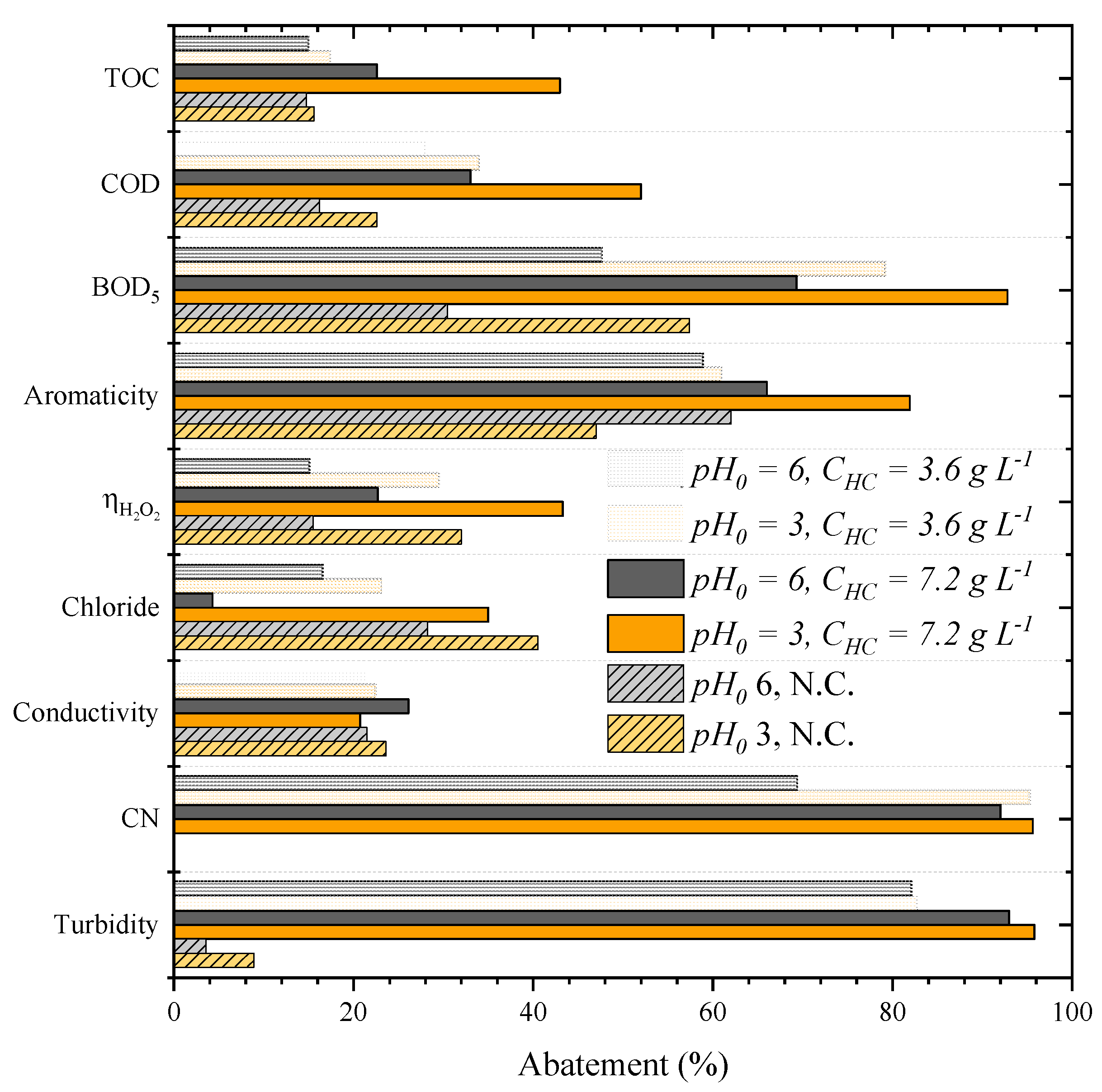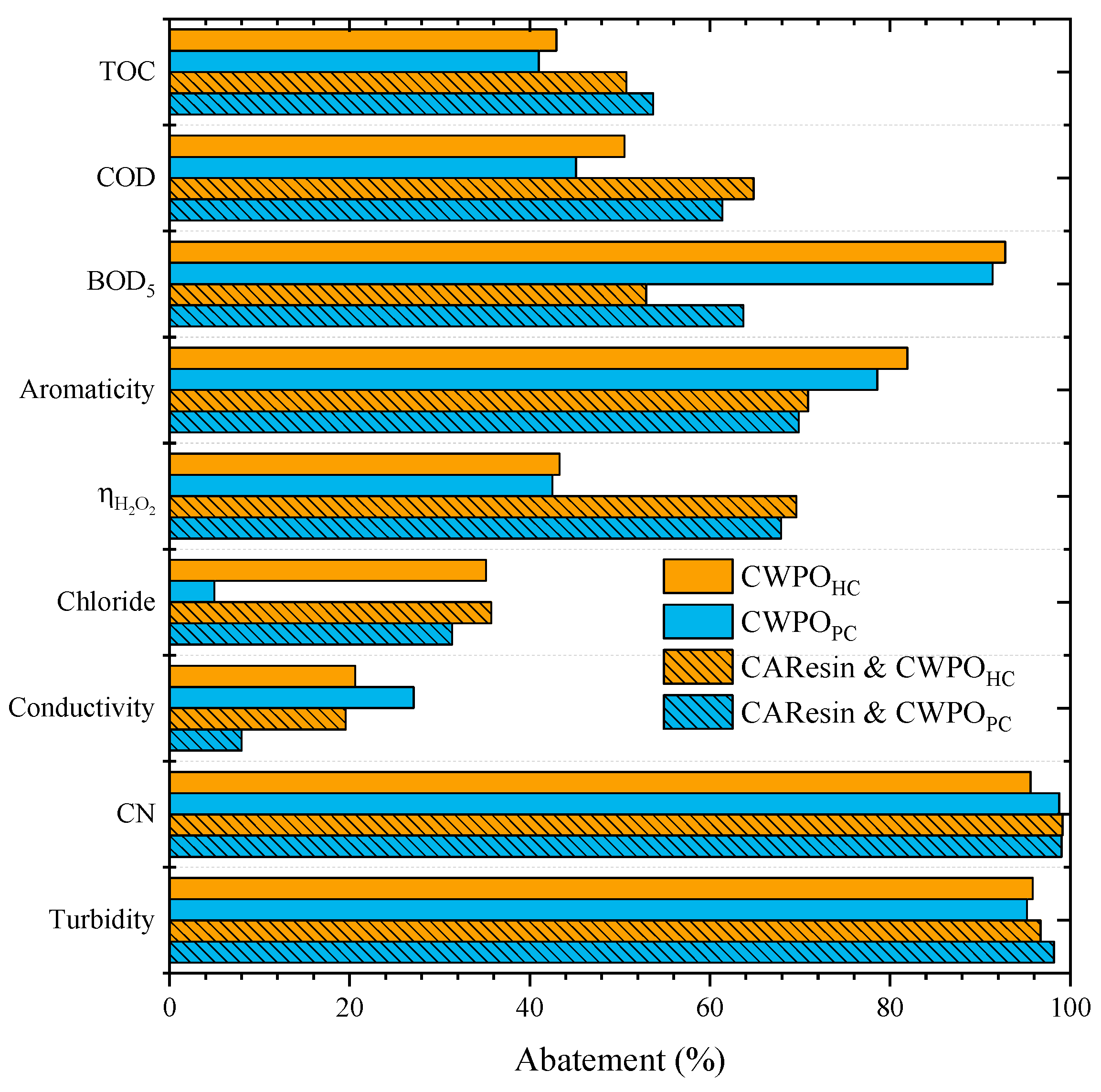Abstract
Matured compost, derived from a mechanical and biological treatment (MBT) plant, was used as a precursor to produce catalysts through hydrothermal and thermal carbonization, HC and PC, respectively. HC and PC displayed suitable properties to act as catalysts in the catalytic wet peroxide oxidation (CWPO) treatment of the highly polluted leachate waters generated in the same MBT plant (TOC0 = 27 g L−1; COD0 = 60 g L−1; BOD5,0 = 23 g L−1). The influence of catalyst loading and pH were studied, considering multiple additions of H2O2. The best experimental conditions found were T = 80 °C, pH0 = 3.0, 7.2 g L−1 of HC catalyst, 85.7 g L−1 of H2O2, added in five batches in one-hour intervals between each addition. Under these experimental conditions, removals of 43%, 52%, 93%, 82%, 35%, 95% and 93% for the COD, TOC, BOD5, aromaticity, chlorides, turbidity and color number (CN) were, respectively, observed. Ion exchange resins and coagulation–flocculation were studied as pretreatment options to reduce the complexity of the leachate waters and enhance the CWPO results. Both strategies resulted in higher mineralization and enhanced the consumption efficiency of H2O2 (ηH2O2). The sequential treatment using coagulation–flocculation and CWPO with PC catalyst showed the best results, achieving abatement of 94%, 70%, 98%, 93%, 31%, 96% and 95% for COD, TOC, BOD5, aromaticity, chlorides, turbidity and CN, respectively.
1. Introduction
Municipal solid waste (MSW) encompasses the waste generated mainly in households and commercial establishments. MSW has a heterogeneous composition depending on the local where it is collected, containing a significant fraction of organic materials (30–50%) [1]. In 2019, each European citizen generated 502 kg of MSW, and MSW generation is expected to amount to 3.4 billion tons by 2050 [2]. MSW can be managed in various ways, including landfilling, incineration, or mechanical and biological treatment (MBT) plants. Landfilling is the most harmful approach, as no pretreatment is performed, causing health-related risks, discarding profitable resources and leading to a landfill leachate [3].
MBT plants can be considered a sustainable alternative. In an MBT plant, the organic matter is separated from the collected waste and further treated by anaerobic digestion, generating three streams: biogas, a liquid stream (leachate water) and a solid stream. The solid stream is then sent to maturation, resulting in compost, commonly sold as a soil fertilizer. Composting from MSW has gradually increased in the EU, rising 278% between 1995 and 2019, from 14 to 53 million tons [2].
In a typical MBT plant, every 100 tons of undifferentiated waste results in 30 tons of compost and allows recovering 5400 kWh of energy, a technological solution aligned with a waste-to-energy perspective. Nonetheless, compost production surpasses its demand, resulting in its discharge at landfill sites. Under this background and applying the directives of end-of-waste criteria, alternatives to add value to compost must be sought, supported by the perspective of a circular economy. One possible strategy is to prepare sustainable, low-cost catalysts through the pyrolysis and hydrothermal carbonization (HTC) of compost [1,4].
