Visible-Light Driven Photocatalytic Degradation of 4-Chlorophenol Using Graphitic Carbon Nitride-Based Nanocomposites
Abstract
:1. Introduction
2. Results and Discussions
2.1. Physical and Chemical Properties of GCN and GCN Composites
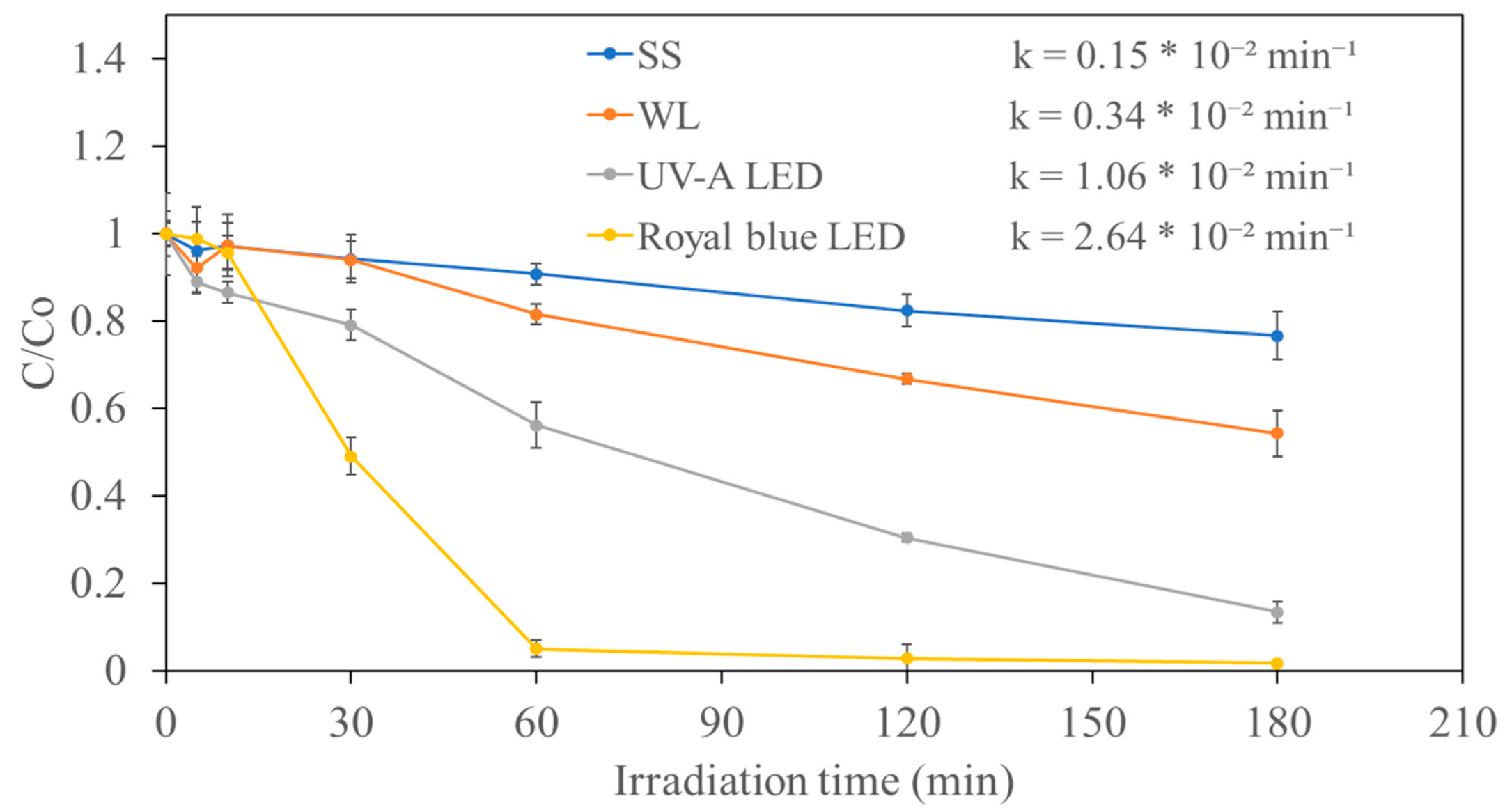
2.2. Photocatalytic Degradation of 4-CP Using GCNs and GCN-Based Nanocomposites
2.3. Photocatalytic Degradation of 4-CP by U-GCN/0.3Ag2CrO4 in Different Photoreactors
2.4. Proposed Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemicals
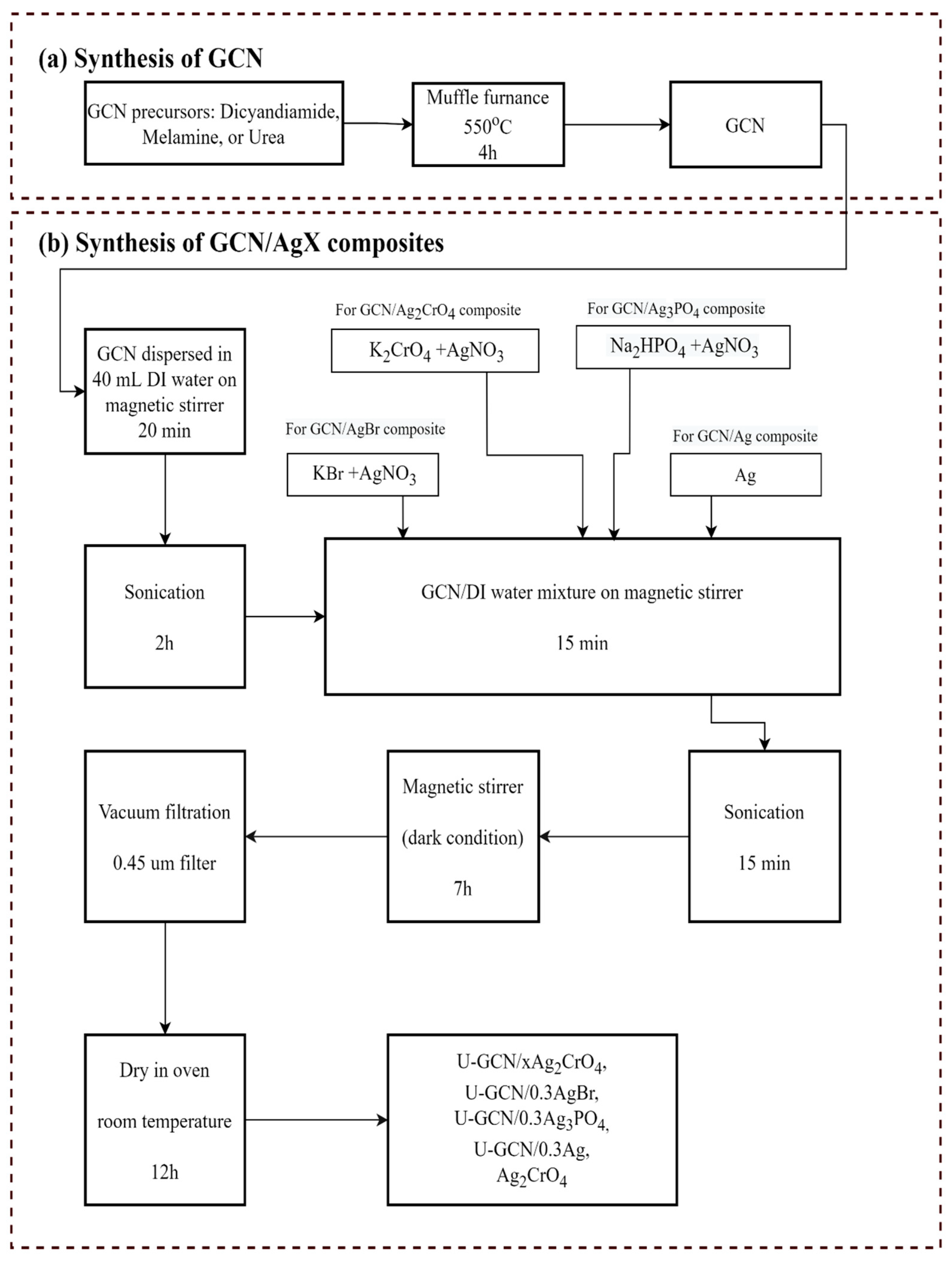
3.2. Synthesis of Graphitic Carbon Nitride (GCNs) and GCN Composites
3.3. Characterization
3.4. Photocatalytic Experiments
3.5. Analysis
3.6. Actinometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, M.; Chen, X.; Pan, B. Simultaneous Determination of 19 Chlorophenols in Water by Liquid Chromatography-Mass Spectrometry with Solid-phase Extraction. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1369–1380. [Google Scholar] [CrossRef]
- Khetan, S.K.; Collins, T.J. Human Pharmaceuticals in the Aquatic Environment: A Challenge to Green Chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef] [PubMed]
- Canada, H. Chlorophenols. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/chlorophenols.html (accessed on 18 June 2021).
