Modelling and Optimisation of the Sol-Gel Conditions for Synthesis of Semi-Hexagonal Titania-Based Nano-Catalyst for Esterification Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Assessment of Synthesised Catalysts
2.2. Optimisation of Parameters in SVM and MLP Models
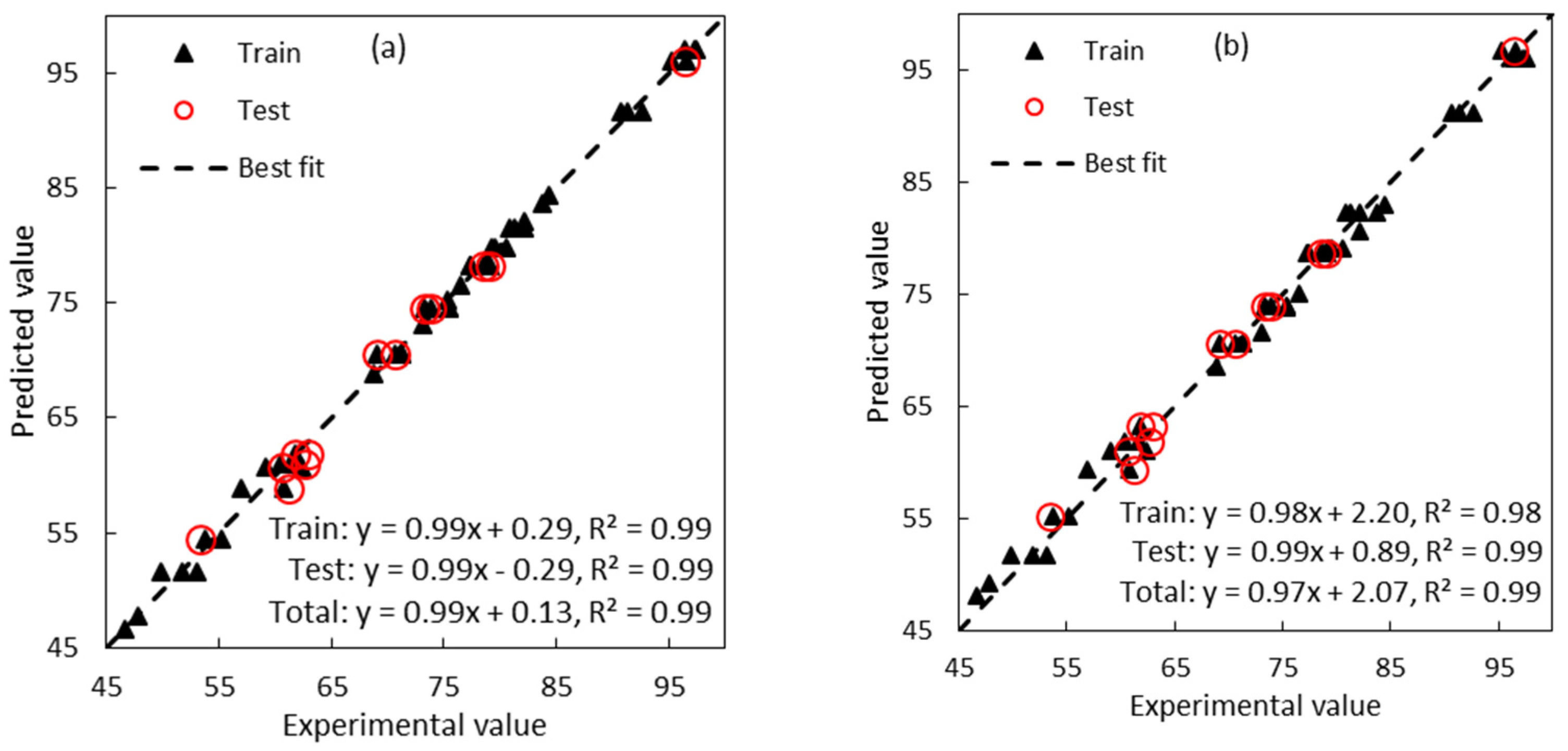
2.3. Assessment of SVM and MLP Models
2.4. Sensitivity Analysis
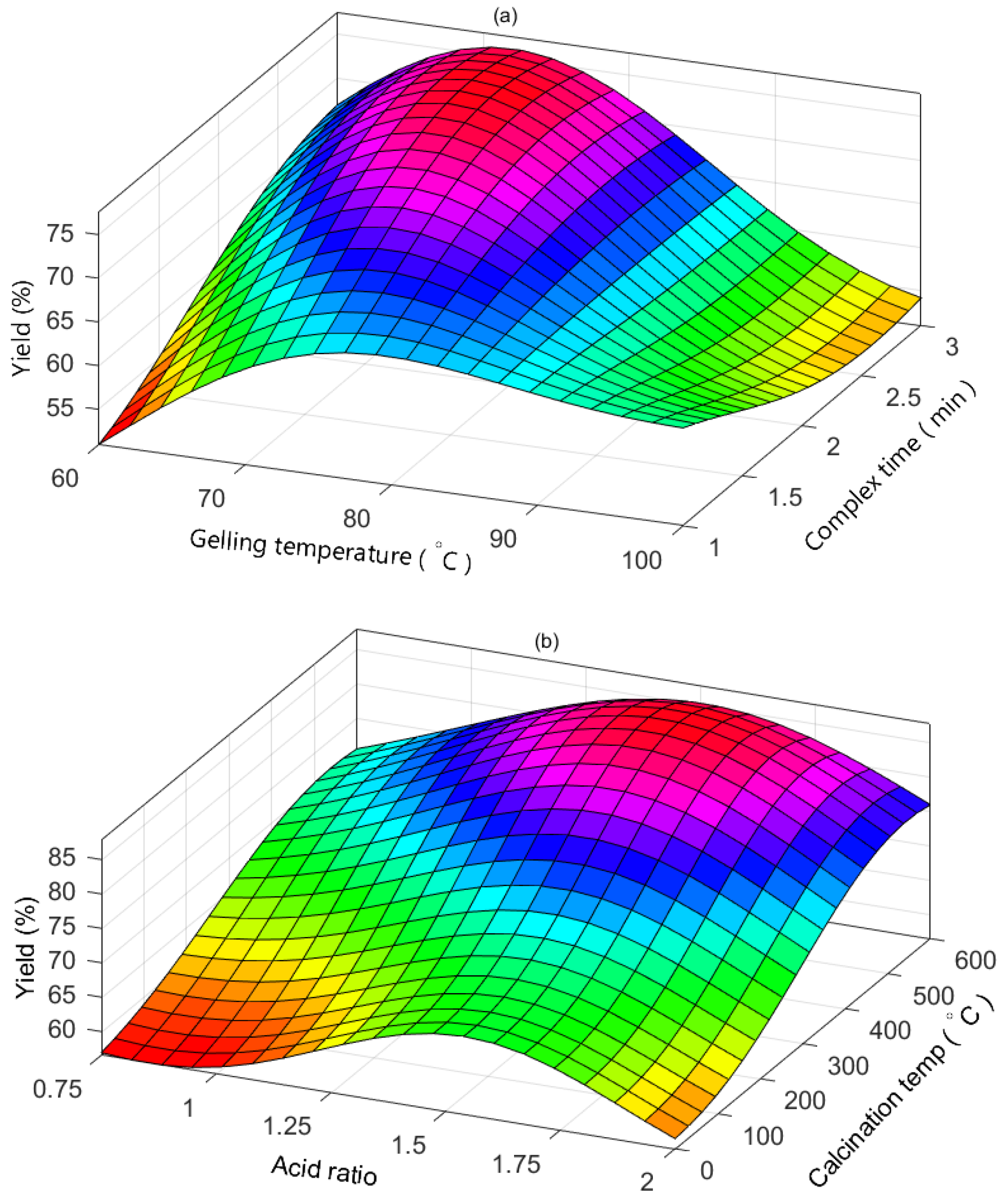
2.5. Assessment of Independent Variable Interaction
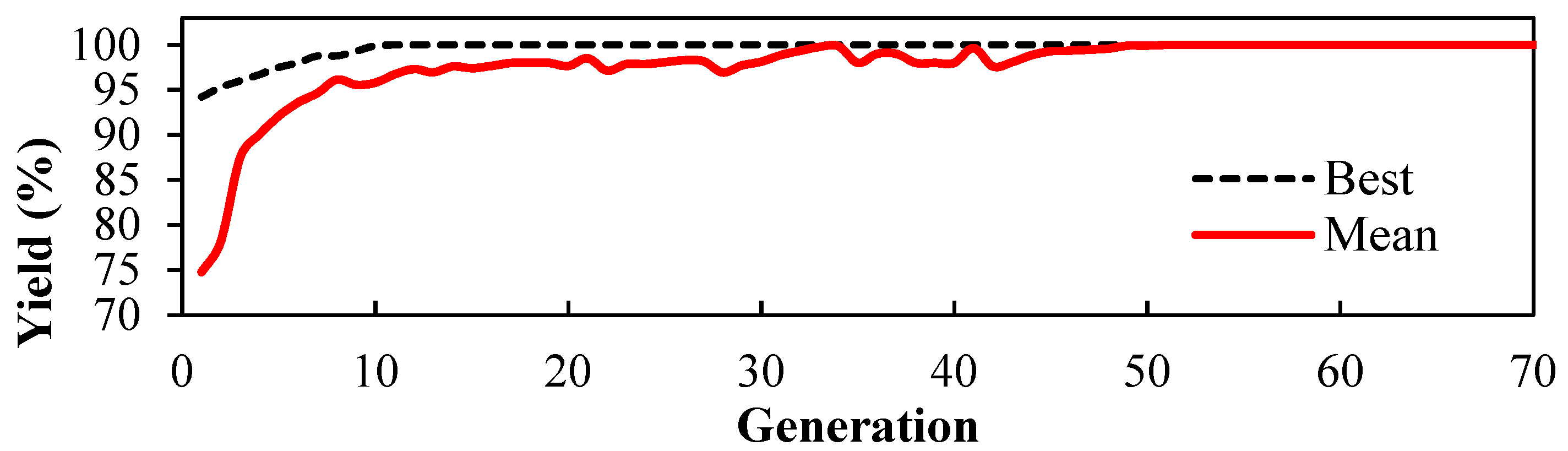
2.6. Optimisation of Catalyst Synthesis Conditions Using Genetic Algorithm
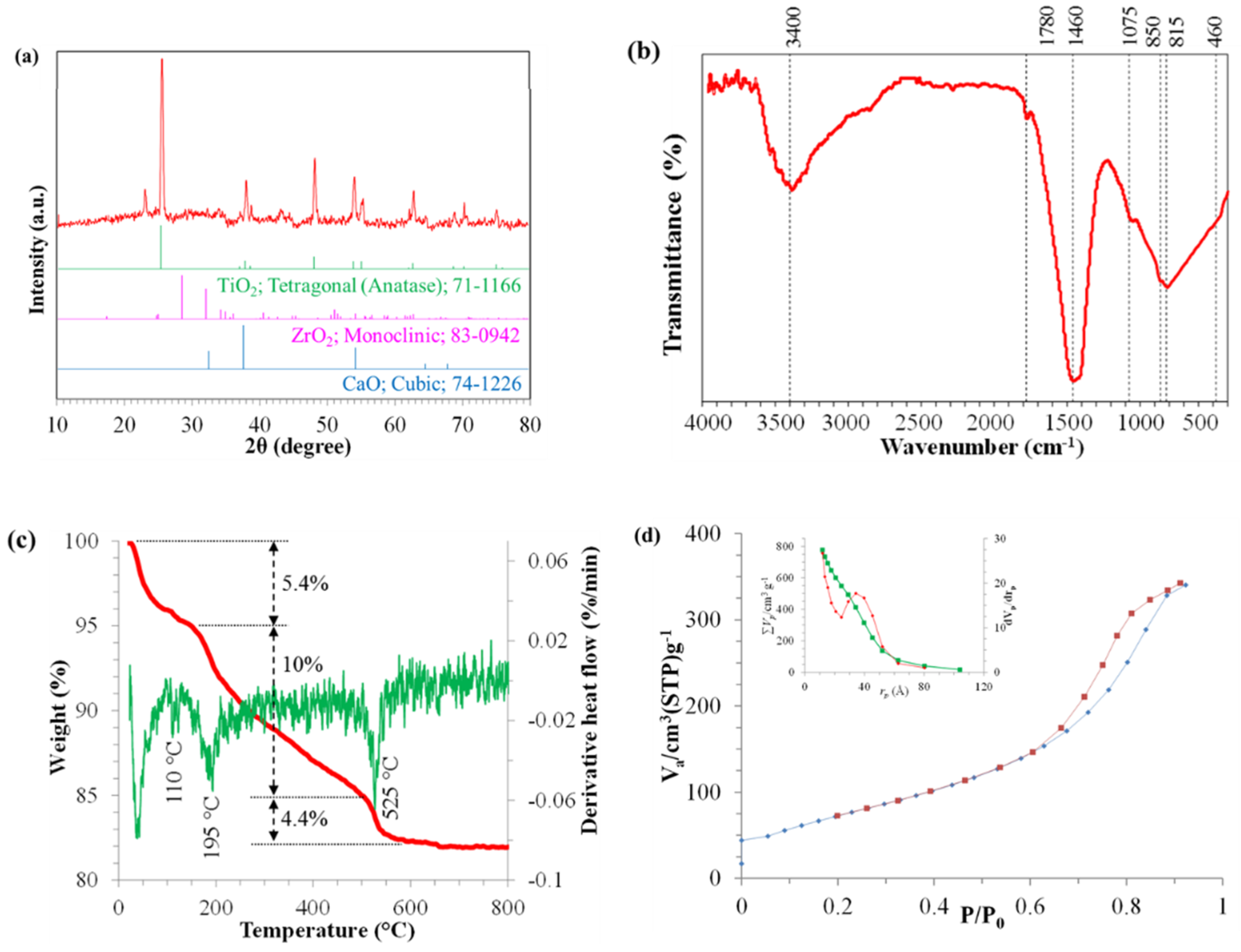
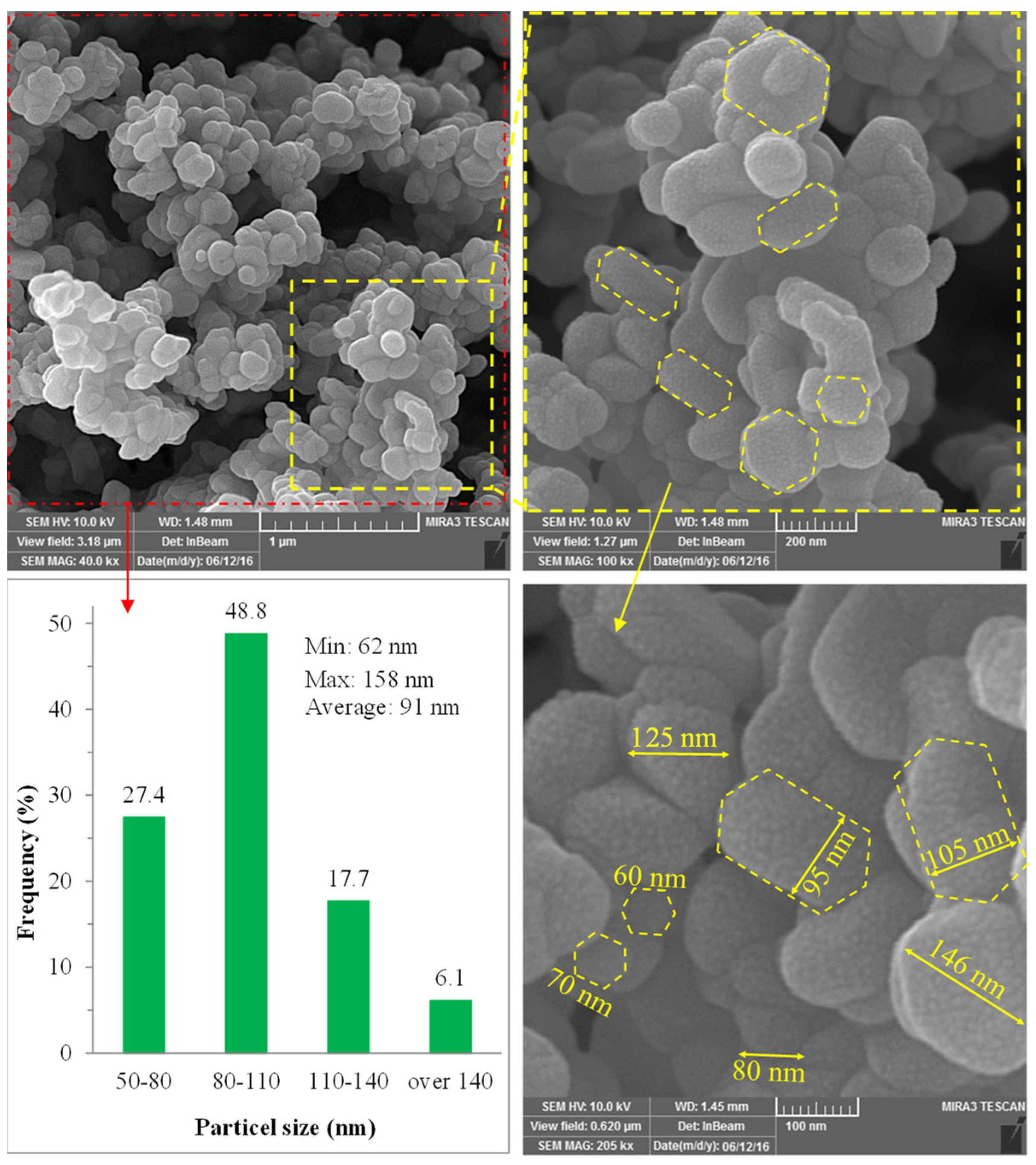
2.7. Structural Assessment of Optimum Catalyst
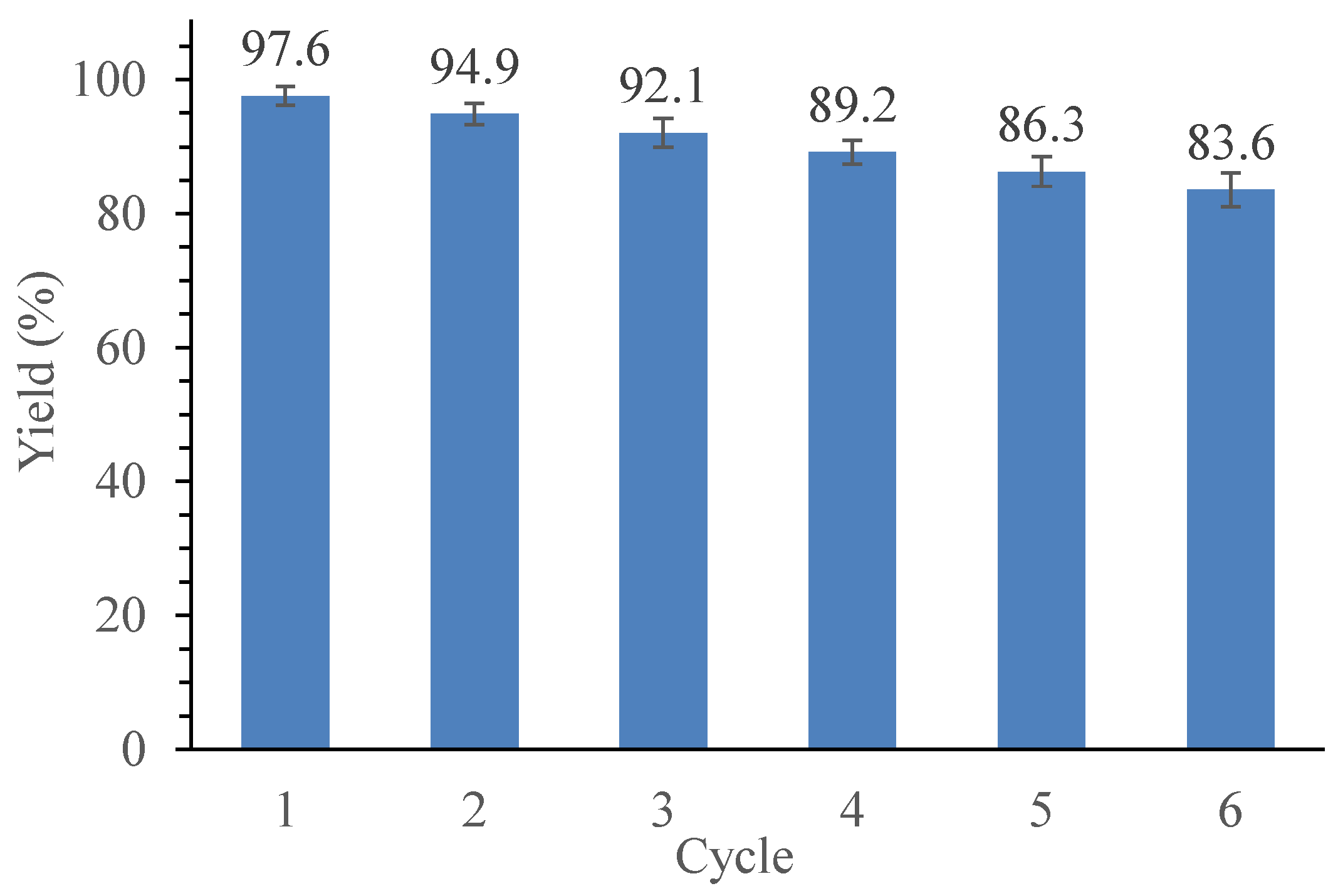
2.8. Reusability Assessment of the Optimum Nano-Catalyst
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterisation
3.3. Catalyst Testing
3.4. Multi-Layer Perceptron (MLP) Model
3.5. Support Vector Machine (SVM) Model
3.6. Genetic Algorithm (GA) for Yield Optimisation
3.7. MLP and SVM Assessment Criteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
Nomenclature
| MLP | Multi-layer perceptron |
| SVM | Support vector machine |
| GA | Genetic algorithm |
| FFA | Free fatty acid |
| TG | Triglyceride |
| ANN | Artificial neural network |
| XRD | X-ray powder diffraction |
| FTIR | Fourier-transform infrared spectroscopy |
| TGA | Thermogravimetric analysis |
| BET | Brunauer–Emmett–Teller |
| FESEM | Field Emission Scanning Electron Microscopy |
| TEM | Transmission electron microscopy |
| R2 | Coefficient of determination |
| RMSE | Root mean square error |
| Trainlm | Training algorithm of Levenberg-Marquardt |
| Traincgf | Training algorithm of Fletcher–Powell Conjugate Gradient |
| Poly1 | Kernel functions of linear |
| Poly2 | Second-order function |
| Poly3 | Third-order function |
| RBF | Radial bias function |
References
- Younis, K.A.; Gardy, J.L.; Barzinji, K.S. Production and characterization of biodiesel from locally sourced sesame seed oil, used cooking oil and other commercial vegetable oils in Erbil-Iraqi Kurdistan. J. Am. J. Appl. Chem. 2014, 2, 105–111. [Google Scholar]
- Lowe, B.; Gardy, J.; Hassanpour, A. The role of sulfated materials for biodiesel production from cheap raw materials. Catalysts 2022, 12, 223. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Elsayed, M.; Esakkimuthu, S.; El-Sheekh, M.; Hanelt, D. Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci. 2020, 81, 100868. [Google Scholar] [CrossRef]
- Gardy, J.L.I.A. Biodiesel Production from Used Cooking Oil Using Novel Solid Acid Catalysts. Ph.D. Thessis, University of Leeds, Leeds, UK, 2017. [Google Scholar]
- Mazubert, A.; Poux, M.; Aubin, J. Intensified processes for FAME production from waste cooking oil: A technological review. Chem. Eng. J. 2013, 233, 201–223. [Google Scholar] [CrossRef] [Green Version]
