O.; Hedhili, M.N.; Costa, P.M. The impact of surface chemistry and texture on the CO2 uptake capacity of graphene oxide. Inorg. Chim. Acta 2018, 482, 470–477. [Google Scholar] [CrossRef]
- Bello, A.; Idem, R.O. Pathways for the formation of products of the oxidative degradation of CO2-loaded concentrated aqueous monoethanolamine solutions during CO2 absorption from flue gases. Ind. Eng. Chem. Res. 2005, 44, 945–969. [Google Scholar] [CrossRef]
- Yun, S.; Oh, S.Y.; Kim, J.K. Techno-economic assessment of absorption-based CO2 capture process based on novel solvent for coal-fired power plant. Appl. Energy 2020, 268, 114933. [Google Scholar] [CrossRef]
- Elhambakhsh, A.; Zaeri, M.R.; Mehdipour, M.; Keshavarz, P. Synthesis of different modified magnetic nanoparticles for selective physical/chemical absorption of CO2 in a bubble column reactor. J. Environ. Chem. Eng. 2020, 8, 104195. [Google Scholar] [CrossRef]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Papa, E.; Landi, E.; Murri, A.N.; Miccio, F.; Vaccari, A.; Medri, V. CO2 adsorption at intermediate and low temperature by geopolymer-hydrotalcite composites. Open Ceram. 2021, 5, 100048. [Google Scholar] [CrossRef]
- Omodolor, I.S.; Otor, H.O.; Andonegui, J.A.; Allen, B.J.; Alba-Rubio, A.C. Dual-Function Materials for CO2 Capture and Conversion: A Review. Ind. Eng. Chem. Res. 2020, 59, 17612–17631. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Ji, X. Carbon dioxide capture. Deep Eutectic Solvents: Synthesis, Properties, and Applications; Wiley VCH Verlag: Weinheim, Germany, 2019. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Z.; Li, C.; Zhang, C.; Genest, A.; Rupprechter, G.; He, L. Emerging applications of MXene materials in CO2 photocatalysis. FlatChem 2021, 28, 100252. [Google Scholar] [CrossRef]
- Li, D.; Rohani, V.; Fabry, F.; Ramaswamy, A.P.; Sennour, M.; Fulcheri, L. Direct conversion of CO2 and CH4 into liquid chemicals by plasma-catalysis. Appl. Catal. B Environ. 2020, 261, 2–9. [Google Scholar] [CrossRef]
- Li, Q.; Fu, J.; Zhu, W.; Chen, Z.; Shen, B.; Wu, L.; Xi, Z.; Wang, T.; Lu, G.; Zhu, J.-J.; et al. Tuning Sn-Catalysis for Electrochemical Reduction of CO2 to CO via the Core/Shell Cu/SnO2 Structure. J. Am. Chem. Soc. 2017, 139, 4290–4293. [Google Scholar] [CrossRef]
- Jia, J.; Qian, C.; Dong, Y.; Li, Y.F.; Wang, H.; Ghoussoub, M.; Butler, K.T.; Walsh, A.; Ozin, G.A. Heterogeneous catalytic hydrogenation of CO2 by metal oxides: Defect engineering-perfecting imperfection. Chem. Soc. Rev. 2017, 46, 4631–4644. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutry, S.; Muller, R.N. Metal Oxide Particles and Their Prospects for Applications. Iron Oxide Nanoparticles for Biomedical Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Iribarren, D.; Petrakopoulou, F.; Dufour, J. Environmental and thermodynamic evaluation of CO2 capture, transport and storage with and without enhanced resource recovery. Energy 2013, 50, 477–485. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry; Marcel Dekker, Inc.: New York, NY, USA, 1997; Volume 1. [Google Scholar]
- Krachuamram, S.; Chanapattharapol, K.C.; Kamonsutthipaijit, N. Synthesis and characterization of NaX-type zeolites prepared by different silica and alumina sources and their CO2 adsorption properties. Microporous Mesoporous Mater. 2021, 310, 110632. [Google Scholar] [CrossRef]
- Bull, O.S.; Bull, I.; Amadi, G.K. Global Warming and Technologies for Carbon Capture and Storage. J. Appl. Sci. Environ. Manag. 2020, 24, 1671–1686. [Google Scholar] [CrossRef]
- Azmi, A.A.; Ruhaimi, A.H.; Aziz, M.A.A. Efficient 3-aminopropyltrimethoxysilane functionalised mesoporous ceria nanoparticles for CO2 capture. Mater. Today Chem. 2020, 16, 100273. [Google Scholar] [CrossRef]
- Cho, M.S.; Lee, S.C.; Chae, H.J.; Kwon, Y.M.; Lee, J.B.; Kim, J.C. Characterization of new potassium-based solid sorbents prepared using metal silicates for post-combustion CO2 capture. Process Saf. Environ. Prot. 2018, 117, 296–306. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Karami, D.; Mahinpey, N. Solution combustion synthesis of zirconia-stabilized calcium oxide sorbents for CO2 capture. Fuel 2020, 269, 117432. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Wang, P.; Sun, J.; Li, W.; Zhao, C.; Lu, P. Magnesium-based basic mixtures derived from earth-abundant natural minerals for CO2 capture in simulated flue gas. Fuel 2019, 243, 298–305. [Google Scholar] [CrossRef]