- Gómez, M.; Murcia, M.D.; Dams, R.; Christofi, N.; Gómez, E.; Gómez, J.L. Removal Efficiency and Toxicity Reduction of 4-Chlorophenol with Physical, Chemical and Biochemical Methods. Environ. Technol. 2012, 33, 1055–1064. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A. Removal of Chlorophenols from Aqueous Solutions by Sorption onto Walnut, Pistachio and Hazelnut Shells. Pol. J. Chem. Technol. 2015, 17, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, L.; Carra, I.; Campo, P.; Soares, A. Treatment Options for Reclaiming Wastewater Produced by the Pesticide Industry. Int. J. Water Wastewater Treat. 2018, 4, 1–15. [Google Scholar] [CrossRef]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, X.; Zhou, C.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Wang, W.; Song, B.; Tang, X.; et al. Recent Advances in Application of Graphitic Carbon Nitride-Based Catalysts for Degrading Organic Contaminants in Water through Advanced Oxidation Processes beyond Photocatalysis: A Critical Review. Water Res. 2020, 184, 116200. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Suriyachai, N.; Chuangchote, S.; Laosiripojana, N.; Champreda, V.; Sagawa, T. Synergistic Effects of Co-Doping on Photocatalytic Activity of Titanium Dioxide on Glucose Conversion to Value-Added Chemicals. ACS Omega 2020, 5, 20373–20381. [Google Scholar] [CrossRef]
- Nashim, A.; Martha, S.; Parida, K.M. Gd2/Ti2O7/In2O3: Efficient Visible-Light-Driven Heterojunction-Based Composite Photocatalysts for Hydrogen Production. ChemCatChem 2013, 5, 2352–2359. [Google Scholar] [CrossRef]
- Zhou, C.; Zeng, Z.; Zeng, G.; Huang, D.; Xiao, R.; Cheng, M.; Zhang, C.; Xiong, W.; Lai, C.; Yang, Y.; et al. Visible-Light-Driven Photocatalytic Degradation of Sulfamethazine by Surface Engineering of Carbon Nitride: Properties, Degradation Pathway and Mechanisms. J. Hazard. Mater. 2019, 380, 120815. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, Z.; Li, C. A Comparison of Graphitic Carbon Nitrides Synthesized from Different Precursors through Pyrolysis. J. Photochem. Photobiol. Chem. 2017, 332, 32–44. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Dong, F.; Zhao, Z. The Multiple Effects of Precursors on the Properties of Polymeric Carbon Nitride. Int. J. Photoenergy 2013, 2013, 685038 . [Google Scholar] [CrossRef] [Green Version]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of G-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Wu, S.-Z.; Chen, C.-H.; Zhang, W.-D. Etching Graphitic Carbon Nitride by Acid for Enhanced Photocatalytic Activity toward Degradation of 4-Nitrophenol. Chin. Chem. Lett. 2014, 25, 1247–1251. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liu, P.; Wang, D.; Li, Y.; Zhao, H. Cross-Linked g-C3N4 /RGO Nanocomposites with Tunable Band Structure and Enhanced Visible Light Photocatalytic Activity. Small 2013, 9, 3336–3344. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin Hexagonal SnS2 Nanosheets Coupled with G-C3N4 Nanosheets as 2D/2D Heterojunction Photocatalysts toward High Photocatalytic Activity. Appl. Catal. B Environ. 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, Q.; Li, X.; Xiong, W.; Tade, M.; Liu, L. 2D Porous Graphitic C3N4 Nanosheets/Ag3PO4 Nanocomposites for Enhanced Visible-Light Photocatalytic Degradation of 4-Chlorophenol. J. Nanopart Res. 2014, 16, 2532. [Google Scholar] [CrossRef]
- Deng, J.; Liang, J.; Li, M.; Tong, M. Enhanced Visible-Light-Driven Photocatalytic Bacteria Disinfection by g-C3N4-AgBr. Colloids Surf. B Biointerfaces 2017, 152, 49–57. [Google Scholar] [CrossRef]
- Faisal, M.; Ismail, A.A.; Harraz, F.A.; Al-Sayari, S.A.; El-Toni, A.M.; Al-Assiri, M.S. Synthesis of Highly Dispersed Silver Doped G-C3N4 Nanocomposites with Enhanced Visible-Light Photocatalytic Activity. Mater. Des. 2016, 98, 223–230. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous Graphitic Carbon Nitride Synthesized via Direct Polymerization of Urea for Efficient Sunlight-Driven Photocatalytic Hydrogen Production. Nanoscale 2012, 4, 5300. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of Thiourea into Carbon Nitride Semiconductors as Visible Light Photocatalysts. J. Mater. Chem. 2012, 22, 8083. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, J.; Fu, X.; Wang, X. Metal-Free Photocatalytic Degradation of 4-Chlorophenol in Water by Mesoporous Carbon Nitride Semiconductors. Catal. Sci. Technol. 2012, 2, 1396. [Google Scholar] [CrossRef]
- Ji, H.; Chang, F.; Hu, X.; Qin, W.; Shen, J. Photocatalytic Degradation of 2,4,6-Trichlorophenol over g-C3N4 under Visible Light Irradiation. Chem. Eng. J. 2013, 218, 183–190. [Google Scholar] [CrossRef]
- Shi, L.; Liang, L.; Wang, F.; Liu, M.; Sun, J. Ag2CrO4 Nanoparticles Loaded on Two-Dimensional Large Surface Area Graphite-like Carbon Nitride Sheets: Simple Synthesis and Excellent Photocatalytic Performance. Dalton Trans. 2016, 45, 5815–5824. [Google Scholar] [CrossRef]
- Shen, J.; Lu, Y.; Liu, J.-K.; Yang, X.-H. Photocatalytic Activity of Silver Chromate Materials by Various Synthesis Methods. J. Exp. Nanosci. 2016, 11, 650–659. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Ma, Y.; Fan, J.; Xue, Y.; Chang, H.; Masubuchi, Y.; Yin, S. Synthesis of Graphitic Carbon Nitride from Different Precursors by Fractional Thermal Polymerization Method and Their Visible Light Induced Photocatalytic Activities. J. Alloys Compd. 2018, 735, 1297–1305. [Google Scholar] [CrossRef]
- De Sousa, J.G.M.; da Silva, T.V.C.; de Moraes, N.P.; Caetano Pinto da Silva, M.L.; da Silva Rocha, R.; Landers, R.; Rodrigues, L.A. Visible Light-Driven ZnO/g-C3N4/Carbon Xerogel Ternary Photocatalyst with Enhanced Activity for 4-Chlorophenol Degradation. Mater. Chem. Phys. 2020, 256, 123651. [Google Scholar] [CrossRef]