- Chozhavendhan, S.; Vijay Pradhap Singh, M.; Fransila, B.; Praveen Kumar, R.; Karthiga Devi, G. A review on influencing parameters of biodiesel production and purification processes. Curr. Res. Green Sustain. Chem. 2020, 1–2, 1–6. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumar Sharma, P.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Gardy, J.; Rehan, M.; Hassanpour, A.; Lai, X.; Nizami, A.-S. Advances in nano-catalysts based biodiesel production from non-food feedstocks. J. Environ. Manag. 2019, 249, 109316. [Google Scholar] [CrossRef]
- Chua, S.Y.; Periasamy, L.A.P.; Goh, C.M.H.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Walvekar, R.; Abdullah, E.C. Biodiesel synthesis using natural solid catalyst derived from biomass waste—A review. J. Ind. Eng. Chem. 2020, 81, 41–60. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Gardy, J.; Osatiashtiani, A.; Céspedes, O.; Hassanpour, A.; Lai, X.; Lee, A.F.; Wilson, K.; Rehan, M. A magnetically separable SO4/Fe-Al-TiO2 solid acid catalyst for biodiesel production from waste cooking oil. J. Appl. Catal. B Environ. 2018, 234, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Gardy, J.; Nourafkan, E.; Osatiashtiani, A.; Lee, A.F.; Wilson, K.; Hassanpour, A.; Lai, X. A core-shell SO4/Mg-Al-Fe3O4 catalyst for biodiesel production. J. Appl. Catal. B Environ. 2019, 259, 118093. [Google Scholar] [CrossRef]
- Lowe, B.; Gardy, J.; Wu, K.; Hassanpour, A. Mixed metal oxide catalysts. In Bio-Diesel Production: Feedstocks, Catalysts and Technologies; Rokhum, S.L., Halder, G., Assabumrun, S., Ngaosuwan., K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 143–166. [Google Scholar]
- Kesserwan, F.; Ahmad, M.N.; Khalil, M.; El-Rassy, H. Hybrid CaO/Al2O3 aerogel as heterogeneous catalyst for biodiesel production. Chem. Eng. J. 2020, 385, 123834. [Google Scholar] [CrossRef]
- Al-Qaysi, K.; Nayebzadeh, H.; Saghatoleslami, N.; Gardy, J. Effect of the loading of di-and tri-valent metal cations on the performance of sulfated silica-titania nano-catalyst in the esterification reaction. J. Nanostruct. 2021, 11, 221–235. [Google Scholar]
- Chen, C.; Cai, L.; Zhang, L.; Fu, W.; Hong, Y.; Gao, X.; Jiang, Y.; Li, L.; Yan, X.; Wu, G. Transesterification of rice bran oil to biodiesel using mesoporous NaBeta zeolite-supported molybdenum catalyst: Experimental and kinetic studies. Chem. Eng. J. 2020, 382, 122839. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ma, X.; Wu, Z.; Cui, P.; Lu, W.; Liu, F.; Chu, H.; Wang, Y. A novel magnetic CaO-based catalyst synthesis and characterization: Enhancing the catalytic activity and stability of CaO for biodiesel production. Chem. Eng. J. 2020, 391, 123549. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, F.; Xu, L.; Peng, R.; Li, Y.; Deng, J. Batch and continuous esterification for the direct synthesis of high qualified biodiesel from waste cooking oils (WCO) with Amberlyst-15/Poly (vinyl alcohol) membrane as a bifunctional catalyst. Chem. Eng. J. 2020, 388, 124214. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A.K. An overview on the recent advancements of sustainable heterogeneous catalysts and prominent continuous reactor for biodiesel production. J. Ind. Eng. Chem. 2020, 88, 58–77. [Google Scholar] [CrossRef]
- Ravi, A.; Gurunathan, B.; Rajendiran, N.; Varjani, S.; Gnansounou, E.; Pandey, A.; You, S.; Raman, J.K.; Ramanujam, P. Contemporary approaches towards augmentation of distinctive heterogeneous catalyst for sustainable biodiesel production. Environ. Technol. Innov. 2020, 19, 100906. [Google Scholar] [CrossRef]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H.; Rehan, M. Biodiesel production from used cooking oil using a novel surface functionalised TiO2 nano-catalyst. J. Appl. Catal. B Environ. 2017, 207, 297–310. [Google Scholar] [CrossRef] [Green Version]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H. Synthesis of Ti(SO4)O solid acid nano-catalyst and its application for biodiesel production from used cooking oil. J. Appl. Catal. A Gen. 2016, 527, 81–95. [Google Scholar] [CrossRef]
- Lowe, B.; Ahmad, A.; Gardy, J.; Hassanpour, A. Nanomaterials used in biorefineries: Types, properties, and synthesis methods. In Nanotechnology for Biorefinery; Ingle, A.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Enesca, A.; Isac, L.; Duta, A. Charge carriers injection in tandem semiconductors for dyes mineralization. J. Appl. Catal. B Environ. 2015, 162, 352–363. [Google Scholar] [CrossRef]
- Nayebzadeh, H.; Saghatoleslami, N.; Rahmani Vahid, B.; Maskooki, A. Effect of calcination temperature on catalytic activity of synthesis SrO/S-ZrO2 by solvent-free method in esterification of oleic acid. Chem. Biochem. Eng. Q. 2013, 23, 267–273. [Google Scholar]
- Vahid, B.R.; Saghatoleslami, N.; Nayebzadeh, H.; Maskooki, A. Preparation of nano-size Al-promoted sulfated zirconia and the impact of calcination temperature on its catalytic activity. Chem. Biochem. Eng. Q. 2012, 26, 71–77. [Google Scholar]
- Pinto, B.F.; Garcia, M.A.S.; Costa, J.C.S.; de Moura, C.V.R.; de Abreu, W.C.; de Moura, E.M. Effect of calcination temperature on the application of molybdenum trioxide acid catalyst: Screening of substrates for biodiesel production. Fuel 2019, 239, 290–296. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Abu-Dahrieh, J.K.; Atia, H.; Armbruster, U.; Ibrahim, A.A.; Khan, W.U.; Abasaeed, A.E.; Fakeeha, A.H. Effect of pre-treatment and calcination temperature on Al2O3-ZrO2 supported Ni-Co catalysts for dry reforming of methane. Int. J. Hydrog. Energy 2019, 44, 21546–21558. [Google Scholar] [CrossRef] [Green Version]