- Dal Pozzo, A.; Armutlulu, A.; Rekhtina, M.; Abdala, P.M.; Müller, C.R. CO2 Uptake and Cyclic Stability of MgO-Based CO2 Sorbents Promoted with Alkali Metal Nitrates and Their Eutectic Mixtures. ACS Appl. Energy Mater. 2019, 2, 1295–1307. [Google Scholar] [CrossRef]
- Li, P.; Chen, R.; Lin, Y.; Li, W. General approach to facile synthesis of MgO-based porous ultrathin nanosheets enabling high-efficiency CO2 capture. Chem. Eng. J. 2021, 404, 126459. [Google Scholar] [CrossRef]
- Ruhaimi, A.H.; Aziz MA, A.; Jalil, A.A. Magnesium oxide-based adsorbents for carbon dioxide capture: Current progress and future opportunities. J. CO2 Util. 2020, 43, 101357. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Yusof, N.; Samitsu, S.; Abdullah, N.; Hamid, M.F.; Nagai, K.; Abidin, M.N.Z.; Azali, M.A.; Ismail, A.F.; Jaafar, J.; et al. Activated carbon nanofibers incorporated metal oxides for CO2 adsorption: Effects of different type of metal oxides. J. CO2 Util. 2021, 45, 101434. [Google Scholar] [CrossRef]
- Kolathodi, M.S.; Hanumantha Rao, S.N.; Natarajan, T.S.; Singh, G. Beaded manganese oxide (Mn2O3) nanofibers: Preparation and application for capacitive energy storage. J. Mater. Chem. A 2016, 4, 7883–7891. [Google Scholar] [CrossRef]
- Wan Isahak, W.N.R.; Ramli, Z.A.C.; Mohamed Hisham, M.W.; Yarmo, M.A. Magnesium oxide nanoparticles on green activated carbon as efficient CO2 adsorbent. AIP Conf. Proc. 2013, 1571, 882–889. [Google Scholar]
- Yusof, S.M.; Othaman, R.; Setiabudi, H.D.; Teh, L.P. Modified fibrous silica for enhanced carbon dioxide adsorption: Role of metal oxides on physicochemical properties and adsorption performance. J. Solid State Chem. 2021, 294, 121845. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Florin, N.H.; Harris, A.T. Reactivity of CaO derived from nano-sized CaCO3 particles through multiple CO2 capture-and-release cycles. Chem. Eng. Sci. 2009, 64, 187–191. [Google Scholar] [CrossRef]
- Li, Y.-J.; Zhao, C.-S.; Qu, C.-R.; Duan, L.; Li, Q.-Z.; Liang, C. CO2 capture using CaO modified with ethanol/water solution during cyclic calcination/carbonation. Chem. Eng. Technol. 2008, 31, 237–244. [Google Scholar] [CrossRef]
- Belova, A.A.G.; Yegulalp, T.M.; Yee, C.T. Feasibility study of In Situ CO2 capture on an integrated catalytic CO2 sorbent for hydrogen production from methane. Energy Procedia 2009, 1, 749–755. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Saxena, S.K. A comparative study of CO2 sorption properties for different oxides. Mater. Renew. Sustain. Energy 2014, 3, 30. [Google Scholar] [CrossRef]
- Duyar, M.S.; Farrauto, R.J.; Castaldi, M.J.; Yegulalp, T.M. In Situ CO2 Capture Using CaO/γ-Al2O3 Washcoated Monoliths for Sorption Enhanced Water Gas Shift Reaction. Ind. Eng. Chem. Res. 2014, 53, 1064–1072. [Google Scholar] [CrossRef]
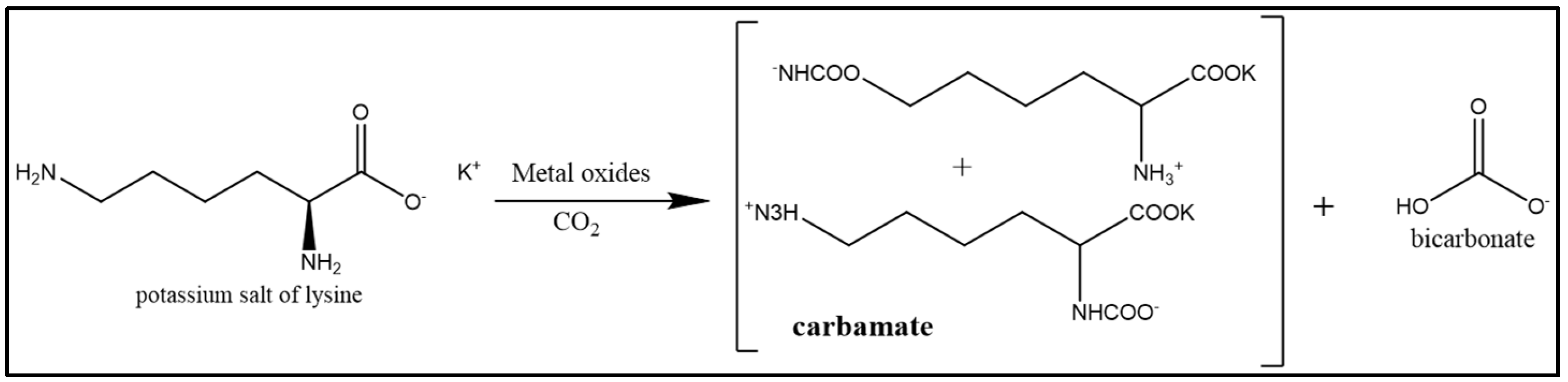
- Dharmalingam, S.; Park, K.T.; Lee, J.Y.; Park, I.G.; Jeong, S.K. Catalytic effect of metal oxides on CO2 absorption in an aqueous potassium salt of lysine. J. Ind. Eng. Chem. 2018, 68, 335–341. [Google Scholar] [CrossRef]
- Liu, G.; Tran-Phu, T.; Chen, H.; Tricoli, A. A Review of Metal- and Metal-Oxide-Based Heterogeneous Catalysts for Electroreduction of Carbon Dioxide. Adv. Sustain. Syst. 2018, 2, 1800028. [Google Scholar] [CrossRef]
- Gawande, M.B.; Pandey, R.K.; Jayaram, R.V. ChemInform Abstract: Role of Mixed Metal Oxides in Catalysis Science—Versatile Applications in Organic Synthesis. Catal. Sci. Technol. 2012, 2, 1113–1125. [Google Scholar] [CrossRef]
- Bhatti, U.H.; Shah, A.K.; Kim, J.N.; You, J.K.; Choi, S.H.; Lim, D.H.; Nam, S.; Park, Y.H.; Baek, I.H. Effects of Transition Metal Oxide Catalysts on MEA Solvent Regeneration for the Post-Combustion Carbon Capture Process. ACS Sustain. Chem. Eng. 2017, 5, 5862–5868. [Google Scholar] [CrossRef]