- Xu, D.; Cao, S.; Zhang, J.; Cheng, B.; Yu, J. Effects of the Preparation Method on the Structure and the Visible-Light Photocatalytic Activity of Ag2CrO 4. Beilstein J. Nanotechnol. 2014, 5, 658–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soofivand, F.; Mohandes, F.; Salavati-Niasari, M. Silver Chromate and Silver Dichromate Nanostructures: Sonochemical Synthesis, Characterization, and Photocatalytic Properties. Mater. Res. Bull. 2013, 48, 2084–2094. [Google Scholar] [CrossRef]
- Shen, Y.; Dos santos-Garcia, A.J.; Martín de Vidales, M.J. Graphitic Carbon Nitride-Based Composite in Advanced Oxidation Processes for Aqueous Organic Pollutants Removal: A Review. Processes 2020, 9, 66. [Google Scholar] [CrossRef]
- Yang, P.; Wang, J.; Yue, G.; Yang, R.; Zhao, P.; Yang, L.; Zhao, X.; Astruc, D. Constructing Mesoporous G-C3N4/ZnO Nanosheets Catalyst for Enhanced Visible-Light Driven Photocatalytic Activity. J. Photochem. Photobiol. Chem. 2020, 388, 112169. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Y.; Liu, X.; Zeng, G.; Shao, B.; Liu, Y.; Liu, Y.; Zhang, W.; Zhang, W.; Yan, M.; et al. Silver Chromate Modified Sulfur Doped Graphitic Carbon Nitride Microrod Composites with Enhanced Visible-Light Photoactivity towards Organic Pollutants Degradation. Compos. Part B Eng. 2019, 173, 106918. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Pal, S.; Licciulli, A. Diatomite/Silver Phosphate Composite for Efficient Degradation of Organic Dyes under Solar Radiation. Bull. Mater. Sci. 2020, 43, 295. [Google Scholar] [CrossRef]
- Ghattavi, S.; Nezamzadeh-Ejhieh, A. GC-MASS Detection of Methyl Orange Degradation Intermediates by AgBr/g-C3N4: Experimental Design, Bandgap Study, and Characterization of the Catalyst. Int. J. Hydrog. Energy 2020, 45, 24636–24656. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Panchal, P.; Nehra, S.P.; Gupta, A.P.; Sharma, A. Synthesis, Characterization and Application of Silver Doped Graphitic Carbon Nitride as Photocatalyst towards Visible Light Photocatalytic Hydrogen Evolution. Int. J. Hydrog. Energy 2020, 45, 23937–23946. [Google Scholar] [CrossRef]
- Zarei, M. Ultrasonic-Assisted Preparation of ZrO2/g-C3N4 Nanocomposites with High Visible-Light Photocatalytic Activity for Degradation of 4-Chlorophenol in Water. Water-Energy Nexus 2020, 3, 135–142. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Z.; Qayum, A.; Zhao, X.; Xia, H.; Lu, F.; Hu, L. Enhanced Photocatalytic Degradation of 4-Chlorophenol under Visible Light over Carbon Nitride Nanosheets with Carbon Vacancies. Nanotechnology 2021, 32, 415704. [Google Scholar] [CrossRef]
- UV LEDs Ramp up the Quiet Side of the LED Market (MAGAZINE). Available online: https://www.ledsmagazine.com/manufacturing-services-testing/research-development/article/16695464/uv-leds-ramp-up-the-quiet-side-of-the-led-market-magazine (accessed on 2 September 2021).
