Preparation of Cordierite Monolith Catalysts with the Coating of K-Modified Spinel MnCo2O4 Oxide and Their Catalytic Performances for Soot Combustion
Abstract
:1. Introduction
2. Results
2.1. Catalytic Performance of As-Prepared Monolith Catalysts for Soot Combustion
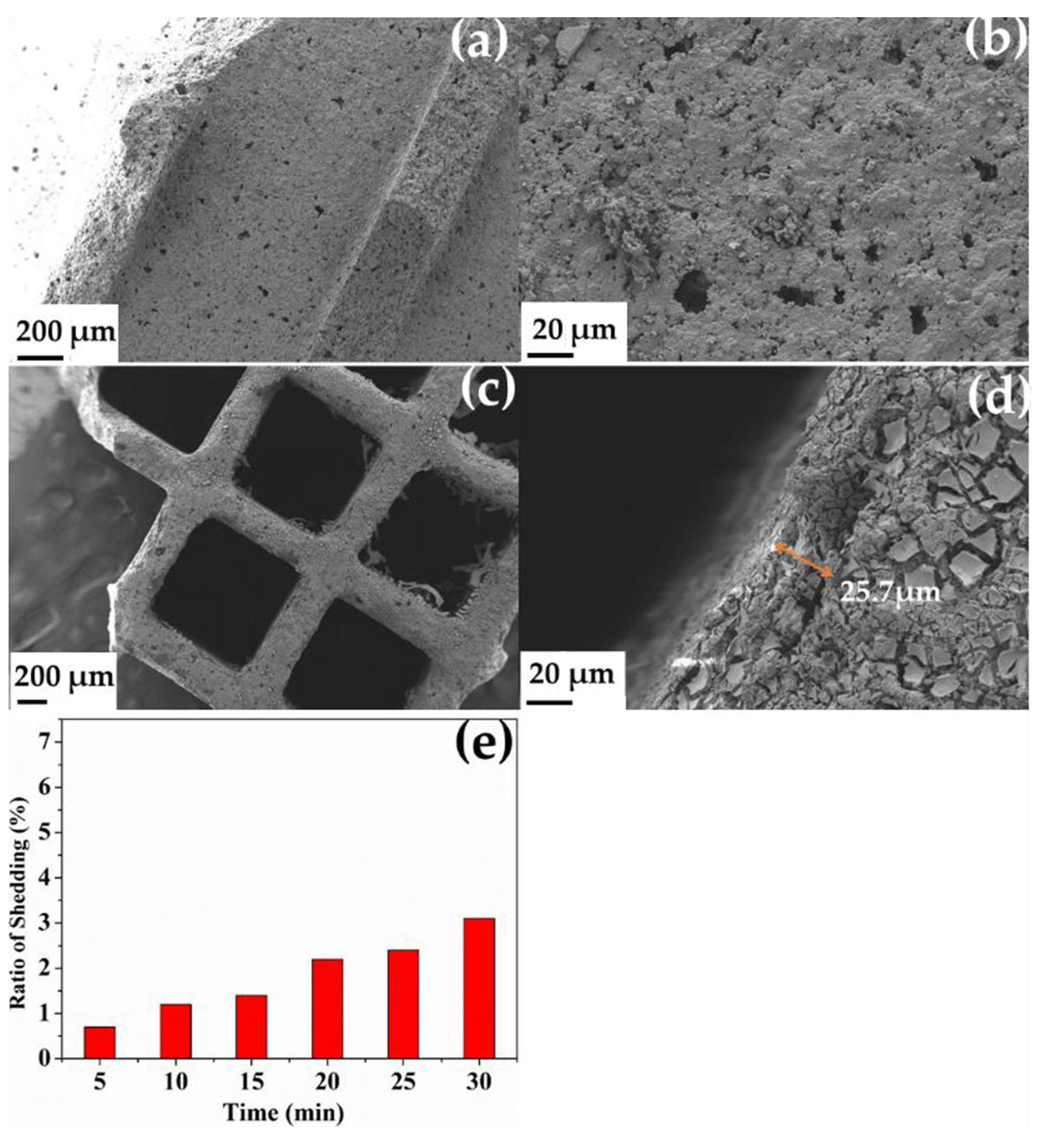
2.2. Resistance versus Sulfur and Water and Stability Test on Monolith Catalysts
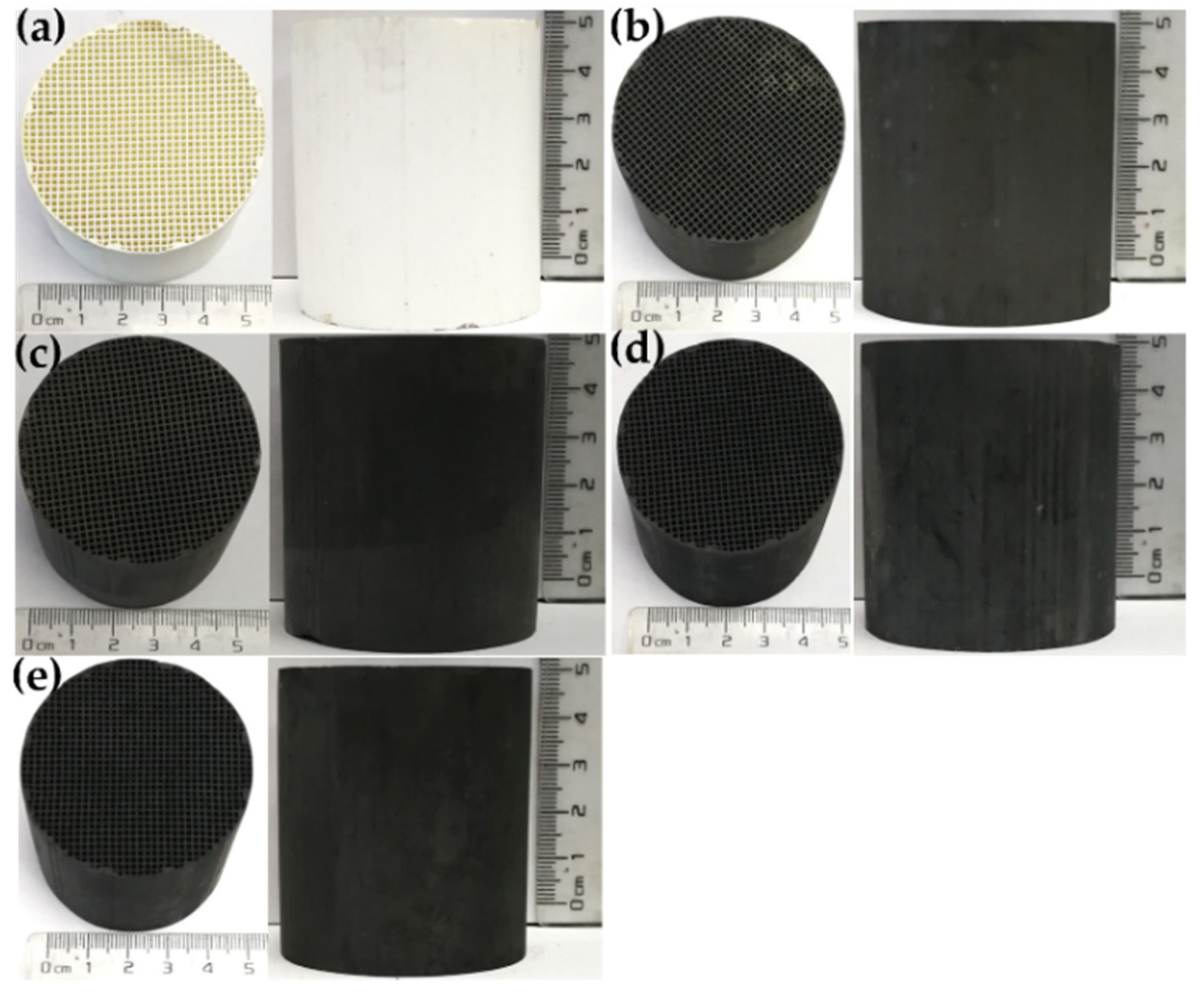
2.3. Morphologies of As-Prepared Monolith Catalysts
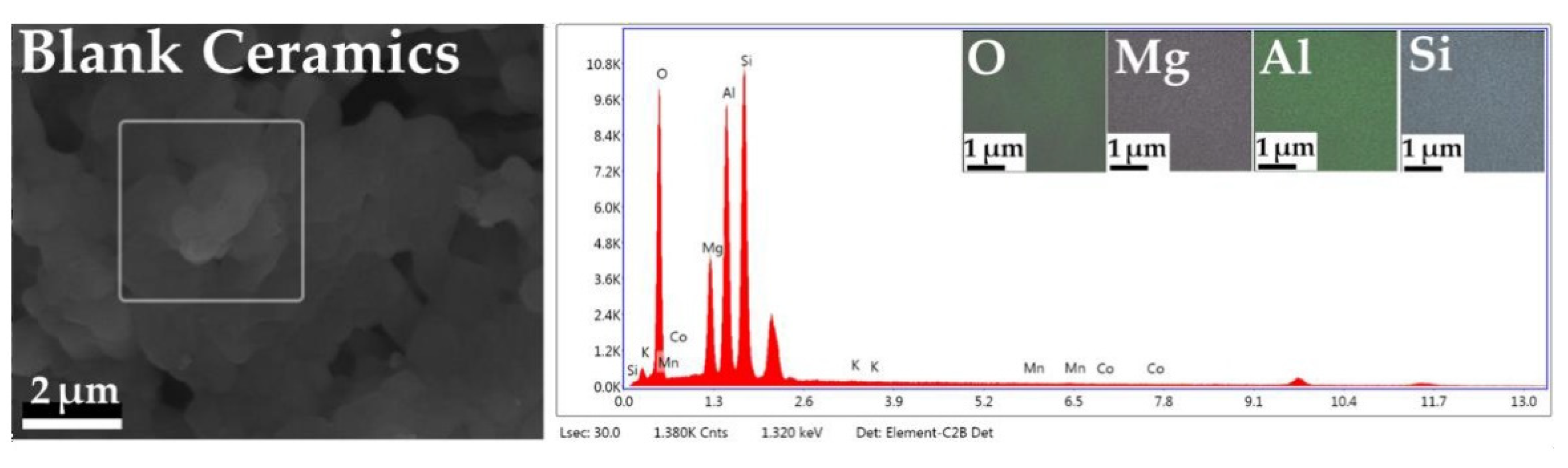
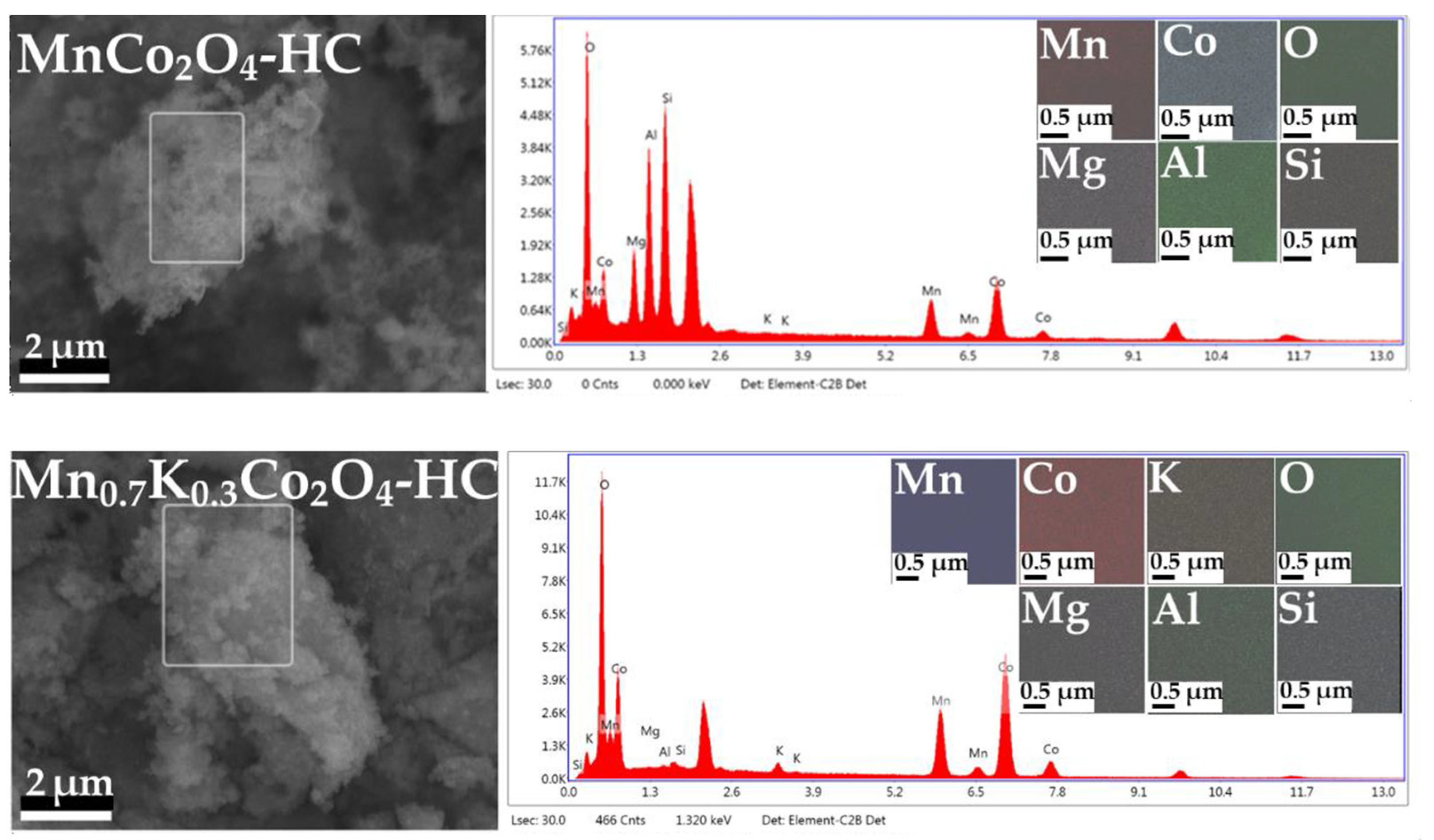
2.4. EDS Mapping and X-ray Fluorescence of As-Prepared Monolith Catalysts
2.5. XRD Patterns of As-Prepared Monolith Catalysts
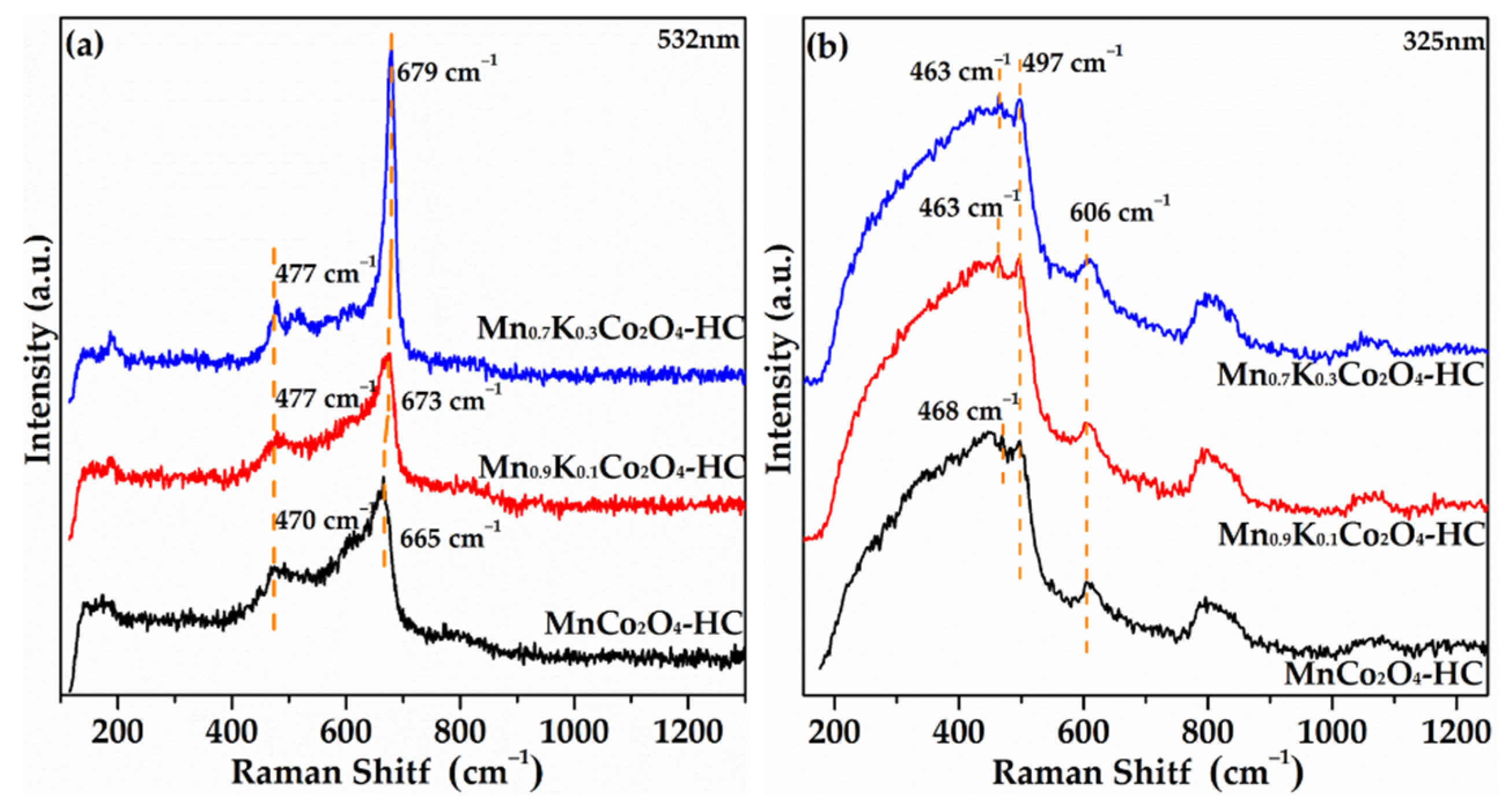
2.6. Raman Spectra of As-Prepared Monolith Catalysts
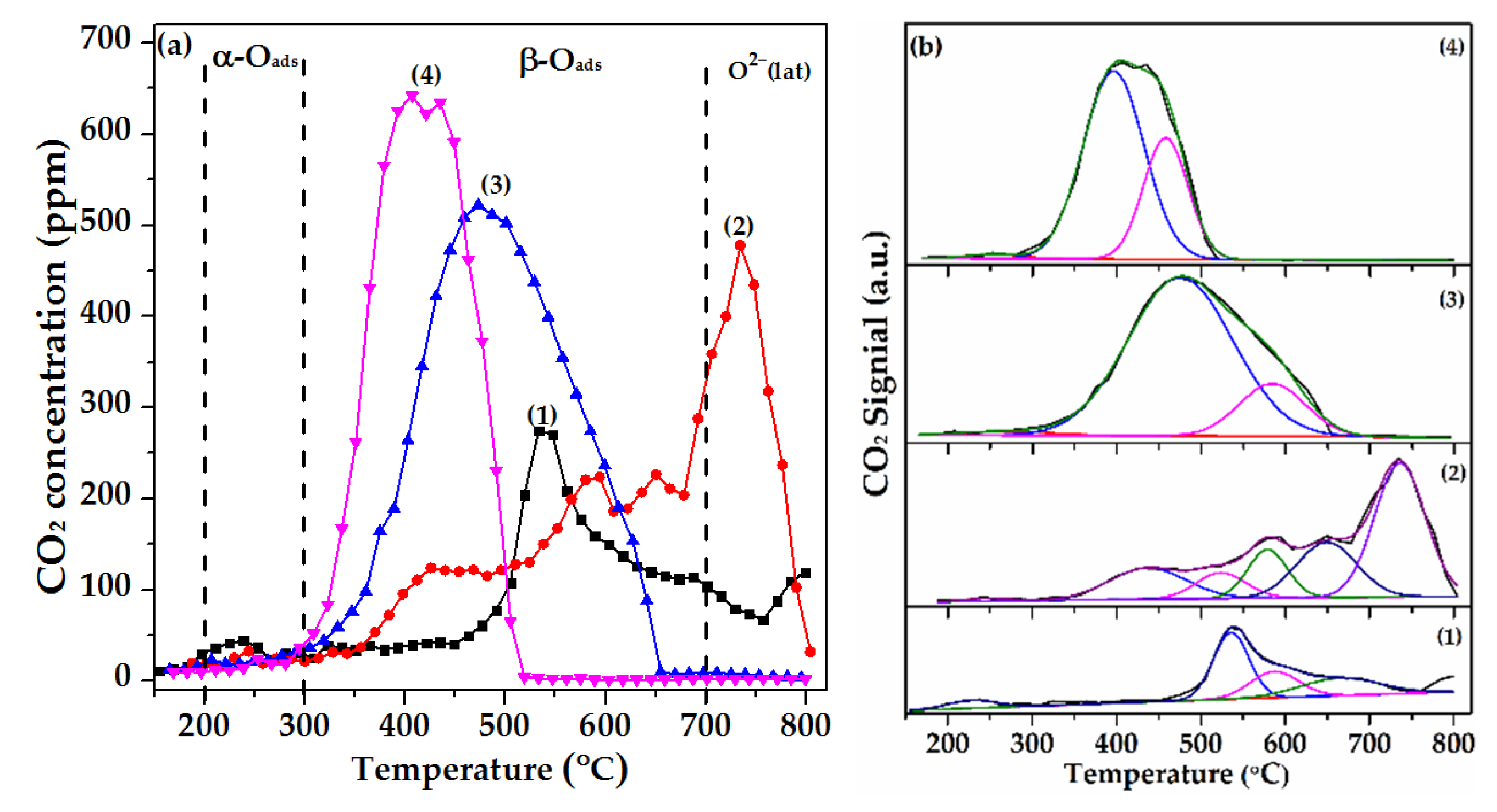
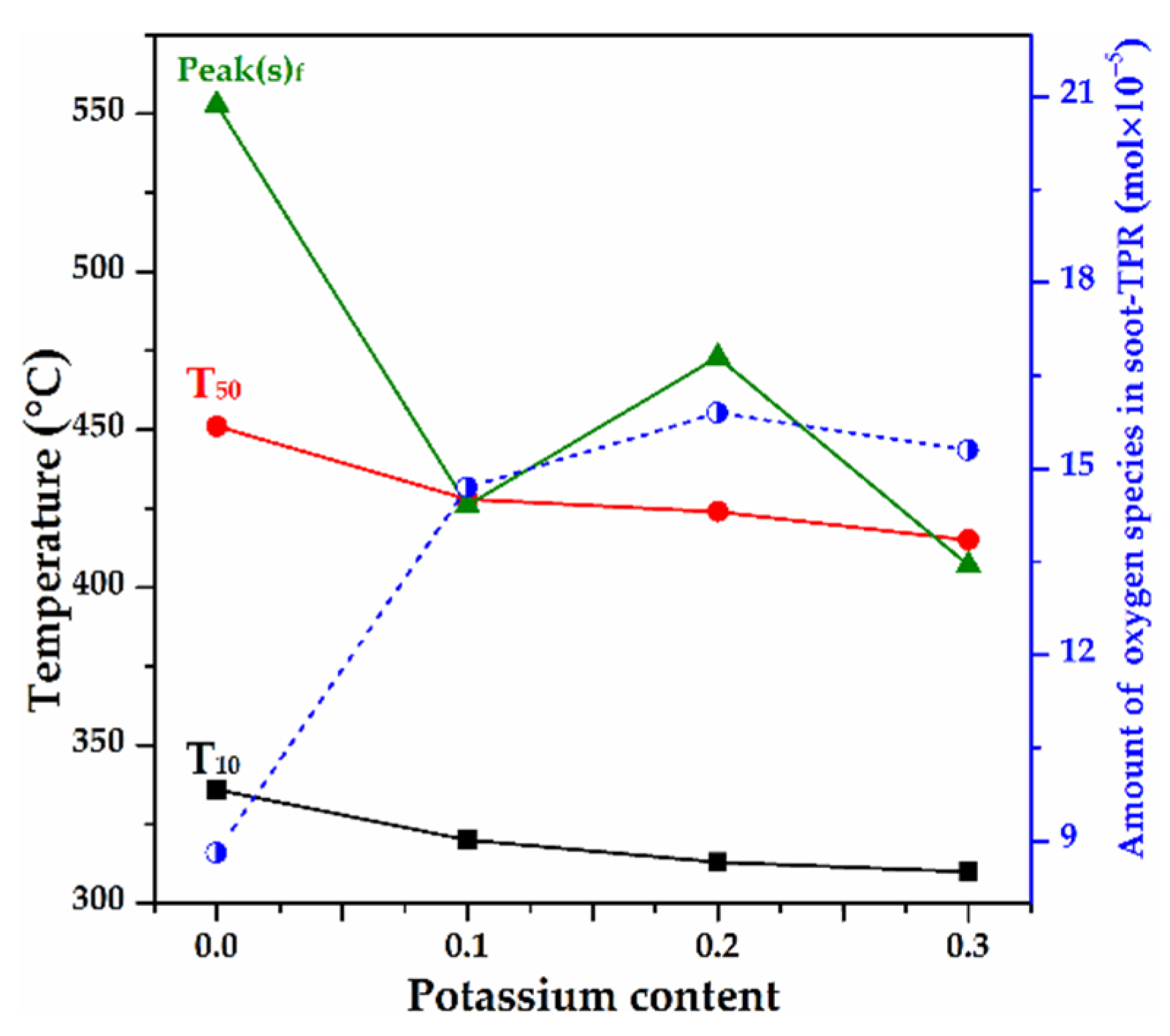
2.7. Soot-TPR
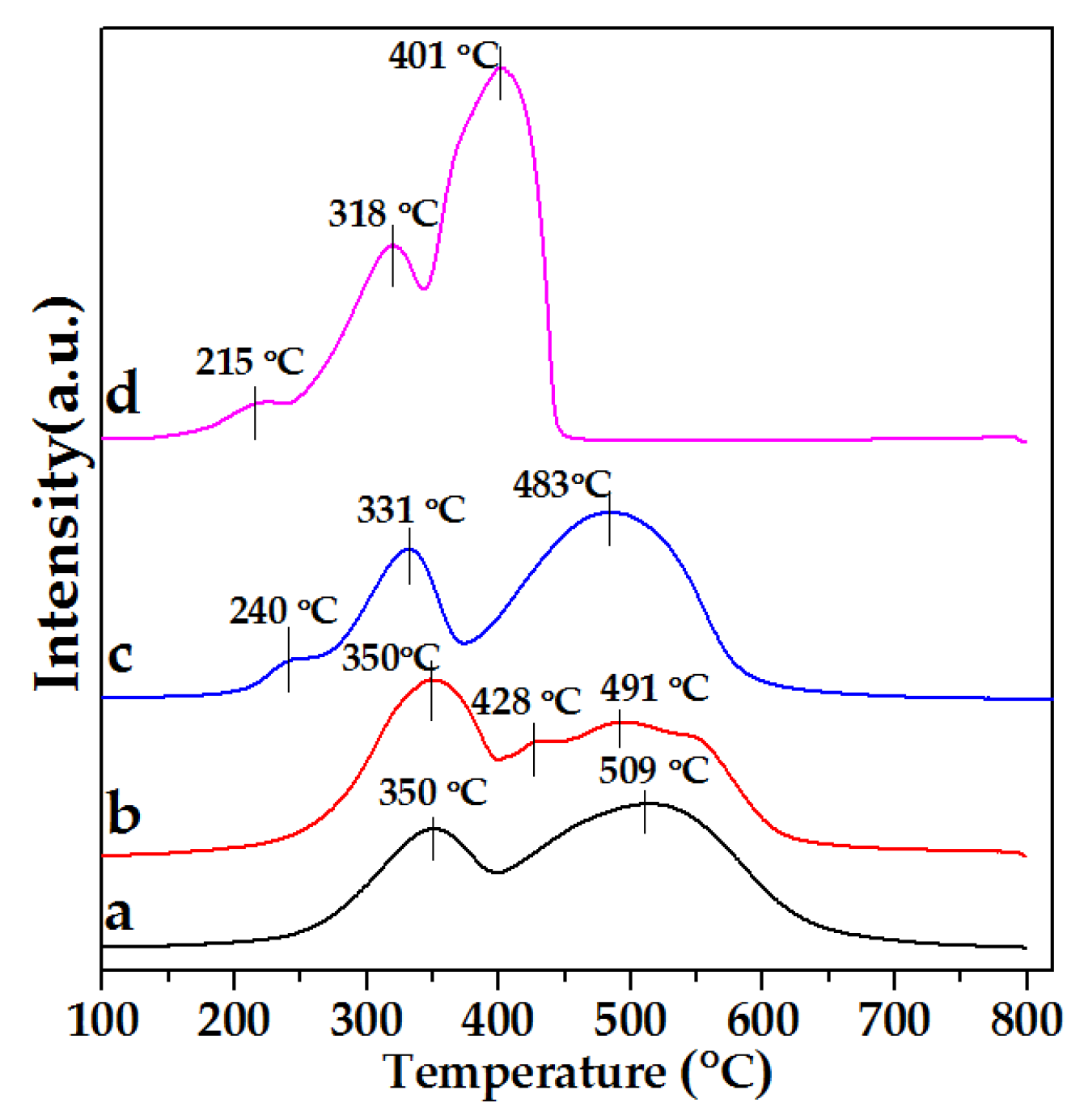
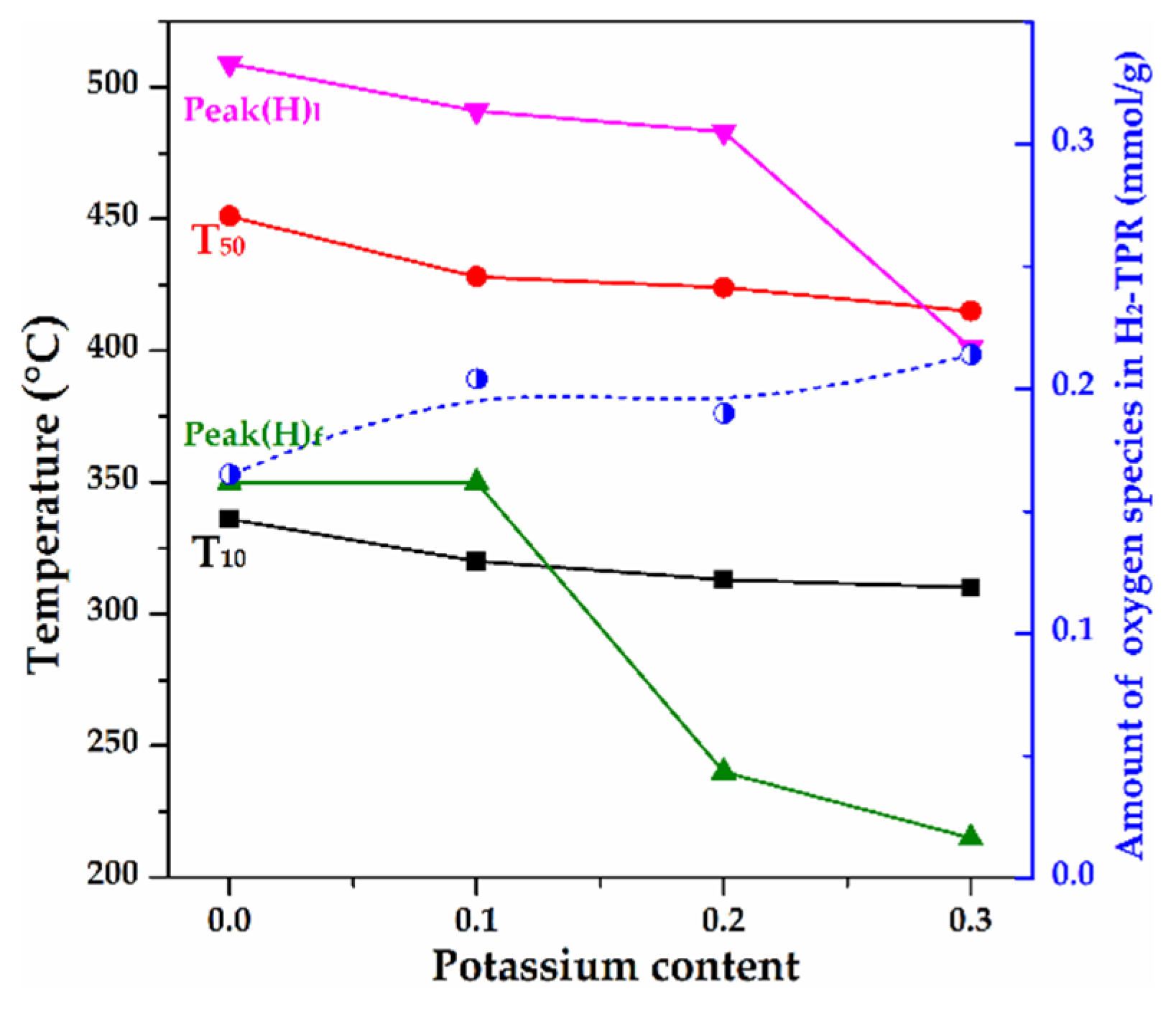
2.8. H2-TPR of As-Prepared Monolith Catalysts
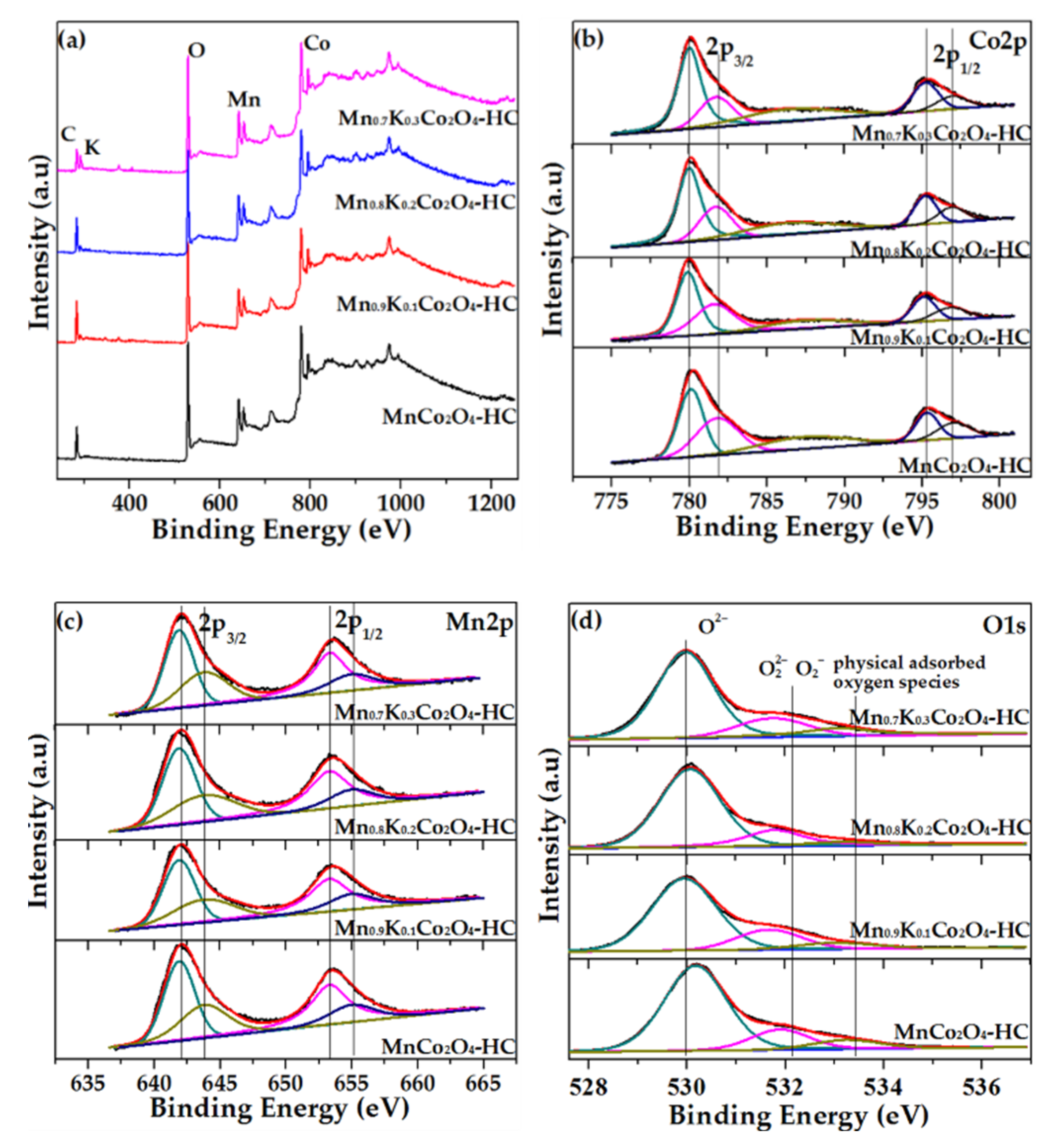
2.9. XPS Results of As-Prepared Monolith Catalysts
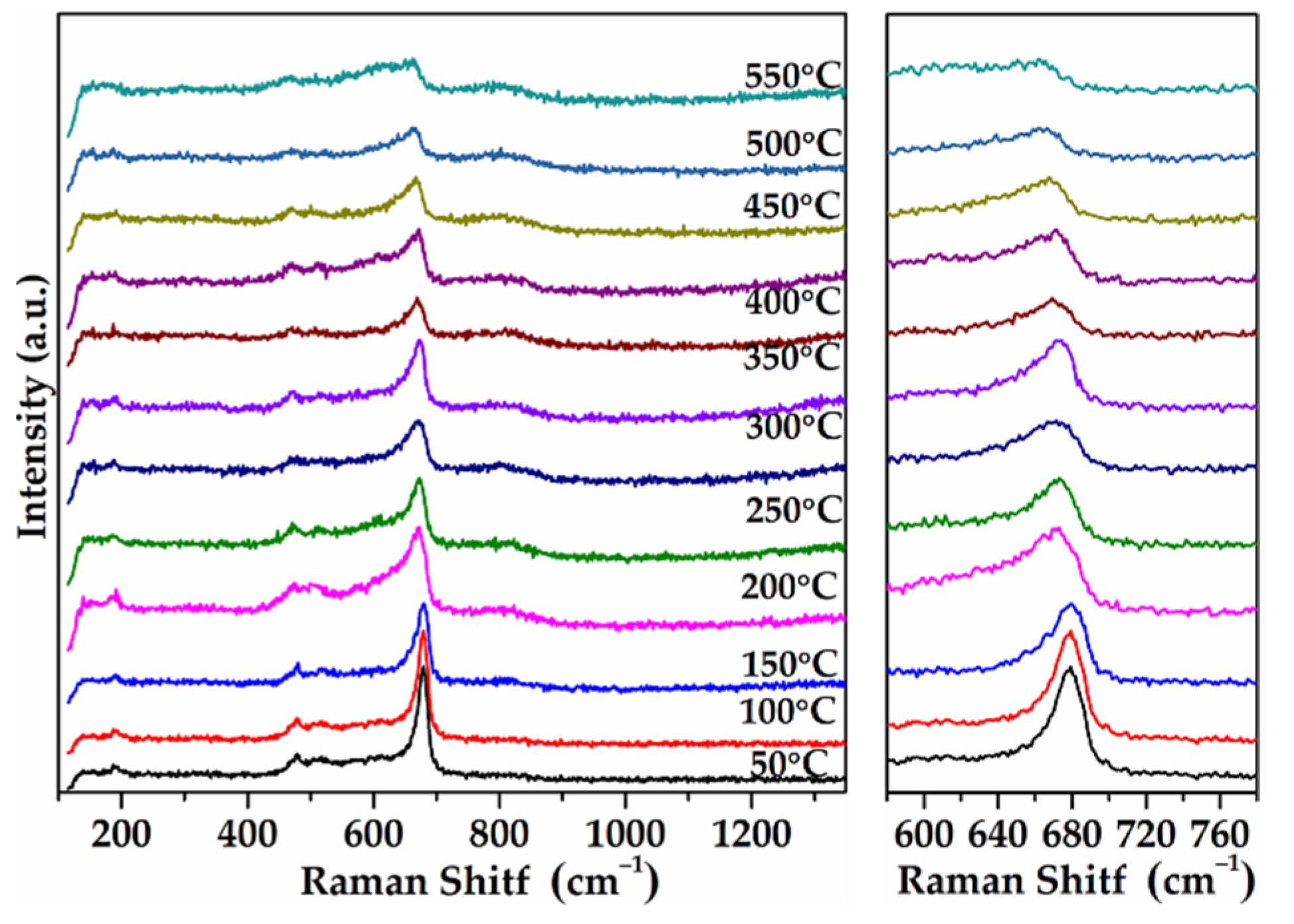
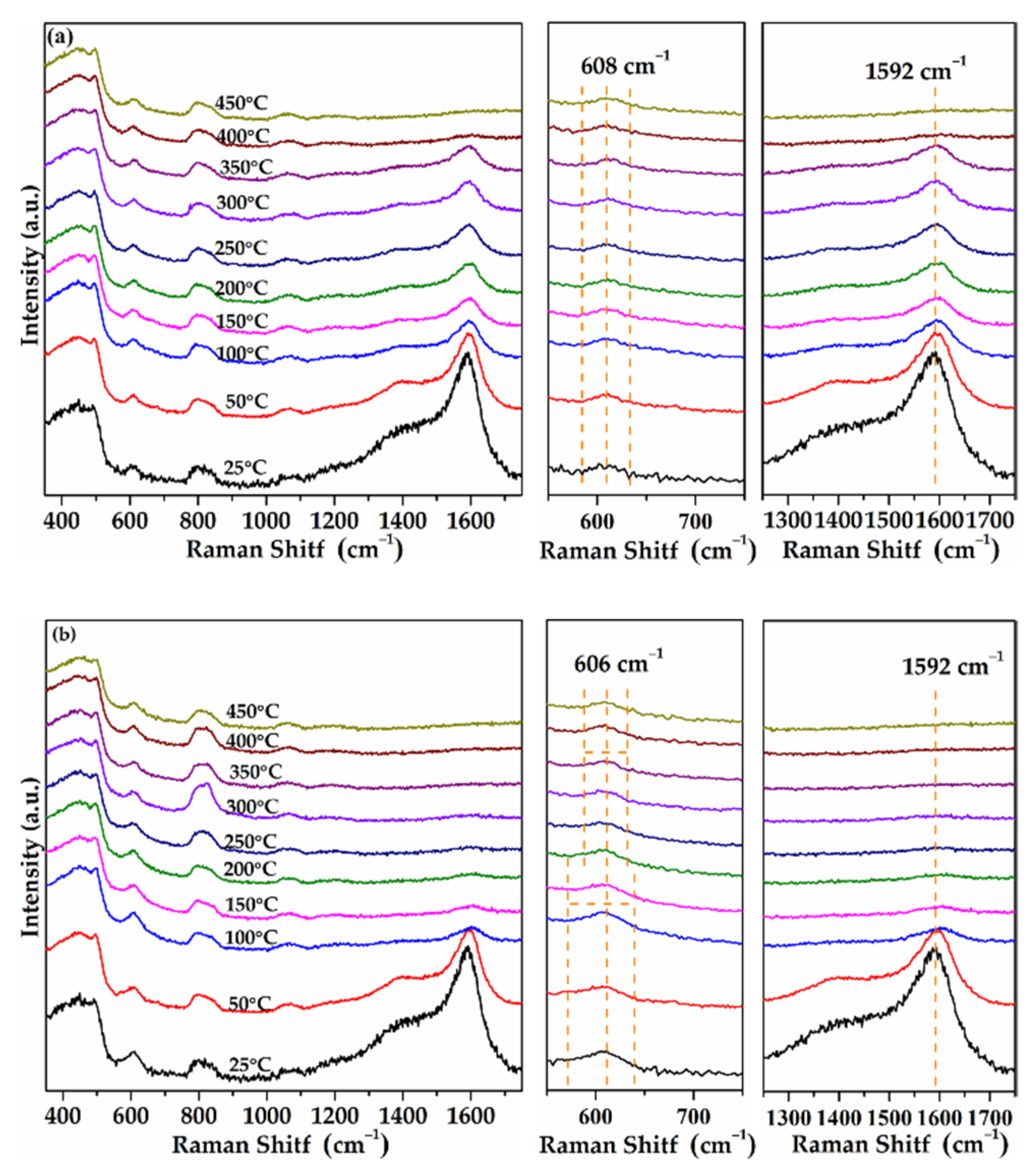
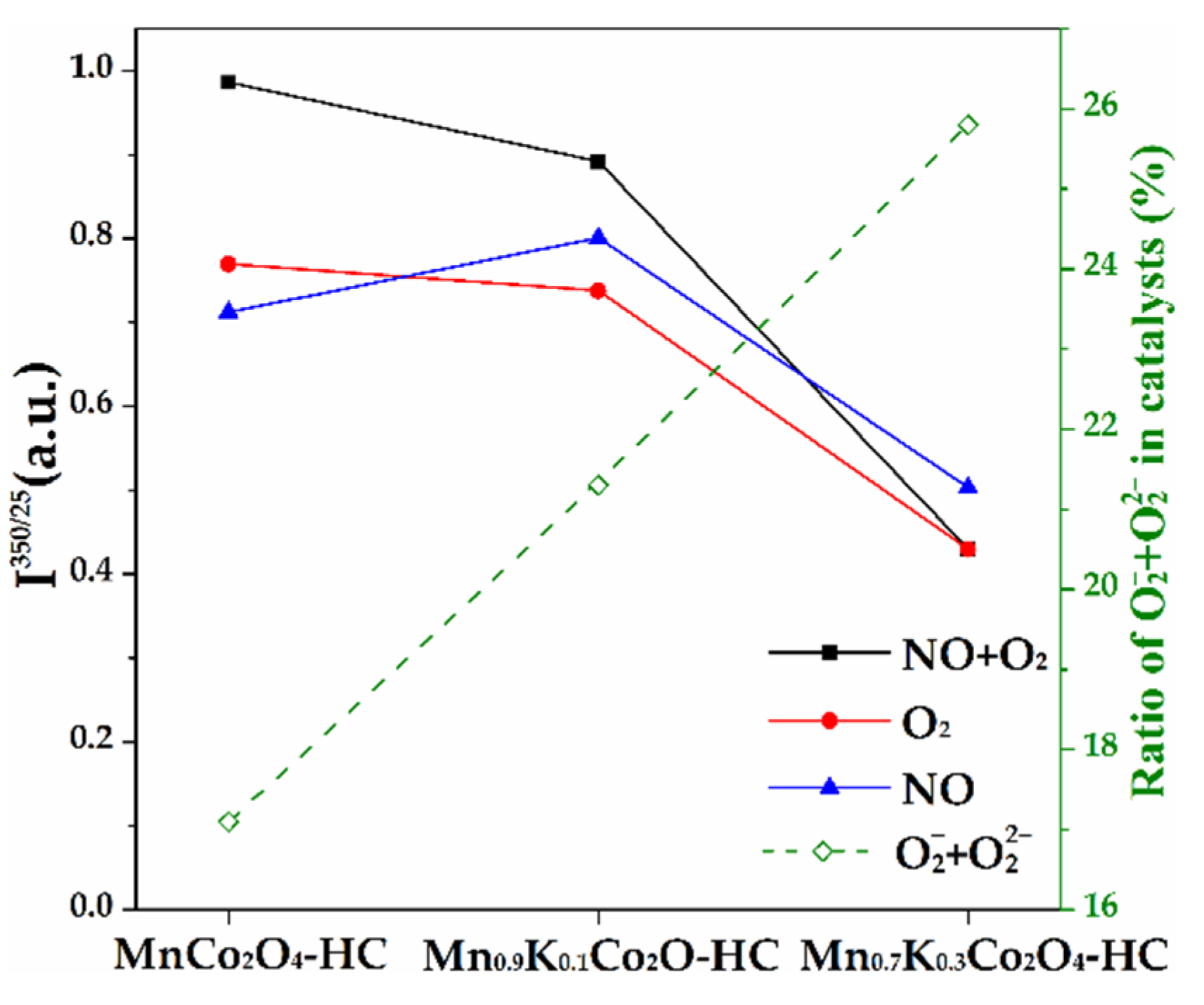
2.10. In-Situ Raman Results of As-Prepared Monolith Catalysts
3. Discussion
3.1. Influencing Factors of Structure for Monolith Catalytic Activity
3.2. The Influence of K Substitution on Monolith Catalytic Performance and Physicochemical Properties
4. Materials and Methods
4.1. Catalyst Preparation
4.1.1. Materials
4.1.2. Synthesis of Honeycomb Ceramics Monolith Catalysts with the Coating of Mn1−nKnCo2O4
4.2. Physical and Chemical Characterizations
4.3. Activity Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Twigg, M.V. Progress and future challenges in controlling automotive exhaust gas emissions. Appl. Catal. B Environ. 2007, 7, 2–15. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Ran, R. Ceria-Based Catalysts for Soot Oxidation: A Review. J. Rare Earths 2015, 33, 567–590. [Google Scholar] [CrossRef]
- Duvvuri, P.P.; Sukumaran, S.; Shrivastava, R.K.; Sreedhara, S. Modeling soot particle size distribution in diesel engines. Fuel 2019, 243, 70–78. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.; Duan, A.; Jiang, G. Comparative Study on Physicochemical Properties and Combustion Behaviors of Diesel Particulates and Model Soot. Energ. Fuels 2010, 24, 3778–3783. [Google Scholar] [CrossRef]
- Johnson, T.V. Review of diesel emissions and control. Int. J. Engine. Res. 2009, 10, 275–285. [Google Scholar] [CrossRef]