- Lippert, C.A.; Liu, K.; Sarma, M.; Parkin, S.R.; Remias, J.E.; Brandewie, C.M.; Odom, S.A.; Liu, K. Improving carbon capture from power plant emissions with zinc- and cobalt-based catalysts. Catal. Sci. Technol. 2014, 4, 3620–3625. [Google Scholar] [CrossRef]
- Widger, L.R.; Sarma, M.; Kelsey, R.A.; Risko, C.; Lippert, C.A.; Parkin, S.R.; Liu, K. Enhancing CO2 absorption for post-combustion carbon capture via zinc-based biomimetic catalysts in industrially relevant amine solutions. Int. J. Greenh. Gas Control 2019, 85, 156–165. [Google Scholar] [CrossRef]
- Seo, S.; Lages, B.; Kim, M. Catalytic CO2 absorption in an amine solvent using nickel nanoparticles for post-combustion carbon capture. J. CO2 Util. 2020, 36, 244–252. [Google Scholar] [CrossRef]
- Bhaduri, G.A.; Alamiry, M.A.H.; Šiller, L. Nickel Nanoparticles for Enhancing Carbon Capture. J. Nanomater. 2015, 2015, 376. [Google Scholar] [CrossRef]
- Pashaei, H.; Ghaemi, A.; Nasiri, M.; Heydarifard, M. Experimental Investigation of the Effect of Nano Heavy Metal Oxide Particles in Piperazine Solution on CO2 Absorption Using a Stirrer Bubble Column. Energy Fuels 2018, 32, 2037–2052. [Google Scholar] [CrossRef]
- Chowdhury, S.; Parshetti, G.K.; Balasubramanian, R. Post-combustion CO2 capture using mesoporous TiO2/graphene oxide nanocomposites. Chem. Eng. J. 2015, 263, 374–384. [Google Scholar] [CrossRef]
- Zhu, X.; Ge, T.; Yang, F.; Lyu, M.; Chen, C.; O’Hare, D.; Wang, R. Efficient CO2 capture from ambient air with amine-functionalized Mg-Al mixed metal oxides. J. Mater. Chem. A 2020, 8, 16421–16428. [Google Scholar] [CrossRef]
- Ravi, N.; Anuar, S.A.; Yusuf, N.Y.M.; Isahak, W.N.R.W.; Masdar, M.S. Amine-mixed oxide hybrid materials for carbon dioxide adsorption from CO2/H2 mixture. Mater. Res. Express 2018, 5, 055501. [Google Scholar] [CrossRef]
- Gao, N.; Chen, K.; Quan, C. Development of CaO-based adsorbents loaded on charcoal for CO2 capture at high temperature. Fuel 2020, 260, 116411. [Google Scholar] [CrossRef]
- Khdary, N.H.; Ghanem, M.A.; Merajuddine, M.G.; Bin Manie, F.M. Incorporation of Cu, Fe, Ag, and Au nanoparticles in mercapto-silica (MOS) and their CO2 adsorption capacities. J. CO2 Util. 2014, 5, 17–23. [Google Scholar] [CrossRef]
- Khdary, N.H.; Ghanem, M.A. Metal-organic-silica nanocomposites: Copper, silver nanoparticles- ethylenediamine-silica gel and their CO2 adsorption behaviour. J. Mater. Chem. 2012, 22, 12032–12038. [Google Scholar] [CrossRef]
- Khdary, N.H.; Ghanem, M.A.; Abdesalam, M.E.; Al-Garadah, M.M. Sequestration of CO2 using Cu nanoparticles supported on spherical and rod-shape mesoporous silica. J. Saudi Chem. Soc. 2018, 22, 343–351. [Google Scholar] [CrossRef]
- Khdary, N.H.; Abdelsalam, M.E. Polymer-silica nanocomposite membranes for CO2 capturing. Arab. J. Chem. 2020, 13, 557–567. [Google Scholar] [CrossRef]
- Bai, S.T.; Zhou, C.; Wu, X.; Sun, R.; Sels, B. Suppressing Dormant Ru States in the Presence of Conventional Metal Oxides Promotes the Ru-MACHO-BH-Catalyzed Integration of CO2Capture and Hydrogenation to Methanol. ACS Catal. 2021, 11, 12682–12691. [Google Scholar] [CrossRef]
- Viter, R.; Iatsunskyi, I. Metal Oxide Nanostructures in Sensing. In Nanomaterials Design for Sensing Applications; Zenkina, O.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–91. [Google Scholar] [CrossRef]
- Toroker, M.C.; Carter, E.A. Strategies to suppress cation vacancies in metal oxide alloys: Consequences for solar energy conversion. J. Mater. Sci. 2015, 50, 5715–5722. [Google Scholar] [CrossRef]
- Sherrill, A.B.; Barteau, M.A. Chapter 10 Principles of reactivity from studies of organic reactions on model oxide surfaces. Chem. Phys. Solid Surf. 2001, 9, 409–442. [Google Scholar]
- Suppiah, D.D.; Daud, W.M.A.W.; Johan, M.R. Supported metal oxide catalysts for CO2 Fischer−Tropsch conversion to liquid fuels—A review. Energy Fuels 2021, 35, 17261–17278. [Google Scholar] [CrossRef]
- Shutilov, A.A.; Zenkovets, G.A.; Pakharukov, I.Y.; Prosvirin, I.P. Influence of CeO2 addition on the physicochemical and catalytic properties of Pd/TiO2 catalysts in CO oxidation. Kinet. Catal. 2014, 55, 111–116. [Google Scholar] [CrossRef]