As abovementioned, leachate waters are also generated in MBT facilities resulting in another issue faced by solid waste management companies. Leachate waters pose a significant threat to the environment. In opposition to leachate waters originating in landfills, there are few literature reports or information regarding the characteristics and subsequent treatment of leachate waters from MBT plants [5].
Leachate waters have a very complex matrix, composed of inorganic (e.g., metallic ions) and organic fractions, resulting in a high content of total organic carbon (TOC), chemical oxygen demand (COD) and five-day biological oxygen demand (BOD5). Furthermore, several ions, such as chlorides, carbonates or sulfates, can also be found, affecting its treatment performance [6,7]. Thus, the need to develop technologies suitable to handle such complex matrices emerges [8,9].
In this context, catalytic wet peroxide oxidation (CWPO) appears as a suitable alternative known to be a low-cost technology with promising results [10]. CWPO relies on the use of powerful oxidants, such as hydroxyl (HO•) and hydroperoxyl radicals (HOO•) obtained from the selective decomposition of H2O2 over a catalyst, to oxidize the organic pollutants in wastewaters. The process is conducted under mild operational conditions (T = 25–130 °C, P = 1–5 atm) [10,11,12]. It is widely known that pH and catalyst load are some of the most influential factors in treating complex matrices by CWPO [5,13,14].
As reported elsewhere [10,15], alkaline conditions result in a decrease in the performance of CWPO for about 50% compared to neutral or acidic conditions. Increasing the catalyst dosage is also expected to improve CWPO performance since higher catalyst doses allow more active sites to promote the degradation of organic matter in wastewaters [12]. On the other hand, excessive catalyst load can cause an agglomeration of catalyst particles, tending to decrease the exposed surface area of the active sites and impacting the efficiency of CWPO [16,17,18]. Low catalyst loads, H2O2 doses and mild operating conditions can be attained by developing stable, active and low-cost heterogeneous catalysts. Catalysts development is one of the main challenges to the industrial application of CWPO [12].
To enhance the performance of the chemical treatment of complex matrixes, such as for the leachate, an interesting strategy is to couple a pretreatment process with CWPO [8,15,19,20]. Coagulation–flocculation is a low-cost technology that can be operated with simple equipment and that has been used as a pretreatment step to remove part of non-biodegradable organic matter and colloidal particles from leachate waters. Ferrous sulfate, ferric chloride and aluminum sulfate are common coagulants [20,21,22]. The use of ion-exchange resins is another pretreatment option. They are usually applied to remove ammonia and metal ions, although their operational costs are higher than coagulation–flocculation [22,23].
Some works report the treatment of real leachate waters by chemical processes [11,16,17,24,25,26,27,28] or the synthesis and application of carbon-based materials in the CWPO of organic pollutants [1,29,30,31]. However, there is a lack of reports combining the application of low-cost carbon-based materials in the CWPO of real leachate wastewaters.
This work aims to assess solutions to treat highly concentrated leachate waters obtained in MBT facilities, exploring the efficiency of CWPO using sustainable, low-cost catalysts prepared from the mature compost obtained in MBT plants and two different pretreatments. Three approaches were evaluated in order to maximize the abatement of the organic content of the effluent: (i) CWPO as a single treatment, (ii) ion exchange resins coupled to CWPO and (iii) a coagulation–flocculation pretreatment step followed by CWPO.
2. Results and Discussion
2.1. Characterization of Catalysts
Table 1 summarizes the elemental composition of the raw precursor (RP) and of catalysts PC and HC. As observed, the elemental composition of PC and HC catalysts was not significantly changed during their preparation when compared to RP. However, the increase in the C/H ratio of the samples (9.2, 44.0 and 11.3 for RP, PC and HC samples, respectively) indicates that carbonization was successful [4]. Higher differences were found in the ashes content, representing inorganic substances in the RP and the catalysts. As observed, pyrolysis resulted in an increase of 26.0% in ashes (from 55.5% to 81.5% for RP and PC, respectively).

Table 1.
Elemental composition of the raw precursor (matured compost, RP) and of the catalysts PC and HC.
On the other hand, hydrothermal carbonization led to a decrease in the ashes content of 22.9% (from 55.5% to 32.6% for RP and HC, respectively). That result may be expected from pyrolysis and hydrothermal carbonization processes since, in general, pyrolysis results in the liberation of volatile carbon compounds from RP, increasing the content of inorganic species. In contrast, hydrothermal treatment in the liquid phase at high temperatures leads to the leaching of inorganic substances from RP [1,31].
It was observed that PC resulted in an increase in all metals analyzed (Mg, K, Si, Al, Ca and Fe) in comparison to the composition of the RP, with the exception of Na, which decreased in comparison with RP. Previous reports indicate that up to 37% of Na can suffer volatilization at temperatures of 800 °C [32]. In contrast, HC has a lower concentration of some metals (K, Na and Mg) in comparison to RP. However, for the case of some minerals (Si, Ca and Al), an enhancement in its concentration has been observed, which is in line with previous reports regarding the HTC of food and municipal waste at 250 °C [33].
These carbonization processes also result in materials with different acid–base properties, as shown in Table 2. On one hand, PC presents strong basicity (2.5 mmol g−1 and pHPZC = 11.0), likely due to the removal of acidic functionalities during pyrolysis and the higher ash content composed of alkali and alkaline-earth metals [4]. On the other hand, HC shows a neutral acid-based character (acidity similar to basicity and pHPZC = 7.5). Pyrolysis or HTC also leads to different surface areas (Table 2). HC and PC have surface areas (SBET) of 12 and 77 m2 g−1, respectively. This difference in the surface area according to the carbonization method was also reported by other authors [31].

Table 2.
Acid–base properties and BET surface area of the catalysts PC and HC.
2.2. CWPO of Leachate Waters as a Single Step
Table 3 summarizes the initial values of all parameters monitored in the experiments of this study, henceforth referred to with a subscript 0 (e.g., TOC0) since those were the values at the initial reaction time. As observed, the leachate water under study contained a high load of organic matter (26.8 g L−1 of TOC and 10.2 g L−1 of aromaticity), which resulted in a high chemical and biological oxygen demand (59.9 g L−1 of COD and 23.3 g L−1 of BOD5, respectively). In addition, the high concentration of chlorides (5.01 g L−1), which is a known HO• radical scavenger, can hinder the performance of CWPO [9,14]. The dose of H2O2 used in the CWPO tests was established as the stoichiometric amount of H2O2 required to theoretically oxidize all organic matter, which was determined from the COD value [34].

Table 3.
Physico-chemical characteristics of the raw leachate.
Figure 1 shows the performance of CWPO applied as a single treatment of the leachate water (without pretreatment) and the effect of the initial pH and catalyst load on CWPO, assessed at 3.0–6.0 and 3.6–7.2 g L−1, respectively, using the catalyst HC.
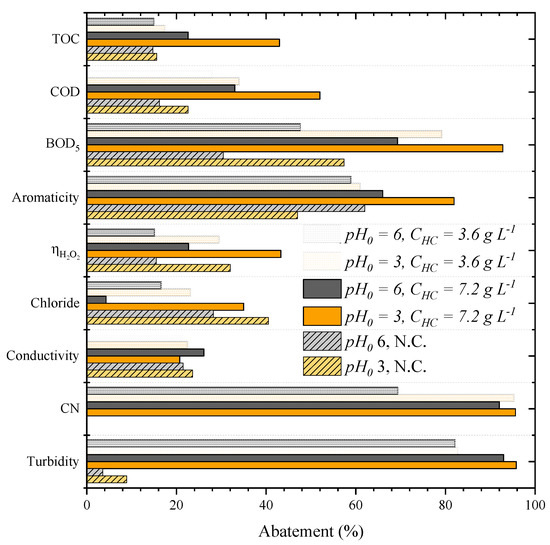
Figure 1.
Effects of the initial pH and HC catalyst load (CHC) on the CWPO of leachate water (Operating conditions: Vleachate = 25 mL, COD0 = 59.9 g L–1, CH2O2 = 85.7 g L–1 and 80 °C). N.C. = non-catalytic run, CN = color number.
The most acidic condition (pH0 = 3) resulted in a better CWPO performance at either catalyst load. For the load of 3.6 g L−1, the treatment at the lowest initial pH (pH0 = 3) resulted in the higher removal of COD and BOD5 (from 28% to 34% and from 47.7% to 80% at pH0 = 6 and 3, respectively). TOC and aromaticity abatement were the only parameters that experienced only a slight improvement at acidic pH (approximately 2% of enhancement for each). It is noteworthy that ηH2O2 duplicated at a more acidic condition, reaching 30%. The same phenomenon was observed for the catalyst load of 7.2 g L−1, meaning that the CWPO for this leachate water performed better at acid pH, in line with previous studies [24,34,35].
For the catalyst load, we observed that, at either pH0, a higher catalyst dosage led to a higher abatement of the studied parameters. For example, at pH0 3, when using 3.6 g L−1 of catalyst load, removals of organic matter, such as the TOC, COD, BOD5 and aromaticities of 17%, 34%, 79% and 61%, respectively, were obtained. When a catalyst load of 7.2 g L−1 was considered, a significant enhancement of the TOC, COD, BOD5 and aromaticity removals were observed (to 43%, 52%, 93% and 82%, respectively). The treatment conducted at pH0 3 and with the catalyst load of 7.2 g L−1 also reduced the turbidity and color number (CN) by more than 95% of its original value.
The considerable improvement in performance generated by the increase of catalyst load is also confirmed by the ηH2O2, reaching 43% of efficiency, almost three-times higher than the value obtained in the CWPO run carried out at pH0 6 and catalyst load of 3.6 g L−1. Similar results were also reported in other studies where the effect of catalyst concentration on CWPO efficiency was analyzed. Diaz de Tuesta et al. (2021) observed that increasing catalyst load from 0.5 g L−1 to 2.5 g L−1 at the same temperature (80 °C) increased caffeine removal by two times [4]. Similar results were observed for phenol abatement using a carbon black catalyst [12], an iron-based catalyst (Fe/γ-Al2O3) [13] and a gold-based catalyst (Au/AC) [36].