- Sadakane, M.; Asanuma, T.; Kubo, J.; Ueda, W. Facile Procedure to Prepare Three-Dimensionally Ordered Macroporous (3DOM) Perovskite-type Mixed Metal Oxides by Colloidal Crystal Templating Method. Chem. Mater. 2005, 17, 3546–3551. [Google Scholar] [CrossRef]
- Sadakane, M.; Takahashi, C.; Kato, N.; Asanuma, T.; Ihara, H.; Ueda, W. Three-dimensionally ordered macroporous mixed iron oxide; Preparation and structural characterization of inverse opals with skeleton structure. Chem. Lett. 2006, 25, 480–481. [Google Scholar] [CrossRef]
- Sadakane, M.; Takahashi, C.; Kato, N.; Ogihara, H.; Nodasaka, Y.; Doi, Y.; Hinatsu, Y.; Ueda, W. Three-dimensionally ordered macroporous (3DOM) materials of spinel-type mixed iron oxides. Synthesis, structural characterization, and formation mechanism of inverse opals with a skeleton structure. Bull. Chem. Soc. Jpn. 2007, 80, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Zhai, G.; Wang, J.; Chen, Z.; Yang, S.; Men, Y. Highly enhanced soot oxidation activity over 3DOM Co3O4-CeO2 catalysts by synergistic promoting effect. J. Hazard. Mater. 2019, 363, 214–226. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Liu, J.; Jiang, G.; Duan, A.; Zheng, J.; Chen, S.; Zhou, R. Three dimensionally ordered macroporous Ce1-xZrxO2 solid solutions for diesel soot combustion. Chem. Commun. 2010, 46, 457–459. [Google Scholar] [CrossRef]
- Sarli, D.V.; Landi, G.; Lisi, L.; Benedetto, A.D. Ceria-Coated Diesel Particulate Filters for Continuous Regeneration. AIChE J. 2017, 63, 3442–3449. [Google Scholar] [CrossRef]
- Sarli, V.D.; Landi, G.; Lisi, L.; Saliva, A.; Benedetto, A.D. Catalytic diesel particulate filters with highly dispersed ceria: Effect of the soot-catalyst contact on the regeneration performance the soot-catalyst contact on the regeneration performance. Appl. Catal. B Environ. 2016, 197, 116–124. [Google Scholar] [CrossRef]
- Sarli, V.D.; Landi, G.; Benedetto, A.D.; Lisi, L. Synergy Between Ceria and Metals (Ag or Cu) in Catalytic Diesel Particulate Filters: Efect of the Metal Content and of the Preparation Method on the Regeneration Performance. Top. Catal. 2021, 64, 256–269. [Google Scholar] [CrossRef]
- Khan, A.U.; Ullah, S.; Yuan, Q.; Ali, S.; Ahmad, A.; Khan, Z.U.H.; Rahman, A.U. In Situ Fabrication of Au–CoFe2O4: An Efficient Catalyst for Soot Oxidation. Appl. Nanosci. 2020, 10, 3901–3910. [Google Scholar] [CrossRef]
- Zou, G.; Fan, Z.; Yao, X.; Zhang, Y.; Zhang, Z.; Chen, M.; Shangguan, W. Catalytic Performance of Ag/Co-Ce Composite Oxides During Soot Combustion in O2 and NOx: Insights into the Effects of Silver. Chin. J. Catal. 2017, 38, 564–572. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, W.; Wu, X.; Liu, S.; Weng, D.; Ran, R. Controllable Synthesis of Supported Platinum Catalysts: Acidic Support Effect and Soot Oxidation Catalysis. Catal. Sci. Technol. 2017, 7, 3268–3274. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Liu, S.; Ogura, M.; Liu, M.; Weng, D. Aggregation and Redispersion of Silver Species on Alumina and Sulphated Alumina Supports for Soot Oxidation. Catal. Sci. Technol. 2017, 7, 3524–3530. [Google Scholar] [CrossRef]
