Synthesis and Photoelectrocatalytic Applications of TiO2/ZnO/Diatomite Composites
Abstract
:1. Introduction
2. Results and Discussion
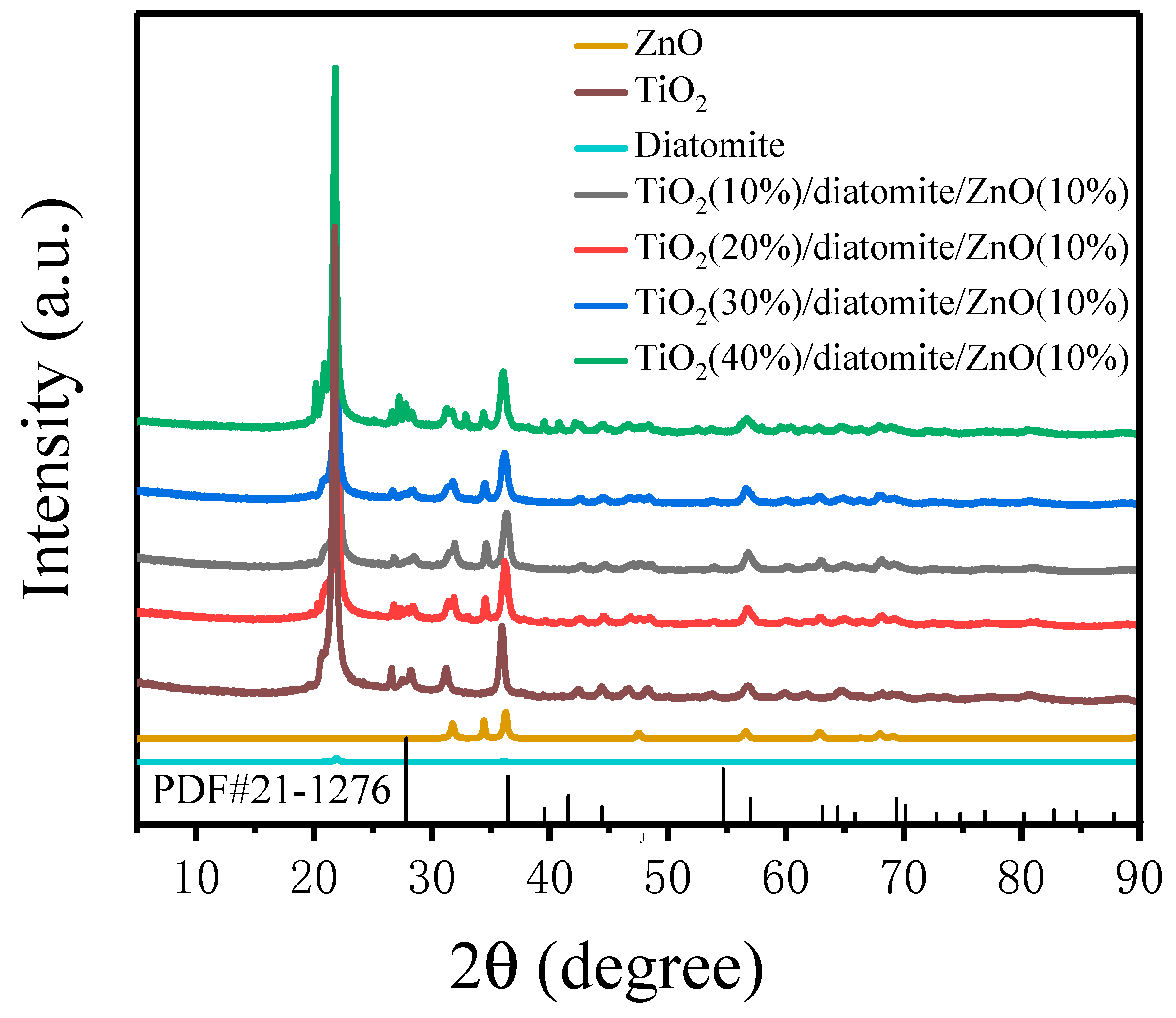
2.1. Phase Analysis
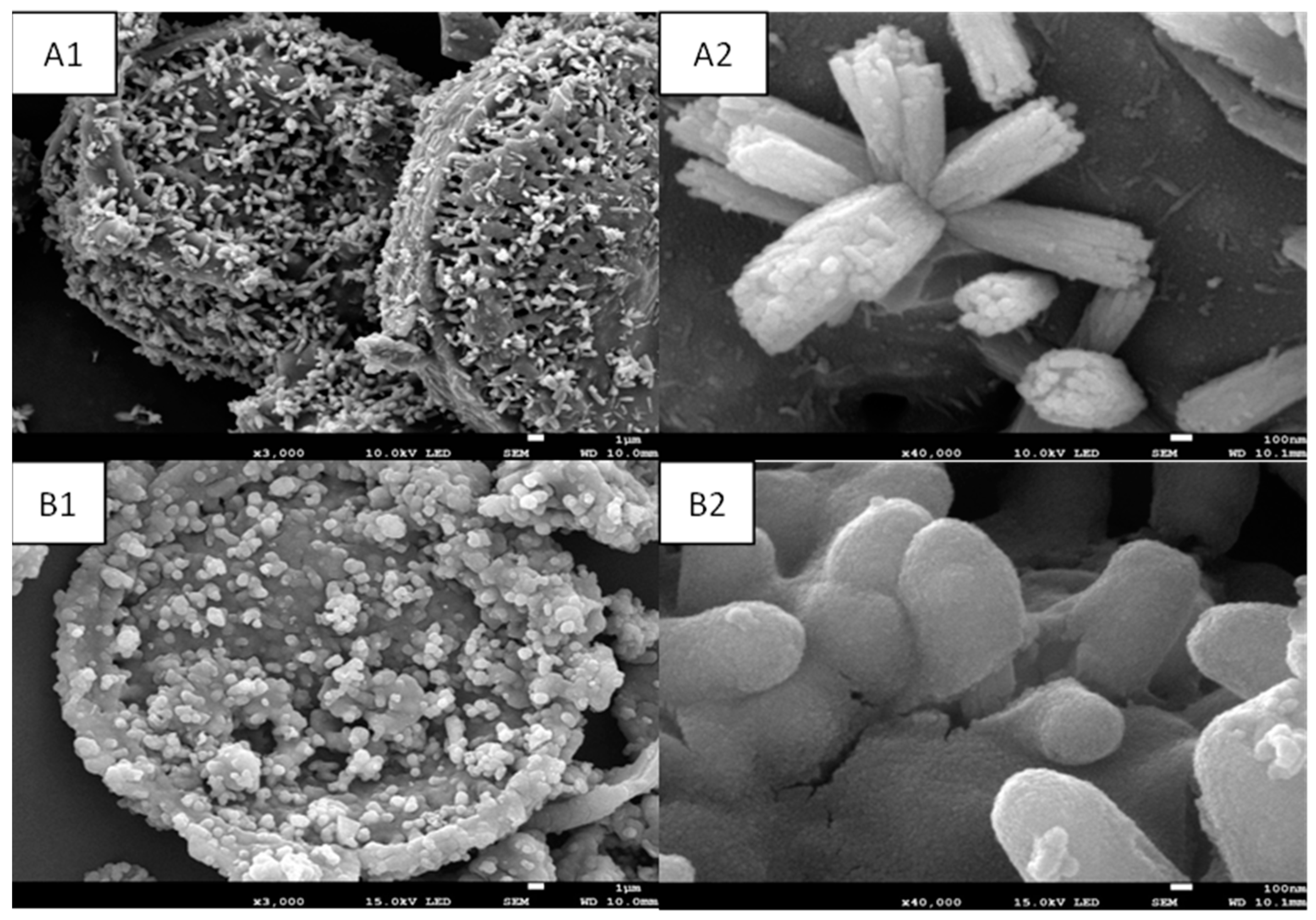
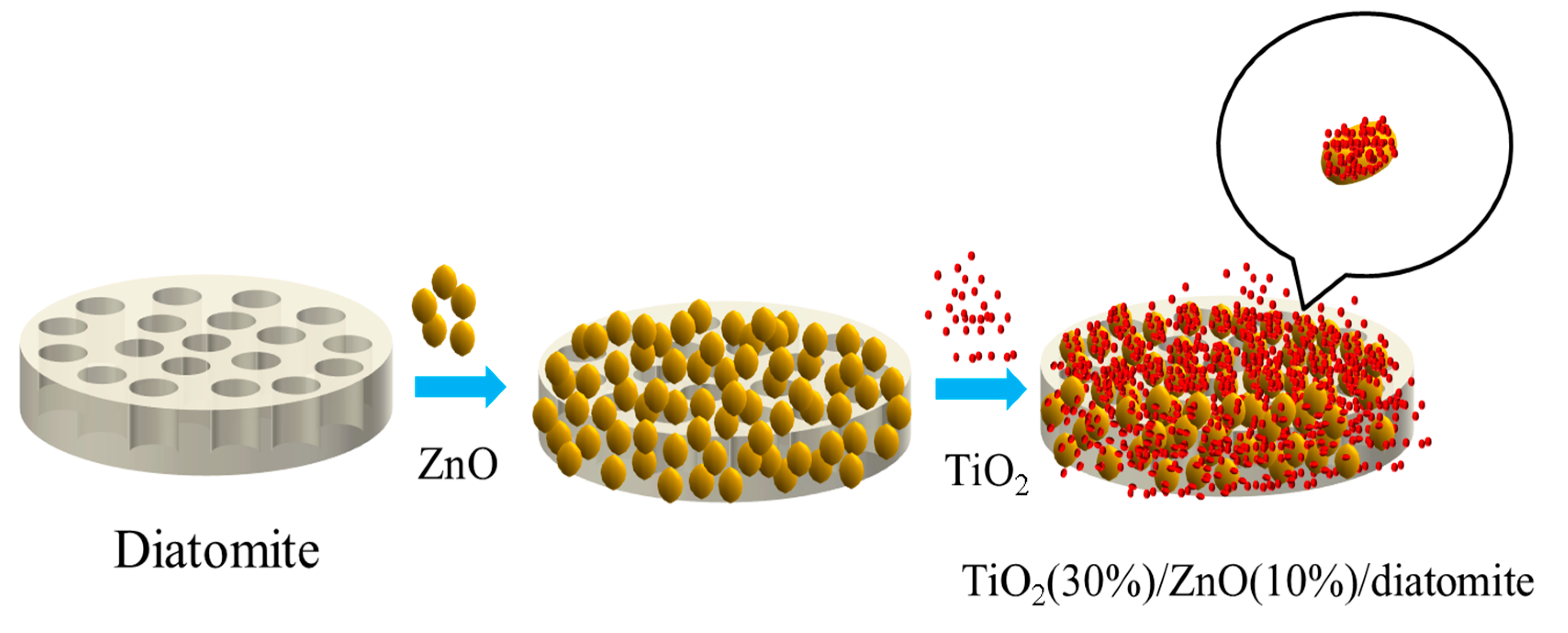
2.2. Morphological Analysis
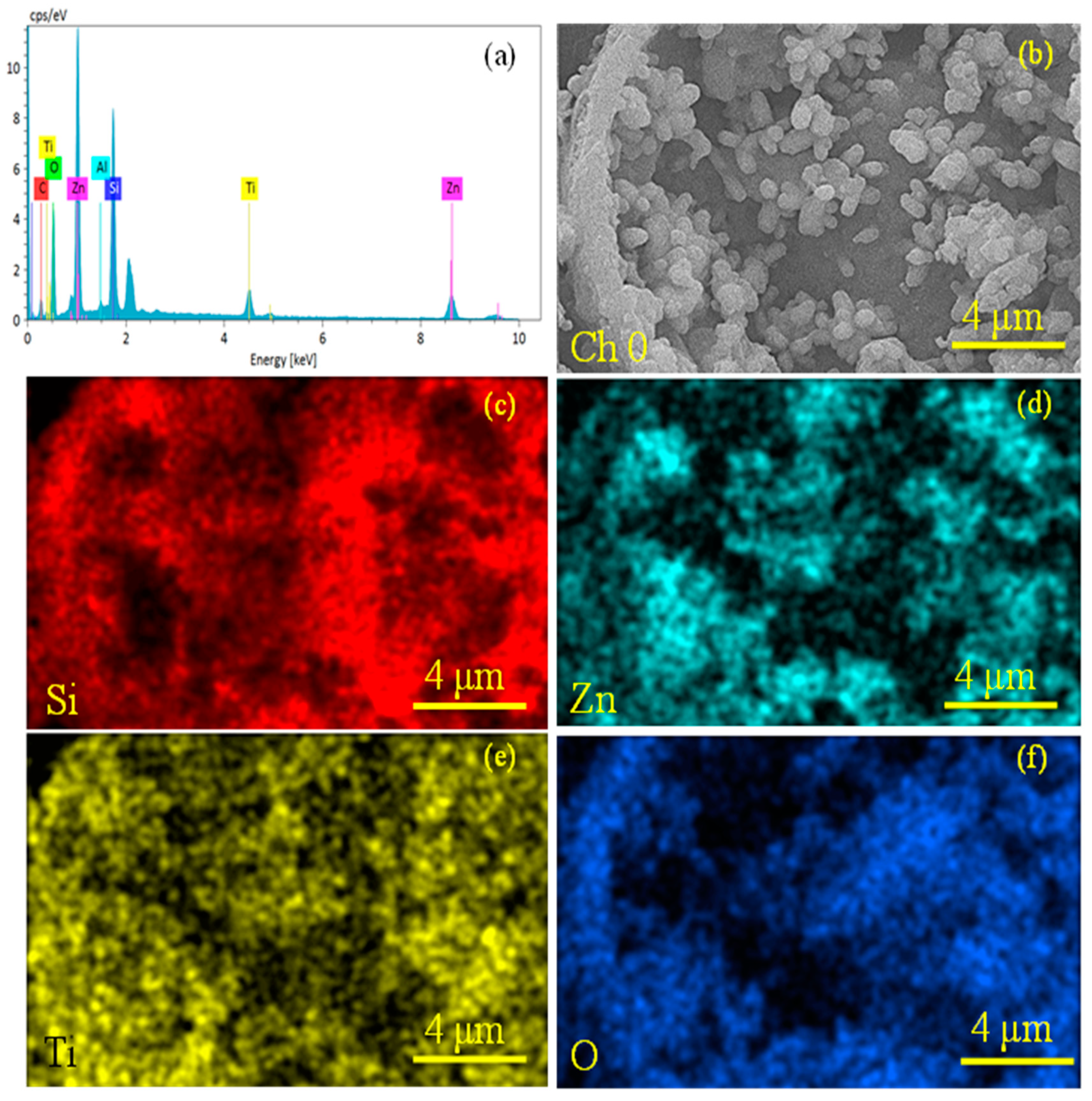
2.3. Elemental Analysis
2.4. XPS Analysis
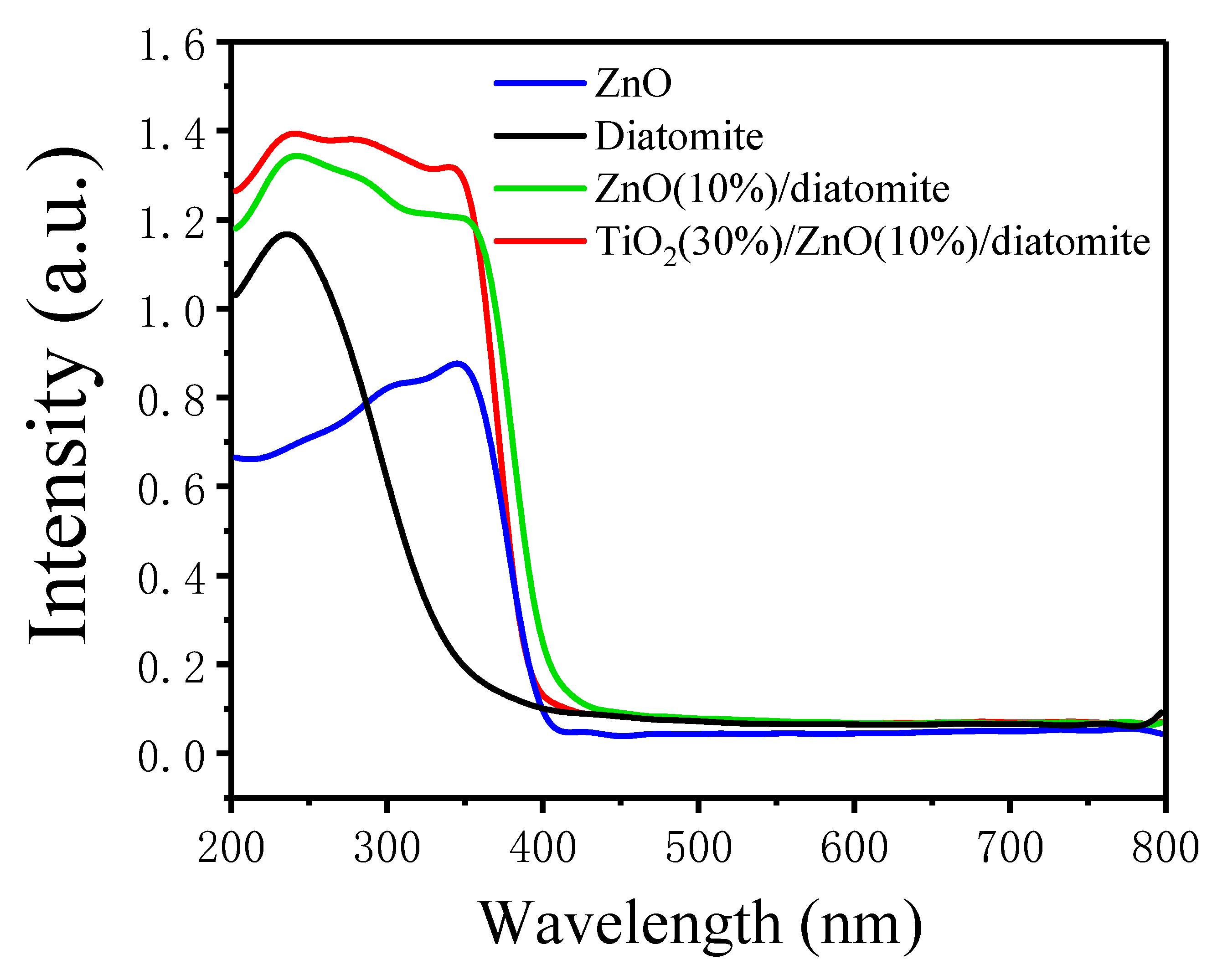
2.5. UV–Vis Diffuse Reflectance Spectra
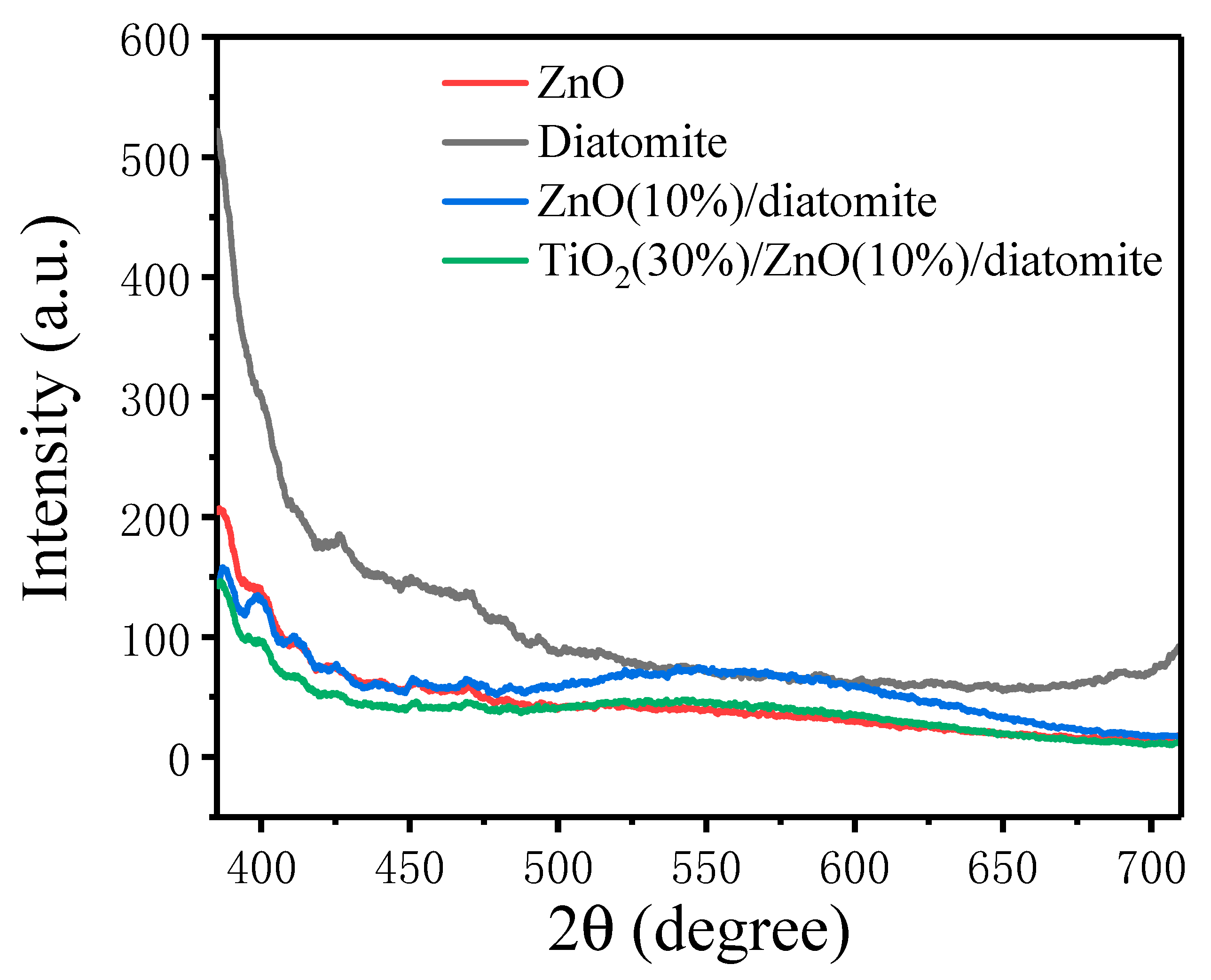
2.6. Photoluminescence (PL) Spectra
2.7. Photocatalytic Performance of Different Catalysts
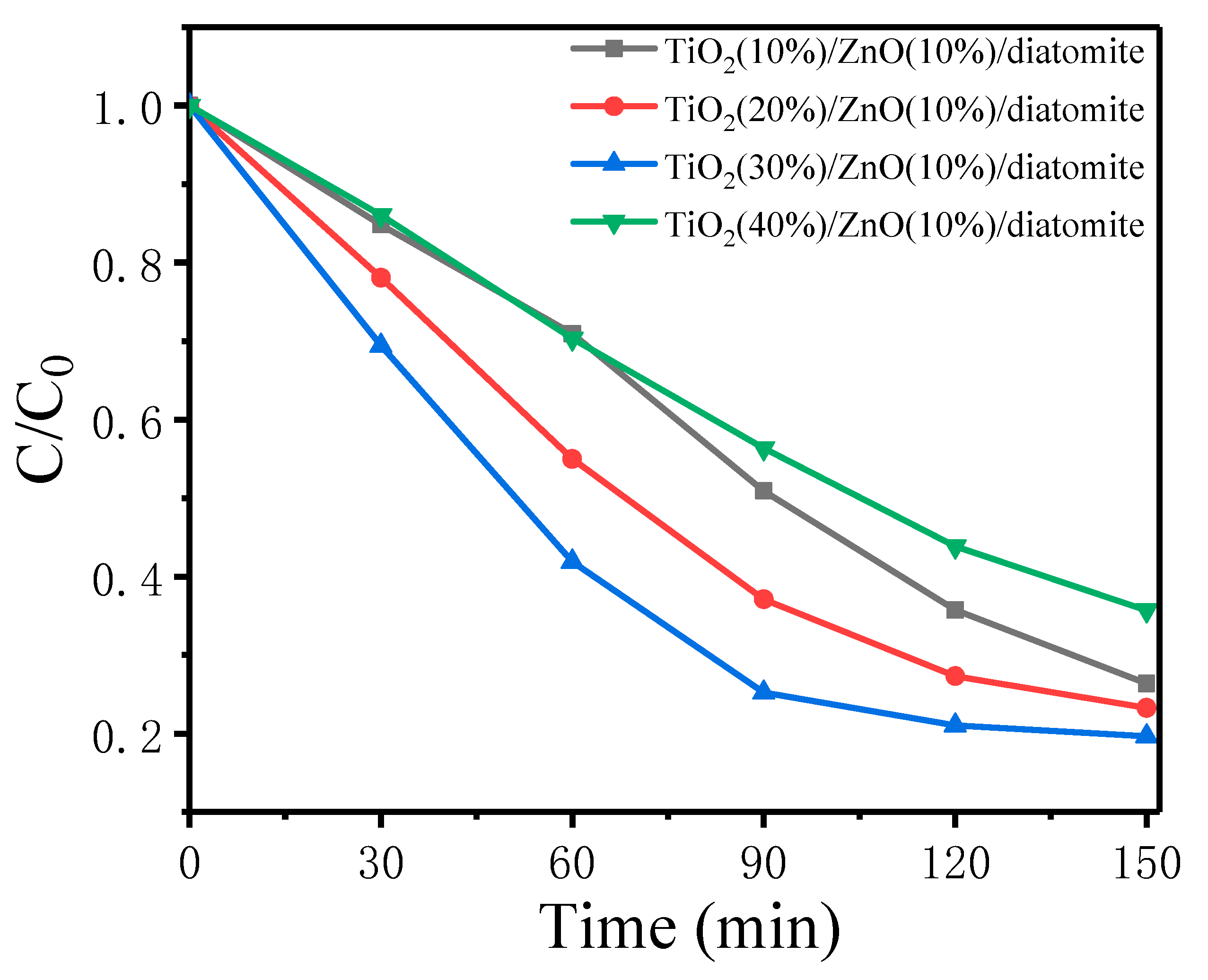
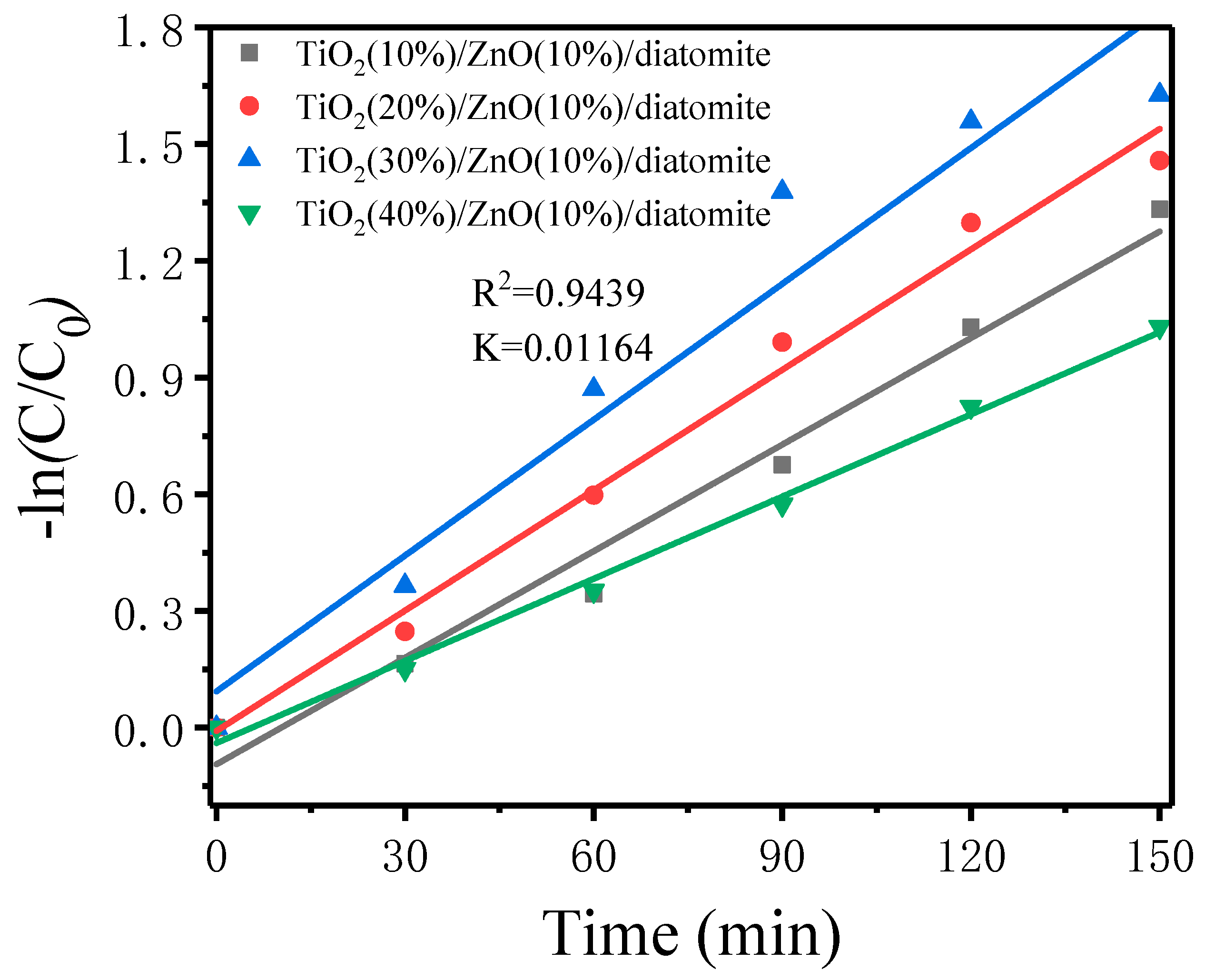
2.7.1. Photocatalytic Degradation of MB by Different Catalysts
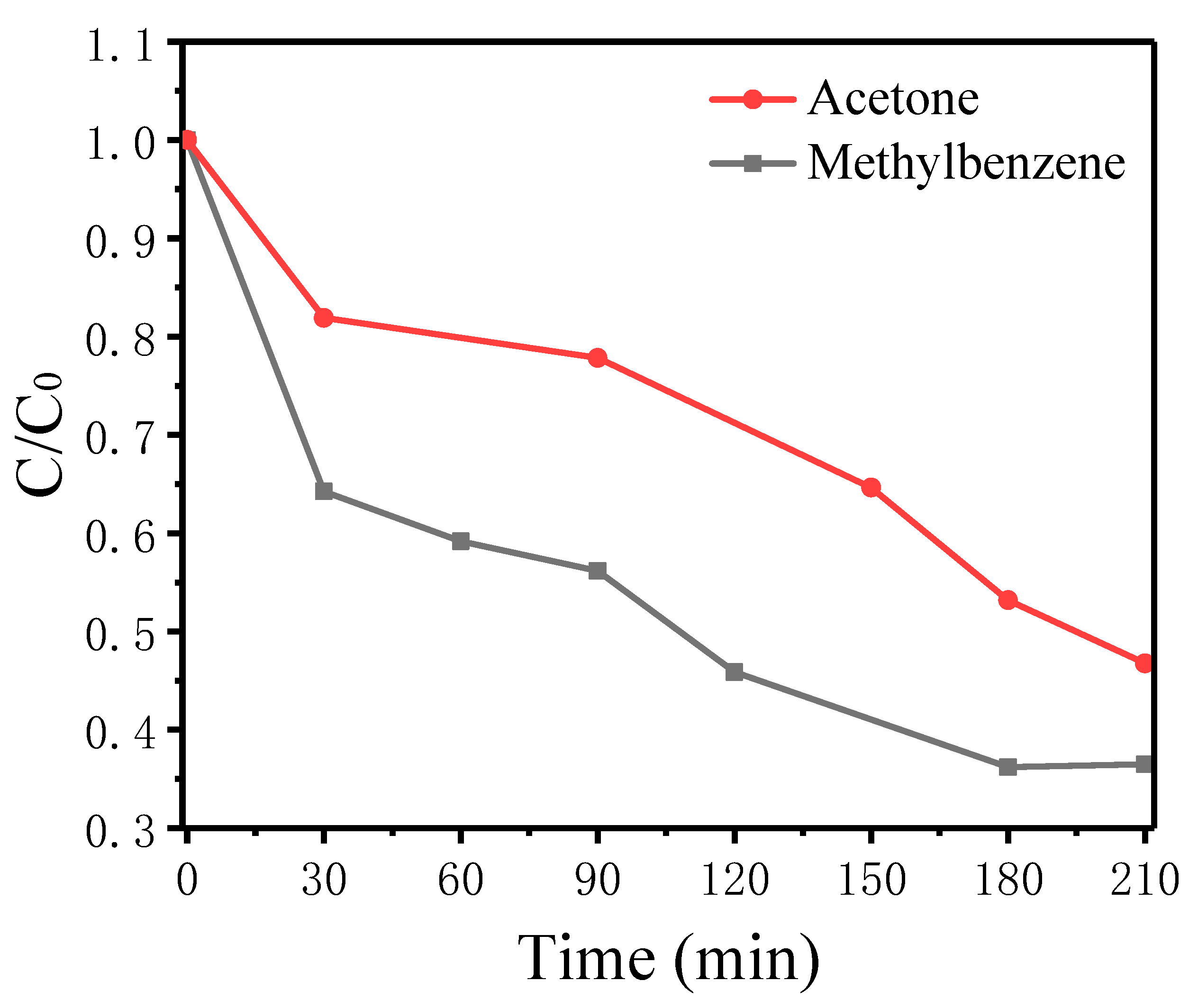
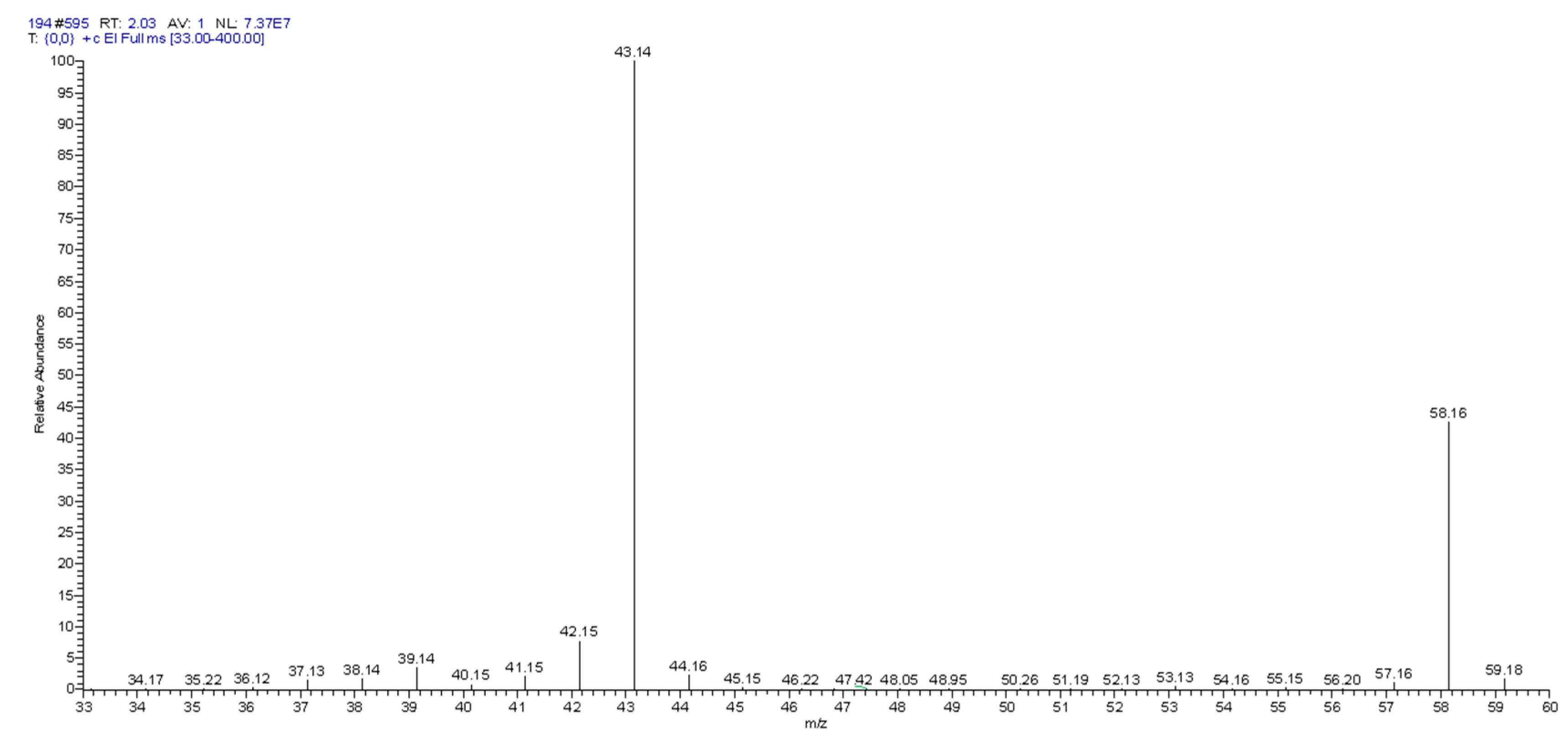
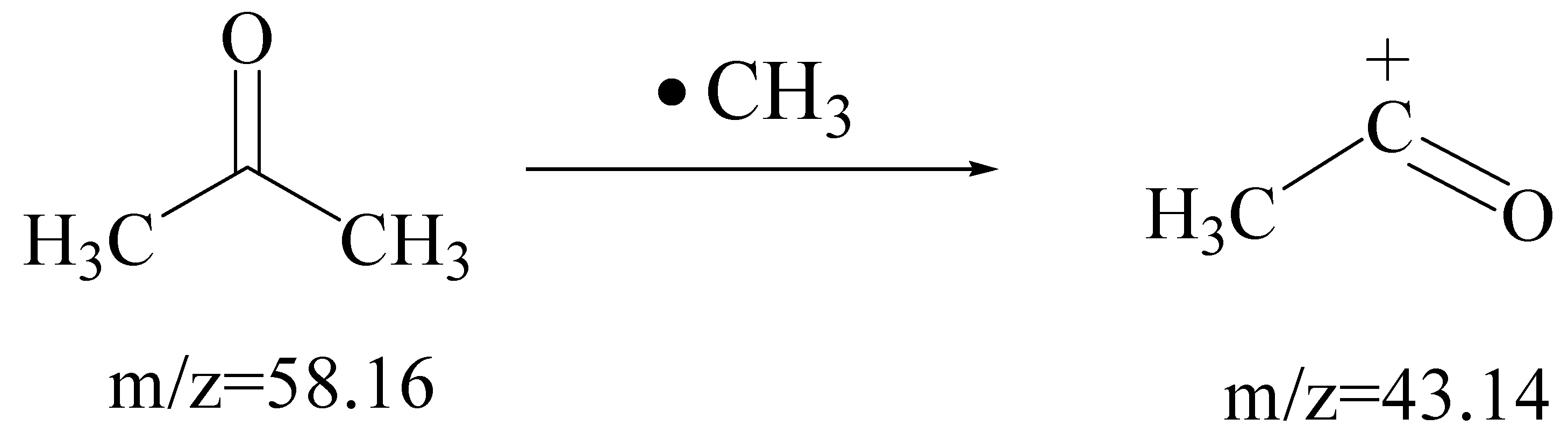
2.7.2. Photocatalytic Degradation of Gaseous Methylbenzene and Gaseous Acetone over TiO2(30%)/ZnO(10%)/Diatomite
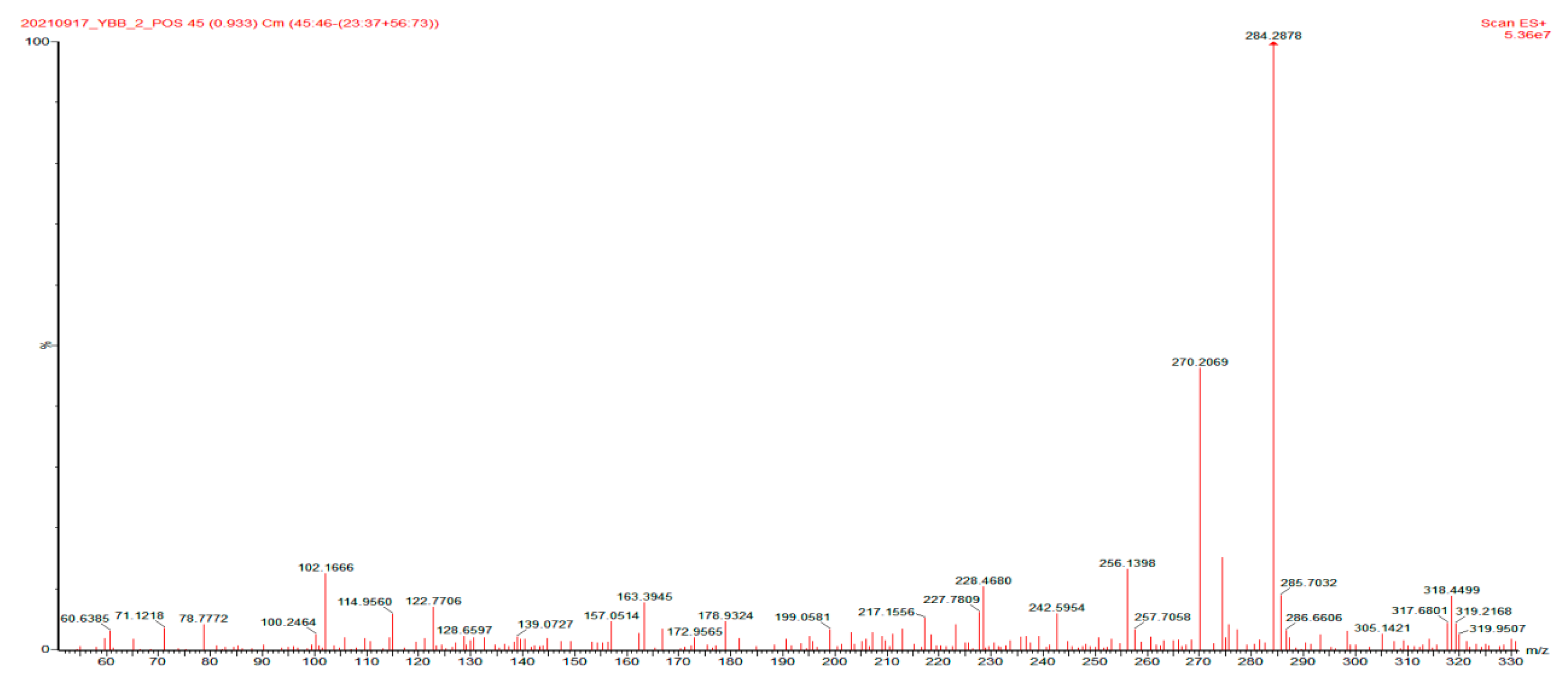
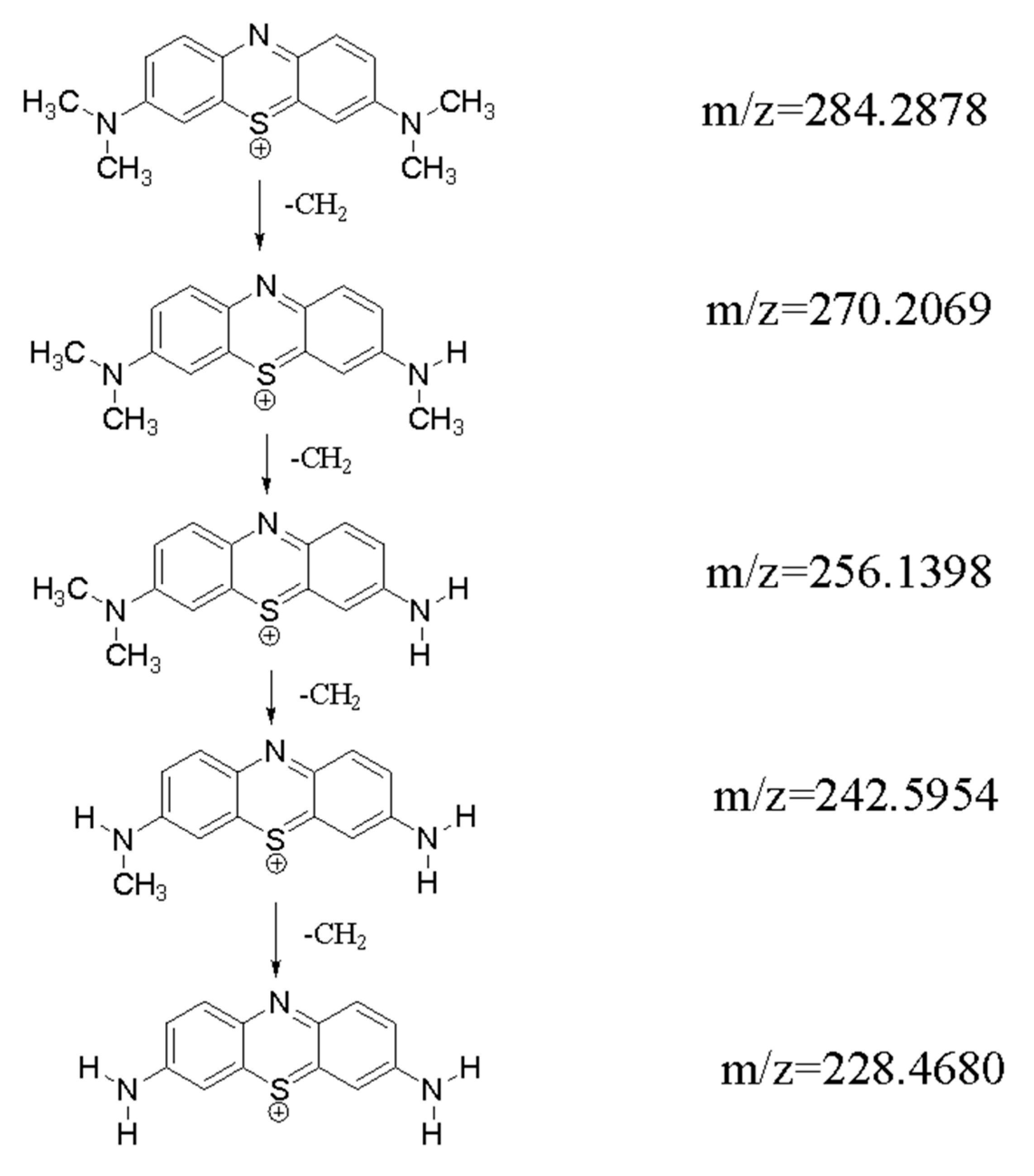
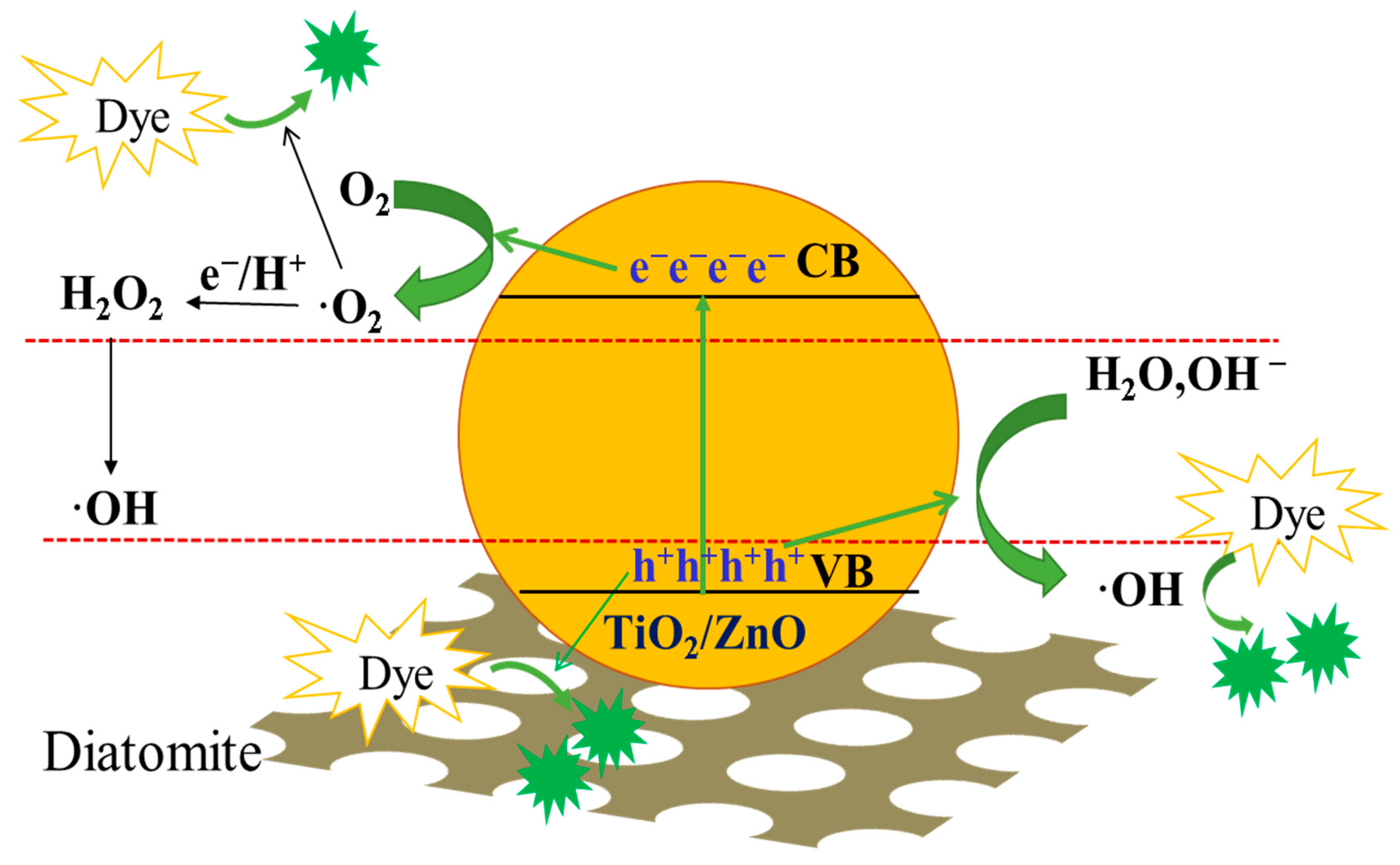
2.7.3. Analysis of the Photocatalytic Mechanism
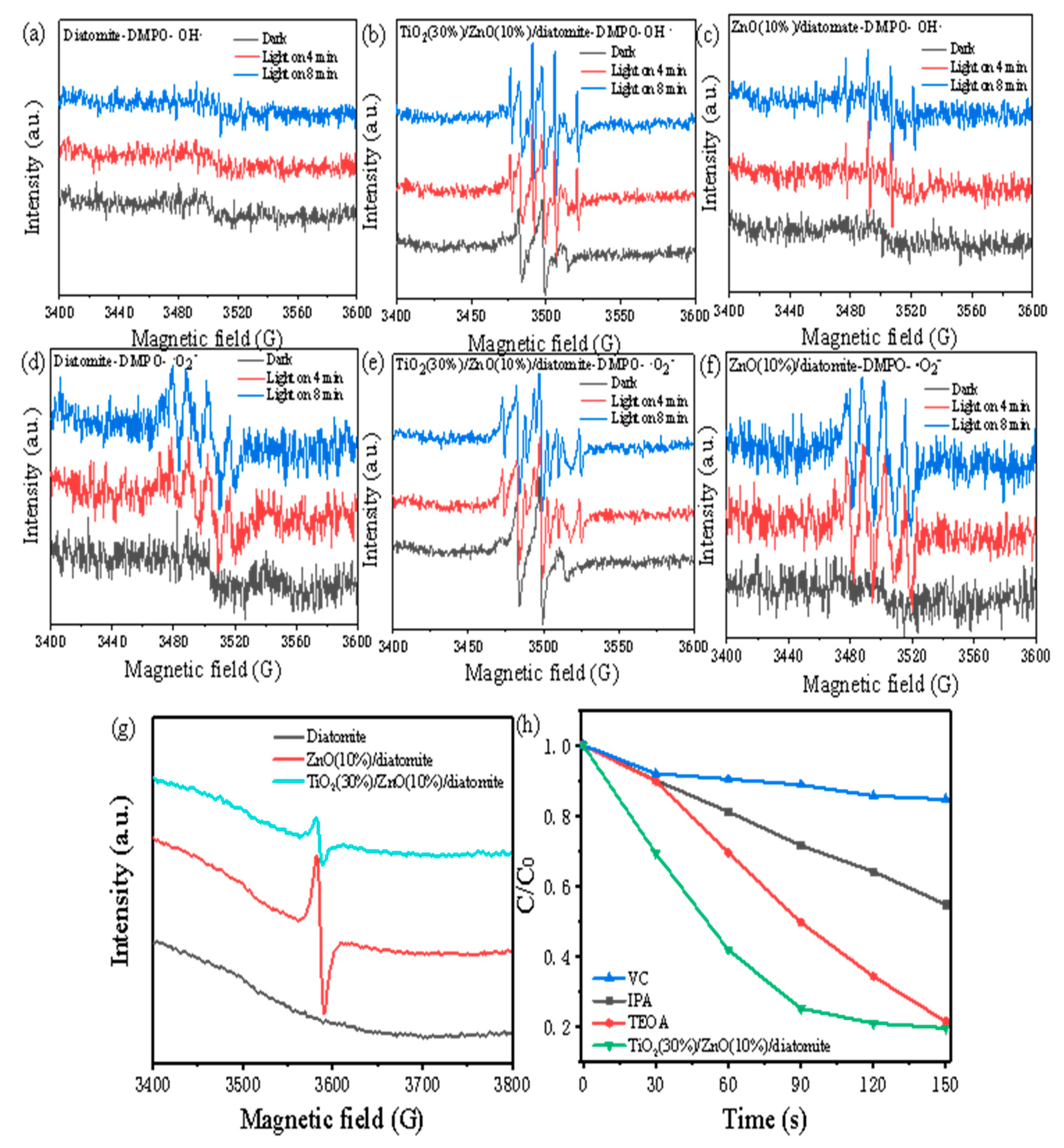