The individual effect of pH0 and catalyst load in the abatement of all parameters monitored in the CWPO process is evident. Furthermore, it is possible to observe that the effect was different for each parameter. For example, the variation in BOD5 and ηH2O2 were more affected by the pH than by the catalyst load. In addition, the effects of both parameters also showed a synergy for certain parameters since the abatement greatly increased, decreasing the pH at the highest catalyst load or increasing the catalyst load at the most acidic pH. That effect was observed for the TOC, COD and aromaticity, demonstrating the higher activity of the catalyst at lower pH. Therefore, pH0 of 3 and a catalyst load of 7.2 g L−1 were found to be the best condition chosen for the subsequent runs.
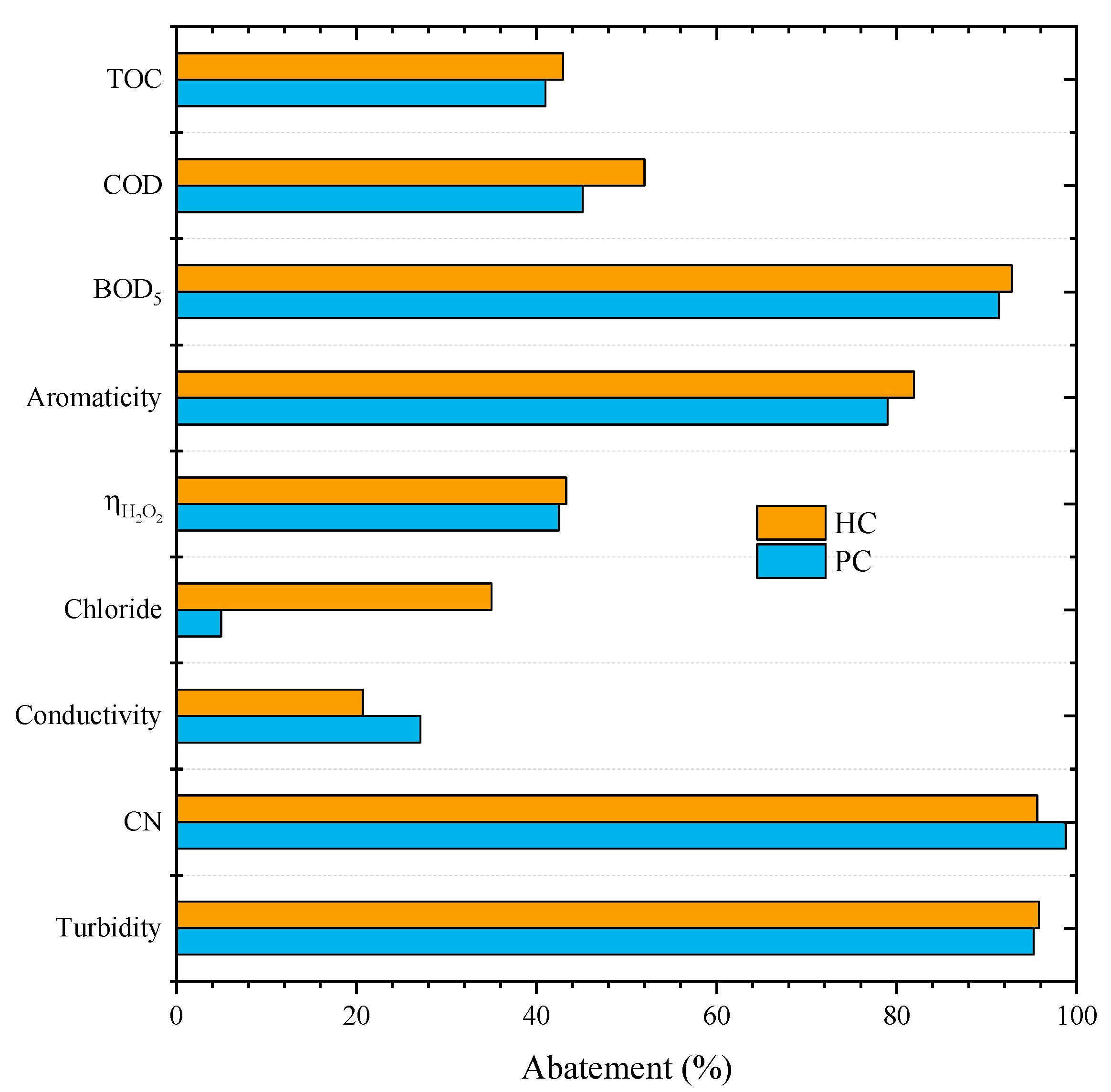
Figure 2 shows the results obtained for HC and PC at the selected conditions of pH0 of 3 and 7.2 g L−1 of catalyst load.
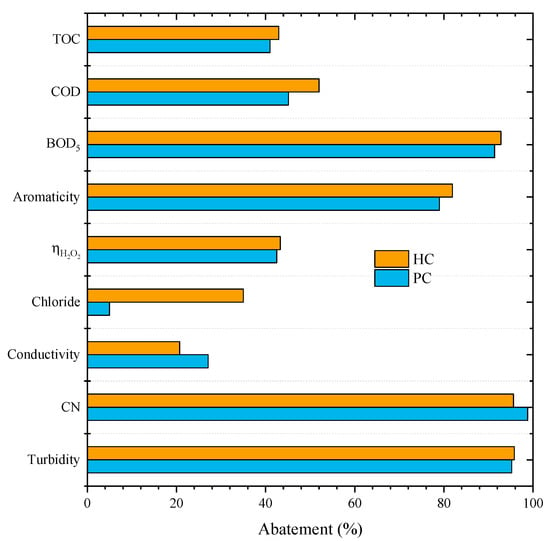
Figure 2.
Effects of the catalyst HC and PC on the CWPO of leachate water (Operating conditions: Vleachate = 25 mL, pH0 = 3, COD0 = 59.9 g L–1, CH2O2 = 85.7 g L–1, Ccatalyst = 7.2 g L−1 and 80 °C).
We observed that both catalysts led to similar conversions since removals of 43% and 41% for TOC, 52% and 45% for COD, 93% and 91% for BOD5, 82% and 79% for aromaticity, and 43% and 42% of ηH2O2 were obtained with HC and PC, respectively. Except for CN and conductivity, the CWPO with HC presented slightly higher removals when compared to PC. The removals achieved in those experiences can be ascribed to pure oxidation since adsorption tests were also conducted at the same operating conditions (pH0 = 3, COD0 = 59.9 g L–1, Ccatalyst = 7.2 g L−1, 80 °C), showing negligible removals (0% and 0.5% with PC and 0% and 2.1% with HC, for TOC and COD, respectively). The adsorption effect was expected to be low as neither of the catalysts has a very developed surface area (Table 2).
It is known that the activity of catalysts in CWPO strongly depends on the capacity of electron exchange of their active sites for the generation of hydroxyl and hydroperoxyl radicals from hydrogen peroxide decomposition and further oxidation of organic substances present in the aqueous matrix [37]. Several works have reported the role of oxygen-containing groups and related acid–base characteristics [38], residual metallic particles [38,39], defect sites [40], the presence of heteroatoms [41,42] and textural properties [43] in the abatement of different pollutants.
It is often difficult to isolate a single site as the main active one as, in most cases, the activity of those materials arises from a synergetic combination of numerous sites in the catalyst particle. In this work, materials that differ significantly in their acid–base character, in the amount of residual metallic particles and textural properties were synthesized, and the activity they displayed was similar, suggesting that a mixture of those different possible sites have relevant contributions to the activity revealed by those materials.
2.3. Ion Exchange Resin and CWPO
In order to enhance the removal of the organic content of the leachate water, a pretreatment with ion exchange resins is herein proposed. The leachate water obtained after the pretreatment was further subject to a CWPO treatment considering the conditions defined above (pH0 = 3 and catalyst load of 7.2 g L−1). Figure 3 shows the results obtained in the CWPO of the leachate water with and without pretreatment by CAResin, using HC and PC, to show the versatility of both catalysts. Since the pretreatment with the resins did not result in any COD removal, no adjustment regarding the dose of H2O2 was needed.
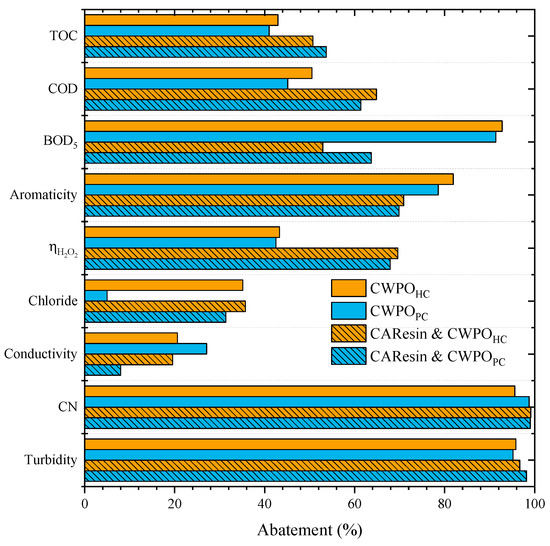
Figure 3.
Effects of the pretreatment of the leachate waters by sequential anion and cation exchange prior to CWPO (Operating conditions: CAResin- pH0 = 3 and 9.5, Cresin = 20 g L−1; CWPO- Vleachate = 25 mL, pH0 = 3, COD0 = 59.9 g L–1, CH2O2 = 85.7 g L–1, Ccatalyst = 7.2 g L−1 and 80 °C).
The combination of pretreatment by resin and subsequent CWPO proved to enhance the treatment of the leachate waters, mainly on the degradation of the organic matter. For example, in the runs conducted with HC catalyst, TOC abatement was enhanced from 43% to 51% and COD removal from 50% to 65%, respectively, for the CWPO single treatment run and the run using both CAResin and CWPO sequentially. However, aromaticity removal decreased from 82%, considering the CWPO single treatment, to 71% in the sequential resin and CWPO treatment, and BOD5 removal changed from 93% to 53%. This decrease in the BOD5 removal combined with an enhancement of COD abatement indicates that the CWPO after the treatment with CAResin enabled the degradation of a higher fraction of organic matter.
However, the resultant effluent cannot be treated biologically compared to the single CWPO treatment process. The main improvement can be noted in the efficiency of hydrogen peroxide consumption. While ηH2O2 reached 43% for the CWPO of leachate water, the ηH2O2 was 70% in the CWPO of the resin-pretreated effluent. The results may be ascribed to removing some compounds during the pretreatment that can act as radical scavengers [9,14]. The pretreatment with the CAResin aimed at minimizing the interference of some radical scavengers, such as bicarbonates and chlorides [5].