- Milt, V.G.; Ulla, M.A.; Miró, E.E. NOx Trapping and Soot Combustion on BaCoO3−y Perovskite: LRS and FTIR Characterization. Appl. Catal. B Environ. 2005, 57, 13–21. [Google Scholar] [CrossRef]
- Feng, N.J.; Meng, J.; Wu, Y.; Chen, C.; Wang, L.; Gao, L.; Wan, H.; Guan, G.F. KNO3 Supported on Three-Dimensionally Ordered Macroporous La0.8Ce0.2Mn1−xFexO3 for Soot Removal. Catal. Sci. Technol. 2016, 6, 2930–2941. [Google Scholar] [CrossRef]
- Shangguan, W.F.; Teraoka, Y.; Kagawa, S. Promotion Effect of Potassium on the Catalytic Property of CuFe2O4 for the Simultaneous Removal of NOx and Diesel Soot Particulate. Appl. Catal. B Environ. 1998, 16, 149–154. [Google Scholar] [CrossRef]
- Shangguan, W.F.; Teraoka, Y.; Kagawa, S. Kinetics of Soot-O2, Soot-No and Soot-O2-NO Reactions over Spinel-Type CuFe2O4 Catalyst. Appl. Catal. B Environ. 1997, 12, 237–247. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, Z.; Huang, Y.; Duan, X.; Cao, Y.; Ye, L.; Jiang, L.; Yuan, Y. Low-Temperature Soot Combustion over Ceria Modified MgAl2O4-Supported Ag Nanoparticles. Catal. Commun. 2018, 111, 26–30. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, X.X.; Pan, L.Y.; Wang, M.; Chen, H.R.; Shi, J.L. Facile Synthesis of Spinel Cu1.5Mn1.5O4 Microspheres with High Activity for the Catalytic Combustion of Diesel Soot. RSC Adv. 2017, 7, 20451–20459. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shen, G.; Lang, Y.; Chen, R.; Jia, L.; Yue, J.; Shen, M.; Du, C.; Shan, B. Promoting Soot Combustion Efficiency by Strengthening the Adsorption of NOx on the 3DOM Mullite Catalyst. J. Catal. 2020, 384, 96–105. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, Q.; Chen, Y.; Zhao, P.; Peng, Q.; Cao, K.; Chen, R.; Shen, M.; Shan, B. Macroporous SmMn2O5 Mullite for NOx-Assisted Soot Combustion. Catal. Sci. Technol. 2017, 7, 838–847. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, J.; Zhao, Z.; Duan, A.; Jiang, G.; Xu, C.; Gao, J.; He, H.; Wang, X. Three-Dimensionally Ordered Macroporous Ce0.8Zr0.2O2-Supported Gold Nanoparticles: Synthesis with Controllable Size and Super-Catalytic Performance for Soot Oxidation. Energy Environ. Sci. 2011, 4, 2959–2970. [Google Scholar] [CrossRef]