- Jeon, K.-W.; Shim, J.-O.; Jang, W.-J.; Lee, D.-W.; Na, H.-S.; Kim, H.-M.; Lee, Y.-L.; Yoo, S.-Y.; Roh, H.-S.; Jeon, B.-H.; et al. Effect of calcination temperature on the association between free NiO species and catalytic activity of Ni−Ce0.6Zr0.4O2 deoxygenation catalysts for biodiesel production. Renew. Energy 2019, 131, 144–151. [Google Scholar] [CrossRef]
- Al-Qaysi, K.; Nayebzadeh, H.; Saghatoleslami, N. Comprehensive Study on the Effect of Preparation Conditions on the Activity of Sulfated Silica–Titania for Green Biofuel Production. J. Inorg. Organomet. Polym. Mater. 2020, in press. [Google Scholar] [CrossRef]
- Sakti La Ore, M.; Wijaya, K.; Trisunaryanti, W.; Saputri, W.D.; Heraldy, E.; Yuwana, N.W.; Hariani, P.L.; Budiman, A.; Sudiono, S. The synthesis of SO4/ZrO2 and Zr/CaO catalysts via hydrothermal treatment and their application for conversion of low-grade coconut oil into biodiesel. J. Environ. Chem. Eng. 2020, 8, 104205. [Google Scholar] [CrossRef]
- Kitajima, H.; Higashino, Y.; Matsuda, S.; Zhong, H.; Watanabe, M.; Aida, T.M.; Smith, R.L. Isomerization of glucose at hydrothermal condition with TiO2, ZrO2, CaO-doped ZrO2 or TiO2-doped ZrO2. Catal. Today 2016, 274, 67–72. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Mahmoud, H.R.; El-Molla, S.A. Influence of support on physicochemical properties of ZrO2 based solid acid heterogeneous catalysts for biodiesel production. Catal. Commun. 2019, 122, 10–15. [Google Scholar] [CrossRef]
- De, A.; Boxi, S.S. Application of Cu impregnated TiO2 as a heterogeneous nanocatalyst for the production of biodiesel from palm oil. Fuel 2020, 265, 117019. [Google Scholar] [CrossRef]
- Salinas, D.; Guerrero, S.; Campos, C.H.; Bustamante, T.M.; Pecchi, G. The Effect of the ZrO2 Loading in SiO2@ZrO2-CaO Catalysts for Transesterification Reaction. Materials 2020, 13, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamad, M.; Ngadi, N.; Wong, S.; Yahya, N.Y.; Inuwa, I.M.; Lani, N.S. Synthesis and Characterization of CaO-TiO2 for Transesterification of Vegetable Palm Oil. Int. J. Eng. 2018, 31, 1326–1333. [Google Scholar]
- Nayebzadeh, H.; Saghatoleslami, N.; Tabasizadeh, M. Optimization of the activity of KOH/calcium aluminate nanocatalyst for biodiesel production using response surface methodology. J. Taiwan Inst. Chem. Eng. 2016, 68, 379–386. [Google Scholar] [CrossRef]
- Mirzaei, M.; Sabbaghi, S.; Zerafat, M.M. Photo-catalytic degradation of formaldehyde using nitrogen-doped TiO2 nano-photocatalyst: Statistical design with response surface methodology (RSM). Can. J. Chem. Eng. 2018, 96, 2544–2552. [Google Scholar] [CrossRef]