These radical scavengers consume hydroxyl radicals and hinder the oxidation of the organic content of the leachate [20]. It can be concluded that the objective was achieved, as in the CWPO reactions conducted with either PC or HC, an improvement in ηH2O2 was observed, which means that fewer parallel reactions other than organic matter oxidation took place. Since in a CWPO unit, H2O2 is the main operational cost [44], conditions that result in a more efficient decomposition of the oxidant source are considered a key step in developing suitable CWPO processes [44].
Chloride ions, conductivity, CN and turbidity abatements had no significant changes when comparing both approaches. Furthermore, no significant differences were found for the runs conducted either with HC or PC.
To the best of our knowledge, there is a lack of reports in the literature that study the combination of ion exchange resins and CWPO to treat leachates. In the work of Oloibiri et al. (2017) [22], the treatment of landfill leachate was performed combining three sequential techniques (coagulation–flocculation, activated carbon adsorption and ion exchange resin). The main purpose of the ion exchange step was the removal of nitrogen compounds as the last step of the treatment [22]. In this work, the ion exchange treatment was rather used as a pretreatment step to reduce the complexity of the leachate and to enhance the performance of CWPO.
Zamri et al. (2017) [23] evaluated the treatment of a stabilized leachate (COD average = 1.8 g L−1 and BOD5 average = 0.21 g L−1) using ion exchange resin. The main focus was the kinetic study and removing metallic ions, COD and NH3. They observed that a higher resin mass enhanced the removal of COD [23]. In this work, the leachate studied has an organic matter concentration noticeably higher (COD and BOD5 values are thirty- and ten-times higher, respectively).
2.4. Coagulation–Flocculation and CWPO
Another strategy to improve the removal of organic matter consists in the application of a coagulation–flocculation process as a pretreatment step before subjecting the effluent to CWPO. For this purpose, four tests considering different loads of coagulants (5 and 10 g L−1) and pH (7 and 8) were conducted. Table 4 summarizes the TOC and COD values obtained after those experiments. It is noteworthy that the experiments at pH 7 could not remove any COD of the leachate water. Considering a pH of 8, removals of 16% and 23% were obtained for coagulant concentrations of 5 and 10 g L−1, respectively.

Table 4.
COD and TOC values of the leachate water after the coagulation–flocculation treatment at different coagulant doses and pH conditions.
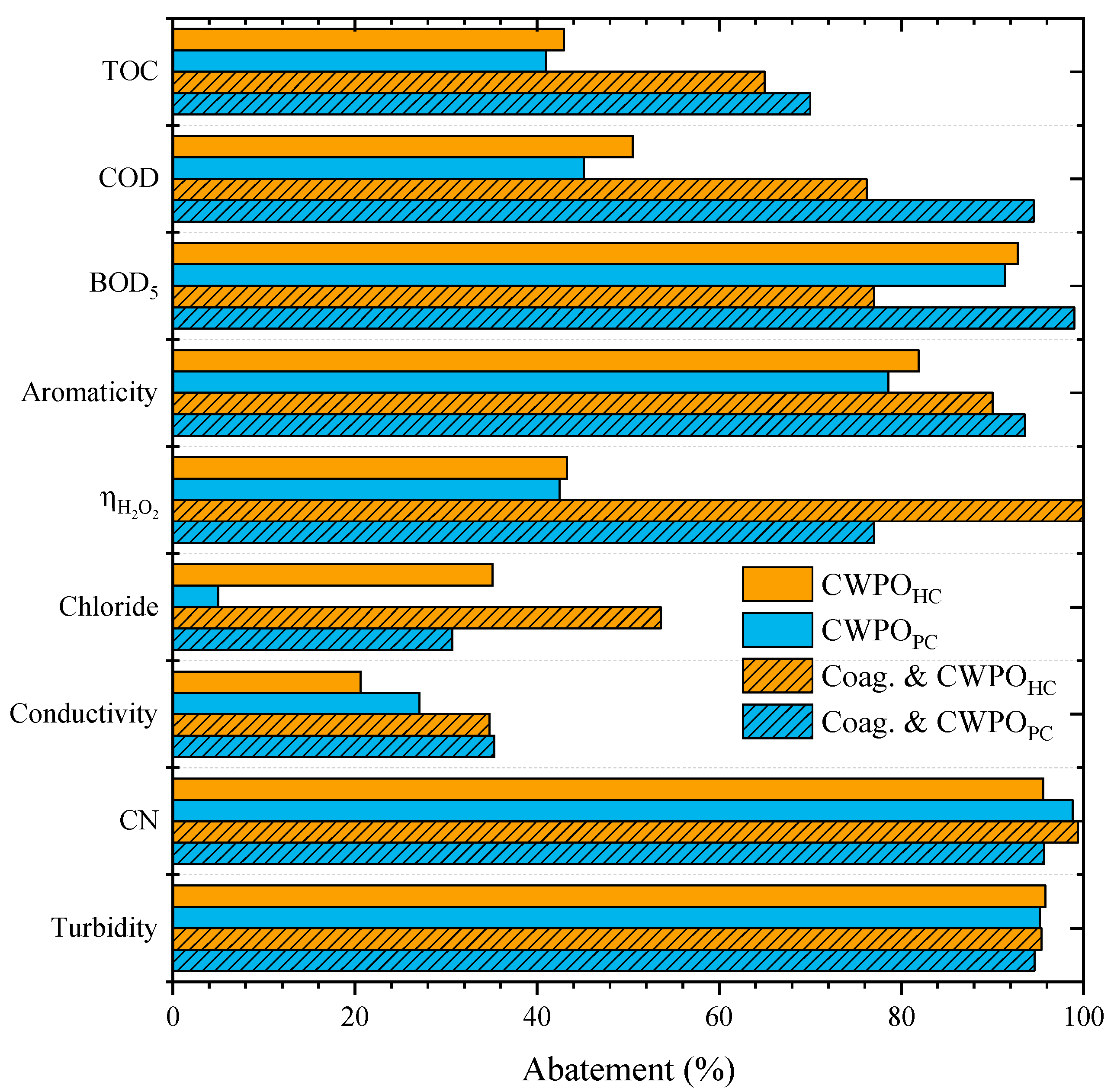
Accordingly, the operational condition that resulted in the leachate water with a lower COD value (pH of 8 and coagulant dosage of 10 g L−1) was chosen, and the effluent obtained was then subjected to a CWPO run at pH0 = 3 and a catalyst load of 7.2 g L−1 (for both HC and PC). The results are depicted in Figure 4. The dose of H2O2 needed was adjusted to the new COD value after coagulation–flocculation.
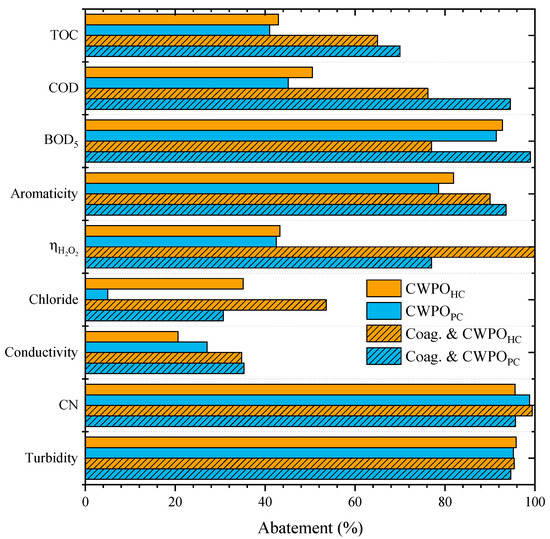
Figure 4.
Effects of the pretreatment of the leachate waters by sequential coagulation and flocculation prior to CWPO (Operating conditions: Coagulation–flocculation-COD0 = 59.9 g L–1, pH0 = 8, CAl2(SO4)3 = 10 g L–1; CWPO-Vleachate = 25 mL, pH0 = 3, COD0 = 46.2 g L–1, CH2O2 = 74.5 g L–1, Ccatalyst = 7.2 g L−1 and 80 °C).
The strategy of combining coagulation–flocculation with CWPO resulted in the highest removal of all analyzed parameters. As observed, the materials HC and PC displayed a very different catalytic activity in the CWPO of the effluent after the coagulation–flocculation treatment when compared to that obtained by single CWPO and by CAResin + CWPO treatments. The use of PC as the catalyst resulted in a higher overall abatement of most parameters when compared to the use of HC.
Overall, the highest mineralization obtained in this work was achieved when combining the coagulation–flocculation step followed by CWPO with PC as the catalyst. This combination increased the organic matter removal to above 70% for all parameters analyzed. The TOC, COD, BOD5 and aromaticity removals were 70%, 94%, 99% and 94%, respectively, proving the capacity of the proposed methodology to mineralize the organic matter of a heavily polluted leachate water. An enhancement was observed for ηH2O2 from 43% (single CWPO treatment process) and 68% (CAResin + CWPO) to 77% (coagulation–flocculation + CWPO).
In addition, it should be highlighted that the H2O2 dose needed for the CWPO of pretreated leachate waters by coagulation–flocculation is lower than in the single CWPO treatment since the amount of H2O2 added is related to the COD of the leachate, and COD decreased during the pretreatment by coagulation–flocculation. Thus, a smaller amount of H2O2 was required, and its consumption was more efficient. In the single CWPO treatment, the abatement of BOD5 was two-times higher than the decrease of COD, showing that most of the organic matter removal was of the biodegradable fraction. However, both BOD5 and COD parameters decreased above 90% (98% and 94%, respectively) by sequential coagulation–flocculation and CWPO treatments, highlighting that this strategy could degrade both non-biodegradable and biodegradable organic matter.