- Wei, Y.C.; Liu, J.A.; Zhao, Z.; Chen, Y.S.; Xu, C.M.; Duan, A.J.; Jiang, G.Y.; He, H. Highly Active Catalysts of Gold Nanoparticles Supported on Three-Dimensionally Ordered Macroporous LaFeO3 for Soot Oxidation. Angew. Chem.-Int. 2011, 50, 2326–2329. [Google Scholar] [CrossRef]
- Li, Y.Z.; Du, Y.H.; Wei, Y.C.; Zhao, Z.; Jin, B.F.; Zhang, X.D.; Liu, J. Catalysts of 3d Ordered Macroporous ZrO2-Supported Core-Shell Pt@CeO2−x Nanoparticles: Effect of the Optimized Pt-CeO2 Interface on Improving the Catalytic Activity and Stability of Soot Oxidation. Catal. Sci. Technol. 2017, 7, 968–981. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.-m.; Duan, A.J.; Meng, T.; Bao, X.J. Simultaneous Removal of NOx and Diesel Soot Particulates over Nanometric La2−xKxCuO4 Complex Oxide Catalysts. Catal. Today 2007, 119, 267–272. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Chen, H.; Xin, Y.; Tian, G.; Lu, C.; Zhang, Z.; Zheng, L.; Zheng, L. K-Supported Catalysts for Diesel Soot Combustion: Making a Balance between Activity and Stability. Catal. Today 2016, 264, 171–179. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, X.; Huang, W.; Pan, L.; Wang, M.; Li, Q.; Shi, J.; Chen, H. Effect of Potassium Nitrate Modification on the Performance of Copper-Manganese Oxide Catalyst for Enhanced Soot Combustion. ChemCatChem 2018, 10, 1455–1463. [Google Scholar] [CrossRef]
- Milt, V.G.; Pissarello, M.L.; Miró, E.E.; Querini, C.A. Abatement of Diesel-Exhaust Pollutants: NOx Storage and Soot Combustion on K/La2O3 Catalysts. Appl. Catal. B Environ. 2003, 41, 397–414. [Google Scholar] [CrossRef]
- Pisarello, M.L.; Milt, V.; Peralta, M.A.; Querini, C.A.; Miró, E.E. Simultaneous Removal of Soot and Nitrogen Oxides from Diesel Engine Exhausts. Catal. Today 2002, 75, 465–470. [Google Scholar] [CrossRef]
- Jiménez, R.; García, X.; Cellier, C.; Ruiz, P.; Gordon, A.L. Soot Combustion with K/MgO as Catalyst. Appl. Catal. A Gen. 2006, 297, 125–134. [Google Scholar] [CrossRef]
- Carrascull, A.; Lick, I.D.; Ponzi, E.N.; Ponzi, M.I. Catalytic Combustion of Soot with a O2/NO Mixture. KNO3/ZrO2 Catalysts. Catal. Commun. 2003, 4, 124–128. [Google Scholar] [CrossRef]
- An, H.; Kilroy, C.; McGinn, P.J. Combinatorial Synthesis and Characterization of Alkali Metal Doped Oxides for Diesel Soot Combustion. Catal. Today 2004, 98, 423–429. [Google Scholar] [CrossRef]
- An, H.; McGinn, P.J. Catalytic Behavior of Potassium Containing Compounds for Diesel Soot Combustion. Appl. Catal. B Environ. 2006, 62, 46–56. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, Z.; Wei, Y.; Liu, J.; Peng, Y.; Li, K. Study on the Coating of Nano-Particle and 3DOM LaCoO3 Perovskite-Type Complex Oxide on Cordierite Monolith and the Catalytic Performances for Soot Oxidation: The Effect of Washcoat Materials of Alumina, Silica and Titania. Catal. Today 2017, 297, 131–142. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, Z.; Li, K.; Yu, X.; Wei, Y.; Liu, J.; Peng, Y.; Li, Y.; Chen, Y. Highly Active Monolith Catalysts of LaKCoO3 Perovskite-Type Complex Oxide on Alumina-Washcoated Diesel Particulate Filter and the Catalytic Performances for the Combustion of Soot. Catal. Today 2020, 339, 159–173. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, D.; Gao, Z.; Tian, Y.; Ding, T.; Zhang, J.; Jiang, Z.; Li, X. Interface Interaction Induced Oxygen Activation of Cactus-Like Co3O4/Oms-2 Nanorod Catalysts in Situ Grown on Monolith Cordierite for Diesel Soot Combustion. Appl. Catal. B Environ. 2021, 286, 119932–119942. [Google Scholar] [CrossRef]
- Nascimento, L.F.; Serra, O.A. Washcoating of Cordierite Honeycomb with Ceria-Copper Mixed Oxides for Catalytic Diesel Soot Combustion. Process Saf. Environ. 2016, 101, 134–143. [Google Scholar] [CrossRef]
- Hernandez-Garrido Juan, C.; Gomez Diana, M.; Gaona, D.; Vidal, H.; Gatica José, M.; Sanz, O.; Rebled José, M.; Peiro, F.; Calvino José, J. Combined (S)TEM-FIB Insight into the Influence of the Preparation Method on the Final Surface Structure of a Co3O4/La-Modified-CeO2 Washcoated Monolith Catalyst. J. Phys. Chem. C. 2013, 117, 13028–13036. [Google Scholar] [CrossRef]
- Hernández-Giménez, A.M.; Lozano-Castelló, D.; Bueno-López, A. Effect of CO2, H2O and SO2 in the Ceria-Catalyzed Combustion of Soot under Simulated Diesel Exhaust Conditions. Appl. Catal. B Environ. 2014, 148-149, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Lei, Z. Incorporation of CoO Nanoparticles in 3D Marigold Flower-Like Hierarchical Architecture MnCo2O4 for Highly Boosting Solar Light Photo-Oxidation and Reduction Ability. Appl. Catal. B Environ. 2018, 237, 1–8. [Google Scholar] [CrossRef]
- Liang, Q.; Qu, X.; Bia, N.; Chen, H.; Zou, X.; Li, G. Alkali metal-incorporated spinel oxide nanofibers enable high performance detection of formaldehyde at ppb level. J. Hazard. Mater. 2020, 400, 123301–123309. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Zhang, T.; Luo, Y.; Lan, Z.; Zhang, K.; Zuo, J.; Jiang, L.; Wang, R. Geometrical-Site-Dependent Catalytic Activity of Ordered Mesoporous Co-Based Spinel for Benzene Oxidation: In Situ Drifts Study Coupled with Raman and XAFS Spectroscopy. ACS Catal. 2017, 7, 1626–1636. [Google Scholar] [CrossRef]
- Bai, B.; Li, J. Positive Effects of K+ Ions on Three-Dimensional Mesoporous Ag/Co3O4 Catalyst for HCHO Oxidation. ACS Catal. 2014, 4, 2753–2762. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Vignesh, A.; Shanmugam, S.; Kalai Selvan, R. Nitrogen-Doped Multi-Walled Carbon Nanotubes-MnCo2O4 Microsphere as Electrocatalyst for Efficient Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2016, 41, 15199–15207. [Google Scholar] [CrossRef]