- Naghibi, S.; Faghihi Sani, M.A.; Hosseini, H.R.M. Application of the statistical Taguchi method to optimize TiO2 nanoparticles synthesis by the hydrothermal assisted sol–gel technique. Ceram. Int. 2014, 40, 4193–4201. [Google Scholar] [CrossRef]
- Mohammad, A.-T.; Abdulhameed, A.S.; Jawad, A.H. Box-Behnken design to optimize the synthesis of new crosslinked chitosan-glyoxal/TiO2 nanocomposite: Methyl orange adsorption and mechanism studies. Int. J. Biol. Macromol. 2019, 129, 98–109. [Google Scholar] [CrossRef]
- Azadi, S.; Karimi-Jashni, A.; Javadpour, S. Modeling and optimization of photocatalytic treatment of landfill leachate using tungsten-doped TiO2 nano-photocatalysts: Application of artificial neural network and genetic algorithm. Process. Saf. Environ. Prot. 2018, 117, 267–277. [Google Scholar] [CrossRef]
- Pereira, S.R.M.; Clerc, F.; Farrusseng, D.; van der Waal, J.C.; Maschmeyer, T.; Mirodatos, C. Effect of the Genetic Algorithm Parameters on the Optimisation of Heterogeneous Catalysts. QSAR Comb. Sci. 2005, 24, 45–57. [Google Scholar] [CrossRef]
- Kim, S.; Sohn, K.-S.; Pyo, M. Genetic Algorithm-Assisted Optimization of Nanoporous TiO2 for Low-Temperature Processable Photoanodes of Dye-Sensitized Solar Cells. ACS Comb. Sci. 2011, 13, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Soleimanzadeh, H.; Niaei, A.; Salari, D.; Tarjomannejad, A.; Penner, S.; Grünbacher, M.; Hosseini, S.A.; Mousavi, S.M. Modeling and optimization of V2O5/TiO2 nanocatalysts for NH3-Selective catalytic reduction (SCR) of NOx by RSM and ANN techniques. J. Environ. Manag. 2019, 238, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Hatte, Q.; Dubos, P.-A.; Guitter, N.; Richard-Plouet, M.; Casari, P. Influence of relative humidity and temperature on the sol-gel transition of a siloxane surface treatment. J. Sol.-Gel Sci. Technol. 2019, 90, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-M.; Zhai, S.-R.; Zhai, B.; An, Q.-D.; Tian, G. Crucial factors affecting the physicochemical properties of sol–gel produced Fe3O4@SiO2–NH2 core–shell nanomaterials. J. Sol.-Gel Sci. Technol. 2012, 64, 347–357. [Google Scholar] [CrossRef]
- Cheng, L.-j.; Liu, Z.; Yuan, S.-l.; Hu, X.; Zhang, B.; Jiang, Y. Preparation of Ag-Mn/γ-Al2O3-TiO2 catalysts by complexation-impregnation process with citric acid and its application in propane catalytic combustion. J. Fuel Chem. Technol. 2019, 47, 1379–1385. [Google Scholar] [CrossRef]
- Sistani, A.; Saghatoleslami, N.; Nayebzadeh, H. Influence of calcination temperature on the activity of mesoporous CaO/TiO2–ZrO2 catalyst in the esterification reaction. J. Nanostructure Chem. 2018, 8, 321–331. [Google Scholar] [CrossRef]
- Silahua-Pavón, A.A.; Espinosa-González, C.G.; Ortiz-Chi, F.; Pacheco-Sosa, J.G.; Pérez-Vidal, H.; Arévalo-Pérez, J.C.; Godavarthi, S.; Torres-Torres, J.G. Production of 5-HMF from glucose using TiO2-ZrO2 catalysts: Effect of the sol-gel synthesis additive. Catal. Commun. 2019, 129, 105723. [Google Scholar] [CrossRef]