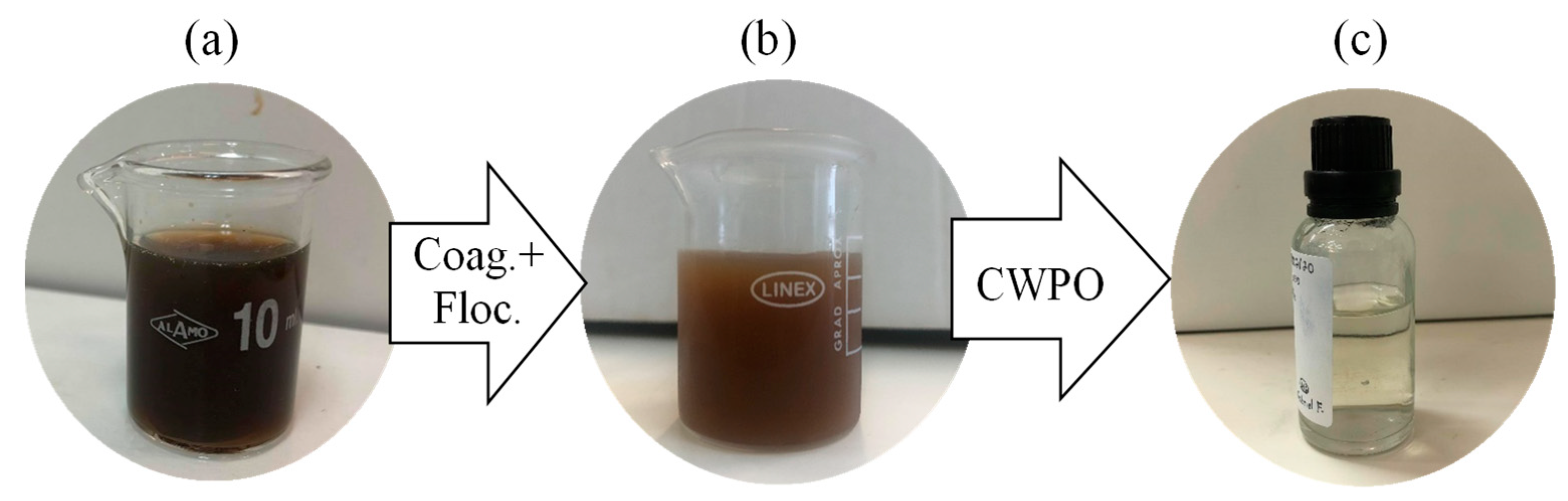
The removal of chloride content was also enhanced, from 5% (the lower abatement) to 36%. Turbidity and CN maintained a high abatement of 95% and 96%, respectively. The treatment was very efficient in removing the color of the effluent (Figure 5), which is crucial for the perception of treatment efficiency.
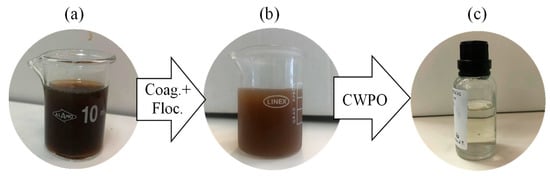
Figure 5.
Color removal after treatments. (a) Original leachate water, (b) after coagulation–flocculation (Coag. + Floc.) step and (c) after sequential coagulation–flocculation and Catalytic Wet Peroxide Oxidation (CWPO) treatment with PC catalyst.
When considering HC as the catalyst, the sequential coagulation–flocculation and CWPO also enhanced the abatement of all analysed parameters, except for BOD5. TOC removal increased from 43% to 65%, COD from 50% to 76% and aromaticity from 80% to 90%. BOD5 decreased from 93% to 77%. However, the most interesting result observed when applying HC is not related to the high mineralization of organic content but to the efficiency in the consumption of H2O2. For the single CWPO treatment, the ηH2O2 obtained was 43%, combining CAResin and CWPO with HC resulted in a ηH2O2 of 70%, and with the coagulation–flocculation treatment and CWPO, ηH2O2 reached ca. 100%, which means that all H2O2 consumed was used to remove the TOC.
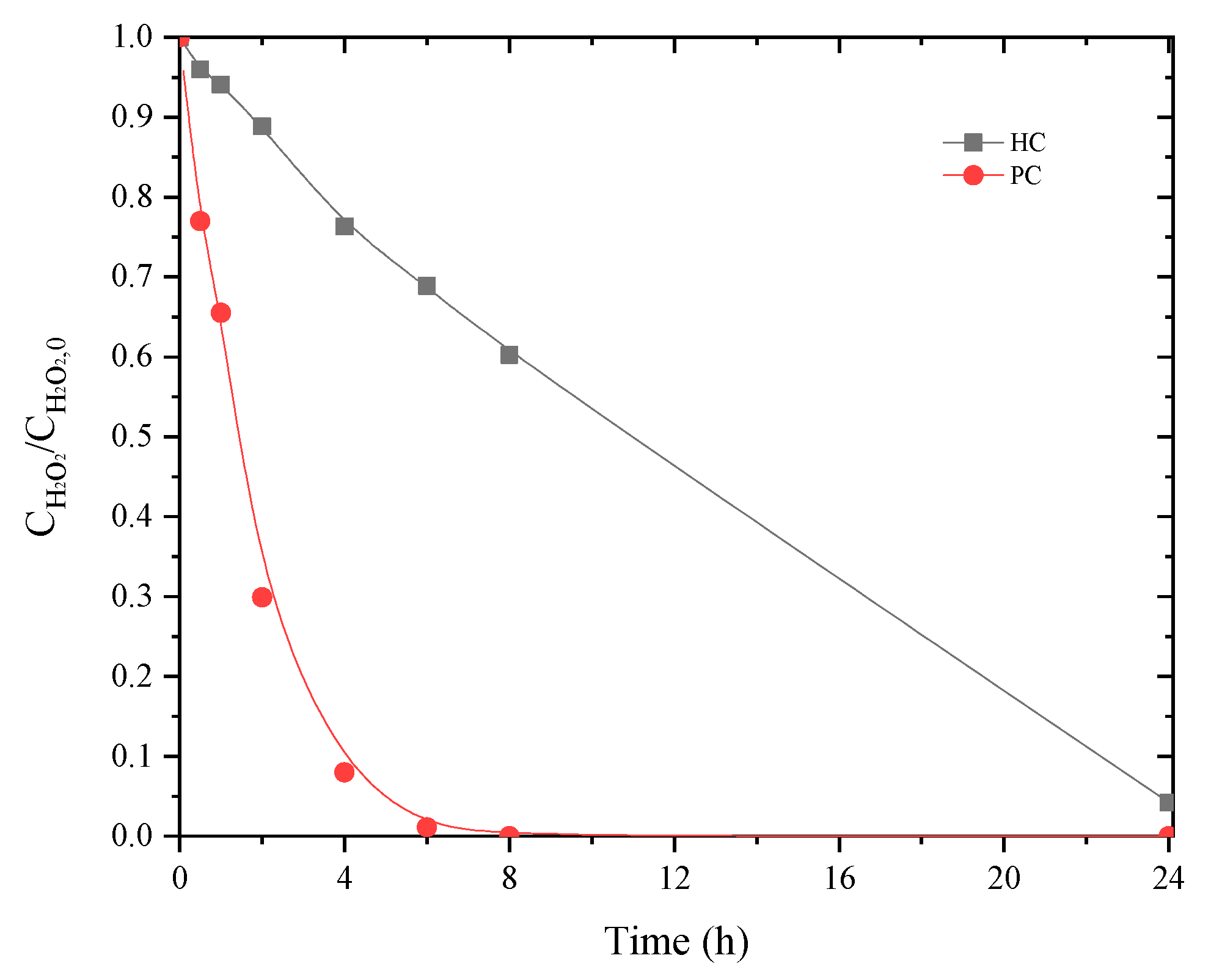
Although mineralization was not itself as high as when using PC, as mentioned before, efficient use of H2O2 may impact the cost of the CWPO unit [44]. The main difference observed with ηH2O2 when using PC or HC catalyst may be ascribed to the slower decomposition of H2O2 when applying HC (as shown in Figure 6). This may be ascribed to the basic character of PC (cf. Table 2) since basic catalysts are known to result in less efficient consumption of hydrogen peroxide for catalytic peroxidation processes [42]. Chlorides and conductivity also presented higher abatements, from 35% and 21% to 54% and 35%, respectively. Removals of turbidity and CN were maintained above 95%.
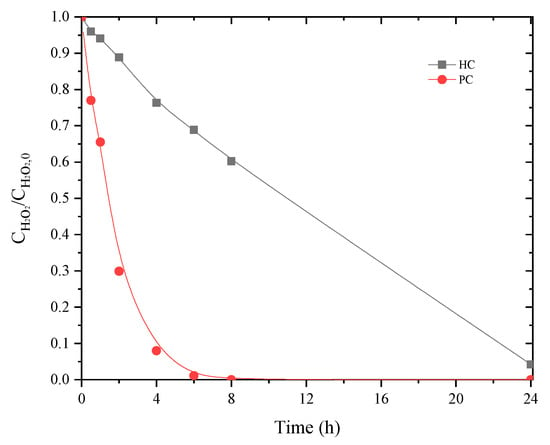
Figure 6.
Decomposition of hydrogen peroxide using PC and HC catalysts considering a single H2O2 addition at the beginning of the reaction. (Operating conditions: pH0 = 3, Ccatalyst = 1.6 g L−1, CH2O2 = 85.7 g L−1, 80 °C).