2.8. Electron Paramagnetic Resonance (EPR) Analysis
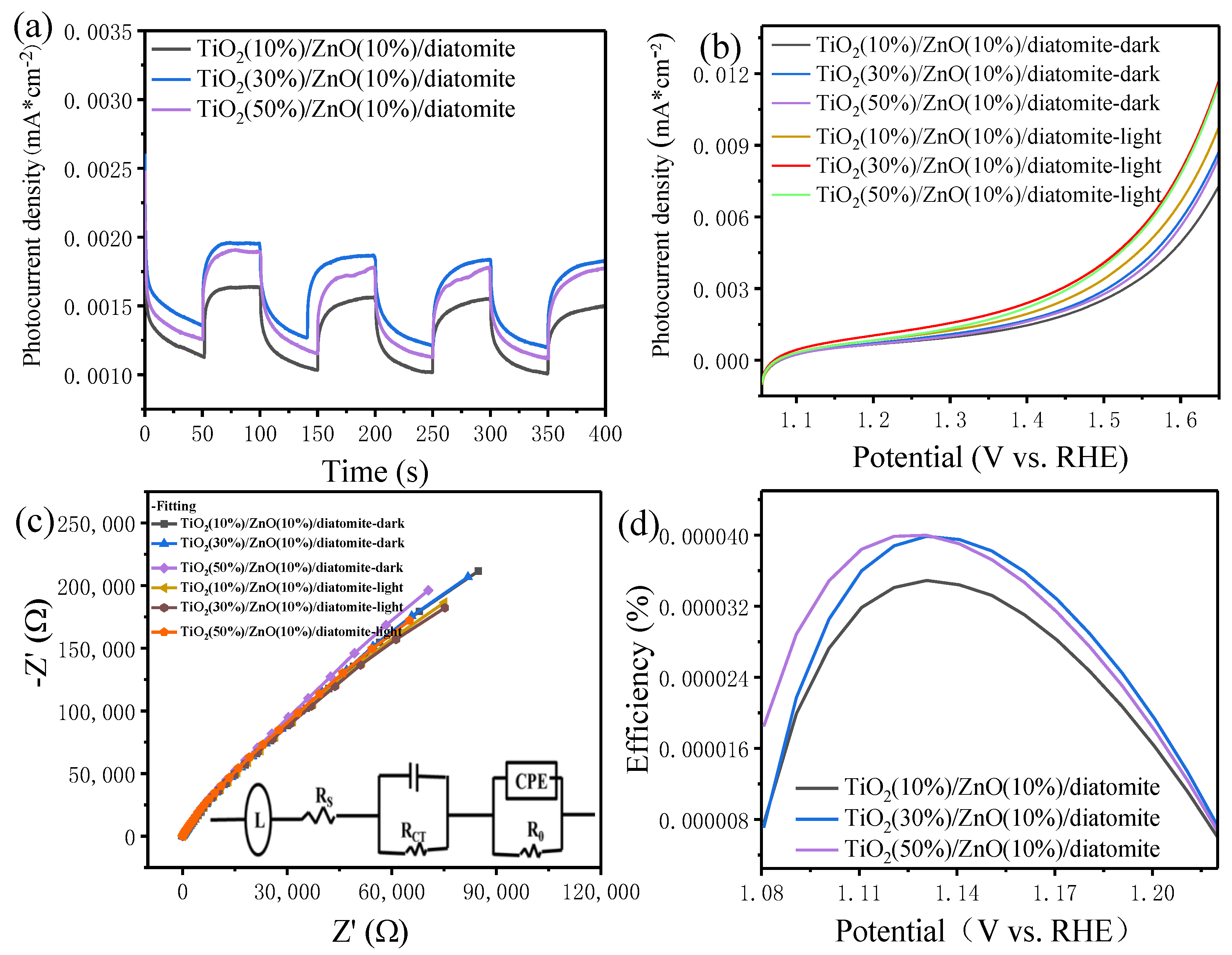
2.9. Photocurrent Analysis
3. Experimental Section
3.1. Chemicals and Materials
3.2. Catalyst Preparation
3.2.1. Synthesis of ZnO(10%)/Diatomite
3.2.2. Synthesis of TiO2(X%)/ZnO(10%)/Diatomite
4. Characterization
4.1. Material Characterization
4.2. Photocatalytic Activity
4.3. Electrochemical Measurements of the Electrocatalysts
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liang, L.; Meng, Y.; Shi, L.; Ma, J.; Sun, J. Enhanced photocatalytic performance of novel visible light-driven Ag–TiO2/SBA-15 photocatalyst. Superlattices Microstruct. 2014, 73, 60–70. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. Recent advances in TiO2 films prepared by sol-gel methods for photocatalytic degradation of organic pollutants and antibacterial activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Rishikesh Manna, A.K.; Soni, R.K. Fabrication of ZnO–TiO2 nanohybrids for rapid sunlight driven photodegradation of textile dyes and antibiotic residue molecules. Opt. Mater. 2020, 107, 110138. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kuk, Y.S.; Kwon, O.H.; Park, Y.W.; Park, M. Synthesis and characterization of ZnO-TiO2/carbon fiber composite with enhanced photocatalytic properties. Nanomaterials 2020, 10, 1960. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Johar, M.A.; Afzal, R.A.; Alazba, A.A.; Manzoor, U. Photocatalysis and bandgap engineering using ZnO nanocomposites. advances in materials science and engineering. Adv. Mater. Sci. Eng. 2015, 2015, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Firdaus, C.M.; Rizam, M.S.B.S.; Rusop, M.; Hidayah, S.R. Characterization of ZnO and ZnO: TiO2 thin films prepared by sol-gel spray-spin coating technique. Procedia Eng. 2012, 41, 1367–1373. [Google Scholar] [CrossRef]
- Kiziltaş, H.; Tekin, T. Increasing of photocatalytic performance of TiO2 nanotubes by doping AgS and CdS. Chem. Eng. Commun. 2017, 204, 852–857. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Huang, W.; Situ, Y.; Huang, H. Multimorphologies nano-ZnO preparing through a simple solvothermal method for photocatalytic application. Mater. Lett. 2015, 141, 294–297. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Kuna, E.; Jakubiak, S.; Michalski, J.; Kurzydłowski, K. Polypropylene nonwoven filter with nanosized ZnO rods: Promising hybrid photocatalyst for water purification. Appl. Catal. B Environ. 2015, 170, 273–282. [Google Scholar] [CrossRef]
- Malakooti, M.H.; Hwang, H.S.; Sodano, H.A. Morphology-controlled ZnO nanowire arrays for tailored hybrid composites with high damping. ACS Appl. Mater. Interfaces 2015, 7, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Stanley, S.L.; Zhao, M.; Zhang, J.; Zhou, H.; Cai, Y.; Huang, F.; Wei, Q. MOF-based C-doped coupled TiO2/ZnO nanofibrous membrane with crossed network connection for enhanced photocatalytic activity. J. Alloys Compd. 2019, 777, 982–990. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Qu, W.; Li, H.; Shi, L.; Zhang, D. Nanodiamond-decorated ZnO catalysts with enhanced photocorrosion-resistance for photocatalytic degradation of gaseous toluene. Appl. Catal. B Environ. 2019, 257, 117880. [Google Scholar] [CrossRef]
- Rachna, R.M.; Shanker, U. Sunlight assisted degradation of toxic phenols by zinc oxide doped prussian blue nanocomposite. J. Environ. Chem. Eng. 2020, 8, 104040. [Google Scholar] [CrossRef]
- Potle, V.D.; Shirsath, S.R.; Bhanvase, B.A.; Saharan, V.K. Sonochemical preparation of ternary rGO-ZnO-TiO2 nanocomposite photocatalyst for efficient degradation of crystal violet dye. Optik 2020, 208, 164555. [Google Scholar] [CrossRef]