- Fan, M.; Si, Z.; Sun, W.; Zhang, P. Sulfonated ZrO2-TiO2 nanorods as efficient solid acid catalysts for heterogeneous esterification of palmitic acid. Fuel 2019, 252, 254–261. [Google Scholar] [CrossRef]
- Li, K.-T.; Wang, C.-K.; Wang, I.; Wang, C.-M. Esterification of lactic acid over TiO2–ZrO2 catalysts. Appl. Catal. A Gen. 2011, 392, 180–183. [Google Scholar] [CrossRef]
- Nayebzadeh, H.; Haghighi, M.; Saghatoleslami, N.; Alaei, S.; Yousefi, S. Texture/phase evolution during plasma treatment of microwave-combustion synthesized KOH/Ca12Al14O33-C nanocatalyst for reusability enhancement in conversion of canola oil to biodiesel. Renew. Energy 2019, 139, 28–39. [Google Scholar] [CrossRef]
- Kazemifard, S.; Nayebzadeh, H.; Saghatoleslami, N.; Safakish, E. Assessment the activity of magnetic KOH/Fe3O4@Al2O3 core–shell nanocatalyst in transesterification reaction: Effect of Fe/Al ratio on structural and performance. Environ. Sci. Pollut. Res. 2018, 25, 32811–32821. [Google Scholar] [CrossRef] [PubMed]
- Rahmani Vahid, B.; Saghatoleslami, N.; Nayebzadeh, H.; Toghiani, J. Effect of alumina loading on the properties and activity of SO42−/ZrO2 for biodiesel production: Process optimization via response surface methodology. J. Taiwan Inst. Chem. Eng. 2018, 83, 115–123. [Google Scholar] [CrossRef]
- Safakish, E.; Nayebzadeh, H.; Saghatoleslami, N.; Kazemifard, S. Comprehensive assessment of the preparation conditions of a separable magnetic nanocatalyst for biodiesel production from algae. Algal Res. 2020, 49, 101949. [Google Scholar] [CrossRef]
- Nayebzadeh, H.; Naderi, F.; Rahmanivahid, B. Assessment the synthesis conditions of separable magnetic spinel nanocatalyst for green fuel production: Optimization of transesterification reaction conditions using response surface methodology. Fuel 2020, 271, 117595. [Google Scholar] [CrossRef]
- Nayebzadeh, H.; Saghatoleslami, N.; Haghighi, M.; Tabasizadeh, M.; Binaeian, E. Comparative assessment of the ability of a microwave absorber nanocatalyst in the microwave-assisted biodiesel production process. Comptes Rendus Chim. 2018, 21, 676–683. [Google Scholar] [CrossRef]
- Nayebzadeh, H.; Saghatoleslami, N.; Tabasizadeh, M. Application of microwave irradiation for fabrication of sulfated ZrO2–Al2O3 nanocomposite via combustion method for esterification reaction: Process condition evaluation. J. Nanostruct. Chem. 2019, 9, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Naderi, F.; Nayebzadeh, H. Performance and stability assessment of Mg-Al-Fe nanocatalyst in the transesterification of sunflower oil: Effect of Al/Fe molar ratio. Ind. Crops Prod. 2019, 141, 111814. [Google Scholar] [CrossRef]