- Cheng, K.; Yang, F.; Zhang, D.; Yin, J.; Cao, D.; Wang, G. Pd nanofilm supported on C@TiO2 nanocone core/shell nanoarrays: A facile preparation of high performance electrocatalyst for H2O2 electroreduction in acid medium. Electrochim. Acta 2013, 105, 115–120. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Q.; Wang, Y.; Xiao, H.; Liu, W.; Zhang, D.; Yang, T. Tailoring performance of Co–Pt/MgO–Al2O3 bimetallic aerogel catalyst for methane oxidative carbon dioxide reforming: Effect of Pt/Co ratio. Int. J. Hydrog. Energy 2019, 44, 19878–19889. [Google Scholar] [CrossRef]
- Chu, S.; Ou, P.; Ghamari, P.; Vanka, S.; Zhou, B.; Shih, I.; Song, J.; Mi, Z. Photoelectrochemical CO 2 Reduction into Syngas with the Metal/Oxide Interface. J. Am. Chem. Soc. 2018, 140, 7869–7877. [Google Scholar] [CrossRef]
- Zhu, N.-N.; Liu, X.-H.; Li, T.; Ma, J.-G.; Cheng, P.; Yang, G.-M. Composite System of Ag Nanoparticles and Metal-Organic Frameworks for the Capture and Conversion of Carbon Dioxide under Mild Conditions. Inorg. Chem. 2017, 56, 3414–3420. [Google Scholar] [CrossRef]
- Lin, A.; Ibrahim, A.A.; Arab, P.; El-Kaderi, H.M.; El-Shall, M.S. Palladium Nanoparticles Supported on Ce-Metal-Organic Framework for Efficient CO Oxidation and Low-Temperature CO2 Capture. ACS Appl. Mater. Interfaces 2017, 9, 17961–17968. [Google Scholar] [CrossRef]
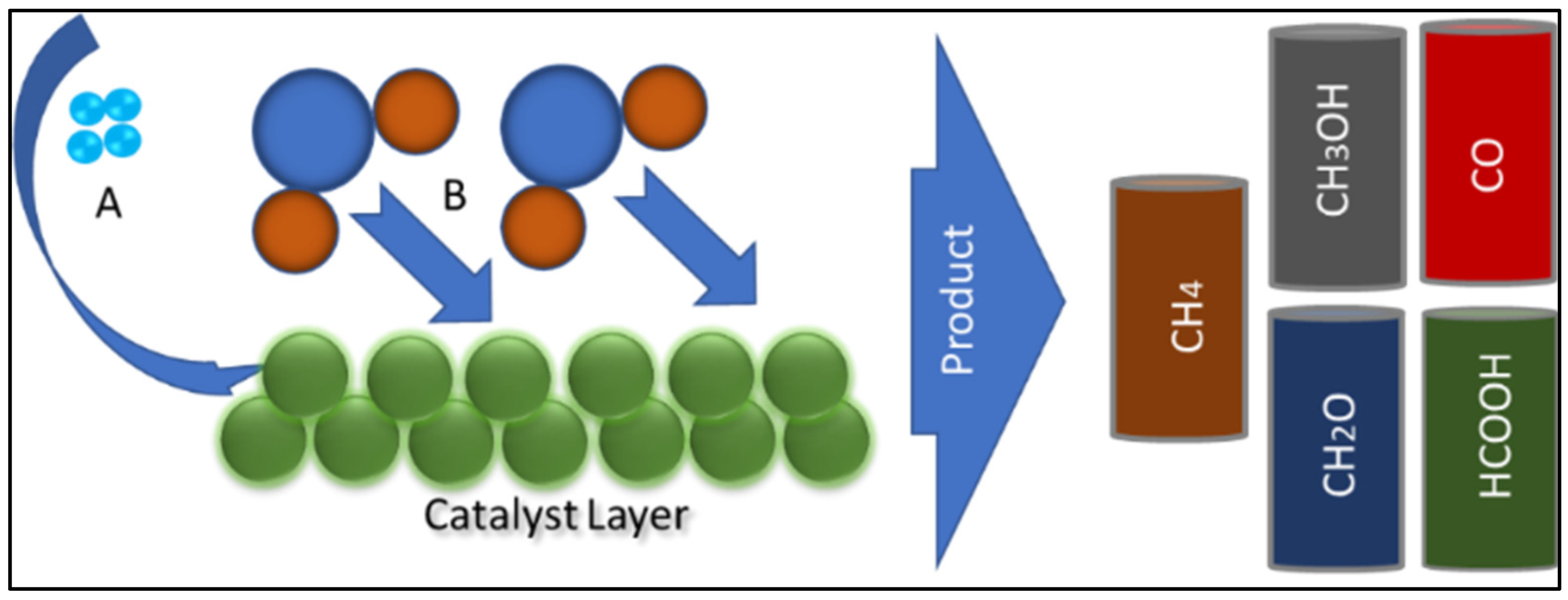
- Yang, B.; Yu, X.; Halder, A.; Zhang, X.; Zhou, X.; Mannie, G.J.A.; Tyo, E.C.; Pellin, M.J.; Seifert, S.; Su, D.; et al. Dynamic Interplay between Copper Tetramers and Iron Oxide Boosting CO2 Conversion to Methanol and Hydrocarbons under Mild Conditions. ACS Sustain. Chem. Eng. 2019, 7, 14435–14442. [Google Scholar] [CrossRef]
- Nolan, M. Adsorption of CO2 on Heterostructures of Bi2O3 Nanocluster-Modified TiO2 and the Role of Reduction in Promoting CO2 Activation. ACS Omega 2018, 3, 13117–13128. [Google Scholar] [CrossRef] [PubMed]
- Zhiani, M.; Kamali, S. Synergistic effect of ceria on the structure and hydrogen evolution activity of nickel nanoparticles grown on reduced graphene oxide. J. Mater. Chem. A 2017, 5, 8108–8116. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, W.; Jia, C.; Ong, S.S.G.; Zhang, C.; Zhang, S.; Lin, H. Eliminating carbon dioxide emissions at the source by the integration of carbon dioxide capture and utilization over noble metals in the liquid phase. J. Catal. 2020, 389, 247–258. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, D.; Liu, T.; Liu, G.; Hong, X. Fabrication of PdZn alloy catalysts supported on ZnFe composite oxide for CO2 hydrogenation to methanol. J. Colloid Interface Sci. 2021, 597, 260–268. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, K.; Zong, L.; Tong, S.; Wang, X.; Zhou, J.; Lu, Z.-H.; Feng, G. Control synthesis of CeO2 nanomaterials supported gold for catalytic oxidation of carbon monoxide. Mol. Catal. 2017, 442, 173–180. [Google Scholar] [CrossRef]
- Qin, Z.; Ren, J.; Miao, M.; Li, Z.; Lin, J.; Xie, K. The catalytic methanation of coke oven gas over Ni-Ce/Al2O3 catalysts prepared by microwave heating: Effect of amorphous NiO formation. Appl. Catal. B Environ. 2015, 164, 18–30. [Google Scholar] [CrossRef]
- Wang, F.; He, S.; Chen, H.; Wang, B.; Zheng, L.; Wei, M.; Evans, D.G.; Duan, X. Active Site Dependent Reaction Mechanism over Ru/CeO2 Catalyst toward CO2 Methanation. J. Am. Chem. Soc. 2016, 138, 6298–6305. [Google Scholar] [CrossRef]
- Li, L.; Wu, Y.; Lu, J.; Nan, C.; Li, Y. Synthesis of Pt–Ni/graphene via in situ reduction and its enhanced catalyst activity for methanol oxidation. Chem. Commun. 2013, 49, 7486–7488. [Google Scholar] [CrossRef]
- Hu, F.; Tong, S.; Lu, K.; Chen, C.-M.; Su, F.-Y.; Zhou, J.; Lu, Z.-H.; Wang, X.; Feng, G.; Zhang, R. Reduced graphene oxide supported Ni-Ce catalysts for CO2 methanation: The support and ceria promotion effects. J. CO2 Util. 2019, 34, 676–687. [Google Scholar] [CrossRef]
- Zhao, K.; Calizzi, M.; Moioli, E.; Li, M.; Borsay, A.; Lombardo, L.; Mutschler, R.; Luo, W.; Züttel, A. Unraveling and optimizing the metal-metal oxide synergistic effect in a highly active Cox(CoO)1−x catalyst for CO2 hydrogenation. J. Energy Chem. 2020, 53, 241–250. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Su, H.; Huang, W.; Dong, X. Binary metal oxide: Advanced energy storage materials in supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar] [CrossRef]