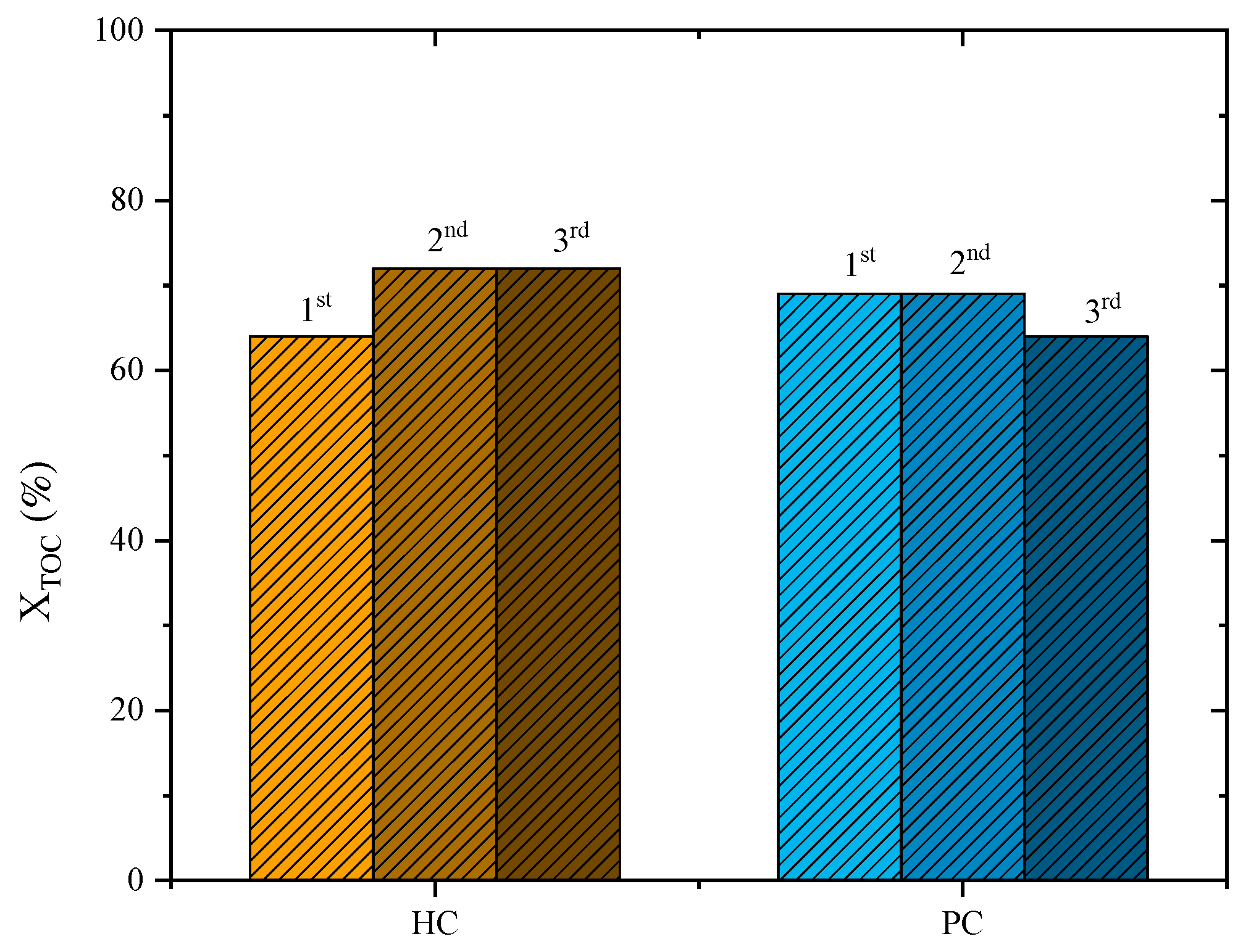
Preliminary tests regarding the reusability of the catalysts were performed, and the results are displayed in Figure 7. For the PC catalyst, no performance loss was observed after the second cycle of reuse (TOC removal of 69% for the first and second cycles). However, on the third cycle of reuse, a slight loss of performance was observed (TOC removal dropped to 64%). On the other hand, for the HC catalyst, an improvement in the abatement of TOC was observed in the second reuse cycle (72%) compared to the first cycle (63%), which was maintained for the third cycle (72%). This behaviour regarding an improvement in the catalytic activity of carbon-based materials was also observed in previous publications [12]. H2O2 promotes an oxidation of the carbon material, increasing its catalytic activity in further runs [12]. Those preliminary results reported here should prompt further studies into the reutilization of those materials.
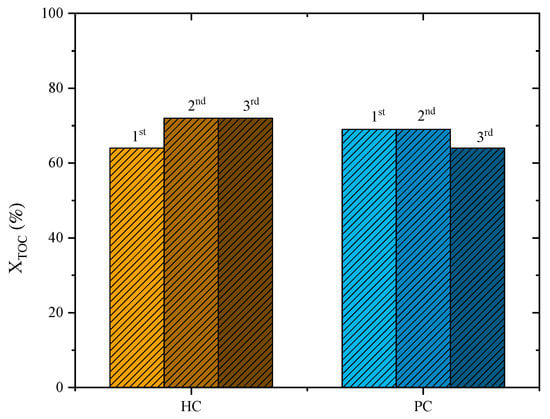
Figure 7.
TOC removal after reutilization runs with PC and HC catalysts for a leachate water previously treated by coagulation–flocculation (Operating conditions: pH0 = 3, COD0 = 46.2 g L–1, CH2O2 = 74.5 g L–1, Ccatalyst = 7.2 g L−1 and 80 °C).
Table 5 displays a comparison of some published works related to the treatment of real leachate waters using a combination of coagulation–flocculation and Advanced Oxidation Process (AOP). Although some works also resulted in high removals of COD (close to 90%), most reports are related to leachate waters with a much lower pollutant load (lower COD and BOD5) than the leachate water treated in this work. To the best of our knowledge, there is no other work dealing with the treatment of leachate waters with high concentrations of pollutants, as reported in this paper, using a combination of two low-cost technologies (coagulation–flocculation and CWPO), especially applying sustainable catalysts obtained from a waste source.

Table 5.
Publications related to the treatment of leachate waters combining a pre-treatment step and an advanced oxidation step.
By the end of their life, the sustainable catalyst produced in this work could be easily managed by being incorporated into construction materials [51,52], or, after thermal or hydrothermal treatments, they could be redirected for soil amendment purposes [53,54], energy recovery [53,55,56] or even for resource recovery [54], such as organic acids, alcohols and other nutrients.
3. Materials and Methods
3.1. Reagents and Materials
The compost and leachate waters used in this work were collected from an MBT plant for MSW located in the north of Portugal. The characteristics of the matured compost were reported in previous works [1,29]. The leachate water was filtered using an analytical paper filter of 25 µm in order to remove the suspended solids that would interfere with the analytical measurements. The leachate properties are summarized in Table 3.
Hydrogen peroxide (H2O2, 30% w/v) and sodium hydroxide (NaOH, 98.73%) were supplied by Fisher Chemical (Waltham, MA, USA). Sulfuric acid (H2SO4, 98%) was supplied by Labkem (Barcelona, Spain). Titanium (IV) oxysulfate (TiOSO4, 99.99 wt.% metal basis, c.a. 15 wt.% solution in dilute sulfuric acid) was supplied by Sigma-Aldrich (Steinhein, Germany). Silver nitrate (AgNO3 for analysis, ACS, ISO), mercury (II) sulfate (HgSO4, 99%), potassium dichromate (K2Cr2O7, 99.5%) and aluminum sulfate octadecahydrate (Al2(SO4)3·18H2O) were obtained from Panreac (Barcelona, Spain). Folin–Ciocalteu’s phenol reagent by Merck (Darmstadt, Germany). All reagents were used as received without further purification. Distilled water was used throughout the research.
Sigma-Aldrich and Alfa Aesar (Kandel, Germany), respectively, supplied Lewatit TP 207 and Amberlite IRA-402(Cl) ion resins.
3.2. Preparation and Characterization of Catalysts
Compost was first washed with distilled water (one liter per 100 g of compost) under strong stirring to homogenize the precursor and suspended solids removed by filtration. The recovered solid was dried overnight at 60 °C and later sieved to obtain particle sizes from 53 to 106 µm, resulting in the raw precursor (RP) used to prepare the catalysts.
Two catalysts were then prepared from RP using pyrolysis and HTC (hydrothermal carbonization) methodologies, resulting in pyrochar (PC) and hydrochar (HC), respectively. The PC catalyst was obtained by thermal treatment at 800 °C under N2 flow (100 Ncm3 min−1) for 4 h, following the procedure described elsewhere [29]. The HC material was obtained by heating the homogenized compost suspended in water (1 g L−1) at 230 °C for 2 h in an autoclave reactor following the procedure described elsewhere [1]. HC was then recovered, abundantly washed with distilled water and dried overnight at 60 °C.
Elemental analysis (EA) of PC and HC were performed in a Carlo Erba EA 1108 Elemental Analyzer (Egelsbach, Germany) in order to quantify their contents of C, H, N and S. For the determination of ashes, PC and HC catalysts were weighed before and after calcination at 800 °C during 4 h in a static air muffle. The composition of the ashes of RP, HC and PC were estimated by microwave-based acid digestion followed by Inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis (Jobin Yvon Activa M.) (Horiba Scientific, Kyoto, Japan).
BET surface areas (SBET) of PC and HC were determined from the analysis of N2 adsorption-desorption isotherms at 77 K, using a Quantachrome NOVA TOUCH LX4 adsorption analyzer (Anton Paar, Graz, Austria). Before determining the adsorption-desorption curves, PC and HC catalysts were degasified under vacuum at 120 °C for 16 h.
3.3. Treatment of the Leachate Waters
3.3.1. Ion-Exchange Resin
For the pretreatment with cationic and anionic ion exchange resins (CAResin), 150 mL of leachate with a pH previously adjusted to 9.5 (as producer recommendation) with a NaOH 1 M solution was mixed with 3.0 g of the cationic ion exchange resin (TP207) in an Erlenmeyer and stirred (240 rpm) for 48 h. Subsequently, the leachate was filtered and its pH adjusted to 3.0 (using an H2SO4 1 M solution) and treated with the anionic resin IRA-402(Cl), under the same conditions discussed above (150 mL of leachate, 3 g of resin, 240 rpm and 48 h of contact time).
3.3.2. Coagulation–Flocculation
Coagulation–flocculation tests were performed in Jar-Test equipment, using Al2(SO4)3 as the coagulant. Briefly, 400 mL of leachate was adjusted to pH = 7 or 8 (using NaOH and H2SO4 1 M solutions). Then, the adequate quantity of Al2(SO4)3 was dosed to reach concentrations of 5 or 10 g L−1 and the solution was stirred at 200 rpm for 5 min, at 20 rpm for 30 min and then left to rest for 5 h. Finally, the suspension was filtered under vacuum using a qualitative paper filter for further treatment by CWPO processes.
3.3.3. CWPO Experiments
The CWPO runs were conducted in a 500 mL glass round-bottom flask, equipped with a condenser, continuously stirred and submerged in a heated oil bath with temperature control. First, the pH of the leachate was adjusted to the desired value (3.0 or 6.0) employing H2SO4 and NaOH 1 M aqueous solutions. Then, 25 mL of the leachate water was transferred to the flask and heated to 80 °C (temperature selected according to previous works [4,35]). The addition of 30% hydrogen peroxide was split into five stepwise additions at 0, 60, 120, 180 and 240 min of reaction time (the same volume of H2O2 was dosed for each load), amounting to a concentration of 85.7 g L−1 (determined based on the theoretical amount needed to mineralize all COD [34]).
After adding the first hydrogen peroxide dose, the selected quantity of either PC or HC was loaded (Ccatalyst = 3.6 or 7.2 g L−1), setting this reaction time as t0 = 0 min. Several samples were then collected at selected reaction times to monitor the experiments for 24 h. At the end of the reaction, the catalyst was separated from the liquid phase by filtration with a membrane filter (0.45 μm). The recovered catalyst was dried in an oven at 60 °C overnight and reutilized without further treatments.