- Viet, T.Q.Q.; Khoi, V.H.; Giang, N.T.H.; Van Anh, H.T.; Dat, N.M.; Phong, M.T.; Hieu, N.H. Statistical screening and optimization of photocatalytic degradation of methylene blue by ZnO–TiO2/rGO nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127464. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, G.; Duan, X.; Liang, X.; Meng, J.; Liang, J. Environmental applications of diatomite minerals in removing heavy metals from water. Ind. Eng. Chem. Res. 2019, 58, 11638–11652. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, G.; Xu, Y.; Odoom-Wubah, T.; Jia, L.; Huang, J.; Sun, D.; Li, Q. Diatomite supported Pt nanoparticles as efficient catalyst for benzene removal. Ind. Eng. Chem. Res. 2019, 58, 14008–14015. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Yang, P.; Yang, Y.; Hu, J.; Wei, J.; Yu, Q. In situ synthesis of diatomite–carbon nanotube composite adsorbent and its adsorption characteristics for phenolic compounds. J. Chem. Eng. Data 2018, 64, 360–371. [Google Scholar] [CrossRef]
- Sun, Q.; Li, H.; Zheng, S.; Sun, Z. Characterizations of nano-TiO2/diatomite composites and their photocatalytic reduction of aqueous Cr (VI). Appl. Surf. Sci. 2014, 311, 369–376. [Google Scholar] [CrossRef]