- Azmoon, A.-H.; Ahmadpour, A.; Nayebzadeh, H.; Saghatoleslami, N.; Heydari, A. Fabrication of nanosized SO42−/Co–Al mixed oxide via solution combustion method used in esterification reaction: Effect of urea-nitrate ratio on the properties and performance. J. Nanostruct. Chem. 2019, 9, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Han, Q.; Jia, X.; Zahid, A.H.; Bi, H. Room-temperature one-step synthesis of tube-like S-scheme BiOBr/BiO (HCOO) Br-x heterojunction with excellent visible-light photocatalytic performance. J. Appl. Surf. Sci. 2020, 530, 147208. [Google Scholar] [CrossRef]
- Dou, L.; Jin, X.; Chen, J.; Zhong, J.; Li, J.; Zeng, Y.; Duan, R. One-pot solvothermal fabrication of S-scheme OVs-Bi2O3/Bi2SiO5 microsphere heterojunctions with enhanced photocatalytic performance toward decontamination of organic pollutants. J. Appl. Surf. Sci. 2020, 527, 146775. [Google Scholar] [CrossRef]
- Hashemzehi, M.; Pirouzfar, V.; Nayebzadeh, H.; Alihosseini, A. Application of response surface methodology to optimize high active Cu-Zn-Al mixed metal oxide fabricated via microwave-assisted solution combustion method. Adv. Powder Technol. 2020, 31, 1470–1479. [Google Scholar] [CrossRef]
- Rohani, A.; Abbaspour-Fard, M.H.; Abdolahpour, S. Prediction of tractor repair and maintenance costs using Artificial Neural Network. Expert Syst. Appl. 2011, 38, 8999–9007. [Google Scholar] [CrossRef]
- Najafi, B.; Akbarian, E.; Lashkarpour, S.M.; Aghbashlo, M.; Ghaziaskar, H.S.; Tabatabaei, M. Modeling of a dual fueled diesel engine operated by a novel fuel containing glycerol triacetate additive and biodiesel using artificial neural network tuned by genetic algorithm to reduce engine emissions. Energy 2019, 168, 1128–1137. [Google Scholar] [CrossRef]
- Taki, M.; Abdanan Mehdizadeh, S.; Rohani, A.; Rahnama, M.; Rahmati-Joneidabad, M. Applied machine learning in greenhouse simulation; new application and analysis. Inf. Process. Agric. 2018, 5, 253–268. [Google Scholar] [CrossRef]
- Miraei Ashtiani, S.-H.; Rohani, A.; Aghkhani, M.H. Soft computing-based method for estimation of almond kernel mass from its shell features. Sci. Hortic. 2020, 262, 109071. [Google Scholar] [CrossRef]
- Zareei, J.; Rohani, A. Optimization and study of performance parameters in an engine fueled with hydrogen. Int. J. Hydrog. Energy 2020, 45, 322–336. [Google Scholar] [CrossRef]
- Amini, S.; Taki, M.; Rohani, A. Applied improved RBF neural network model for predicting the broiler output energies. Appl. Soft Comput. 2020, 87, 106006. [Google Scholar] [CrossRef]






| Divide Data Set | MLP | SVM | |||||
|---|---|---|---|---|---|---|---|
| Train Ratio | Test Ratio | Train | Test | Total | Train | Test | Total |
| 80 | 20 | 1.09 | 1.11 | 1.11 | 1.18 | 0.97 | 1.14 |
| 60 | 40 | 1.59 | 4.78 | 3.26 | 2.10 | 1.57 | 1.89 |
| 40 | 60 | 2.34 | 6.37 | 5.16 | 2.10 | 3.34 | 2.85 |