- Muramoto, Y.; Kimura, M.; Nouda, S. Development and Future of Ultraviolet Light-Emitting Diodes: UV-LED Will Replace the UV Lamp. Semicond Sci. Technol. 2014, 29, 084004. [Google Scholar] [CrossRef] [Green Version]
- Elghniji, K.; Hentati, O.; Mlaik, N.; Mahfoudh, A.; Ksibi, M. Photocatalytic Degradation of 4-Chlorophenol under P-Modified TiO2/UV System: Kinetics, Intermediates, Phytotoxicity and Acute Toxicity. J. Environ. Sci. 2012, 24, 479–487. [Google Scholar] [CrossRef]
- Li, X.; Cubbage, J.W.; Tetzlaff, T.A.; Jenks, W.S. Photocatalytic Degradation of 4-Chlorophenol. 1. The Hydroquinone Pathway. J. Org. Chem. 1999, 64, 8509–8524. [Google Scholar] [CrossRef]
- Li, X.; Cubbage, J.W.; Jenks, W.S. Photocatalytic Degradation of 4-Chlorophenol. 2. The 4-Chlorocatechol Pathway. J. Org. Chem. 1999, 64, 8525–8536. [Google Scholar] [CrossRef]
- Abeish, A.M.; Ang, H.M.; Znad, H. Solar Photocatalytic Degradation of 4-Chlorophenol: Mechanism and Kinetic Modelling. Desalination Water Treat. 2015, 53, 2915–2923. [Google Scholar] [CrossRef]
- Shi, L.; Liang, L.; Wang, F.; Liu, M.; Sun, J. Facile Synthesis of a G-C3N4 Isotype Composite with Enhanced Visible-Light Photocatalytic Activity. RSC Adv. 2015, 5, 101843–101849. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Y.; Dong, F.; Zhang, W.; Duan, S.; Zhang, Q. Facile Synthesis of Organic–Inorganic Layered Nanojunctions of g-C3N4 /(BiO)2CO3 as Efficient Visible Light Photocatalyst. Dalton Trans. 2014, 43, 12026–12036. [Google Scholar] [CrossRef]
- Che, Y.; Lu, B.; Qi, Q.; Chang, H.; Zhai, J.; Wang, K.; Liu, Z. Bio-Inspired Z-Scheme g-C3N4/Ag2CrO4 for Efficient Visible-Light Photocatalytic Hydrogen Generation. Sci. Rep. 2018, 8, 16504. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Y.; Cao, B.; Xin, S.; Guo, L.; Zhang, J.; Li, F. Ag3PO4/Reduced Graphite Oxide Sheets Nanocomposites with Highly Enhanced Visible Light Photocatalytic Activity and Stability. Appl. Catal. B Environ. 2013, 132–133, 45–53. [Google Scholar] [CrossRef]
- Obregón, S. Improved H2 Production of Pt-TiO2/g-C3N4-MnOx Composites by an Efficient Handling of Photogenerated Charge Pairs. Appl. Catal. B Environ. 2014, 144, 775–782. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Peng, S.; Lu, G.; Li, S. Eosin Y-Sensitized Graphitic Carbon Nitride Fabricated by Heating Urea for Visible Light Photocatalytic Hydrogen Evolution: The Effect of the Pyrolysis Temperature of Urea. Phys. Chem. Chem. Phys. 2013, 15, 7657. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Chauhan, N.S.; Mittal, A.; Sharma, S. TiO2 and Its Composites as Promising Biomaterials: A Review. BioMetals 2018, 31, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Hatchard, C.G.; Parker, C.A. A New Sensitive Chemical Actinometer—II. Potassium Ferrioxalate as a Standard Chemical Actinometer. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1956, 235, 518–536. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsuda, S.; Cuevas, C.A.; Saiz-Lopez, A.; Yabushita, A.; Nakano, Y. Experimental Determination of the Photooxidation Of Aqueous I as a Source of Atmospheric I2. ACS Earth Space Chem. 2019, 3, 669–679. [Google Scholar] [CrossRef]