- Xiao, B.; Zhao, K.; Zhang, L.; Cai, T.; Zhang, X.; Wang, Z.; Yuan, J.; Yang, L.; Gao, P.; He, D. A Green and Facile Synthesis of Co3O4 Monolith Catalyst with Enhanced Total Oxidation of Propane Performance. Catal. Commun. 2018, 116, 1–4. [Google Scholar] [CrossRef]
- Hastuti, E.; Subhan, A.; Amonpattaratkit, P.; Zainuri, M.; Suasmoro, S. The Effects of Fe-Doping on MnO2: Phase Transitions, Defect Structures and Its Influence on Electrical Properties. RSC Adv. 2021, 11, 7808–7823. [Google Scholar] [CrossRef]
- Fernandez, J.F.; Caballero, A.C.; Villegas, M.; Khatib, S.J.; Banares, M.A.; Fierro, J.L.G.; Costa-Kramer, J.L.; Lopez-Ponce, E.; Mart’ ın-Gonzalez, M.S.; Briones, F.; et al. Structure and Magnetism in the Zn–Mn–O System: A Candidate for Room Temperature Ferromagnetic Semiconductor. J. Eur. Ceram. Soc. 2006, 26, 3017–3025. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M.; Poinsignon, C. Lattice Vibrations of Manganese Oxides: Part I. Periodic Structures. Spectrochim. Acta A 2004, 60, 689–700. [Google Scholar] [CrossRef]
- Fu, F.; Zhang, Y.; Zhang, Y.; Chen, Y. Synthesis of Mn-Doped and Anatase/Rutile Mixed-Phase TiO2 Nanofibers for High Photoactivity Performance. Catal. Sci. Technol. 2021, 11, 4181–4195. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.-m.; Duan, A.J. Simultaneous Removal of NOx and Diesel Soot over Nanometer Ln-Na-Cu-O Perovskite-Like Complex Oxide Catalysts. Appl. Catal. B Environ. 2008, 78, 61–72. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Li, Q.; Wang, Z.; Zhang, Z. Identification of Active Oxygen Species for Soot Combustion on LaMnO3 Perovskite. Catal. Sci. Technol. 2012, 2, 1822–1824. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Cheng, L.; Shin, E.W.; Men, Y. Three-Dimensionally Ordered Macroporous Spinel-Type MCr2O4 (M = Co, Ni, Zn, Mn) Catalysts with Highly Enhanced Catalytic Performance for Soot Combustion. Catal. Sci. Technol. 2015, 5, 4594–4601. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, H.; Lu, G.; Wang, P.; Xing, L.; Wang, J.; Wang, G. Enhanced Water Splitting Electrocatalysis over MnCo2O4 Via Introduction of Suitable Ce Content. ACS Sustain. Chem. Eng. 2019, 7, 1169–1177. [Google Scholar] [CrossRef]