- Zhou, B.; Ou, P.; Pant, N.; Cheng, S.; Vanka, S.; Chu, S.; Rashid, R.T.; Botton, G.; Song, J.; Mi, Z. Highly efficient binary copper?iron catalyst for photoelectrochemical carbon dioxide reduction toward methane. Proc. Natl. Acad. Sci. USA 2020, 117, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, H.; Lamaison, S.; Qi, Z.; Koshy, D.M.; Stevens, M.B.; Wakerley, D.; Zeledón, J.A.Z.; King, L.A.; Zhou, L.; et al. Article Bimetallic effects on Zn-Cu electrocatalysts enhance activity and selectivity for the conversion of CO2 to CO Bimetallic effects on Zn-Cu electrocatalysts enhance activity and selectivity. Chem. Catal. 2021, 1, 663–680. [Google Scholar] [CrossRef]
- Cai, Z.; Wu, Y.; Wu, Z.; Yin, L.; Weng, Z.; Zhong, Y.; Xu, W.; Sun, X.; Wang, H. Unlocking bifunctional electrocatalytic activity for CO2 reduction reaction by win-win metal-oxide cooperation. ACS Energy Lett. 2018, 3, 2816–2822. [Google Scholar] [CrossRef]
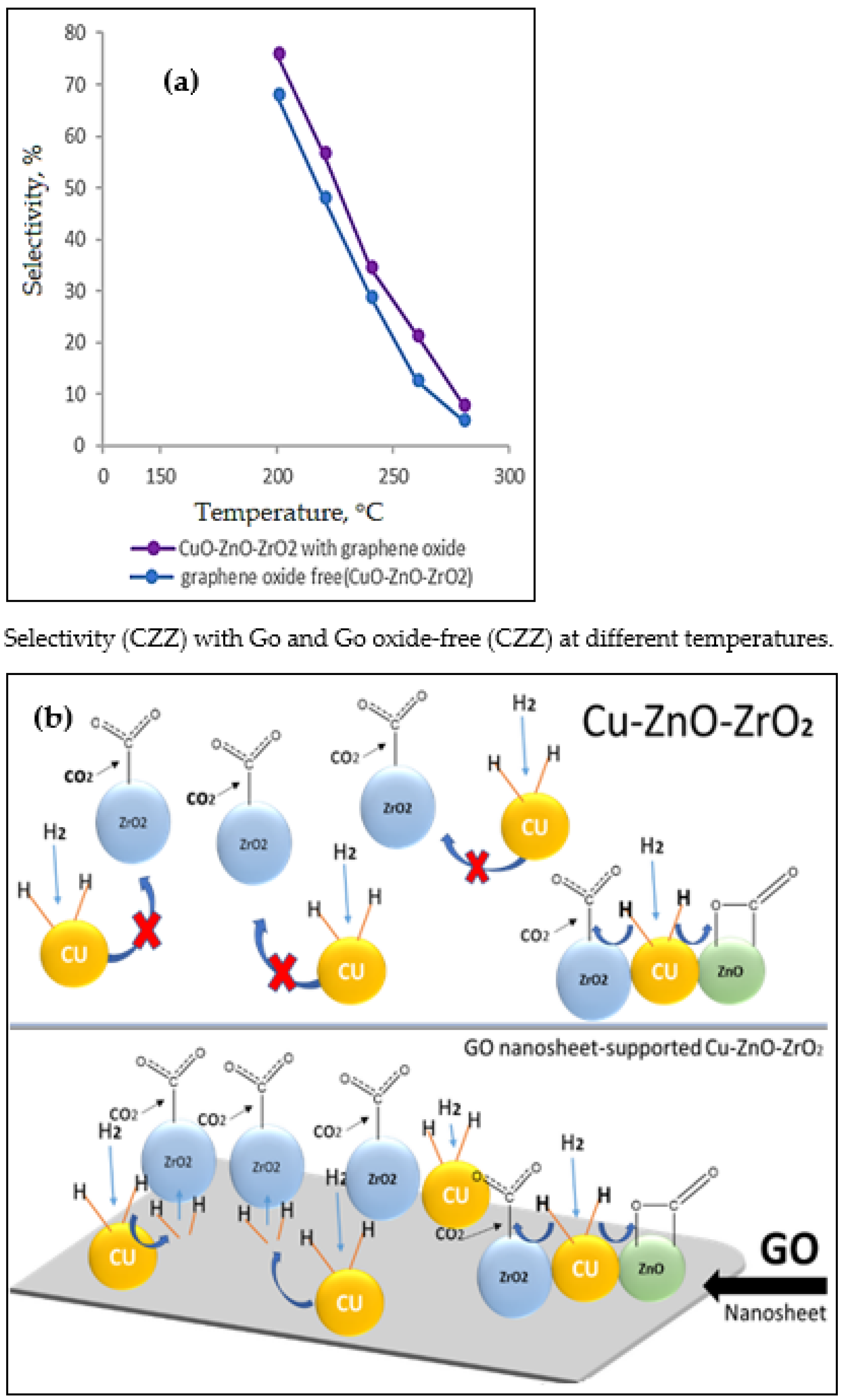
- Witoon, T.; Numpilai, T.; Phongamwong, T.; Donphai, W.; Boonyuen, C.; Warakulwit, C.; Chareonpanich, M.; Limtrakul, J. Enhanced activity, selectivity and stability of a CuO-ZnO-ZrO2 catalyst by adding graphene oxide for CO2 hydrogenation to methanol. Chem. Eng. J. 2018, 334, 1781–1791. [Google Scholar] [CrossRef]
- Biradar, A.V.; Umbarkar, S.B.; Dongare, M.K. Transesterification of diethyl oxalate with phenol using MoO3/SiO2 catalyst. Appl. Catal. A Gen. 2005, 285, 190–195. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Corr, S.A. Metal oxide nanoparticles. In Nanoscience: Volume 1: Nanostructures through Chemistry; Royal Society of Chemistry: London, UK, 2012; pp. 180–207. [Google Scholar] [CrossRef]
- Kim, D.; Kley, C.S.; Li, Y.; Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proc. Natl. Acad. Sci. USA 2017, 114, 10560–10565. [Google Scholar] [CrossRef]
- Jeong, K.; Hong, T.; Kim, J. Development of a CO2 emission benchmark for achieving the national CO2 emission reduction target by 2030. Energy Build. 2018, 158, 86–94. [Google Scholar] [CrossRef]
- Santos, G. Road transport and CO2 emissions: What are the challenges? Transp. Policy 2017, 59, 71–74. [Google Scholar] [CrossRef]
- Sharma, A.; Gore, P.M.; Kandasubramanian, B. Reduction of carbon dioxide (CO2) using ‘ p ’ & ‘ d ’ block electro-catalysts: A review. J. Environ. Chem. Eng. 2021, 9, 104798. [Google Scholar]
- Garba, M.D.; Usman, M.; Khan, S.; Shehzad, F.; Galadima, A.; Ehsan, M.F.; Ghanem, A.S.; Humayun, M. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons. J. Environ. Chem. Eng. 2020, 9, 104756. [Google Scholar] [CrossRef]
- Du, C.; Wang, X.; Chen, W.; Feng, S.; Wen, J.; Wu, Y. CO2 transformation to multicarbon products by photocatalysis and electrocatalysis. Mater. Today Adv. 2020, 6, 100071. [Google Scholar] [CrossRef]
- Khalil, M.; Gunlazuardi, J.; Ivandini, T.A.; Umar, A. Photocatalytic conversion of CO2 using earth-abundant catalysts: A review on mechanism and catalytic performance. Renew. Sustain. Energy Rev. 2019, 113, 109246. [Google Scholar] [CrossRef]
- Wang, X.; Xuan, X.; Wang, Y.; Li, X.; Huang, H.; Zhang, X.; Du, X. Nano-Au-modified TiO2 grown on dendritic porous silica particles for enhanced CO2 photoreduction. Microporous Mesoporous Mater. 2021, 310, 110635. [Google Scholar] [CrossRef]
- Akple, M.S.; Low, J.; Liu, S.; Cheng, B.; Yu, J.; Ho, W. Fabrication and enhanced CO2 reduction performance of N-self-doped TiO2 microsheet photocatalyst by bi-cocatalyst modification. J. CO2 Util. 2016, 16, 442–449. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Vo, D.-V.N.; Trinh, Q.T.; Bae, S.-R.; Chae, S.Y.; Kim, S.Y.; Van Le, Q. Recent advances in TiO2-based photocatalysts for reduction of CO2 to fuels. Nanomaterials 2020, 10, 337. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Jung, H.S.; Kim, J.M.; Kang, Y.T. Photocatalytic CO2 conversion on highly ordered mesoporous materials: Comparisons of metal oxides and compound semiconductors. Appl. Catal. B Environ. 2018, 224, 594–601. [Google Scholar] [CrossRef]