- Xiao, L.; Pang, B. Preparation of diatomite supported nano zinc oxide composite photocatalytic material and study on its formaldehyde degradation. IOP Conf. Ser. Mater. Sci. Eng. 2017, 242, 012016. [Google Scholar] [CrossRef]
- Vasilaki, E.; Katsarakis, N.; Dokianakis, S.; Vamvakaki, M. rGO functionalized ZnO–TiO2 core-shell flower-like architectures for visible light photocatalysis. Catalysts 2021, 11, 332. [Google Scholar] [CrossRef]
- Hua, C.; Liu, X.; Ren, S.; Zhang, C.; Liu, W. Preparation of visible light-responsive photocatalytic paper containing BiVO4@diatomite/MCC/PVBCFs for degradation of organic pollutants. Ecotoxicol. Environ. Saf. 2020, 202, 110897. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Ren, Q.; Liu, X.; Jin, Z.; Ding, Y.; Zhu, H.; Li, J.; Chen, R. Fenton activity on RhB degradation of magnetic g-C3N4/diatomite/Fe3O4 composites. Appl. Surf. Sci. 2021, 543, 148844. [Google Scholar] [CrossRef]
- Liu, X.; He, Y.; Yang, B.; Yan, Q.; Yang, J. Highly efficient photo-degradation of gaseous organic pollutants catalyzed by diatomite-supported titanium dioxide. Catalysts 2020, 10, 380. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Synthesis of highly monodispersed teardrop-shaped core–shell SiO2/TiO2 nanoparticles and their photocatalytic activities. Appl. Surf. Sci. 2015, 351, 320–326. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Zhang, J.; Guo, W.; Li, L.; Liu, H.; Wang, Z.L. One-step synthesis of ultrathin nanobelts-assembled urchin-like anatase TiO2 nanostructures for highly efficient photocatalysis. CrystEngComm 2017, 19, 129–136. [Google Scholar] [CrossRef]
- Najibi Ilkhechi, N.; Mozammel, M.; Yari Khosroushahi, A. Antifungal effects of ZnO, TiO2 and ZnO-TiO2 nanostructures on Aspergillus flavus. Pestic. Biochem. Physiol. 2021, 176, 104869. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Guo, L.; Shang, L.; Ge, S.; Song, G.; Naik, N.; Shao, Q.; Lin, J.; Guo, Z. Metal organic framework-derived C-doped ZnO/TiO2 nanocomposite catalysts for enhanced photodegradation of Rhodamine B. J. Colloid Interface Sci. 2021, 599, 566–576. [Google Scholar] [CrossRef]
- Tyagi, J.; Gupta, H.; Purohit, L.P. Mesoporous ZnO/TiO2 photoanodes for quantum dot sensitized solar cell. Opt. Mater. 2021, 115, 111014. [Google Scholar] [CrossRef]