- Qiu, M.; Zhan, S.; Yu, H.; Zhu, D.; Wang, S. Facile Preparation of Ordered Mesoporous MnCo2O4 for Low-Temperature Selective Catalytic Reduction of NO with NH3. Nanoscale 2015, 7, 2568–2577. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, Q.; Mei, X.; Liu, J.; Wei, Y.; Zhao, Z.; Wu, D.; Li, J. Fabrication of Spinel-Type PdxCo3-xO4 Binary Active Sites on 3D Ordered Meso-Macroporous Ce-Zr-O2 with Enhanced Activity for Catalytic Soot Oxidation. ACS Catal. 2018, 8, 7915–7930. [Google Scholar] [CrossRef]
- Cai, N.; Fu, J.; Chan, V.; Liu, M.; Chen, W.; Wang, J.; Zeng, H.; Yu, F. MnCo2O4@Nitrogen-Doped Carbon Nanofiber Composites with Meso-Microporous Structure for High-Performance Symmetric Supercapacitors. J. Alloy Compd. 2019, 782, 251–262. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, J.; Yang, B.; Shi, X.; Lu, C.; Yin, J.; Yu, Y.; Hu, X. Peanut Shaped MnCo2O4 Winded by Multi-Walled Carbon Nanotubes as an Efficient Cathode Catalyst for Li-O2 Batteries. J. Alloy Compd. 2018, 749, 433–440. [Google Scholar] [CrossRef]
- Zou, G.; Xu, Y.; Wang, S.; Chen, M.; Shangguan, W. The synergistic effect in Co-Ce oxides for catalytic oxidation of diesel soot. Catal. Sci. Technol. 2015, 5, 1084–1092. [Google Scholar] [CrossRef]
- Lunsford, J.H.; Yang, X.; Haller, K.; Laane, J.; Mestl, G.; Knoezinger, H. In Situ Raman Spectroscopy of Peroxide Ions on Barium/Magnesium Oxide Catalysts. J. Phys. Chem. 1993, 97, 13810–13813. [Google Scholar] [CrossRef]
- Li, M.; Feng, Z.; Xiong, G.; Ying, P.; Xin, Q.; Li, C. Phase Transformation in the Surface Region of Zirconia Detected by UV Raman Spectroscopy. J. Phys. Chem. B 2001, 105, 8107–8111. [Google Scholar] [CrossRef]
- Cao, C.; Xing, L.; Yang, Y.; Tian, Y.; Ding, T.; Zhang, J.; Hu, T.; Zheng, L.; Li, X. Diesel Soot Elimination over Potassium-Promoted Co3O4 Nanowires Monolithic Catalysts under Gravitation Contact Mode. Appl. Catal. B Environ. 2017, 218, 32–45. [Google Scholar] [CrossRef]
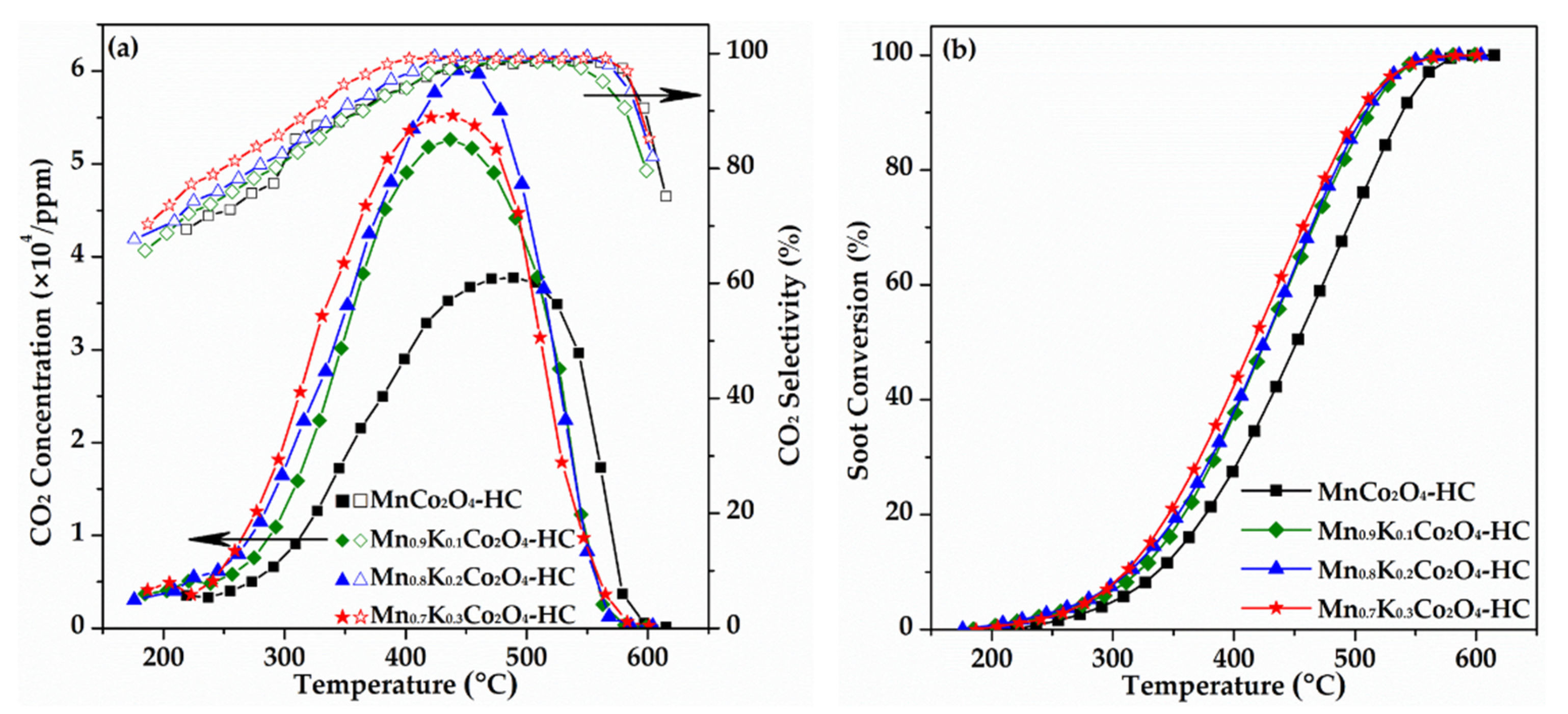


| Title 1 | T10/°C | T50/°C | T90/°C | Tm/°C | |
|---|---|---|---|---|---|
| Soot (without catalyst) | 451 | 562 | 607 | 588 | 38.9 |
| MnCo2O4-HC | 336 | 451 | 538 | 489 | 98.2 |
| Mn0.9K0.1Co2O4-HC | 320 | 428 | 511 | 436 | 97.3 |
| Mn0.8K0.2Co2O4-HC | 313 | 424 | 508 | 442 | 99.4 |
| Mn0.7K0.3Co2O4-HC | 310 | 415 | 504 | 439 | 99.1 |
| Mn0.7K0.3Co2O4-HC-cycle 2 | 311 | 415 | 503 | 429 | 98.9 |
| Mn0.7K0.3Co2O4-HC-cycle 3 | 318 | 421 | 506 | 469 | 99.2 |
| Mn0.7K0.3Co2O4-HC-cycle 4 | 311 | 423 | 516 | 453 | 98.2 |
| Mn0.7K0.3Co2O4-HC-cycle 5 | 327 | 431 | 513 | 445 | 98.5 |
| Mn0.7K0.3Co2O4-HC (SO2) | 312 | 414 | 496 | 438 | 99.3 |
| Mn0.7K0.3Co2O4-HC (H2O) | 370 | 480 | 573 | 486 | 98.9 |
| Element | Blank Ceramics (wt %) | MnCo2O4-HC (wt %) | Mn0.7K0.3Co2O4-HC (wt %) |
|---|---|---|---|
| MnO | -- | 0.64 | 0.44 |
| Co3O4 | -- | 0.96 | 0.91 |
| K2O | 0.21 | 0.23 | 0.42 |
| SiO2 | 45.71 | 48.23 | 45.71 |