| 20 | 80 | 3.61 | 7.31 | 6.72 | 2.05 | 5.31 | 4.80 |
| MLP | SVM | |||||
|---|---|---|---|---|---|---|
| Train | Test | Total | Train | Test | Total | |
| All independent variables | 1.09 | 1.11 | 1.11 | 1.18 | 0.97 | 1.14 |
| All excluding gelling temp | 4.74 | 3.84 | 4.57 | 5.15 | 3.11 | 4.81 |
| All excluding complex time | 3.54 | 4.06 | 3.65 | 3.87 | 4.66 | 4.04 |
| All excluding acid ratio | 3.19 | 3.89 | 3.35 | 2.99 | 3.84 | 3.18 |
| All excluding calcination temp | 9.28 | 6.42 | 8.77 | 7.12 | 4.59 | 6.67 |
| Acid Ratio (mol/mol) | Gelling Temp. (°C) | Complex Time (h) | Calcination Temp. (°C) | Yield (%) | |
|---|---|---|---|---|---|
| Experimental data | 1.5 | 70 | 3 | 400 | 97.1 |
| First solution of GA | 1.42 | 72 | 2.65 | 487 | 100 |
| Second solution of GA | 1.40 | 75 | 2.50 | 500 | 99.8 |
| Variables | Unit | Levels | ||||
|---|---|---|---|---|---|---|
| Acid ratio | mol/mol | 0.75 | 1 | 1.5 | 2 | |
| Complex time | h | 1 | 1.5 | 2 | 3 | |
| Gelling temperature | °C | 60 | 70 | 80 | 100 | |
| Calcination temperature | °C | 0 | 200 | 300 | 400 | 500 |
| Function | Algorithm |
|---|---|
| trainlm | Levenberg-Marquardt |
| trainrp | Resilient Backpropagation |
| traingdm | Gradient Descent with Momentum |
| trainbfg | BFGS Quasi-Newton |
| traingdx | Variable Learning Rate Gradient Descent |
| trainscg | Scaled Conjugate Gradient |
| trainoss | One Step Secant |
| traincgb | Conjugate Gradient with Powell/Beale Restarts |
| trainbr | Bayesian Regularisation |
| traingd | Gradient Descent |
| traincgp | Polak–Ribiére Conjugate Gradient |
| traincgf | Fletcher–Powell Conjugate Gradient |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayebzadeh, H.; Rohani, A.; Sistani, A.; Hassanpour, A.; Gardy, J. Modelling and Optimisation of the Sol-Gel Conditions for Synthesis of Semi-Hexagonal Titania-Based Nano-Catalyst for Esterification Reaction. Catalysts 2022, 12, 239. https://doi.org/10.3390/catal12020239
Nayebzadeh H, Rohani A, Sistani A, Hassanpour A, Gardy J. Modelling and Optimisation of the Sol-Gel Conditions for Synthesis of Semi-Hexagonal Titania-Based Nano-Catalyst for Esterification Reaction. Catalysts. 2022; 12(2):239. https://doi.org/10.3390/catal12020239
Chicago/Turabian StyleNayebzadeh, Hamed, Abbas Rohani, Aliakbar Sistani, Ali Hassanpour, and Jabbar Gardy. 2022. "Modelling and Optimisation of the Sol-Gel Conditions for Synthesis of Semi-Hexagonal Titania-Based Nano-Catalyst for Esterification Reaction" Catalysts 12, no. 2: 239. https://doi.org/10.3390/catal12020239
APA StyleNayebzadeh, H., Rohani, A., Sistani, A., Hassanpour, A., & Gardy, J. (2022). Modelling and Optimisation of the Sol-Gel Conditions for Synthesis of Semi-Hexagonal Titania-Based Nano-Catalyst for Esterification Reaction. Catalysts, 12(2), 239. https://doi.org/10.3390/catal12020239