3.4. Analytical Techniques
TOC was determined using a Shimadzu TOC-L CSN analyzer (Kyoto, Japan). The H2O2, COD and aromaticity concentrations were determined by UV-VIS colorimetric methods (T70 UV/Vis Spectrometer, PG Instruments, Leicestershire, UK,) at the wavelengths of 405, 440 and 254 nm, respectively, following the methodology described elsewhere [5,34,57]. The interference of H2O2 in COD measurements was considered [58] to build a calibration curve to subtract the COD of H2O2 on COD measurements (R2 = 0.9935). The apparent COD value obtained (CODapp) was corrected as described in Equation (1).
COD (g L−1) = CODapp (g L−1) − 0.3305 CH2O2 (g L−1) + 12.387 g L−1
The BOD5 was determined by the standardized respirometric OxiTop method (WTW, Xylem, Weilheim, Germany), and the apparent obtained BOD5 (BOD5,app) was corrected using the theoretical interference of H2O2, using Equation (2) as given elsewhere [5].
BOD5 (g L−1) = BOD5,app (g L−1) − 0.4706 CH2O2 (g L−1)
The conductivity, pH and turbidity were measured at room temperature using WTW InoLab Cond Level 1, PHS-3BW Bench TOP pH/mV/°C meter (Bante Instruments, Shanghai, China) and WTW Turb 550 equipment (Xylem, Weilheim, Germany), respectively. The concentration of chlorides was determined using potassium chromate as an indicator by Mohr titration with silver nitrate. The color number (CN) was determined by a UV-VIS methodology, following the methodology described elsewhere [59,60]. Briefly, each spectral absorption coefficient (SACi) was determined by absorption (Absi) at the selected wavelengths of 436, 525 and 620 nm (Equation (3)), where x stands for the path length of the cuvette (0.01 m). The color number was then determined from the SACs as detailed in Equation (4).
The efficiency of H2O2 consumption (ƞH2O2) was calculated considering the amount of H2O2 necessary to oxidize 1 mol of carbon, as described in a previous work related to CWPO [44] (Equation (5)).
where CTOC,0 and CH2O2,0 are the initial molar concentrations of total organic carbon and hydrogen peroxide, respectively, CTOC and CH2O2 are those concentrations at the end of the treatment, and XTOC is the conversion of TOC.
4. Conclusions
The combination of physicochemical pretreatments with CWPO, using low-cost sustainable catalysts prepared from matured compost, proved to be a viable alternative for the treatment of real leachate waters containing high organic pollutant concentration (TOC = 27 g L−1, COD = 60 g L−1, BOD5 = 23 g L−1 and aromaticity = 10 g L−1). The CWPO treatment of the leachate water was strongly influenced by the pH and catalyst load, both parameters showing a synergy that led to obtaining higher removals at the minimal pH tested (T = 80 °C, pH0 = 3).
The leachate water may be treated directly by CWPO obtaining removals of 43%, 52%, 93% and 82% for TOC, COD, BOD5 and aromaticity, respectively, at T = 80 °C, pH0 = 3, Ccatalyst = 7.2 g L−1 and using the stoichiometric dose of hydrogen peroxide for the mineralization of COD (CH2O2 = 85.7 g L−1).
The sequential treatment of the leachate by ion-exchange resin or coagulation–flocculation followed by CWPO enhanced the removal of organic matter and improved the efficiency of H2O2 consumption in the latter. The highest mineralization of organic matter was obtained by sequential treatment of coagulation–flocculation pretreatment at pH 8 using Al2(SO4)3 = 10 g L−1, followed by CWPO (at the conditions as mentioned earlier) using the catalyst obtained from pyrolysis (PC), resulting, respectively, in TOC, COD, BOD5 and aromaticity removals of 70%, 95%, 99% and 94%.
On the other hand, when applying HC as the catalyst after pretreatment by coagulation–flocculation, the best result in terms of the efficiency in degrading H2O2 was achieved (ca. 100% efficiency), likely due to a more neutral character of this catalyst. Using either PC or HC as catalysts, an efficient color abatement was observed, which is a particularly important parameter for the perception of the quality of treatment and the final effluent. Preliminary tests regarding the reusability of the catalysts were performed, and no evident loss of performance was observed, although further studies should be conducted.
Author Contributions
Conceptualization, F.F.R., J.L.D.d.T. and H.T.G.; methodology, F.F.R. and J.L.D.d.T.; investigation, G.d.F.B. and F.F.R.; resources, P.P. and H.T.G.; writing—original draft preparation, G.d.F.B. and F.F.R.; writing—review and editing, J.L.D.d.T., R.V.M. and H.T.G.; visualization, G.d.F.B., F.F.R. and J.L.D.d.T.; supervision, J.L.D.d.T., R.V.M. and H.T.G.; project administration, H.T.G.; funding acquisition, H.T.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by project “VALORCOMP-Valorización de compost y otros desechos procedentes de la fracción orgánica de los residuos municipales”, with reference 0119_VALORCOMP_2_P, through FEDER under Program INTERREG; Base Funding-UIDB/50020/2020 of the Associate Laboratory LSRE-LCM-funded by national funds through FCT/MCTES (PIDDAC); CIMO (UIDB/00690/2020) through FEDER under Program PT2020 and national funding by FCT, Foundation for Science and Technology and European Social Fund, FSE, through the individual research grant SFRH/BD/143224/2019 of Fernanda Fontana Roman.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roman, F.F.; de Tuesta, J.L.D.; Praça, P.; Silva, A.M.; Faria, J.L.; Gomes, H.T. Hydrochars from compost derived from municipal solid waste: Production process optimization and catalytic applications. J. Environ. Chem. Eng. 2021, 9, 104888. [Google Scholar] [CrossRef]
- Eurostat—Statistics Explained. Municipal Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Municipal_waste_statistics (accessed on 3 November 2019).
- Wei, Y.; Li, J.; Shi, D.; Liu, G.; Zhao, Y.; Shimaoka, T. Environmental challenges impeding the composting of biodegradable municipal solid waste: A critical review. Resour. Conserv. Recycl. 2017, 122, 51–65. [Google Scholar] [CrossRef]
- De Tuesta, J.L.D.; de Almeida, F.V.; Oliveira, J.R.; Praça, P.; Guerreiro, M.C.; Gomes, H.T. Kinetic insights on wet peroxide oxidation of caffeine using EDTA-functionalized low-cost catalysts prepared from compost generated in municipal solid waste treatment facilities. Environ. Technol. Innov. 2021, 24, 101984. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Rodrigues, R.O.; Silva, A.M.; Tavares, P.B.; Carvalho, A.M.; Figueiredo, J.L.; Faria, J.; Gomes, H.T. Hybrid magnetic graphitic nanocomposites towards catalytic wet peroxide oxidation of the liquid effluent from a mechanical biological treatment plant for municipal solid waste. Appl. Catal. B Environ. 2017, 219, 645–657. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total Environ. 2020, 703, 135468. [Google Scholar] [CrossRef]
- Gautam, P.; Kumar, S.; Lokhandwala, S. Advanced oxidation processes for treatment of leachate from hazardous waste landfill: A critical review. J. Clean. Prod. 2019, 237, 117639. [Google Scholar] [CrossRef]
- Ishak, A.R.; Hamid, F.S.; Mohamad, S.; Tay, K.S. Removal of organic matter from stabilized landfill leachate using Coagulation-Flocculation-Fenton coupled with activated charcoal adsorption. Waste Manag. Res. 2017, 35, 739–746. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Rueda Márquez, J.J.; Levchuk, I.; Sillanpää, M. Application of Catalytic Wet Peroxide Oxidation for Industrial and Urban Wastewater Treatment: A Review. Catalysts 2018, 8, 673. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.; Figueiredo, J.; Faria, J.; Gomes, H.T. Catalytic wet peroxide oxidation: A route towards the application of hybrid magnetic carbon nanocomposites for the degradation of organic pollutants. A review. Appl. Catal. B Environ. 2016, 187, 428–460. [Google Scholar] [CrossRef]
- De Tuesta, J.D.; Quintanilla, A.; Casas, J.; Rodriguez, J. Kinetic modeling of wet peroxide oxidation with a carbon black catalyst. Appl. Catal. B Environ. 2017, 209, 701–710. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. Highly stable Fe/γ-Al2O3 catalyst for catalytic wet peroxide oxidation. J. Chem. Technol. Biotechnol. 2011, 86, 497–504. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Q.; Hua, T. Removal of Organic Matter from Landfill Leachate by Advanced Oxidation Processes: A Review. Int. J. Chem. Eng. 2010, 2010, 270532. [Google Scholar] [CrossRef]
- Torretta, V.; Ferronato, N.; Katsoyiannis, I.A.; Tolkou, A.K.; Airoldi, M. Novel and Conventional Technologies for Landfill Leachates Treatment: A Review. Sustainability 2016, 9, 9. [Google Scholar] [CrossRef]
- Galeano, L.A.; Vicente, M.Á.; Gil, A. Treatment of municipal leachate of landfill by Fenton-like heterogeneous catalytic wet peroxide oxidation using an Al/Fe-pillared montmorillonite as active catalyst. Chem. Eng. J. 2011, 178, 146–153. [Google Scholar] [CrossRef]
- Sruthi, T.; Gandhimathi, R.; Ramesh, S.; Nidheesh, P. Stabilized landfill leachate treatment using heterogeneous Fenton and electro-Fenton processes. Chemosphere 2018, 210, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Mena, I.F.; Diaz, E.; Rodriguez, J.J.; Mohedano, A.F. CWPO of bisphenol A with iron catalysts supported on microporous carbons from grape seeds activation. Chem. Eng. J. 2017, 318, 153–160. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Pang, Y.; Li, X.; Chen, F.; Liao, X.; Lei, M.; Song, Y. Landfill leachate treatment by coagulation/flocculation combined with microelectrolysis-Fenton processes. Environ. Technol. 2019, 40, 1862–1870. [Google Scholar] [CrossRef]