- Salaices, M.; Serrano, B.; De Lasa, H.I. Experimental evaluation of photon absorption in an aqueous TiO2 slurry reactor. Chem. Eng. J. 2002, 90, 219–229. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, H.I.; Shon, J.K.; Hur, J.Y.; Kang, M.S.; Park, S.S.; Kong, S.S.; Yu, J.A.; Seo, M.; Li, D.; et al. Preparation of highly ordered mesoporous TiO2 materials with crystalline framework from different mesostructured silica templates via nanoreplication. Chem. Lett. 2008, 37, 140–141. [Google Scholar] [CrossRef]
- Serhan, M.; Sprowls, M.; Jackemeyer, D.; Long, M.; Perez, I.D.; Maret, W.; Tao, N.; Forzani, E. Total iron measurement in human serum with a smartphone. In Proceedings of the 2019 AIChE Annual Meeting. American Institute of Chemical Engineers, Orlando, FL, USA, 10–15 November 2019. [Google Scholar]
- Izadpanah Ostad, M.; Niknam Shahrak, M.; Galli, F. Photocatalytic carbon dioxide reduction to methanol catalyzed by ZnO, Pt, Au, and Cu nanoparticles decorated zeolitic imidazolate framework-8. J. CO2 Util. 2021, 43, 101373. [Google Scholar] [CrossRef]
- Maina, J.W.; Schutz, J.; Grundy, L.; Ligneris, E.D.; Yi, Z.; Kong, L.; Pozo-Gonzalo, C.; Ionescu, M.; Dumée, L.F. Inorganic Nanoparticles/Metal Organic Framework Hybrid Membrane Reactors for Efficient Photocatalytic Conversion of CO2. ACS Appl. Mater. Interfaces 2017, 9, 35010–35017. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Zhou, C.; Cao, Z.; Zhang, R. ZnO rod/reduced graphene oxide sensitized by α-Fe2O3 nanoparticles for effective visible-light photoreduction of CO2. J. Colloid Interface Sci. 2019, 554, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Suleman Tahir, M.; Manzoor, N.; Sagir, M.; Tahir, M.B.; Nawaz, T. Fabrication of ZnFe2O4 modified TiO2 hybrid composites for photocatalytic reduction of CO2 into methanol. Fuel 2021, 285, 119206. [Google Scholar] [CrossRef]
- Moualkia, H.; Rekhila, G.; Izerrouken, M.; Mahdjoub, A.; Trari, M. Influence of the film thickness on the photovoltaic properties of chemically deposited CdS thin films: Application to the photodegradation of orange II. Mater. Sci. Semicond. Process. 2014, 21, 186–193. [Google Scholar] [CrossRef]
- Ghosh, A.; Mangalvedhe, N.; Ratasuk, R.; Mondal, B.; Cudak, M.; Visotsky, E.; Thomas, T.A.; Andrews, J.G.; Xia, P.; Jo, H.S.; et al. Heterogeneous cellular networks: From theory to practice. IEEE Commun. Mag. 2012, 50, 54–64. [Google Scholar] [CrossRef]
- Bharath, G.; Rambabu, K.; Hai, A.; Othman, I.; Ponpandian, N.; Banat, F.; Show, P.L. Hybrid Pd 50 -Ru 50/MXene (Ti3 C2 Tx) nanocatalyst for effective hydrogenation of CO2 to methanol toward climate change control. Chem. Eng. J. 2021, 414, 128869. [Google Scholar] [CrossRef]
- Chen, G.; Godfroid, T.; Britun, N.; Georgieva, V.; Delplancke-Ogletree, M.-P.; Snyders, R. Plasma-catalytic conversion of CO2 and CO2/H2O in a surface-wave sustained microwave discharge. Appl. Catal. B Environ. 2017, 214, 114–125. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Leys, C.; Van Langenhove, H. Combining non-thermal plasma with heterogeneous catalysis in waste gas treatment: A review. Appl. Catal. B Environ. 2008, 78, 324–333. [Google Scholar] [CrossRef]
- Ghorbanzadeh, A.M.; Lotfalipour, R.; Rezaei, S. Carbon dioxide reforming of methane at near room temperature in low energy pulsed plasma. Int. J. Hydrogen Energy 2009, 34, 293–298. [Google Scholar] [CrossRef]
- Tu, X.; Whitehead, J.C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: Understanding the synergistic effect at low temperature. Appl. Catal. B Environ. 2012, 125, 439–448. [Google Scholar] [CrossRef]
- George, A.; Shen, B.; Craven, M.; Wang, Y.; Kang, D.; Wu, C.; Tu, X. A A Review of Non-Thermal Plasma Technology: A novel solution for CO2 conversion and utilization. Renew. Sustain. Energy Rev. 2021, 135, 109702. [Google Scholar] [CrossRef]