| Al2O3 | 35.24 | 34.79 | 35.24 |
| MgO | 16.82 | 13.44 | 16.83 |
| other | 2.02 | 1.71 | 0.45 |
| Catalyst | Soot-TPR a | H2-TPR b | ||||||
|---|---|---|---|---|---|---|---|---|
| Peak 1 (°C) | Peak 2 (°C) | Peak 3 (°C) | R1 (mol × 10−5) | Peak 1 (°C) | Peak 2 (°C) | Peak 3 (°C) | R2 (mmol/g) | |
| MnCo2O4 | 533 | 687 | - | 8.81 | 350 | 509 | - | 0.165 |
| Mn0.9K0.1Co2O4 | 426 | 594 | 734 | 14.7 | 350 | 428 | 491 | 0.204 |
| Mn0.8K0.2Co2O4 | 473 | - | - | 16.9 | 240 | 331 | 483 | 0.180 |
| Mn0.7K0.3Co2O4 | 407 | 435 | - | 15.1 | 215 | 318 | 401 | 0.214 |
| Catalysts | Mn Species | Co Species | O Species | K Species | |||||
|---|---|---|---|---|---|---|---|---|---|
| Atomic a | Mn2+b | Mn3+b | Atomic a | Co2+c | Co3+c | O2−+ O22−d | O2−d | Atomic a | |
| MnCo2O4-HC | 15.3% | 62.2% | 37.8% | 15.9% | 48.5 % | 51.5% | 17.1% | 82.9% | |
| Mn0.9K0.1Co2O4-HC | 14.3% | 62.7% | 37.3% | 15.2% | 41.9% | 58.1% | 20.6% | 79.4% | 2.2% |
| Mn0.8K0.2Co2O4-HC | 17.3% | 63.7% | 36.3% | 17.0% | 34.6% | 65.4% | 21.3% | 78.7% | 3.1 % |
| Mn0.7K0.3Co2O4-HC | 14.7% | 64.1% | 35.9% | 15.4% | 30.9% | 69.1% | 25.8% | 74.2% | 4.3% |
| Catalyst | mHC/g | mfre/g | Δm/g |
|---|---|---|---|
| MnCo2O4-HC | 55.67 | 58.29 | 2.62 |
| Mn0.9K0.1Co2O4-HC | 56.31 | 58.78 | 2.47 |
| Mn0.8K0.2Co2O4-HC | 57.39 | 59.92 | 2.53 |
| Mn0.7K0.3Co2O4-HC | 55.65 | 58.24 | 2.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Li, J.; Wang, L.; Li, D.; Liu, B.; Li, R.; Yu, X.; Wei, Y.; Liu, J.; Zhao, Z. Preparation of Cordierite Monolith Catalysts with the Coating of K-Modified Spinel MnCo2O4 Oxide and Their Catalytic Performances for Soot Combustion. Catalysts 2022, 12, 295. https://doi.org/10.3390/catal12030295
Zhao K, Li J, Wang L, Li D, Liu B, Li R, Yu X, Wei Y, Liu J, Zhao Z. Preparation of Cordierite Monolith Catalysts with the Coating of K-Modified Spinel MnCo2O4 Oxide and Their Catalytic Performances for Soot Combustion. Catalysts. 2022; 12(3):295. https://doi.org/10.3390/catal12030295
Chicago/Turabian StyleZhao, Kun, Jianmei Li, Lanyi Wang, Dong Li, Bonan Liu, Renjie Li, Xuehua Yu, Yuechang Wei, Jian Liu, and Zhen Zhao. 2022. "Preparation of Cordierite Monolith Catalysts with the Coating of K-Modified Spinel MnCo2O4 Oxide and Their Catalytic Performances for Soot Combustion" Catalysts 12, no. 3: 295. https://doi.org/10.3390/catal12030295
APA StyleZhao, K., Li, J., Wang, L., Li, D., Liu, B., Li, R., Yu, X., Wei, Y., Liu, J., & Zhao, Z. (2022). Preparation of Cordierite Monolith Catalysts with the Coating of K-Modified Spinel MnCo2O4 Oxide and Their Catalytic Performances for Soot Combustion. Catalysts, 12(3), 295. https://doi.org/10.3390/catal12030295