- Bakraouy, H.; Souabi, S.; Digua, K.; Dkhissi, O.; Sabar, M.; Fadil, M. Optimization of the treatment of an anaerobic pretreated landfill leachate by a coagulation–flocculation process using experimental design methodology. Process Saf. Environ. Prot. 2017, 109, 621–630. [Google Scholar] [CrossRef]
- Oloibiri, V.; De Coninck, S.; Chys, M.; Demeestere, K.; Van Hulle, S.W. Characterisation of landfill leachate by EEM-PARAFAC-SOM during physical-chemical treatment by coagulation-flocculation, activated carbon adsorption and ion exchange. Chemosphere 2017, 186, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Zamri, M.F.M.A.; Kamaruddin, M.A.; Yusoff, M.S.; Aziz, H.A.; Foo, K.Y. Semi-aerobic stabilized landfill leachate treatment by ion exchange resin: Isotherm and kinetic study. Appl. Water Sci. 2017, 7, 581–590. [Google Scholar] [CrossRef]
- Niveditha, S.; Gandhimathi, R. Flyash augmented Fe3O4 as a heterogeneous catalyst for degradation of stabilized landfill leachate in Fenton process. Chemosphere 2020, 242, 125189. [Google Scholar] [CrossRef] [PubMed]
- Orkun, M.O.; Kuleyin, A. Treatment performance evaluation of chemical oxygen demand from landfill leachate by electro-coagulation and electro-fenton technique. Environ. Prog. Sustain. Energy 2012, 31, 59–67. [Google Scholar] [CrossRef]
- Moravia, W.G.; Amaral, M.C.; Lange, L.C. Evaluation of landfill leachate treatment by advanced oxidative process by Fenton’s reagent combined with membrane separation system. Waste Manag. 2013, 33, 89–101. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, H.J.; Huang, C.-P. Optimization of Fenton process for the treatment of landfill leachate. J. Hazard. Mater. 2005, 125, 166–174. [Google Scholar] [CrossRef]
- Tizaoui, C.; Mansouri, L.; Bousselmi, L. Ozone catalysed with solids as an advanced oxidation process for landfill leachate treatment. Water Sci. Technol. 2007, 55, 237–243. [Google Scholar] [CrossRef]
- De Tuesta, J.L.D.; Pantuzza, G.F.; Silva, A.M.T.; Praça, P.; Faria, J.L.; Gomes, H.T. Catalysts Prepared with Matured Compost Derived from Mechanical-Biological Treatment Plants for the Wet Peroxide Oxidation of Pollutants with Different Lipophilicity. Catalysts 2020, 10, 1243. [Google Scholar] [CrossRef]
- Gomes, H.; Miranda, S.; Sampaio, M.J.; Silva, A.; Faria, J. Activated carbons treated with sulphuric acid: Catalysts for catalytic wet peroxide oxidation. Catal. Today 2010, 151, 153–158. [Google Scholar] [CrossRef]
- de Tuesta, J.L.D.; Saviotti, M.C.; Roman, F.F.; Pantuzza, G.F.; Sartori, H.J.; Shinibekova, A.; Kalmakhanova, M.S.; Massalimova, B.K.; Pietrobelli, J.M.; Lenzi, G.G.; et al. Assisted hydrothermal carbonization of agroindustrial byproducts as effective step in the production of activated carbon catalysts for wet peroxide oxidation of micro-pollutants. J. Environ. Chem. Eng. 2021, 9, 105004. [Google Scholar] [CrossRef]
- Wang, C.; Jin, X.; Wang, Y.; Yan, Y.; Cui, J.; Liu, Y.; Che, D. Release and Transformation of Sodium during Pyrolysis of Zhundong Coals. Energy Fuels 2014, 29, 78–85. [Google Scholar] [CrossRef]
- Smith, A.; Singh, S.; Ross, A.B. Fate of inorganic material during hydrothermal carbonisation of biomass: Influence of feedstock on combustion behaviour of hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef]
- Huaccallo-Aguilar, Y.; de Tuesta, J.D.; Álvarez-Torrellas, S.; Gomes, H.; Larriba, M.; Ovejero, G.; García, J. New insights on the removal of diclofenac and ibuprofen by CWPO using a magnetite-based catalyst in an up-flow fixed-bed reactor. J. Environ. Manag. 2021, 281, 111913. [Google Scholar] [CrossRef] [PubMed]
- De Tuesta, J.L.D.; Quintanilla, A.; Casas, J.A.; Morales-Torres, S.; Faria, J.L.; Silva, A.M.; Gomes, H.T. The pH effect on the kinetics of 4-nitrophenol removal by CWPO with doped carbon black catalysts. Catal. Today 2020, 356, 216–225. [Google Scholar] [CrossRef]
- Domínguez, C.; Quintanilla, A.; Casas, J.A.; Rodriguez, J. Kinetics of wet peroxide oxidation of phenol with a gold/activated carbon catalyst. Chem. Eng. J. 2014, 253, 486–492. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Rey, A.; Faraldos, M.; Bahamonde, A.; Casas, J.A.; Zazo, J.A.; Rodríguez, J.J. Role of the Activated Carbon Surface on Catalytic Wet Peroxide Oxidation. Ind. Eng. Chem. Res. 2008, 47, 8166–8174. [Google Scholar] [CrossRef]
- Pinho, M.T.; Ribeiro, R.S.; Gomes, H.T.; Faria, J.L.; Silva, A.M.T. Screening of Activated Carbons for the Treatment of Highly Concentrated Phenol Solutions Using Catalytic Wet Peroxide Oxidation: The Effect of Iron Impurities on the Catalytic Activity. Catalysts 2020, 10, 1318. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Liu, P.; Yu, Y.; Zhao, Y.; Li, X.; Jiang, W.; Wang, J.; Yang, X.; Sun, C. Effect of structural defects on activated carbon catalysts in catalytic wet peroxide oxidation of m-cresol. Catal. Today 2015, 258, 120–131. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Ribeiro, R.S.; Machado, B.F.; Serp, P.; Morales-Torres, S.; Silva, A.M.T.; Figueiredo, J.; Faria, J.L.; Gomes, H.T. Role of Nitrogen Doping on the Performance of Carbon Nanotube Catalysts: A Catalytic Wet Peroxide Oxidation Application. ChemCatChem 2016, 8, 2068–2078. [Google Scholar] [CrossRef]
- De Tuesta, J.D.; Quintanilla, A.; Casas, J.; Rodriguez, J. P-, B- and N-doped carbon black for the catalytic wet peroxide oxidation of phenol: Activity, stability and kinetic studies. Catal. Commun. 2017, 102, 131–135. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.; Pinho, M.T.; Figueiredo, J.L.; Faria, J.; Gomes, H.T. Development of glycerol-based metal-free carbon materials for environmental catalytic applications. Catal. Today 2015, 240, 61–66. [Google Scholar] [CrossRef]
- De Tuesta, J.L.D.; Quintanilla, A.; Moreno, D.; Ferro, V.R.; Casas, J.A. Simulation and Optimization of the CWPO Process by Combination of Aspen Plus and 6-Factor Doehlert Matrix: Towards Autothermal Operation. Catalysts 2020, 10, 548. [Google Scholar] [CrossRef]
- Vedrenne, M.; Vasquez-Medrano, R.; Prato, D.; Frontana-Uribe, B.A.; Ibanez, J.G. Characterization and detoxification of a mature landfill leachate using a combined coagulation–flocculation/photo Fenton treatment. J. Hazard. Mater. 2012, 205-206, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gu, Z.; Wen, P.; Li, Q. Degradation of refractory organic contaminants in membrane concentrates from landfill leachate by a combined coagulation-ozonation process. Chemosphere 2019, 217, 411–422. [Google Scholar] [CrossRef]
- Amor, C.; De Torres-Socías, E.; Peres, J.; Maldonado, M.I.; Oller, I.; Malato, S.; Lucas, M.S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, W.; Shi, P.; Guo, J.; Cheng, J. Characterization of dissolved organic matter in landfill leachate during the combined treatment process of air stripping, Fenton, SBR and coagulation. Waste Manag. 2015, 41, 111–118. [Google Scholar] [CrossRef]
- Segundo, I.D.B.; Gomes, A.I.; Souza-Chaves, B.M.; Park, M.; dos Santos, A.B.; Boaventura, R.A.; Moreira, F.C.; Silva, T.F.; Vilar, V.J. Incorporation of ozone-driven processes in a treatment line for a leachate from a hazardous industrial waste landfill: Impact on the bio-refractory character and dissolved organic matter distribution. J. Environ. Chem. Eng. 2021, 9, 105554. [Google Scholar] [CrossRef]
- Tejera, J.; Miranda, R.; Hermosilla, D.; Urra, I.; Negro, C.; Blanco, Á. Treatment of a Mature Landfill Leachate: Comparison between Homogeneous and Heterogeneous Photo-Fenton with Different Pretreatments. Water 2019, 11, 1849. [Google Scholar] [CrossRef]
- Muthulakshmi, S.; Uma, S.; Hemalatha, G. Feasibility study on compost as partial replacement for fine aggregate in concrete. Mater. Today Proc. 2021, 46, 3775–3778. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Corpas-Iglesias, F.A.; Pérez-Villarejo, L.; Iglesias-Godino, F.J. Recycling of sawdust, spent earth from oil filtration, compost and marble residues for brick manufacturing. Constr. Build. Mater. 2012, 34, 275–284. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Halder, P.; Ratnnayake, N.; Marzbali, M.H.; Aktar, S.; Selezneva, E.; Paz-Ferreiro, J.; Surapaneni, A.; de Figueiredo, C.C.; et al. A critical literature review on biosolids to biochar: An alternative biosolids management option. Rev. Environ. Sci. Bio/Technol. 2020, 19, 807–841. [Google Scholar] [CrossRef]
- Munir, M.T.; Mansouri, S.S.; Udugama, I.A.; Baroutian, S.; Gernaey, K.V.; Young, B.R. Resource recovery from organic solid waste using hydrothermal processing: Opportunities and challenges. Renew. Sustain. Energy Rev. 2018, 96, 64–75. [Google Scholar] [CrossRef]
- Basso, D.; Weiss-Hortala, E.; Patuzzi, F.; Castello, D.; Baratieri, M.; Fiori, L. Hydrothermal carbonization of off-specification compost: A byproduct of the organic municipal solid waste treatment. Bioresour. Technol. 2015, 182, 217–224. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.; Pastrana-Martínez, L.M.; Figueiredo, J.; Faria, J.; Gomes, H. Graphene-based materials for the catalytic wet peroxide oxidation of highly concentrated 4-nitrophenol solutions. Catal. Today 2015, 249, 204–212. [Google Scholar] [CrossRef]
- De Tuesta, J.L.D.; García-Figueruelo, C.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Application of high-temperature Fenton oxidation for the treatment of sulfonation plant wastewater. J. Chem. Technol. Biotechnol. 2015, 90, 1839–1846. [Google Scholar] [CrossRef]
- Tizaoui, C.; Bousselmi, L.; Mansouri, L.; Ghrabi, A. Landfill leachate treatment with ozone and ozone/hydrogen peroxide systems. J. Hazard. Mater. 2007, 140, 316–324. [Google Scholar] [CrossRef]
- Krull, R.; Döpkens, E. Recycling of dyehouse effluents by biological and chemical treatment. Water Sci. Technol. 2004, 49, 311–317. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).