- Yang, B.; Liu, X.; Ma, Z.; Wang, Q.; Yang, J. Synthesis of nano-ZnO/diatomite composite and research on photoelectric application. Catalysts 2021, 11, 1232. [Google Scholar] [CrossRef]
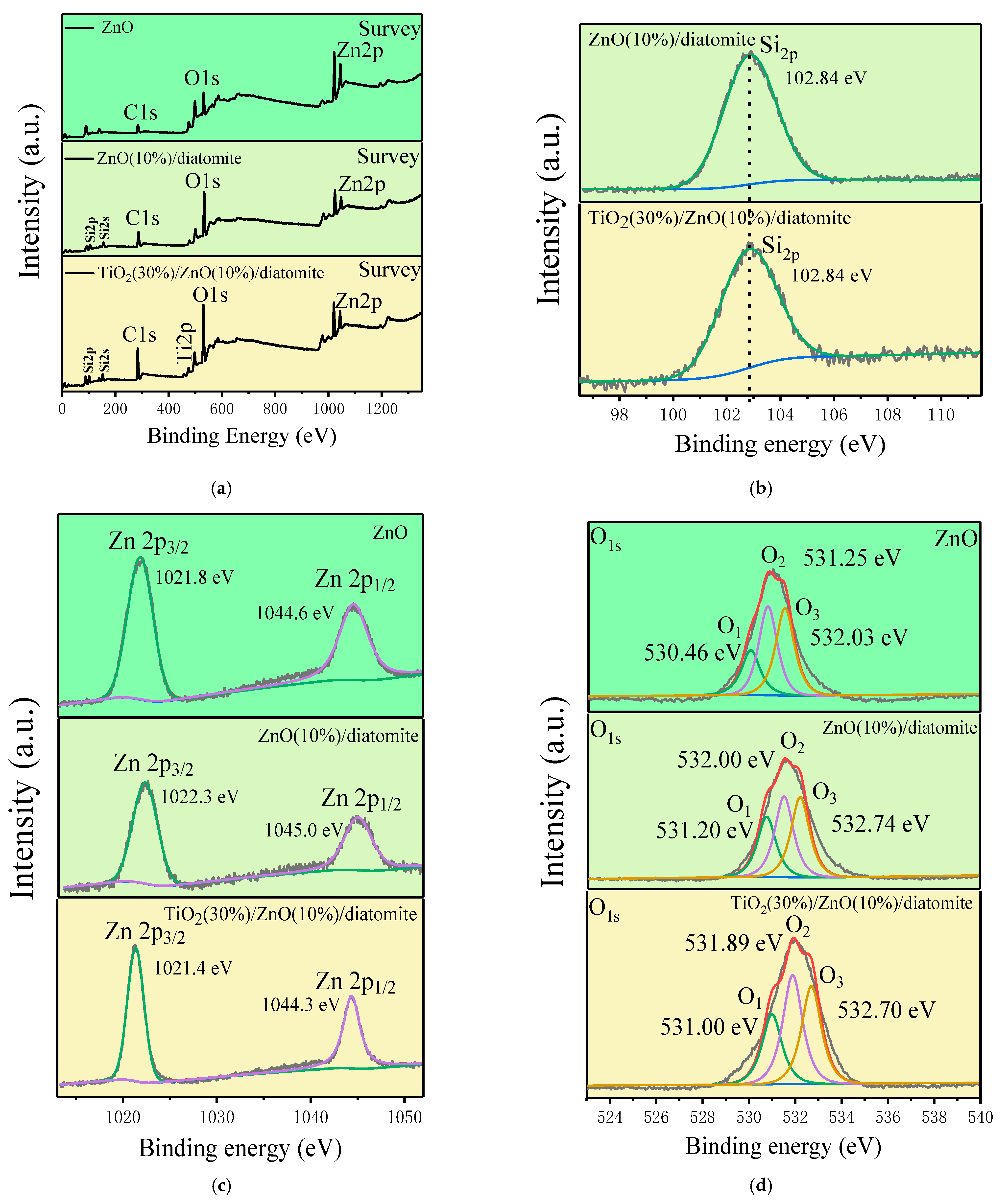
| Oxygen Species | O1 | O2 | O3 | |
|---|---|---|---|---|
| Sample | ||||
| ZnO | 28.02% | 47.81% | 24.17% | |
| ZnO(10%)/diatomite | 26.77% | 44.67% | 28.56% | |
| TiO2(30%)/ZnO(10%)/diatomite | 25.27% | 39.43% | 35.30% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Ma, Z.; Wang, Q.; Yang, J. Synthesis and Photoelectrocatalytic Applications of TiO2/ZnO/Diatomite Composites. Catalysts 2022, 12, 268. https://doi.org/10.3390/catal12030268
Yang B, Ma Z, Wang Q, Yang J. Synthesis and Photoelectrocatalytic Applications of TiO2/ZnO/Diatomite Composites. Catalysts. 2022; 12(3):268. https://doi.org/10.3390/catal12030268
Chicago/Turabian StyleYang, Beibei, Zixu Ma, Qian Wang, and Junjiao Yang. 2022. "Synthesis and Photoelectrocatalytic Applications of TiO2/ZnO/Diatomite Composites" Catalysts 12, no. 3: 268. https://doi.org/10.3390/catal12030268
APA StyleYang, B., Ma, Z., Wang, Q., & Yang, J. (2022). Synthesis and Photoelectrocatalytic Applications of TiO2/ZnO/Diatomite Composites. Catalysts, 12(3), 268. https://doi.org/10.3390/catal12030268