- Xu, S.; Chansai, S.; Shao, Y.; Xu, S.; Wang, Y.-c.; Haigh, S.; Mu, Y.; Jiao, Y.; Stere, C.E.; Chen, H.; et al. Mechanistic study of non-thermal plasma assisted CO2 hydrogenation over Ru supported on MgAl layered double hydroxide. Appl. Catal. B Environ. 2020, 268, 118752. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, L.; Wu, C.; Wang, J.; Shen, B.; Tu, X. Low temperature reforming of biogas over K-, Mg- and Ce-promoted Ni/Al2O3 catalysts for the production of hydrogen rich syngas: Understanding the plasma-catalytic synergy. Appl. Catal. B Environ. 2018, 224, 469–478. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; Wu, C.; Ashford, B.; Williams, P.; Tu, X. Plasma-photocatalytic conversion of CO2 at low temperatures: Understanding the synergistic effect of plasma-catalysis. Appl. Catal. B Environ. 2016, 182, 525–532. [Google Scholar] [CrossRef]
- Ye, R.-P.; Li, Q.; Gong, W.; Wang, T.; Razink, J.J.; Lin, L.; Qin, Y.-Y.; Zhou, Z.; Adidharma, H.; Tang, J.; et al. High-performance of nanostructured Ni/CeO2 catalyst on CO2 methanation. Appl. Catal. B Environ. 2020, 268, 118474. [Google Scholar] [CrossRef]
- Ray, D.; Chawdhury, P.; Bhargavi, K.; Thatikonda, S.; Lingaiah, N.; Subrahmanyam, C. Ni and Cu oxide supported γ-Al2O3 packed DBD plasma reactor for CO2 activation. J. CO2 Util. 2021, 44, 101400. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; He, Y.L.; Yan, J.D.; Tu, X. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: Understanding the effect of packing materials. Plasma Sources Sci. Technol. 2015, 24, 15011. [Google Scholar] [CrossRef]
- Ray, D.; Chawdhury, P.; Subrahmanyam, C. A facile method to decompose CO2 using a g-C3N4-assisted DBD plasma reactor. Environ. Res. 2020, 183, 109286. [Google Scholar] [CrossRef]
- Woodward, J.; Dunning, S.; Westoby, M.; Sugden, D. Northumbria Research Link. Earth Planet. Sci. Lett. 2018, 501, 56–66. [Google Scholar]
- Liu, C.J.; Xu, G.H.; Wang, T. Non-thermal plasma approaches in CO2 utilization. Fuel Process. Technol. 1999, 58, 119–134. [Google Scholar] [CrossRef]
- Ashford, B.; Wang, Y.; Poh, C.K.; Chen, L.; Tu, X. Plasma-catalytic conversion of CO2 to CO over binary metal oxide catalysts at low temperatures. Appl. Catal. B Environ. 2020, 276, 119110. [Google Scholar] [CrossRef]
- Bouchoul, N.; Fourré, E.; Tatibouët, J.-M.; Duarte, A.; Tanchoux, N.; Batiot-Dupeyrat, C. Structural modifications of calcium based catalysts by non-thermal plasma in the CO2 reforming of CH4 and the influence of water. J. CO2 Util. 2020, 35, 79–89. [Google Scholar] [CrossRef]
- Liang, X.; Shen, Y.; Liu, Y.; Wang, J.; Gao, Y.; Li, S.; Wang, M.; Gao, S. Investigations on the basic electrical properties of Polyurethane foam material. In Proceedings of the 2015 IEEE 11th International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Sydney, Australia, 19–22 July 2015; pp. 863–866. [Google Scholar]
- Argin, M.; Karady, G.G. Characterization of polyurethane foam dielectric strength. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 350–355. [Google Scholar] [CrossRef]
- Taghvaei, H.; Pirzadeh, E.; Jahanbakhsh, M.; Khalifeh, O.; Rahimpour, M.R. Polyurethane foam: A novel support for metal oxide packing used in the non-thermal plasma decomposition of CO2. J. CO2 Util. 2021, 44, 101398. [Google Scholar] [CrossRef]
- Li, S.; Ongis, M.; Manzolini, G.; Gallucci, F. Non-thermal plasma-assisted capture and conversion of CO2. Chem. Eng. J. 2021, 410, 128335. [Google Scholar] [CrossRef]
- Li, R.; Li, C. Photocatalytic Water Splitting on Semiconductor-Based Photocatalysts. Advances in Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 60. [Google Scholar]
- Xu, L.; Xiu, Y.; Liu, F.; Liang, Y.; Wang, S. Research progress in conversion of CO2 to valuable fuels. Molecules 2020, 25, 3653. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.X.; Gandionco, K.A.; Bond, A.M.; Zhang, J. Electrocatalytic carbon dioxide reduction: From fundamental principles to catalyst design. Mater. Today Adv. 2020, 7, 100074. [Google Scholar] [CrossRef]
- Fu, L.; Wang, R.; Zhao, C.; Huo, J.; He, C.; Kim, K.-H.; Zhang, W. Construction of Cr-embedded graphyne electrocatalyst for highly selective reduction of CO2 to CH4: A DFT study. Chem. Eng. J. 2021, 414, 128857. [Google Scholar] [CrossRef]
- Le, M.; Ren, M.; Zhang, Z.; Sprunger, P.T.; Kurtz, R.L.; Flake, J.C. Electrochemical Reduction of CO2 to CH3OH at Copper Oxide Surfaces. J. Electrochem. Soc. 2011, 158, E45–E49. [Google Scholar] [CrossRef]