- Yu, L.; Achari, G.; Langford, C.H. LED-Based Photocatalytic Treatment of Pesticides and Chlorophenols. J. Environ. Eng. 2013, 139, 1146–1151. [Google Scholar] [CrossRef]



| Photocatalyst | BET Surface Area (m2/g) | BJH Pore Volume (cm3/g) | BJH Average Pore Size (nm) |
|---|---|---|---|
| M-GCN | 2.95 | 0.02 | 25.92 |
| D-GCN | 8.79 | 0.05 | 20.76 |
| U-GCN | 57.68 | 0.30 | 23.78 |
| Ag2CrO4 | 1.32 | 0.01 | 97.96 |
| U-GCN/0.1Ag2CrO4 | 60.38 | 0.35 | 27.15 |
| U-GCN/0.3Ag2CrO4 | 42.68 | 0.23 | 26.78 |
| U-GCN/0.5Ag2CrO4 | 44.67 | 0.25 | 24.52 |
| U-GCN/0.3Ag | 41.85 | 0.21 | 23.87 |
| U-GCN/0.3AgBr | 48.02 | 0.24 | 23.70 |
| U-GCN/0.3Ag3PO4 | 67.38 | 0.39 | 30.29 |
| Photocatalyst | Bandgap Energy (eV) | Wavelength (nm) |
|---|---|---|
| M-GCN | 2.68 | 462 |
| D-GCN | 2.67 | 464 |
| U-GCN | 2.75 | 450 |
| Ag2CrO4 | 1.80 | 689 |
| U-GCN/0.1Ag2CrO4 | 2.75 | 450 |
| U-GCN/0.3Ag2CrO4 | 2.74, 1.78 | 452, 698 |
| U-GCN/0.5Ag2CrO4 | 2.73, 1.75 | 454, 708 |
| U-GCN/0.3Ag | 2.73 | 454 |
| U-GCN/0.3AgBr | 2.74 | 452 |
| U-GCN/0.3Ag3PO4 | 2.72 | 455 |
| S/N | Photocatalyst | Synthesis Method | Light Source | Catalyst Amount (mg) | Conc. of 4-CP in Solution (mg/L) | Degradation Time (min) | Degradation Efficiency (%) | Rate Constant (min−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | mpg-CN | Template | 300 W Xe lamp | 40 | 15.4 | 60 | ~100 | 5.26 × 10−2 | [26] |
| 2 | CN/APO | Precipitation | 500 W Xe lamp | 50 | 20 | 180 | 96.5 | 1.80 × 10−2 | [20] |
| 3 | ZrO2/g-C3N4 | Ultrasonication | 300 W Xe lamp | 60 | 30 | 120 | ~90 | 1.73 × 10−2 | [40] |
| 4 | ZnO/g-C3N4/carbon xerogel | Precipitation | Solar simulator: 300 W lamp Visible light: 400 W lamp | 100 | 10 | 300 | 92 72 | 1.20 × 10−2 0.51 × 10−2 | [31] |
| 5 | Vc-GCN | Calcination | 300 W Xe lamp | 50 | 10 | 120 | 60.8 | 0.79 × 10−2 | [41] |
| 6 | U-GCN/0.3Ag2CrO4 | Precipitation | 460 nm Royal blue LED | 40 | 20 | 180 | 98.26 | 2.64 × 10−2 | Current study |
| Photoreactor | Ephoton (J) |
|---|---|
| Solar simulator | 8960 |
| Cool white light | 1366 |
| UV-A LED | 514 |
| Royal blue LED | 1364 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akintunde, O.O.; Yu, L.; Hu, J.; Kibria, M.G.; Achari, G. Visible-Light Driven Photocatalytic Degradation of 4-Chlorophenol Using Graphitic Carbon Nitride-Based Nanocomposites. Catalysts 2022, 12, 281. https://doi.org/10.3390/catal12030281
Akintunde OO, Yu L, Hu J, Kibria MG, Achari G. Visible-Light Driven Photocatalytic Degradation of 4-Chlorophenol Using Graphitic Carbon Nitride-Based Nanocomposites. Catalysts. 2022; 12(3):281. https://doi.org/10.3390/catal12030281
Chicago/Turabian StyleAkintunde, Olufemi Oluseun, Linlong Yu, Jinguang Hu, Md Golam Kibria, and Gopal Achari. 2022. "Visible-Light Driven Photocatalytic Degradation of 4-Chlorophenol Using Graphitic Carbon Nitride-Based Nanocomposites" Catalysts 12, no. 3: 281. https://doi.org/10.3390/catal12030281
APA StyleAkintunde, O. O., Yu, L., Hu, J., Kibria, M. G., & Achari, G. (2022). Visible-Light Driven Photocatalytic Degradation of 4-Chlorophenol Using Graphitic Carbon Nitride-Based Nanocomposites. Catalysts, 12(3), 281. https://doi.org/10.3390/catal12030281