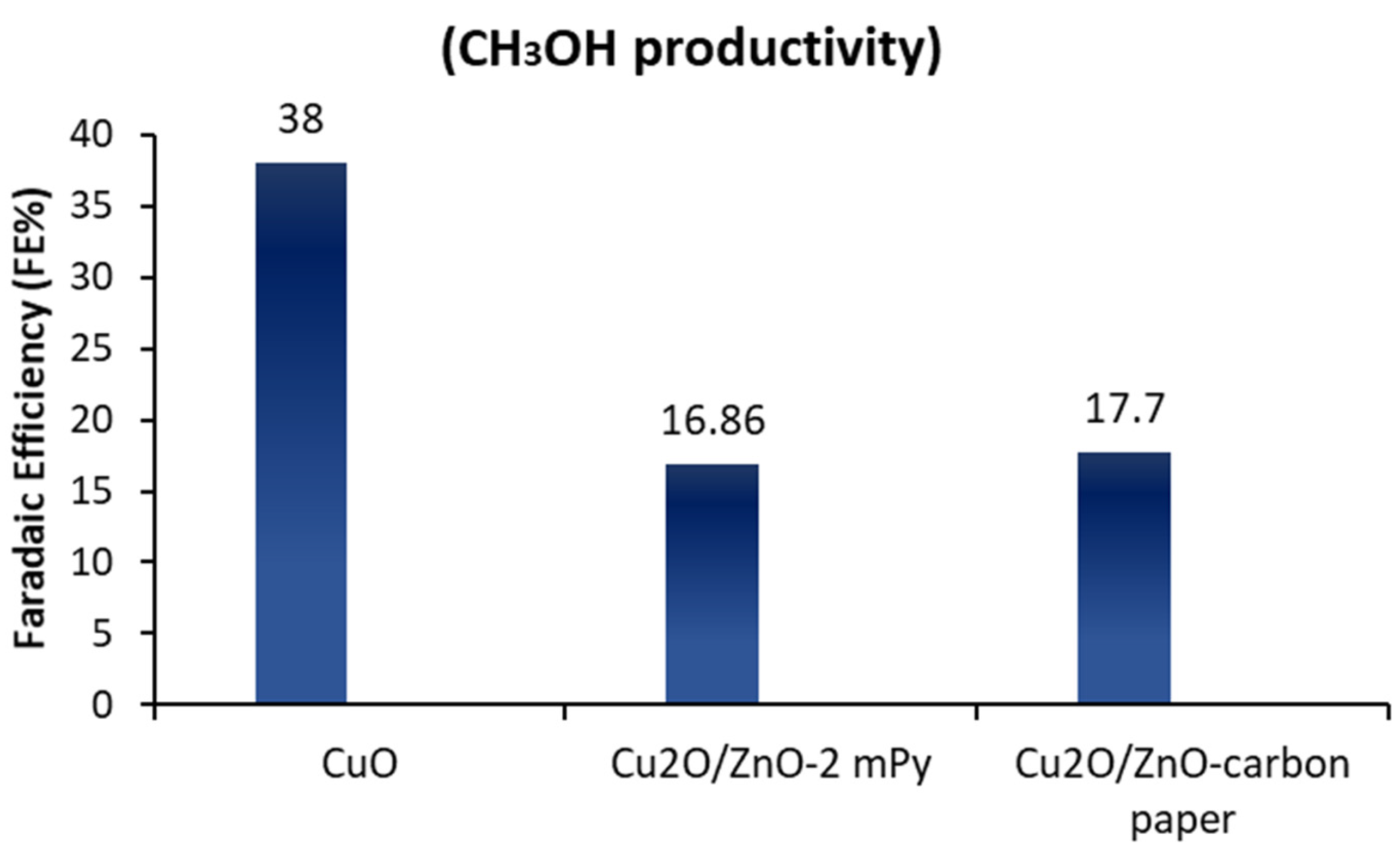
- Albo, J.; Beobide, G.; Castaño, P.; Irabien, A. Methanol electrosynthesis from CO2 at Cu2O/ZnO prompted by pyridine-based aqueous solutions. J. CO2 Util. 2017, 18, 164–172. [Google Scholar] [CrossRef]
- Albo, J.; Sáez, A.; Solla-Gullón, J.; Montiel, V.; Irabien, A. Production of methanol from CO2 electroreduction at Cu2O and Cu2O/ZnO-based electrodes in aqueous solution. Appl. Catal. B Environ. 2015, 176–177, 709–717. [Google Scholar] [CrossRef]
- Lu, Z.-J.; Bao, S.-J.; Gou, Y.-T.; Cai, C.-J.; Ji, C.-C.; Xu, M.-W.; Song, J.; Wang, R. Nitrogen-doped reduced-graphene oxide as an efficient metal-free electrocatalyst for oxygen reduction in fuel cells. RSC Adv. 2013, 3, 3990–3995. [Google Scholar] [CrossRef]
- Kamat, P.V. Graphene-based nanoarchitectures. Anchoring semiconductor and metal nanoparticles on a two-dimensional carbon support. J. Phys. Chem. Lett. 2010, 1, 520–527. [Google Scholar] [CrossRef]
- Liu, A.; Gao, M.; Ren, X.; Meng, F.; Yang, Y.; Gao, L.; Yang, Q.; Ma, T. Current progress in electrocatalytic carbon dioxide reduction to fuels on heterogeneous catalysts. J. Mater. Chem. A 2020, 8, 3541–3562. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Adegoke, R.O.; Ibrahim, A.O.; Adegoke, S.A.; Bello, O.S. Electrocatalytic conversion of CO2 to hydrocarbon and alcohol products: Realities and prospects of Cu-based materials. Sustain. Mater. Technol. 2020, 25, e00200. [Google Scholar] [CrossRef]
- Fazel Zarandi, R.; Rezaei, B.; Ghaziaskar, H.S.; Ensafi, A.A. Modification of copper electrode with copper nanoparticles@ reduced graphene oxide–Nile blue and its application in electrochemical CO2 conversion. Mater. Today Energy 2020, 18, 100507. [Google Scholar] [CrossRef]
- Li, Q.; Sun, S. Recent advances in the organic solution phase synthesis of metal nanoparticles and their electrocatalysis for energy conversion reactions. Nano Energy 2016, 29, 178–197. [Google Scholar] [CrossRef]



| Particularity | Physical Absorption | Chemical Absorption |
|---|---|---|
|
The Reaction Occurs | Absorption occurs at high CO2 partial pressure and low temperature | Absorption occurs between CO2 and chemical solvent, and a weakly-bonded intermediate compound is formed, which is dissociated by heat, after which CO2 |
| Solvent Used | Dimethyl ether, polyethylene glycol, and cold methanol | Monoethanolamine (MEA), diethanolamine (DEA), and triethanolamine (TEA) |
| Features | Low energy and less solvent sensitivity to feed impurities | Requires high energy |
| Disadvantages | Low carbon dioxide absorption capacity | High carbon dioxide adsorption capacity |
| Adsorbent Material | Method | Temperature (K) | Uptake CO2 mmolg−1 | Ref. |
|---|---|---|---|---|
| TiO2/GO | Volumetric analysis | 298 | 1.88 | [56] |
| Mixed metal oxides (MMOs) | TGA/PB | 298 | 2.27 | [57] |
| Diethanolamine (DEA)/CuO–MgO | Facile hydrothermal | 298 | 21.2 | [58] |
| CaO-loaded charcoal | sol−gel | 298 | 15.1 | [59] |
| MgO-FS | sol−gel | 298 | 3.43 | [40] |
| CaO-FS | sol−gel | 298 | 3.11 | [40] |
| CeO2-FS | impregnation | 298 | 7.85 | [40] |
| MgO/C | thermaldecomposition | 300 | 32 | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khdary, N.H.; Alayyar, A.S.; Alsarhan, L.M.; Alshihri, S.; Mokhtar, M. Metal Oxides as Catalyst/Supporter for CO2 Capture and Conversion, Review. Catalysts 2022, 12, 300. https://doi.org/10.3390/catal12030300
Khdary NH, Alayyar AS, Alsarhan LM, Alshihri S, Mokhtar M. Metal Oxides as Catalyst/Supporter for CO2 Capture and Conversion, Review. Catalysts. 2022; 12(3):300. https://doi.org/10.3390/catal12030300
Chicago/Turabian StyleKhdary, Nezar H., Alhanouf S. Alayyar, Latifah M. Alsarhan, Saeed Alshihri, and Mohamed Mokhtar. 2022. "Metal Oxides as Catalyst/Supporter for CO2 Capture and Conversion, Review" Catalysts 12, no. 3: 300. https://doi.org/10.3390/catal12030300
APA StyleKhdary, N. H., Alayyar, A. S., Alsarhan, L. M., Alshihri, S., & Mokhtar, M. (2022). Metal Oxides as Catalyst/Supporter for CO2 Capture and Conversion, Review. Catalysts, 12(3), 300. https://doi.org/10.3390/catal12030300








